Abstract
Purpose of review:
To review the recent findings that small ‘drug-like’ compounds block disease-specific HLA molecules in type 1 diabetes (T1D).
Recent findings:
The predominant genetic risk for developing T1D, the immune mediated form of diabetes, is conferred through human leukocyte antigen (HLA) genes. One such gene, termed HLA-DQ8, is present in 50–60% of patients with T1D and those at-risk. DQ8 presents disease-relevant peptides to T cells, which mediate tissue specific destruction of pancreatic islets. Using a structure-based approach to evaluate the ‘druggability’ of the DQ8 molecule, methyldopa, a clinically well-established oral anti-hypertensive agent, was discovered to bind DQ8. Methyldopa blocked the activation of DQ8 specific T cells responding to self-antigens such as insulin but not influenza. In a proof-of-concept clinical trial (NCT01883804), methyldopa was administered to recent-onset T1D patients with the DQ8 gene that confirmed the mechanism of action and diminished inflammatory T cell responses towards insulin.
Summary:
Methyldopa blocks the diabetes-specific function of HLA-DQ8, which represents a personalized medicine approach to treat the underlying autoimmunity in T1D. Clinical trials are warranted and underway to evaluate methyldopa in potentially preserving residual beta-cell function in those with new-onset and at-risk for T1D.
Keywords: Type 1 Diabetes, Immunotherapy, Methyldopa, T cells, HLA
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease that results in the loss of insulin producing beta cells within pancreatic islets (1–3). There is a significant amount of understanding regarding the natural history of T1D development, which comes from prospective birth cohort studies following at-risk children, those with a first degree relative with T1D or high risk human leukocyte antigen (HLA) genes, to the development of islet autoimmunity and eventual clinical T1D onset (4). From the Diabetes Autoimmunity Study in the Young (DAISY) (5), Type 1 Diabetes Prediction and Prevention Study (DIPP) (6), and BABYDIAB study (7), it is now appreciated that T1D is predictable with the measurement of serum islet autoantibodies directed against insulin, glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2), and zinc transporter 8 (ZnT8). The presence of two or more autoantibodies confers ~85% risk of developing diabetes within 15 years and nearly 100% over the lifetime of an at-risk individual (8). With the ability to accurately assess T1D risk, large randomized clinical trials have been completed to delay and prevent disease onset (9, 10). Unfortunately, none have been successful and T1D prevention has proven more difficult than originally thought (11). In our opinion, personalized therapies directed against specific molecular targets involved in disease pathogenesis are needed to improve prevention efforts including preserving residual beta cell function in new-onset T1D patients. In this review, we focus on recent findings that small ‘drug-like’ molecules can specifically block the function of T1D-risk HLA molecules.
HLA-DQ8 as a molecular target in type 1 diabetes
HLA genes confer significant genetic risk for T1D development, especially the class II genes DQ and DR (and to a lesser extent DP) (12). It is by T1D risk HLA DQ and DR genes that subjects have been selected for birth cohort studies including The Environmental Determinants of Diabetes in Youth (TEDDY), an ongoing multicenter international study (13). HLA class II genes encode major histocompatibility complex (MHC) class II proteins expressed on B cells, dendritic cells and macrophages collectively termed antigen presenting cells. MHC proteins function to present peptide from processed proteins to CD4 T lymphocytes, thus forming a trimolecular complex: MHC class II – peptide – T cell receptor (14, 15). In the case of autoimmunity, self-proteins are taken up by antigen presenting cells then presented as peptide/MHC complexes on the cell surface that can activate self-reactive T cells to target a given tissue, e.g. pancreatic islets in T1D (16, 17). In T1D, approximately 50–60% of all patients have the HLA-DR4/DQ8 haplotype with the DQ8 gene providing an odds ratio for disease development of 6.5–11 (12, 18, 19). This is in contrast to DQ6 (DQB1*06:02), which confers an odds ratio of only 0.03 for T1D development (12, 20). The stark contrast in risk highlights the importance of DQ8 in T1D development and DQ6 in providing dominant protection. Furthermore, DQ8 has been shown to present peptides of (pro)insulin to activate CD4 T cells isolated from the remaining islets of recent-onset T1D organ donors, implicating DQ8 responding T cells in T1D pathogenesis (21–24). The evidence that DQ8 is (1) common in T1D, (2) confers significant genetic risk, and (3) is involved in disease pathogenesis, makes the DQ8 molecule an attractive therapeutic target to treat the underlying autoimmunity in T1D.
Structure-based approach to select DQ8 binding small molecules
Analyzing the crystal structure of an insulin peptide bound to DQ8 revealed that the peptide formed hydrogen bonds to DQ8 within four structural pockets (P1, P4, P6 and P9) in the peptide-binding groove (25). These structural pockets have the appropriate chemistry and geometry to bind small molecules (molecular weight <500 Daltons), but are very unlikely to be contact sites for monoclonal antibodies (26). Although anti-HLA class II antibodies exist, e.g. those that bind either pan-DQ, DR, or DP molecules, it is difficult to generate allele-specific antibodies as key amino acid differences that distinguish closely related molecules are at sites within the peptide-binding groove that are unreachable by antibodies. Monoclonal antibodies for peptide/MHC molecules have been generated (27–29), but are only specific for single epitopes, while there can be immune responses to many different self-antigens in T1D (30). As such, we favored a small molecule approach targeting pockets within the DQ8 peptide-binding groove (31).
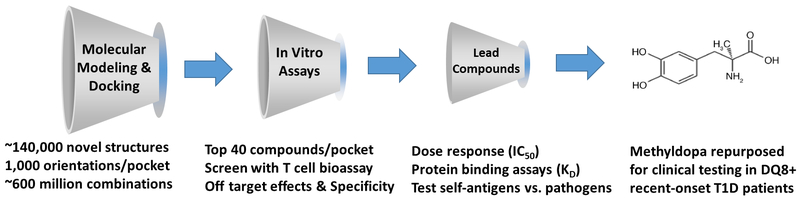
We rationalized that drug binding to DQ8 within structural pockets in the peptide-binding groove would block peptide binding and subsequent T cell activation. Therefore, we used a supercomputer to perform molecular modeling and docking to screen the P1, P4, P6 and P9 pockets using a library of ~140,000 small molecules (Fig. 1) (32). Each compound in the repository was docked in 1000 orientations within a single pocket and scored based on the estimated free energy of binding. The top 40 scoring compounds were then tested with an in vitro T cell bioassay (31). A T cell readout was utilized in the initial screening assay, as opposed to peptide binding, as effector T cells mediated tissue-specific damage in T1D and other autoimmune diseases. Remarkably ~75% of the tested compounds blocked an insulin specific T cell response, especially those compounds targeting the middle of the peptide-binding groove, P4 and P6 (31). Compounds with structural similarity were further evaluated for specificity and ‘off-target’ effects. One such identified compound, TATD, was able to block both DQ8 and the structurally similar MHC class II molecule (I-Ag7) in the nonobese diabetic (NOD) mouse model of spontaneous autoimmune diabetes (33). TATD was administered to NOD mice in preclinical studies and able to prevent diabetes onset, block the formation of insulin autoantibodies, lessen insulitis and maintain normal blood glucose levels when administered at later stages of diabetes development.
Figure 1.
Structure-based approach to identify small ‘drug-like’ compounds binding to the type 1 diabetes risk HLA-DQ8 molecule. Using the crystal structure of DQ8, molecular modeling and docking was performed with a library of small molecules into each of the four pockets (P1, P4, P6, and P9) in the peptide-binding groove. Free energy of binding was estimated and the top 40 scoring compounds were further tested with an in vitro T cell bioassay. Lead compounds were selected based upon off target, specificity, and in vivo testing. Methyldopa (Aldomet), which is an oral FDA approved drug to treat hypertension in both children and adults, was identified through this approach. Methyldopa was repurposed and used to treat recent-onset T1D patients with DQ8 in a proof-of-mechanism clinical trial.
Methyldopa as a specific DQ8 blocker
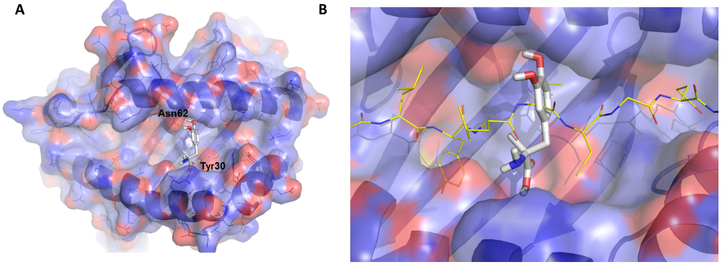
Having success identifying DQ8 blocking compounds and altering diabetes onset in a preclinical animal model, we then asked whether a known drug could be repurposed to block DQ8. Using the approach outlined in figure 1, we performed molecular modeling and docking of FDA approved drugs to bind within pockets in the DQ8 peptide-binding groove (31). By screening the top ‘hits’, we discovered methyldopa (Aldomet), a clinically well-established oral antihypertensive drug used to treat both children and adults (34), specifically blocked DQ8. Further testing revealed methyldopa bound DQ8 in the middle of the peptide-binding groove with a dissociation constant, KD ≈ 26 μM (Fig. 2A) (31). A model of insulin peptide binding to methyldopa/DQ8 indicates that there are significant interactions between the drug and peptide binding to DQ8 (Fig. 2B). Functional testing of T cells showed that methyldopa blocked both insulin (T1D) and gliadin (Celiac disease) reactive T cells restricted to DQ8 (35, 36), but those T cells restricted to peptides presented by other MHC class II molecules (e.g. DR4) were not inhibited. These data indicate that methyldopa is specific for DQ8 and blocks binding to a number of peptides and subsequent T cell responses. Interestingly, methyldopa blocked self-antigen T cell responses to DQ8 but not responses to a high affinity immunodominant peptide from influenza presented by DQ8. These data suggest that cytotoxic T cells may protect from influenza virus infection during methyldopa treatment, which blocks interactions between lower affinity self-peptides to DQ8.
Figure 2.
Molecular models of methyldopa in the DQ8 peptide-binding groove. (A) Methyldopa is shown within pocket 6 of DQ8. There are hydrogen bonds between methyldopa and asparagine (ASN) at position 62 of the DQ8 alpha chain and tyrosine (Tyr) at position 30 of the DQ8 beta chain. These hydrogen bonds were confirmed in structure activity relationship testing using modified methyldopa compounds. (B) Methyldopa in the DQ8 peptide-binding groove clashing with the binding of an insulin peptide. Images were generated using PYMOL software.
Since methyldopa did not cross-react with the NOD mouse MHC class II molecule, in vivo studies were conducted using DQ8 transgenic mice. These studies indicated that orally dosed methyldopa engaged DQ8 and blocked the ability to activate T cells in a dose dependent manner (31). Furthermore, frequency of dosing was critical for an in vivo effect as the half-life of methyldopa is only 2-hours. As methyldopa has been used clinically for over 50 years, the safety profile is well characterized and is on the World Health Organization’s list of Essential Medicines, making it an attractive drug candidate to repurpose as a personalized disease modifying therapy in T1D patients with the DQ8 gene.
Methyldopa treatment in recent-onset type 1 diabetes
We translated our preclinical findings into an open-label phase 1b dose escalation trial treating recent-onset T1D patients with the DQ8 gene having residual beta cell function (NCT01883804). Twenty DQ8 positive T1D participants (median age 22 years, median T1D duration 90 days, and all positive for at least one islet autoantibody) received methyldopa at three separate dosages titrated over 6-weeks to prevent hypotension and correlate different dosages to DQ8 blocking (31). Methyldopa was well tolerated, did not lower systolic or diastolic blood pressure, and there were no serious adverse events. Treatment resulted in the specific inhibition of DQ8 presentation by peripheral blood mononuclear cells (PBMCs). The assay used to measure DQ8 function utilized cryopreserved PBMCs from each participant over time as antigen presenting cells to stimulate an engineered T cell, T cell receptor transductant (37), responding to a specific peptide presented by an MHC class II molecule (DQ8, DR4, or DQ2). DQ8 was blocked in 17/20 (85%) participants compared to baseline levels and there was no change in DR4 and DQ2 presentation, indicating that oral methyldopa treatment is indeed specific for blocking DQ8. Six weeks after stopping treatment, DQ8 function returned to baseline levels showing that the effects of methyldopa are reversible (31).
We also evaluated the ability of methyldopa to decrease primary insulin-specific T cell responses in treated patients, as opposed to the effects on antigen presenting cells described above. In those individuals with detectable insulin-specific T cell responses restricted to DQ8 at baseline, as measured by an IFN-ƴ ELISPOT assay (38), all of the responses decreased during the course of the 12-week study (31). Importantly, the studied insulin peptide is presented by DQ8 to activate islet infiltrating CD4 T cells obtained from the pancreatic islets of recent-onset T1D organ donors (21). As a control, T cell responses directed towards tetanus toxin, a common vaccine, and peptides within tetanus toxin presented by DR4 remained unaffected. This indicates that methyldopa treatment reduced inflammatory insulin-specific T cell responses while leaving responses to a commonly received vaccine intact.
Metabolic measurements among the participants included hemoglobin A1c (HbA1c), insulin use/Kg of body weight, and C-peptide area under the curve (AUC) following a mixed meal tolerance test conducted at baseline and end of the study (3 months). Overall, there was excellent blood glucose control at study completion with an average HbA1c of 6.5% without a significant change in self-administered insulin. C-peptide AUC, which is a measure of residual beta cell function, was preserved over the 3-month study (31). These results indicate that methyldopa may preserve residual beta cell function; however, longer treatment in placebo-controlled trials are needed to fully evaluate the metabolic effects.
With the clinical proof-of-concept in place that methyldopa specifically blocks DQ8, lessens inflammatory T cell responses towards insulin, and may limit loss of residual beta cell function, longer-term placebo controlled trials are warranted to evaluate the ability of methyldopa to modify the disease course in those with new-onset and at-risk for T1D. As such, a multicenter randomized, double-blinded, placebo-controlled crossover trial, ‘Methyldopa for reduction of DQ8 antigen presentation in at-risk subjects for type 1 diabetes mellitus’ (NCT03396484), is being planned by the NIH sponsored Type 1 Diabetes TrialNet clinical trials network. This mechanistic study aims to assess the safety, mechanism of action (DQ8 blockade), and immunologic effects of methyldopa in DQ8 positive patients at-risk for T1D.
HLA as a target to treat autoimmune diseases
In addition to T1D, HLA class II genes confer significant risk for many autoimmune diseases including Celiac disease (gluten sensitivity), rheumatoid arthritis, multiple sclerosis, narcolepsy, and autoimmune thyroid disease (39, 40). HLA-DQ2 is present in ~90% of those with Celiac disease, while DQ8 is present in the remaining 10% of patients (41). Narcolepsy only develops in those with DQ6 (DQB*06:02) (42). Specific DR4 subtypes confer risk for rheumatoid arthritis (43); DR2 (now named DR15) is present in 50–60% of patients with multiple sclerosis (44). In many of these disorders, there is understanding of how self-peptides are presented by HLA class II molecules to activate T cells. In Celiac disease, gliadin peptides are deamidated (Glutamine → Glutamic acid) by tissue transglutaminase, which then bind DQ2 and/or DQ8 with high affinity to activate T cells (36). Specific DR4 subtypes present post-translationally citrullinated antigens to self-reactive CD4 T cells in rheumatoid arthritis (45).
From these studies, it is appreciated that many different HLA class II genes predispose risk towards different autoimmune diseases, indicating a disease specific HLA genes does not produce a globally abnormal MHC class II molecule, rather the disease associated MHC class II molecule favors production of self-antigen specific T cells that leads to tissue destruction. These insights render HLA molecules attractive therapeutic targets not only for T1D but for many autoimmune diseases. Notably, others have described small molecule compounds that bind DR3, which is common in autoimmune thyroid disease. Using a viritual screening approach, a compound (cepharanthine), was identified to have in vitro effects on blocking thyroglobulin peptide binding and in vivo activity using transgenic animal models (46). Similarly, DR2 binding small molecules have been described in the context of multiple sclerosis with effects both in vitro and in preclinical animal models (47).
There are potential concerns with blocking specific MHC class II molecules in autoimmunity. First, there may be negative effects on immune system function by creating a hole in the immune repertoire, i.e. blocking T cells specific for a given MHC class II molecule. However, this is unlikely given that there are other class II molecules available to process and present peptides to T cells. In the case of methyldopa, DQ8 is blocked but there are DR and DP molecules available for normal immune system functioning. Furthermore, we showed that methyldopa blocks insulin-specific T cell responses restricted to DQ8 but not those of tetanus toxin, a common vaccination. Second, drugs targeting MHC molecules may trigger unwanted immune responses, such as hypersensitivity syndromes by changing the peptide repertoire that can be presented by a given MHC molecule (48). T cell mediated hypersensitivity has been described for the anti-retroviral drug, abacavir, binding to the class I molecule HLA-B57 (49, 50). However, this drug binds with a strong affinity in the low nanomolar range (~0.2nM) (51), compared to the affinity for methyldopa to DQ8 in the low micromolar range (~26μM), which is 100-fold less. By repurposing existing drugs, the safety-profile and off-target effects are well-characterized. As methyldopa has over 50 years of clinical use and is indicated for the treatment of pregnancy induced hypertension, this concern is lessened and methyldopa should be considered safe.
Conclusions
HLA genes provide significant genetic risk for the development of autoimmune diseases. For T1D, HLA-DQ8 confers this genetic risk, is common in the patient population, and involved in disease pathogenesis by activating islet-resident T cells. Using a rationale structure-based approach, we identified methyldopa as a drug capable of binding DQ8 and blocking self-antigen T cell responses. With a clinical proof-of-mechanism in place, methyldopa represents personalized medicine with the potential to modify the disease course and prevent T1D. Furthermore, there is broad applicability to targeting HLA molecules with safe and specific small drug-like compounds to develop disease-modifying therapies for the treatment of many autoimmune disorders.
Key Points.
The HLA-DQ8 gene confers significant risk for developing type 1 diabetes and is present in 50–60% of all patients.
The HLA-DQ8 molecule is remarkably amenable to binding small molecules within pockets in the peptide-binding groove.
A clinically well-established small molecule drug, Methyldopa, blocks DQ8 binding to disease-specific antigens in type 1 diabetes
Acknowledgements
We thank the late George S. Eisenbarth (1942–2012) for helpful scientific discussions, inspiration, and encouragement.
Financial support and sponsorship
This work was supported by NIH Grants (DK095995, DK108868, DK110845, DK032083); the Juvenile Diabetes Research Foundation (17–2010-744, 2014–143-C-R); the Children’s Diabetes Foundation; and the Barbara Davis Center Translational Research Unit.
Footnotes
Conflicts of Interest
A.W. Michels and D.A. Ostrov are inventors on a patent, Compounds That Modulate Autoimmunity and Methods of Using the Same, licensed to ImmunoMolecular Therapeutics. A.W. Michels and P.A. Gottlieb are scientific cofounders of ImmunoMolecular Therapeutics and own shares in the company.
References
■ of special interest
■■ of outstanding interest
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Excellent review on the current understanding of type 1 diabetes and potential future directions for research and clinical care.
- 3.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonifacio E Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015;38(6):989–96. [DOI] [PubMed] [Google Scholar]
- 5.Barker JM, Barriga KJ, Yu L, Miao D, Erlich HA, Norris JM, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab. 2004;89(8):3896–902. [DOI] [PubMed] [Google Scholar]
- 6.Kupila A, Keskinen P, Simell T, Erkkila S, Arvilommi P, Korhonen S, et al. Genetic risk determines the emergence of diabetes-associated autoantibodies in young children. Diabetes. 2002;51(3):646–51. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48(3):460–8. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skyler JS. Prevention and reversal of type 1 diabetes--past challenges and future opportunities. Diabetes Care. 2015;38(6):997–1007. [DOI] [PubMed] [Google Scholar]
- 10.Simmons KM, Gottlieb PA, Michels AW. Immune Intervention and Preservation of Pancreatic Beta Cell Function in Type 1 Diabetes. Curr Diab Rep 2016;16(10):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michels AW, Gottlieb PA. Learning From Past Failures of Oral Insulin Trials. Diabetes. 2018;67(7):1211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Perspective on the clinical trials using oral insulin to prevent type 1 diabetes and the need for personalized medicine therapies to modify the disease course in type 1 diabetes.
- 12.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark A, Hagopian WA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michels AW. Targeting the trimolecular complex. Clin Immunol 2013;149(3):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michels AW. Targeting the trimolecular complex: the pathway towards type 1 diabetes prevention. Diabetes Technol Ther 2013;15 Suppl 2:S2-8–s2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugliese A Autoreactive T cells in type 1 diabetes. J Clin Invest 2017;127(8):2881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Outstanding review on self-reactive T cells and their role in type 1 diabetes pathogenesis.
- 17.Nakayama M, Simmons KM, Michels AW. Molecular Interactions Governing Autoantigen Presentation in Type 1 Diabetes. Curr Diab Rep 2015;15(12):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM, et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet 2015;47(8):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360(16):1646–54. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese A, Boulware D, Yu L, Babu S, Steck AK, Becker D, et al. HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 Haplotype Protects Autoantibody-Positive Relatives From Type 1 Diabetes Throughout the Stages of Disease Progression. Diabetes. 2016;65(4):1109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels AW, Landry LG, McDaniel KA, Yu L, Campbell-Thompson M, Kwok WW, et al. Islet-Derived CD4 T Cells Targeting Proinsulin in Human Autoimmune Diabetes. Diabetes. 2017;66(3):722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This manuscript describes CD4 T cells obtained from the pancreatic islets of recent-onset type 1 diabetes organ donors being activated by proinsulin peptides presented by DQ8 or DQ8-trans molecules.
- 22.Pathiraja V, Kuehlich JP, Campbell PD, Krishnamurthy B, Loudovaris T, Coates PT, et al. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes. 2015;64(1):172–82. [DOI] [PubMed] [Google Scholar]
- 23.Babon JA, DeNicola ME, Blodgett DM, Crevecoeur I, Buttrick TS, Maehr R, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 2016;22(12):1482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent SC, Mannering SI, Michels AW, Babon JAB. Deciphering the Pathogenesis of Human Type 1 Diabetes (T1D) by Interrogating T Cells from the “Scene of the Crime”. Curr Diab Rep 2017;17(10):95. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Outstanding review on the progress made to characterize T cell reactivity from the inflamed pancreatic islets of type 1 diabetes organ donors.
- 25.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol 2001;2(6):501–7. [DOI] [PubMed] [Google Scholar]
- 26.Schmidtke P, Bidon-Chanal A, Luque FJ, Barril X. MDpocket: open-source cavity detection and characterization on molecular dynamics trajectories. Bioinformatics. 2011;27(23):3276–85. [DOI] [PubMed] [Google Scholar]
- 27.Spanier JA, Frederick DR, Taylor JJ, Heffernan JR, Kotov DI, Martinov T, et al. Efficient generation of monoclonal antibodies against peptide in the context of MHCII using magnetic enrichment. Nature communications. 2016;7:11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Crawford F, Yu L, Michels A, Nakayama M, Davidson HW, et al. Monoclonal antibody blocking the recognition of an insulin peptide-MHC complex modulates type 1 diabetes. Proc Natl Acad Sci U S A. 2014;111(7):2656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahan R, Gebe JA, Preisinger A, James EA, Tendler M, Nepom GT, et al. Antigen-specific immunomodulation for type 1 diabetes by novel recombinant antibodies directed against diabetes-associates auto-reactive T cell epitope. J Autoimmun. 2013;47:83–93. [DOI] [PubMed] [Google Scholar]
- 30.Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med 2012;2(4):a007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrov DA, Alkanani A, McDaniel KA, Case S, Baschal EE, Pyle L, et al. Methyldopa blocks MHC class II binding to disease-specific antigens in autoimmune diabetes. J Clin Invest 2018;128(5):1888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This is the original report of identifying and characterizing small ‘drug-like’ molecules binding to HLA-DQ8 including the proof-of-concept trial using methyldopa in recent-onset type 1 diabetes patients with the DQ8 gene.
- 32.Shoichet BK, McGovern SL, Wei B, Irwin JJ. Lead discovery using molecular docking. Curr Opin Chem Biol 2002;6(4):439–46. [DOI] [PubMed] [Google Scholar]
- 33.Michels AW, Ostrov DA, Zhang L, Nakayama M, Fuse M, McDaniel K, et al. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J Immunol 2011;187(11):5921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mah GT, Tejani AM, Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev 2009(4):Cd003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eerligh P, van Lummel M, Zaldumbide A, Moustakas AK, Duinkerken G, Bondinas G, et al. Functional consequences of HLA-DQ8 homozygosity versus heterozygosity for islet autoimmunity in type 1 diabetes. Genes Immun 2011;12(6):415–27. [DOI] [PubMed] [Google Scholar]
- 36.Tollefsen S, Arentz-Hansen H, Fleckenstein B, Molberg O, Raki M, Kwok WW, et al. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest 2006;116(8):2226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams T, Krovi HS, Landry LG, Crawford F, Jin N, Hohenstein A, et al. Development of T cell lines sensitive to antigen stimulation. J Immunol Methods. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama M, McDaniel K, Fitzgerald-Miller L, Kiekhaefer C, Snell-Bergeon JK, Davidson HW, et al. Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proc Natl Acad Sci U S A. 2015;112(14):4429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol 2018;18(5):325–39. [DOI] [PubMed] [Google Scholar]; ■ Excellent review focused on HLA molecules and their role in autoimmune diseases.
- 40.Tsai S, Santamaria P. MHC Class II Polymorphisms, Autoreactive T-Cells, and Autoimmunity. Front Immunol 2013;4:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green PH, Cellier C. Celiac disease. N Engl J Med 2007;357(17):1731–43. [DOI] [PubMed] [Google Scholar]
- 42.Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet 2009;41(6):708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet 2012;44(3):291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt H, Williamson D, Ashley-Koch A. HLA-DR15 haplotype and multiple sclerosis: a HuGE review. Am J Epidemiol 2007;165(10):1097–109. [DOI] [PubMed] [Google Scholar]
- 45.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med 2013;210(12):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li CW, Menconi F, Osman R, Mezei M, Jacobson EM, Concepcion E, et al. Identifying a Small Molecule Blocking Antigen Presentation in Autoimmune Thyroiditis. J Biol Chem 2016;291(8):4079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji N, Somanaboeina A, Dixit A, Kawamura K, Hayward NJ, Self C, et al. Small molecule inhibitor of antigen binding and presentation by HLA-DR2b as a therapeutic strategy for the treatment of multiple sclerosis. J Immunol 2013;191(10):5074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michels AW, Ostrov DA. New approaches for predicting T cell-mediated drug reactions: A role for inducible and potentially preventable autoimmunity. J Allergy Clin Immunol 2015;136(2):252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109(25):9959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486(7404):554–8. [DOI] [PubMed] [Google Scholar]
- 51.Pompeu YA, Stewart JD, Mallal S, Phillips E, Peters B, Ostrov DA. The structural basis of HLA-associated drug hypersensitivity syndromes. Immunol Rev 2012;250(1):158–66. [DOI] [PubMed] [Google Scholar]