Abstract
Objective
This paper presents the first study where a dynamic neck brace was used to characterize the head motion of ALS patients while concurrently recording the surface electromyography (EMG) of the neck muscles.
Methods
Eleven ALS patients and 10 age‐matched healthy controls consented and participated in an experiment. Each participant was asked to perform three single‐plane motions of the head‐neck that included flexion–extension in the sagittal plane, lateral bending in the coronal plane, and axial rotation in the transverse plane. Each motion was performed in a cycle and was repeated five times at self‐selected speeds.
Results
During single‐plane flexion–extension under gravity, compared to healthy peers, ALS patients showed a shorter duration to reach the maximum flexion and an earlier EMG onset in the neck extensors starting from the neutral. The brace measures in activation of the neck muscles in ALS patients were well correlated with clinically measured scores, such as the ALSFRS‐r and the FVC. The activation duration of sternocleidomastoid, used to rotate the head, correlated well with the ALSFRS‐r and FVC in ALS patients during axial rotation.
Interpretation
The ability to synchronously activate a pair of muscles to execute single‐plane motions in ALS patients seems to have been compromised due to the disease and potentially results in head drop. The neck brace measures can be adapted in the clinic to complement self‐reporting in ALS patients and used to assess the head drop and progress of the disease.
Introduction
Amyotrophic lateral sclerosis (ALS)1, 2, 3 is an adult‐onset neurodegenerative disease characterized by progressive loss of upper and lower motor neurons. The disease leads to limb and bulbar paralysis and respiratory failure. Patients with ALS show variability in their clinical course with durations ranging from several months to more than 10 years, although the majority of the patients die within a few years after symptom onset. At present, the disease is clinically diagnosed based on an array of symptoms and laboratory testing while excluding other definable diseases.
Dropped head is one of the features of the disease due to progressive loss of neck muscle strength.4, 5, 6, 7 Eventually, the patients lose mobility of the head completely, settling in chin‐on‐chest posture with complications in speech, breathing, and swallowing. Patients with neck weakness often use braces to keep the head‐neck in a neutral configuration, for example, Miami J or a Head Master.4, 6 The neck braces progressively become uncomfortable to wear and constrict breathing, swallowing, and speech.7, 8 There is a paucity of literature on new neck braces for this user group7, 8 to facilitate activities of daily living.
Recent studies have shown that weakness of neck muscles is a significant prognostic marker9, 10 for milestones such as loss of speech, loss of swallowing, loss of upper limb function, difficulty in turning on the bed, loss of walking, and ultimately the death.1, 2, 11, 12 In addition to moving and supporting the head‐neck, some of the neck muscles, such as sternocleidomastoid (SCM), also play an important role in respiratory functions. The neck muscles and respiratory muscles are innervated by motor neurons in the cervical cord at levels C3 through C5. SCM is innervated by the spinal accessory nerve – cranial nerve XI. This postulates why the weakness of the neck muscles is correlated with the weakness of respiratory muscles.
While studies of neck movements in ALS are limited, a recent study concluded that the head motion of ALS patients is more jerky compared to age‐matched healthy controls.13 However, the study did not record concurrent muscle activity to explain at the physiology level. Our group at Columbia University has developed a robotic neck brace and its use has been validated on healthy adults. A pilot study with the brace, with simultaneous measurements from a motion capture system, validated the measurement accuracy of the brace. The study concluded that the brace allows roughly 70% of the active range of motion of the human head.14 Using a joystick interface, the brace can actively control the head with motors mounted on it.15 Additionally, by substituting motors with compliant springs at the joints, it can provide assistive forces to keep the head in a neutral configuration.16
This paper presents the first study where the dynamic neck brace was used to characterize the head motion of ALS patients with concurrent recordings of surface electromyography (EMG) of the neck muscles.
Materials and Methods
Experiment setup
The robotic neck brace, while having the ability to make active head‐neck movements,15, 16 was used as a measurement tool in this study. Angle sensors are mounted on the brace joints which allow to compute the orientation of the head relative to the shoulders.14 In the present study (Figure 1 right), the muscle activity was measured by a wireless EMG system (Noraxon DTS, Noraxon USA Inc., Scottsdale, AR). A microcontroller (NI myRIO‐1900; National Instruments, Austin, TX) was used to send a digital trigger to the neck brace and the EMG system for synchronization. The data were recorded and viewed in real‐time on a laptop, as shown in Figure 1 (right).
Figure 1.
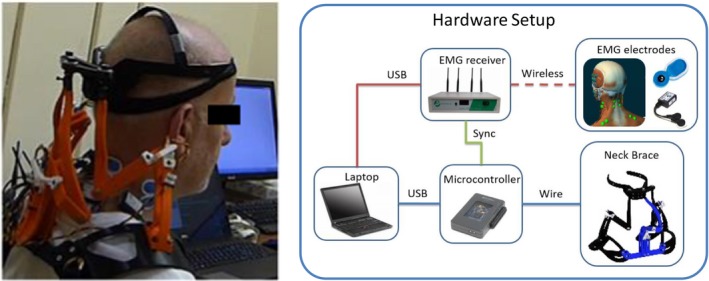
A study participant and the system overview. (left) A participant with ALS wearing the neck brace (Movie S1), (right) System overview – the head angles of the participant are measured by the robotic neck brace. Muscle activations of the major surface neck muscles, SCM, and SC are measured using a wireless EMG system. The EMG measurements are synchronized with the movement of the neck brace via a trigger input from the microcontroller. The data from the two systems are recorded on a laptop and a Graphical User Interface displays the recorded data.
Protocol
Eleven ALS patients, diagnosed and treated at the Eleanor and Lou Gehrig MDA/ALS Research Center, Columbia University, and 10 age‐matched healthy controls consented and participated in this experiment (Tables 1 and 2). The diagnoses were based on El Escorial ALS Diagnostic Criteria,17, 18 clinical judgement,19, 20 and supported by lab testing.20 This study was approved by the Institutional Review Board (IRB) of Columbia University.
Table 1.
Characteristics of subject groups.
| Gender | Age (year) | Height (cm) | Weight (kg) | |
|---|---|---|---|---|
| ALS (11) |
Female (3) Male (8) |
60.3 ± 12.8 | 171.8 ± 9.2 | 75.1 ± 10.2 |
| Control (10) |
Female (3) Male (7) |
54.3 ± 10.6 | 173.4 ± 10.1 | 78.6 ± 11.6 |
Table 2.
Characteristics of ALS patients.
| ID | Gender | Age (yr) | Height (cm) | Weight (kg) | FVC (%) | ALSFRS‐r |
|---|---|---|---|---|---|---|
| 1 | F | 76 | 150 | 50 | 75 | 37 |
| 2 | F | 69 | 173 | 82 | 21 | 23 |
| 3 | F | 57 | 167 | 69 | 75 | 31 |
| 4 | M | 77 | 178 | 66 | 61 | 38 |
| 5 | M | 64 | 170 | 82 | 109 | 42 |
| 6 | M | 61 | 182 | 82 | 91 | 42 |
| 7 | M | 60 | 170 | 74 | 108 | 36 |
| 8 | M | 51 | 180 | 75 | 65 | 26 |
| 9 | M | 49 | 175 | 83 | 47 | n/a |
| 10 | M | 33 | 180 | 83 | 60 | 36 |
| 11 | M | 67 | 165 | 80 | 68 | 42 |
For subject 9, the ALSFRS‐r score was not available.
The patients enrolled in this study were examined and referred by a treating physician. All these subjects had the ability to keep the head upright and return the head back to neutral without external support. The strength of the neck extensor and flexor muscles were measured by the clinical team and the minimum score among the subjects was 4 (on a 0–5 scale, with 5 being normal). Healthy controls were recruited to match the age of the ALS subjects. The control subjects did not have a history of neck injury. The age (P = 0.192), height (P = 0.887), weight (P = 0.306), and sex (P = 0.730, chi‐square = 0.120) were not significantly different between the two groups.
All procedures and data handling followed the approved protocol. The inclusion criteria required participants to have the ability to keep the head in upright neutral without external support. All participants remained seated throughout the experiment. The skin around the neck and shoulder areas was cleaned with alcohol pads. A pair of electrodes was then attached along the fibers of each SCM and splenius capitis (SC) muscle. A transmitter unit for each channel was wired to the electrode and taped on the body. The neck brace was then firmly attached to the shoulders and the end effector of the brace was tied to the forehead (Fig. 1). Padding was added to the head and the shoulders to increase comfort and avoid sliding. A static trial was recorded where each participant was asked to relax and self‐support the head in upright neutral position for 5 sec. This static trial calibrated the brace position to the neutral and recorded baseline muscle activations. The entire setup time for the experiment was between 5 and 10 min, depending on the brace fit to the subject.
Each participant was then asked to perform three single‐plane motions that included flexion–extension in the sagittal plane, lateral bending in the coronal plane, and axial rotation in the transverse plane. Each motion was performed in a cycle and was repeated five times at self‐selected speeds. The subjects were asked to move as far as they could or what the brace allows. At the end of the experiment, each patient was asked to evaluate their experience with the neck brace during movements. A comfort scale was designed (Table 3). Each patient was asked to select a number between 0 (intolerable) and 8 (unnoticeable).
Table 3.
Scale used in subjective evaluation.
| 8 | Un‐noticeable | |
| 7 | Occasionally noticeable | Psychologically discomfort |
| 6 | Constantly noticeable | |
| 5 | Occasionally annoying | |
| 4 | Constantly annoying | |
| 3 | Itchy irritant | Physical discomfort |
| 2 | Concerning pressure | |
| 1 | Hurts | |
| 0 | Painful |
For ALS patients, ALS Functional Rating Scale‐Revised (ALSFRS‐r) and Forced Vital Capacity (FVC) were also measured to correlate outcomes measured from the brace and EMG recordings. ALSFRS‐r is an estimate of the patient’s functional impairment and objectively assesses response to a treatment or progression of the disease. FVC is an index of respiratory function that is often used to indicate potential respiratory compromise in ALS. Figure 1 shows a study participant and an overview of the experiment with simultaneous motion of the head‐neck and the corresponding activity of the neck muscles.
Data processing and analysis
The neck brace was sampled at 100 Hz and the EMG system at 1.5 kHz. An electronic trigger was used to synchronize the data from both systems. As an example, shown in Figure 2, the head angles were low‐pass filtered (fourth order Butterworth) at 6 Hz to reduce noise. The processing of the EMG signal from each channel followed these steps: (1) remove the DC offset, (2) band‐pass filter (10–450 Hz), (3) rectify, (4) envelope using an RMS window approach, and (5) normalize each channel using the largest value recorded in that channel during experiment with the participant.
Figure 2.
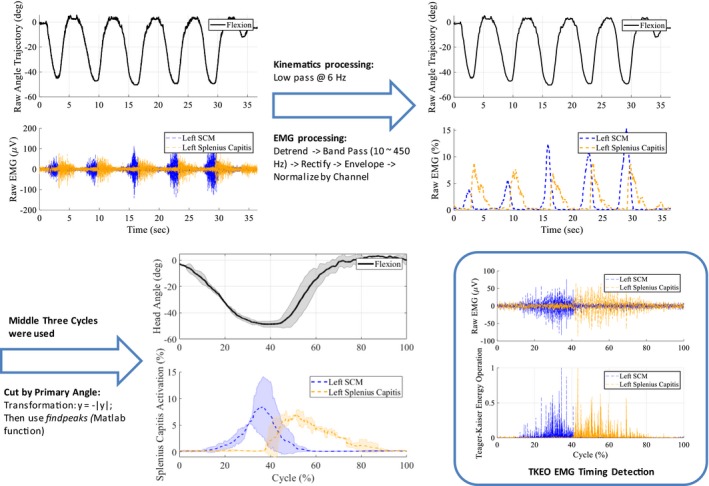
Data processing. The raw data of a subject during a session were filtered and smoothened. The middle three cycles were segmented and averaged based on the primary head angle. The EMG onset times were computed using Teague‐Kaiser Energy Operation.
The middle three cycles during a motion were averaged and used for computing the outcomes. The cycles were segmented based on the primary angle of motion and normalized with respect to time. Teague‐Kaiser Energy Operation (TKEO) was used for EMG onset detection following the steps introduced.21 These onset times of EMG, along with timing of head motions, were used in data analysis to investigate the muscle coordination differences between the healthy and ALS groups.
The normality of the samples was tested using one‐sample Kolmogorov–Smirnov test. Due to small sample size, measurement variables in the two groups, that is, ALS and control, were not normally distributed. Wilcoxon rank‐sum test was hence used to compare the variables between the two groups. Spearman rank correlation was used to examine the relationship between the variables and the clinical evaluation scores, that is, ALSFRS‐r and FVC. Data analysis was performed using Matlab (The MathWorks Inc., Natick, MA) and the statistical significance was set at P < 0.05.
Results
Representative results from a single healthy control – rope analogy
This section summarizes the results of head‐neck motion, along with EMGs from left and right SCM and SC muscles, in a single anatomical plane. This motion establishes the physiology to compare abnormal motions observed in ALS patients. We use the muscle activity to develop an analogy where each of the four muscles represents a rope between the shoulders and the head. We interpret the start, peak, and end of the muscle activity as a pattern for corresponding force pull by the rope. While head drop is an abnormality during flexion–extension, motions such as axial rotations and bending are simpler to understand and their physiology are described first.
The salient features of axial neck rotations are shown in Figure 3: (1) During right axial rotation of the head‐neck, the left SCM and right SC are active together, (2) During left axial rotation, the right SCM and left SC are active together, (3) The temporal patterns of activity of the left SCM/right SC muscles and right SCM/left SC muscles are similar and the muscle peaks precede axial rotation motion peak. Effectively, the axial rotation can be visualized as being caused by a contralateral pair of ropes attached between the shoulders and head. The lateral bending of the head‐neck similarly is caused by an ipsilateral pair of ropes attached between the shoulders and the head (Fig. 4).
Figure 3.
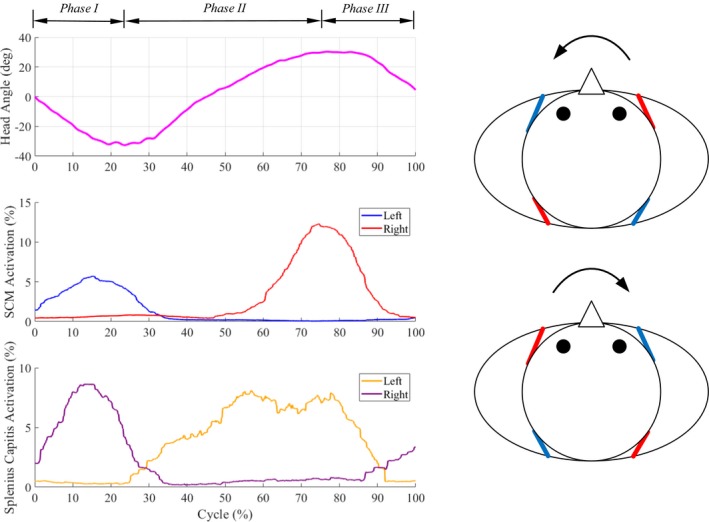
Single plane axial rotation (Movie S2). (left) Motion (primary head angle) and EMG patterns of neck muscles of a healthy subject during a movement cycle. (right) Axial rotation is caused by a contralateral pair of muscles. For example, the simultaneous actuation of left SCM rope and right SC rope results in right axial rotation. The arrows indicate the directions of motion, blue (inactive) and red (active) lines indicate the activation of corresponding muscles during motion.
Figure 4.
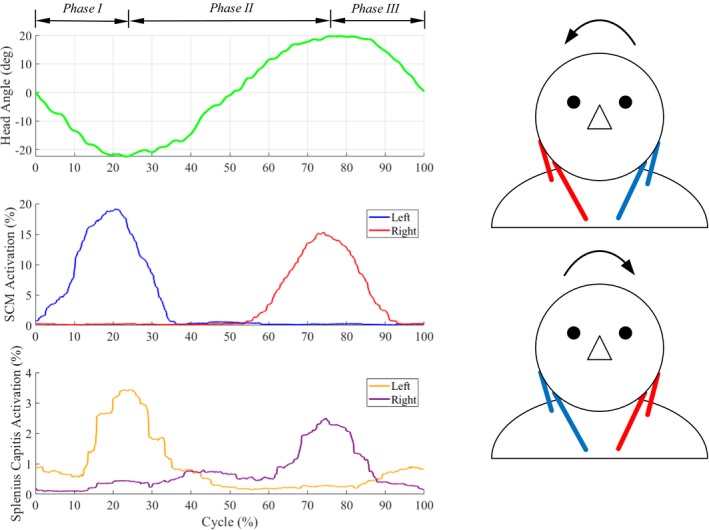
Single plane lateral bending (Movie S3). (left) Motion (primary head angle) and EMG patterns of neck muscles of a healthy subject during a movement cycle. (right) Lateral bending is caused by an ipsilateral pair of muscles. For example, the simultaneous actuation of left SCM rope and left SC rope results in left lateral bending. The arrows indicate the directions of motion, blue (inactive) and red (active) lines indicate the activation of corresponding muscles during motion.
Gravity plays an important role in flexion–extension compared to lateral bending and axial rotation. As seen in Fig. 5, flexion–extension is coordinated by a pair of ropes at the front or back attached between the shoulders and head. As the head starts to fall forward under gravity, the ropes at the back apply forces to prevent the head from falling forward uncontrollably. During extension from fully flexed position of the head, ropes at the back pull to restore the head to the neutral position.
Figure 5.
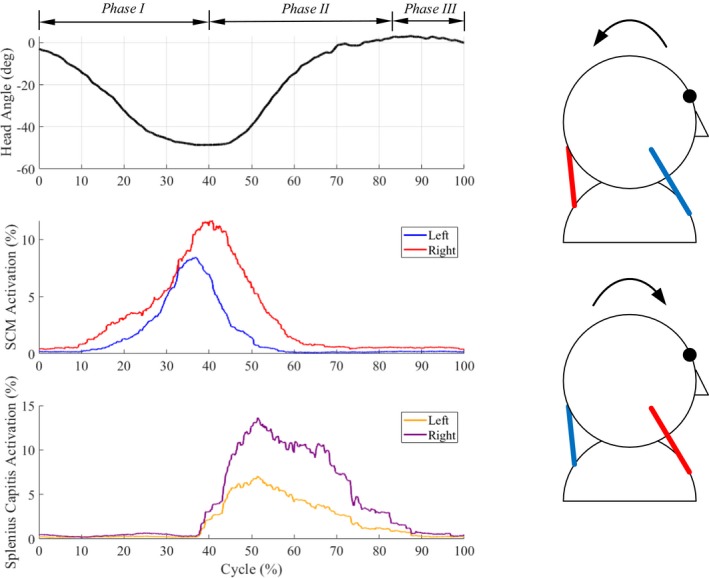
Single‐plane flexion–extension (Movie S4). (left) Motion (primary head angle) and EMG patterns of neck muscles of a healthy subject during a movement cycle. (right) Flexion–extension is caused by simultaneous actuation of the front/back pair of muscles. For example, the actuation of front SCM ropes results in flexion and the actuation of back SC ropes results in extension. The arrows indicate the directions of motion, blue (inactive) and red (active) lines indicate the activation of corresponding muscles during motion.
Group results
In contrast with healthy subjects, as detailed in Tables 4 and 5, the head‐neck movements in ALS patients showed salient differences. These could suggest that the ability to activate a pair of muscles to execute single‐plane motions, in contrast to healthy subjects, has been compromised in the patient group.
Table 4.
Onset time and duration of activation of each muscle as a fraction of the cycle during movements in three anatomical planes of ALS and control group.
| Onset time | Duration of muscle activation | |||||||
|---|---|---|---|---|---|---|---|---|
| Left SCM | Right SCM | Left SC | Right SC | Left SCM | Right SCM | Left SC | Right SC | |
| Axial rotation | ||||||||
| Control | 0.114 (0.13) | 0.585 (0.08) | 0.265 (0.21) | 0.046 (0.12) | 0.312 (0.29) | 0.256 (0.08) | 0.646 (0.27) | 0.479 (0.30) |
| ALS | 0.101 (0.17) | 0.413 (0.27) | 0.216 (0.24) | 0.030 (0.08) | 0.493 (0.33) | 0.419 (0.28) | 0.723 (0.29) | 0.717 (0.33) |
| Lateral bending | ||||||||
| Control | 0.083 (0.12) | 0.474 (0.23) | 0.033 (0.08) | 0.234 (0.25) | 0.504 (0.32) | 0.424 (0.28) | 0.779 (0.29) | 0.627 (0.33) |
| ALS | 0.047 (0.06) | 0.409 (0.25) | 0.035 (0.07) | 0.125 (0.21) | 0.363 (0.22) | 0.426 (0.27) | 0.828 (0.30) | 0.828 (0.27) |
| Flexion–extension | ||||||||
| Control | 0.086 (0.11) | 0.131 (0.15) | 0.264 (0.19) | 0.298 (0.19) | 0.506 (0.29) | 0.472 (0.36) | 0.612 (0.24) | 0.580 (0.24) |
| ALS | 0.085 (0.14) | 0.121 (0.21) | 0.097 (0.15) | 0.121 (0.16) | 0.510 (0.32) | 0.596 (0.32) | 0.792 (0.31) | 0.811 (0.23) |
Table 5.
Head angles during movements in three anatomical planes of ALS and control group.
| Phase I | Phase II | Phase III | |
|---|---|---|---|
| Axial rotation | |||
| Control | 0.245 (0.07) | 0.526 (0.08) | 0.229 (0.09) |
| ALS | 0.231 (0.07) | 0.539 (0.10) | 0.230 (0.05) |
| Lateral bending | |||
| Control | 0.290 (0.07) | 0.501 (0.08) | 0.209 (0.05) |
| ALS | 0.264 (0.09) | 0.516 (0.08) | 0.220 (0.06) |
| Flexion–extension | |||
| Control | 0.345 (0.08) | 0.481 (0.09) | 0.174 (0.07) |
| ALS | 0.282 (0.08) | 0.510 (0.08) | 0.208 (0.08) |
During axial rotation in Phase II, where subjects moved from axially rotated one extreme position to the other, right SCM was found to have a longer duration of activation in ALS subjects (P = 0.013). Additionally, this variable seems to have a correlation with the clinical scores, that is, ALSFRS‐r (r = −0.62, n = 10, P = 0.08) and FVC (r = −0.56, n = 11, P = 0.09). With a lower clinical score, it is possible that a patient has a shorter activation in SCM during Phase II in axial rotation. However, no statistical significance was found.
During lateral bending in Phase II, where subjects moved from laterally bent one extreme position to the other, right SC was found to have a longer duration of activation in ALS subjects (P = 0.012). During flexion–extension, the length of Phase I, where the subjects flexed their head under gravity from the neutral to the maximum, was found to be longer in ALS than the control group (P = 0.001). The onset times of SC of these patients, however, suggested that their neck extensors tend to activate much earlier (P < 0.001).
In the control group, we found strong correlations between temporal variables of neck muscle activations and head angles, as illustrated using scatter plots in Figure 6. Results of Spearman correlation indicated that there was a positive association between the average EMG onset times of all four neck muscles and the time of maximum flexion during single‐plane flexion–extension motion (r = 0.903, n = 10, P = 0.0009). A positive correlation was also found between the average EMG onset time of the contralateral SCM muscle and the time of maximum rotation during single‐plane axial rotation (r = 0.891, n = 10, P = 0.0014). This suggests that the head movements are coordinated by certain muscles consistently in the healthy controls. However, such relationships were not found in the patient group (P > 0.05).
Figure 6.
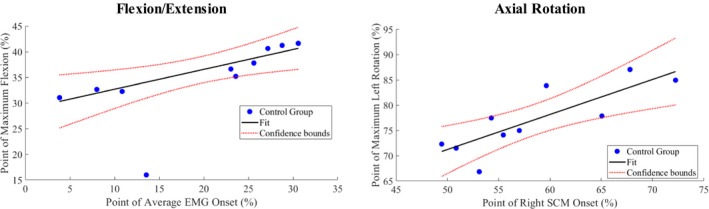
Correlating muscle EMG onsets with motion peaks in the group of healthy subjects. (left) Scatter plot of the time of maximum flexion against the average onset times of all four SCM and SC during a cycle. (right) Scatter plot of the time of maximum left rotation against the average onset time of the right SCM muscles during the cycle. Each dot represents a subject and the straight line represents the least‐square fit of the data.
Discussion
We observed the kinematics and surface EMGs of the head‐neck during simple single‐plane motions. An important goal of this paper was to identify features that may be valuable clinically. The brace was evaluated by ALS subjects as highly wearable and comfortable (average self‐evaluation score of 7.18 ± 0.8 out of a maximum of 8). We developed a “four‐rope” analogy to explain the head movement activated by four neck muscles. The pairs of SCM and SC on the two sides of the neck form four ropes connecting the head and the shoulders. A pair in these four ropes pull synergistically to generate a specific movement. We found that this muscle‐motion pattern was clear and consistent in healthy controls but were absent in ALS patient group.
During single‐plane flexion–extension, under the gravity, ALS patients showed a shorter duration to reach the maximum flexion starting from the neutral, whereas their neck extensor muscles activated much sooner during flexion phase. This is likely to protect the head from falling and thus co‐contracted with flexor muscles during flexion motion. This strategy may have been adopted to increase cervical joint stiffness during the flexion motion. The head weighs about 5 kg and the gravity plays an important role during flexion–extension motion and may have biased the control of muscles in patients.
The duration of muscle activity to go from one extreme to the other, that is, SC in lateral bending and SCM in axial rotation, were found to be longer in patient group. This may result in excessive fatigue in those who have head drop in early stages and could justify the use of neck braces.
We found that the brace measures in ALS patients were correlated with the clinically measured scores, such as the ALSFRS‐r and the FVC used in clinic. Hence, this procedure can be adopted in a clinic to complement self‐reporting. The activation duration of SCM plays a role in respiration and this was correlated with the ALSFRS‐r and FVC in ALS patients during axial rotation.
The movement of the head is achieved not only by surface muscles, such as SCM and SC used in this study but also by deeper muscles. However, obtaining signals from deeper muscles requires invasive methods. We kept our procedure to be non‐invasive and easy to conduct so that it can be potentially adapted in a clinic. Our data shows that differences in head‐neck coordination can still be extracted in ALS patients using surface neck muscles.
Temporal variables of neck EMG were compared between the patient and control group. These variables, in conjunction with the timing of the head angles, reveal the muscle‐movement pattern in head‐neck. Additionally, temporal information are more reliable when comparing surface EMG among different subjects as there usually are slight differences in sensor placements and skin preparation.
The sample size of this study was small and the group characteristic of the patients was heterogeneous. Additionally, the brace used in this experiment had only one size, and fitted subjects within a range of anatomical sizes. However, this study demonstrated the feasibility of using this robotic brace for ALS patients. The brace could allow relatively a large range of motion of the head and was comfortable to wear. The procedure used in this study could be adapted to complement current clinic examinations. In the future, more subjects will be enrolled and braces with different sizes will be developed to fit a broader range of users. Future research will also evaluate other modalities of this robotic brace, including providing active assistance through motors, with ALS patients.
Conclusion
This paper presented a procedure to study head movements and concurrent neck muscle activations in ALS patients using a robotic neck brace. Patients with ALS showed different head movement and muscle activation patterns during single‐plane motions compared to age‐matched healthy controls. During flexion–extension, the head of ALS patients falls forward quicker and requires early activation of the extensor muscles to decelerate the head to the fully flexed position. These findings suggest that the nature of head‐neck movements may have been altered even when the ALS patients still have relatively large range of motion and strength in the neck.
Conflict of Interest
None declared.
Supporting information
Movie S1. An example of a video recording of an ALS patient during the flexion‐extension motion.
Movie S2. An animation of four‐rope model during a head‐neck axial rotation cycle. The animation was generated using data from Figure 2. The colors of the ropes indicate the muscle activation levels (red – active, blue – inactive).
Movie S3. An animation of four‐rope model during a head‐neck lateral bending cycle. The animation was generated using data from Figure 3. The colors of the ropes indicate the muscle activation levels (red – active, blue – inactive).
Movie S4. An animation of four‐rope model during a head‐neck flexion/extension cycle. The animation was generated using data from Figure 4. The colors of the ropes indicate the muscle activation levels (red – active, blue – inactive).
Acknowledgments
Some of the authors were funded with partial support from the following grants: NSF IIS‐1527087, NYS Translational Grant # C31290GG. The authors would like to thank Dr. Victor Santamaria for his help in data analysis.
Funding Information
The authors do not have any financial or non‐financial interest in the materials discussed in this manuscript.
References
- 1. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med 2001;344:1688–1700. [DOI] [PubMed] [Google Scholar]
- 2. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942–955. [DOI] [PubMed] [Google Scholar]
- 3. Brown RH, Al‐Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med 2017;2:162–172. [DOI] [PubMed] [Google Scholar]
- 4. Smith RA. Symptomatic care of patients with amyotrophic lateral sclerosis. JAMA J Am Med Assoc 1975;234:715. [PubMed] [Google Scholar]
- 5. Petheram TG, Hourigan PG, Emran IM, et al. Dropped head syndrome a case series and literature review. Spain 2008;33:47–51. [DOI] [PubMed] [Google Scholar]
- 6. Gourie‐Devi M, Nalini A, Sandhya S. Early or late appearance of "dropped head syndrome" in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2003;74:683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pancani S, Rowson J, Tindale W, et al. Assessment of the Sheffield Support Snood, an innovative cervical orthosis designed for people affected by neck muscle weakness. Clin Biomech 2016;32:201–206. [DOI] [PubMed] [Google Scholar]
- 8. Glazener P. Pilot study to determine the effectiveness of a new neck brace design for patients with amyotrophic lateral sclerosis. J Nurs Educ Pract 2014;4:1–5. [Google Scholar]
- 9. Pinto S, de Carvalho M. Motor responses of the sternocleidomastoid muscle in patients with amyotrophic lateral sclerosis. Muscle Nerve 2008;38:1312–1317. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura R, Atsuta N, Watanabe H, et al. Neck weakness is a potent prognostic factor in sporadic amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry 2013;84(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 11. Radunović A, Mitsumoto H, Leigh N. Clinical care of patients with amyotrophic lateral sclerosis. Lancet Neurol 2007;6:913–925. [DOI] [PubMed] [Google Scholar]
- 12. Mitsumoto H, Bromberg M, Johnston W, et al. Promoting excellence in end‐of‐life care in ALS. Amyotrophic Lateral Scler 2005;6:145–154. [DOI] [PubMed] [Google Scholar]
- 13. Pancani S, Tindale W, Shaw PJ, et al. An objective functional characterisation of head movement impairment in individuals with neck muscle weakness due to amyotrophic lateral sclerosis. PLoS ONE 2017;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Agrawal SK. Kinematic design of a dynamic brace for measurement of head/neck motion. IEEE Robot Autom Lett 2017;2:1428–1435. [Google Scholar]
- 15. Zhang H, Agrawal SK. An active neck brace controlled by a joystick to assist head motion. IEEE Robot Autom Lett 2018;3:37–43. [Google Scholar]
- 16. Zhang H, Albee K, Agrawal SK. A spring‐loaded compliant neck brace with adjustable supports. Mech Mach Theory 2018;125:34–44. [Google Scholar]
- 17. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 18. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1994;124:96–107. [DOI] [PubMed] [Google Scholar]
- 19. Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol 2014;10:661–670. [DOI] [PubMed] [Google Scholar]
- 20. Kim WK, Liu X, Sandner J, et al. Study of 962 patients indicates progressive muscular atrophy is a form of ALS. Neurology 2009;17:1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Zhou P, Aruin AS. Teager‐Kaiser energy operation of surface EMG improves muscle activity onset detection. Ann Biomed Eng 2007;35:1532–1538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. An example of a video recording of an ALS patient during the flexion‐extension motion.
Movie S2. An animation of four‐rope model during a head‐neck axial rotation cycle. The animation was generated using data from Figure 2. The colors of the ropes indicate the muscle activation levels (red – active, blue – inactive).
Movie S3. An animation of four‐rope model during a head‐neck lateral bending cycle. The animation was generated using data from Figure 3. The colors of the ropes indicate the muscle activation levels (red – active, blue – inactive).
Movie S4. An animation of four‐rope model during a head‐neck flexion/extension cycle. The animation was generated using data from Figure 4. The colors of the ropes indicate the muscle activation levels (red – active, blue – inactive).


