Abstract
Aim of the study:
Over the past fifty years, the application of mammography – an X-ray of the breast – to screen healthy women has been a successful strategy to reduce breast cancer mortality. The aim of this study was to review the literature on novel imaging approaches that have the potential to replace mammography.
Methods:
An online literature search was carried out using PubMed, Google Scholar, ScienceDirect and Google Patents. The search keywords included “breast cancer”, “imaging” and “screening”, with 51 journal articles and five United States patents being selected for review. Seventeen relevant online sources were also identified and referenced.
Results:
In addition to full-field digital mammography (FFDM), a further nine imaging modalities were identified for review. These included: digital breast tomosynthesis (DBT); breast computed tomography (BCT); automated breast ultrasound (ABUS); fusion of FFDM and ABUS; fusion of DBT and ABUS; magnetic resonance imaging (MRI); optical imaging; radio-wave imaging; and tactile sensor imaging. Important parameters were considered: diagnostic success (sensitivity and specificity), especially in dense breasts; time to acquire the images; and capital cost of the equipment.
Conclusions:
DBT is rapidly replacing FFDM although it still misses invasive cancers in dense tissue. The fusion of ABUS, either with FFDM or DBT, will lead to sensitivity and specificity approaching 100%. The fusion of opto-acoustic imaging with ultrasound holds considerable promise for the future.
Keywords: Breast cancer, Screening, Imaging, Dense breast tissue
1. Introduction: Full-Field Digital Mammography (FFDM)
The potential for mammography – an X-ray of the breast – to screen women for breast cancer was first described six decades ago by Egan [1]. Over the following thirty years, screen-film mammography was implemented in both Europe [2] and the USA [3] and proved to be a successful strategy for reducing breast cancer mortality. In the early 1990s, it was recognised that traditional mammography based on film had to fulfil three separate functions: image acquisition; image storage; and image display. The limitations in film mammography, especially the poor contrast for women with dense breast tissue, led to the development of full-field digital mammography (FFDM) by various manufacturers [4].
Once FFDM systems had been accepted by the regulatory authorities, they began to replace film mammography in many breast screening programmes [5]. However, it was still not certain that the new digital technology was outperforming its analogue counterpart. A research group called the Digital Mammographic Imaging and Screening Trial (DMIST), funded by the National Cancer Institute in the USA, came together to answer this question [6]. Based on a multi-centre study of almost 50,000 asymptomatic women who underwent both FFDM and film mammography, they concluded that while the overall diagnostic accuracy was similar, FFDM was more accurate in women under 50, women with radiographically dense breasts, and pre-menopausal women.
By analysing a national database known as SEER (Surveillance, Epidemiology, and End Results), Hendrick et al. [7] showed that the age-adjusted breast cancer mortality rates for women in the USA aged 40 to 84 decreased steadily from 1989 onwards. They estimated that between 384,00 and 614,500 breast cancer deaths had been averted through the use of mammography screening and improved treatment. Although FFDM has clearly reduced mortality, the imaging modality performs poorly in women who have dense breast tissue, with sensitivity dropping to less than 50% [8]. One way in which FFDM diagnostic performance can be improved is to inject an iodinated intra-venous contrast agent, with recent studies suggesting this approach may have some potential for screening [9].
2. Digital Breast Tomosynthesis (DBT)
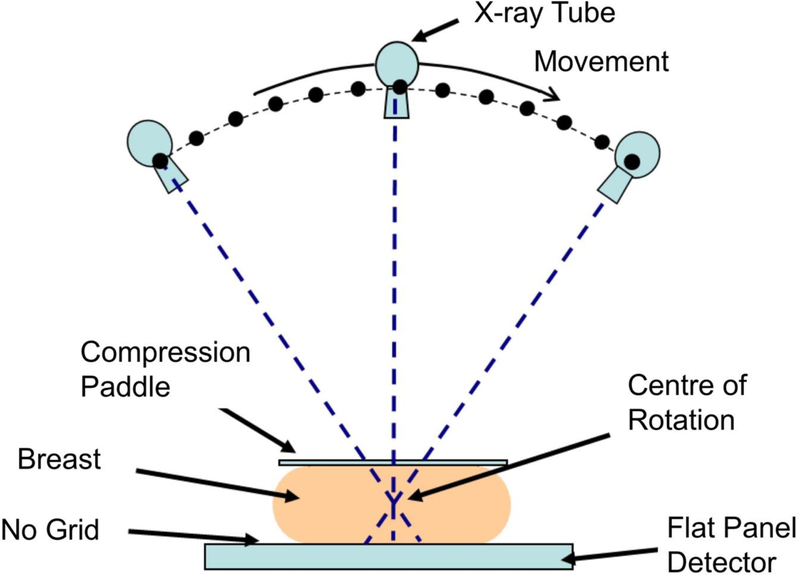
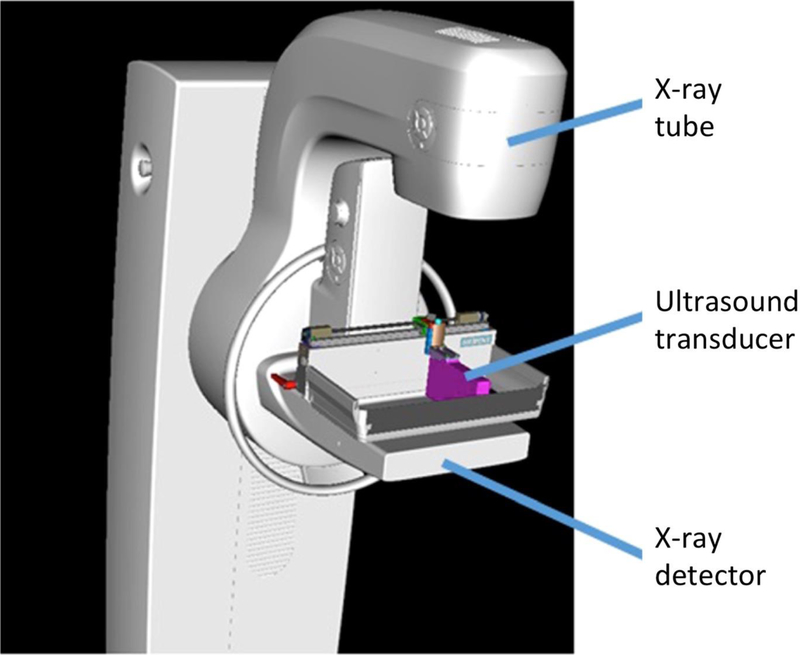
FFDM performs poorly in dense breasts because an X-ray is created from the superposition of overlapping tissue structures, and a malignant lesion shows up white on a background of dense parenchymal tissue that is also white. The problem has therefore been characterised as akin to “looking for a polar bear in a snowstorm” [10]. In the mid-1990s, the technique of digital breast tomosynthesis (DBT) was developed [11], in which a set of low-dose images is acquired while an X-ray tube rotates in an arc across the stationary breast (see Figure 1). A computer algorithm then reconstructs a series of slices through the breast, enabling the removal of overlapping tissues and allowing tumours to become more conspicuous and their morphology more apparent (see Figure 2).
Figure 1.
Standard geometry for a digital breast tomosynthesis (DBT) system, illustrating how the X-ray tube moves through an arc while the flat panel detector acquires a series of low dose images of the breast.
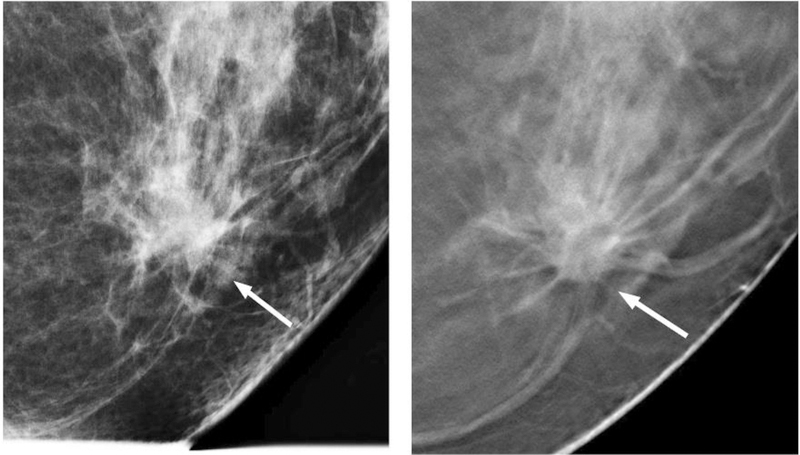
Figure 2.
A low-grade invasive ductal carcinoma, comparing full-field digital mammography (left) and digital breast tomosynthesis (right). Images courtesy of Dr Steven Poplack.
Hologic was the first manufacturer in 2010 to secure regulatory approval from the Food and Drug Administration (FDA) for its DBT system [12], and during the past decade the technology has become ubiquitous in breast-screening clinics, particularly in the USA. In the past decade there have been numerous studies comparing DBT with FFDM, although few have been designed as randomised, controlled trials. Among the first reports was by Friedewald et al. [13] who conducted a retrospective multi-centre analysis of over 400,000 examinations. They showed that by adding DBT to FFDM, the recall rate was decreased while the cancer detection rate increased. More recently, in a prospective cohort study of almost 100,000 women, Hofvind et al. [14] have shown that DBT – including synthesized 2D images – increased the detection rate of histologically favourable tumours compared to FFDM.
These successes have led Pisano and Yaffe [15] to wonder whether DBT should replace FFDM for screening purposes. Their response has been to launch the Tomosynthesis Mammography Imaging Screening Trial (TMIST), a massive randomised trial in which they will have to recruit 165,000 women [16]. This is an ambitious project, and will take at least five years to complete, but will answer an important question. It is pertinent to point out that despite its success, DBT still misses a substantial number of invasive cancers in women with dense breasts [17].
3. Breast Computed Tomography (BCT)
Some radiologists and companies such as Hologic have been referring to DBT as “3D mammography” although, strictly speaking, this is incorrect [18]. Real 3D mammography is the domain of breast computer tomography (BCT). While there are similarities between DBT and BCT, there are also some important differences. First, both DBT and BCT use X-rays to acquire an image of the breast, so the patient is exposed to ionising radiation. Second, both systems take images of the breast over an angular range – typically 30 degrees for DBT but the full 360 degrees for BCT. Finally, the breast is compressed for DBT while for BCT it is not.
Following the pioneering work of Cormack [19] and Hounsfield [20] during the 1960s, CT systems became commercially available in the mid-1970s, and the first application of BCT was reported by Chang et al. [21] at the University of Kansas. Using a conventional CT system, they performed examinations on 1625 patients and established that BCT had a breast cancer detection rate of 94% compared to 77% for mammography. BCT appeared to be especially superior to mammography for detecting cancers in dense, pre-menopausal dysplastic breasts, and seemed to be a better test for identifying pre-cancerous high-risk lesions. It is unclear why, but BCT fell out of favour over the next two decades.
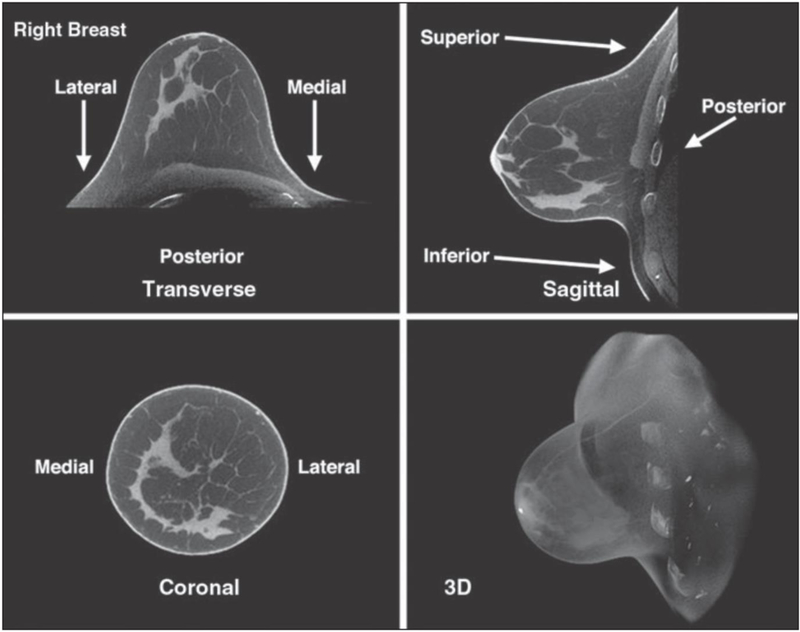
Boone et al. [22] at the University of California Davis were the first to design and test a custom BCT system based on cone-beam computed tomography in which the woman lies in a prone position with her breast suspended through an opening in the examination table. A team from the University of Rochester conducted a series of studies addressing the issues of radiation dose, breast coverage and image quality [23, 24] that enabled the spin-out company Koning to secure FDA clearance for its scanner [25]. Koning’s BCT system, which enables true 3D images of the breast to be constructed (Figure 3), has only been approved as a diagnostic device, and not for screening, which means that it may only be used as an adjunct to FFDM or DBT.
Figure 3.
Breast computer tomography (BCT) images of a 44-year-old woman with heterogeneously dense breasts and normal findings [23]. © American Roentgen Ray Society.
Kalender et al. [26] at the University of Erlangen designed their BCT system around a photon-counting detector and demonstrated that for an average glandular dose of 5 mGy, they were able to achieve high 3D spatial resolution with reliable detectability of calcifications and soft tissue delineation. Their spin-out company AB-CT received the CE mark in 2018 for its BCT system [27], and a limited clinical trial has recently been published [28]. While the BCT systems from Koning and AB-CT have successfully addressed the important issues of radiation dose and image quality, they do have challenges to address from a screening perspective: the high cost of their systems (over $1 million); inability to image the axillary region where lymph nodes are located; and the difficulty of elderly women to position their breasts in the imaging volume.
4. Automated Breast Ultrasound (ABUS)
Dense fibro-glandular tissue masks underlying tumours in both FFDM and DBT and a false negative diagnosis can have devastating consequences for the patient: more costly treatment and a poorer prognosis [29]. Although ultrasound lacks the spatial resolution of X-rays, it is capable of penetrating and distinguishing tissues of different densities remarkably well and does not suffer the problems of ionising radiation [30]. For over five decades, hand-held ultrasound (HHUS) has been used as an adjunct to mammography and continues to play a vital role in the diagnosis of breast cancer, especially in younger women with dense breast tissue, and is also employed during subsequent biopsies [31]. However, from a screening point of view, HHUS is time-consuming – taking 20 to 30 minutes per patient – and, since it relies on the skill of the operator, suffers from repeatability problems [32].
In the past fifteen years, dedicated automated breast ultrasound (ABUS) devices have been developed and commercialized. In these devices, the patient lies on a bed – either in a supine position with her breasts naturally compressed under gravity [33], or in a prone position with her breast protruding through an aperture in the bed [34]. With these two ABUS designs, 3D volumetric data is acquired, either by a linear B-mode ultrasound probe that scans across the breast in the frontal plane [33] or by a circular transducer immersed in water that moves upwards from the nipple to the chest wall [34].
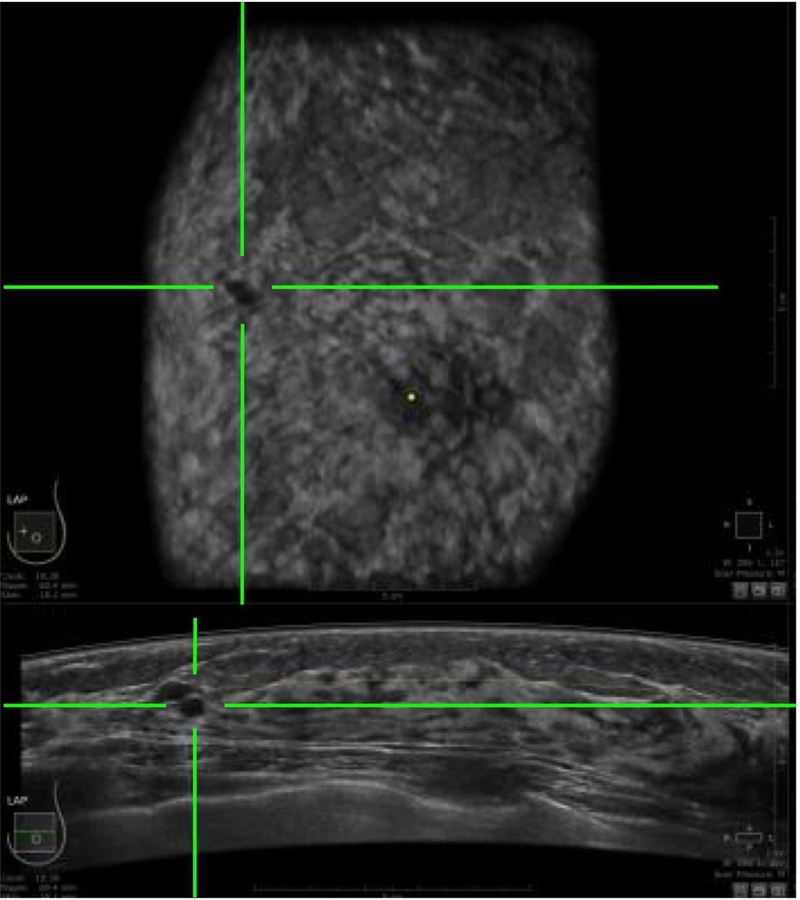
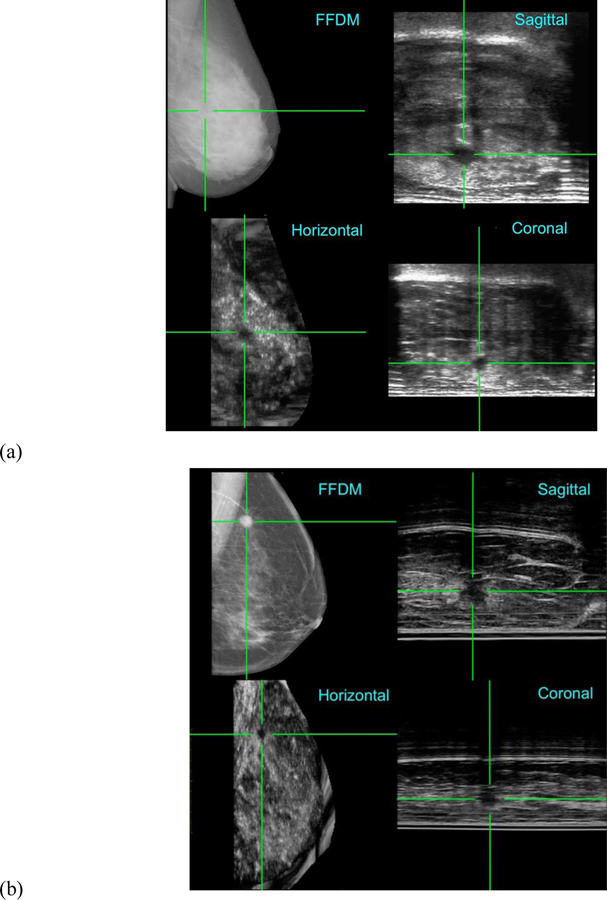
Three companies – GE [35], Siemens [36] and SonoCiné [37 – whose designs are based on the supine position, and another two manufacturers – Delphinus [38] and QT Ultrasound [39] – whose designs are based on the prone position, have secured FDA clearance for their systems to be deployed as an adjunct to mammography. Whereas the supine designs make use of reflected ultrasound signals, the prone designs record not only reflected signals but also the attenuated transmitted signals plus the speed of sound. Figure 4 shows a co-registered ABUS image in the horizontal and coronal planes where the green crosshairs indicate the location of a cancerous lesion.
Figure 4.
An automated breast ultrasound (ABUS) image in the horizontal plane (bottom) and coronal plane (top), in which the green crosshairs identify the location of a cancerous lesion.
5. Fusion of FFDM and ABUS
The most compelling evidence to date supporting the application of FFDM followed by ABUS as screening strategy was reported by Giuliano and Giuliano [40] who studied 3,418 women whose breasts were mammographically dense. They showed the addition of ABUS led to the detection of 12.3 breast cancers per 1,000 women screened compared to 4.6 per 1,000 for FFDM alone. Sensitivity increased from 76.0% to 97.7%, while specificity increased from 98.2% to 99.7%. However, this approach employed two separate imaging systems, required more than 30 minutes to acquire the images, and the breast was in a completely different orientation and degree of compression for the FFDM and ABUS images.
Over the past 25 years, there have been a number of patents issued that describe the fusion of FFDM and ABUS in a single platform [41, 42, 43]. While a system based on one of these patents was built [44], it never progressed beyond the prototype stage because the developers were unable to solve the problem of acoustically coupling the ultrasound probe to the breast. A group from the University of Cape Town [45] solved the problem by locating the X-ray camera and ultrasound probe beneath the breast in a hermetically-sealed platform filled with mineral oil [46]. The dual-modality Aceso system, named after the Greek goddess of healing, is seen in Figure 5 [47].
Figure 5.
A frontal view of the dual-modality Aceso system that combines full-field digital mammography (FFDM) and automated breast ultrasound (ABUS) in a hermetically-sealed breast platform filled with mineral oil [49]. The ultrasound probe is seen on the left and the digital X-ray camera on the right. Prior to image acquisition, both transducers are located out of the field of view on the right-hand side, and the ABUS and FFDM images are acquired simultaneously as the transducers move from right to left.
Two clinical trials of Aceso were conducted on 83 women – 65 healthy volunteers and 18 patients referred by the breast clinic at Groote Schuur Hospital – and the findings demonstrated the potential of the system to be used in a breast screening clinic [48, 49]. The average time spent by each woman in the imaging room for the radiographer to gather a full set of FFDM and ABUS images was just 10 minutes. Since the FFDM and ABUS images had a common origin and coordinate system and were acquired simultaneously with the breast in the identical degree of compression and orientation, co-registration of the images was straightforward.
In the first trial, one of the volunteers had extremely dense breasts and no prior history of breast pathology [48]. The FFDM image for the left medio-lateral oblique view confirmed no pathology but when the ABUS images in the sagittal plane were viewed as a video, a dark well-defined lesion was revealed. Figure 6a shows co-registration of the images and the green crosshairs in the three ABUS views identify the location of the lesion that is clearly occult in the FFDM image. Follow-up evaluation revealed a benign cyst. In the second trial, one of the patients presented with a 2cm suspicious, irregular mass when examined clinically [49]. The FFDM image revealed a spiculated lesion which was co-registered with the three ABUS images (Figure 6b). Based on the clinical trials and further independent testing, the Aceso system was subsequently awarded the CE mark [50].
Figure 6.
Co-registration of the FFDM images in the horizontal plane and the ABUS images in the horizontal, coronal and sagittal planes, as acquired by the Aceso system. (a) A 42-year-old healthy volunteer with extremely dense breast tissue where a benign cyst is highlighted by green crosshairs in the ABUS images, but the lesion is occult in the FFDM image [48]. A 61-year-old patient in which a malignant tumour is clearly co-registered in the ABUS and FFDM images [49]. Note: for the ABUS images, the sagittal plane view is the acquired image, whereas the coronal and horizontal plane views have been reconstructed from the sagittal plane slices.
6. Fusion of DBT and ABUS
As highlighted above in sections 2 and 4, digital breast tomosynthesis (DBT) and automated breast ultrasound (ABUS) have independently demonstrated the ability to improve cancer detection, especially in women with dense breast tissue. The next logical step is to combine DBT and ABUS in a single platform and the research group that first built a successful prototype was based at the University of Michigan. With the financial support of the National Institutes of Health and GE Healthcare, their first system incorporated an early DBT prototype from GE, a hand-held ultrasound probe located on a motorized transducer carriage which in turn was built into a TPX paddle that compressed the breast [51].
This first system was used clinically on 27 patients and, despite the length of time to acquire a full set of dual-modality images (30 minutes), plus the difficulty in locating lesions close to the chest wall, 3D fusion of the DBT and ABUS images was shown to be feasible. More recently, the Michigan group has built and tested a second prototype that combined the GE SenoClaire DBT system and the GE Invenia ABUS system, where the compression paddle that incorporates the ultrasound transducer was made from a flexible polyester mesh [52]. In a clinical study of 13 patients, acquisition time for two DBT views and one ABUS view was reduced to 15 minutes, while successful co-registration of lesions was demonstrated.
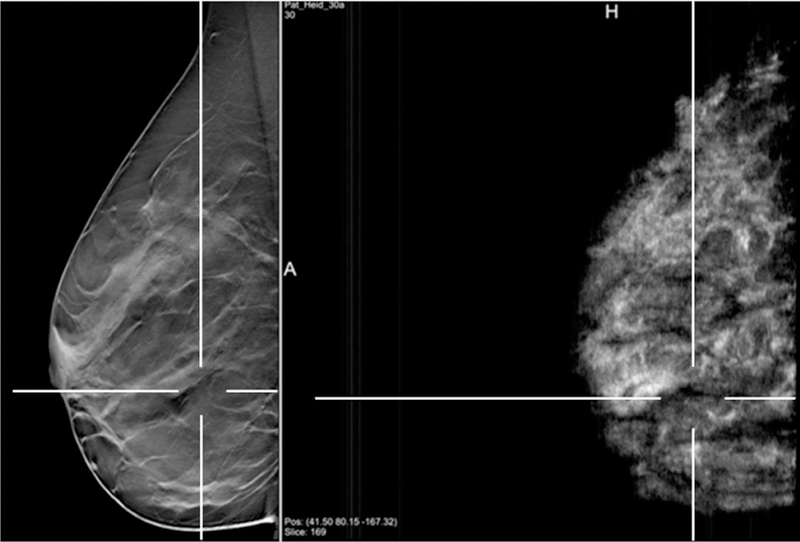
There are two separate groups in Germany, one based in Erlangen [53] and the other in Heidelberg [54], that have developed similar prototypes based on Siemens technology – the Mammomat Inspiration DBT system plus the Acuson S2000 ABUS system. As with the University of Michigan prototype, the ultrasound transducer is built into a modified compression paddle and is located above the breast (Figure 7). The Heidelberg group studied 23 patients, demonstrating co-registration of lesions in both imaging modalities (Figure 8) although, of the 6 malignant lesions, all were identified with DBT but only 4 with ABUS [54]. Coverage with ABUS was only 66% of DBT, a direct result of the ultrasound transducer being located above the breast which limited the acoustic coupling. In contrast, by locating the ultrasound probe beneath the breast, the University of Cape Town group is able to achieve ABUS coverage of over 90% [49].
Figure 7.
Fusion of DBT and ABUS with the Siemens Mammomat Inspiration and Acuson S2000 systems [54]. Note: the ultrasound transducer is built into the compression paddle and is located above the breast. © European Society of Radiology.
Figure 8.
Fusion of DBT and ABUS images in the sagittal plane, showing co-registration of a cancerous lesion [54]. © European Society of Radiology.
7. Magnetic Resonance Imaging (MRI)
Among current clinical imaging modalities, magnetic resonance imaging (MRI) of the breast has the highest sensitivity for early detection of cancer, especially in women with dense breast tissue [55]. However, its application has been primarily for diagnosis, with its use a screening tool largely confined to women with a lifetime risk of greater than 20%. In a landmark paper published in The Lancet, a group from Aachen in Germany advocated the use of MRI to detect breast cancer at its earliest stage, recommending the modality as a replacement for, rather than as an adjunct to mammography [56]. But, from a screening point of view, MRI has four major drawbacks compared to mammography: the time spent in the scanner; the need to inject a contrast agent such as gadolinium; poor specificity; and the high capital cost of the equipment.
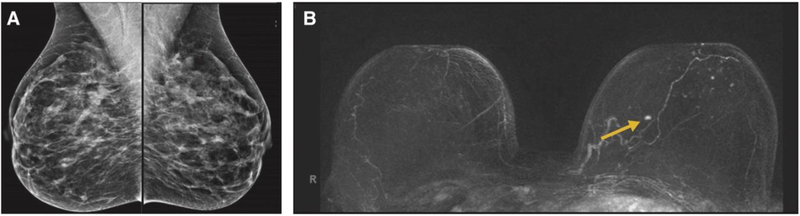
To address the first drawback, the Aachen group developed an abbreviated protocol where the acquisition time was reduced from 17 to 3 minutes [57]. They studied 443 women who had a normal or benign mammogram and, for those with heterogeneously dense or extremely dense breasts, also had a normal or benign ultrasound. As seen in Figure 9, an invasive ductal carcinoma that was mammographically occult was detected with the abbreviated MRI protocol. Reading time was less than 30 seconds for the abbreviated protocol, which also resulted in an additional cancer yield of 18.2 per 1,000. In a recent review article, Kuhl [58] provided the rationale to use MRI in general, and abbreviated MRI in particular, for breast cancer screening.
Figure 9.
Comparison of FFDM images (A) and MRI images (B) of both breasts of a 45-year-old woman with a moderate history of breast cancer [57]. An invasive ductal carcinoma in the left breast was clearly evident in the MRI image but was occult in the FFDM image. © American Society of Clinical Oncology.
To address the second drawback of MRI – the need for a contrast agent with the associated risk and cost – a group from the University of Washington in Seattle has been exploring the application of diffusion-weighted imaging or DWI [59]. DWI uses motion-sensitizing gradients to measure the random movement of water molecules and provides information on tissue cellularity and microstructure and has the potential to differentiate benign and malignant breast lesions. Although DWI lacks the spatial resolution of dynamic contrast-enhanced MRI, which looks at areas of altered vascularity, the researchers are optimistic that recent technical advances in DWI will lead to improved sensitivity [60].
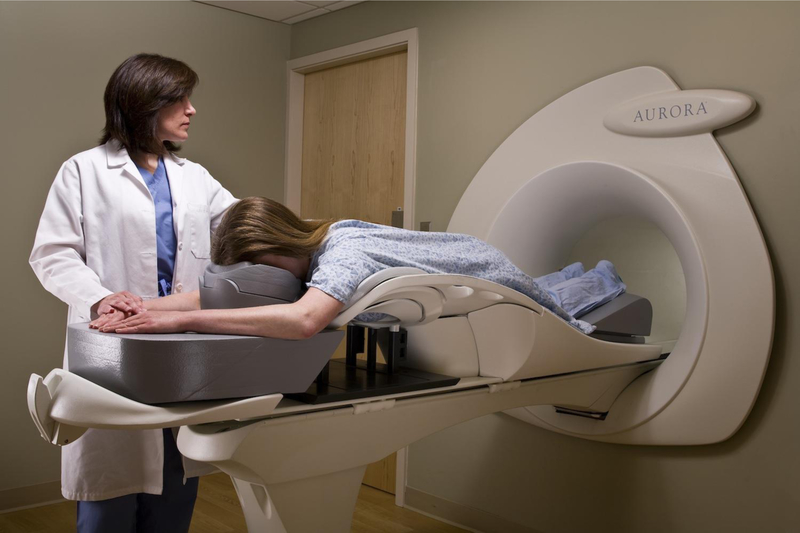
With regard to the poor specificity of MRI, Kuhl [58] has argued that positive predictive value (PPV), which is the rate of true-positive diagnoses over all positive diagnoses (true and false), is a better measure of diagnostic performance and the PPV for MRI is equal to that of mammographic screening. Only one company – Aurora – has developed a dedicated 1.5 Tesla breast MRI scanner [61], although the cost is still significantly greater than FFDM and DBT systems from other manufacturers. With sensitivity and specificity of 92% and 89% respectively, the diagnostic performance of the Aurora system (Figure 10) was comparable to other MRI scanners [62].
Figure 10.
A dedicated 1.5 Tesla breast MRI system manufactured by Aurora Healthcare Corporation [61].
8. Optical Imaging
In a wide-ranging review of optical breast imaging by near-infrared (NIR) spectroscopy, Grosenick et al. [63] have explored the optical properties of healthy breast tissue and of malignant and benign lesions, and the various methods to improve the contrast between healthy and diseased tissue. They concluded that sensitivity and specificity of optical mammography were likely too low for its application as a screening tool, primarily because the poor spatial resolution of diffuse optical imaging prevented early detection of breast cancer. However, a group from Boston has presented data on a multi-modal system that incorporates both DBT and co-registered dynamic diffuse optical tomography (DOT). They demonstrated successful fusion of the anatomical location of a tumour in the DBT image with the functional location of increased haemoglobin uptake in the DOT image [64]. Although this hybrid system is not yet in routine clinical use as a screening tool, the authors believe their first patient images hold considerable promise.
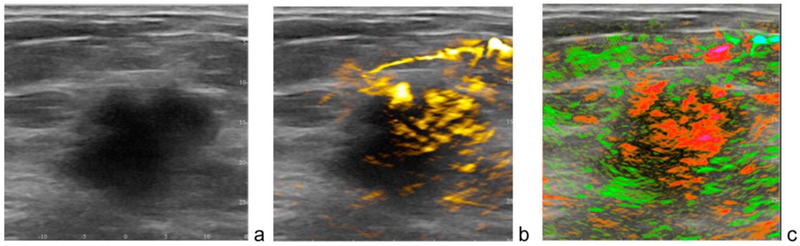
A dual-modality system that integrates opto-acoustic (OA) imaging with conventional ultrasound for co-registered functional and anatomical mapping of breast tumours has been developed and tested clinically in a multi-centre trial of more than 2,000 patients [65]. The Imagio system, designed and manufactured by Seno Medical Instruments [66], takes advantage of the OA effect – the conversion of laser light energy into sound energy. A hand-held probe that incorporates both ultrasound and NIR lasers is able to detect the presence of tumour angiogenesis, taking advantage of the fact that malignant and benign lesions have different levels of haemoglobin and oxygen concentration. As seen in Figure 11, a malignant mass is seen in the gray scale ultrasound image, with increased haemoglobin due to angiogenesis shown in yellow, and diffuse internal blood oxygenation shown in red. The developers of Imagio envision a next-generation system that will support three-dimensional OA tomography that could enable automated screening for breast cancer [65].
Figure 11.
Dual-modality opto-acoustic and ultrasound images of a patient acquired with the Imagio system manufactured by Seno Medical Instruments [66]. (a) A 2.6 cm malignant mass seen on gray-scale ultrasound. (b) Increased haemoglobin due to high density of angiogenesis. (c) Diffuse internal blood deoxygenation [65]. © Alexander Oraevsky.
9. Radio-Wave Imaging
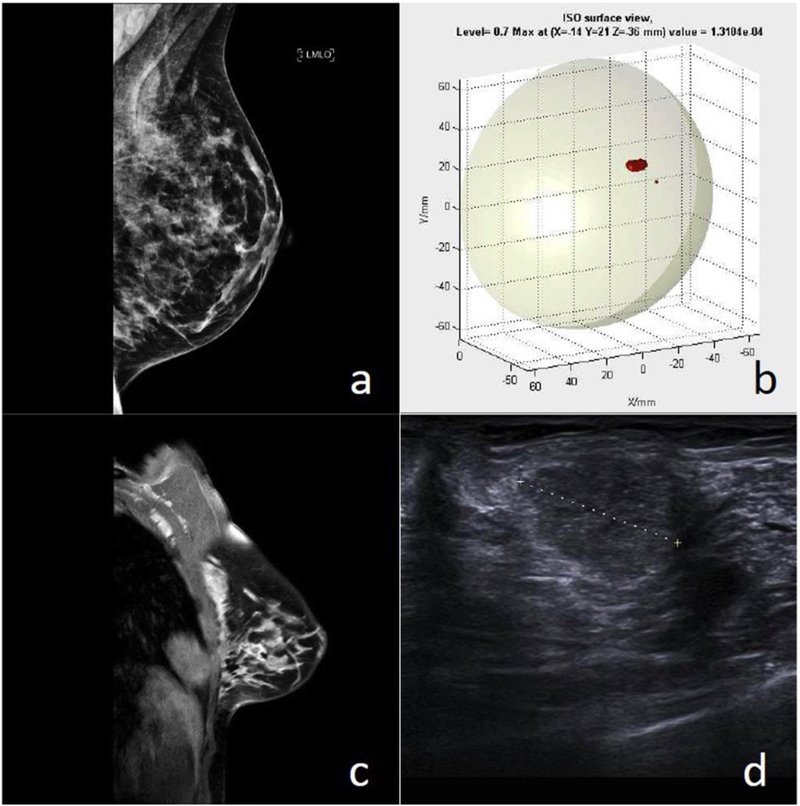
Over the past 15 years, engineers at Bristol University in the UK have developed a breast imaging system called MARIA that is based on radio-waves [67]. Similar to the ABUS system from Delphinus described earlier in Section 4 [38], the patient lies prone on a bed with her breast hanging under the influence of gravity in the transducer. The MARIA transducer is a concave cup that can accommodate a range of breast sizes (310 to 850 cm3) and incorporates an array of 60 radio-frequency antennae operating in the 3 to 8 GHz range that surround the breast. Each antenna transmits sequentially and the other 59 record the signals reflected from the underlying tissue, enabling the device to construct a 3D map of the breast [68].
MARIA takes less than 5 minutes to scan each breast and captures variations in three tissue parameters – impedance, permittivity and conductance – that enable a clinician to differentiate between normal tissue and lesions. Two clinical trials of MARIA have been reported, the first with 86 symptomatic patients recruited from a breast clinic [67], while the second was a multi-centre study of 225 patients who had presented with either malignant or benign lesions [68]. In some of the cases, patients were also imaged using FFDM, hand-held ultrasound (HHUS) and MRI. As illustrated in Figure 12, for one of the patients the FFDM image was negative, whereas MARIA, MRI and HHUS all revealed a lesion that later biopsy confirmed as a Grade 2 carcinoma.
Figure 12.
Patient with a Grade 2 carcinoma [68]. (a) FFDM image which was reported as normal. (b) MARIA image, showing a lesion in the upper outer quadrant (UOQ). (c) Contrast-enhanced MRI, showing a 16 mm irregular mass in the UOQ. (d) Ultrasound image, confirming the lesion. © European Journal of Radiology.
Results from both clinical trials showed an overall sensitivity of 76% which was similar across benign and malignant findings, as well as in dense breasts. The technology has been commercialized by a company called Micrima which received the CE mark for MARIA in 2015 [69]. Because radio-waves produce no ionizing radiation and MARIA does not require breast compression, the company is optimistic their technology will be a competitor to FFDM. However, they will still need to address their relatively low sensitivity and the limitation on breast cup sizes (32A to 42DD).
10. Tactile Sensor Imaging
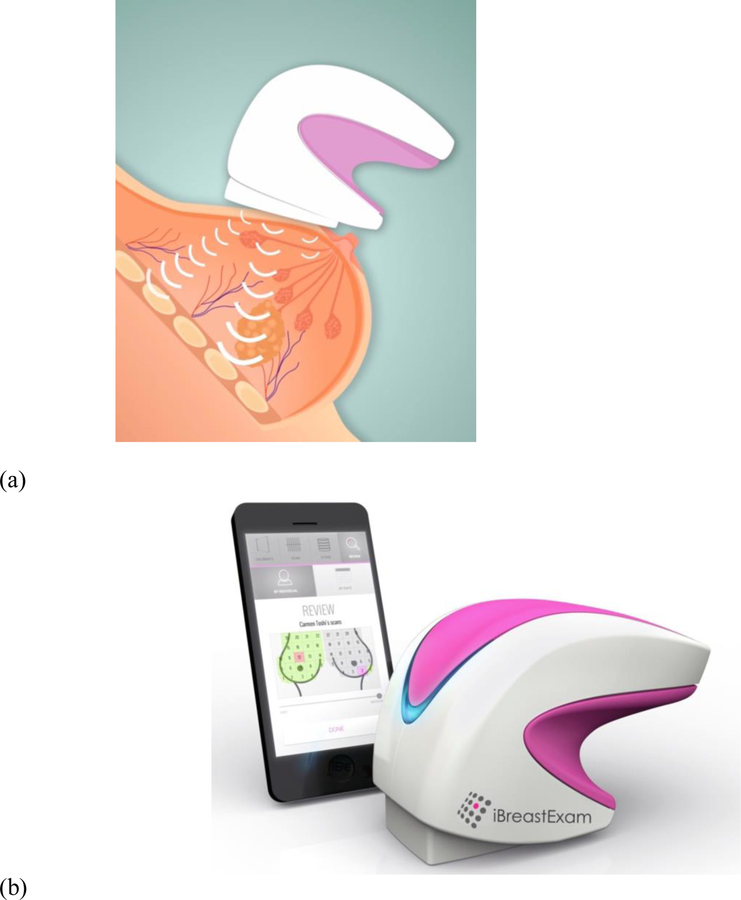
In developing countries contemplating the introduction of a breast screening programme, the cost of FFDM, DBT or ABUS systems – priced between $100,000 and $500,000 – can be a significant impediment. Scientists at Drexel University in Philadelphia have developed a hand-held and battery-operated breast scanner that addresses the problem (see Figure 13). The iBreastExam [70], which has secured both CE mark and FDA approvals, has the potential to make an important contribution to early detection of breast cancer in low-resource environments. The tactile sensors, for which a patent is pending [71], are based on the principle of piezo-electric detectors that generate quantitative information – such as elastic and shear modulus – regarding tissue compression and stiffness. Breast cancer tumours tend to be hard and stiff when compared to normal breast tissue.
Figure 13.
The hand-held iBreastExam [70]. (a) Based on the piezo-electric principle, the 4×4 array of tactile pressure sensors measure the stiffness of the underlying tissues. (b) The device communicates wirelessly with a mobile phone that displays and records the data in real time.
The iBreastExam (iBE) has been tested in two clinical trials [72, 73]. Broach et al. [72] studied 78 patients who, as part of their diagnostic work-up, underwent an examination with both HHUS and iBE. In addition, 52 of the patients had diagnostic mammography while 39 had follow-up biopsies. In 60 patients, various masses – such as fibroadenomas, cysts, fat necrosis, DCIS or tumours – were confirmed, with 12 women being diagnosed with breast cancer. The iBE correctly identified 66 lesions with sensitivity of 86% and specificity of 89%, confirming the device’s potential as a low-cost screening tool. A second clinical trial of 1,300 women in rural India established similar values for diagnostic success [73], which has led to the iBE now being offered in more than 60 sites throughout the country.
Highlights.
Full-field digital mammography performs poorly in dense breast tissue
Automated breast ultrasound holds promise when fused with mammography
Magnetic resonance imaging has excellent sensitivity but is still expensive
Fusion of opto-acoustic imaging with ultrasound has significant potential for screening
Radio-wave imaging still requires extensive clinical trials before use as a screening tool
Acknowledgments
I acknowledge the grant funding from the National Institutes of Health (R21 CA101705) for the early development of the dual-modality Aceso system [45, 50], and the Cancer Association of South Africa (CANSA) that supported the clinical trials [48, 49] which led to the award of the CE mark for Aceso.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
I am an employee of, and a board member and shareholder in CapeRay Medical (Pty) Ltd, a company whose product called Aceso is described in this review paper.
CLV is a board member and shareholder in CapeRay.
Ethical approval
Not required.
References
- [1].Egan RL, “Experience with mammography in a tumor institution. Evaluation of 1,000 studies”, Radiology, 75: 894–900, 1960. [DOI] [PubMed] [Google Scholar]
- [2].Larsson LG, Nyström L, Wall S, Rutqvist L, Andersson I, Bjurstam N, Fagerberg G, Frisell J, Tabár L, “The Swedish randomised mammography screening trials: analysis of their effect on the breast cancer related excess mortality”, Journal of Medical Screening, 3(3) :129–132, 1996. [DOI] [PubMed] [Google Scholar]
- [3].Hendrick RE, Smith RA, Rutledge JH, Smart CR, “Benefit of screening mammography in women aged 40–49: a new meta-analysis of randomized controlled trials”, Journal of the National Cancer Institute Monographs, 22: 87–92, 1997. [DOI] [PubMed] [Google Scholar]
- [4].Pisano ED, “Current status of full-field digital mammography”, Radiology, 214: 26–28, 2000. [DOI] [PubMed] [Google Scholar]
- [5].Kopans DB, “Breast cancer screening: Where have we been and where are we going? A personal perspective based on history, data and experience”, Clinical Imaging, 50: 91–95, 2018. [DOI] [PubMed] [Google Scholar]
- [6].Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, Conant EF, Fajardo LL, Bassett L, D’Orsi C, Jong R, Rebner M, “Diagnostic performance of digital versus film mammography for breast-cancer screening”, New England Journal of Medicine, 353(17): 1773–1783, 2005. [DOI] [PubMed] [Google Scholar]
- [7].Hendrick RE, Baker JA, Helvie MA, “Breast cancer deaths averted over 3 decades”, Cancer, 125(9): 1482–1488, 2019. [DOI] [PubMed] [Google Scholar]
- [8].Kelly KM, Dean J, Comulada WS, Lee S-J. “Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts”, European Radiology, 20(3): 734–742, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim G, Phillips J, Cole E, Brook A, Mehta T, Slanetz P, Fishman MDC, Karimova E, Mehta R, Lotfi P, Resteghini N, Raj S, Dialani V, “Comparison of contrast-enhanced mammography with conventional digital mammography in breast cancer screening: a pilot study”, Journal of the American College of Radiology, doi: 10.1016/j.jacr.2019.04.007, 2019. [DOI] [PubMed] [Google Scholar]
- [10].“Looking for a polar bear in a snow storm”, http://bit.ly/2M0Lj0V, accessed 31 May 2019.
- [11].Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong BH, Landberg CE, Slanetz PJ, Giardino AA, Moore R, Albagli D, DeJule MC, Fitzgerald PF, Fobare DF, Giambattista BW, Kwasnick RF, Liu J, Lubowski SJ, Possin GE, Richotte JF, Wei CY, Wirth RF, “Digital tomosynthesis in breast imaging”, Radiology, 205(2): 399–406, 1997. [DOI] [PubMed] [Google Scholar]
- [12].“Hologic receives FDA approvable letter for Selenia Dimensions (3-D) digital mammography tomosynthesis system”, http://bit.ly/2VBvEop, 23 November 2010.
- [13].Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, Hayes MK, Copit DS, Carlson KL, Cink TM, Barke LD, Greer LN, Miller DP, Conant EF, “Breast cancer screening using tomosynthesis in combination with digital mammography”, Journal of the American Medical Association, 311(24): 2499–2507, 2014. [DOI] [PubMed] [Google Scholar]
- [14].Hofvind S, Hovda T, Holen ÅS, Lee CI, Albertsen J, Bjørndal H, Brandal SHB, Gullien R, Lømo J, Park D, Romundstad L, Suhrke P, Vigeland E, Skaane P, “Digital breast tomosynthesis and synthetic 2D mammography versus digital mammography: evaluation in a population-based screening program”, Radiology, 287(3): 787–794, 2018. [DOI] [PubMed] [Google Scholar]
- [15].Pisano ED, Yaffe MJ, “Breast cancer screening: should tomosynthesis replace digital mammography?”, Journal of the American Medical Association, 311(24): 2488–2489, 2014. [DOI] [PubMed] [Google Scholar]
- [16].“A question of equipoise”, http://bit.ly/2K2MRFh, accessed 31 May 2019.
- [17].Berg WE, “Current status of supplemental screening in dense breasts”, Journal of Clinical Oncology, 34(16): 1840–1843, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].“True 3D X-ray breast images”, http://bit.ly/2HtPWw6, accessed 31 May 2019.
- [19].Vaughan CL, Imagining the Elephant: A Biography of Allan MacLeod Cormack, ISBN 978–1-86094–988-3, Imperial College Press, London, 304 pages, 2008. [Google Scholar]
- [20].Hounsfield GN, “Computerized transverse axial scanning (tomography): Part I. Description of system”, British Journal of Radiology, 46(552): 1016–1022, 1973. [DOI] [PubMed] [Google Scholar]
- [21].Chang CH, Sibala JL, Fritz SL, Dwyer SJ, Templeton AW, Lin F, Jewell WR, “Computed tomography in detection and diagnosis of breast cancer”, Cancer, 46(4 Suppl): 939–946, 1980. [DOI] [PubMed] [Google Scholar]
- [22].Boone JM, Kwan AL, Seibert JA, Shah N, Lindfors KK, Nelson TR, “Technique factors and their relationship to radiation dose in pendant geometry breast CT”, Medical Physics, 32(12): 3767–3776, 2005. [DOI] [PubMed] [Google Scholar]
- [23].O’Connell A, Conover DL, Zhang Y, Seifert P, Logan-Young W, Lin CF, Sahler L, Ning R “Cone-beam CT for breast imaging: radiation dose, breast coverage, and image quality”, American Journal of Roentgenology, 195(2): 496–509, 2010. [DOI] [PubMed] [Google Scholar]
- [24].O’Connell AM, Karellas A, Vedantham S, “The potential role of dedicated 3D breast CT as a diagnostic tool: review and early clinical examples”, The Breast Journal, 20(6): 592–605, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].“A better way of breast imaging”, http://bit.ly/2X4VfaY, accessed 31 May 2019.
- [26].Kalender WA, Koldit D, teiding C, Ruth V, Lück F, ler AC R, Wenkel E, “Technical feasibility proof for high-resolution low-dose photon-counting CT of the breast”, European Radiology, 27(3): 1081–1086, 2017. [DOI] [PubMed] [Google Scholar]
- [27].“Discover true isotropic 3D resolution”, https://www.ab-ct.com/, accessed 31 May 2019.
- [28].Berger N, Marcon M, Saltybaeva N, Kalender WA, Alkadhi H, Frauenfelder T, Boss A, “Dedicated breast computed tomography with a photon-counting detector: initial results of clinical in vivo imaging”, Investigative Radiology, doi: 10.1097/RLI.0000000000000552, 2019. [DOI] [PubMed] [Google Scholar]
- [29].Nelson HD, O’Meara E , Kerlikowske K, Balch S, Miglioretti D, “Factors associated with rates of false-positive and false-negative results from digital mammography screening: An analysis of registry data”, Annals of Internal Medicine, 164: 226–235, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang W, Dempsey PJ, “Diagnostic breast ultrasound: Current status and future directions”, Ultrasound Clinics, 4: 117–133, 2009. [DOI] [PubMed] [Google Scholar]
- [31].Dempsey PJ, “The history of breast ultrasound”, Journal of Ultrasound in Medicine, 23: 887–894, 2004. [DOI] [PubMed] [Google Scholar]
- [32].Yaffe MJ, Jong RA “Adjunctive ultrasonography in breast cancer screening”, Lancet, 387: 313–314, 2016. [DOI] [PubMed] [Google Scholar]
- [33].Wilczek B, Wilczek HE, Rasouliyan L, Leifland K, “Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program”, European Journal of Radiology, 85: 1554–1563, 2016. [DOI] [PubMed] [Google Scholar]
- [34].Sak M, Duric N, Littrup P, Bey-Knight L, Ali H, Vallieres P, Sherman ME, Gierach GL, “Using speed of sound imaging to characterize breast density”, Ultrasound in Medicine and Biology, 43: 91–103, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].“Look differently at dense breast tissue”, http://bit.ly/2SfxyLb, accessed 13 July 2019.
- [36].“A comprehensive solution for breast ultrasound”, http://bit.ly/2JFaEZT, accessed 13 July 2019.
- [37].“Automated whole breast ultrasound”, http://bit.ly/2LQ8DwM, accessed 13 July 2019.
- [38].“It’s time to talk about breast health”, http://bit.ly/2XMPSwn, accessed 13 July 2019.
- [39].“Quantitative transmission ultrasound: an evolution in breast imaging”, http://bit.ly/2YM6ztf, accessed 13 July 2019.
- [40].Giuliano V, Giuliano C, “Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts”, Clinical Imaging, 37(3): 480–486, 2013. [DOI] [PubMed] [Google Scholar]
- [41].hmulewit A, “Methods and apparatus for performing sonomammography”, United States Patent and Trademark Office, Patent Number 5,474,072, issued 12 December 1995.
- [42].Dines KA, Kelly-Fry E, Romilly AP, “Mammography method and apparatus”, United States Patent and Trademark Office, Patent Number 6,574,499, issued 3 June 2003.
- [43].Besson GM, Nields MW, “Integrated X-ray and ultrasound medical imaging system”, United States Patent and Trademark Office, Patent Number 6,846,289, issued 25 January 2005. [Google Scholar]
- [44].uri J , Danielson T, Guo Y, Janer R. Fischer’s fused full field digital mammography and ultrasound system (FFDMU )”. In: Bos L, editor. Medical and Care Compunetics, 2 IOS Press, pp. 177–200, 2005. [PubMed] [Google Scholar]
- [45].Vaughan CL, Evans MD, “Diagnosing breast cancer: an opportunity for innovative engineering”, South African Medical Journal, 102(6): 562–564, 2012. [DOI] [PubMed] [Google Scholar]
- [46].Evans MD, mith RV, Vaughan CL “Dual-modality mammography”, United States Patent and Trademark Office, Patent Number 9,636,073, issued 2 May 2017.
- [47].Vaughan CL, “Detecting early breast cancer by integrating full-field digital mammography and automated breast ultrasound”, Diagnostic Imaging Europe, 33(5): 62–64, 2017. [Google Scholar]
- [48].Vaughan CL, Douglas TS, Said-Hartley Q, Baasch RV, Boonzaier JA, Goemans BC, Harverson J, Mingay MW, Omar , mith RV, Venter NC, Wilson H , “Testing a dual-modality system that combines full-field digital mammography and automated breast ultrasound”, Clinical Imaging, 40(3): 498–505, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Padia K, Douglas T , Cairncross LL, Baasch RV, Vaughan CL, “Detecting breast canscer with a dual-modality device”, Diagnostics, 7(1): 17, 2017. http://bit.ly/2sVr7mk, accessed 25 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].“Aceso dual-modality imaging: early detection of cancer in dense breasts, http://bit.ly/2HlVybm , accessed 25 August 2019.
- [51].Sinha SP, Roubidoux MA, Helvie MA, Nees AV, Goodsitt MM, LeCarpentier GL, Fowlkes JB, Chalek CL, Carson PL, “Multi-modality 3D breast imaging with X-ray tomosynthesis and automated ultrasound”, Proceedings of the 29th International Conference of the IEEE EMBS, Lyon, France, pp. 1335–1338, 23–26 August 2007. [DOI] [PubMed] [Google Scholar]
- [52].Larson ED, Lee WM, Roubidoux MA, Goodsitt MM, Lashbrook C, Davis CE, Kripfgans OD, Carson PL, “Preliminary clinical experience with a combined automated breast ultrasound and digital breast tomosynthesis system”, Ultrasound in Medicine & Biology, 44(3): 734–742, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schulz-Wendtland R, Jud SM, Fasching PA, Hartmann A, Radicke M, Rauh C, Uder M, Wunderle M, Gass P, Langemann H, Beckmann MW, Emons J, “A standard mammography unit - standard 3D ultrasound probe fusion prototype: first results”, Geburtshilfe und Frauenheilkunde, 77(6): 679–685, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schaefgen B, Heil J, Barr RG, Radicke M, Harcos A, Gomez C, Stieber A, Hennigs A, von Au A, Spratte J, Rauch G, Rom J, Schütz F, Sohn C, Golatta M, “Initial results of the FUSION-X-US prototype combining 3D automated breast ultrasound and digital breast tomosynthesis”, European Radiology, 28(6):2499–2506, 2018. [DOI] [PubMed] [Google Scholar]
- [55].Mann RM, Cho N, Moy L, “Breast MRI: state of the art”, Radiology, 292: 520–536, 2019 [DOI] [PubMed] [Google Scholar]
- [56].Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, Kuhn W, Schild HH, “MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study”, The Lancet, 370(9586):485–492, 2007. [DOI] [PubMed] [Google Scholar]
- [57].Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB, “Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI”, Journal of Clinical Oncology, 32(22): 2304–2310, 2014. [DOI] [PubMed] [Google Scholar]
- [58].Kuhl CK, “Abbreviated magnetic resonance imaging (MRI) for breast cancer screening: rationale, concept, and transfer to clinical practice”, Annual Reviews of Medicine, 70: 501–519, 2019. [DOI] [PubMed] [Google Scholar]
- [59].Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund, “Diffusion-weighted breast MRI: clinical applications and emerging techniques”, Journal of Magnetic Resonance Imaging, 45(2): 337–355, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gagnon L, “Abbreviated protocol may address barriers to breast MRI screening”, http://bit.ly/2HuiF3x, accessed 27 August 2019. [Google Scholar]
- [61].“The Aurora 1.5T dedicated breast MRI system is designed specifically for breast imaging”, http://bit.ly/2ZrrTUf, accessed 27 August 2019. [Google Scholar]
- [62].Hillman BJ, Harms SE, Stevens G, Stough RG, Hollingsworth AB, Kozlowski KF, Moss LJ, “Diagnostic performance of a dedicated 1.5-T breast MR imaging system”, Radiology, 265(1): 51–58, 2012. [DOI] [PubMed] [Google Scholar]
- [63].Grosenick D, Rinneberg H, Cubeddu R, Taroni P, “Review of optical breast imaging and spectroscopy”, Journal of Biomedical Optics, 21(9): 091311, 2016. [DOI] [PubMed] [Google Scholar]
- [64].Zimmermann BB, Deng B, Singh B, Martino M, Selb J, Fang Q, Sajjadi AY, Cormier J, Moore RH, Kopans DB, Boas DA, Saksena MA, Carp SA, “Multimodal breast cancer imaging using coregistered dynamic diffuse optical tomography and digital breast tomosynthesis”, Journal of Biomedical Optics, 22(4): 46008, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oraevsky AA, Clingman B, Zalev J, Stavros AT, Yang WT, Parikh JR, “Clinical optoacoustic imaging combined with ultrasound for coregistered functional and anatomical mapping of breast tumors”, Photoacoustics, 12: 30–45, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].“Opto-acoustic imaging represents a breakthrough in diagnostic imaging technology”, http://bit.ly/2UeUDPg, accessed 27 August 2019.
- [67].Preece AW, Craddock I, Shere M, Jones L, Winton HL, “MARIA M4: clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection”, Journal of Medical Imaging, 3(3): 033502, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shere M, Lyburn I, Sidebottom R, Massey H, Gillett C, Jones L, “MARIA® M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system to detect lesions in the symptomatic breast”, European Journal of Radiology, 116: 61–67, 2019. [DOI] [PubMed] [Google Scholar]
- [69].“Micrima: Developing technology for breast cancer screening”, http://bit.ly/2NDWYC2, accessed 27 August 2019.
- [70].“Making breast exams quick & painless: radiation-free and easy for women everywhere”, http://bit.ly/2zpynZx, accessed 27 August 2019.
- [71].Shih WY, Shih W-H, Xu X, “Depth measurement in tissue using piezoelectric sensors having different probe sizes”, United States Patent and Trademark Office, Patent Application Number US20160331300, filed 7 January 2015. [Google Scholar]
- [72].Broach RB, Geha R, Englander BS, DeLaCruz L, Thrash H, Brooks AD, “A cost-effective handheld breast scanner for use in low-resource environments: a validation study”, World Journal of Surgical Oncology, 14:277, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Khandelwal R, “Health worker led breast examination in rural India using electro-mechanical hand-held breast palpation device”, Journal of Cancer Prevention & Current Research, 9(3): 138–141, 2018. [Google Scholar]