Abstract
Purpose of Review.
The purpose of this review is to highlight recent research advances in noninvasive prenatal diagnostic methods.
Recent Findings.
Recent studies developing noninvasive prenatal diagnostic (NIPD) methods have been focused on either fetal nucleated red blood cells (fNRBCs) or circulating trophoblasts (cTBs). Enriched cTBs were successfully utilized for whole genome profiling and short tandem repeat (STR) identification to confirm feto-maternal relationship. However, further analysis of isolated fNRBCs remains confined to examining fetal cytogenetics.
Summary.
Invasive prenatal diagnostic procedures, amniocentesis and chorionic villus sampling, are the gold standard for the diagnosis of fetal chromosomal abnormalities and genetic disorders. Meanwhile, noninvasive techniques of analyzing circulating cell-free fetal DNA (cffDNA) have been limited to screening tools and are highly fragmented and confounded by maternal DNA. By detecting circulating fetal nucleated cells (CFNCs) we are able to noninvasively confirm fetal chromosomal abnormalities, truly realizing the concept of “noninvasive prenatal diagnostics”. The primary technical challenge is the enrichment of the low abundance of CFNCs in maternal peripheral blood. For any cell-based NIPD method, both fetal whole genome profiling and confirmation of the feto-parental relationship are essential. This has been successfully performed using enriched and isolated cTBs, making cTB a better candidate for NIPD. cTB enumeration also correlates with abnormal fetal or placental development. On the other hand, downstream analysis of fNRBCs remains limited to examining fetal sex and aneuploidies. Furthermore, trophoblast-based NIPD via an endocervical sample is also promising because of reduced dilution from hematologic cells.
Keywords: Noninvasive Prenatal Diagnostic, Circulating Fetal Nucleated Red Blood Cell, Circulating Trophoblast, Whole Genome Amplification, Array Comparative Genomic Hybridization, Short Tandem Repeat
Introduction
The current gold standard for prenatal diagnosis of chromosomal abnormalities and genetic disorders involves an invasive procedure[1, 2], namely chorionic villus sampling (CVS, performed between 10–13 weeks gestation) or amniocentesis (performed between 15–18 weeks gestation), when either amnioctyes or chorionic villi are collected for karyotyping, microarray analysis, or sequencing[3]. These two procedures provide accurate diagnostic information for clinical decision-making. However, there are concerns regarding procedural risk, which includes an increased risk of pregnancy loss (0.6–2%)[4] and other complications (i.e. limb-reduction defects and rupture of membranes). To implement prenatal diagnosis without these risks, significant endeavors have been devoted into developing paradigm shifting non-invasive prenatal diagnostic (NIPD) technologies (Table 1).
Table 1.
Benefits of the circulating trophoblast(cTB)-based NIPD technology (using maternal blood) and alternative trophoblast(TB)-based NIPD technology (using endocervical swab samples) in comparison to the conventional invasive diagnostic procedures (i.e., amniocentesis and CVS) and cffDNA-based NIPT screening.
| Invasive Prenatal Diagnosis |
cffDNA-based NIPT | cTB-based NIPD | TB-based NIPD | ||
|---|---|---|---|---|---|
| Amniocentesis | Chorionic Villus Sampling (CVS) | ||||
| Sample | Fetal cells | Placental tissues | Mixtures of fetal and maternal DNA | Circulating trophoblasts (cTBs) in peripheral blood | Trophoblasts (TBs) in cervix |
| Coverage | High | High | Low (chromosome 21/18/13/X/Y) | High | High |
| Accuracy | High | High | High for trisomy 21 | High | High |
| Timing (GA) | 15 weeks | 10 weeks | >9 weeks (only for screening) | >8 weeks (potential to be >6 weeks) | >5 weeks |
Cell-free fetal DNA (cffDNA)
Dr. Lo first reported[5] the presence of cell-free fetal DNA (cffDNA) in maternal blood in 1997. Two sequencing methods have been developed to detect fetal aneuploidies: one is massive parallel shotgun sequencing (MPSS)[6, 7], while the other is single-nucleotide polymorphisms (SNP)-based targeted sequencing[8]. Both methods detect the most commonly seen fetal aneuploidies including trisomy 21, 18, 13, and sex chromosome abnormality with high sensitivity. Currently, in the United States, there are multiple Clinical Laboratory Improvement Amendments (CLIA) labs providing cffDNA-based noninvasive prenatal tests (NIPT), including Sequenom, Ariosa, Illumina, and Natera, among others. However, cffDNA is highly fragmented and compounded by a significant background of maternal DNA. Due to their various limitations (e.g., high false positive rates[9], low sensitivity to less common aneuploidies, and an inability to detect shorter copy number variations CNVs and microdeletions/duplications)[10], these cffDNA-based methods only served as screening tools, rather than diagnostic tools. Patients with positive NIPT results require diagnostic confirmation by invasive prenatal diagnostic procedures, i.e., CVS and amniocentesis.
Circulating fetal nucleated cells (CFNCs) for NIPD
In contrast to cffDNA, CFNCs[11, 12] in maternal blood possess intact fetal genomic DNA, making these cells an ideal candidate for NIPD. Fetal-maternal cellular trafficking[13] is the bidirectional passage of cells that is responsible for CFNCs in maternal circulation. The potential to use CFNCs during the first and second trimester for prenatal diagnosis was described[11] in 1969 (before cffDNA). Unfortunately, it has thus far not been possible to develop a routine clinical prenatal test despite extensive commercial and academic research efforts bover the past 49 years. This hurdle can be attributed to the technical challenges associated with the detection, isolation, and characterization of CFNCs in maternal blood samples. These cells are both fragile and low in abundance[14] (compared to 109 hematologic cells/mL of maternal blood in a normal pregnancy). Throughout the past half century[15, 12], several different technologies for enriching CFNCs have been studied, including: gradient centrifugation,[16, 17] magnetic-activated cell sorting (MACS),[18, 19] fluorescence-activated cell sorting (FACS),[20, 21] microchip technologies,[15, 22] filtration,[23] high-throughput microscopy[14, 24], and various combinations of the aforementioned methods. Yet, none of these technologies have demonstrated sufficient sensitivity and reliability to support clinical implementation.
Among CFNCs[12] that have been identified in maternal circulation, e.g., fetal nucleated red blood cells (fNRBCs),[25] leukocytes, and circulating trophoblasts (cTBs)[24], the majority of efforts have focused on developing NIPD methods using fNRBCs and cTBs. A key milestone was achieved by a prospective, multicenter clinical project, i.e. the National Institute of Child Health and Human Development Fetal Cell Isolation Study (NIFTY)[26], led by Dr. Bianchi to develop fNRBC-based NIPD methods. In this study, both MACS and FACS techniques were employed to isolate fNRBCs from maternal blood samples, and were followed by fluorescence in situ hybridization (FISH) to detect aneuploidy and fetal sex. Unfortunately, the results of the NIFTY trial[26] demonstrated the rates of detecting at least one cell with a Y-chromosome signal in women carrying singleton male fetuses and of identifying at least one aneuploid cell in cases of fetal aneuploidy to be insufficient for clinical use. Furthermore, the high false-positive rate in determining fetal sex (based on Y-chromosome signal) made this approach inappropriate for clinical use.
Circulating trophoblasts (cTBs) for NIPD
Recent studies[19, 24, 27] suggest that cTB[24] may be a better target CFNC, given their i) short lifespan (a few days), which excludes the possibility of isolating cTBs from prior pregnancies, ii) representation of fetal karyotype and genotype (except for the rare circumstances where discrepancy can result from confined placenta mosaicism), and iii) expression of specific biomarker signatures[28] (e.g., CD105[19], EpCAM[27], CK7[19, 24, 27] and HLA-G[27]) that can be used for both enrichment and identification. In 2016, two studies[19, 24] led by Dr. Beaudet at Baylor College of Medicine demonstrated that cTBs could be recovered by either a single-cell isolation technique[24] or an optimized MACS approach[19] from maternal blood (as early as 10 weeks’ gestation) for whole genome profiling by array comparative genomic hybridization (aCGH) and/or next-generation sequencing (NGS). The feasibility of conducting aCGH-based whole genome profiling of cTB was independently validated by Vestergaard et al[29] in Denmark. They reported results from five clinical cases of first trimester high-risk pregnancies (those with a >1:300 risk of a chromosomal abnormality). In all of these, cTBs were enriched by a MACS-based approach, followed by single-cell isolation.
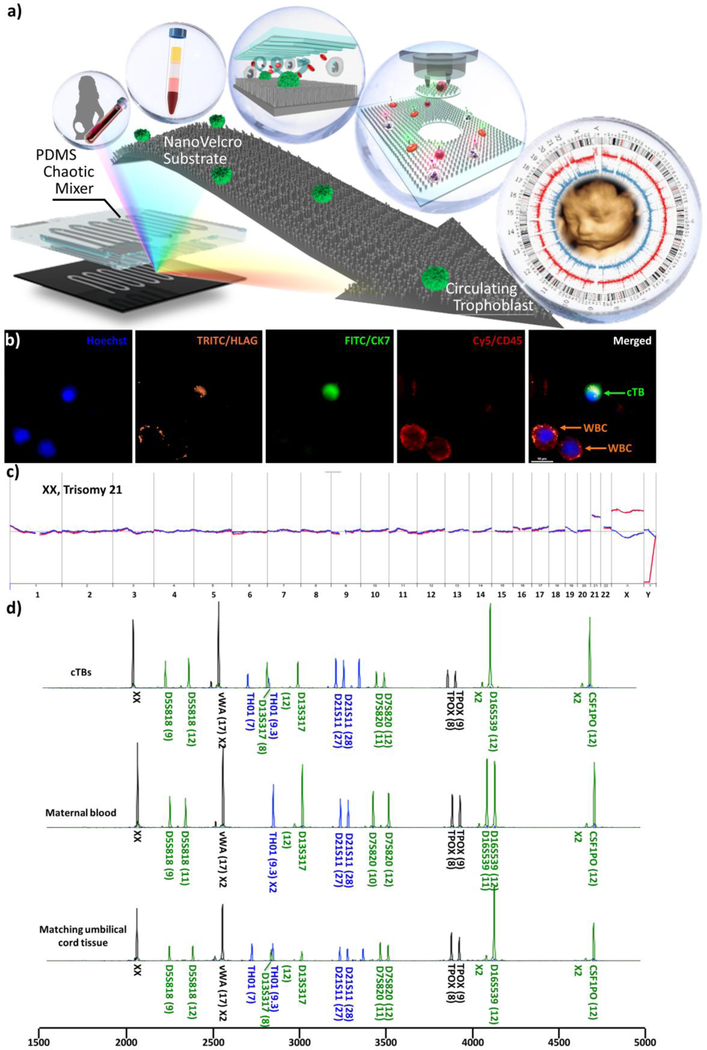
In 2017, a joint team across three institutions, including the University of California, Los Angeles (UCLA), Cedars Sini Medical Center (CSMC), and Cathay General Hospital in Taipei, demonstrated highly precise single cTB isolation (Figure 1a) using a new class of NanoVelcro Rare-Cell Assays[30–32]. This technology was originally developed for the detection and characterization of circulating tumor cells (CTCs) in the cancer diagnostics field. Compared with existing rare-cell enrichment technologies, the uniqueness of NanoVelcro Assays stems from their use of nanosubstrates – specifically, nanostructured substrates based on silicon,[33, 34] TiO2,[35] and polymer[36, 37] nanostructures – which allow for enhanced local topographic interactions[38–40] between the nanostructured substrates and nanoscale cellular surface components (e.g., microvilli), resulting in vastly improved cell-capture affinities compared to those observed for non-structured (i.e., flat) substrates. To explore NanoVelcro Assays’ application in the field of NIPD, we utilized our 2nd-generation NanoVelcro Chips,[27] in which laser capture microdissection (LCM)-compatible nanosubstrates were prepared via nanoimprinting in a cost-efficient and scalable manner. A cTB-specific capture agent (anti-EpCAM) was grafted onto the NanoVelcro substrates to confer specificity for cTB enrichment in maternal blood samples. From there, a 4-channel immunocytochemistry (ICC) protocol for parallel staining of Hoechst, anti-CK7, anti-HLAG, and anti-CD45 was developed to stain cTBs. In parallel, high-resolution microscopy imaging was adopted to identify and register individual cTBs (Hoechst+/CK7+/HLAG+/CD45−, 15 µm>sizes>7 µm, Figure 1b) from nonspecifically captured white blood cells (WBCs) and cellular debris on the NanoVelcro substrates. Subsequently, an LCM system was employed to isolate the identified cTBs, followed by downstream fetal whole genome profiling by aCGH and short tandem repeat (STR) assays for confirming feto-parental relationship. A streamlined workflow[27] (Figure 1a) is illustrated starting from single cTB identification and isolation to whole genome profiling of cTBs by aCGH and STR. Accurate detection of fetal sex and chromosomal aberrations has been demonstrated (Figure 1c) in 15 clinical samples.
Figure 1.
Isolation and examination of cTBs from maternal blood, using the 2nd-generation NanoVelcro assay, for genome-wide detection. a) The workflow of cTB enrichment and single cTB isolation. After centrifugation of the maternal blood, the peripheral blood mononuclear cell (PBMC) layer is extracted to capture cTBs with NanoVelcro Chips for further genetic analysis. b) Immunostaining of cTB by specific markers. cTBs are identified by Hoechst+ (blue), HLAG+ (orange), CK7 + (green), and CD45− (red). c) cTB-derived aCGH revealed trisomy 21 from three cTBs of a female fetus. d) STR genomic mapping verified the feto-parental relationship between cTBs and their matching maternal and paternal white blood cells. Adapted with permission from Hou, S. et al.[27] Copyright (2018) American Chemical Society.
The four publications[19, 24, 29, 27] described above validated the potential of cTB-based NIPD to diagnose the most common chromosomal abnormalities: aneuploidies potentially accompanied by mosaicism, unbalanced translocations, deletions, and/or duplications. Understanding the potential impact, multiple scientific and biotechnology news outlets, e.g., GenomeWeb and Chemical & Engineering News, have highlighted these results[19, 24, 27], opening the possibility of high-resolution detection of CNVs, and even whole genome or exome sequencing to detect both inherited and de novo mutations.
With these milestones in place, expectations for a practical cTB-based NIPD prenatal technology must include i) sufficient sensitivity and specificity to recover pure cTBs from maternal blood during the first trimester, ii) sufficient reproducibility and scalability to conduct fetal whole genome profiling using the recovered cTBs, and iii) confirmation of cTB identity by checking feto-parental (or at least feto-maternal) relationship using STR fingerprints. Due to the low numbers of cTBs found in the first trimester, a practical cTB-based NIPD technology would need to be capable of highly effective cTB isolation and of handling a volume of blood (>10 milliliters) sufficient for isolating cTBs for whole genome profiling and STR fingerprints, in order to be reproducible enough for clinical use. Second, cTBs need to be isolated in a pure and intact form[19, 24, 27] in order to be subjected to whole genome amplification (WGA), followed by fetal whole genome profiling via aCGH and/or NGS. To reproducibly amplify the low quantity of cTB-derived DNA represents a crucial technical hurdle for whole genome profiling. Extensive tests conducting WGA on DNA isolated from single and pooled cTBs have been performed in order to credibly demonstrate the feasibility of cTB-based fetal whole genome profiling. Third, to confirm the feto-parental relationship of the isolated cTBs, their matching maternal/paternal STR fingerprints have to be evaluated.[19, 24, 27] This confirmatory test serves as a crucial checkpoint, preventing potential misidentification of maternal cells as female cTBs. Moreover, it is well documented that trophoblast cells may be mosaic, leading to false positive results. Mosaicism rates in potentially viable embryos range between 4.8% – 16.9% and 1.3% at CVS.[41–44] Large-scale clinical validation studies on individual cTB-based NIPT technologies will have to be conducted in order to assess fetal mosaicism rates.
Latest progress on fNRBC-based NIPD
Recently, extensive research efforts have also been devoted to exploring the use of different rare-cell enrichment technologies to identify and isolate fNRBCs for NIPD applications. Byeon et al.[45] utilized a two-step enrichment workflow composed of an enrichment process based on red blood cell (RBC) hyper-aggregation in tubes, and immunomagnetic WBC depletion in microfluidic chips. The enriched fNRBCs were further characterized by ICC and PCR-based detection of SRY gene (associated with fetal Y chromosome). He et al.[46] exploited a nanostructure microchip to isolate fNRBCs, followed by on-chip fNRBCs analysis by ICC (covering DAPI, CD71, and ε-HbF) and FISH (to detect trisomy). The frequency-enhanced transferrin receptor antibody-labelled microfluidic chip (FETAL-Chip) was designed by Zhang et al.[47] for enrichment of circulating fNRBCs. Subsequently, ICC and SRY gene detection were carried out to identify fNRBCs. Compared to the fNRBC enrichment approaches employed in the NIFTY trial[26], there might have been some technical improvements in more recent studies. However, the scope of the downstream analysis remains confined to either traditional cytogenetic techniques (e.g., karyotyping and FISH) or polymerase chain reaction (PCR), which are of limited capacity for detecting CNVs. The challenges encountered in the NIFTY trial[26] remained unsolved. fNRBC-based NIPD has yet to demonstrate its potential to adopt more powerful molecular analysis technologies capable of performing fetal whole genome profiling (e.g., aCGH and NGS). Furthermore, STR identification for confirming feto-maternal relationship (trios) was not performed in fNRBC-based NIPD either. A noteworthy concern for fNRBC-based NIPD is the presence of maternal NRBCs in circulation and the challenges associated with rigorously distinguishing these cells from fNRBCs[12] (i.e., fNRBCs of a female fetus without any chromosomal abnormality cannot be differentiated from maternal NRBCs by FISH or PCR of SRY).
Other CFNC-based NIPD
A new approach has also been developed by the Beijing Genomics Institute[48] (BGI) to address the urgent need for a highly sensitive and accurate method of isolating CFNCs for NIPD. The BGI team developed a double negative selection (DNS) protocol coupled with positive genetic identification for CFNCs. First, CD45-negative cells were collected by depleting WBCs using MACS, these CD45- cells were then incubated with Hoechst and propidium iodide (PI). Isolation of CD45−/Hoechst+/PI− fetal cells was performed by FACS. The isolated cells were further processed by WGA using quantitative PCR (qPCR) and STR detection, identifying several disease-associated variants. An inertial microfluidic device was demonstrated by Winter et al.[49] in 2018 for efficient isolation of trophoblasts from maternal peripheral blood by filtering for cells with sizes greater than approximately 15 μm. After optimizing the inertial microfluidic device using a cell line, they conducted a pilot study with a blood sample from a woman carrying a fetus suspected to have trisomy 21. Subsequent ICC and FISH results agreed with a diagnosis of trisomy 21, which was confirmed by amniocentesis for karyotyping. Still, more clinical samples and further WGA are necessary to validate the feasibility of this size-based device.
Other potential utilities
Trophoblast (TB)-based NIPD solution using endocervical swab samples as a complimentary approach to maximize success.
An alternative approach for achieving fetal cell-based NIPD is to utilize trophoblasts[50] that migrate from the placenta into the reproductive tract.[51] It has been demonstrated that hundreds of trophoblasts can be recovered safely and non-invasively in the cervical canal using a Papanicolaou (Pap) procedure.[52] Since the discovery of circulating fetal cells in 1971, numerous approaches, including immunomagnetic enrichment with anti-HLAG,[53] and filtration followed by NGS[28] and single-cell STR genotyping[54] have been developed. Extravillous trophoblast (EVT) cells purified by trophoblast retrieval and isolation from the cervix (TRIC) at 5–20 weeks of gestation contain the fetal genome (for genetic assessment of the embryo/fetus), as well as the fetal transcriptome, which may reveal placental abnormalities as early as the first trimester. TRIC may one day provide a method of risk assessment for maternal and fetal disease, paving the way for a diagnostic tool in obstetric precision medicine.
CFNC enumeration predicts abnormal fetal development, prognosis, and outcomes.
Since CFNCs can be detected in maternal circulation as early as the sixth week of gestation [23], and increased CFNC count suggests escalated feto-maternal cellular trafficking - an indication of potential abnormal fetal development[55, 56, 27] (e.g., fetal aneuploidy and adherent placentation including placenta accreta), CFNC enumeration may be used to screen for abnormal fetal/placental development. Because CFNC enumeration can be conveniently carried out within the existing workflow once the cells are captured on the NanoVelcro chips[27, 32], it can be used as a complementary diagnostic screening tool while performing the CFNC-based fetal whole genome profiling. The CFNC-based enumeration assay might serve as a powerful assay capable of assessing abnormal trophoblast invasion into the endometrium which may be used for diagnosing and monitoring diseases related to abnormal placental development, including highly morbid conditions such as placenta accreta.
Conclusions
Of the current non-invasive prenatal genetic testing methods, cffDNA-based NIPT remains solely as a screening technology because of the fragmented nature of fetal genomic information and the maternal cell-free DNA background naturally found in maternal blood. As for CFNCs, isolation of fNRBCs for further analysis is limited by the presence of maternal NRBCs, low detection rates, and high false-positive rates. Another CFNC-based approach, i.e., cTB-based analysis, has demonstrated several utilities as a potential NIPD. The cTB-derived aCGH data correctly identified fetal sex, and chromosomal and subchromosomal abnormalities, which had been confirmed by invasive procedures (e.g., amniocentesis and CVS). In parallel, the STR fingerprints from cTBs were correlated with maternal/paternal blood cells, which confirmed the feto-parental relationships. These studies support the feasibility of cTB as a good candidate for NIPD. With ongoing and anticipated large-scale clinical studies in the pipeline, this cTB-based NIPD holds great potential to evolve into a truly noninvasive prenatal diagnostic solution. Progress in genomic analysis should make it feasible to detect copy number variations in the megabase range in the near future and perhaps even to detect de novo point mutations. In addition, potential TB-based NIPD using endocervical swab samples could provide assessments for fetal genomic aberrations as well as fetal and maternal risks in pregnancy. Overall, circulating and endocervical fetal nucleated cell enumeration may serve as a complementary screening tool for abnormal placental development while performing fetal whole genome profiling. Such technology would transform the practice of prenatal care and diagnosis. While the feasibility of performing single-cTB genetic testing has been demonstrated by our team[27] and others[19, 24], the biggest remaining challenge to becoming a real NIPD is to demonstrate acceptable reproducibility and scalability of this test.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Pin-Jung Chen, Pai-Chi Teng, Yazhen Zhu, Yu Jen Jan, Yalda Afshar, Li-Ching Chen, Margareta D. Pisarska, and Hsian-Rong Tseng declare no conflict of interest. Dr. Smalley reports personal fees from CytoLumina.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
References
- 1.Dimaio MS, Fox JE, Mahoney MJ. Prenatal Diagnosis: Cases and Clinical Challenges 1st ed. Wiley-Blackwell; 2010. [Google Scholar]
- 2.Beckmann CRB, Herbert W, Laube D, Ling F, Smith R. Obstetrics and Gynecology Lippincott Williams & Wilkins ed. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 3.Society for Maternal-Fetal Medicine. Electronic address pso, Dugoff L, Norton ME, Kuller JA The use of chromosomal microarray for prenatal diagnosis. Am J Obstet Gynecol 2016;215(4):B2–9. 10.1016/j.ajog.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Mujezinovic F, Alfirevic Z. Procedure-related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstet Gynecol 2007;110(3):687–94. 10.1097/01.AOG.0000278820.54029.e3. [DOI] [PubMed] [Google Scholar]
- 5.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350(9076):485–7. 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 6.Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proceedings of the National Academy of Sciences of the United States of America 2008;105(51):20458–63. 10.1073/pnas.0810641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP et al. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol 2012;119(5):890–901. 10.1097/AOG.0b013e31824fb482. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides KH, Syngelaki A, Gil M, Atanasova V, Markova D. Validation of targeted sequencing of single-nucleotide polymorphisms for non-invasive prenatal detection of aneuploidy of chromosomes 13, 18, 21, X, and Y. Prenat Diagn 2013;33(6):575–9. 10.1002/pd.4103. [DOI] [PubMed] [Google Scholar]
- 9.Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015;372(17):1589–97. 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 10.Williams J 3rd, Rad S, Beauchamp S, Ratousi D, Subramaniam V, Farivar S et al. Utilization of noninvasive prenatal testing: impact on referrals for diagnostic testing. Am J Obstet Gynecol 2015;213(1):102 10.1016/j.ajog.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Walknowska J, Conte FA, Grumbach MM. Practical and theoretical implications of fetal-maternal lymphocyte transfer. Lancet 1969;1(7606):1119–22. [DOI] [PubMed] [Google Scholar]
- 12.•.Beaudet AL. Using fetal cells for prenatal diagnosis: History and recent progress. Am J Med Genet C 2016;172(2):123–7. 10.1002/ajmg.c.31487. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi DW. Robert E. Gross Lecture. Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. Journal of pediatric surgery 2007;42(1):12–8. 10.1016/j.jpedsurg.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Emad A, Bouchard EF, Lamoureux J, Ouellet A, Dutta A, Klingbeil U et al. Validation of automatic scanning of microscope slides in recovering rare cellular events: application for detection of fetal cells in maternal blood. Prenat Diagn 2014;34(6):538–46. 10.1002/pd.4345. [DOI] [PubMed] [Google Scholar]
- 15.Kantak C, Chang CP, Wong CC, Mahyuddin A, Choolani M, Rahman A. Lab-on-a-chip technology: impacting non-invasive prenatal diagnostics (NIPD) through miniaturisation. Lab on a chip 2014;14(5):841–54. 10.1039/c3lc50980j. [DOI] [PubMed] [Google Scholar]
- 16.Bhat NM, Bieber MM, Teng NN. One-step enrichment of nucleated red blood cells. A potential application in perinatal diagnosis. Journal of immunological methods 1993;158(2):277–80. [DOI] [PubMed] [Google Scholar]
- 17.Kwon KH, Jeon YJ, Hwang HS, Lee KA, Kim YJ, Chung HW et al. A high yield of fetal nucleated red blood cells isolated using optimal osmolality and a double-density gradient system. Prenat Diagn 2007;27(13):1245–50. 10.1002/pd.1888. [DOI] [PubMed] [Google Scholar]
- 18.Mavrou A, Kouvidi E, Antsaklis A, Souka A, Kitsiou Tzeli S, Kolialexi A. Identification of nucleated red blood cells in maternal circulation: a second step in screening for fetal aneuploidies and pregnancy complications. Prenat Diagn 2007;27(2):150–3. 10.1002/pd.1640. [DOI] [PubMed] [Google Scholar]
- 19.••.Kolvraa S, Singh R, Normand EA, Qdaisat S, van den Veyver IB, Jackson L et al. Genome-wide copy number analysis on DNA from fetal cells isolated from the blood of pregnant women. Prenat Diagn 016;36(12):1127–34. 10.1002/pd.4948. [DOI] [PubMed] [Google Scholar]
- 20.Herzenberg LA, Bianchi DW, Schroder J, Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proceedings of the National Academy of Sciences of the United States of America 1979;76(3):1453–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Wit H, Nabbe KC, Kooren JA, Adriaansen HJ, Roelandse-Koop EA, Schuitemaker JH et al. Reference values of fetal erythrocytes in maternal blood during pregnancy established using flow cytometry. American journal of clinical pathology 2011;136(4):631–6. 10.1309/AJCPHL3VXY0VMLXL. [DOI] [PubMed] [Google Scholar]
- 22.He ZB, Guo F, Feng C, Cai B, Lata JP, He RX et al. Fetal nucleated red blood cell analysis for non-invasive prenatal diagnostics using a nanostructure microchip. Journal of Materials Chemistry B 2017;5(2):226–35. 10.1039/c6tb02558g. [DOI] [PubMed] [Google Scholar]
- 23.Mouawia H, Saker A, Jais JP, Benachi A, Bussieres L, Lacour B et al. Circulating trophoblastic cells provide genetic diagnosis in 63 fetuses at risk for cystic fibrosis or spinal muscular atrophy. Reprod Biomed Online 2012;25(5):508–20. 10.1016/j.rbmo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.••.Breman AM, Chow JC, U’Ren L, Normand EA, Qdaisat S, Zhao L et al. Evidence for feasibility of fetal trophoblastic cell-based noninvasive prenatal testing. Prenat Diagn 2016;36(11):1009–19. 10.1002/pd.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganshirt D, Smeets FW, Dohr A, Walde C, Steen I, Lapucci C et al. Enrichment of fetal nucleated red blood cells from the maternal circulation for prenatal diagnosis: experiences with triple density gradient and MACS based on more than 600 cases. Fetal diagnosis and therapy 1998;13(5):276–86. 10.1159/000020854. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi DW, Simpson JL, Jackson LG, Elias S, Holzgreve W, Evans MI et al. Fetal gender and aneuploidy detection using fetal cells in maternal blood: analysis of NIFTY I data. National Institute of Child Health and Development Fetal Cell Isolation Study. Prenat Diagn 2002;22(7):609–15. 10.1002/pd.347. [DOI] [PubMed] [Google Scholar]
- 27.••.Hou S, Chen JF, Song M, Zhu Y, Jan YJ, Chen SH et al. Imprinted NanoVelcro Microchips for Isolation and Characterization of Circulating Fetal Trophoblasts: Toward Noninvasive Prenatal Diagnostics. ACS Nano 2017;11(8):8167–77. 10.1021/acsnano.7b03073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain CV, Kadam L, van Dijk M, Kohan-Ghadr HR, Kilburn BA, Hartman C et al. Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Science translational medicine 2016;8(363):363re4 10.1126/scitranslmed.aah4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vestergaard EM, Singh R, Schelde P, Hatt L, Ravn K, Christensen R et al. On the road to replacing invasive testing with cell-based NIPT: Five clinical cases with aneuploidies, microduplication, unbalanced structural rearrangement, or mosaicism. Prenat Diagn 2017;37(11):1120–4. 10.1002/pd.5150. [DOI] [PubMed] [Google Scholar]
- 30.Lin M, Chen JF, Lu YT, Zhang Y, Song J, Hou S et al. Nanostructure embedded microchips for detection, isolation, and characterization of circulating tumor cells. Acc Chem Res 2014;47(10):2941–50. 10.1021/ar5001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JF, Zhu Y, Lu YT, Hodara E, Hou S, Agopian VG et al. Clinical Applications of NanoVelcro Rare-Cell Assays for Detection and Characterization of Circulating Tumor Cells. Theranostics 2016;6(9):1425–39. 10.7150/thno.15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•.Jan YJ, Chen JF, Zhu Y, Lu YT, Chen SH, Chung H et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv Drug Deliv Rev 2018;125:78–93. 10.1016/j.addr.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Wang H, Jiao J, Chen KJ, Owens GE, Kamei K et al. Three-dimensional nanostructured substrates toward efficient capture of circulating tumor cells. Angew Chem Int Ed Engl 2009;48(47):8970–3. 10.1002/anie.200901668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Liu K, Liu J, Yu ZT, Xu X, Zhao L et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angewandte Chemie 2011;50(13):3084–8. 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang N, Deng Y, Tai Q, Cheng B, Zhao L, Shen Q et al. Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Advanced materials 2012;24(20):2756–60. 10.1002/adma.201200155. [DOI] [PubMed] [Google Scholar]
- 36.Hou S, Zhao L, Shen Q, Yu J, Ng C, Kong X et al. Polymer nanofiber-embedded microchips for detection, isolation, and molecular analysis of single circulating melanoma cells. Angewandte Chemie 2013;52(12):3379–83. 10.1002/anie.201208452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou S, Zhao H, Zhao L, Shen Q, Wei KS, Suh DY et al. Capture and stimulated release of circulating tumor cells on polymer-grafted silicon nanostructures. Advanced materials 2013;25(11):1547–51. 10.1002/adma.201203185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer KE, Aleman BJ, Tao SL, Daniels RH, Li EM, Bunger MD et al. Biomimetic Nanowire Coatings for Next Generation Adhesive Drug Delivery Systems. Nano Letters 2009;9(2):716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtis ASG, Varde M. Control of Cell Behavior - Topological Factors. J Natl Cancer I 1964;33(1):15-&. [PubMed] [Google Scholar]
- 40.Liu WF, Chen CS. Cellular and multicellular form and function. Advanced Drug Delivery Reviews 2007;59(13):1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledbetter DH, Zachary JM, Simpson JL, Golbus MS, Pergament E, Jackson L et al. Cytogenetic results from the U.S. Collaborative Study on CVS. Prenat Diagn 1992;12(5):317–45. [DOI] [PubMed] [Google Scholar]
- 42.Munne S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril 2017;108(1):62–71 e8. 10.1016/j.fertnstert.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Schattman GL. Chromosomal mosaicism in human preimplantation embryos: another fact that cannot be ignored. Fertil Steril 2018;109(1):54–5. 10.1016/j.fertnstert.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Huang A, Adusumalli J, Patel S, Liem J, Williams J 3rd, Pisarska MD. Prevalence of chromosomal mosaicism in pregnancies from couples with infertility. Fertil Steril 2009;91(6):2355–60. 10.1016/j.fertnstert.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 45.Byeon Y, Ki CS, Han KH. Isolation of nucleated red blood cells in maternal blood for Non-invasive prenatal diagnosis. Biomed Microdevices 2015;17(6):118 10.1007/s10544-015-0021-3. [DOI] [PubMed] [Google Scholar]
- 46.He Z, Guo F, Feng C, Cai B, Lata JP, He R et al. Fetal nucleated red blood cell analysis for non-invasive prenatal diagnostics using a nanostructure microchip. Journal of Materials Chemistry B 2017;5(2):226–35 10.1039/C6TB02558G. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Yang Y, Li X, Shi Y, Hu B, An Y et al. Frequency-enhanced transferrin receptor antibody-labelled microfluidic chip (FETAL-Chip) enables efficient enrichment of circulating nucleated red blood cells for non-invasive prenatal diagnosis. Lab Chip 2018;18(18):2749–56. 10.1039/c8lc00650d. [DOI] [PubMed] [Google Scholar]
- 48.Chen F, Liu P, Gu Y, Zhu Z, Nanisetti A, Lan Z et al. Isolation and whole genome sequencing of fetal cells from maternal blood towards the ultimate non-invasive prenatal testing. Prenat Diagn 2017;37(13):1311–21. 10.1002/pd.5186. [DOI] [PubMed] [Google Scholar]
- 49.Winter M, Hardy T, Rezaei M, Nguyen V, Zander-Fox D, Ebrahimi Warkiani M et al. Isolation of Circulating Fetal Trophoblasts Using Inertial Microfluidics for Noninvasive Prenatal Testing. Advanced Materials Technologies 2018;3(7). 10.1002/admt.201800066. [DOI] [Google Scholar]
- 50.Moser G, Drewlo S, Huppertz B, Armant DR. Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum Reprod Update 2018;24(4):484–96. 10.1093/humupd/dmy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imudia AN, Kumar S, Diamond MP, DeCherney AH, Armant DR. Transcervical retrieval of fetal cells in the practice of modern medicine: a review of the current literature and future direction. Fertil Steril 2010;93(6):1725–30. 10.1016/j.fertnstert.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imudia AN, Suzuki Y, Kilburn BA, Yelian FD, Diamond MP, Romero R et al. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: a pilot study. Hum Reprod 2009;24(9):2086–92. 10.1093/humrep/dep206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolnick JM, Kilburn BA, Bajpayee S, Reddy N, Jeelani R, Crone B et al. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil Steril 2014;102(1):135–42 10.1016/j.fertnstert.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfeifer I, Benachi A, Saker A, Bonnefont JP, Mouawia H, Broncy L et al. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta 2016;37:56–60. 10.1016/j.placenta.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Falcidia E, Parano E, Grillo A, Pavone P, Takabayashi H, Trifiletti RR et al. Fetal cells in maternal blood: a six-fold increase in women who have undergone amniocentesis and carry a fetus with Down syndrome: a multicenter study. Neuropediatrics 2004;35(6):321–4. 10.1055/s-2004-830365. [DOI] [PubMed] [Google Scholar]
- 56.Parano E, Falcidia E, Grillo A, Takabayashi H, Trifiletti RR, Pavone P. Fetal nucleated red blood cell counts in peripheral blood of mothers bearing Down syndrome fetus. Neuropediatrics 2001;32(3):147–9. 10.1055/s-2001-16612. [DOI] [PubMed] [Google Scholar]