Abstract
Autism spectrum disorder (ASD) affects more boys than girls. Recent animal studies found that early life exposure to ambient particles caused autism-like behaviors only in males. However, there has been little study of sex-specificity of effects on ASD in humans. We evaluated ASD risk associated with prenatal and first year of life exposures to particulate matter less than 2.5 µm in aerodynamic diameter (PM2.5) by child sex. This retrospective cohort study included 246,420 singleton children born in Kaiser Permanente Southern California (KPSC) hospitals between 1999 and 2009. The cohort was followed from birth through age five to identify 2471 ASD cases from the electronic medical record. Ambient PM2.5 and other regional air pollution measurements (PM less than 10 µm, ozone, nitrogen dioxide) from regulatory air monitoring stations were interpolated to estimate exposure during each trimester and first year of life at each geocoded birth address. Hazard ratios (HRs) were estimated using Cox regression models to adjust for birth year, KPSC medical center service areas, and relevant maternal and child characteristics. Adjusted HRs per 6.5 µg/m3 PM2.5 were elevated during entire pregnancy [1.17 (95% confidence interval (CI), 1.04–1.33)]; first trimester [1.10 (95% CI, 1.02–1.19)]; third trimester [1.08 (1.00–1.18)]; and first year of life [1.21 (95% CI, 1.05–1.40)]. Only the first trimester association remained robust to adjustment for other exposure windows, and was specific to boys only (HR = 1.18; 95% CI, 1.08–1.27); there was no association in girls (HR = 0.90; 95% CI, 0.76–1.07; interaction p-value 0.03). There were no statistically significant associations with other pollutants. PM2.5-associated ASD risk was stronger in boys, consistent with findings from recent animal studies. Further studies are needed to better understand these sexually dimorphic neurodevelopmental associations.
Keywords: autism, air pollution, sex difference
Graphical Abstract
Introduction
Autism spectrum disorder (ASD) prevalence increased dramatically during 2000–2012 from 0.7% to 1.7% in the United States (Baio et al., 2018). The increase can only partly be explained by better ascertainment (Baio, 2014); causes of ASD, in prenatal and early postnatal life, are likely multi-factorial (Lyall et al., 2014). A particularly strong risk factor is male sex. ASD is about four times more likely to occur in boys than in girls (Baio et al., 2018), for reasons that are not well understood.
Recent epidemiological studies have found associations between ASD and ambient particulate matter < 2.5 µm in aerodynamic diameter (PM2.5) during gestation and the first year of life (Lam et al., 2016). Exposure to particles causes neuroinflammatory effects, perhaps mediated by particle-induced oxidative stress and systemic inflammation (Block and Calderón-Garcidueñas, 2009; Campbell et al., 2009; Campbell et al., 2014; Rossignol and Frye, 2014). Recent toxicological studies showed that PM2.5 exposure during gestation and early life causes ASD-related behaviors in male mice only, including communication deficits, poor social interaction and novelty avoidance; and causes neuroinflammation, including microglial cell activation (Church et al., 2017; Li et al., 2017).
Two previous epidemiologic studies found stronger associations of ASD with PM2.5 in boys than girls (Raz et al., 2015; Ritz et al., 2018). However, the difference in effect was not statistically significant (Raz et al., 2015) or was not reported (Ritz et al., 2018). Two other recently published studies reported similar associations of ASD with PM2.5 in boys and in girls (Kaufman et al., 2019; Pagalan et al., 2018). Many studies have had small sample sizes and hence were underpowered to identify differences in particle-associated risk by sex. Therefore, additional research in large sample sizes is needed to determine whether the sex differences in behavioral effects in animal models is relevant for human ASD.
Another uncertainty to epidemiologic studies to date is the vulnerable developmental window of exposure, which has not been extensively examined with mutual adjustment for multiple exposure windows. Previous studies generally found stronger ASD-PM2.5 associations during the third trimester and/or first year of life than earlier in pregnancy (Flores-Pajot et al., 2016).
Based on the evidence from animal toxicological studies, we hypothesized that boys would have a higher risk of ASD associated with PM2.5 exposure during early development than girls. In a large, population-based pregnancy cohort from Kaiser Permanente Southern California (KPSC), we examined sex-specific associations of ASD with prenatal and first year of life PM2.5 and other regional pollutant exposures.
Materials and Methods
Study design and population.
This retrospective cohort study included mother-child pairs with singleton deliveries in KPSC hospitals between January 1, 1999, and December 31, 2009) in 14 geographically located service areas across Southern California (Fig. S1 in Supplementary Material). Residential addresses extracted from birth certificate records were linked by a unique KPSC membership identifier. The primary analysis included 246,420 mother-child pairs with children still enrolled as KPSC plan members at age one year, as previously described (Xiang et al., 2015), after excluding children with birth certificate addresses outside Southern California (n = 636) or addresses that could not be accurately geocoded (n = 4406). Follow-up was accrued until the first occurrence of 1) clinical diagnosis of ASD; 2) last date of continuous KPSC plan membership; 3) death from any cause; or 4) age five. Children were censored at age five to ensure the same follow-up time for the entire cohort, regardless of birth date. Thus, the youngest children, born in 2009, were followed through 2014. Both outcome and covariate data were extracted from the KPSC electronic medical records (EMR), as previously described (Xiang et al., 2015). Both the KPSC and the University of Southern California Institutional Review Boards approved this study.
Outcome data on ASD.
KPSC neurodevelopment screening procedures included an abbreviated Checklist for Autism in Toddlers (CHAT) (Baron-Cohen et al., 2000) administered at 18- and 24-month well child visits. Children failing the screening were referred to a pediatric developmental specialist for further evaluation and ASD diagnosis (Xiang et al., 2018; Xiang et al., 2015). The presence or absence of ASD during follow-up was identified by ICD-9 codes 299.x or equivalent KPSC codes from the EMR from at least two separate visits, an approach validated previously (Coleman et al., 2015; Xiang et al., 2018).
Exposure assessment.
Birth certificate residential addresses were geocoded using MapMarker USA Version 28.0.0.11. Exposure metrics at each geocoded address included regional pollutant PM2.5, PM≤10 µm in diameter (PM10), nitrogen dioxide (NO2), and ozone (O3). Monthly averages for each pollutant between 1998–2009 were obtained from data compiled from the EPA regional air quality monitoring network. Exposure at each address was assigned based on the monthly inverse distance-squared weighted average from up to four closest regional monitoring stations within 50 km for each pollutant. For geocoded address locations within 0.25 km of a monitor, data only from that monitoring station were used. Although the distance-weighted approach has limited accuracy in areas with sparse monitoring networks, performance is acceptable in Southern California due to the dense geographical network of historical measurements covering the region. In a previous Southern California study evaluating this method using leave-one-out validation for monthly monitoring station data, the coefficients of determination (r2) were 0.76, 0.73, 0.53, and 0.46 for O3, NO2, PM2.5 and PM10, respectively, with lower R2 values for PM attributed to the local (primary emission) dust component that is not regional (Eckel et al., 2016). Bias was less than 1 ppb or 1 µg/m3. Each address was assigned the monthly average of the 24-hour concentrations of PM2.5, PM10, and NO2. For O3, the monthly average of daily maximum 8-hour concentrations was estimated. Based on the mother’s last menstrual period, averages of the monthly concentrations were calculated during each trimester, the entire pregnancy and the first year of life. First trimester exposure was defined as 0–12 weeks, second trimester as 13–26 weeks, and third trimester as 27 weeks to birth. The monthly average exposures during months overlapping these different time periods were weighted by the number of days in each period.
Covariates.
Potential confounders chosen a priori, based on previous associations with ASD in this cohort (Xiang et al., 2015), included child sex and maternal race/ethnicity, and maternal age at delivery, parity, education, maternal history of comorbidity [≥1 diagnosis of heart, lung, kidney, or liver disease; cancer], and median family household income in the census tract of residence. An indicator variable was created for missing covariates (parity [n = 4,125], education [n = 2,222], and household income [n = 1,850]). Features of the design of the study included year of birth and KPSC service area. To account for correlated temporal changes in ASD incidence rate and pollution levels, we adjusted for birth year, and to account for broad geographical characteristics associated with ASD, we adjusted for the 14 KPSC service areas.
Additional pregnancy-related covariates potentially in PM2.5-ASD causal pathways included maternal pre-eclampsia/eclampsia and diabetes (none, gestational diabetes mellitus, pre-existing type 2 diabetes), and preterm birth (< 37 weeks vs. ≥ 37 weeks).
Statistical analyses.
Partial Pearson correlations were calculated between pollutants within each exposure window and across multiple exposure windows, adjusted for birth year and KPSC service areas. Cox proportional hazards models were used to estimate the ASD hazard ratios (HRs) associated in separate models with each pollutant exposure, adjusting for potential confounders and for potential correlation due to multiple siblings born to the same mother by specifying family as a random effect.Additionally adjusting for covariates potentially on the causal pathway did not change estimated effects by >10%, so these variables were not included in the final models. Restricted cubic splines identified no evidence of non-linear associations of ASD with pollutants, so pollutants were treated as continuous variables and modeled linearly. Because the analysis of pollutant effects on ASD was adjusted for year and service areas, we scaled each HR to be representative of exposure contrasts both within-service area and within-year. For each pollutant, this effect estimate was scaled to the difference between the 95th and the 5th percentile of the distribution of deviations of each child’s pregnancy exposure from the average for children born in the same service area in the same year. Deviations were calculated as each residential pollutant exposure value minus the within-service area, within-year mean exposure. For example, for each of the 14 service areas and 11 years (154 in total) the average PM2.5 residential exposure and the deviations of individual PM2.5 from this average were calculated. The 95th percentile (3.0 µg/m3) minus the 5th percentile (−3.5 µg/m3) of PM2.5 deviation distributions resulted in the within-service area and within-year scale of 6.5 µg/m3 for PM2.5. The same procedure was used to calculate the within-service area, within-year scales for other pollutants: 16.1 µg/m3 for PM10, 10.4 ppb for NO2, and 15.7 ppb for O3. For any pollutant found to be associated with ASD, we conducted sensitivity analyses mutually adjusted for multiple exposure windows, including each trimester during pregnancy and first year of life in a single model. To assess the hypothesis that effects of PM2.5 would be larger in boys, we tested for exposure interactions with sex. We also stratified by sex and estimated the HRs separately for boys and girls. For exposure windows for which significant interactions with sex were observed, we also examined the joint effects by fitting a model with an 8-category exposure (sex by pollutant quartiles), using the first quartile of exposure in girls as reference.
Two-sided statistical tests were conducted at an alpha level of 0.05, and precision was measured using 95% confidence intervals (CIs). Data analyses were conducted using SAS 9.4 (SAS Institute, Inc, Cary, NC) and R, version 3.0.2 (64 bit).
Results
During the follow-up period, 2471 children were diagnosed with ASD in the cohort. Unadjusted annual ASD incidence rates (per 1,000 person-years) increased with birth year from 1.93 in 1999 to 4.03 in 2009 (Fig. 1), a period during which national prevalence rates of ASD were also increasing (Baio, 2014).
Fig. 1.
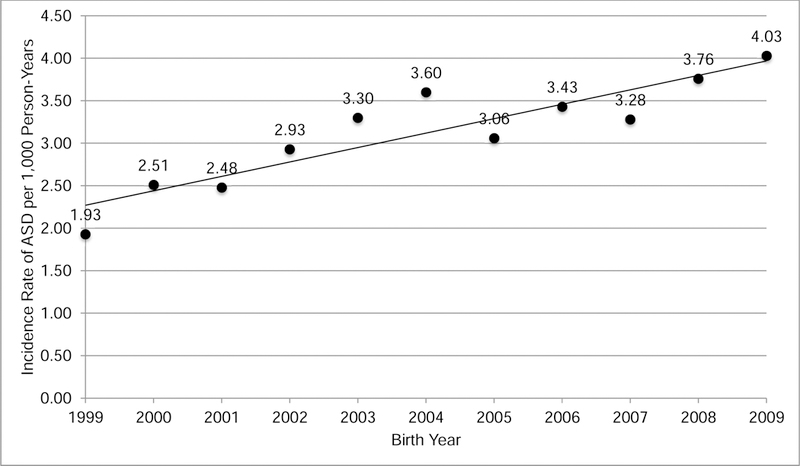
Crude incidence rate of ASD by birth year, based on 2471 children diagnosed with ASD during follow-up through age 5 in the cohort of 246,420.
Children with ASD were substantially more likely to be boys (n = 2,030) than girls (n = 441; Table 1). Mothers of cases were older at delivery (31.0 years; standard deviation (SD) 5.7) than mothers of children without ASD (29.7 years, SD 5.8). Mothers of cases were also more likely have more than a high school education and to have a history of other comorbidities.
Table 1.
Characteristics of the cohort by ASD status
| Characteristics | ASD N (column %)a,b | No ASD N (column %)a,b | ASD Mean (SD)a,b | No ASD Mean (SD)a,b |
|---|---|---|---|---|
| Child | ||||
| Male | 2030 (82.2) | 124,278 (50.9) | ||
| Female | 441 (17.8) | 119,671 (49.1) | ||
| Maternal | ||||
| Age (years) | 31.0 (5.7) | 29.7 (5.8) | ||
| Parity | ||||
| 0 | 1123 (45.5) | 95,841 (39.3) | ||
| 1 | 802 (32.5) | 77,692 (31.9) | ||
| ≥2 | 511 (20.7) | 66,326 (27.2) | ||
| Unknown | 35 (1.4) | 4090 (1.7) | ||
| Education | ||||
| High school or lower | 811 (32.8) | 100,959 (41.4) | ||
| Some college | 761 (30.8) | 67,968 (27.9) | ||
| College graduate or higher | 888 (35.9) | 72,811 (29.9) | ||
| Unknown | 11 (0.5) | 2211 (0.9) | ||
| Household annual incomec | ||||
| <$30,000 | 223 (9.0) | 19,846 (8.1) | ||
| $30,000-$49,999 | 845 (34.2) | 81,793 (33.5) | ||
| $50,000-$69,999 | 810 (32.8) | 78,116 (32.0) | ||
| $70,000-$89,999 | 365 (14.8) | 39,393 (16.2) | ||
| ≥$90,000 | 228 (9.2) | 24,801 (10.2) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 569 (23.0) | 62,205 (25.5) | ||
| Non-Hispanic black | 266 (10.8) | 23,589 (9.7) | ||
| Hispanic | 1216 (49.2) | 124,907 (51.2) | ||
| Asian/Pacific Islander | 374 (15.1) | 29,400 (12.1) | ||
| Other | 46 (1.9) | 3848 (1.6) | ||
| History of maternal comorbidityd | 301 (12.2) | 21,753 (8.9) |
Based on 2471 ASD cases and 243,949 without ASD
All characteristics were significantly different based on the χ2–squared test for proportions and analysis of variance for means (p<0.001)
Based on census tract median
≥1 diagnosis of heart, lung, kidney, or liver disease; cancer
Mean levels of PM2.5, PM10, NO2, and O3 during entire pregnancy across all years were 17.9 micrograms per meter-cubed (µg/m3), 38.1 µg/m3, 25.1 parts per billion (ppb), and 41.6 ppb, respectively. However, mean levels of both PM2.5 and NO2 decreased across years from 1999–2009 (Fig. 2). PM10 levels fluctuated across time, potentially reflecting varying levels of precipitation across the years. O3 levels remained relatively stable across years.
Fig. 2.
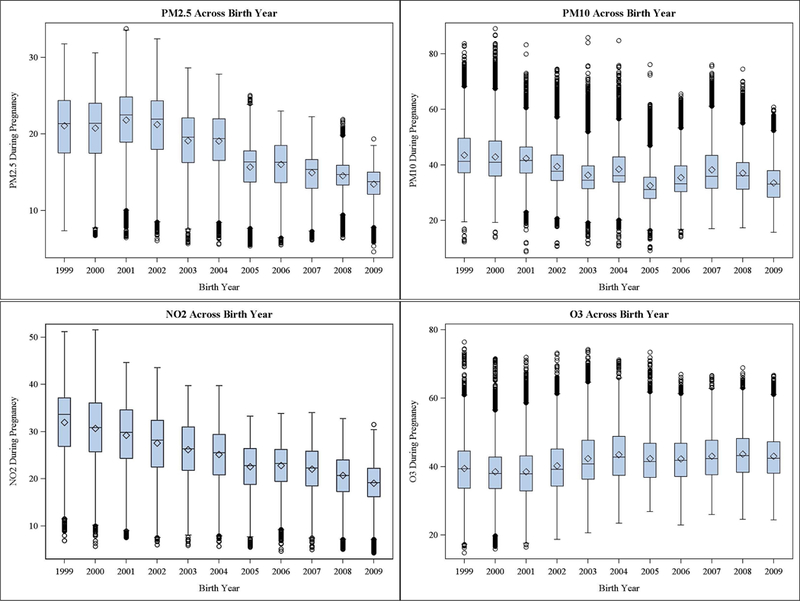
Distribution of pollutant concentrations during pregnancy across birth year 1999–2009.
Mean levels of pollutants also varied between KPSC service areas, with highest levels of mean 1999–2009 PM2.5, PM10 and NO2 in Ontario (21.6 µg/m3, 49.7 µg/m3, 31.2 ppb, respectively), and lowest levels of PM2.5, PM10, and NO2 in San Diego (13.1 µg/m3, 30.7 µg/m3, 18.0 ppb). Highest mean levels of O3 were in Moreno Valley (52.5 ppb), and lowest mean levels of O3 were in Downey (31.8 ppb).
An increase in the adjusted ASD risk was associated with PM2.5 exposure during the entire pregnancy (HR = 1.17 per 6.5 µg/m3, 95% CI: 1.04, 1.33), the first trimester (HR = 1.10, 95% CI: 1.02, 1.19) and the third trimester (HR = 1.08, 95% CI: 1.00, 1.18; Table 2) in models adjusted for potential confounders. Increased risk of ASD was also associated with first year of life PM2.5 exposure (HR = 1.21, 95% CI: 1.05, 1.40). Adjusting for birth year and KPSC service areas generally substantially increased the strength of the associations. Adding other potential confounders (maternal age, parity, maternal race/ethnicity, maternal education, census tract median household income, maternal history of comorbidities before pregnancy, and child sex) did not result in any further appreciable change in the effect estimates. There were generally weak associations of ASD with other pollutants, none of which was statistically significant.
Table 2.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for ASD associated with each pollutant birth address exposure.
| PM2.5 | PM10 | NO2 | O3 | |
|---|---|---|---|---|
| Exposure window | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Entire pregnancy | 1.17 (1.04–1.33) | 1.01 (0.89–1.15) | 1.05 (0.91–1.20) | 1.10 (0.95–1.26) |
| First trimester | 1.10 (1.02–1.19) | 1.00 (0.92–1.10) | 1.03 (0.95–1.11) | 0.97 (0.91–1.02) |
| Second trimester | 1.06 (0.97–1.14) | 1.02 (0.93–1.12) | 1.01 (0.93–1.09) | 1.03 (0.97–1.10) |
| Third trimester | 1.08 (1.00–1.18) | 1.00 (0.91–1.10) | 1.02 (0.94–1.11) | 1.05 (0.99–1.11) |
| First year of life | 1.21 (1.05–1.40) | 1.06 (0.92–1.22) | 1.12 (0.97–1.30) | 0.94 (0.80–1.11) |
Separate models were estimated for each time window, adjusted for birth year, KPSC medical center service areas, maternal age, parity, maternal race/ethnicity, maternal education, census tract median household income, maternal history of comorbidities before pregnancy (≥1 diagnosis of heart, lung, kidney, liver disease or cancer), child sex, and family specified as a random effect. Hazard ratios were scaled per 6.5 µg/m3 PM2.5; per 16.1µg/m3 PM10; per 10.4 ppb NO2; and per 15.7 ppb O3. ASD = autism spectrum disorder; PM2.5 = particulate matter <2.5 µm in aerodynamic diameter; PM10 = particulate matter <10 µm, NO2 = nitrogen dioxide; O3 = ozone
Based on moderate partial correlations of PM2.5 exposures across trimesters (R=0.33–0.54) and between trimester-specific exposures and first year of life (R=0.66–0.67; Table S1 in Supplementary Material), we fit a multiple exposure window model that co-adjusted all 3 trimester-specific and first year of life PM2.5 exposures. The ASD risk associated with PM2.5 during the first trimester was still elevated, but the estimate was less precise (HR = 1.09 per 6.5 µg/m3, 95% CI: 0.99–1.19; p = 0.07) after adjusting for exposures in other time windows, which were markedly attenuated (Table S2 in Supplementary Material). ASD HR associated with PM2.5 during the first year of life was also elevated, but was not statistically significant.
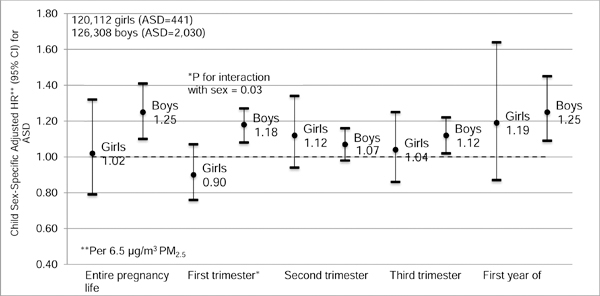
The interaction between PM2.5 exposure during first trimester and child sex was statistically significant (p = 0.03) in a model adjusted for potential confounders. Increased ASD risk was associated with PM2.5 in boys (HR = 1.18 per 6.5 µg/m3, 95% CI: 1.08, 1.27; Fig. 3). No associations were observed among girls (HR = 0.90 per 6.5 µg/m3, 95% CI: 0.76, 1.07). The adjusted sex-specific PM2.5 effect estimates were generally larger in boys during the other exposure windows (except for the second trimester), but none of these statistical interactions was significant. To compare the relative effects of sex and first trimester PM2.5, we fit a model with an 8-category exposure (sex by quartiles of PM2.5). There was almost a 4-fold increased risk of ASD among boys in the lowest quartile of exposure as compared to girls with low exposure (Table S3 in Supplementary Material). For boys, there was a further 1.30-fold increased HR with an exposure-response relationship across four quartiles of first trimester exposure averaged across all years (<14.0 µg/m3, 14.0-<17.3 µg/m3, 17.3-<21.6 µg/m3, and ≥21.6 µg/m3, respectively). There was little evidence for an exposure gradient in risk in girls.
Fig. 3.
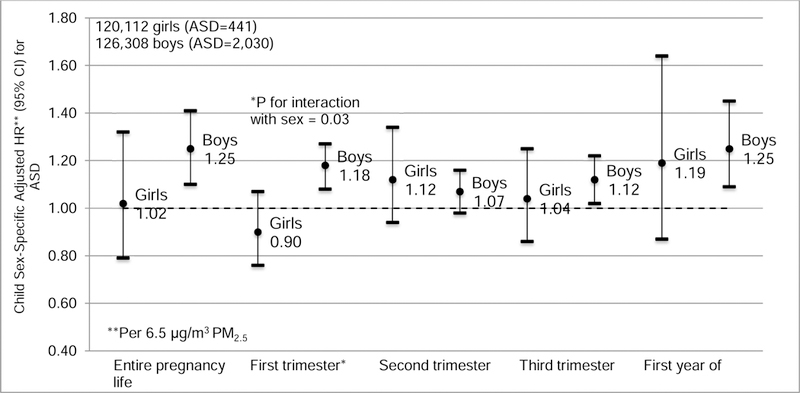
Child sex-specific adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for ASD per 6.5 g/m3 PM2.5 during each exposure period. Separate models were estimated for each time window, and estimates were adjusted for birth year, KPSC medical center service areas, maternal age, parity, maternal race/ethnicity, maternal education, census tract median household income, maternal history of comorbidities before pregnancy, and family specified as a random effect. Circles represent the hazard ratios and whiskers represent the 95% CIs.
Discussion
In this large cohort study, increased risk of ASD was associated with maternal exposure to PM2.5 during pregnancy, specific to the first and third trimester, and during the first year of the child’s life. Only first trimester exposure was robust to adjustment for other windows of PM2.5 exposure. A key strength of the study was the large sample size and high quality EMR data that made it possible to test a hypothesis that PM2.5 effects would be stronger in boys, based on evidence from recent animal studies (Church et al., 2017; Li et al., 2017). Associations were generally stronger for boys than for girls across time windows of gestation, and this difference was statistically significant for first trimester PM2.5 exposure. The approach could also be used in future hypothesis-generating studies examining synergistic effects of multiple early-life risk factors with exposure to airborne particles to understand better the environmental origin of ASD.
Oxidative stress and associated systemic inflammation during pregnancy that have adverse affects on the developing fetal brain may contribute to the pathogenesis of ASD (Block et al., 2012). The microglial cell response to inflammatory insults during embryonic brain development may lead to synapse dysfunction involved in the development of ASD (Hammond et al., 2018). Inflammatory insults such as PM2.5 exposure may have larger effects in males, because they have significantly more microglia than females during gestational and early postnatal periods (Bolton et al., 2017; Hanamsagar and Bilbo, 2016). This potentially explains the higher male-prevalence of ASD diagnosed during early life, and the susceptibility of boys to PM2.5 (Hanamsagar and Bilbo, 2016). Other potential contributing explanations include greater fetal testosterone during male brain development that leads to more activated microglia compared to females and therefore to greater vulnerability to PM or other insults that increase risk of ASD (Baron-Cohen et al., 2011; McCarthy, 2016). Newborn males also have lower glutathione and sulfate levels, which are involved in detoxifying oxidant environmental insults such as PM2.5, compared to newborn females (Kern, 2017). In male mice, an autism-like behavioral phenotype was caused by PM2.5 exposure during early postnatal life (equivalent to the human third trimester) (Church et al., 2017; Li et al., 2017), in contrast to our findings of more robust ASD associations with first trimester exposure. Given these male-specific results across developmental windows, experimental studies designed to specify more precisely the window(s) of vulnerability to these sexually dimorphic effects are needed.
Our results are generally consistent with the few previous epidemiological studies that have assessed sex differences in PM2.5-ASD susceptibility. In the Nurses Health Study, stronger ASD associations with PM2.5 during pregnancy were observed among boys (odds ratio (OR) 1.73 per 4.42 µg/m3, 95% CI: 1.29, 2.31) than among girls (OR 1.12, 95% CI: 0.59, 2.12; interaction p-value = 0.17); however, the power of the study to detect interactions was limited by a small number of ASD cases in girls (n = 23) (Raz et al., 2015). A Danish study with the largest number of ASD cases in both boys and girls to date (n = 11,853; n = 3,534, respectively) reported that the association of PM2.5 exposure during 9 months after birth with ASD was modestly stronger among boys (OR 1.07 per 3.61 µg/m3, 95%CI: 1.01, 1.13) than among girls (OR 1.02 per 3.61 µg/m3, 95% CI: 0.92, 1.12) (Ritz et al., 2018). In contrast, a study from Canada reported similar PM2.5 pregnancy exposure associations with ASD among boys (OR 1.04 per 1.5 µg/m3, 95% CI: 0.98, 1.10) and among girls (OR 1.03 per 1.5 µg/m3, 95% CI: 0.90, 1.18) (Pagalan et al., 2018). A recent study in Ohio reported similar PM2.5 associations with ASD during pregnancy and early life when restricted to boys and in the entire study population; this study did not report PM2.5 associations for the 78 girls with ASD (Kaufman et al., 2019). Our study included a larger number of ASD cases in girls (n = 441) than some of these previous studies, and to our knowledge it is the only study to date to report a statistically significant difference between PM2.5-ASD associations in boys and girls. Because ASD is a rare disease, a large sample size is required to assess interactions of sex with modifiable ASD risk factors such as PM2.5.
Large population studies provide an opportunity to study the role of multifactorial causal pathways that might identify substantial attributable proportions of ASD. It is unlikely that PM2.5 by itself contributed substantially to the epidemic of ASD reflected in the KPSC temporal trends, because ambient concentrations of PM2.5 were decreasing during this same period (Figure 2). However, in the context of synergistic effects with another risk factor such as maternal obesity (Flegal et al., 2012) or gestational diabetes mellitus (Bardenheier et al., 2015), which were increasing during this period, PM2.5 could contribute to the epidemic even in the context of stable or decreasing regional PM2.5 levels over the period of increasing ASD rates.
Identifying the window of vulnerability to PM2.5 during pregnancy can provide clues to the pathogenesis of ASD, based on brain development during this window, with potential implications for prevention. We observed associations of increased ASD risk with several exposure windows in early life, but the first trimester was most robust to co-adjustment for other exposure windows. Although the ASD-PM2.5 association during the first year of life was also elevated, this HR was markedly attenuated after mutually adjusting for other exposure windows. Previous epidemiological studies have observed ASD-PM2.5 associations mainly during the third trimester and first year of life (Becerra et al., 2013; Raz et al., 2015). Three recently published studies have mutually adjusted for PM2.5 exposure across trimesters and/or early life (Pagalan et al., 2018; Raz et al., 2015; Ritz et al., 2018). While one found no differences in PM2.5 associations across trimesters of pregnancy (Pagalan et al., 2018), one found robust PM2.5 association during the third trimester (Raz et al., 2015), and one found robust PM2.5 association during 9 months after birth (Ritz et al., 2018). Also, one recently published study that found positive associations of PM2.5 with ASD, mutually adjusted for pairwise combinations of trimester-specific and early life exposures, but no effects were statistically significant (Kaufman et al., 2019). Another study that found null ASD associations with trimester-specific PM2.5 exposure reported an increased risk of ASD with cumulative exposure from 3 months before pregnancy to the second year of life (Talbott et al., 2015). The date of last menstrual period determined from self-report at first prenatal visit or from gestational age determined by ultrasound in the KPSC EMR likely more accurately specified the window of exposure than most previous studies that calculated trimesters based on self-reported gestational age at birth and child’s birth date (Becerra et al., 2013; Kaufman et al., 2019; Raz et al., 2015; Ritz et al., 2018).
A recent meta-analysis of observational studies of ASD-PM2.5 associations during pregnancy reported a pooled OR of 2.32 per 10 µg/m3 (Lam et al., 2016). We observed substantially smaller estimates of risk. For example, the first trimester HR 1.10 per 6.5 µg/m3 was equivalent to HR 1.16 per 10 µg/m3, and the first trimester HR 1.18 per 6.5 µg/m3 for males was equivalent to 1.29 per 10 µg/m3. However, the pooled estimate was based on only 3 studies, which had substantial heterogeneity, with a range of ORs from 1.15 to 2.77 per 10 µg/m3, that could not be explained by differences in study design and methods. Although our ASD-PM2.5 associations were in the same direction as in these other studies, unexplained differences in magnitude of the associations could potentially be resolved with additional studies. Additionally, comparing results across studies with different designs and measures of association remains a challenge.
We found no associations of ASD with other pollutants. Our results are consistent with the human epidemiological and animal toxicological evidence that has largely converged on the neurotoxicological effects of PM2.5, although there is some uncertainty to the epidemiological associations (Church et al., 2017; Lam et al., 2016; Li et al., 2017). A cohort study from Europe reported no PM2.5 association with autistic behavioral traits (Guxens et al., 2015). Various other studies have examined associations with other pollutants (PM10, O3, NO2, and sulfur dioxide), with indicators of exposure to the near-roadway mixture, and with hazardous air pollutants, including gaseous, traffic-related air pollutants, metals and other volatile organic compounds (Lam et al., 2016). However, associations of ASD with these pollutants have not been consistent.
This study has several strengths. It is one of the few cohort studies to examine risk of ASD associated with air pollution. Selection bias was unlikely to have influenced the results, because the cohort did not self-select and also had a high annual average 95% retention through age 5 after cohort enrollment (Xiang et al., 2015). The large study sample provided statistical power to assess interaction of PM2.5 with sex. Unlike many health systems that have only recently adopted an EMR, the KPSC EMR system has developed since its implementation in the early 1990’s. Incident ASD ascertainment occurred in a clinical setting using standardized diagnostic algorithms developed through the EMR (Coleman et al., 2015). The analysis controlled for various individual-level covariates that were available through the EMR, such as gestational diabetes, that are difficult to obtain without standardized procedures in a single healthcare system. The KPSC membership is similar in age, racial/ethnic, and socioeconomic distribution to the census reference population, and comprises approximately 16% of that population (Koebnick et al., 2012). Because KPSC is a health maintenance organization offered widely through employer plans, results are generalizable to the population of working Southern California families. Because Southern California has large pollutant exposure gradients representative of ranges and extremes across the U.S., the results are broadly relevant to the U.S. and other countries with similar exposures.
There were some limitations. Although there were large exposure gradients in the cohort, exposure concentrations estimated at the birth address were used as a proxy for personal exposure. Residential mobility during pregnancy and the first year of life, and time of mother or child away from home would have resulted in exposure measurement error. During pregnancy 9–34% of mothers move, resulting in corresponding changes in exposure that may not be recalled well (Bell and Belanger, 2012; Chen et al., 2010; Chen et al., 2016). If the effect of this bias were non-differential with respect to the outcome, then the true effect of exposure could likely have been larger than we observed (Rothman et al., 2008). Future work is planned to improve exposure assignment resulting from change in residence and corresponding exposures during pregnancy and early life, ASD phenotype and severity, and to assess joint effects of air pollution with other risk factors, based on information in the EMR.
Conclusions
We conclude that boys may be at increased risk of ASD induced by maternal PM2.5 exposure during the first trimester of pregnancy. This study provides potential clues to the reasons for the sex-specificity of ASD, effects that were predictable based on animal studies. Further toxicological study is needed to better understand the biological basis and timing of susceptibility during neurodevelopment and of sex differences in neurobehavioral effects of particle exposures.
Supplementary Material
Capsule: First trimester pregnancy exposure to fine particles was robustly associated with subsequent increased risk of autism spectrum disorder that was specific to boys.
Highlights.
Greater first trimester pregnancy exposure to ambient fine particles was robustly associated with subsequent increased risk of autism spectrum disorder (ASD) that was specific to boys.
The male-specific results were consistent with findings from recent animal studies.
There were no statistically significant associations between other ambient pollutants and ASD.
The hypothesis-driven approach, based on results from animal experimental results, in a large population sample is a promising paradigm for studying environmental influences on sex-related differences in ASD.
Acknowledgements:
The authors thank the patients of Kaiser Permanente for helping us improve care through the use of information collected through our electronic health record systems.
Funding: This research was supported by Kaiser Permanente Southern California Direct Community Benefit Funds; National Institutes of Environmental Health Sciences (#5F31ES027340 (Jo); #R56ES028121 (Xiang); 5P30ES007048 (Gilliland); P01ES022845 and U.S. Environmental Protection Agency RD-83544101 (McConnell); and University of Southern California Provost Scholarship Award (Jo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare they have no actual or potential competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests
The authors declare that they have no competing interests.
Appendix A. Supplementary data
References
- Baio J, 2014. Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ 63, 1–21. [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Rosenberg C. Robinson, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF, 2018. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardenheier BH, Imperatore G, Gilboa SM, Geiss LS, Saydah SH, Devlin HM, Kim SY, Gregg EW, 2015. Trends in Gestational Diabetes Among Hospital Deliveries in 19 U.S. States, 2000–2010. Am J Prev Med 49, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R, 2011. Why are autism spectrum conditions more prevalent in males? PLoS Biol 9, e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Cox A, Baird G, Charman T, Swettenham J, Drew A, Doehring P, 2000. Early identification of autism by the CHecklist for Autism in Toddlers (CHAT). J R Soc Med 93, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B, 2013. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect 121, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Belanger K, 2012. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol 22, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, Diaz-Sanchez D, Dorman DC, Gold DR, Gray K, Jeng HA, Kaufman JD, Kleinman MT, Kirshner A, Lawler C, Miller DS, Nadadur SS, Ritz B, Semmens EO, Tonelli LH, Veronesi B, Wright RO, Wright RJ, 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD, 2017. Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Front Synaptic Neurosci 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Araujo JA, Li H, Sioutas C, Kleinman M, 2009. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol 9, 5099–5104. [DOI] [PubMed] [Google Scholar]
- Campbell A, Daher N, Solaimani P, Mendoza K, Sioutas C, 2014. Human brain derived cells respond in a type-specific manner after exposure to urban particulate matter (PM). Toxicol In Vitro 28, 1290–1295. [DOI] [PubMed] [Google Scholar]
- Chen L, Bell EM, Caton AR, Druschel CM, Lin S, 2010. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res 110, 162–168. [DOI] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD, 2016. Ambient Air Pollutants Have Adverse Effects on Insulin and Glucose Homeostasis in Mexican Americans. Diabetes Care 39, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Tijerina PB, Emerson FJ, Coburn MA, Blum JL, Zelikoff JT, Schwartzer JJ, 2017. Perinatal exposure to concentrated ambient particulates results in autism-like behavioral deficits in adult mice. Neurotoxicology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, Lynch FL, Madden JM, Owen-Smith A, Pearson JA, Pearson KA, Rusinak D, Quinn VP, Croen LA, 2015. Validation of Autism Spectrum Disorder Diagnoses in Large Healthcare Systems with Electronic Medical Records. J Autism Dev Disord 45, 1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel SP, Cockburn M, Shu YH, Deng H, Lurmann FW, Liu L, Gilliland FD, 2016. Air pollution affects lung cancer survival. Thorax 71, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL, 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307, 491–497. [DOI] [PubMed] [Google Scholar]
- Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ, 2016. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ Res 151, 763–776. [DOI] [PubMed] [Google Scholar]
- Guxens M, Ghassabian A, Gong T, Garcia-Esteban R, Porta D, Giorgis-Allemand L, Almqvist C, Aranbarri A, Beelen R, Badaloni C, Cesaroni G, de Nazelle A, Estarlich M, Forastiere F, Forns J, Gehring U, Ibarluzea J, Jaddoe VW, Korek M, Lichtenstein P, Nieuwenhuijsen MJ, Rebagliato M, Slama R, Tiemeier H, Verhulst FC, Volk HE, Pershagen G, Brunekreef B, Sunyer J, 2015. Air Pollution Exposure during Pregnancy and Childhood Autistic Traits in Four European Population-Based Cohort Studies: The ESCAPE Project. Environ Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Robinton D, Stevens B, 2018. Microglia and the Brain: Complementary Partners in Development and Disease. Annu Rev Cell Dev Biol [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Bilbo SD, 2016. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol 160, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JA, Wright JM, Rice G, Connolly N, Bowers K, Anixt J, 2019. Ambient ozone and fine particulate matter exposures and autism spectrum disorder in metropolitan Cincinnati, Ohio. Environ Res 171, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JKG, D. A.; Homme KG; King PG; Bjørklund G; Chirumbolo S; Geier MR, 2017. Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol Exp (Wars) 77, 269–296. [PubMed] [Google Scholar]
- Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ, 2012. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 16, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, Lawler C, Davidson L, Daniels N, Newschaffer C, Woodruff T, 2016. A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder. PLoS One 11, e0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Li L, Cui B, Gai Z, Li Q, Wang S, Yan J, Lin B, Tian L, Liu H, Liu X, Xi Z, 2017. Early postnatal exposure to airborne fine particulate matter induces autism-like phenotypes in male rats. Toxicol Sci [DOI] [PubMed] [Google Scholar]
- Lyall K, Schmidt RJ, Hertz-Picciotto I, 2014. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol 43, 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, 2016. Sex differences in the developing brain as a source of inherent risk. Dialogues in Clinical Neuroscience 18, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagalan L, Bickford C, Weikum W, Lanphear B, Brauer M, Lanphear N, Hanley GE, Oberlander TF, Winters M, 2018. Association of Prenatal Exposure to Air Pollution With Autism Spectrum Disorder. JAMA Pediatr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, Weisskopf MG, 2015. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect 123, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Liew Z, Yan Q, Cuia X, Virk J, Ketzel M, Raaschou-Nielsen O, 2018. Air pollution and autism in Denmark. Environmental Epidemiology 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE, 2014. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K, Greenland S, Lash TL, 2008. Modern Epidemiology, 3rd Edition. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Talbott EO, Arena VC, Rager JR, Clougherty JE, Michanowicz DR, Sharma RK, Stacy SL, 2015. Fine particulate matter and the risk of autism spectrum disorder. Environ Res 140, 414–420. [DOI] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK, 2018. Maternal Type 1 Diabetes and Risk of Autism in Offspring. JAMA 320, 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, Buchanan TA, Coleman KJ, Getahun D, 2015. Association of maternal diabetes with autism in offspring. JAMA 313, 1425–1434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


