Perivascular adipose tissue (PVAT) is a local fat depot surrounding most blood vessels. “Outside-to-inside” signaling from PVAT to the underlying vessel wall actively contributes to vascular homeostasis, as well as to pathogenesis of cardiovascular diseases including hypertension, arterial stiffness, atherosclerosis, neointima formation and aneurysm.1–3 Crosstalk between PVAT and blood vessels happens partly through paracrine effects of adipokines, such as adiponectin, leptin, IGFBP2 (insulin like growth factor binding protein 2), as well as inflammatory cytokines, which may act on the vascular wall to induce smooth muscle cell (SMC) and endothelial cell proliferation, migration and differentiation. In addition, PVAT produces vessel contracting and relaxing factors to regulate vascular tone and affect blood pressure.4, 5 Mouse thoracic PVAT shares most of the defined characteristics of brown adipose tissue, including cellular morphology and expression of thermogenic genes.6–9 Its distinctive function in thermogenesis, in response to cold environment or β3-adrenergic receptor agonists, is critical to maintaining blood and body temperature.1, 6 Additionally, as seen in other adipose depots, PVAT undergoes a “whitening” process under obese or thermoneutral conditions, while circulating hormones from other tissues such as irisin, FGF21(fibroblast growth factor 21) or ANP (atrial natriuretic peptide) etc. might convert “whitening” PVAT back to “brown-like” PVAT.10–12 Crosstalk between PVAT and blood vessels also happens through trans-differentiation of cells in the vessel wall. We reported that PVAT may share the same precursors with SMCs in blood vessels.6 Consistent with our findings, Long et al reported that beige adipocytes expressed a smooth muscle-like gene signature while ectopic expression PRDM16 (PR/SET domain 16) converts bona fide vascular SMCs into brown-like adipocytes in vitro.13 However, it remains unknown which groups of cells in the blood vessels will differentiate into adipocytes or other cell types and which are the specific stimuli driving the process in each case. A recent study suggests that PVAT harbors multipotent mesenchymal stem cells (MSCs) capable of differentiating into multiple cell types, including cells from the adipogenic, osteogenic and chondrogenic lineages.14 Whether the MSCs in PVAT play any role in vascular development and remodeling remained to be addressed, largely due to technical limitations imposed by their small numbers.
The assessment of the single-cell transcriptome through single-cell RNA sequencing (scRNA-seq) provides us the opportunity to reveal and directly visualize the heterogeneity of gene expression at the single cell resolution in certain organs and tissues.15, 16 For example, Xiong et al uncovered disruptions of the vascular signaling network during nonalcoholic steatohepatitis using single cell secretome gene analysis;17 Cochain and Winkels et al revealed the transcriptional landscape of aortic immune cells in atherosclerosis.18, 19 Furthermore, regarding the power of this emerging technology is highlighted by the finding one sub-type of disease associated macrophages, TREM2 (triggering receptor expressed on myeloid cells 2) high expressing macrophages in the atherosclerotic plaque, fatty liver, obese adipose tissues and central nervous system with neurodegenerative disease.19–21 Therefore, monitoring and targeting this particular macrophage sub-type may help better understand the cellular heterogeneity responsible for the development of diseases and aid in development of new treatments.
Pan and colleagues demonstrated last year that there are resident PVAT stromal cells (PVASCs) in mouse thoracic PVAT.22 Through scRNA-seq analysis of primary cultured PVASCs (with >80% cells Sca1+/CD90+/CD146+/CD29+), they detected enormous transcriptional heterogeneity in PVASCs. There are 10 to 12 distinct sub-populations, including adipogenic, endothelial, epithelial, neural and cardiomyocyte lineages, in PVASCs both from young and old mice. Gene ontology (GO) analysis revealed that pathways related to vasculature development were enriched in PVASCs, which suggests the PVASCs may play a critical role in vascular remodeling. In addition, they identified and characterized the human PVASCs (hPVASCs) with a similar strategy. Using the mouse carotid artery ligation model, transplanted hPVASCs were found to be involved in vascular remodeling and neointima formation. In this ATVB issue, Gu and colleagues used multiple methods providing unique direct evidences that mesenchymal stem cells (CD29+/Sca1+/PLIN1−/PECAM1−) reside in the PVAT, hence called perivascular adipose tissue-derived mesenchymal stem cells (PV-ADSCs), and that they contribute to vascular remodeling through migration and SMC differentiation. This work, for the first time, demonstrated the existence of PV-ADSCs by performing the scRNA-seq assays in enzymatically digested, no-cultured primary PV-ADSCs, which preserves the in vivo characteristics of these cells. After applying stringent quality control filters, two distinct sub-populations of PV-ADSCs were identified in the mouse thoracic PVAT with specific signature gene expression patterns. The cluster 2 PV-ADSCs, similar to adventitial Sca1+ cells,23 displayed enriched myogenesis and TGF-β signaling pathways, which indicates that it favors differentiation to the SMC lineage and is involved in vascular remodeling in the disease condition. In addition, scRNA-seq combined with pseudotime analysis were performed in cultured PV-ADSCs at low passages, which revealed their SMC differentiation trajectory. Using the vein graft model, the authors further indicate that, in vivo, PV-ADSCs transplanted into the adventitia side of the vein graft, significantly promoted neointima formation through migration and differentiation to SMCs. Through metabolic profiling during the differentiation of PV-ADSCs to SMC, this study uncovered that SMC differentiation induced by TGFβ1 and miR-378a-3p is associated with reprogramming of lipid metabolism.
There are a few limitations in this study. The relatively small cell numbers used for the initial scRNA-seq profiling from no-passaged PV-ADSCs may not be enough to fully reveal the actual heterogeneity of PV-ADSCs phenotypes in vivo. Additionally, the evidence for the contribution of PV-ADSCs to vascular remodeling via migration and SMC differentiation in vivo are to a certain degree preliminary. It has been suggested that in vitro expansion of the progenitor cells will at least cause partial loss of its signature characteristics. Therefore, PVAT transplantation and lineage tracing may help strengthen the conclusion.
In summary (Figure), the current study uses the power of the rapidly emerging and evolving scRNA-seq technology combined with metabolomics to uncover the unexpected heterogeneity of PV-ADSCs and propose the novel concept that the PV-ADSCs contribute to vascular remodeling through regulation of their metabolic reprogramming. These results support a model where PVAT is an intrinsic layer of the vascular wall and highlight the need to further explore the adipose-vascular wall crosstalk and the role of PV-ADSCs in vascular remodeling in various metabolic diseases, such as atherosclerosis and diabetes mellitus. Furthermore, scRNA-seq could lead to development of innovative strategies for vascular disease diagnosis, including those with atypical clinical manifestations, strong gender or age association, and rare syndromes and for treatment by uncovering and targeting specific cell sub-populations or metabolic pathways.
Cartoon showing the crosstalk between PVAT and aorta.
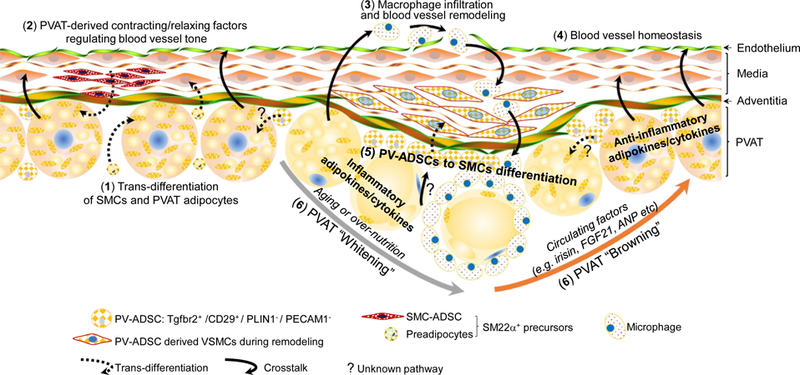
1) SM22α+ precursors from PVAT and blood vessels contribute to PVAT adipogenesis; 2) PVAT-derived contracting or relaxing factors regulate blood vessel tone and homeostasis; 3) PVAT-derived inflammatory adipokines/cytokines promote macrophage infiltration; 4) PVAT-derived anti-inflammatory adipokines/cytokines maintain blood vessel homeostasis; 5) ADSCs in PVAT contribute to blood vessel remodeling; 6) PVAT “Browning”/”Whitening” characteristics affect PVAT-aorta crosstalk to promote homeostasis or disease, respectively.
Acknowledgments
Sources of Funding
This work was partially supported by National Institutes of Health grants HL138139 (J. Zhang), HL068878 and HL134569 (Y.E. Chen), HL122664 (L. Chang).
Footnotes
Disclosure
None.
References:
- 1.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE and Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang L, Milton H, Eitzman DT and Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J 2013;77:11–18. [DOI] [PubMed] [Google Scholar]
- 3.Qi XY, Qu SL, Xiong WH, Rom O, Chang L and Jiang ZS. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol 2018;17:134. doi: 10.1186/s12933-018-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD and Chen YE. Bmal1 in Perivascular Adipose Tissue Regulates Resting-Phase Blood Pressure Through Transcriptional Regulation of Angiotensinogen. Circulation 2018;138:67–79. doi: 10.1161/CIRCULATIONAHA.117.029972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villacorta L and Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig 2015;21:137–147. doi: 10.1515/hmbci-2014-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R and Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang L, Zhao X, Garcia-Barrio M, Zhang J and Chen YE. MitoNEET in Perivascular Adipose Tissue Prevents Arterial Stiffness in Aging Mice. Cardiovasc Drugs Ther 2018;32:531–539. doi: 10.1007/s10557-018-6809-7. [DOI] [PubMed] [Google Scholar]
- 8.Xiong W, Zhao X, Villacorta L, Rom O, Garcia-Barrio MT, Guo Y, Fan Y, Zhu T, Zhang J, Zeng R, Chen YE, Jiang Z and Chang L. Brown Adipocyte-Specific PPARgamma (Peroxisome Proliferator-Activated Receptor gamma) Deletion Impairs Perivascular Adipose Tissue Development and Enhances Atherosclerosis in Mice. Arterioscler Thromb Vasc Biol 2018;38:1738–1747. doi: 10.1161/ATVBAHA.118.311367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan XX, Cao JM, Cai F, Ruan CC, Wu F and Gao PJ. Loss of miR-146b-3p Inhibits Perivascular Adipocyte Browning with Cold Exposure During Aging. Cardiovasc Drugs Ther 2018;32:511–518. doi: 10.1007/s10557-018-6814-x. [DOI] [PubMed] [Google Scholar]
- 10.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R and Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012;122:1022–1036. doi: 10.1172/JCI59701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuevas-Ramos D, Mehta R and Aguilar-Salinas CA. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front Physiol 2019;10:37. doi: 10.3389/fphys.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ and Tang D. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014;63:514–525. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 13.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr., Rosen ED and Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran KV, Fitzgibbons T, Min SY, DeSouza T and Corvera S. Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice. Mol Metab 2018;9:199–206. doi: 10.1016/j.molmet.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lescroart F, Wang X, Lin X, Swedlund B, Gargouri S, Sanchez-Danes A, Moignard V, Dubois C, Paulissen C, Kinston S, Gottgens B and Blanpain C. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 2018;359:1177–1181. doi: 10.1126/science.aao4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu D and Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 2019. June 11. doi: 10.1038/s41569-019-0218-x [Epub ahead of print] [DOI] [PubMed]
- 17.Xiong X, Kuang H, Ansari S, Liu T, Gong J, Wang S, Zhao X, Ji Y, Li C, Guo L, Zhou L, Chen Z, Leon-Mimila P, Chung MT, Kurabayashi K, Opp J, Campos-Pérez F, Villamil-Ramírez H, Canizales-Quinteros S, Lyons R, Lumeng CN, Zhou B, Qi L, Huertas-Vazquez A, Lusis AJ, Xu XZS, Li S, Yu Y, Li J, Lin JD. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Molecular Cell 2019. (It will be published on Aug. 8, 2019) [DOI] [PMC free article] [PubMed]
- 18.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K and Wolf D. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res 2018;122:1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE and Zernecke A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ Res 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509. [DOI] [PubMed] [Google Scholar]
- 20.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E and Amit I. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019;178:686–698 e14. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M and Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017;169:1276–1290 e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Pan XX, Ruan CC, Liu XY, Kong LR, Ma Y, Wu QH, Li HQ, Sun YJ, Chen AQ, Zhao Q, Wu F, Wang XJ, Wang JG, Zhu DL and Gao PJ. Perivascular adipose tissue-derived stromal cells contribute to vascular remodeling during aging. Aging Cell 2019;18:e12969. doi: 10.1111/acel.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu W, Ni Z, Tan YQ, Deng J, Zhang SJ, Lv ZC, Wang XJ, Chen T, Zhang Z, Hu Y, Jing ZC and Xu Q. Adventitial Cell Atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-Deficient Mice Defined by Single-Cell RNA Sequencing. Arterioscler Thromb Vasc Biol 2019;39:1055–1071. doi: 10.1161/ATVBAHA.119.312399. [DOI] [PMC free article] [PubMed] [Google Scholar]


