Abstract
Objective
To establish individually expandable primary fibroblast and keratinocyte cultures from 3‐mm skin punch biopsies for patient‐derived in vitro skin models to investigate of small fiber pathology.
Methods
We obtained 6‐mm skin punch biopsies from the calf of two patients with small fiber neuropathy (SFN) and two healthy controls. One half (3 mm) was used for diagnostic intraepidermal nerve fiber density (IENFD). From the second half, we isolated and cultured fibroblasts and keratinocytes. Cells were used to generate patient‐derived full‐thickness three‐dimensional (3D) skin models containing a dermal and epidermal component. Cells and skin models were characterized morphologically, immunocyto‐ and ‐histochemically (vimentin, cytokeratin (CK)‐10, CK 14, ki67, collagen1, and procollagen), and by electrical impedance.
Results
Distal IENFD was reduced in the SFN patients (2 fibers/mm each), while IENFD was normal in the controls (8 fibers/mm, 7 fibers/mm). Two‐dimensional (2D) cultured skin cells showed normal morphology, adequate viability, and proliferation, and expressed cell‐specific markers without relevant difference between SFN patient and healthy control. Using 2D cultured fibroblasts and keratinocytes, we obtained subject‐derived 3D skin models. Morphology of the 3D model was analogous to the respective skin biopsy specimens. Both, the dermal and the epidermal layer carried cell‐specific markers and showed a homogenous expression of extracellular matrix proteins.
Interpretation
Our protocol allows the generation of disease‐specific 2D and 3D skin models, which can be used to investigate the cross‐talk between skin cells and sensory neurons in small fiber pathology.
Introduction
Small fiber neuropathy (SFN) is a subgroup of sensory neuropathies, which selectively impairs thinly myelinated A‐delta and unmyelinated C nerve fibers and characteristically leads to acral burning pain, par‐ and dysesthesias. The peripheral nerve endings of the small fibers terminate in the epidermis as nociceptors, where they are exposed to direct influences from the environment, but also from the surrounding skin cells. It is striking, that intraepidermal innervation is reduced in SFN patients, while pain is a key symptom. Thus, hyperexcitability of the dorsal root ganglion sensory neurons or the remaining peripheral nociceptors is assumed. Indeed, we found an increase in pro‐inflammatory cytokine gene expression in skin punch biopsies of SFN patients that may contribute to nociceptor sensitization.1 Interestingly, this increase was not based on cutaneous inflammatory cell infiltration, which raises the question of a potential role of surrounding skin cells as a source for algesic mediators.
Keratinocytes secrete neuroactive molecules capable of modulating sensory neurons such as neurotrophins, adenose intriphosphate, and cytokines, and carry nociception‐associated ion channels, growth factor, and cytokine receptors.2, 3 In a recent study using human two‐dimensional (2D) culture models, the interaction of keratinocytes and sensory nerve fibers via formation of sensory units was shown.4 Another study applying optogenetics in mice revealed that activation of epidermal keratinocytes elicits action potentials in sensory neurons.5 These studies suggest an active role of keratinocytes in sensory processing and support the novel concept of cutaneous nociception. Furthermore, skin cells release axon guidance cues like netrins and semaphorins, which may direct nerve fiber growth.6, 7 Thus, evidence is increasing for a potential pathophysiological role of keratinocytes in peripheral innervation and sensory neuron sensitization, and novel experimental approaches are needed for translational in‐depth analysis of the underlying mechanisms, which is hampered by the limited availability of human biomaterial.
A promising tool to study interactions between peripheral nociceptors and skin cells is three‐dimensional (3D) full‐thickness skin model (FTSM).8 FTSM enables the investigation of human cell–cell and cell–matrix interactions which is particularly useful when suitable animal models are lacking. However, published protocols require large biopsies of several cm2 area,9 highly proliferative juvenile cells,10 or a combination of both, and are therefore not applicable when only few millimeter skin samples of adult patients are available as obtained for routine diagnostics. Our aim was to establish individually expandable primary keratinocyte and fibroblast 2D and 3D cell culture models from 3‐mm skin punch biopsies and to generate patient‐derived FTSM as a new in vitro tool for the investigation of the pathophysiological impact of skin cells on small fiber pathology.
Methods
Patient recruitment
Patients with SFN were prospectively recruited at the Department of Neurology, University of Würzburg, Germany, according to current criteria11 and after exclusion of large fiber polyneuropathy using clinical examination and nerve conduction studies. Additionally, age‐ and sex‐matched healthy controls were recruited. For the establishment of subject‐derived skin models, skin punch biopsies were obtained from two patients with SFN and two healthy controls (Table 1).
Table 1.
Patient characteristics.
| SFN‐1 | SFN‐2 | Ctr‐1 | Ctr‐2 | |
|---|---|---|---|---|
| Sex | Male | Male | Male | Male |
| Age | 54 | 44 | 69 | 29 |
| SFN etiology | Idiopathic | Idiopathic | N/A | N/A |
| Current pain intensity (on numeric rating scale 0–10) | 8/10 | 4/10 | N/A | N/A |
| Pain localization and character | Acral burning | Acral burning | N/A | N/A |
| pain attacks | Yes | Yes | N/A | N/A |
| Pain duration | 6 years | 6 years | N/A | N/A |
| IENFD at calf (fibers/mm) | 2 | 2 | 7 | 8 |
Abbreviations: Ctr, control; IENFD, intraepidermal nerve fiber density; N/A, not applicable; SFN, small fiber neuropathy.
Isolation of adult skin cells
Skin punch biopsy
Figure 1 depicts our experimental setup. A 6‐mm skin punch biopsy (apparatus by Stiefel GmbH, Offenbach, Germany) was taken in local anesthesia from the calf 10 cm above the lateral malleolus of two patients with idiopathic SFN and two healthy controls following a standard procedure.1 Each skin sample was divided in two 3‐mm parts: one part was used for immunohistochemical determination of the intraepidermal nerve fiber density (IENFD) as practiced in clinical routine12; the second part was processed for the isolation of adult human dermal fibroblasts (hDF) and epidermal keratinocytes (hEK). The epidermis was manually separated from the dermis using a scalpel (B. Braun, Melsungen, Germany). Both halves were further processed for primary cell cultures of hDF and hEK immediately after partitioning.
Figure 1.
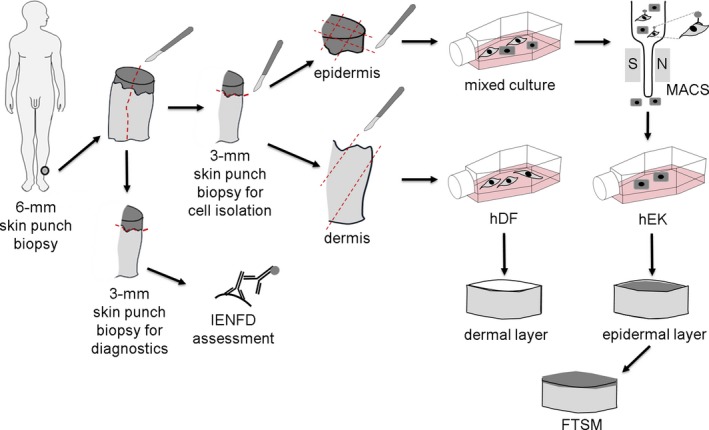
Experimental setup. 6‐mm skin punch biopsies were taken from the patients with small fiber neuropathy and the healthy controls and were divided into two 3‐mm parts each. One half was used for intraepidermal nerve fiber density (IENFD) assessment with specific antibodies against protein gene product‐9.5. In the second half, the epidermis was separated manually from the dermis. Both parts were then cut into small pieces and placed into cell culture flasks. Magnetic‐activated cell sorting (MACS) was used to purify the initially mixed skin cell culture and to gain human epidermal keratinocytes (hEK) for epidermis formation. Human dermal fibroblasts (hDF) were used to build a dermal layer. hEK were seeded on top of the dermal layer to generate a full‐thickness skin equivalents (FTSE).
hDF culture
To obtain hDF, the dermis was cut into three small pieces placed in a T25 flask which were covered with 10 µL of Dulbecco`s Modified Eagle Medium, F‐12 Nutrient Mixture (DMEM/F‐12) with 1% penicillin/streptomycin, and with 10% fetal calf serum (fibroblast medium, Thermo Fischer Scientific, Carlsbad, USA), and incubated for 1h at 37°C, 5% CO2 to ensure cell attachment. Thereafter, 1.5‐mL fibroblast medium was carefully added. Medium was changed every 2–3 days until cell outgrowth was observed. Subsequently, cells were expanded and further processed at passage four.
hEK culture
For the extraction of hEK, the epidermis was cut into nine small pieces and placed into three T25 cell culture bottles (three pieces per bottle) with the basal layer facing down toward the cell culture surface. To ensure cell attachment, samples were covered with 10 µL of fibroblast medium and incubated for 1 h at 37°C, 5% CO2. Thereafter, 1.5‐mL fibroblast medium was carefully added. Cells were inspected daily until keratinocyte outgrowth was observed. Then, medium was changed to keratinocyte medium (EpiLife Medium supplemented with 1% Epilife defined growth supplement, and 1% penicillin/streptomycin, Thermo Fischer Scientific, Carlsbad, USA). After passage two, magnetic‐activated cell sorting (MACS) with human Anti‐Fibroblast MicroBeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) was used according to the manufacturer’s protocol for purification of the hEK primary cell culture. Medium was changed every 2–3 days. Cells were further processed at passage four.
Cellular impedance
Measurements of cellular real‐time impedance for hEK and hDF were performed with xCELLigence® RTCA DP (Roche, Basel, Switzerland). About 3000 cells/well (n = 3/cell type) were seeded in 200 µL of the respective medium into an E‐plate 96 (ACEA Biosciences Inc., San Diego; USA). Cell‐based impedance (as reflected by the cell index) was measured hourly in a time interval of 50–100 h after cell seeding. Cellular doubling time (i.e. period of time that is needed for doubling a cell population) was calculated from the obtained data by fitting the curve to an exponential equation. Each cell line was measured in triplicate and RTCA Data Analysis Software 1.0 (Roche, Basel, Switzerland) was used for analysis. Data are illustrated as line charts or bar graphs (GraphPad Prism 7 software, San Diego, USA), representing the mean and standard deviation.
Generation of FTSE
Full‐thickness skin equivalents (FTSE) were generated using a Corning® Costar® well plate. First, hDF (4.5 × 104 cells) were embedded in collagen I hydrogel isolated in‐house from rat tails to build the dermal layer of the FTSE. Care was taken to avoid the formation of air bubbles in the highly viscous collagen I hydrogel. The dermal layer was cultivated in Epilife® medium supplemented with 1X human keratinocyte growth supplement (both Thermo Fisher Scientific, Waltham, MA USA), 100 U/mL penicillin‐streptomycin, 1.5‐mmol/L CaCl2 (both Sigma‐Aldrich, St. Louis, USA), 10 ng/mL keratinocyte growth factor, and 73 μg/mL l‐ascorbic acid 2‐phosphate (both Sigma‐Aldrich, St. Louis, USA). Medium was changed every 2–3 days.
After 1 week of culture, hEK were seeded on the dermal layer (5 × 105 cells). Medium was removed from the epidermal layer after 24 h. At the air–liquid interface, hEK formed an epidermis with vital cell layers and a stratum corneum. FTSE were cultured for 14 days. Medium was changed every 2–3 days.
Immunocytochemistry and immunohistochemistry
For immunocytochemistry, cells were fixed with 50% ethanol, 50% acetone mixture for 10 min at room temperature. FTSE and native skin for immunohistochemistry were fixed with Roti®‐Histofix 4% (Carl Roth, Karlsruhe, Germany) for 1 h room temperature, washed in tap water for 1 h, and embedded in paraffin. Afterwards, 4‐µm histological cross sections were prepared using a microtome SM 2010 R (Leica, Wetzlar, Germany). For morphological assessment, skin sections were stained with hematoxylin and eosin (HE). Immunoreactions with antibodies against the intermediate filament of epithelial cells cytokeratin (CK)‐10 (DAKO Cytomation, Hamburg, Germany, 1:100), the marker for basal keratinocytes CK‐14 (Sigma‐Aldrich, St. Louis, USA, 1:1000), nuclear proliferation marker ki67 (Abcam, Cambridge, UK 1:100), the intermediate filament of mesenchymal cells vimentin (Abcam, Cambridge, UK, 1:2000), the structural protein collagen 1 (Acris Antibodies, Herford, Germany, 1:100), and the collagen precursor procollagen (Merck Millipore, Burlington, USA 1:200) were performed. Briefly, paraffin‐embedded skin sections were deparaffinized, rehydrated, and rinsed in demineralized water. Slides were incubated at +4°C over night with the primary antibodies. Donkey anti‐mouse IgG (Life Technologies, Carlsbad, USA, 1:400), donkey anti‐rabbit IgG (Life Technologies, Carlsbad, USA, 1:400), or goat anti‐rat IgG (Life Technologies, Carlsbad, USA, 1:200) were used as secondary antibodies. For IENFD determination, skin sections were embedded in Tissue Tek, OCT medium (optimal cutting temperature; Sakura, Staufen, Germany) and frozen in 2‐methylbutane cooled in liquid nitrogen. Forty micrometer cryosections were prepared using a cryostat (Leica, Blenheim, Germany). Sections were immunoreacted with antibodies against the panaxonal marker protein gene product 9.5 (PGP 9.5; Zytomed, Berlin, Germany, 1: 1000). As secondary antibody, goat anti‐rabbit IgG (Amersham, Kingsport, TN, 1:100) was applied. Positive nerve fibers were quantified using a fluorescent microscope (Axiophot 2, Zeiss, Oberkochen, Germany) by two investigators blinded to the diagnosis.
Results
Patient characteristics
Details about demographic and clinical data of two patients with SFN (SFN‐1, and SFN‐2) and two healthy controls (Ctr‐1, and Ctr‐2) are given in Table 1. The SFN diagnosis was made according to current criteria and after exclusion of large fiber polyneuropathy using clinical examination and nerve conduction studies.11 The control subjects showed no symptoms or signs of neuropathy. Both patients with SFN had reduced IENFD, while IENFD at the lower leg was normal in healthy controls (SFN‐1, Ctr‐2; Fig. 2).
Figure 2.
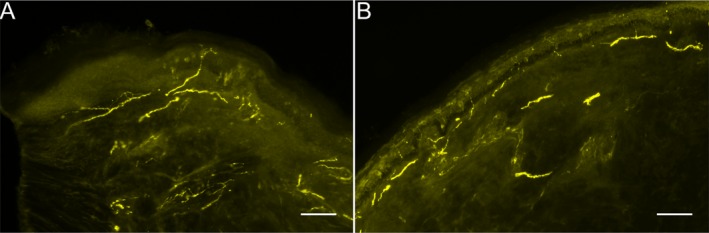
Representative photomicrographs of skin biopsy sections immunoreacted with antibodies against protein gene product‐9.5 for the detection of intraepidermal nerve fibers (IENF). IENF (indicated by white arrows) were reduced in the skin sample obtained from the lateral calf of a patient with small fiber neuropathy (SFN‐1, A) compared to a healthy control (Ctr‐2, B). Scale bar represents 100 μm.
Primary fibroblasts and keratinocyte 2D cell culture
We succeeded in isolating hDF and hEK cells from the 3‐mm skin specimens of the SFN patient (SFN‐1) and the healthy control (Ctr‐2), and established stable hDF (Fig. 3)A and B and hEK (Fig. 3C and D) cell cultures for subsequent application in FTSM. Morphological assessment showed a typical spindle‐shape appearance of the hDF, whereas hEK displayed a cobblestone morphology. For cellular characterization, immunoreactions with cell‐specific markers were performed. hDF of the SFN patient and the healthy control showed homogeneous expression of the intermediate filament of mesenchymal cells vimentin (Fig. 4A and E). hEK isolated from the SFN patient and the healthy control expressed the basal epidermal marker CK‐14 in 2D cell culture but were negative for the intermediate filament of differentiated keratinocytes CK‐10 (Fig. 4B, C, F, and G). The nuclear proliferation marker ki67 was equally expressed in hEK of the SFN patient and the healthy control (Fig. 4D and H). We did not detect morphological or immunocytochemical differences between skin cells obtained from the SFN patient and the healthy control.
Figure 3.
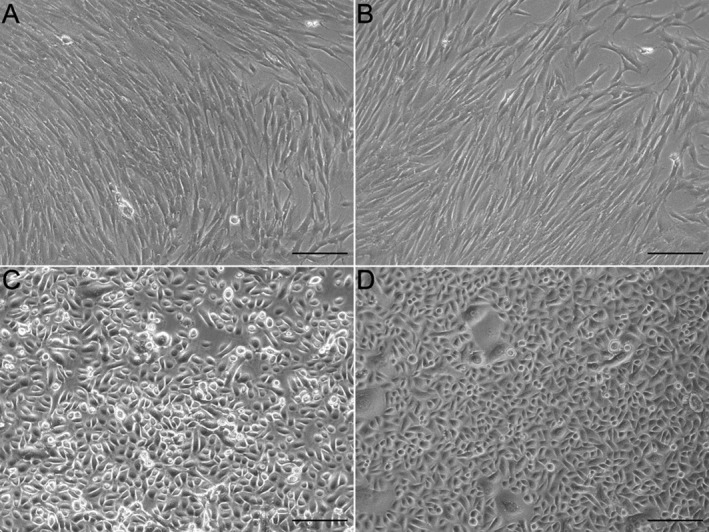
2D culture of primary human dermal fibroblasts (hDF) and epidermal keratinocytes (hEK). Phase contrast images of hDF culture of a patient (SFN‐1) with small fiber neuropathy (SFN, A), a healthy control (Ctr‐2, B), and hEK culture of a patient with SFN (C) and a healthy control (D). The morphology was normal in the hDF and hEK of the SFN patient and the healthy control. Scale bar represents 100 μm.
Figure 4.
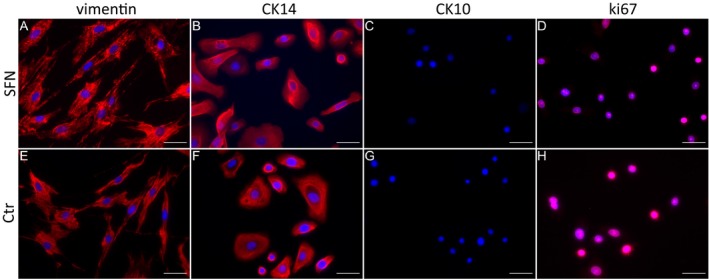
Expression of cell‐specific markers in fibroblasts and keratinocytes. Skin cells of a patient (SFN‐1) with small fiber neuropathy (SFN, A–D) and a healthy control (Ctr‐2, E–H) were immunoreacted with specific antibodies against vimentin (A, E), cytokeratin (CK)‐14, CK‐10, and ki67. Human dermal fibroblasts (hDF) expressed vimentin (A, E), whereas human epidermal keratinocytes (hEK) expressed the epithelial marker CK‐14 (B, F), but not CK‐10 (C, G). hEK also expressed the nuclear proliferation marker ki67 (D, H). Scale bar represents 50 μm.
Adequate cellular viability and constant proliferation of the SFN patient and the healthy control
To assess the viability and proliferation of skin cells in 2D culture, real‐time impedance was determined in hEK and hDF of the patient (SFN‐1) and the healthy control (Ctr‐2). The cell index of both cell types constantly increased indicating adequate viability and constant proliferation without relevant difference between the SFN patient and the healthy control (Fig. 5A and B). In contrast, the doubling time of hDF and hEK obtained from the SFN patient was lower (Fig. 5C, hDF: 217.47 h; hEK: 96.27 h) compared to those from the healthy control (hDF: 89.11 h; hEK: 57.65 h).
Figure 5.
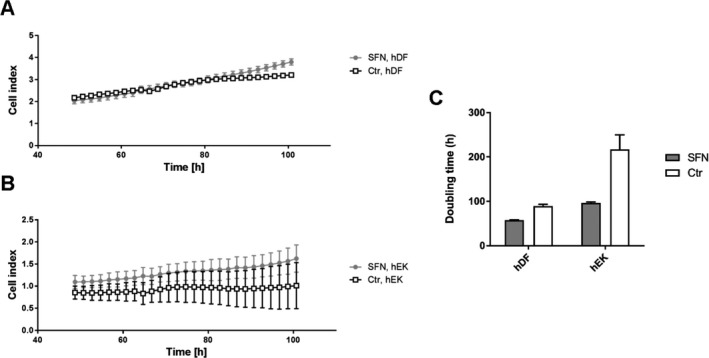
Real‐time impedance measurements using xCELLigence® instrument. Line charts display the cell index within the time frame of 50–100 h after seeding human dermal fibroblasts (hDF, A) and human epidermal keratinocytes (hEK, B) of the patient with small fiber neuropathy (SFN‐1) and the healthy control (Ctr‐2). Both cell types showed increase in the cell index, without differences between the cells of the SFN patient and the healthy control. Bar graphs show the doubling time (C) of hDF and hEK isolated from the patient with SFN and the healthy control. The doubling time of both cell types from the patient with SFN was lower compared to the healthy control.
Characterization of the FTSM
After 3 weeks, a FTSM was generated in skin cells obtained from two SFN patients (Fig. 6A, B) and two healthy controls (Fig. 6C, D). We first used HE staining to assess the morphological structure of the FTSM. The overall morphology of the FTSM was analogous to the respective HE stains performed on skin punch biopsy sections both in the SFN patients (Fig. 6A′ and A″; 6B′ and B″) and the healthy controls (Fig. 6C′ and C″; 6D′ and D″) consisting of a dermis and a multilayered epidermis with stratum corneum. To further investigate the composition of the FTSM of one patient with SFN (SFN‐1) and one healthy control (Ctr‐2), we performed immunoreactions with antibodies against selected tissue‐specific markers. Both models expressed intermediate filament markers, CK‐10 and CK‐14, in the epidermal layer. Differentiation between the basal and suprabasal strata of the epidermis was not possible in both models due to the low number of epidermal layers. The dermal layer showed a homogeneous distribution of vimentin, collagen 1, and procollagen (Fig. 7). We did not detect morphological or immunohistochemical differences between the FTSM of the SFN patient and the healthy control. We also performed immunoreactions on native skin from a healthy control subject embedded in paraffin (Ctr.2; Fig. 7). Expression of the markers CK‐10, CK‐14, vimentin, collagen 1, and procollagen was similar to the FTSM.
Figure 6.
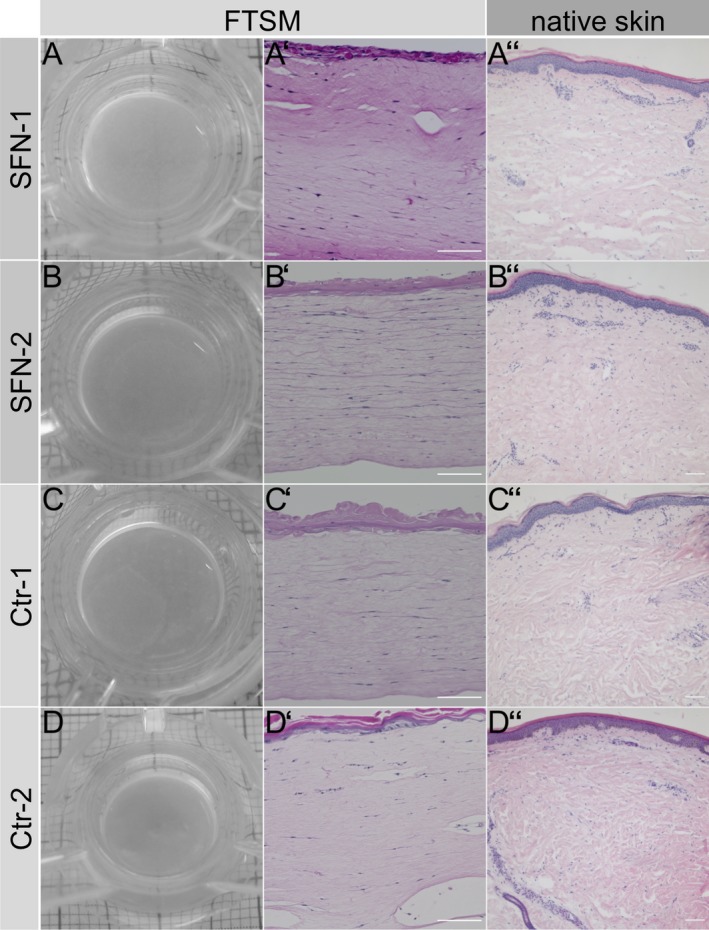
Morphological structure of the full‐thickness skin model (FTSM). Macroscopic images of the FTSM of two patients with small fiber neuropathy (SFN, A, B) and two healthy controls (Ctr, C, D). Hematoxylin and eosin (HE) stainings of the FTSM (A′‐D′), the respective skin punch biopsy (A″–D″) of the patients with SFN, and the healthy control. Morphology was normal in the FTSM of the SFN patients and the healthy controls, and was similar to that of native skin. Scale bar represents 100 μm.
Figure 7.
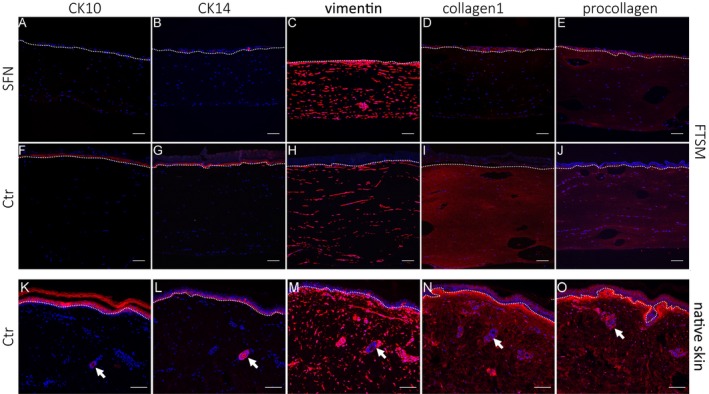
Cellular composition of the full‐thickness skin model (FTSM) and native skin. FTSM of a patient (SFN‐1) with small fiber neuropathy (SFN, A–E), FTSM and native skin of a healthy control (Ctr‐2, F–J and K–O) were immunoreacted with antibodies against cytokeratin (CK)‐10, CK‐14, vimentin, collagen 1, and procollagen. CK‐10 (A, F, K), and CK‐14 (B, G, L) are expressed in the epidermal layer. Both FTSM express vimentin (C, H, M), collagen 1 (D, I, N), and procollagen (E, J, O) in the dermal layer. Native skin sections contain blood vessels in the dermis (indicated by arrows). The dotted line marks the epidermis‐dermis border. Scale bar represents 100 μm.
Discussion
We set out to individually isolate and expand hDF and hEK cell cultures and provide a protocol to isolate both cell types from a single 3‐mm skin punch biopsy obtained from adult subjects. We have succeeded in generating 2D cell cultures and a 3D in vitro skin model from individual subject‐derived cells which can now be used for in‐depth pathophysiological analysis of small fiber damage. We showed that both cell types have the typical morphology, express cell‐specific markers, and display constant proliferation as determined by impedance measurement.
SFN is a subgroup of sensory neuropathies, which is characterized by burning pain and dysesthesias in hands and feet, while skin punch biopsies typically reveal a reduction in IENFD.11 The traditional view, that neurodegenerative processes in DRG sensory neurons may underlie SFN pain and peripheral denervation, is increasingly challenged by studies providing evidence for an active role of skin cells in sensory processing and cutaneous nociception. As the main skin cell population, keratinocytes and fibroblasts are in close and active contact with sensory nerve fiber endings, and human and experimental data are pointing toward an intensive interaction between skin cells and nociceptors.13 This novel concept of skin cell involvement in sensory signaling is supported by the expression of nociception‐associated receptors14, 15, 16 and the release of pain‐related mediators17, 18, 19 by fibroblasts and keratinocytes. In addition, keratinocytes release axon guidance cues like netrins and semaphorins,6, 7 which may act as local pathfinders.
Several studies have shown the interplay between skin cells and sensory neurons. Overexpression of neurotrophins like nerve growth factor or neurotrophin 3 by keratinocytes prolonged neuronal survival.20 Axonal outgrowth was strongly stimulated when cocultivating neurons with keratinocytes, but was absent in pure neuronal cultures indicating the influence of a potential soluble factor secreted by keratinocytes.21 Using mice expressing channelrhodopsin in keratinocytes, a recent study revealed that mere activation of the epidermis already evokes action potentials in sensory neurons.5 In transgenic mice, selective transient receptor potential vanilloid (TRPV) 3 overexpression and heat activation of keratinocytes induced prostaglandin E2 release causing nocifensive behavior.22 After conditioned expression of the capsaicin receptor TRPV1 in keratinocytes of TRPV1‐knockout mice, exclusive stimulation of keratinocytes was sufficient to evoke pain behavior.23
While data from experimental studies are encouraging, the limited availability of patient biomaterial remains the main translational roadblock to transfer knowledge from bench to bedside. To overcome this hurdle, 3D skin models have been established. One group developed a hybrid model of human skin with axonal growth of rat DRG neurites into the dermis.24 In another study, the authors developed a skin model using neonatal foreskin fibroblasts and keratinocytes, coated with human‐induced neural stem cells.25 Recently, a fully human‐derived and innervated skin model using fibroblasts and keratinocytes from breast reductive surgeries was developed.26 The major drawbacks for the methodological transfer into clinical studies are the need of large skin samples, juvenile and highly proliferative cells as prerequisites,9, 25 or hybrid models obtained from different species,27 which are either not practicable in clinical routine or does not reflect real‐life conditions. Moreover, these tools do not model disease conditions since skin cells and neurons derived from healthy donors were included. Here, we established a protocol, which enables the expansion of patient‐derived skin cells under 2D and 3D conditions using only few millimeter of skin from adult subjects obtained under conditions used in clinical practice.
Our study has some limitations. Although keratinocytes and fibroblasts are the most abundant cell types in the skin, various other cells (e.g. immune cells and Schwann cells) were not included in the current model. Also, cutaneous structures, such as blood vessels and nerve fibers, are missing. Future developments improving and extending 2D and 3D cell culture protocols will help refining our model.
Our protocol provides a promising in vitro method for generating disease‐specific 2D cultures and 3D skin models from a single 3‐mm skin punch biopsy. 2D cultures enable gene expression analysis of distinct skin cells rather than using the entire skin samples. Likewise, our patient‐derived 3D skin models facilitate individualized in‐depth analysis of cutaneous cell–cell interactions as a potential contributor to small fiber pathology. Our method also provides a solid basis for future developments toward innervated 3D‐skin models serving as novel tools in personalized medicine.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author Contributions
NÜ, FGB, and FK contributed to the conception and design of the study. FK, MW, LK, TM, and PF were involved in acquisition and analysis of data. FK and NÜ drafted the manuscript and figures. NÜ, FGB, and MW revised the manuscript critically for important intellectual content.
Acknowledgments
We thank Alina Hohmuth (Department of Neurology, University of Würzburg) for technical help during the early phases of our 2D skin cell protocol establishment.
Funding Information
The study was supported by the Interdisciplinary Center for Clinical Research (Interdisziplinäres Zentrum für klinische Forschung, IZKF) University of Würzburg (N.Ü.: N353). N.Ü. was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG: UE171‐5/1).
Funding Statement
This work was funded by Interdisziplinäres Zentrum für klinische Forschung grant N353; Deutsche Forschungsgemeinschaft grant UE171‐5/1.
References
- 1. Üçeyler N, Kafke W, Riediger N, et al. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 2010;74:1806–1813. [DOI] [PubMed] [Google Scholar]
- 2. Olah A, Szollosi AG, Biro T. The channel physiology of the skin. Rev Physiol Biochem Pharmacol 2012;163:65–131. [DOI] [PubMed] [Google Scholar]
- 3. Hanel KH, Cornelissen C, Luscher B, Baron JM. Cytokines and the skin barrier. Int J Mol Sci 2013;14:6720–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krishnan‐Kutty V, Bigliardi PL, Dykas MM, et al. Peripheral nerve fibres form multifacet interactions with keratinocytes in a novel complete human 2D culture model. Exp Dermatol 2017;26:281–284. [DOI] [PubMed] [Google Scholar]
- 5. Baumbauer KM, DeBerry JJ, Adelman PC, et al. Keratinocytes can modulate and directly initiate nociceptive responses. Elife 2015;4 10.7554/eLife.09674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tominaga M, Ogawa H, Takamori K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br J Dermatol 2008;158:842–844. [DOI] [PubMed] [Google Scholar]
- 7. Botchkarev VA, Yaar M, Peters EM, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol 2006;126:1719–1727. [DOI] [PubMed] [Google Scholar]
- 8. Groeber F, Holeiter M, Hampel M, et al. Skin tissue engineering–in vivo and in vitro applications. Adv Drug Deliv Rev 2011;63:352–366. [DOI] [PubMed] [Google Scholar]
- 9. Johansen C. Generation and culturing of primary human keratinocytes from adult skin. J Vis Exp 2017;130 10.3791/56863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reuter C, Walles H, Groeber F. Preparation of a three‐dimensional full thickness skin equivalent. Methods Mol Biol 2017;1612:191–198. [DOI] [PubMed] [Google Scholar]
- 11. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lauria G, Cornblath DR, Johansson O, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:747–758. [DOI] [PubMed] [Google Scholar]
- 13. Keppel Hesselink JM, Kopsky DJ, Bhaskar AK. Skin matters! The role of keratinocytes in nociception: a rational argument for the development of topical analgesics. J Pain Res 2017;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao P, Barr TP, Hou Q, et al. Voltage‐gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain 2008;139:90–105. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Fang Q, Wang Z, et al. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J Biol Chem 2016;291:10252–10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lakoma J, Donadio V, Liguori R, Caprini M. Characterization of human dermal fibroblasts in Fabry disease. J Cell Physiol 2016;231:192–203. [DOI] [PubMed] [Google Scholar]
- 17. Li WW, Guo TZ, Li XQ, et al. Fracture induces keratinocyte activation, proliferation, and expression of pro‐nociceptive inflammatory mediators. Pain 2010;151:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept 2013;186:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paish HL, Kalson NS, Smith GR, et al. Fibroblasts promote inflammation and pain via IL‐1alpha induction of the monocyte chemoattractant chemokine (C‐C Motif) ligand 2. Am J Pathol 2018;188:696–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albers KM, Davis BM. The skin as a neurotrophic organ. Neuroscientist 2007;13:371–382. [DOI] [PubMed] [Google Scholar]
- 21. Ulmann L, Rodeau JL, Danoux L, et al. Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur J Neurosci 2007;26:113–125. [DOI] [PubMed] [Google Scholar]
- 22. Huang SM, Lee H, Chung MK, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci 2008;28:13727–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pang Z, Sakamoto T, Tiwari V, et al. Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain 2015;156:656–665. [DOI] [PubMed] [Google Scholar]
- 24. Martorina F, Casale C, Urciuolo F, et al. In vitro activation of the neuro‐transduction mechanism in sensitive organotypic human skin model. Biomaterials 2017;113:217–239. [DOI] [PubMed] [Google Scholar]
- 25. Vidal SEL, Tamamoto KA, Nguyen H, et al. 3D biomaterial matrix to support long term, full thickness, immuno‐competent human skin equivalents with nervous system components. Biomaterials 2019;198:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller Q, Beaudet MJ, De Serres‐Berard T, et al. Development of an innervated tissue‐engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater 2018;82:93–101. [DOI] [PubMed] [Google Scholar]
- 27. Roggenkamp D, Falkner S, Stab F, et al. Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J Invest Dermatol 2012;132:1892–900. [DOI] [PubMed] [Google Scholar]


