Abstract
Communication between cancer cells enables cancer progression and metastasis. While cell–cell communication in cancer has primarily been examined through chemical mechanisms, recent evidence suggests that mechanical communication through cell–cell junctions and cell–ECM linkages is also an important mediator of cancer progression. Cancer and stromal cells remodel the ECM through a variety of mechanisms, including matrix degradation, cross-linking, deposition, and physical remodeling. Cancer cells sense these mechanical environmental changes through cell–matrix adhesion complexes and subsequently alter their tension between both neighboring cells and the surrounding matrix, thereby altering the force landscape within the microenvironment. This communication not only allows cancer cells to communicate with each other, but allows stromal cells to communicate with cancer cells through matrix remodeling. Here, we review the mechanisms of intercellular force transmission, the subsequent matrix remodeling, and the implications of this mechanical communication on cancer progression.
Keywords: Mechanotransduction, Extracellular matrix, Mechanosensing, Cell mechanics, Intercellular force
Introduction
Cell–cell communication has primarily been investigated through chemical mechanisms, as cancer cells secrete soluble signals into the environment to communicate with recipient cells.1,40,69,75,86,160 More recently, mechanical interactions between cells have also been described as a mode of cell–cell communication.61,120,135 Mechanotransduction, or mechanically-induced cell signaling, can be triggered by externally applied forces, flows, and pressure; however, cells are also able to exert forces that change the physical landscape in the microenvironment to affect other cells. While the mechanisms by which cells exert force are increasingly well understood, the resulting effects on the cell itself and neighboring cells are less well understood. Here, we focus on cell–cell mechanical communication, specifically how forces and changes in mechanical properties of cells and the surrounding extracellular matrix (ECM) created by the cells themselves can induce changes in the behaviors of neighboring cells to promote cancer progression.
Cell–cell mechanical communication involves the transmission of forces between cells through both cell–ECM and cell–cell linkages as cells both transmit and receive mechanical signals from the ECM and adjacent cells through these linkages.17,25,36,42,61,62,81,89,99,120,135,144,150 Mechanical changes to the tumor microenvironment are mediated through a variety of factors, including matrix degradation, cross-linking, deposition, and physical remodeling.30 Numerous cell types within the tumor microenvironment, including cancer cells and cancer-associated fibroblasts, contribute to these mechanical changes via the secretion of remodeling factors and physical contact.19,45,80,96,158 Cells transduce these mechanical changes into enhanced cellular contractility and matrix remodeling efforts, thus generating a feedback loop for further mechanical changes to the tumor microenvironment.51,135 Importantly, these reciprocal cell–ECM interactions facilitate mechanical communication within the tumor stroma where cancer cells transmit intercellular forces to adjacent cells directly via cell–cell junctions or to neighboring cells through the ECM via traction forces to coordinate cancer-related behaviors.42,61,89,99,120,135,144 Matrix remodeling and mechanical communication ultimately promote numerous cancerous phenotypes including angiogenesis, mechanical competition, collective migration, and cancer metastasis.14,15,19,31,34,38,53,82,90,117,136,149
Mechanical Communication Through the ECM
Cancer cells mechanically communicate with neighboring cells without direct cell–cell contact by exerting forces through the ECM. This mode of mechanical communication involves both the reception and transmission of forces through the ECM. Cells bind to the matrix through cell–matrix adhesion complexes (CMACs), composed of integrin ECM receptors that bind ECM ligands, including collagen and fibronectin, and adaptor molecules that link integrins with the actin cytoskeleton (Fig. 1).11,59,100,123,150,162,163 Cells in contact with the ECM also receive mechanical signals from the surrounding ECM through these CMACs. More specifically, integrins within CMACs sense both the chemical composition of the surroundings (i.e., which ECM ligands are present) and the mechanical properties of the surrounding matrix (i.e., ECM stiffness).26,150 The composition of ligands in the ECM dictates which signaling pathways will be activated based on integrin signaling; the spatial architecture of ECM fibers determines the stability and size of the CMACs.22,57,100,128 Specifically, the chemical composition and physical properties of the ECM can regulate integrin-mediated cytoskeletal assembly and tyrosine phosphorylation to generate different types of adhesions with different downstream pathways.71 The transmission of mechanical signals from the ECM is additionally dependent upon matrix mechanical properties. Different ECM proteins, including collagen I and fibronectin, can transmit or inhibit mechanical forces depending upon matrix tension, subsequently regulating downstream signaling events.129
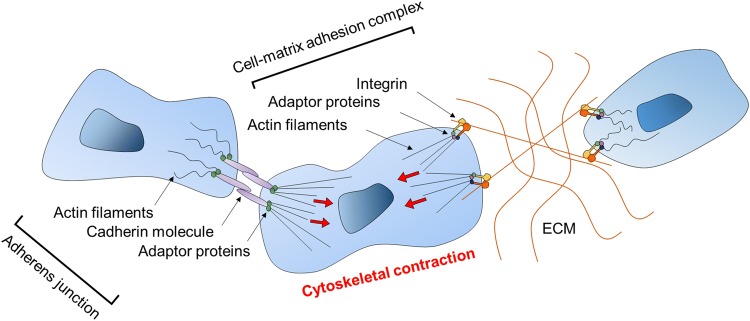
Figure 1.
Cellular transmission and reception of mechanical signals. Cancer cells transmit mechanical signals to neighboring cells through two mechanisms. Cancer cells can directly transmit forces to adjacent cells through cell–cell adhesions, specifically adherens junctions. Cancer cells can also transmit forces to nearby cells without direct contact through cell–matrix adhesion complexes (CMACs). Increased cellular contractility allows cells to exerts forces on neighboring cells through adherens junctions or on the ECM through CMACs. Cellular forces exerted onto the ECM can remodel the ECM and induce fiber alignment. Other cells in contact with the matrix sense these changes through their CMACs, resulting in phenotypic changes.
Cells within the tumor microenvironment transmit mechanical forces by directly altering the mechanical landscape of the surrounding ECM through numerous mechanisms including physical reorganization, matrix degradation, cross-linking, and deposition (Fig. 2). Matrix remodeling alters the local mechanical properties surrounding cells, resulting in direct changes to cell behavior as well as altering mechanical communication between cells within the matrix.
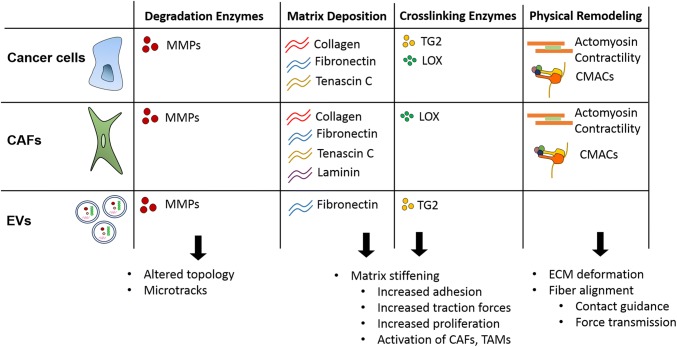
Figure 2.
Players, mechanisms, and implications of ECM remodeling in cancer. Cancer cells, cancer-associated fibroblasts (CAFs), and extracellular vesicles (EVs) are the major players involved in cancer ECM remodeling. All three players have large roles in ECM degradation through the release of matrix metalloproteinases (MMPs), leading to altered ECM topography and the generation of tracks in the ECM. Additionally, cancer cells, CAFs, and EVs have all been implicated in matrix deposition of various proteins, leading to matrix stiffening. Cancer cells, CAFs, and EVs are involved in matrix crosslinking to stiffen the matrix through tissue transglutaminase (TG2) and lysyl oxidase (LOX). Both cancer cells and CAFs are highly involved in physical remodeling of the ECM, both through actomyosin contractility and cell–matrix adhesion complexes (CMACs).
Physical Remodeling
Cells transmit forces through the ECM by reorganizing their actin cytoskeleton, controlled by activation of Rho GTPase and Rho-associated protein kinase (ROCK) signaling.25,60,107,115,122,153,157 Activation of ROCK, downstream of Rho GTPase, results in the phosphorylation of myosin light chain II.5,68,122 This pathway promotes the contraction of actin fibers which pull on the ECM through CMACs and transmit traction forces through the ECM (Fig. 1).4,28,70,110 Two classes of adhesion complexes have been reported that exhibit differential force–size relationships.139 For adhesions greater than 1 μm2 in area, the size of focal adhesions positively correlates with the force generated at the adhesion. Adhesions smaller than 1 μm2 in area generate substantial forces that inversely correlates with the adhesion size.139
These cell-generated contractile forces are used by cancer and stromal cells to remodel the ECM in two ways: deformation and fiber alignment. Physical deformation of the matrix is used by invading cancer cells to maneuver dense ECM without using ECM degrading proteases, and has been shown to be dependent on cell contractility through the ROCK pathway.158 However, cancer cells also physically deform collagen fibers with protease activity present. Thus, physical deformation and matrix degradation can be used in concert. Additionally, stromal cells physically deform the matrix to assist in cancer cell migration. It was recently shown that cancer-associated fibroblasts (CAFs) are able to deform the basement membrane to promote cancer cell invasion.47
The physical alignment of collagen fibers has also been shown to enhance cancer cell invasion. Collagen fibers aligned normal to the tumor boundary were identified as a tumor-associated collagen signature (Fig. 2).114 In these regions of aligned fibers, groups of cancer cells migrating away from the tumor boundary were observed, indicating local invasion through collective cell migration. The alignment of collagen fibers into bundles parallel to the contractile force exerted by cancer cells provides contact guidance for migrating cancer cells and enhances migration persistence in the direction of the aligned collagen.116,121 Additionally, this alignment of fibers has been shown to facilitate long range cell–cell communication. It has been reported that mammary acini can interconnect by aligning collagen fibers that coordinate and accelerate the transition of acini to an invasive state.131 More recently, mechanical signaling resulting from ECM fiber alignment was shown to promote cancer cell protrusion frequency, persistence, and lengthening along the alignment axis to promote migration efficiency, thus facilitating metastatic cell invasion through the ECM during metastasis (Fig. 2).20,54
Physical remodeling of the matrix can have additional consequences in long distance force transmission. Cell traction forces on polyacrylamide gels induce deformation in the matrix that can be sensed by nearby cells (Fig. 2).120 Additionally, cancer cell contraction stiffens the surrounding ECM, forming a stress gradient radiating away from the cell, extending far into the matrix.55 Similarly, cell-induced matrix strains on fibrin matrices can alter the local mechanical properties of fibrin gels that can be sensed by cells over longer distances.155 Computational modeling investigating long range force transmission through the ECM indicates that tension-driven fiber alignment allows forces to propagate further into fibrous matrices and allows for further mechanical communication between cells.148 Physical remodeling provides contact guidance for invading cancer cells, longer distance force transmission, and a method to deform and reorganize the ECM, resulting in a protease-independent mechanism of traversing the ECM.
Matrix Stiffening
Cancer and stromal cells transmit mechanical signals to the matrix in the forms of matrix crosslinking and matrix deposition, resulting in increased ECM stiffness in cancerous tissue compared to healthy tissue.117 Enzymatic crosslinking can alter the structural integrity of the ECM without greatly altering the overall organization and composition of the proteins in the matrix. The two main enzymes responsible for ECM crosslinking in the tumor microenvironment are lysyl oxidase (LOX) and tissue transglutaminase 2 (TG2) (Fig. 2). LOX is an extracellular copper-dependent enzyme, secreted from a variety of cells including fibroblasts and endothelial cells, that can crosslink collagen and elastin molecules via an oxidation reaction.151 LOX is overexpressed in the tumor microenvironment of several cancer types including oral and oropharyngeal squamous cell carcinoma (OSCC), gastric cancer, and breast cancer.3,74,85 Furthermore, high LOX expression has been correlated with poor prognosis in OSCCs and estrogen receptor negative (ER−) breast cancer patients and has become an attractive target for cancer therapies.3,37 Additionally, an orthotopic breast cancer mouse model revealed that the downregulation of LOX expression with shRNAs significantly decreases metastases in tumor-bearing mice.37 Similarly, TG2 is multifaceted enzyme expressed in cancer cells that participates in protein crosslinking, ATP/GTP hydrolysis, signal transduction, and even displays protein disulfide isomerase activity.27 TG2 adds proteolytic resistant e(g-glutamyl)lysine cross-linking bonds to a number of proteins.27
In conjunction with enzymatic crosslinking, the mechanical properties of ECM can change due to alterations in ECM deposition by cells within the tumor microenvironment. Both cancer and stromal cells upregulate matrix protein expression to secrete increased matrix components into the surrounding environment resulting in desmoplasia.39,95,96,117 CAFs deposit significant amounts of fibronectin, collagen, tenascin C, and laminin, to contribute to the dense tumor stromal matrix (Fig. 2).24,66,95,124 While matrix protein secretion is dependent upon cancer cell type, it has been shown that malignant cells deposit significant amounts of collagen, fibronectin, and tenascin C (Fig. 2).96 Through the deposition of various ECM components, CAFs and cancer cells construct a fibrotic stroma, leading to altered tissue mechanical properties and altered mechanically-induced signaling in cells.
Matrix stiffness alters the way cancer and stromal cells interact with and communicate through the ECM. Lo et al. (2000) reported the first evidence of durotaxis, or the cellular preference for stiffer substrates.84 From this, it was determined that the direction of cell migration can be manipulated by changing the mechanical properties of the substrate. With increased mechanical tension, integrins and downstream mechanosensing equipment become activated and further strengthen focal adhesion and actin stress fiber formation.126,137 While changes in ECM stiffness can make the matrix more resistant to cell-mediated physical reorganization, increased matrix stiffening can also alter cellular contractility.76 As cellular contractility is the main driving force of physical reorganization of matrix fibers, changes in matrix stiffness can also result in changes in the ability of cells to reorganize matrix. Ultimately, this increased matrix stiffness has been associated with increased F-actin bundling, the formation of stress fibers, mature focal adhesions, increased cancer cell adhesion, traction forces, and proliferation.50,76,119,127,142,161 Importantly, this increased stiffness can differentiate both fibroblasts and macrophages into their cancer-supporting counterparts, CAFs and tumor-associated macrophages (TAMs), respectively.2,43 In summary, matrix stiffening resulting from increased matrix crosslinking and matrix deposition mechanically signals to both cancer cells and stromal cells to promote cancer progression.
Matrix Degradation
Matrix degradation in the tumor microenvironment primarily occurs through proteolytic enzymes. Importantly, remodeling via proteolytic degradation results in alterations to the physical properties of the ECM, including changes in topography, which directly influence cell behavior. Various matrix-degrading proteases are upregulated in cancer and stromal cells and degrade a variety of matrix proteins found in the basement membrane and ECM to facilitate cancer cell invasion (Fig. 2).23,63,72,102 Here, we focus on the most prominent protease family involved in mechanical communication in cancer progression: the metalloproteinases.
Matrix metalloproteinases (MMPs) are typically secreted into the ECM and digest numerous ECM proteins to allow cells to breach the basement membrane and traverse the ECM.45,63,72,102 Both cancer cells and CAFs are major sources of secreted MMPs in the tumor microenvironment. MMP-2, as one example, is expressed in several cancer cell lines and primarily degrades collagen to promote cancer cell migration (Fig. 2).159 Alternatively, MMP-9 has little to no expression in cancer cells, but is secreted from CAFs and endothelial cells and is involved in both matrix degradation and vascular remodeling (Fig. 2).97,167 MMPs can be released directly by cells or they can be contained within extracellular vesicles (EVs).35,79 Numerous cancer types have been shown to release EVs containing MMPs. As one example, melanoma cells release EVs containing enzymatically active MT1-MMP capable of matrix degradation.52 Similarly, EVs released from prostate cancer cells have been shown to contain enzymatically active MMP2 and MMP9.7,33 Notably, the presence of matrix degradation enzymes in EVs likely results in matrix remodeling far from the primary cell since EVs can travel far distances before rupturing.8,29,109
A subset of MMPs, termed membrane-type metalloproteinases (MT-MMPs), are anchored to the cell membranes. MT-MMPs have been identified on invadopodia structures of migrating cancer cells.93,164 These protease rich invadopodia degrade the matrix as the cell invades to form tube-like microtracks (Fig. 3).9,156 Utilizing microfabricated 3D collagen microtracks to emulate paths left by invasive cancer cells, Kraning-Rush et al. (2013) showed that cancer cells can migrate independently of MMP activity when using the microtracks compared to through 3D collagen matrices.77 Further investigation revealed that cancer cells in these tracks did not require cell–matrix mechanocoupling but were more dependent on internal cytoskeletal dynamics to drive migration through the microtracks.21 Thus, cells in contact with these microtracks may use them as easy passage through the ECM to the bloodstream to eventually colonize a secondary site. Stromal fibroblasts have also been implicated in leading collective cancer cell invasion using protease-dependent pathways (Fig. 3). As fibroblasts remodel the matrix through Rho-mediated myosin light chain activity and MMP-dependent matrix degradation, cancer cells can retain an epithelial phenotype and invade away from the primary tumor.41,47 In summary, matrix degradation is routinely used to remodel the ECM during cancer progression, and degradation-based remodeling modifies physical properties of the ECM, including altered topology such as microtracks, which is sensed by cancer and stromal cells within the tumor microenvironment to promote cancer progression and metastasis.
Figure 3.
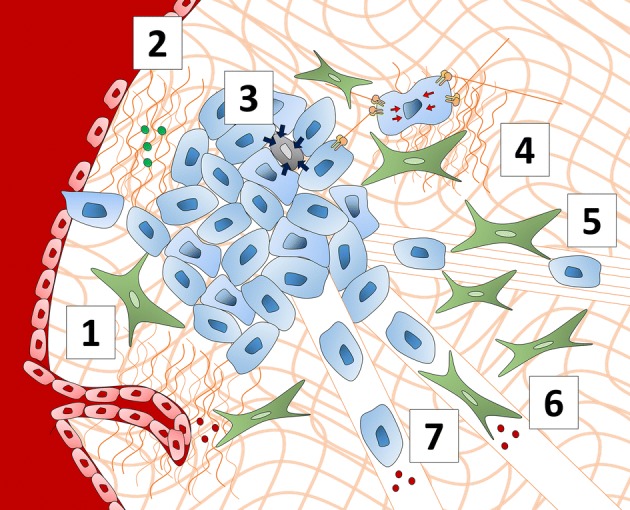
Consequences of cell–cell mechanical communication in cancer. Cell–cell mechanical communication in cancer results in a variety of cancer-promoting behaviors. (1) Increased ECM crosslinking via LOX and ECM remodeling via proteases enhance sprouting angiogenesis and enable endothelial cells to migrate through the ECM to form capillaries. (2) Increased ECM rigidity decreases the structural integrity and barrier function of blood vessels. (3) Cancer cells exhibit mechanical competition as they must outcompete less-mechanically fit neighboring cells via compressive forces that induce apoptosis. (4) Cancer cells sense increased matrix stiffness through CMACs and transmit these mechanical signals to nearby cells by exerting traction forces on the matrix. (5) CAFs in the tumor stroma align matrix fibers which cancer cells use as tracks to invade away from the primary tumor. (6) Fibroblasts act as leader cells, using matrix-degrading proteases to form microtracks in the ECM, which cancer cells use to invade away from the primary tumor in a form of collective migration. (7) Cancer cells secrete matrix-degrading proteases to form microtracks in the ECM to invade away from the primary tumor.
Mechanical Communication at Cell–Cell Contacts
Cytoskeletal dynamics drive cell protrusion, adhesion, and contraction, allowing cancer cells to migrate.104 However, intercellular cytoskeletal forces generated by cancer cells are also transmitted to adjacent cells as a form of mechanical communication. Epithelial cells directly transmit intercellular forces to neighboring cells through adherens junctions (AJs) (Fig. 1). AJs mechanically link the cytoskeletons of adjacent cells and are the primary mechanism of cell contact-mediated intercellular force transmission.145 The extracellular domain of cadherins on opposing cells interact to form a stable adhesion between cells.133 Intercellular domains of cadherins are linked to the actomyosin cytoskeleton through a complex supramolecular interface of adaptor proteins, including α-catenin, β-catenin, and vinculin, which add mechanical integrity to the junction and act as mechanotransducers.13,145 The vinculin interface and a-catenin binding are important to mechanotransduction mechanisms of E-cadherin based adhesions and these proteins change conformation under applied force to induce signaling pathways and cytoskeletal remodeling.13 The alignment of the actomyosin bundles relative to the junction allows for normal and shear stresses to be applied across the junctions between cells.48 Additionally, cells can coordinate tissue-level contractile forces through these mechanical linkages.87,88
The contractile forces generated by actomyosin bundles are transmitted across the mechanical linkages and sensed by cadherins and adapter proteins on adjacent cells. Cadherins sense tensile forces and rigidity of contacts.48 Different types of cadherins, including E-, N-, and P-cadherin, are expressed on distinct cell types and play a range of roles in intercellular force transmission in cancer. In an epithelial state, cancer cells predominantly express E-cadherin with low expressions of N- and P-cadherin.154 Single molecule analysis of cadherin bonds has revealed differential mechanics between E- to E-cadherin bonds and N- to N-cadherin bonds.103 The E- to E-cadherin bonds are able to withstand larger forces before breaking when compared to the N- to N-cadherin bonds.103 Upon epithelial-to-mesenchymal transition, cancer cells reduce E-cadherin expression and increase N- and P-cadherin expression, supporting the hypothesis that cell–cell adhesions decrease after EMT.154 However, while investigating the adhesion strength between epithelial cell pairs before (MCF-10A) and after EMT (MDA-MB-231 and MDA-MB-436), Pawlizak et al. (2015) found that MCF-10A cells displayed the highest cadherin density and highest E-cadherin expression, but MDA-MB-231 cells had the highest cell–cell adhesion strength as measured by an AFM-based method.108 This result may be explained by differential spatiotemporal dynamics of adhesion and intracellular signaling responses to applied force. Through investigation of epithelial monolayer dynamics, Bazellières et al. have shown that P-cadherin expression can predict the magnitude of intercellular tension across the monolayer, while E-cadherin expression can predict the build-up rate of the intercellular tension.12 Furthermore, by pulling on the apical layer of the epithelial monolayers with cadherin coated beads, Bazellières et al. found that E-cadherin mediated adhesions become structurally reinforced in response to external force whereas P-cadherin mediated adhesions do not.12 Heterotypic adhesions between the cadherins are also possible and the strength of these adhesions are similar to the homophilic adhesions.113 Furthermore, CAFs and cancer cells are able to form E-cadherin/N-cadherin adhesions which transmit intercellular forces and aid in cancer cell invasion.78 Thus, it is possible that both the composition of intercellular contacts and the ratio of the different cadherins expressed are important regulators of cell–cell adhesion strength. Nonetheless, these studies highlight cadherins as mediators of mechanical communication at cell–cell contacts through the transmission of intercellular forces.
Other varieties of cell–cell junctions exist, including tight junctions, desmosomes, and gap junctions. Tight junctions are the most apical junctions found in epithelial cells and composed of transmembrane proteins claudins that are linked to the cytoskeleton via several adaptor proteins including ZO proteins and cingulin.134 Tight junctions are predominantly associated with modulating barrier function and maintaining cell polarity; however, recent evidence suggests they play a role in mechanical communication. The deletion of ZO-1 and GEF-H1, important tight junction associated proteins, leads to higher global tension across adherens junctions which leads to cytokinesis defects.56 This result implies that coordinated intercellular forces are required for proper cell division in epithelial tissues and highlights the importance of tight junctions in modulating these intercellular forces and possibly preventing tumor initiation via cell division defects. The opposite effect was found in endothelial cells as the deletion of ZO-1 decreased the tension across VE-cadherin adhesions in endothelial cells.140 This difference may indicate a cadherin or cell type dependence.
Desmosomes are slow forming adhesions that mechanically couple adjacent cells and are anchored to intermediate filament cytoskeletal networks.106 In desmosomes, intercellular linkages are formed by members of the cadherin family and predominantly linked to intermediate filaments by armadillo proteins and desmoplakin.65 Recent evidence implicates desmosomes in a role outside of mechanical integrity of the epithelia.16 By expressing various forms of desmoplakin, Broussard et al. (2017) found that decoupling the desmosomes and intermediate filaments resulted in lower traction forces and cell–cell tugging forces, while enhancing desmosome to intermediate filament linkages increased traction forces and cell–cell tugging forces. This effect is highly dependent on actomyosin contractility but still implicates the importance of desmosomes in regulating intercellular forces. Furthermore, intermediate filaments themselves play a regulatory role in organizing cell–cell junctions, as intermediate filaments control actin dynamics at adherens junctions, indicating a role in modulating direct cell–cell mechanical communication.105 While some desmosomal proteins have implications in cancer progression, the evidence underlying these claims focus on alterations in biochemical signaling due to increased/decreased desmosomal protein expression.166 Since intercellular forces drive tissue formation and help coordinate collective migration,46,73 it is likely that the desmosomes have important roles in mechanical communication during cancer progression; however, direct evidence remains to be uncovered.
Gap junctions connect the cytoplasm of adjacent cells together via the pore forming proteins connexins.125 While gap junctions are not directly linked to cytoskeletal elements, they may still play a role mechanical communication. Gap junctions are canonically known to facilitate intercellular signaling through chemical messengers. Thus, while gap junctions themselves do not appear to directly transmit mechanical stimuli, they are able to indirectly facilitate mechanical communication by facilitating downstream signaling of mechanical stimuli to adjacent cells. For example, gap junctions between human astrocytes and glioma cells can transmit intracellular calcium upon mechanical stimulation of a single cell.165 Similarly, when a single Hela cell expressing Connexin-43 is mechanically stimulated with a glass pipette, the intracellular calcium levels are increased in the stimulated and surrounding cells.67 These data provide evidence of the mechanosensitivity of connexins.
Consequences of Intercellular Force Transmission and Matrix Remodeling on Tumor Progression
Sensing of the mechanical changes induced by cancer and stromal cells on the matrix and at cell–cell junctions by neighboring cells results in a variety of pro-tumor consequences, including the promotion of mechanical competition, angiogenesis, and cancer cell migration.
Mechanical Competition
While cellular competition is well-described, until recently, it has been mostly focused on competition for nutrients.32 The concept of mechanical competition has recently emerged, in which winner cells eliminate less-mechanically fit neighboring cells via compressive forces that induce apoptosis.15,53,90 It has been best-described relative to cell proliferation. Uncontrolled proliferation is a hallmark of cancer and as cells proliferate, cell density increases and available tissue space may begin to diminish as cells are confined by tissue boundaries. In the classical model of cell competition, winner cells must replace loser cells as they compete for limited space and resources.90 This is a highly conserved process with important roles in tissue development and homeostasis.6,90 Cancer cells are viewed as super-competitors as they are able to overwhelm surrounding wild-type cells and expand to form tumors.32 In mechanical competition, cell survival and apoptosis is dictated by compressive forces. Epithelial cell studies have revealed Piezo1 and p53 as important mediators of loser cell elimination via density driven compressive forces; however, much remains unclear about the molecular mechanisms underlying mechanical cell competition.49,147 Because cancer cells must outcompete the surrounding cells, it is likely that they are more mechanically fit to form solid tumors (Fig. 3). This may reveal novel therapeutic strategies either to mechanically weaken cancer cells or strengthen surrounding stromal cells to prevent cancer progression.
Angiogenesis
Growing tumors must stimulate angiogenesis to recruit blood vessels that deliver nutrients and oxygen to support the continued growth of proliferating cancer cells. Potent pro-angiogenic factors such as vascular endothelial growth factor (VEGF) are released from cancer cells to attract endothelial cells from nearby vessels to stimulate endothelial cell proliferation and migration into the tumor microenvironment where they encounter an altered ECM. While the chemical composition of the ECM has been the primary target of tumor angiogenesis research, the mechanical properties of the altered ECM also play a role. The tumor microenvironment can be significantly stiffer than normal tissue due to crosslinking via LOX, among other stiffening mechanisms discussed in this review.10 Endothelial cells are sensitive to ECM rigidity, where ECM crosslinking in the tumor microenvironment enhances sprouting angiogenesis while diminishing the structural integrity of newly formed vessels (Fig. 3).14 Contrary to stiffening via crosslinking, enhanced matrix density via excessive matrix deposition can inhibit angiogenesis as it acts as a physical barrier to endothelial cell migration.34 During the initial steps of angiogenesis, a single cell branches out from a pre-existing vessel to migrate into the ECM and this tip cell begins forming a new vessel branch.112 Canonically, lateral Delta-like ligand 4 (Dll4) signaling through Notch 1 has been the primary mechanism of controlling tip cell designation during angiogenesis.58 However, recent evidence supports intercellular tension as a regulator of tip cell formation.152 Using pharmacological inhibitors of cellular contractility (Y27632) and Notch receptor cleavage (DAPT), Wang et al. (2017a) showed that reducing intercellular tension enhanced the formation of tip cells in a similar manner to reduced Notch1-Dll4 signaling in endothelial cells. Furthermore, reducing intercellular tension and Notch signaling together did not result in an additive effect on tip cell formation, suggesting that cellular contractility mediates endothelial tip cell formation by regulating Notch signaling. Thus, intercellular contractility may be required to mechanically pull on Notch to expose its cleavage site and initiate signaling and thus reducing cellular contractility reduces the ability of cells to initiate Notch signaling. However, an alternative explanation may be that downstream effectors of cadherin-dependent force transmission inhibit downstream Notch-signaling.
Endothelial cell contractility also plays a role in mechanical communication through the ECM during angiogenesis. Mechanical models have been proposed that show that endothelial cells exert forces on the ECM which creates tension, alters ECM fiber alignment, and clusters the ECM to trigger nearby endothelial cells to reorient in the direction of alignment and migrate towards higher concentration of ECM to form vascular networks.98,101 More recently, a hybrid cellular Potts and finite element model mimicking endothelial cell–ECM mechanical communication and network formation suggested that interactions between endothelial cells, both direct and through the ECM, lead to vascular-like network formation and sprouting of endothelial spheroids in vitro.144
ECM remodeling via proteases enables endothelial cells to migrate through the ECM and form capillaries (Fig. 3).44 Endothelial cells grown in 3D fibrin matrices are unable to form capillaries without the aid of proteases secreted by co-cultured lung fibroblasts or mesenchymal stem cells.44 Interestingly, CAFs are also able to enhance vascularization in a 3D in vitro blood vessel formation assay via mechanical deformations.130 When CAFs were transduced with shRNAs to knockdown proteins important for contractility and mechanotransduction (Rho, ROCK, SN1, & YAP), their ability to deform the matrix and enhance vascular growth was decreased. To isolate the effect of mechanical deformations, thrombin-coated magnetic beads were added to the fibrin matrices and manipulated with a magnet to deform the matrix. Even in the absence of fibroblasts, the magnetically induced deformations were sufficient to increase vessel growth. These studies reveal the influence of mechanical communication driving angiogenesis and the ability of altered ECM rigidity, intercellular tension, proteolysis, and cellular contractility to affect vessel formation and integrity.
Cancer Cell Migration
The degradation, stiffening, and physical remodeling of the ECM, initiated by both stromal and cancer cells, contributes to cancer cell migration. Cancer cells exhibit two modes of migration during invasion: single cell migration or collective migration. The increased matrix stiffness associated with increased contractility, matrix deposition and crosslinking has been shown to promote single cell migration. Although stiff matrices often have smaller pores, cancer cells can remodel the matrix by exerting elevated traction forces.76 Previously, Fritz et al. (1999) discovered elevated Rho/ROCK activity in stiff tumors induces tumor dissemination. This increased tumor dissemination in stiff tumors was later found to be due to increased RhoA activation, focal adhesion assembly, and contractility of the actin cytoskeleton.18,64 Additionally, increased ECM stiffness alters cell–matrix adhesions to promotes tumor cell metastatic potential and invasiveness through increased integrin clustering and subsequently enhanced integrin signaling through focal adhesion proteins such as paxillin and vinculin.82,92 With this, stiff matrices increase the number of focal adhesions and traction force generated compared to compliant matrices thereby altering cell–ECM mechanical communication (Fig. 3).92,111 As such, tissue stiffness can drive single cell migration by increasing Rho/ROCK signaling, focal adhesion assembly, and cellular contractility.
During collective migration, an aggregate of cells coupled through cell–cell contacts migrate as a unit with leader cells at the front of the pack and follower cells behind them. While the single cell migratory response to mechanical cues has received attention, there is still much to learn about the chemical and mechanical mechanisms driving collective motions. This is an inherently more complicated process as cellular forces are transmitted to the matrix and to numerous adjacent cells and there exist a limited number of techniques to measure and perturb those forces. Studies investigating monolayer dynamics have revealed the importance of intercellular force transmission through cell–cell contacts in coordinating collective migration. Coordination of traction forces via intercellular forces is evident in cell monolayers. The highest traction forces can be found towards the leading edge, where leader cells are mechanically coupled via actin cables where they exert strong traction forces that propagate into the monolayer and help orient migration direction of follower cells.83,118 The dynamics of intercellular stresses distributed throughout a cellular monolayer also help coordinate the migration of cells in a phenomena termed plithotaxis.141 Plithotaxis describes the guidance mechanism specific to collective migration where cells migrate in the direction that minimizes the local shear stresses.138 Because cells are mechanically linked during collective migration, they are able to exert forces directly onto one another and redistribute forces throughout the monolayer. Interestingly, mechanical interactions of follower cells, including a mechanical pull on the future leader, have been implicated in the selection of leader cells as the mechanical pull induced by follower cells aids in leader cell polarization and protrusion.146 Another emerging mechanism of collective cell guidance is collective durotaxis which describes the ability of groups of cells to follow gradients in substrate rigidity.136 Interestingly, cells that do not undergo durotaxis as individuals still may utilize collective durotaxis.136 The ability of cells to follow rigidity gradients as a group is dependent on local stiffness sensing at the periphery and long-range force transmission through cell–cell mechanical linkages.136 Atomic force microscopy (AFM) measured local mechanical changes generated by cells in collagen matrices and observed strain stiffening at the leading edge of cancer cells in collective migration.143 This finding highlights the reciprocal nature of invasion, as cells sense the “traveling wave” of stiffened substrate as they invade.143 These studies reveal the contribution of matrix mechanics and mechanical signals to both single cell and collective migration in cancer progression.
Conclusion
Traditional cell–cell communications rely upon chemical signals that trigger receptors or directly enter the cell. Mechanical cell–cell communication lies outside of these traditional methods. Instead, the signals that constitute mechanical communication are mechanical signals that cells exert and detect through adhesion complexes linked to the cytoskeleton and altered physical properties of the ECM that result from physical forces or enzymatic activities. Cancer and cancer-associated cells have been shown to utilize a range of mechanical communication methods during cancer progression. Cancer cells, CAFs, and EVs carry a repertoire of enzymes and matrix components that remodel the native ECM and produce an altered mechanical environment. Additionally, cancer and cancer-associated cells all possess the ability to directly exert contractility-driven forces onto each other, and cell–cell adhesion complexes can directly transduce these forces through complex mechanotransduction systems. The transmission of these changes in the mechanical environment and physical forces give rise to cellular behaviors that promote cancer progression. Specifically, cell–cell mechanical communication in cancer has been shown to create inter-cellular mechanical competition, induce and modulate angiogenesis, and facilitate individual and collective cell migration. The mechanisms outlined in this review underline the importance of holistic in vitro models for cancer research that accurately recapitulate the matrix components, stiffness, and stromal cells that play important roles in many of the hallmarks of cancer.
While there has been significant progress into the investigation of cell–cell mechanical communication and its contribution to cancer progression, the field is still new and holds many questions to be answered. Novel mechanosensing mechanisms are continuously being discovered and thus research efforts must be placed to understand how these mechanisms fit into current cell–cell mechanical communication schemes. Furthermore, research should aim to determine how cells integrate numerous mechanical signals as cancer cells exist in a complex environment and must interpret many signals simultaneously. While mechanical communication likely plays numerous roles during cancer progression, this review highlighted only three consequences of mechanical communication in cancer: mechanical competition, angiogenesis, and cell migration. In the future, it will be important to fully understand how mechanical communication can impact additional systems, including cancer immune response and colonization of the metastatic site. The tumor microenvironment has been shown to influence the phenotype of immune cells, and with efforts towards immunotherapies for cancer treatment growing, important information may lie in how cancer and immune cells mechanically communicate with each other.91,132 At the metastatic site, cancer cells originating from the mechanically distinct tumor environment encounter a more native ECM and must interact with healthy cells. Thus, it will be important to understand the mechanical interaction between cancer and healthy cells in the metastatic site. Lastly, a majority of mechanical communication research is focused at the single cell level. As it is important to understand biology at all levels, it will be important for ongoing research to address how mechanical communication is conveyed at the tissue scale and the consequences of tissue-level mechanical interactions.
A continued hurdle within the mechanobiology field is the limited number of tractable techniques that can be employed by researchers in vitro and/or in vivo. As additional techniques are developed to measure and perturb cell-initiated mechanical cues, our understanding of cell–cell mechanical communication will grow significantly. Development of platforms that can measure and manipulate forces in realistic, physiologically relevant environments are critical to progress in mechanobiology. Recent work to develop platforms that image mechanical perturbations more deeply into tissue, more quickly, and with less bleaching are emerging.94 As these techniques become adaptable for use in biological labs, our ability to connect mechanobiology to clinical translation will be significantly strengthened.
Acknowledgment
This work was supported by awards from the NIH (Award Number HL127499) and NSF (1738345, 1740900) to C.A.R-K.
Animal Studies
No animal studies were carried out by the authors for this article.
Conflict of Interest
Samantha Schwager, Paul Taufalele, and Cynthia Reinhart-King have no conflicts of interest to disclose.
Human Studies
No human studies were carried out by the authors for this article.
References
- 1.Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. Gap junctions and cancer: communicating for 50 years. Nat. Rev. Cancer. 2016;16:775–788. doi: 10.1038/nrc.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acerbi I, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. Quant. Biosci. Nano Macro. 2015;7:1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albinger-Hegyi A, et al. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC) Int. J. Cancer. 2010;126:2653–2662. doi: 10.1002/ijc.24948. [DOI] [PubMed] [Google Scholar]
- 4.Alexander NR, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. CB. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 6.Amoyel M, Bach EA. Cell competition: how to eliminate your neighbours. Development. 2014;141:988–1000. doi: 10.1242/dev.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelucci A, et al. Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin. Exp. Metastasis. 2000;18:163. doi: 10.1023/a:1006778000173. [DOI] [PubMed] [Google Scholar]
- 8.Antonyak MA, Cerione RA. Microvesicles as Mediators of Intercellular Communication in Cancer. In: Robles-Flores M, editor. Cancer Cell Signaling: Methods and Protocols. New York: Springer; 2014. pp. 147–173. [DOI] [PubMed] [Google Scholar]
- 9.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 10.Baker A-M, Bird D, Lang G, Cox TR, Erler JT. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 11.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 12.Bazellières E, et al. Control of cell–cell forces and collective cell dynamics by the intercellular adhesome. Nat. Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertocchi C, et al. Nanoscale architecture of cadherin-based cell adhesions. Nat. Cell Biol. 2017;19:28–37. doi: 10.1038/ncb3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordeleau F, et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brás-Pereira C, Moreno E. Mechanical cell competition. Curr. Opin. Cell Biol. 2018;51:15–21. doi: 10.1016/j.ceb.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Broussard JA, et al. The desmoplakin–intermediate filament linkage regulates cell mechanics. Mol. Biol. Cell. 2017;28:3156–3164. doi: 10.1091/mbc.E16-07-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 18.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 19.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey SP, Goldblatt ZE, Martin KE, Romero B, Williams RM, Reinhart-King CA. Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr. Biol. Quant. Biosci. Nano Macro. 2016;8:821–835. doi: 10.1039/c6ib00030d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey SP, et al. Comparative mechanisms of cancer cell migration through 3D matrix and physiological microtracks. Am. J. Physiol. Cell Physiol. 2015;308:C436–C447. doi: 10.1152/ajpcell.00225.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalcanti-Adam EA, Micoulet A, Blümmel J, Auernheimer J, Kessler H, Spatz JP. Lateral spacing of integrin ligands influences cell spreading and focal adhesion assembly. Eur. J. Cell Biol. 2006;85:219–224. doi: 10.1016/j.ejcb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Cawston TE, Young DA. Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res. 2010;339:221. doi: 10.1007/s00441-009-0887-6. [DOI] [PubMed] [Google Scholar]
- 24.Chang HY, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. U.S.A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri O, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014;13:970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 26.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin–cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 27.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 28.Connell LE, Helfman DM. Myosin light chain kinase plays a role in the regulation of epithelial cell survival. J. Cell Sci. 2006;119:2269–2281. doi: 10.1242/jcs.02926. [DOI] [PubMed] [Google Scholar]
- 29.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat. Cell Biol. 2015;17:276–287. doi: 10.1038/ncb3115. [DOI] [PubMed] [Google Scholar]
- 32.Di Gregorio A, Bowling S, Rodriguez TA. Cell competition and its role in the regulation of cell fitness from development to cancer. Dev. Cell. 2016;38:621–634. doi: 10.1016/j.devcel.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Di Vizio D, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar LT, Underwood CJ, Guilkey JE, Hoying JB, Weiss JA. Extracellular matrix density regulates the rate of neovessel growth and branching in sprouting angiogenesis. PLoS ONE. 2014;9:e85178. doi: 10.1371/journal.pone.0085178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endres M, Kneitz S, Orth MF, Perera RK, Zernecke A, Butt E. Regulation of matrix metalloproteinases (MMPs) expression and secretion in MDA-MB-231 breast cancer cells by LIM and SH3 protein 1 (LASP1) Oncotarget. 2016;7:64244–64259. doi: 10.18632/oncotarget.11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat. Cell Biol. 2014;16:587–594. doi: 10.1038/ncb2973. [DOI] [PubMed] [Google Scholar]
- 37.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 38.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Fullár A, et al. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer. 2015;15:256. doi: 10.1186/s12885-015-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fusek M, Vetvickova J, Vetvicka V. Secretion of cytokines in breast cancer cells: the molecular mechanism of procathepsin D proliferative effects. J. Interferon Cytokine Res. 2007;27:191–199. doi: 10.1089/jir.2006.0105. [DOI] [PubMed] [Google Scholar]
- 41.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 42.Ganz A, et al. Traction forces exerted through N-cadherin contacts. Biol. Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 43.Georges PC, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 44.Ghajar CM, et al. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp. Cell Res. 2010;316:813–825. doi: 10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 46.Gjorevski N, Piotrowski AS, Varner VD, Nelson CM. Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci. Rep. 2015;5:11458. doi: 10.1038/srep11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glentis A, et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017;8(1):924. doi: 10.1038/s41467-017-00985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell–cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Gudipaty SA, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017;543:118–121. doi: 10.1038/nature21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo W, Frey MT, Burnham NA, Wang Y. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J. 2014;28:3589–3599. doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 52.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell. Biochem. 2008;105:1211–1218. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- 53.Hall MS, et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl. Acad. Sci. 2016;113:14043–14048. doi: 10.1073/pnas.1613058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han W, et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl. Acad. Sci. 2016;113:11208–11213. doi: 10.1073/pnas.1610347113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han YL, et al. Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc. Natl. Acad. Sci. U.S.A. 2018;115(16):4075–4080. doi: 10.1073/pnas.1722619115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatte G, Prigent C, Tassan J-P. Tight junctions negatively regulate mechanical forces applied to adherens junctions in vertebrate epithelial tissue. J. Cell Sci. 2018 doi: 10.1242/jcs.208736. [DOI] [PubMed] [Google Scholar]
- 57.Heino J, Käpylä J. Cellular receptors of extracellular matrix molecules. Curr. Pharm. Des. 2009;15:1309–1317. doi: 10.2174/138161209787846720. [DOI] [PubMed] [Google Scholar]
- 58.Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 59.Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin—a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 60.Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humphries DL, Grogan JA, Gaffney EA. Mechanical cell–cell communication in fibrous networks: the importance of network geometry. Bull. Math. Biol. 2017;79:498–524. doi: 10.1007/s11538-016-0242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F–actin interface. J. Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 63.Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21–36. doi: 10.1042/bse0380021. [DOI] [PubMed] [Google Scholar]
- 64.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 65.Johnson JL, Najor NA, Green KJ. Desmosomes: regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harb. Perspect. Med. 2014;4(11):a015297. doi: 10.1101/cshperspect.a015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 67.Kameritsch P, Khandoga N, Pohl U, Pogoda K. Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis. 2013;4:e584. doi: 10.1038/cddis.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaneko-Kawano T, et al. Dynamic regulation of myosin light chain phosphorylation by Rho-kinase. PLoS ONE. 2012;7:e39269. doi: 10.1371/journal.pone.0039269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kano A. Tumor cell secretion of soluble factor(s) for specific immunosuppression. Sci. Rep. 2015;5:8913. doi: 10.1038/srep08913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kassianidou E, Hughes JH, Kumar S, Wang Y-L. Activation of ROCK and MLCK tunes regional stress fiber formation and mechanics via preferential myosin light chain phosphorylation. Mol. Biol. Cell. 2017;28:3832–3843. doi: 10.1091/mbc.E17-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katz B-Z, Zamir E, Bershadsky A, Kam Z, Yamada KM, Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell–matrix adhesions. Mol. Biol. Cell. 2000;11:1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J-H, Dooling LJ, Asthagiri AR. Intercellular mechanotransduction during multicellular morphodynamics. J. R. Soc. Interface. 2010;7:S341–S350. doi: 10.1098/rsif.2010.0066.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirschmann DA, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- 75.Klinke DJ. Eavesdropping on altered cell-to-cell signaling in cancer by secretome profiling. Mol. Cell. Oncol. 2015;3:e1029061. doi: 10.1080/23723556.2015.1029061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraning-Rush CM, Califano JP, Reinhart-King CA. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE. 2012;7(2):e32572. doi: 10.1371/journal.pone.0032572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraning-Rush CM, Carey SP, Lampi MC, Reinhart-King CA. Microfabricated collagen tracks facilitate single cell metastatic invasion in 3D. Integr. Biol. Quant. Biosci. Nano Macro. 2013;5:606–616. doi: 10.1039/c3ib20196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labernadie A, et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017;19:224–237. doi: 10.1038/ncb3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laghezza Masci V, Taddei AR, Gambellini G, Giorgi F, Fausto AM. Microvesicles shed from fibroblasts act as metalloproteinase carriers in a 3-D collagen matrix. J. Circ. Biomark. 2016 doi: 10.1177/1849454416663660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 81.le Duc Q, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li L, et al. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell. Mol. Life Sci. 2012;69:2779–2789. doi: 10.1007/s00018-012-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma L-J, et al. Expression of LOX and MMP-2 in gastric cancer tissue and the effects of LOX and MMP-2 on tumor invasion and metastasis. Chin. J. Oncol. 2011;33:37–41. [PubMed] [Google Scholar]
- 86.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell–cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J. Cell Biol. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell–ECM traction force modulates endogenous tension at cell–cell contacts. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maruyama T, Fujita Y. Cell competition in mammals—novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol. 2017;48:106–112. doi: 10.1016/j.ceb.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 91.McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015;72:1303–1316. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mekhdjian AH, et al. Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix. Mol. Biol. Cell. 2017;28:1467–1488. doi: 10.1091/mbc.E16-09-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monsky WL, et al. A potential marker protease of invasiveness, separase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–5710. [PubMed] [Google Scholar]
- 94.Mulligan JA, Bordeleau F, Reinhart-King CA, Adie SG. Measurement of dynamic cell-induced 3D displacement fields in vitro for traction force optical coherence microscopy. Biomed. Opt. Express. 2017;8:1152–1171. doi: 10.1364/BOE.8.001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muranen T, et al. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 2017;8:13989. doi: 10.1038/ncomms13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteomics (MCP) 2012;11:M111.014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol. Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 98.Namy P, Ohayon J, Tracqui P. Critical conditions for pattern formation and in vitro tubulogenesis driven by cellular traction fields. J. Theor. Biol. 2004;227:103–120. doi: 10.1016/j.jtbi.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Ng MR, Besser A, Brugge JS, Danuser G. Mapping the dynamics of force transduction at cell–cell junctions of epithelial clusters. eLife. 2014;3:e03282. doi: 10.7554/eLife.03282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oria R, et al. Force loading explains spatial sensing of ligands by cells. Nature. 2017;552:219–224. doi: 10.1038/nature24662. [DOI] [PubMed] [Google Scholar]
- 101.Oster GF, Murray JD, Harris AK. Mechanical aspects of mesenchymal morphogenesis. J. Embryol. Exp. Morphol. 1983;78:83–125. [PubMed] [Google Scholar]
- 102.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panorchan P, Thompson MS, Davis KJ, Tseng Y, Konstantopoulos K, Wirtz D. Single-molecule analysis of cadherin-mediated cell–cell adhesion. J. Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- 104.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pascalis CD, et al. Intermediate filaments control collective migration by restricting traction forces and sustaining cell–cell contacts. J. Cell Biol. 2018;217(9):3031–3044. doi: 10.1083/jcb.201801162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pasdar M, Nelson WJ. Kinetics of desmosome assembly in Madin–Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell–cell contact. II. Morphological analysis. J. Cell Biol. 1988;106:687–695. doi: 10.1083/jcb.106.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 108.Pawlizak S, et al. Testing the differential adhesion hypothesis across the epithelial–mesenchymal transition. New J. Phys. 2015;17:083049. [Google Scholar]
- 109.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pelham RJ, Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-Rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 113.Prakasam AK, Maruthamuthu V, Leckband DE. Similarities between heterophilic and homophilic cadherin adhesion. Proc. Natl. Acad. Sci. 2006;103:15434–15439. doi: 10.1073/pnas.0606701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling, and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Provenzano PP, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reffay M, et al. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat. Cell Biol. 2014;16:217–223. doi: 10.1038/ncb2917. [DOI] [PubMed] [Google Scholar]
- 119.Reid SE, et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017;36:2373–2389. doi: 10.15252/embj.201694912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reinhart-King CA, Dembo M, Hammer DA. Cell–cell mechanical communication through compliant substrates. Biophys. J. 2008;95:6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Riching KM, et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014;107:2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riveline D, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc. Natl Acad. Sci. U. S. A. 2009;106:16245–16250. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodemann HP, Müller GA. Characterization of human renal fibroblasts in health and disease: II. In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am. J. Kidney Dis. 1991;17:684–686. doi: 10.1016/s0272-6386(12)80352-0. [DOI] [PubMed] [Google Scholar]
- 125.Salameh A, Dhein S. Effects of mechanical forces and stretch on intercellular gap junction coupling. Biochim. Biophys. Acta (BBA) 2013;1828:147–156. doi: 10.1016/j.bbamem.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 126.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schrader J, et al. Matrix stiffness modulates proliferation, chemotherapeutic response and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction. J. Cell Sci. 2012;125:3051–3060. doi: 10.1242/jcs.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seong J, Wang N, Wang Y. Mechanotransduction at focal adhesions: from physiology to cancer development. J. Cell Mol. Med. 2013;17:597–604. doi: 10.1111/jcmm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sewell-Loftin MK, et al. Cancer-associated fibroblasts support vascular growth through mechanical force. Sci. Rep. 2017;7:12574. doi: 10.1038/s41598-017-13006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi Q, et al. Rapid disorganization of mechanically interacting systems of mammary acini. Proc. Natl. Acad. Sci. 2014;111:658–663. doi: 10.1073/pnas.1311312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sica A, et al. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 133.Sivasankar S, Gumbiner B, Leckband D. Direct measurements of multiple adhesive alignments and unbinding trajectories between cadherin extracellular domains. Biophys. J. 2001;80:1758–1768. doi: 10.1016/S0006-3495(01)76146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sluysmans S, Vasileva E, Spadaro D, Shah J, Rouaud F, Citi S. The role of apical cell–cell junctions and associated cytoskeleton in mechanotransduction. Biol. Cell. 2017;109:139–161. doi: 10.1111/boc.201600075. [DOI] [PubMed] [Google Scholar]
- 135.Stachowiak MR, et al. A mechanical-biochemical feedback loop regulates remodeling in the actin cytoskeleton. Proc. Natl Acad. Sci. U.S.A. 2014;111:17528–17533. doi: 10.1073/pnas.1417686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sunyer R, et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353:1157–1161. doi: 10.1126/science.aaf7119. [DOI] [PubMed] [Google Scholar]
- 137.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 138.Tambe DT, et al. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tornavaca O, et al. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015;208:821–838. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol. 2011;21:638–646. doi: 10.1016/j.tcb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Helvert S, Friedl P. Strain stiffening of fibrillar collagen during individual and collective cell migration identified by AFM nanoindentation. ACS Appl. Mater. Interfaces. 2016;8:21946–21955. doi: 10.1021/acsami.6b01755. [DOI] [PubMed] [Google Scholar]
- 144.van Oers RFM, Rens EG, LaValley DJ, Reinhart-King CA, Merks RMH. Mechanical cell–matrix feedback explains pairwise and collective endothelial cell behavior in vitro. PLoS Comput. Biol. 2014;10(8):e1003774. doi: 10.1371/journal.pcbi.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vasquez CG, Martin AC. Force transmission in epithelial tissues. Dev. Dyn. 2016;245:361–371. doi: 10.1002/dvdy.24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vishwakarma M, Russo JD, Probst D, Schwarz US, Das T, Spatz JP. Mechanical interactions among followers determine the emergence of leaders in migrating epithelial cell collectives. Nat. Commun. 2018;9:3469. doi: 10.1038/s41467-018-05927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wagstaff L, et al. Mechanical cell competition kills cells via induction of lethal p53 levels. Nat. Commun. 2016;7:11373. doi: 10.1038/ncomms11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang H, Abhilash AS, Chen CS, Wells RG, Shenoy VB. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys. J. 2014;107:2592–2603. doi: 10.1016/j.bpj.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang K, Andresen Eguiluz RC, Wu F, Seo BR, Fischbach C, Gourdon D. Stiffening and unfolding of early deposited-fibronectin increase proangiogenic factor secretion by breast cancer-associated stromal cells. Biomaterials. 2015;54:63–71. doi: 10.1016/j.biomaterials.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 151.Wang T-H, Hsia S-M, Shieh T-M. Lysyl oxidase and the tumor microenvironment. Int. J. Mol. Sci. 2016;18(1):100. doi: 10.3390/ijms18010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S, Sun J, Xiao Y, Lu Y, Zhang DD, Wong PK. Intercellular tension negatively regulates angiogenic sprouting of endothelial tip cells via Notch1-Dll4 signaling. Adv. Biosyst. 2017;1:1600019. doi: 10.1002/adbi.201600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat. Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 154.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J. Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 155.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS ONE. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wolf K, et al. Compensation mechanism in tumor cell migration: mesenchymal–amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 159.Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65:130–136. [PubMed] [Google Scholar]
- 160.Xu WW, et al. Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression. Nat. Commun. 2017;8:14399. doi: 10.1038/ncomms14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yeh Y-C, Ling J-Y, Chen W-C, Lin H-H, Tang M-J. Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: reciprocal regulation of caveolin-1 and β1 integrin. Sci. Rep. 2017;7:15008. doi: 10.1038/s41598-017-14932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell–matrix adhesion complexes. Biochem. Soc. Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 163.Zamir E, et al. Dynamics and segregation of cell–matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 164.Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small GTPases. 2014;5:e983869. doi: 10.4161/sgtp.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang W, Couldwell WT, Simard MF, Song H, Lin JH-C, Nedergaard M. Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res. 1999;59:1994–2003. [PubMed] [Google Scholar]
- 166.Zhou G, et al. The role of desmosomes in carcinogenesis. Onco Targets Ther. 2017;10:4059–4063. doi: 10.2147/OTT.S136367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu X, et al. Galectin-1 knockdown in carcinoma-associated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim. Biophys. Sin. 2016;48:462–467. doi: 10.1093/abbs/gmw019. [DOI] [PMC free article] [PubMed] [Google Scholar]