Abstract
Purpose of review:
Uromodulin (UMOD), also known as Tamm-Horsfall protein, is the most abundant protein in human urine. UMOD has multiple functions such as protection against urinary tract infections (UTIs) and nephrolithiasis. This review outlines recent progress made in UMOD’s role in renal physiology, tubular transport, and mineral metabolism.
Recent findings:
UMOD is mostly secreted in the thick ascending limb (TAL) and to a lesser degree in the distal convoluted tubule (DCT). UMOD secretion is regulated by the calcium sensing receptor. UMOD upregulates ion channels (e.g. ROMK, TRPV5, and TRPM6) and cotransporters (e.g. NKCC2 and NCC) in the TAL and DCT. Higher serum UMOD concentrations have been associated with higher renal function and preserved renal reserve. Higher serum UMOD has also been linked to a lower risk of cardiovascular disease and diabetes mellitus.
Summary:
With better serum UMOD detection assays the extent of different functions for UMOD is still expanding. Urinary UMOD regulates different tubular ion channels and cotransporters. Variations of urinary UMOD secretion can so contribute to common disorders such as hypertension or nephrolithiasis.
Keywords: Uromodulin, Tamm-Horsfall protein, thick ascending limb, distal convoluted tubule, NKCC2, ROMK, NCC, TRPV5, TRPM6
Introduction
Uromodulin, also known as Tamm Horsfall protein, is the most abundant protein in human urine. The protein was first described by Tamm and Horsfall as a urinary mucoprotein which prevents viral hemagglutination (1). Over thirty years later a protein was purified from the urine of pregnant women which due to its immunosuppressive characteristics was named Uromodulin (UMOD) (2). Subsequently, UMOD and Tamm Horsfall protein were found to be identical (3). In this review I will refer to this protein as UMOD. Different functions have been described for UMOD: Protection against urinary tract infections (UTIs) (4–6), and calcium (Ca2+)-containing kidney stones (7–9), contribution to urinary concentration (10, 11), and modification of tubular ion transport for potassium (K+), sodium (Na+), Ca2+, and magnesium (Mg2+) (12–15). Moreover, UMOD modifies innate immunity (16–20). Heterozygous mutations in the UMOD gene cause a tubulointerstital nephropathy called autosomal dominant tubulointerstital kidney disease (ADTKD-UMOD) (21). This review describes the latest findings regarding the physiological significance of UMOD in the regulation of minerals in the human body, also known as mineral metabolism.
UMOD trafficking and secretion
The UMOD gene is localzied on chromosome 16p12.3 and encodes a 640 amino acid protein (22). The UMOD protein consists of an N-terminal signal peptide, followed by three epidermal growth factor-like (EGF-like) domains, which are important for protein-protein interaction, a D8C domain of unknown significance, a fourth EGF-like domain, a long zona pellucida domain, which is crucial for UMOD polymerization (23–26), and a C-terminal GPI-anchor (27) (Fig. 1A). UMOD has a high number of 48 cysteine residues forming 24 intramolecular disulfide bridges. About 60% of all UMOD mutations affect one of these 48 conserved cysteine residues and cause protein misfolding and accumulation of the mutant UMOD protein in the endoplasmic reticulum (ER) (28). Recent publications have linked UMOD mutations to increased inflammation, fibrosis, altered unfolded protein response and mitochondrial function (29–32). Regarding further information on ADTKD-UMOD I refer the interested reader to a comprehensive review given the space limitations (33).
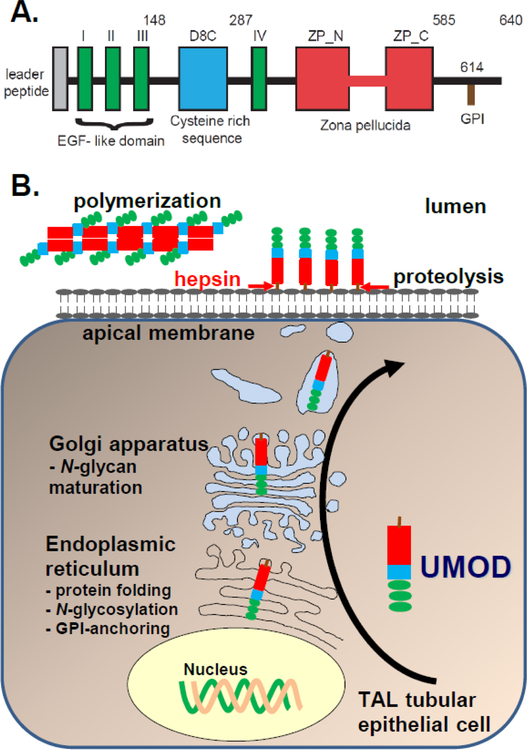
Figure 1:
UMOD synthesis and secretion. A. UMOD contains EGF-like domains (green) which are important for protein-protein interaction, a cysteine-rich domain (D8C) (blue) of unknown significance, and a zona pellucida domain (red) which is crucial for UMOD multimerization. B. During UMOD synthesis the molecule is translocated to the ER where the signal peptide is cleaved, N-glycosylation and glycosylphosphatidylinositol (GPI)-anchoring occur, and disulfide bridges are formed. In the Golgi apparatus N-glycans are further modified. UMOD is then sorted to the apical membrane where it is linked to the cell membrane by it’s GPI- anchor. Hepsin, a type II transmembrane serine protease, cleaves UMOD and releases it into urine.
The UMOD precursor enters the cellular secretory pathway resulting in a 85 kDa glycoprotein (34) (Fig. 1B). Apical UMOD is then cleaved by Hepsin, a type II transmembrane serine protease, and released UMOD into urine (Fig. 1B) (23). UMOD homopolymers contribute to the protective effect of UMOD against UTIs and kidney stones, and modify innate immunity (5, 9, 27, 35). Interaction between the internal hydrophobic patch (IHP) within the UMOD ZP domain and the external hydrophobic patch (EHP), which is between the cleavage site and the transmembrane domain, prevents intracellular UMOD polymerization (25). Crystallography further narrowed down the region within UMOD that mediates self-polymerization to an interdomain linker conneting the ZP-N and ZP-C domains (36). Additionally, UMOD is also sorted to the basolateral membrane to a lesser degree, where it is released in the interstitium and enters the circulation as monomeric UMOD (37–39). It was thought that UMOD is exclusively secreted in the thick ascending limb (TAL) (40). Recent findings have shown that UMOD is also secreted in DCT1 (up to approximately 10% of the TAL UMOD secretion) (41).
It has remained unclear how tubular UMOD secretion is modified. The Ca2+ sensing receptor (CaSR) reduces UMOD secretion. In mouse models Casr activating mutations lowered UMOD secretion, whereas inactivating mutations increased UMOD secretion (42). Humans treated with cinacalcet also had reduced urinary UMOD secretion (42).
Physiological variations of UMOD secretion
An association between urinary UMOD secretion and eGFR has been suggested (37, 43–45). Twenty-four hour urinary UMOD secretion has been linked to urine volume and kidney size, while age and diabetes mellitus have been associated inversely with UMOD secretion, suggesting that urinary UMOD reflects tubular activity (46). Common variants near the UMOD gene have also been associated with increased renal UMOD expression, higher urinary UMOD secretion, hypertension, eGFR, and decline of renal function (46–53).
Urinary UMOD may estimate nephron mass because a significant reduction of urinary UMOD has been demonstrated in living kidney donors (54). Urinary UMOD may also serve as a predictor for acute kidney injury (AKI) after cardiac surgery. A preoperative lower spot urine UMOD/creatinine ratio implicated a higher odds of more severe AKI postoperatively (55, 56). Lower urinary and serum UMOD levels have also been described in patients with earlier kidney allograft failure (57–59). How urinary UMOD protects against AKI and progressive kidney disease is still unclear. In addition, there is still conflicting data whether high urinary UMOD is protective or aggravating towards CKD. While one hypothesis suggests that urinary UMOD enhances tubular salt absorption and worsens hypertension and subsequent CKD, another line of thought suggests that UMOD modifies the immune system (39, 53, 60–63). There is evidence that UMOD deficiency results in a state of inflammation (61). UMOD modifies the abundance and phagocytic activity of mononuclear phagocytic cells and granulopoesis which affects the response to AKI (39). A cross-talk between the TAL and the proximal tubule has been suggested with UMOD inhibiting chemokine signaling and thereby reducing the risk of adjacent proximal tubule injury (62, 64). Regarding the impact of UMOD on the immune system I refer the interested reader due to space limitation to comprehensive reviews about this topic (60, 65).
Uromodulin in tubular ion transport
Uromodulin modifies salt absorption in the TAL and contributes to hypertension
Mice lacking UMOD (Umod−/−) display altered expression of renal transporters in the TAL and in the distal nephron (11). Specifically, UMOD stimulates NKCC2 by phosphorylation, reducing intracellular Cl- concentration (13). Umod−/− mice respond to furosemide with a blunted response regarding Na+, K+, and Cl- excretion but not regarding urine volume (13). These findings are consistent with an intracellular role of UMOD regarding upregulation of NKCC2 activity (Fig. 2). The significance of NKCC2 stimulation by UMOD in mice and humans can be exemplified by higher urinary UMOD secretion in individuals with the UMOD single nucleotide polymorphism SNP4293393 (53). Mice overexpressing wild-type UMOD (TgUmodwt/wt had a 80% higher Umod secretion than WT mice) show higher systolic blood pressures, left ventricular hypertrophy, and had higher phosphorylated NKCC2 in total kidney lysate. In TgUmodwt/wt animals a low salt diet improves blood pressures and furosemide treatment leads to a higher natriuretic effect compared to WT animals. NKCC2 activation by UMOD seems to be at least partially mediated through the SPAK/OSR1 pathway. Graham et al confirmed the role of UMOD in modulating blood pressure in Umod−/− mice by demonstrating lower blood pressures and higher urinary Na+ excretion compared to control mice (66).
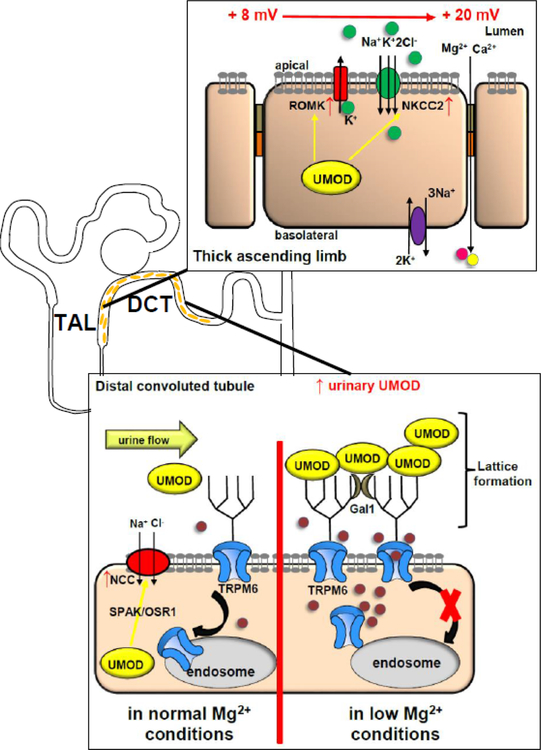
Figure 2:
UMOD modifies ion channels and cotransporters along the TAL and DCT. In the TAL UMOD stimulates from the intracellular space NKCC2 and ROMK cell surface abundance. In the DCT UMOD stimulates NCC possibly via intracellular SPAK/OSR1 signaling. Luminal UMOD enhances TRPM6 cell surface abundance by forming a urinary multi-protein complex. Urinary galectin-1 stabilizes lattice formation between TRPM6 N-glycan and UMOD, thereby impairing dynamin-2 dependent TRPM6 endocytosis. In low Mg2+ conditions more urinary UMOD is secreted, possibly to compensate for the low Mg2+ state by increasing tubular Mg2+ absorption via TRPM6. UMOD increases apical TRPV5 abundance in a similar fashion as TRPM6 by lattice formation and impairing endocytosis.
ROMK forms a functional unit with NKCC2 in the TAL and is crucial for Na+ absorption by recycling K+ into the tubular lumen which has been absorbed via NKCC2 (Fig. 2). Physical interaction between ROMK and UMOD increases ROMK current and surface expression (12). These findings are consistent with Umod−/− mice displaying urinary K+ losses (66).
In the TAL of WT and Umod−/− mice RNA-seq data were analyzed when provided with a high salt diet (67). In WT mice a significant upregulation of heat-shock proteins with Hspa1b and a (aka. Hsp70), and other members of the heat-shock family was found but not in Umod−/− mice. Hsp70 has been implicated in hypertension (68). Additional evidence for a role of UMOD polymers in hypertension was described in the stroke-prone spontaneously hypertensive rat (SHRSP) which serves as a model for maternal chronic hypertension indicating a possible role of UMOD in pre-eclampsia (69). Elevated urinary UMOD levels have been described in pre-eclampsia patients (70). The signifcance of UMOD for hypertension and its relationship to renal dysfunction has been exemplified in a human cohort by the SNP rs12917707 (G/G allele) which was associated with lower renal function and left atrial remodeling (71).
An additional role for UMOD was recently shown for the sodium-chloride cotransporter (NCC) (Fig. 2). The authors showed in in vivo and in vitro experiments that UMOD upregulates the sodium-chloride cotransporter (NCC) via phosphorylation (41). The authors speculated about possible involvement of the SPAK-OSR1 pathway in this mechanism.
Serum UMOD concentrations are associated with hypertension, cardiovascular events, CKD, and diabetes mellitus
More reliable assays measuring serum UMOD levels in humans have complemented our understanding of UMOD in the development of hypertension beyond genome-wide association studies (GWAS). In a cohort of 529 patients with coronary artery disease a protective effect of higher serum UMOD concentrations on mortality was identified (72). A larger study of 3316 individuals referred for angiography confirmed this protective effect by showing that patients who have a higher serum UMOD have a more a favorable metabolic profile, lower rates of hypertension, diabetes mellitus, heart failure, and a lower risk for 10-year mortality (38).
Regarding CKD development higher serum UMOD levels associated with higher kidney function supporting the notion that plasma UMOD is a biomarker of renal function and represents residual renal mass (73–75). Serum UMOD concentrations have been shown to reliably correlate with renal function and have even allowed to distinguish between CKD0 and CKD1 stages (76). One wonders if serum UMOD concentration may be sensitive enough to replace serum creatinine as a more sensitive marker for early CKD. An additional study found an association between UMOD SNPs and CKD in diabetic patients and showed that higher serum UMOD concentrations are associated with a lower CKD risk (77). For a more detailed review regarding the relationship between UMOD and CKD risk in larger populations, I refer the interested reader to a comprehensive review (78).
Evidence shows an association between UMOD and the risk for diabetes mellitus (79, 80). Lower serum UMOD levels in humans correlate inversely with HgbA1c and serum glucose levels (38, 79, 81, 82). Reduced urinary and serum UMOD levels were described in patients with T1DM, T2DM, and diabetic nephropathy (83–89). How UMOD is linked to diabetes mellitus is not clear. One wonders how these findings can be reconciled with the results outlining higher CKD risk and mortality due to higher urinary UMOD levels. Is the UMOD function in the blood and the urine different? Could the beneficial effect of a higher UMOD plasma level be a systemic immunomodulatory effect improving systemic inflammation which is common with metabolic syndrome?
Uromodulin functions as a macromolecule and enhances Ca2+ absorption in the DCT to reduce the risk for hypercalciuria and kidney stones
Umod−/− mice develop spontaneoulsy intrarenal Ca2+-containing crystals resulting in renal calcinosis, ureteral obstruction, and hydronephrosis (7–9). We also found large bladder stones in Umod−/− mice (Fig. 3, unpublished data). In these animals crystals were detected in deep medulla and renal papillae and compared to the Randall’s plaques in humans (90). Renal papillary calcification was found as early as 2 months (9). The crystals contained mostly Ca2+-phosphate in the form of hydroxyapatite. Urinary supersaturation was elevated for Ca2+ and Ca2+-oxalate. Umod−/− mice showed a compensatory increase of the urinary protein osteopontin, another inhibitor of Ca2+ crystal formation (7). Urinary osteopontin and UMOD work synergistically in preventing renal Ca2+ crystals. Previously, UMOD had been shown to inhibit Ca2+-oxalate stone aggregation as a polyanionic macromolecule (91). UMOD and osteopontin are thought to coat the crystal surface and thereby prevent crystal nucleation and excert an anti-crystal effect (92–94).
Figure 3:
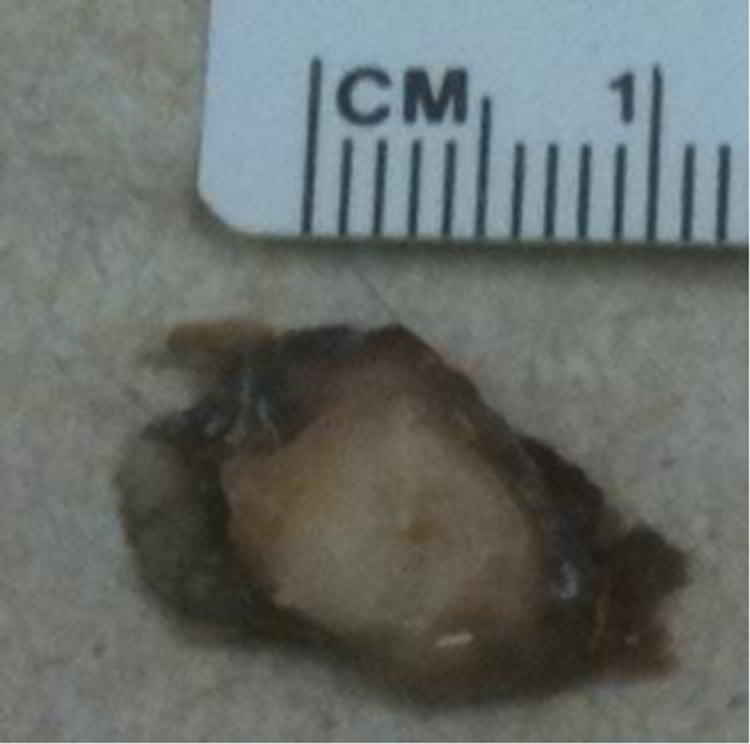
Umod−/− mice form bladder stones. We confirmed large bladder stones in Umod−/− mice.
An additional mechanism to prevent kidney stones is the upregulation of the apical Ca2+ channel TRPV5 by UMOD (14). Extracellular UMOD, secreted in the TAL stimulated TRPV5 current density by increasing the number TRPV5 channels in the apical membrane. UMOD required dynamin-2 dependent endocytosis for TRPV5 upregulation. The significance of endocytosis for TRPV5 stimulation by UMOD was confirmed in fibroblasts lacking caveolin-1 (Cav1−/−), another key protein for endocytosis, whereas co-transfection of WT Cav1 plasmid in Cav1−/− fibroblasts rescued the stimulation of TRPV5 by UMOD. In immunofluorescent (IF) studies of the kidney, Umod−/− mice displayed a lower TRPV5 abundance compared to WT mice (14). The literature on UMOD in human stone-formers is conflicting due to heterogenous and not well characterized cohorts. Some of the conflicting data may also stem from different preparations or storage conditions of the urine (95).
Calcium and uric acid stone formers excrete a lower urinary UMOD concentration (96). Whereas in control individuals a linear correlation between urinary Ca2+ and UMOD was found, this correlation was not present in Ca2+-stone formers suggesting that these individuals may be unable to secret more UMOD with a higher urinary Ca2+ concentration (96). In a recent study of different kidney stone-formers, stone-formers had 32% lower urinary UMOD concentration and a lower sialic acid content compared to controls, supporting the significance of correct UMOD glycosylation in the prevention of nephrolithiasis (97). Moreover, a GWAS identified a SNP within UMOD that protected against formation of Ca2+-containing kidney stones (98).
Uromodulin increases Mg2+ absorption in the DCT via TRPM6.
In order to better understand what genes are upregulated in hypomagnesemia the transcriptome of the DCT of mice fed with a low or high Mg2+ diet was studied (99). UMOD was described as of one of the highest upregulated mRNAs in the low Mg2+ diet cohort but it remained unclear how UMOD compensates for a low Mg2+ state. One study showed that Umod−/− mice have hypermagnesuria and increased expression of genes involved in Mg2+ homeostasis, suggesting that UMOD contributes to maintenance of Mg2+ balance in a low Mg2+ state (15). The TAL is responsible for the majority of tubular Mg2+ absorption. One would hypothesize a blunted response to furosemide in the Umod−/− mice if the Mg2+ wasting would be due to a dysfunctional TAL. Previously, in Umod−/− mice a blunted response of the TAL after furosemide was published (13, 53, 100). While we found hypercalciuria and hypermagnesuria in Umod−/− mice at baseline, we found no significant difference between Umod−/− and WT mice after furosemide regarding urine volume, natriuresis, kaliuresis, Ca2+, or Mg2+ excretion, thus making the TAL as the source for the hypermagnesuria unlikely (15). The different response to furosemide compared to the other groups may be due to differences in age, furosemide dose, genetic background, mouse models, urine collection method, and corrections for urine volume or body weight. Umod−/− mice have less apical TRPM6 abundance in IF studies of the DCT compared to WT mice. UMOD enhances the cell surface abundance of the Mg2+ channel TRPM6 and thereby increases thereby TRPM6 current density (Fig. 2) (15). UMOD excerts its effect on TRPM6 from the extracellular space but requires the ubiquitously secreted urinary protein galectin-1 (15). TRPM6 and UMOD physically interact, allowing for lattice formation between the apical TRPM6 channels and UMOD polymers thereby impairing TRPM6 endocytosis (15). We identified a novel TRPM6 N-glycan site which is required for the UMOD upregulation of TRPM6 current density. Finally, urinary UMOD secretion was upregulated in animals fed a low Mg2+ diet, suggesting a feedback mechanism with higher urinary UMOD secretion as a compensatory mechanism to upregulate TRPM6 in a low Mg2+ state and to support tubular Mg2+ absorption (Fig. 2).
Conclusion and Future Directions:
UMOD has been known as a urinary protein for over a century. With the help of improved detection tools we still learn more about the multi-faceted roles of this protein. UMOD modifies multiple apical tubular ion channels from the intracellular and extracellular space. New insight has shown that not only pathological mutations but also physiological variations of normal urinary UMOD secretion contribute to common diseases. Measurement of urinary UMOD secretion could serve as an inexpensive and noninvasive test to assess nephron mass, predict AKI risk, and kidney transplant longevity. In addition, measurement of serum UMOD may serve as a screening tool for evaluating CKD risk, cardiovascular complications, diabetes mellitus, diabetic nephropathy, and possibly overall mortality. Further studies will have to confirm UMOD’s role as a serum marker for early CKD. Future human studies investigating the effect of UMOD on nephrolithiasis should focus on well characterized subpopulations of stone formers and incorporate detailed dietary information.
Supplementary Material
Key points:
UMOD has multi-faceted roles in hypertension, the formation of Ca2+-containing kidney stones, and renal K+ and Mg2+ homeostasis.
These effects are due to direct effects of UMOD on ion channels (ROMK, TRPV5, TRPM6) and cotransporters (NKCC2 and NCC) along the TAL and DCT.
Pathological mutations in UMOD result in autosomal-dominant tubulo-interstitial kidney disease (ADTKD).
Variable secretion of wild-type (WT) UMOD also contributes to different common diseases by exposing individuals with higher urinary UMOD to a higher risk of hypertension and individuals with a lower urinary UMOD to a higher risk of kidney stones, acute kidney injury, chronic kidney disease, and diabetes mellitus.
Acknowledgements:
Disclosure of funding: Funding for this manuscript has been provided by the National Institutes of Health (NIH R03DK111776, NIH P30 DK079328–11) and Children’s Clinical Research Advisory Committee (CCRAC), Children’s Health System, Dallas.
Financial support : Funding for this manuscript has been provided by the National Institutes of Health (NIH R03DK111776, NIH P30 DK079328–11) and Children’s Clinical Research Advisory Committee (CCRAC), Children’s Health System, Dallas.
Footnotes
Conflicts of interest: none
References
* indicates a publication of special interest
* * indicates a publication of outstanding interest
- 1.Tamm I, Horsfall FL Jr. Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med 1950;74(1):106–8. [PubMed] [Google Scholar]
- 2.Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science 1985;229(4712):479–81. [DOI] [PubMed] [Google Scholar]
- 3.Pennica D, Kohr WJ, Kuang WJ, Glaister D, Aggarwal BB, Chen EY, et al. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science 1987;236(4797):83–8. [DOI] [PubMed] [Google Scholar]
- 4.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 2004;65(3):791–7. [DOI] [PubMed] [Google Scholar]
- 5.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol 2004;286(4):F795–802. [DOI] [PubMed] [Google Scholar]
- 6.Ghirotto S, Tassi F, Barbujani G, Pattini L, Hayward C, Vollenweider P, et al. The Uromodulin Gene Locus Shows Evidence of Pathogen Adaptation through Human Evolution. J Am Soc Nephrol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 2004;66(3):1159–66. [DOI] [PubMed] [Google Scholar]
- 8.Mo L, Liaw L, Evan AP, Sommer AJ, Lieske JC, Wu XR. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol Renal Physiol 2007;293(6):F1935–43. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR, et al. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol 2010;299(3):F469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiggins RC. Uromucoid (Tamm-Horsfall glycoprotein) forms different polymeric arrangements on a filter surface under different physicochemical conditions. Clin Chim Acta 1987;162(3):329–40. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, et al. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 2005;288(3):F559–67. [DOI] [PubMed] [Google Scholar]
- 12.Renigunta A, Renigunta V, Saritas T, Decher N, Mutig K, Waldegger S. Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem 2011;286(3):2224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, et al. Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 2011;286(34):30200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf MT, Wu XR, Huang CL. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int 2013;84(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Nie M, Bal MS, Liu J, Yang Z, Rivera C, Wu XR, et al. Uromodulin regulates renal magnesium homeostasis through the ion channel transient receptor potential melastatin 6 (TRPM6). J Biol Chem 2018.This is the first report outlining a mechanism how UMOD enhances tubular Mg2+ absorption.
- 16.Rhodes DC, Hinsman EJ, Rhodes JA. Tamm-Horsfall glycoprotein binds IgG with high affinity. Kidney Int 1993;44(5):1014–21. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes DC. Binding of Tamm-Horsfall protein to complement 1q measured by ELISA and resonant mirror biosensor techniques under various ionic-strength conditions. Immunology and cell biology 2000;78(5):474–82. [DOI] [PubMed] [Google Scholar]
- 18.Hession C, Decker JM, Sherblom AP, Kumar S, Yue CC, Mattaliano RJ, et al. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science 1987;237(4821):1479–84. [DOI] [PubMed] [Google Scholar]
- 19.Schmid M, Prajczer S, Gruber LN, Bertocchi C, Gandini R, Pfaller W, et al. Uromodulin facilitates neutrophil migration across renal epithelial monolayers. Cell Physiol Biochem 2010;26(3):311–8. [DOI] [PubMed] [Google Scholar]
- 20.Saemann MD, Weichhart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest 2005;115(2):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, et al. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 2002;39(12):882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scolari F, Puzzer D, Amoroso A, Caridi G, Ghiggeri GM, Maiorca R, et al. Identification of a new locus for medullary cystic disease, on chromosome 16p12. Am J Hum Genet 1999;64(6):1655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, et al. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife 2015;4:e08887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santambrogio S, Cattaneo A, Bernascone I, Schwend T, Jovine L, Bachi A, et al. Urinary uromodulin carries an intact ZP domain generated by a conserved C-terminal proteolytic cleavage. Biochem Biophys Res Commun 2008;370(3):410–3. [DOI] [PubMed] [Google Scholar]
- 25.Schaeffer C, Santambrogio S, Perucca S, Casari G, Rampoldi L. Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol Biol Cell 2009;20(2):589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaeffer C, Cattaneo A, Trudu M, Santambrogio S, Bernascone I, Giachino D, et al. Urinary secretion and extracellular aggregation of mutant uromodulin isoforms. Kidney Int 2012;81(8):769–78. [DOI] [PubMed] [Google Scholar]
- 27.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 2011;80(4):338–47. [DOI] [PubMed] [Google Scholar]
- 28.Rampoldi L, Caridi G, Santon D, Boaretto F, Bernascone I, Lamorte G, et al. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet 2003;12(24):3369–84. [DOI] [PubMed] [Google Scholar]
- *29.Trudu M, Schaeffer C, Riba M, Ikehata M, Brambilla P, Messa P, et al. Early involvement of cellular stress and inflammatory signals in the pathogenesis of tubulointerstitial kidney disease due to UMOD mutations. Sci Rep 2017;7(1):7383.In this publication the authors describe upregulation of inflammatory and fibrosis pathways in UMOD mutant mice.
- *30.Kemter E, Frohlich T, Arnold GJ, Wolf E, Wanke R. Mitochondrial Dysregulation Secondary to Endoplasmic Reticulum Stress in Autosomal Dominant Tubulointerstitial Kidney Disease - UMOD (ADTKD-UMOD). Sci Rep 2017;7:42970.This group describes the involvement of the unfolded protein reponse and mitochondiral dysregulation in the kidneys of UMOD mutant mice.
- *31.Schaeffer C, Merella S, Pasqualetto E, Lazarevic D, Rampoldi L. Mutant uromodulin expression leads to altered homeostasis of the endoplasmic reticulum and activates the unfolded protein response. PLoS One 2017;12(4):e0175970.Involvement of the unfolded protein response in the kidneys of mutant mice is reported.
- **32.Johnson BG, Dang LT, Marsh G, Roach AM, Levine ZG, Monti A, et al. Uromodulin p.Cys147Trp mutation drives kidney disease by activating ER stress and apoptosis. J Clin Invest 2017;127(11):3954–69.Using a new UMOD mutant mouse model the authors investigated several important pathways and identified ER stress and apoptosis in the mutant UMOD mice. Pharmacological blockade of TNF alpha improved renal outcome of these mice.
- **33.Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 2017;13(9):525–44.This is a very comprehensive review about the physiology, population genetics, and effects of mutant UMOD.
- 34.Serafini-Cessi F, Malagolini N, Hoops TC, Rindler MJ. Biosynthesis and oligosaccharide processing of human Tamm-Horsfall glycoprotein permanently expressed in HeLa cells. Biochem Biophys Res Commun 1993;194(2):784–90. [DOI] [PubMed] [Google Scholar]
- 35.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 2003;42(4):658–76. [DOI] [PubMed] [Google Scholar]
- 36.Bokhove M, Nishimura K, Brunati M, Han L, de Sanctis D, Rampoldi L, et al. A structured interdomain linker directs self-polymerization of human uromodulin. Proc Natl Acad Sci U S A 2016;113(6):1552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 1985;68(5):529–35. [DOI] [PubMed] [Google Scholar]
- *38.Delgado GE, Kleber ME, Scharnagl H, Kramer BK, Marz W, Scherberich JE. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J Am Soc Nephrol 2017;28(7):2201–10.This study describes the association of higher serum UMOD levels with favorable metabolic profile and lower prevalence of hypertension, diabetes mellitus and heart failure.
- *39.Micanovic R, Khan S, Janosevic D, Lee ME, Hato T, Srour EF, et al. Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney. J Am Soc Nephrol 2018;29(3):841–56.This publications studied the effect of UMOD on monocytes in the kidney and the effect of UMOD on AKI.
- 40.Bachmann S, Metzger R, Bunnemann B. Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle’s loop in rat kidney. Histochemistry 1990;94(5):517–23. [DOI] [PubMed] [Google Scholar]
- *41.Tokonami N, Takata T, Beyeler J, Ehrbar I, Yoshifuji A, Christensen EI, et al. Uromodulin is expressed in the distal convoluted tubule, where it is critical for regulation of the sodium chloride cotransporter NCC. Kidney Int 2018.The expression and secretion of UMOD in the early DCT is described.
- *42.Tokonami N, Olinger E, Debaix H, Houillier P, Devuyst O. The excretion of uromodulin is modulated by the calcium-sensing receptor. Kidney Int 2018;94(5):882–6.The authors have investigated the inhibitory effect of the CaSR on UMOD secretion.
- 43.Ollier-Hartmann MP, Pouget-Abadie C, Bouillie J, Hartmann L. Variations of urinary Tamm-Horsfall protein in humans during the first thirty years of life. Nephron 1984;38(3):163–6. [DOI] [PubMed] [Google Scholar]
- 44.Reinhart HH, Obedeanu N, Robinson R, Korzeniowski O, Kaye D, Sobel JD. Urinary excretion of Tamm-Horsfall protein in elderly women. J Urol 1991;146(3):806–8. [DOI] [PubMed] [Google Scholar]
- 45.Pruijm M, Ponte B, Ackermann D, Paccaud F, Guessous I, Ehret G, et al. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin J Am Soc Nephrol 2016;11(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olden M, Corre T, Hayward C, Toniolo D, Ulivi S, Gasparini P, et al. Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J Am Soc Nephrol 2014;25(8):1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 2009;41(6):712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kottgen A, Hwang SJ, Larson MG, Van Eyk JE, Fu Q, Benjamin EJ, et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J Am Soc Nephrol 2010;21(2):337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chambers JC, Zhang W, Lord GM, van der Harst P, Lawlor DA, Sehmi JS, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat Genet 2010;42(5):373–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J, Chen Y, Liu Y, Liang Y, Wang X, Liu L, et al. Common variants of the UMOD promoter associated with blood pressure in a community-based Chinese cohort. Hypertens Res 2012;35(7):769–74. [DOI] [PubMed] [Google Scholar]
- 51.Han J, Liu Y, Rao F, Nievergelt CM, O’Connor DT, Wang X, et al. Common genetic variants of the human uromodulin gene regulate transcription and predict plasma uric acid levels. Kidney Int 2013;83(4):733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, et al. Genome-wide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet 2010;6(10):e1001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 2013;19(12):1655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Pivin E, Ponte B, de Seigneux S, Ackermann D, Guessous I, Ehret G, et al. Uromodulin and Nephron Mass. Clin J Am Soc Nephrol 2018;13(10):1556–7.This paper describes the correlation between renal mass and UMOD.
- *55.Garimella PS, Jaber BL, Tighiouart H, Liangos O, Bennett MR, Devarajan P, et al. Association of Preoperative Urinary Uromodulin with AKI after Cardiac Surgery. Clin J Am Soc Nephrol 2017;12(1):10–8.The authors investigate the utility of urinary UMOD to predict AKI after cardiac surgery.
- 56.Bennett MR, Pyles O, Ma Q, Devarajan P. Preoperative levels of urinary uromodulin predict acute kidney injury after pediatric cardiopulmonary bypass surgery. Pediatr Nephrol 2018;33(3):521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bostom A, Steubl D, Garimella PS, Franceschini N, Roberts MB, Pasch A, et al. Serum Uromodulin: A Biomarker of Long-Term Kidney Allograft Failure. Am J Nephrol 2018;47(4):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Steubl D, Block M, Herbst V, Schlumberger W, Nockher A, Angermann S, et al. Serum uromodulin predicts graft failure in renal transplant recipients. Biomarkers 2017;22(2):171–7.Serum UMOD levels are used to study longevity of kidney transplants.
- 59.Bostom AG, Steubl D, Friedman AN. Hypothesis: Potential Utility of Serum and Urine Uromodulin Measurement in Kidney Transplant Recipients? Transplantation direct 2017;3(11):e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Achkar TM, Dagher PC. Tubular cross talk in acute kidney injury: a story of sense and sensibility. Am J Physiol Renal Physiol 2015;308(12):F1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micanovic R, Chitteti BR, Dagher PC, Srour EF, Khan S, Hato T, et al. Tamm-Horsfall Protein Regulates Granulopoiesis and Systemic Neutrophil Homeostasis. J Am Soc Nephrol 2015;26(9):2172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 2013;304(8):F1066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bleyer AJ, Kmoch S. Tamm Horsfall Glycoprotein and Uromodulin: It Is All about the Tubules! Clin J Am Soc Nephrol 2016;11(1):6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem 2012;287(20):16365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *65.Micanovic R, LaFavers K, Garimella PS, Wu XR, El-Achkar TM. Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis. Nephrol Dial Transplant 2019.Comprehensive review about UMOD and its effect on the immunse system and how it ameliorates AKI.
- 66.Graham LA, Padmanabhan S, Fraser NJ, Kumar S, Bates JM, Raffi HS, et al. Validation of uromodulin as a candidate gene for human essential hypertension. Hypertension 2014;63(3):551–8. [DOI] [PubMed] [Google Scholar]
- *67.Graham LA, Aman A, Campbell DD, Augley J, Graham D, McBride MW, et al. Salt stress in the renal tubules is linked to TAL-specific expression of uromodulin and an upregulation of heat shock genes. Physiol Genomics 2018;50(11):964–72.The authors identified the effects of a high salt diet in WT and UMOD KO mice and found that in WT mice heat shock proteins are upregulated.
- 68.Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegard J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 2002;20(9):1815–20. [DOI] [PubMed] [Google Scholar]
- *69.Mary S, Small HY, Siwy J, Mullen W, Giri A, Delles C. Polymerization-Incompetent Uromodulin in the Pregnant Stroke-Prone Spontaneously Hypertensive Rat. Hypertension 2017;69(5):910–8.UMOD may be involved in pre-eclampsia.
- 70.Carty DM, Siwy J, Brennand JE, Zurbig P, Mullen W, Franke J, et al. Urinary proteomics for prediction of preeclampsia. Hypertension 2011;57(3):561–9. [DOI] [PubMed] [Google Scholar]
- 71.Algharably EAH, Bolbrinker J, Lezius S, Reibis R, Wegscheider K, Voller H, et al. Uromodulin associates with cardiorenal function in patients with hypertension and cardiovascular disease. J Hypertens 2017;35(10):2053–8. [DOI] [PubMed] [Google Scholar]
- *72.Leiherer A, Muendlein A, Saely CH, Ebner J, Brandtner EM, Fraunberger P, et al. Serum uromodulin is a predictive biomarker for cardiovascular events and overall mortality in coronary patients. Int J Cardiol 2017;231:6–12.The association between serum UMOD and cardiovascular events is investigated.
- 73.Leiherer A, Muendlein A, Saely CH, Brandtner EM, Geiger K, Fraunberger P, et al. The value of uromodulin as a new serum marker to predict decline in renal function. J Hypertens 2018;36(1):110–8. [DOI] [PubMed] [Google Scholar]
- 74.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, et al. Plasma Uromodulin Correlates With Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine (Baltimore) 2016;95(10):e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lv L, Wang J, Gao B, Wu L, Wang F, Cui Z, et al. Serum uromodulin and progression of kidney disease in patients with chronic kidney disease. J Transl Med 2018;16(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, et al. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant 2018;33(2):284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sjaarda J, Gerstein HC, Yusuf S, Treleaven D, Walsh M, Mann JFE, et al. Blood HER2 and Uromodulin as Causal Mediators of CKD. J Am Soc Nephrol 2018;29(4):1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *78.Devuyst O, Pattaro C. The UMOD Locus: Insights into the Pathogenesis and Prognosis of Kidney Disease. J Am Soc Nephrol 2018;29(3):713–26.The effects of UMOD variants on population genetics are outlined.
- 79.Ahluwalia TS, Lindholm E, Groop L, Melander O. Uromodulin gene variant is associated with type 2 diabetic nephropathy. J Hypertens 2011;29(9):1731–4. [DOI] [PubMed] [Google Scholar]
- 80.Deshmukh HA, Palmer CN, Morris AD, Colhoun HM. Investigation of known estimated glomerular filtration rate loci in patients with type 2 diabetes. Diabet Med 2013;30(10):1230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Leiherer A, Muendlein A, Saely CH, Kinz E, Brandtner EM, Fraunberger P, et al. Serum uromodulin is associated with impaired glucose metabolism. Medicine (Baltimore) 2017;96(5):e5798.Serum UMOD levels were found to be associated with the risk of diabetes mellitus.
- 82.Dahlstrom E, Sandholm N. Progress in Defining the Genetic Basis of Diabetic Complications. Curr Diab Rep 2017;17(9):80. [DOI] [PubMed] [Google Scholar]
- 83.Schlatzer D, Maahs DM, Chance MR, Dazard JE, Li X, Hazlett F, et al. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes Care 2012;35(3):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holmquist P, Torffvit O, Jorgensen PE, Torring N, Nexo E, Sjoblad S. Early urinary changes in Tamm-Horsfall protein and epidermal growth factor in diabetic children. Pediatr Nephrol 2001;16(6):488–92. [DOI] [PubMed] [Google Scholar]
- 85.Torffvit O, Agardh CD, Thulin T. A study of Tamm-Horsfall protein excretion in hypertensive patients and type 1 diabetic patients. Scand J Urol Nephrol 1999;33(3):187–91. [DOI] [PubMed] [Google Scholar]
- 86.Zheng M, Ye S, Chen Y, Chen M. A study of urinary Tamm-Horsfall protein excretion in adult type 2 diabetes mellitus. Clin Nephrol 2018;90(1):40–5. [DOI] [PubMed] [Google Scholar]
- 87.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int 2015;88(5):1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bjornstad P, Wiromrat P, Johnson RJ, Sippl R, Cherney DZI, Wong R, et al. Serum Uromodulin Predicts Less Coronary Artery Calcification and Diabetic Kidney Disease Over 12 Years in Adults With Type 1 Diabetes: The CACTI Study. Diabetes Care 2019;42(2):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Troyanov S, Delmas-Frenette C, Bollee G, Youhanna S, Bruat V, Awadalla P, et al. Clinical, Genetic, and Urinary Factors Associated with Uromodulin Excretion. Clin J Am Soc Nephrol 2016;11(1):62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu XR. Interstitial calcinosis in renal papillae of genetically engineered mouse models: relation to Randall’s plaques. Urolithiasis 2015;43 Suppl 1:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Rooijen JJ, Kamerling JP, Vliegenthart JF. Sulfated di-, tri- and tetraantennary N-glycans in human Tamm-Horsfall glycoprotein. Eur J Biochem 1998;256(2):471–87. [DOI] [PubMed] [Google Scholar]
- 92.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens 2006;15(4):374–80. [DOI] [PubMed] [Google Scholar]
- 93.Kumar V, Pena de la Vega L, Farell G, Lieske JC. Urinary macromolecular inhibition of crystal adhesion to renal epithelial cells is impaired in male stone formers. Kidney Int 2005;68(4):1784–92. [DOI] [PubMed] [Google Scholar]
- 94.Hess B, Nakagawa Y, Coe FL. Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol 1989;257(1 Pt 2):F99–106. [DOI] [PubMed] [Google Scholar]
- 95.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O. Determination of uromodulin in human urine: influence of storage and processing. Nephrol Dial Transplant 2014;29(1):136–45. [DOI] [PubMed] [Google Scholar]
- 96.Glauser A, Hochreiter W, Jaeger P, Hess B. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant 2000;15(10):1580–7. [DOI] [PubMed] [Google Scholar]
- 97.Argade S, Chen T, Shaw T, Berecz Z, Shi W, Choudhury B, et al. An evaluation of Tamm-Horsfall protein glycans in kidney stone formers using novel techniques. Urolithiasis 2015. [DOI] [PubMed] [Google Scholar]
- 98.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet 2010;6(7):e1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Baaij JH, Groot Koerkamp MJ, Lavrijsen M, van Zeeland F, Meijer H, Holstege FC, et al. Elucidation of the distal convoluted tubule transcriptome identifies new candidate genes involved in renal Mg(2+) handling. Am J Physiol Renal Physiol 2013;305(11):F1563–73. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Goldfarb D, El-Achkar TM, Lieske JC, Wu XR. Tamm-Horsfall Protein/Uromodulin Deficiency Elicits Tubular Compensatory Responses Leading to Hypertension and Hyperuricemia. Am J Physiol Renal Physiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.