Abstract
Background
Colon cancer is one of the most common malignancies worldwide. Because of the side effects and defects in tolerance of chemotherapy, it is necessary to discover new drugs for colon cancer treatment. Columbamine has been identified as an effective anti-osteosarcoma compound with only minor side effects. In this study, we analyzed the anticancer effect of columbamine on colon cancer.
Methods
Human colon cancer cell lines were treatment with columbamine. MTT assay, colony formation assay, apoptosis detection and tumorigenicity assay were performed to detect the protective effect of columbamine on colon cancer development. Western blot assay and luciferase reporter assay were conducted to investigate the potential mechanism of the columbamine treatment.
Results
Columbamine significantly inhibited the proliferation, migration, invasion process of colon cancer cells, and dramatically promoted the apoptosis rate of colon cancer cells to further suppress the development of colon cancer to tumor. Both the signaling transducing and key factors expression of Wnt/β-catenin signaling pathway were obviously repressed by columbamine treatment in a dose-dependent manner.
Conclusion
The present study indicated that columbamine exerts its anti-tumor effect in colon cancer cells through abolishing Wnt/β-catenin signaling pathway. Columbamine may be a new therapy compound for colon cancer.
Keywords: colon cancer, cells growth, apoptosis, HCT116, LoVo
Introduction
Colon cancer is a digestive tract malignancy that occurs in the colon and has already been one of the most common malignancies worldwide.1 Although the incidence and mortality of colon cancer in the United States has been declining in recent decades, there are still about 140,000 new cases and about 50,000 death every year.2 In China, the incidence and mortality of colon cancer remains high and maintains an upward trend in recent years, which seriously threatens human health.3 The occurrence and development of colon cancer is a multi-step and multi-stage process, which is regulated by a number of genes and proteins contributing to the persistent pathogenesis.4 Inherited or acquired mutations in the key molecules involved in signaling pathway including Transforming growth factor beta (TGF-β), Wnt/β-catenin, Phosphoinositide 3-kinase (PI3K)/Akt, p53 and Phosphatase and tensin homolog (PTEN) may contribute to the pathogenesis of colon cancer.5,6 Epigenetic alteration, such as direct hypermethylation or hypomethylation of CpG islands within promoter region, is another genetic factor involved in the development of colon cancer.7,8 The occurrence and development of colon cancer may be the result of a combination of genetic, environmental factors and lifestyle, but the specific pathogenesis still remains unclear.9
At present, surgical treatment, radiotherapy, chemotherapy, immunotherapy and other methods are commonly used for the clinical treatment of colon cancer. However, many patients in the late stage of the disease have lost the indications for surgical treatment. Radiotherapy and chemotherapy have a large side effect on patients. Additionally, the chemotherapy has defects in tolerance, efficacy and cross-resistance,10 which is not conducive to improving the prognosis of patients. Therefore, it is urgent to explore new drugs with relatively low toxicity and highly therapeutic efficiency.11
Chinese medicine resources are abundant and have been applied as treatment for thousands of years. Discovering anti-tumor ingredients from traditional Chinese medicine will help to improve the chemotherapy effect of colon cancer and improve the comprehensive curative effect of tumors. Rhizoma Coptidis is a natural perennial herb belonging to the genus Ranunculaceae. Berberine extracted from Rhizoma Coptidis has anti-bacterial, anti-viral, anti-inflammatory, hypoglycemic and lipid-lowering effects.12 The active constituents of Rhizoma Coptidis are mainly alkaloids with isoquinoline structure, including berberine (BBR), coptisine (COP), palmatine (PAL), and berberine (Epiberberine, EPI), columbamine (COL), jatrorrhizine (JAT).12 Columbamine (COL) is a tetrahydroisoquinoline alkaloid derived from the rhizome of Chinese herbal medicine Rhizoma Coptidis. Previous experiments have shown that COL play roles in reducing high glycine, protecting cells from oxidative stress, relieving inflammation and pain.12–15 Due to its minor side effects, COL has been identified as an effective anti-osteosarcoma compound.16 Despite these studies, whether COL exerts its antitumor effect on colon cancer is still unknown. Here, we aim to verify the action of COL on colon cancer cells in vitro and in vivo, and explore the potential mechanism of action of COL in colon cancer.
In the present study, we found COL functioned as an inhibitor for colon cancer cells growth, invasion and migration, thereby inhibiting the development of colon cancer. Mechanism studies proved COL suppressed tumor development by inhibiting the Wnt/β-catenin signaling pathway.
Materials and methods
Cell culture and reagents
Human colorectal cancer cell lines LoVo, HCT-116 and SW480 were purchased from the Shanghai Cell Collection (Shanghai, China). The LoVo, HCT-116, SW480 cells were maintained in RPMI 1640, McCoy’s 5A medium and L-15 medium, respectively, supplemented with 10% fetal calf serum, 2 mM glutamine, 100 units/mL penicillin and 100 mg/mL streptomycin (Invitrogen, Carlsbad, CA). All cells were cultured in humidified air containing 5% CO2 at 37°C. Columbamine (P1191) used in this research was purchased from Shanghai PureOne Biotechnology (Shanghai, China).
Plasmid construction and transfection
Flag-tagged β-catenin (S33Y) was amplified from pcDNA3-S33Y b-catenin (Addgene, MA, USA), and cloned between the XbaI and BamHI sites of plvx-IRES-zsGreen (Clontech Laboratories, CA, USA). The plasmid construction was verified by DNA sequencing. The mutant plasmid and blank vector were transiently transfected into HCT116 cells at 70% confluence in accordance with manufacturer’s instruction of LipoFectamine Plus Reagent (Invitrogen, Carlsbad, CA). After 24 hrs post transfection, cells were incubated with indicated dosage of COL for further assay.
MTT assay
The HCT116, LoVo, SW480 cells were seeded into a 96-well plate at a density of 2×103 cells per well 1 day prior to experiments. All the cells were treated with COL at concentrations of 0 mM, 20 mM, 30 mM, 40 mM and 50 mM for time-course assay (24 hrs, 48 hrs and 72 hrs). During the last 4 hrs of culture, cells were added with MTT of 50 mg per well (Sigma-Aldrich, MO, USA). Formazan was dissolved by using dimethyl sulfoxide, and the absorbance was measured at 450 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Tek Instruments, VT, US).
Cell growth assay
Equal number of control and test cells were seeded in 12-well plates and maintained in medium supplemented with 10% FBS at a density of 1×103 cells per well. Medium was changed every other day. After ten days of standard culture, the medium was removed and all the cells were stained with 0.5% crystal violet solution in 20% methanol. After staining for 10 mins, the fixed cells were gently washed with phosphate-buffered saline (PBS) twice. The plates were let to air-dry overnight and then photographed to count the colony number for plating efficiency (PE) calculation.
Scratch wound assay
The cells were seeded into 24-well tissue culture dishes containing coverslips pre-coated with collagen type I (40 μg/mL) for 2 hrs at 37°C, at a concentration of 3×105 cells/mL. Cells were cultured in medium containing 10% FBS to nearly confluent cell monolayers. Then, a linear wound was generated with a sterile 100 μL plastic pipette tip. Cellular debris was removed by washing the coverslips with PBS. The cells were fixed with 4% paraformaldehyde for 15 mins. Three representative images from each coverslip of the scratched areas under each condition were photographed to estimate the relative migration cells. The data were analyzed using ImageJ software of version 1.41 (National Institutes of Health, Bethesda, MD). The experiments were performed at least in duplicate.
Cell invasion and migration assay
A transwell chamber of 8 µm (BD, NJ, US) (the chamber for invasion detection was coated with Matrigel, and the chamber for migration detection was coated without Matrigel) was placed into a 24-well plate. The volume of 600 µL medium containing 10% FBS was added to the bottom chamber. The prepared cell suspension was seeded in a transwell chamber at density of 1×103 cells per well. After an appropriate number of cells had passed through the cell pores, the cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet for 30 mins at room temperature. After washing twice by PBS, the images of plate were captured.
FACS analysis
After treatment with COL for 24 hrs, the HCT116 cells were collected for apoptosis assay by commercial kit (Abcam, Cambridge, UK) following instructions of manufacturers. Harvested cells were re-suspended in 500 μL of 1× Binding Buffer, and then were incubated with 5 μL of Annexin V-FITC and 5 μL of propidium iodide at room temperature for 5 mins in the dark. Flow cytometry was performed through Fluorescence-activated cell sorting (FACS) Calibur (BD, NJ, US).
TUNEL staining
TUNEL staining was performed using an apoptosis detection kit S7100 (EMD Millipore, MA, USA) according to the manufacturer’s protocol. The nuclei of TUNEL-positive cells containing apoptotic bodies were stained blue and were identified as apoptotic. The apoptotic cells were counted under high-power magnification (×400) and the percentage of TUNEL-positive cells among the total cells was calculated.
Tumorigenicity assay
Six-weeks-old male BALB/c nude mice were purchased from Shanghai SLAC laboratory animal company (Shanghai, China) for the study. Total number of 5×106 colon cancer cells HCT116 and control cells were subcutaneously injected on the opposite flanks of the same mouse. Different doses (5, 10, 20 mg/kg body weight) of COL were administered to experimental mice for 23 days. The tumor sizes of each mouse were measured every day from day 7 to day 23. All procedures performed in this study were approved by the Medical Ethics Committee of the Fifth Hospital in Wuhan and were performed according to institutional protocols.
Western blot assay
The cells were maintained on ice in radioimmunoprecipitation assay buffer (RIPA) for 2 hrs before being collected with a cell scraper. The sample was then centrifuged at 12,000 rpm, 4°C for 15 mins to remove cell debris and the supernatant was stored at 80°C until use. The protein concentration was detected by bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical Company, IL, USA) and was adjusted to 5 μg/μL with lysis buffer. Electrophoresis of 10% SDS-PAGE gel was conducted to separate protein extracts, followed by transferring protein onto PVDF membranes (Millipore, Watford, UK). The membrane was blocked at room temperature with 5% BSA for 1 hr and then incubated with the corresponding primary antibody at room temperature for 2 hrs. After washing thrice in 1×TBST, the membrane was probed with corresponding HRP-labeled secondary antibodies (Santa Cruz Biotechnology, CA, USA) at room temperature for 1 hr. Finally, protein bands were detected using an ECL Western blotting detection system (Amersham, Little Chalfont, UK). The primary antibodies including anti-E-cadherin, anti-N-Cadherin, anti-BCL2, anti-BAD, anti-MMP2, anti-MMP7, anti-Caspase3, anti-cleaved caspase3 were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-β-actin was brought from Abmart (Shanghai, China). Anti-PAPR, anti-PAPR and anti-β-catenin were from Abcam (Cambridge, UK).
Luciferase reporter assay
HCT116 cells were transfected with Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, MA, US) according to the instructions. Briefly, 2.5×104 cells were transfected with 500 ng of luciferase reporter construct pGL3-LEF/TCF and 50 ng internal control plasmid Renilla luciferase vector. After 24 hrs, the medium was removed and promoter activity was analyzed using a commercial dual-luciferase assay kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions.
Image J assay
The proteins of interest in all Western blots presented in the present study were quantified by Image J software (National Institutes of Health, MD, USA). The gray value of protein band was normalized against its internal control β-actin, and was expressed as values relative to the control from three independent experiments.
Statistical analysis
All data values are expressed as mean±standard deviation (SD). Statistical analysis was performed by software package GraphPad Prism 6.0 (GraphPad Software, CA, USA). Significant differences were analyzed by one-way ANOVA or student’s t-test. p<0.05 was considered statistically significant.
Results
Columbamine inhibits the proliferation of colon cancer cells
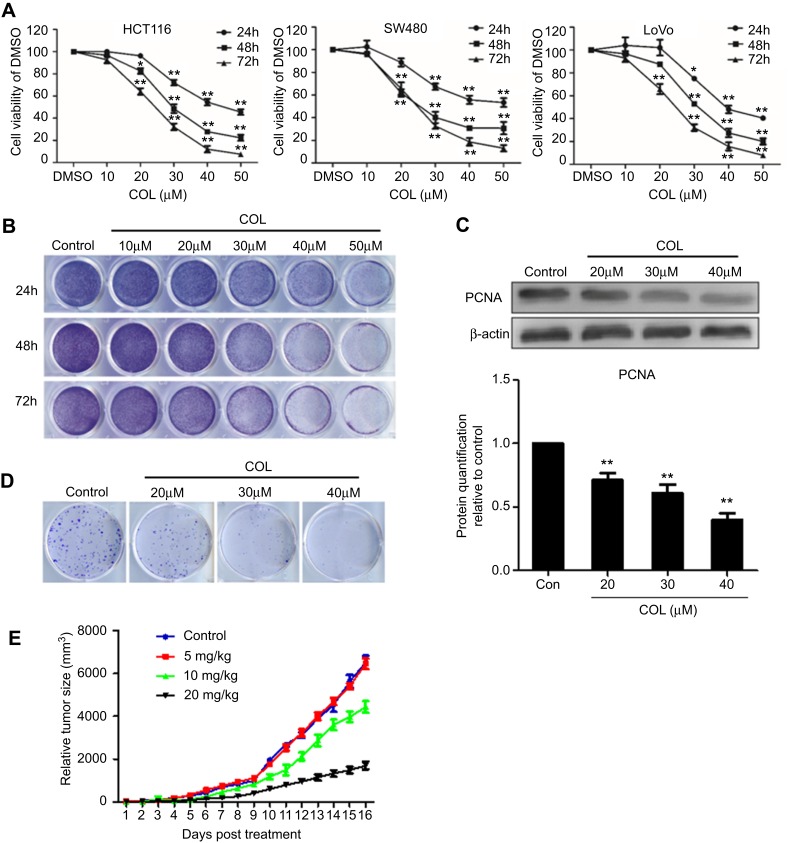
To assess its anticancer efficiency, COL was applied to HCT116, SW480 and Lovo cells for 24 hrs, 48 hrs and 72 hrs. The MTT results showed that COL inhibited the growth of the colon cells, which were proportional to treatment time and dosage (Figure 1A). Similar results were obtained by colony-formation assay to examine the activity of COL on the clonogenicity of cancer cells. As shown in Figure 1B, COL suppressed clone formation of HCT116 cells in a concentration and time-dependent manner. Notably, incubating HCT116 cells with COL at concentrations of 20 μM, 30 μM and 40 μM could obviously inhibit the growth of colon cancer cells (Figure 1C and D). According to the results, we selected the concentration of 20 μM, 30 μM and 40 μM for the subsequent in vitro experiments. We also examined explanted tumor size in mice subcutaneously injected with HCT116 cells after treatment of COL. Compared with the control group, COL treatment significantly decreased the tumor volumes in a dose-dependent manner (Figure 1E).
Figure 1.
Columbamine inhibits the proliferation of colon cancer cells in vitro and in vivo. HCT116, SW480 and LoVo cells were treated with different concentration of columbamine. Samples were harvested at the indicated time points to detect the effect of COL on colon cancer cells. (A) The proliferation of HCT116, SW480 and LoVo cells were detected by MTT assay. Data were representatives of three independent experiments with similar results. The error bars indicated the SD of duplicates. (B and D) The proliferation of HCT116 cells was determined by colony formation assay. (C) PCNA protein expression was detected by WB. The gray value of protein band was normalized to its internal control β-actin, and was expressed as values relative to the control from three independent experiments (lower panel). The error bars represented standard deviations of quantification from three independent experiments. (E) The tumor size of nude mice with HCT116 injection after columbamine treatment was recorded. The error bars indicated the standard deviations. Data were representatives of three independent experiments with similar results.
Notes: **P<0.01; *P<0.05, compared to control group.
Abbreviations: SD, standard deviations; WB, Western blotting; COL, columbamine.
Columbamine inhibits the invasion and migration of colon cancer cells
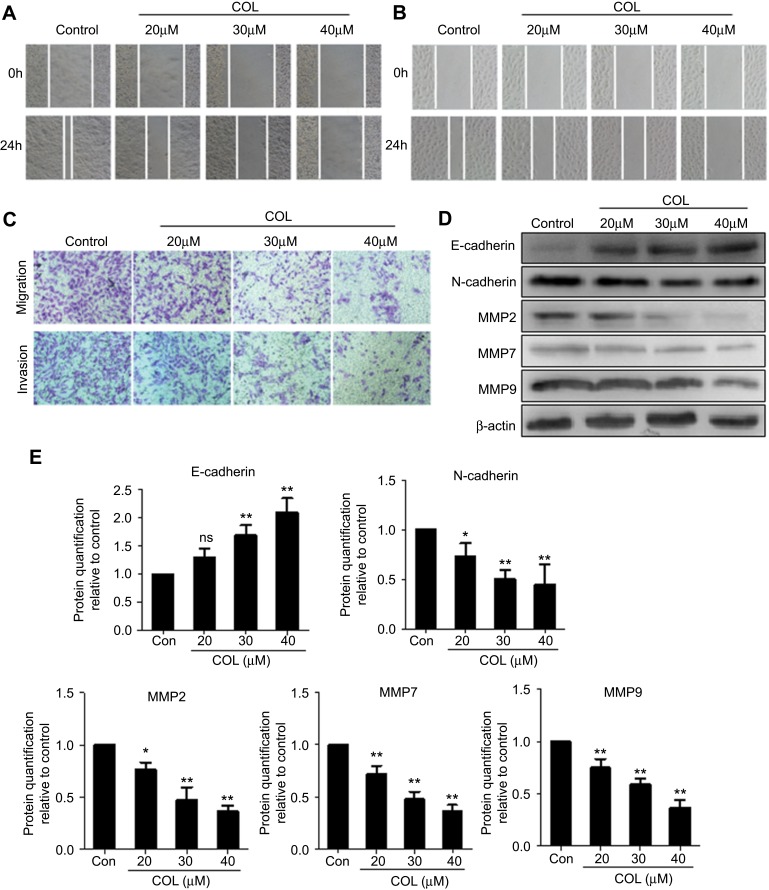
The invasion and migration of colon cancer cells were examined using scratch assay and transwell assay, respectively. COL significantly inhibited the migration and invasion of HCT116 and Lovo cells compared with the control cells (Figure 2A and B). Transwell assay showed treatment with COL greatly impaired the invasion and migration of HCT116 cells in dose-dependent manner (Figure 2C). To further study the functions of COL in colon cancer cells, we explored the expression of metastasis-related proteins E-cadherin, N-Cadherin, MMP2, MMP7 and MMP9 in HCT116 cells (Figure 2D and E). COL suppressed the expression of N-Cadherin and promoted the expression of E-cadherin, resulting in the inhibition of epithelial-mesenchymal transition (EMT) of colon cancer cells. The expressions of MMP2, MMP7 and MMP9 proteins were also attenuated by COL incubation (Figure 2D and E). In summary, these results suggested that COL impaired the migration and invasion ability of the colon cancer cells.
Figure 2.
Columbamine attenuates the invasion and migration of colon cancer cells. Colon cancer cells were incubated with different concentration of columbamine. The action of COL on invasion and migration of colon cancer cells were investigated at indicated time points. Cell samples were harvested for Western blot to detect protein expression. (A and B) The abilities of invasion and migration in HCT116 (A) and LoVo cells (B) were detected by scratch wound assay at 0 hr, 24 hrs post COL treatment. (C) The invasion and migration of HCT116 cells were confirmed by transwell experiment. Data were representatives of three independent experiments with similar results. (D) HCT116 cells were harvested at 24 hrs post treatment for Western blotting to detect invasion-related proteins expression. (E) The gray value of protein band was normalized to its internal control β-actin, and was expressed as values relative to the control from three independent experiments. The error bars represented standard deviations of quantification from three independent experiments.
Notes: ns, P>0.05; **P<0.01; *P<0.05, compared to control group.
Abbreviations: MMP, matrix metallopeptidase; COL, columbamine.
Columbamine induces apoptosis in colon cancer cells
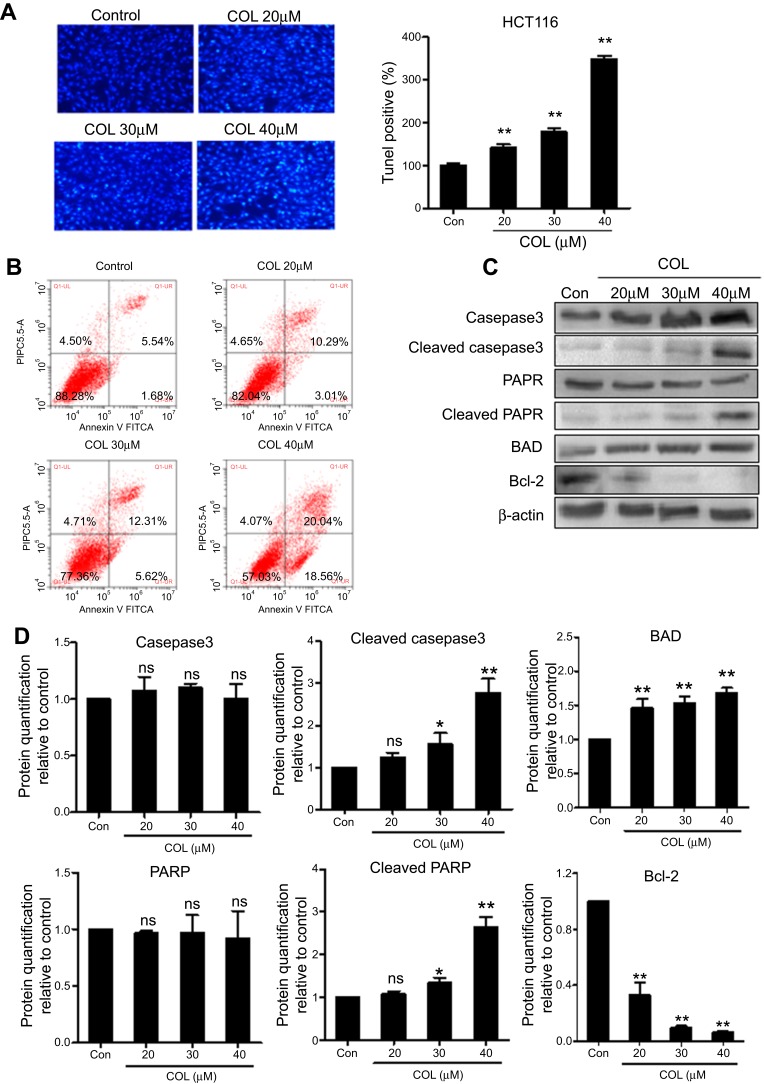
To investigate the effect of COL on apoptosis of colon cancer cells, we analyzed the population of apoptotic HCT116 cells under the COL treatment. TUNEL staining image revealed that treatment with COL at concentration from 20 μM to 40 μM significantly prevented cell growth and promoted cell death (Figure 3A). Flow cytometry results exhibited that COL addition increased the population of Annexin V-positive cells (Figure 3B), suggesting COL induced apoptosis in HCT116 cells. To further determine the action of COL in apoptosis of colon cancer cells, we examined the expression of apoptosis-related proteins by Western blotting. As shown in Figure 3C and D, the cleaved form of caspase-3 and PARP were increased without affecting the expression of caspase-3 and PARP by treatment with COL in dose-dependent manner. COL treatment also promoted pro-apoptosis factor BAD expression and repressed anti-apoptosis factor Bcl-2 expression. Taken together, columbamine triggered apoptosis by inhibiting a caspase-dependent mechanism.
Figure 3.
Columbamine induces apoptosis in colon cancer cells. HCT116 cells were incubated with COL at concentrations of 20 μM, 30 μM and 40 μM for 48 hrs. TUNEL staining (A) and flow cytometry (B) were performed to quantify the apoptotic of colon cancer cells. Data were representatives of three independent experiments with similar results. (C) The protein level of apoptosis-related molecules including Caspase3, cleaved Caspase3, PARP, cleaved PARP, BAD, Bcl-2 were detected by Western blotting. (D) Protein levels were quantified by ImageJ software. The gray value of protein band was normalized to its internal control β-actin, and was expressed as values relative to the control from three independent experiments. The error bars represented standard deviations of quantification from three independent experiments.
Notes: ns, P>0.05; **P<0.01; *P<0.05, compared to control group.
Abbreviations: PARP, poly ADP ribose polymerase; BAD, Bcl-2-associated death promoter; Bcl-2,B-cell lymphoma 2; COL, columbamine.
Columbamine inhibits the proliferation of colon cancer cells via suppressing Wnt/β-catenin signaling pathway
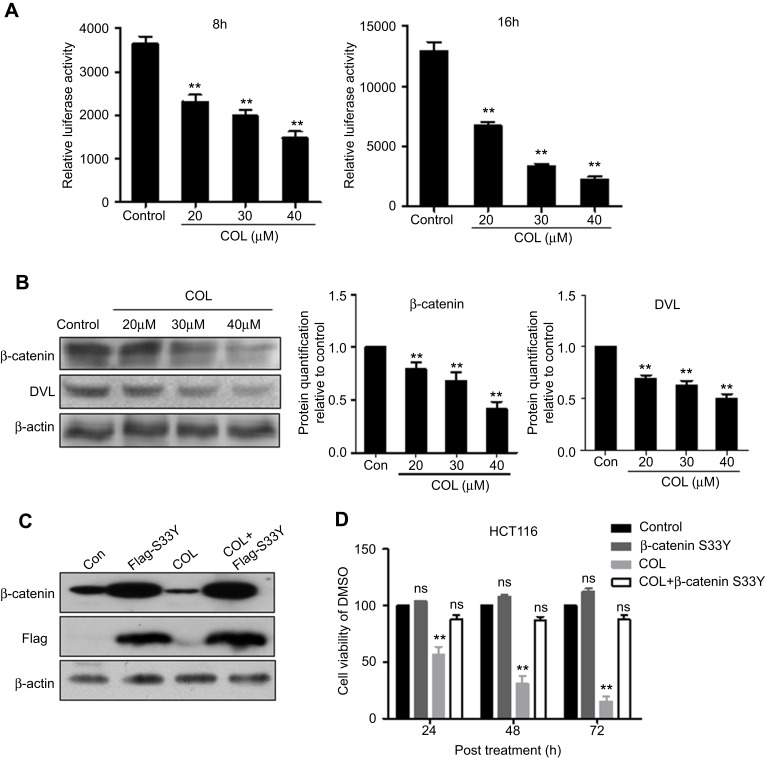
Previous studies demonstrated that Wnt/β-catenin signaling pathway is involved in colon cancer cell proliferation. Therefore, we speculated that COL may exert its influence on colon cancer cells via Wnt/β-catenin signaling pathway. The plasmid harboring TCF/LEF responsive element was transfected into HCT116 cells added with COL at indicated concentrations. At 8 hrs and 16 hrs post treatment, COL induced decrease in luciferase activity, indicating Wnt/β-catenin signaling pathway was negatively regulated by COL (Figure 4A).
Figure 4.
Columbamine inhibits the proliferation of colon cancer cells via suppressing Wnt/β-catenin signaling pathway. pGL3-LEF/TCF harboring Wnt/β-catenin responsive element and internal control plasmid were transfected into HCT116 cells. (A) Cells transfected with reporter plasmids for 24 hrs were harvested at 8 hrs and 16 hrs post columbamine (COL) incubation. Luciferase activity was quantified to determine the effect of COL on Wnt/β-catenin signaling. Data were representatives of three independent experiments with similar results. The error bars indicated the SD of duplicates. (B) The protein expression of factors involved in Wnt/β-catenin pathway was analyzed by Western blotting. Protein levels of β-catenin and DVL under COL treatment were quantified by Image J software. The gray value of protein band was normalized to its internal control β-actin, and was expressed as values relative to the control from three independent experiments. The error bars represented standard deviations of quantification from three independent experiments. (C and D) Flag-tagged β-catenin (S33Y) and blank vector were transfected into HCT116 cells following commercial instruction. Endogenous β-catenin and Flag-tagged mutant β-catenin (S33Y) were detected by Western blotting (C). After 24 hrs post transfection, COL was added into cells and cell viability was quantified at 24 hrs, 48 hrs and 72 hrs post COL incubation (D).
Notes: **P<0.01; ns, P>0.05, compared to control group.
At 36 hrs post treatment, the transfected cells were harvested for protein detection. The increased protein level of β-catenin represented Wnt/β-catenin signaling activation. As shown in Figure 4B, treatment of COL reduced β-catenin expression in dose-dependent manner. We also detected the expression of Wnt/β-catenin key regulators including DVL in HCT116 cells after COL incubation. Similarly, COL treatment also significantly down-regulated DVL expression. These results gave strong evidences that columbamine played a critical role in repressing Wnt/β-catenin signaling pathway in colon cancer cells.
Next, we investigated whether the effect of COL on Wnt/β-catenin signaling pathway affected the proliferation of colon cancer cells. The Flag-tagged β-catenin (S33Y)17 was transfected into HCT116 cells to reconstitute stably β-catenin expression, and the ectopic protein was verified by Western blotting (Figure 4C). As shown in Figure 4D, restoration of β-catenin greatly abrogated the inhibitory effect of COL on HCT116 cells proliferation, indicating that COL inhibited colon cancer proliferation, at least partially, through disrupting Wnt/β-catenin signaling pathway.
Discussion
Colon cancer is one of the most common malignancies of digestive tract worldwide. At present, surgical resection is still the main method in clinical treatment. However, only about 70% of patients are suitable for surgical treatment, and the survival rate after surgery is 75%.18 A large number of patients urgently require adjuvant chemotherapy. However, the drug treatment regimen has defects in tolerance, efficacy and cross-resistance.10 Besides, economic burden and side effects also limit the use of chemotherapy. Therefore, it is urgent to seek a mild and effective chemotherapy for clinical treatment. Columbamine (COL), similar with berberine, is a tetrahydroisoquinoline alkaloid derived from the rhizome of Chinese herbal medicine Rhizoma Coptidis. Previous experiments showed that COL has been identified as an effective anti-osteosarcoma compound with minor side effects.16 But the biological effect of COL in colon cancer is still unclear. In this study, we provided several lines of evidence to show that COL treatment restricted the malignant development of colon cancer by abolishing the activation of Wnt/β-catenin signaling pathway and promoting apoptosis.
First, COL inhibited colon cancer cells proliferation in dose-dependent manner (Figure 1). Second, COL significantly abolished colon cancer cells migration and invasion ability, and reduced related genes expression in dose-dependent manner (Figure 2). Third, COL was able to trigger colon cancer cells apoptosis to impede the process to malignant (Figure 3). Finally, through luciferase reporter assay, we found that the anti-tumor effect of COL may be exerted through blocking Wnt/β-catenin signaling pathway (Figure 4). Taken together, these results demonstrated that COL may abolish colon cancer development through negatively regulating Wnt/β-catenin signaling pathway.
PCNA expression increases significantly in the late G1 phase. After reaching its peak in the S phase, PCNA expression decreases in the G2 to M phase.19,20 Therefore, PCNA is considered as a biomolecular index reflecting the biological activity and malignancy of tumors. We examined the expression of PCNA in columbamine-treated colon cancer cell lines by Western blot assay. As shown in Figure 1C, columbamine inhibited the expression of PCNA in colon cells in a dose-dependent manner, which confirmed columbamine was able to inhibit the proliferation of colon cancer cells. Further, animal experiments were performed to investigate the anti-tumor effect of COL in vivo. The tumor size in nude mice injected with colon cancer cells revealed treatment with COL of 20 μM successfully retarded tumor growth in vivo (Figure 1E).
Invasion and metastasis are the main biological characteristics of malignant tumors. In this study, whether COL treatment affected invasion and migration of colon cells were determined by transwell assay (with Matrigel EMC) (Figure 2A–C). EMT is a process by which epithelial cells gain migratory and invasive properties. It usually occurs in the initiation of metastasis in cancer progression. Loss of E-cadherin and gain of N-cadherin are considered to be fundamental events in EMT.21,22 In the present study, we found that columbamine inhibited N-Cadherin expression, and promoted E-cadherin expression, thus inhibiting EMT in colon cancer cells. Degradation of extracellular matrix (ECM) is an important pathway for tumors to infiltrate normal tissues and begin to metastasize. Matrix metalloproteinases (MMPs) can promote tumor cells to break through the tissue barrier composed of basement membrane (BM) and ECM by destroying the degradation balance of the matrix.23–25 Meanwhile, COL reduced the expression of invasion-related proteins MMP2, MMP7 and MMP9 (Figure 2D and E). These data proved that COL inhibited the invasion and metastasis of colon cancer cells by impairing EMT process and protecting ECM from degradation.
The Wnt/β-catenin signaling pathway is a classical cascade involved in the regulation of cell growth, differentiation, apoptosis and energy metabolism. Wnt/β-catenin signaling pathway also participates in the development and progression of a variety of tumors.26 When Wnt/β-catenin signaling pathway is activated, β-catenin will be protected from the degradation mediated by the proteasome,27,28 resulting in the translocation of β-catenin into nuclei to form a complex with its co-activators and initiates Wnt target genes transcription.29 In more than 80% of colon cancers cases, accumulation of β-catenin in the nucleus (marker of Wnt/β-catenin signaling activation) can be observed. Colon cancer cell resistance is partially mediated by Wnt/β-catenin signaling,30 suggesting that this pathway may be associated with poor prognosis in colon cancer patients.31 In the present study, COL repressed Wnt/β-catenin signaling pathway activation in a dose-dependent manner (Figure 4A). Protein detection showed β-catenin protein level was significantly reduced in HCT116 cells treated with COL (Figure 4B). Expression of other key molecules in Wnt/β-catenin pathway including DVL was also negatively affected by columbamine, demonstrating that columbamine negatively regulated Wnt/β-catenin signaling pathway. To further explore the relationship between the inhibitory effect of COL on Wnt/β-catenin signaling pathway and colon cancer proliferation, we transfected β-catenin (S33Y) into HCT116 cells. The β-catenin (S33Y), one potent stimulatory form of β-catenin, in which Ser33 was replaced by Tyr33, is resistant to proteolytic degradation.17 In the present study, ectopical expression of β-catenin (S33Y) markedly restored colon cancer cells proliferation (Figure 4C and D). These results gave evidence that COL is able to inhibit colon cancer malignization by disrupting Wnt/β-catenin signaling pathway.
Nevertheless, there are still many questions required to be continuously studied. More clinical evidence or in vivo data are required to confirm the clinical therapeutic effect of COL on colon cancer development. We will probe the molecule involved in Wnt/β-catenin signaling pathway, which is targeted by COL in colon cancer cells, and further investigate the possible mechanism of the effect of COL on other critical signaling pathways. The beneficial action of COL on other tumors will be also explored to expand the clinical therapy.
In conclusion, we found that columbamine significantly inhibited the proliferation, migration, invasion and promoted apoptosis of colon cancer cells, thereby inhibiting the malignant development of colon cancer. Besides, COL exerted its anti-tumor effect on colon cancer cells possibly through abolishing the Wnt/β-catenin signaling pathway.
Conclusion
Present findings suggested columbamine exerts its anti-tumor effect in colon cancer cells through abolishing Wnt/β-catenin signaling pathway. Columbamine may be a new therapy method for colon cancer.
Acknowledgment
This study was supported by Hubei Province health and family planning scientific research project (NO.WJ2015MA018). The Flag-β-catenin (S33Y) was designed and reconstructed by Bin Shen from Department of Hepatobiliary Surgery, Jiaxing Hospital of Traditional Chinese Medicine.
Abbreviations
COL, Columbamine; TGF-β, Transforming growth factor beta; PI3K/Akt, Phosphoinositide 3-kinase/Akt; PTEN, Phosphatase and tensin homolog; FACS, Fluorescence-activated cell sorting; ELISA, Enzyme-Linked Immunosorbent Assay; MMP, Matrix Metallopeptidase; PARP, Poly ADP ribose polymerase; BAD, Bcl-2-associated death promoter; Bcl-2, B-cell lymphoma 2.
Ethics statement
All procedures performed in this study were approved by the Medical Ethics Committee of the Fifth Hospital in Wuhan and were performed according to institutional protocols.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Binefa G, Rodríguez-Moranta F, Teule À, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20(22):6786–6808. doi: 10.3748/wjg.v20.i22.6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):104–117. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anti Cancer Res. 2004;24(5A):2783. [PubMed] [Google Scholar]
- 5.Reynolds A, Wharton N, Parris A, et al. Canonical Wnt signals combined with suppressed TGFβ/BMP pathways promote renewal of the native human colonic epithelium. Gut. 2014;63(4):610–621. doi: 10.1136/gutjnl-2012-304067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361(25):2449–2460. doi: 10.1056/NEJMra0804588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanwal R, Gupta S. Epigenetic modifications in cancer. Clin Genet. 2012;81(4):303–311. doi: 10.1111/j.1399-0004.2011.01809.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnekenburger M, Diederich M. Epigenetics offer new horizons for colorectal cancer prevention. Curr Colorectal Cancer Rep. 2012;8(1):66–81. doi: 10.1007/s11888-011-0116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta T, Vercruysse K, Johnson T, Ejiofor AO, Myles E, Quick QA. Violacein induces p44/42 mitogen-activated protein kinase-mediated solid tumor cell death and inhibits tumor cell migration. Mol Med Rep. 2015;12(1):1443–1448. doi: 10.3892/mmr.2015.3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 11.Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21(3):207–213. doi: 10.1111/jtm.12115 [DOI] [PubMed] [Google Scholar]
- 12.Wright CW, Marshall SJ, Russell PF, et al. In vitro antiplasmodial, antiamoebic, and cytotoxic activities of some monomeric isoquinoline alkaloids. J Nat Prod. 2000;63(12):1638–1640. [DOI] [PubMed] [Google Scholar]
- 13.Chen HY, Ye XL, Cui XL, et al. Cytotoxicity and antihyperglycemic effect of minor constituents from Rhizoma Coptis in HepG2 cells. Fitoterapia. 2012;83(1):67–73. doi: 10.1016/j.fitote.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 14.Xiaohua L, Zhenlin H, Qirong S, et al. Anti-inflammatory and anti-nociceptive activities of compounds from Tinospora sagittata (Oliv.) Gagnep. Arch Pharm Res. 2010;33(7):981. doi: 10.1007/s12272-010-0702-7 [DOI] [PubMed] [Google Scholar]
- 15.Misík V, Bezáková L, Máleková L, Kostálová D. Lipoxygenase inhibition and antioxidant properties of protoberberine and aporphine alkaloids isolated from Mahonia aquifolium. Planta Med. 1995;61(04):372–373. doi: 10.1055/s-2006-958107 [DOI] [PubMed] [Google Scholar]
- 16.Bao M, Cao Z, Yu D, et al. Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity. Toxicol Lett. 2012;215(3):174–180. doi: 10.1016/j.toxlet.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 17.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787 [DOI] [PubMed] [Google Scholar]
- 18.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 19.Qiu X, Mei J, Yin J, Wang H, Wang J, Xie M. Correlation analysis between expression of PCNA, Ki-67 and COX-2 and X-ray features in mammography in breast cancer. Oncol Lett. 2017;14(3):2912–2918. doi: 10.3892/ol.2017.6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo JL, Gu SQ, Li Y, Zhang XY. Evaluation of clinical significance of endoglin expression during breast cancer and its correlation with ER and PCNA. Eur Rev Med Pharmacol Sci. 2017;21(23):5402. [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vergara D, Simeone P, Franck J, et al. Translating epithelial mesenchymal transition markers into the clinic: novel insights from proteomics. EuPA Open Proteom. 2016;10(C):S2212968516300034. doi: 10.1016/j.euprot.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastroianni CM, Liuzzi GM, D’Ettorre G, et al. Matrix Metalloproteinase-9 and tissue inhibitors of matrix Metalloproteinase-1 in plasma of patients co-infected with HCV and HIV. HIV Clin Trials. 2002;3(4):310–315. doi: 10.1310/U9LJ-MFF9-ARE1-257H [DOI] [PubMed] [Google Scholar]
- 24.Duffy MJ, Maguire TM, Hill A, Mcdermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2(4):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enzo MV, Rastrelli M, Rossi CR, et al. The Wnt/β-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target. Mol Cell Ther. 2015;3(1):1. doi: 10.1186/s40591-015-0038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart M, Concordet JP, Lassot I, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol. 1999;9(4):207–210. doi: 10.1016/s0960-9822(99)80091-8 [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-TrCP couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A. 1999;96(11):6273–6278. doi: 10.1073/pnas.96.11.6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884 [DOI] [PubMed] [Google Scholar]
- 30.Teng W, Zhen C, Yifei Z, et al. Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. J Biochem. 2014;290(1):448–456. doi: 10.1074/jbc.M114.590364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanitsuwan W, Kanngurn S, Boonpipattanapong T, Sangthong R, Sangkhathat S. Overall expression of beta-catenin outperforms its nuclear accumulation in predicting outcomes of colorectal cancers. World J Gastroenterol. 2008;14(39):6052–6059. doi: 10.3748/wjg.14.6052 [DOI] [PMC free article] [PubMed] [Google Scholar]