Abstract
Introduction
We characterized tobacco use, cessation patterns, and patient satisfaction with a cessation support program at an NCI Designated Comprehensive Cancer Center following a mandatory tobacco assessment and automatic referral.
Methods:
A 3-month follow-up survey (via web, paper, or telephone) was administered between March 2013 and November 2013 for all patients referred to and contacted by a cessation support service, and who consented to participation three months prior to administration. Patients were asked about their perceived importance and self-efficacy to quit smoking, quit attempts, and satisfaction with the cessation service.
Results:
Fifty-two percent (257/499) of patients who participated in the cessation support service, and consented to be contacted again, completed a follow-up survey. Of those who participated, 9.7% were referred to the service as having recently quit tobacco (in the past 30 days) and 23.6% reported having quit at the time of first contact. At the 3-month follow-up, 48.1% reported being smoke-free for the previous seven days. When patients were asked about their experience with the cessation service, 86.4% reported being very or mostly satisfied with the service, and 64.3% reported that their experience with the service increased their satisfaction with the care received at the cancer centre.
Conclusions:
Our findings suggest that recently diagnosed cancer patients are aware that quitting tobacco is important, are making attempts to quit, and are amenable to an opt-out automatic referral cessation support service as part of their cancer care.
Introduction
Despite being the most important risk factor for the development of cancer, tobacco use is rarely assessed after a cancer diagnosis (Gritz, Dresler, & Sarna, 2005; Toll et al., 2013; Warren, Alberg, Kraft, & Cummings, 2014). Tobacco use frequently remains high among patients after a diagnosis with cancer, even in those cancers that are smoking related (Cataldo, Dubey, & Prochaska, 2010; Duffy, Louzon, & Gritz, 2012). Few cancer centres offer comprehensive evidence-based cessation support services for patients (Toll et al., 2013; Warren, Alberg, et al., 2014). The health-care setting, especially in oncology, is a readily available, but vastly underutilised, setting for systematic tobacco use assessment and comprehensive cessation support (Boyle, Solberg, & Fiore, 2011; Gritz et al., 2005). Inadequate clinician training for providing cessation support and structure of the clinical setting are two examples of barriers to widespread integration of evidence-based cessation support into cancer patient care (Warren, Marshall, Cummings, Toll, Gritz, Hutson, Dibaj, Herbst, & Dresler, 2013a; Warren, Marshall, Cummings, Toll, Gritz, Hutson, Dibaj, Herbst, Mulshine, et al., 2013b; Weaver et al., 2012).
The American Society for Clinical Oncology (ASCO) has emphasised the need for standardised tobacco use assessments and evidence-based cessation support to become integrated into standard clinical practice (Hanna, Mulshine, Wollins, Tyne, & Dresler, 2013). A cancer diagnosis presents a unique opportunity as a teachable moment with the potential to improve patient receptivity to smoking cessation messages (Duffy et al., 2012; Gritz et al., 2006). Despite the belief that cancer patients are not interested in smoking cessation, or that smoking cessation after a cancer diagnosis is useless, literature suggests that cancer patients have a high level of interest, so it is important that they are not overlooked (Gritz, 2000; Gritz et al., 2006; Warren, Marshall, et al., 2014). The availability and use of cessation support, including integrating steps from the 5A’s of smoking cessation (Ask, Advise, Assess, Assist, and Arrange) from the U.S. Public Health Service Clinical Practice Guidelines has been shown to improve cessation efforts and increase patient satisfaction with the health care they receive (Conroy et al., 2005; Fiore et al., 2008).
The main objective of this program evaluation is to assess smoking cessation outcomes among patients at an NCI designated national comprehensive cancer centre who are current or recent tobacco users (within the past 30 days), referred to a dedicated cessation support service and are successfully contacted at least once by the cessation service. Four specific research objectives for this study include characterizing: (1) the participation rates for the 3-month follow-up survey, (2) the quit rates after 3-months of contact by the cessation service, (3) factors associated with quitting, and (4) patient satisfaction with the cessation service.
Methods
The cessation support service began providing cessation assistance to thoracic centre patients in the fall of 2011 and expanded to screening and referring all tobacco using patients at RPCI in July 2013. The electronic medical record (EMR) is utilised to provide mandatory tobacco use assessments with standardised questions and automatic electronic referrals to the dedicated cessation service. Nurses complete the tobacco use assessment as part of each intake interview, and approximately every 30 days at subsequent visits. All patients who report current or recent tobacco use (within the last 30 days) in a tobacco related or high-risk clinic (i.e. thoracic [including lung cancer screening], head and neck, bone marrow transplant, haematology/oncology, genitourinary, or undiag-nosed) are automatically referred to the cessation service at any stage of their treatment.
A cessation specialist attempts to interview referred patients by telephone to evaluate their readiness to quit and to assist them in making individual plans for quitting or staying tobacco free. Strategies used by the specialists to promote cessation include coaching patients in ways to reduce behaviours that promote tobacco use, implementing strategies for dealing with urges to smoke, and obtaining pharmacologic support if desired. During the initial contact, the tobacco cessation specialist asked patients for permission to be contacted in the future with opportunities to participate in other activities that will help improve the program. The cessation specialist follows up with patients to provide additional support and tips as they go through the quit process, unless the patient specifies that the cessation service not contact them again. Detailed descriptions of referral questions and patterns, as well as the design of the cessation support service have been described elsewhere (Warren et al., 2014b).
Patients may participate in anywhere from one to eight cessation support calls, which may occur weekly, bi-weekly, monthly or at a greater interval, depending on the preference of the patient rather than a set schedule. Patients participating in this follow-up survey completed an average of 4.47 counselling calls (standard deviation= 2.18; range: 1–8), with an average of 60.33 days between referral and the first successful contact by cessation support service (standard deviation = 125.01 days).
A 3-month follow-up survey was conducted between March 2013 and November 2013 in order to assess short-term self-reported quit rates for patients referred to, contacted by, and consented by the cessation support service three months prior to survey administration. Consent to be contacted in the future may have been obtained on a follow-up call; these patients were eligible to participate in the service, but resulted in variable follow-up time. 61% of patients consented on the first counselling call, 15% at the first follow-up, and 24% at subsequent contacts (mean = 1.91 calls, median = 1 call, range: 1–8 calls). Cessation support service participants were mailed an invitation letter to complete the follow-up survey either by mail or online approximately 3-months after their first contact by the cessation service. The letter included instructions and a unique user ID and password. The mailing also included a paper copy of the survey and a prepaid envelope for returning the completed survey. Participants were informed that the survey would require about 10–15 minutes of their time and that their participation is voluntary. Two weeks after the follow-up survey was mailed, non-responders were followed-up to attempt to complete the survey by telephone; up to 15 call attempts were made in order to reach participants. The average time between survey completion and consent, which was not necessarily the first cessation support call, was 113.71 days (standard deviation = 27.49 days; range: 67–208 days).
Patients eligible for inclusion in the 3-month follow-up were adults (age 18+) referred to the cessation support service as current or recent (within the past 30 days) tobacco users, participated in at least once by the cessation support telephone call, and gave permission to be contacted again in the future with opportunities to help improve the program. Patients were not compensated for survey participation.
Data were obtained from the EMR and the 3-month follow-up survey. As an indicator of disease severity, Eastern Cooperative Oncology Group performance score (ECOG PS) was extracted from the EMR and dichotomised as 0 (no limitations) versus ≥ 1 (any health limitation resulting from disease) (Oken et al., 1982). The denominator for certain survey items varies depending upon missing data and incomplete surveys.
Patients were considered to be quit if they self-reported being smoke-free for the seven consecutive days prior to completing the 3-month follow-up survey; however, self-reported quit for the previous 24 hours and 30 days prior to survey completion were also assessed. Participants were asked to indicate their perceived importance of quitting, perceived self-efficacy to quit, intentions to quit in the next 30 days, and quit attempts made in the last 3 months that lasted 24 hours or 7 days; response categories are noted in the figures. Patients were also asked to indicate how satisfied they were with the cessation service, if their needs had been met, and how their experience with the cessation service changed their satisfaction with the care received. The RPCI IRB approved this study.
Descriptive statistics were used to characterise the participation rates, quit rates, and responses to the patient satisfaction questions. Bivariate analyses were used to explore associations between participation rates, quit rates, and responses to patient satisfaction questions with demographic, health behaviour and smoking-related potential confounders. To explore differences in cessation outcomes between clinics, data were stratified on clinic referring to the cessation support service: thoracic, head and neck, and other referring clinics (ambulatory, bone marrow transplant, breast surgery, colorectal, dermatology, gynaecology, leukaemia, lymphoma, neurology, pain, radiation therapy, undiagnosed, upper gastrointestinal, and urology centres).
Results
Participation Rates for the 3-Month Follow-up Survey
Between March 2013 and November 2013, a total of 499 surveys were mailed to patients referred to and contacted at least once by the cessation support service, and who also gave consent to be contacted in the future with opportunities to help improve the program three months prior to survey administration. Of note, during the consenting period, only 4.1% (n = 22/532) declined future participation in program improvement opportunities. Eleven of the 510 eligible participants were not mailed surveys. 51.5% (n = 257/499) of patients participated in the 3-month follow-up survey (Table 1). 59.1% (n = 152/257) of participants completed the survey via telephone, 38.9% (n = 100/257) were completed by mail, and 1.9% (n = 5/257) were completed on the web. Among the 242 non-responders, 0.4% (n 1/242) refused the first question and left all subsequent=questions blank, 8.7% (n = 21/242) were deceased, 21.9% (n = 53/242) refused, and 69.0% (n = 167/242) could not be reached for the entire study duration. Not all participants responded to every question, leading to varying denominators for questions in each section of the results.
Table 1.
Characteristics of participants in the 3-month follow-up survey compared to non-participants (n = 499)
| Participation in 3-Month Follow-up | |||||
|---|---|---|---|---|---|
| Participant (n = 257) | Non-participant (n = 242) | ||||
| Characteristics | Mean | Std. Dev. | Mean | Std. Dev. | p-Value |
| Age | 59.4 | 10.9 | 55.8 | 12.8 | 0.001* |
| Pack-years | 12.1 | 16.9 | 12.7 | 13.4 | 0.666 |
| Counselling call consent was obtained | 2.0 | 1.5 | 1.8 | 1.5 | 0.311 |
| Completed counselling calls | 4.5 | 2.2 | 3.5 | 2.0 | <0.001* |
| N | % | N | % | p-value | |
| Sex | |||||
| Female | 132 | 55.0 | 108 | 45.0 | 0.132 |
| Male | 125 | 48.3 | 134 | 51.7 | |
| Race | |||||
| Caucasian | 211 | 51.7 | 197 | 48.3 | 0.840 |
| Other | 46 | 50.5 | 45 | 49.5 | |
| Ethnicity | |||||
| Non-Hispanic | 242 | 50.7 | 235 | 49.3 | 0.109 |
| Other | 15 | 68.2 | 7 | 31.8 | |
| Marital status | |||||
| Single | 81 | 43.3 | 106 | 56.7 | 0.001* |
| Married | 130 | 61.0 | 83 | 39.0 | |
| Other | 46 | 46.5 | 53 | 53.5 | |
| Referring clinica | |||||
| Thoracic | 104 | 52.3 | 95 | 47.7 | 0.963 |
| Head & neck | 52 | 51.0 | 50 | 49.0 | |
| Other | 101 | 51.0 | 97 | 49.0 | |
| ECOG PS | |||||
| 0 | 74 | 52.1 | 68 | 47.9 | 0.864 |
| ≥1 | 183 | 51.3 | 174 | 48.7 | |
| Quit status at referral | |||||
| Current | 232 | 51.8 | 216 | 48.2 | 0.708 |
| Quit | 25 | 49.0 | 26 | 51.0 | |
| Quit at first contact by cessation support service | |||||
| Current | 197 | 51.4 | 186 | 48.6 | 0.957 |
| Quit | 60 | 51.7 | 56 | 48.3 | |
Statistically significant at p<0.05.
Other clinic includes: Ambulatory referred, bone marrow transplant, breast surgery, colorectal, dermatology, gynaecology, leukaemia, lymphoma, neurology, pain, radiation therapy, undiagnosed, upper GI, and urology.
Participants were generally similar to patients who did not participate in the 3-month follow-up survey. As shown in Table 1, participants differed from non-participants on age and marital status. Participants also participated in approximately one more counselling call than non-participants did (4.5 calls vs. 3.5 calls, respectively; p<0.001). Among participants, 40.5% (n = 104/257) were referred to the cessation support service from the thoracic centre, 20.2% (n = 52/257) from the head and neck centre, and 39.3% (n = 101/257) from other clinics (bone marrow transplant, colorectal, dermatology, leukaemia, lymphoma, neurology, pain, radiation therapy, undiagnosed, upper gastrointestinal, and urology clinics).
Quit Rates Three Months after Initial Contact by the Cessation Service
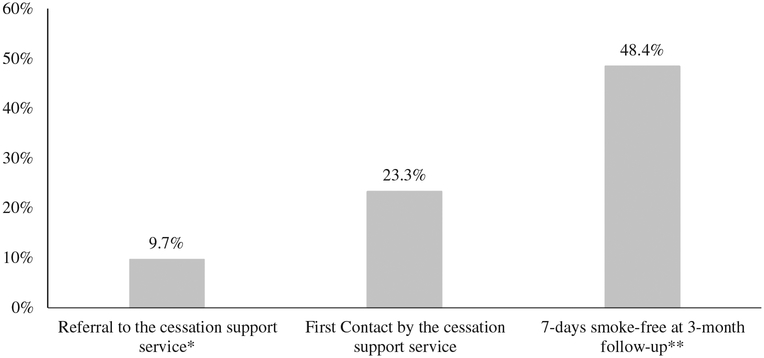
As shown in Figure 1, 9.7% (n = 25/257) of patients were referred to the cessation support service as former smokers, and 23.3% (n = 60/257) self-reported quitting at first contact by the cessation support service. Three months after initial contact, 48.4% (n = 124/256) were smoke-free for the past seven days (Figure 1); n = 1 left this question blank.
Figure 1. Self-reported quit rates selected time points among respondents to the 3-month follow-up survey (n 257).
Note: *There were no statistically significant differences of quit rates between referring clinic, except for the quit rate at referral: 17.3% of thoracic (n = 18/104), 5.8% of head & neck (n = 3/52), and 4.0% of patients from other clinics (n = 4/101) were referred to RPCI TACS as recent quitters (p = 0.003). **n = 256 participants responded to this question; n = 1 left it blank.
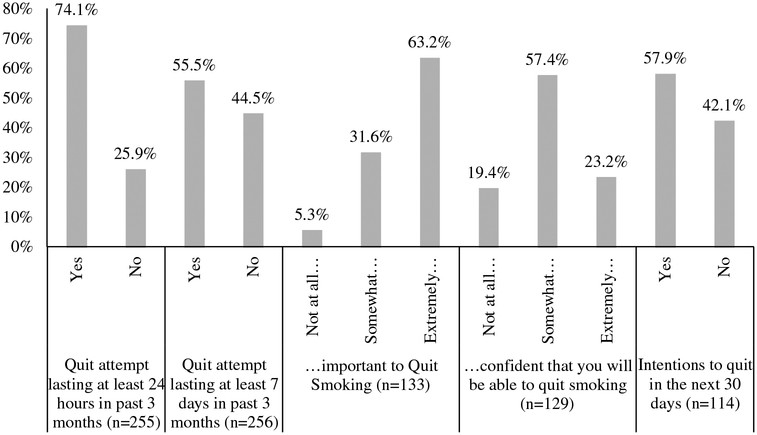
Overall, 74.1% (n = 189/255) of participants reported a quit attempt lasting at least 24 hours in the three months prior to contact (Figure 2); while 55.5% (n = 142/256) of patients reported a quit attempt lasting at least 7 days. The 133 participants reporting current tobacco use also indicated their perceived importance of quitting. 63.2% (n = 84/133) of patients reported perceiving quitting smoking as extremely important; 31.6% (n = 42/133) reported that it is somewhat important; and only 5.3% (n = 7/132) reported that it is not at all important to quit smoking. In contrast, the majority of current users reported they were somewhat confident in their ability to quit smoking (57.4%; 74/129). Only 23.3% (n = 30/129) were extremely confident in their ability to quit=smoking and19.4% (n = 25/129) were not at all confident. Despite the low perceived confidence in quitting, 57.9% (n = 66/114) of patients reported intending to quit in the next 30 days. There were no differences in reported perceived importance to quit, self-efficacy to quit or quit attempts by clinic.
Figure 2. Self-reported quit behaviour and intentions to quit among smokers referred to cessation counselling service.
Note: Patients who responded ‘yes’ to ‘Have you smoked, even a puff, in the last 24 hours?’ were asked the last three questions. There were no statistically significant differences for any question between thoracic, head and neck, and other clinics.
Characteristics Associated with Quitting
In general, few demographic or disease characteristics were significantly associated with participants self-reporting cessation for the 7-days prior to completion of the 3-month follow-up survey. Participants who self-reported 7-day abstinence at the 3-month follow-up participated in more counselling calls compared to participants who indicated continued tobacco use (4.9 calls vs. 4.1 calls, respectively; p = 0.007; Table 2). Caucasians were most likely to report cessation than those of other races (51.2%, n = 108/211, vs. 34.8, n = 5/15, respectively; p = 0.044). Participants who had quit smoking at time of initial referral to RPCI TACS were more likely to report being quit for the last 7 days at the 3-month follow-up than current users at referral (88.0%, n = 22/25, vs. 44.0%, n = 102/232; p<0.001). Similarly, patients reporting being quit at the first contact by the cessation service were more likely to report 7-day abstinence at the 3-month follow-up than current users at first contact (83.3%, n = 50/60, vs. 37.6%, n = 74/197; p<0.001). There were no differences in self-reported 7-day abstinence by referring clinic, other patient demographics or performance status.
Table 2.
Characteristics associated with self-reporting 7-days smoke-free prior to follow-up survey completion (n = 257)
| Smoke-free for Past 7 Days | |||||
|---|---|---|---|---|---|
| No (n = 133) | Yes (n = 124) | ||||
| Characteristics | Mean | Std. Dev. | Mean | Std. Dev. | p-Value |
| Age (in years) | 58.4 | 10.5 | 60.4 | 11.2 | 0.148 |
| Pack-years | 10.4 | 12.2 | 14.0 | 20.6 | 0.085 |
| Completed counselling calls | 4.1 | 2.3 | 4.9 | 2.0 | 0.007* |
| N | % | N | % | p-value | |
| Sex | |||||
| Female | 68 | 51.5 | 64 | 48.5 | 0.938 |
| Male | 65 | 52.0 | 60 | 48.0 | |
| Race | |||||
| Caucasian | 103 | 48.8 | 108 | 51.2 | 0.044* |
| Other | 30 | 65.2 | 16 | 34.8 | |
| Ethnicity | |||||
| Non-Hispanic | 123 | 50.8 | 119 | 49.2 | 0.234 |
| Other | 10 | 66.7 | 5 | 33.3 | |
| Marital status | |||||
| Single | 49 | 60.5 | 32 | 39.5 | 0.095 |
| Married | 59 | 45.4 | 71 | 54.6 | |
| Other | 25 | 54.3 | 21 | 45.7 | |
| Referring clinic | |||||
| Thoracic | 52 | 50.0 | 52 | 50.0 | 0.618 |
| Head & neck | 25 | 48.1 | 27 | 51.9 | |
| Other | 56 | 55.4 | 45 | 44.6 | |
| ECOG P.S. | |||||
| 0 | 37 | 50.0 | 37 | 50.0 | 0.721 |
| ≥1 | 96 | 52.5 | 87 | 47.5 | |
| Quit status at referral | |||||
| Current | 130 | 56.0 | 102 | 44.0 | < 0.001* |
| Quit | 3 | 12.0 | 22 | 88.0 | |
| Quit at cessation support service first contact | |||||
| Current | 123 | 62.4 | 74 | 37.6 | < 0.001* |
| Quit | 10 | 16.7 | 50 | 83.3 | |
Statistically significant at p<0.05.
Patient Satisfaction with the Cessation Service
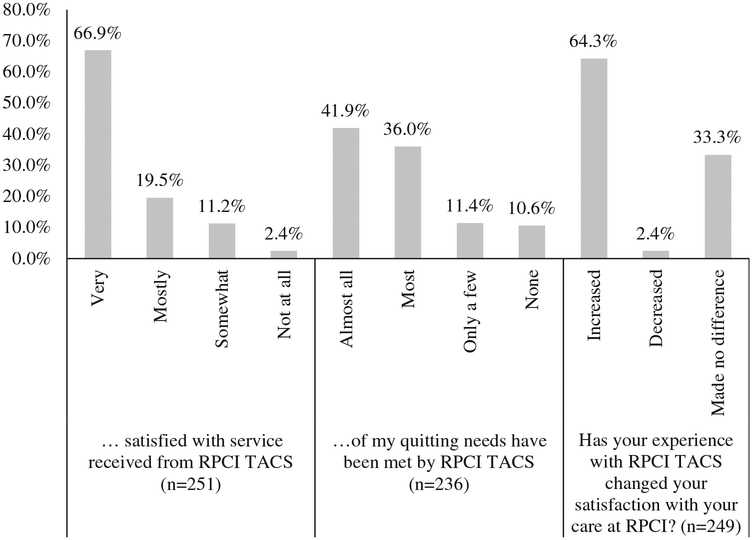
The majority of participants were ‘very satisfied’ or ‘mostly satisfied’ with the service received by the cessation service (66.9%, n = 168/251 and 19.5%, n = 49/251, respectively; Figure 3). Nearly 78% of participants reported that ‘almost all’ or ‘most’ of their needs had been met (41.9%, n = 99/236, and 36.0%, n = 85/236, respectively; Figure 3). 11.4% (n = 27/236) reported that only a few of their needs had been met, and 10.6% (n = 25/236) reported that none of their quitting needs had been met. Participants reporting that none of their needs had been met also had low perceived self-efficacy to quit and did not have any successful quit attempts.
Figure 3. Patient satisfaction with the cessation support service.
Note: n=251/258 responded to the first question, n=3 said they do not know, and n=4 left this question blank; n=236 responded to the second question, n=10 left this question blank, and n=12 said they do not know; and n=249 responded to the final question, n=5 left this question blank, and n=4 said they do not know.
The majority of participants reported that the experience with the RPCI Cessation Service increased their satisfaction with the care received (64.1%, n = 159/248; Figure 3). Few reported that it decreased their satisfaction with the care received (2.4%, n = 6/248), while 33.5% (n = 83/248) reported that the service received from the cessation service made no difference with regard to their satisfaction with medical care received.
Discussion
Cancer patients appear to be interested in and receptive to a free tobacco cessation service following a mandatory tobacco use screen and automatic referral to the program (Dobson Amato et al., 2015; Warren, Marshall, et al., 2014). The majority of patients who participated were ‘very’ or ‘mostly’ satisfied with the care they received with the free cessation service. The service also appears to improve patient satisfaction with their overall care received at the hospital.
The 7-day point prevalence of abstinence self-reported in this subset of a non-random sample of cancer centre patients (48.4%) who received counselling support was lower than the 65% reported at 3-months in another cross-sectional survey of lung cancer patients (Cooley et al., 2012). However, it was higher than the 7-day point prevalence abstinence of lung cancer patients at 3-months after receiving behavioural counselling and varenicline (34.4%) (Park et al., 2011). The NYS Smokers’ Quitline conducts similar 3- follow-up surveys to smok ing participants from New York State. In 2009, the NYS Smokers’ Quitline reported a 3-month follow-up 7-day point prevalence abstinence rate of 34% for their clients, which was notably lower than the 48.4% quit rate our cancer centre patient participants reported, as well (NYS Smokers’ Quitline, 2009). Quit rates in the cancer patient population may be higher than the general healthy population due to the impact of a cancer diagnosis on personal health behaviours. A cancer diagnosis may serve as a strong teachable moment where the patient is more receptive to quit messages and more motivated to quit (Gritz et al., 2006). The assumption that the cessation service resulted in a higher quit rate than standard of care treatment cannot be made due to the lack of a control group.
Improving cancer centre patient perceived self-efficacy to quit is important. High motivation to quit has been associated with high perceived self-efficacy to quit in a similar study of lung cancer patients after participation in a brief intense cessation trial (Schnoll et al., 2004). Similar results are shown with this study, where perceived confidence to quit improves as importance to quit also increases among the entire group of participants. Among patients who are ‘extremely’ confident they can quit, 83.3% (n = 25/30) also think it is ‘extremely’ important to quit, compared to 13.3% (n = 4/30) of those who think quitting is ‘somewhat’ important and 3.3% (n = 1/30) of those who think it is ‘not at all’ important (p<0.001).
The cessation service design is similar to an ‘Ask-Advise-Connect’ approach, which has been shown to be more effective than an opt-in ‘Ask-Advise-Refer’ program (Richter & Ellerbeck, 2015; Vidrine, Shete, Cao, et al., 2013; Vidrine, Shete, Li, et al., 2013). The cessation service was designed to be fluid and tailored to the individual patient with regard to every aspect of cessation support from timing, content, and length and duration. Specific tailoring to the cancer patient’s individual needs has been shown to improve quit rates (Gritz, 2000). This study supports that patients participating in a free, opt-out, telephone based cessation service, based on systematic assessments of smoking, are also very satisfied with their cancer care, as well as have interest in cessation, motivation to quit, and are making quit attempts.
One strength of this study is that the response rate of 50% among a non-random group of patients who smoke and participated in the cessation service is similar to that of the NYS Smokers’ Quitline follow-up surveys with the healthy general population of smokers. Low response rate is one concern with tobacco cessation programs for cancer patients; however, this study has demonstrated that cancer patients are willing to participate in a brief survey 3-months after a diagnosis at the same rate of the healthy general population of smokers. This rate is more than double that of a previous study, which reported a 22.7% response rate for mailed follow-up surveys sent to smoking-related cancer survivors (Berg, Carpenter, Jardin, & Ostroff, 2013). Another strength was that, in general, there were no major demographic or disease differences among survey participants. However, this study did note a greater participation rate among married patients (Table 1, p = 0.001). Lastly, multiple survey modalities allowed patients the opportunity to participate in the manner in which they were most receptive. For example, most patients preferred telephone contact, with very few patients participating via web and about 40% responding via mail.
One limitation with this study is the relatively small sample size from a single institution across multiple clinical centres. The statistical power and scope of the analyses, as well as the generalizability of the results, are therefore limited. Relying on self-reported smoking status is another limitation, which may lead to inaccurate quit rates. Patients, and specifically lung cancer patients, often mis-represent their smoking status to clinicians (Morales et al., 2013). Similarly, healthier and/or more motivated patients may have self-selected into the cessation services program and opted to participate in this follow-up survey, which may inflate the quit rates compared to that of all patients at this cancer centre. The short follow-up interval and variable time frame for responses, represents other limitations of this study. The wide range of days between consent and survey completion may be due to non-response via mail or web followed by attempts to complete the survey via telephone, or due to a long response time for survey completion via mail. To demonstrate the effectiveness of this cessation service, quit rates should be examined beyond 3-months. Quit rates examined over a longer period of time will also allow for patterns of quitting to be explored, as well as the relation of long-term quit status with survival and various other cancer outcomes. Lastly, the lack of a control group is an inherent limitation that results from using an existing clinical program as the basis for this study. The generalization of these results is limited to the patient population referred to and participating in the tobacco cessation service. It cannot be statistically shown that the participants in this study had a higher quit rate compared to tobacco using patients not participating in the tobacco cessation service.
This study will serve as the foundation for future work. Important factors that need to be explored further include a longer and more precise follow-up time such as 6- or 12-months, a longer accrual period to increase the same size and biochemical confirmation of quit status. These results support that a larger follow-up study would be feasible in the cancer patient population referred to the cessation support program.
Conclusion
Participants in a structured cessation support program, reported improved quit rates that from the time of initial referral. Patients view cessation as important but lack confidence in their ability to quit smoking. However, intentions to quit in the near term are high. Patients report that the opt-out, free telephone-based tobacco cessation service improved their satisfaction with their overall cancer care. Continued research efforts in this area are necessary; our result demonstrate that cancer patients are willing to participate in follow-up surveys and that a larger study in the future is feasible.
Acknowledgements
We would like to acknowledge and thank Patricia Hysert, Robert Hysert, and Stephanie Segal for their hard work and dedication in helping patients to become tobacco free; Gary Giovino, PhD, Robert Reed, MPH, Michael Zevon, PhD, and Graham Warren, MD, PhD for their contributions to support the cessation service; and the Roswell Park Cancer Institute Survey Research Shared Resource team, including Danielle Smith, MPH and Cheryl Rivard, MPH, for conducting the follow-up survey.
Financial Support
This work was supported in part by the Roswell Park Alliance Foundation and NCI R25CA113951.
Footnotes
Conflicts of Interest
Dr Cummings and Dr Mahoney serve as expert witnesses for the plaintiffs in tobacco litigation cases. Dr Mahoney is also the speaker of bureau of Pfizer and manufacturer of Chantix/varenicline.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki declaration of 1975, as revised in 2008.
References
- Berg CJ, Carpenter MJ, Jardin B, & Ostroff JS (2013). Harm reduction and cessation efforts and interest in cessation resources among survivors of smoking-related cancers. Journal of Cancer Survivorship, 7(1), 44–54. doi: 10.1007/s11764-012-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle R, Solberg L, & Fiore M (2011). Use of electronic health records to support smoking cessation. The Cochrane Database of Systematic Reviews, (12), CD008743. doi: 10.1002/14651858.CD008743.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo JK, Dubey S, & Prochaska JJ (2010). Smoking cessation: An integral part of lung cancer treatment. Oncology, 78(5–6), 289–301. doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy MB, Majchrzak NE, Regan S, Silverman CB, Schneider LI, & Rigotti NA (2005). The association between patient-reported receipt of tobacco intervention at a primary care visit and smokers’ satisfaction with their health care. Nicotine & Tobacco Research, 7(Suppl. 1), S29–S34. doi: 10.1080/14622200500078063. [DOI] [PubMed] [Google Scholar]
- Cooley ME, Wang Q, Johnson BE, Catalano P, Haddad RI, Bueno R, & Emmons KM (2012). Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung Cancer, 76(2), 144–149. doi: 10.1016/j.lungcan.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson Amato KA, Hyland A, Reed R, Mahoney MC, Marshall J, Giovino G, et al. (2015). Tobacco cessation may improve lung cancer patient survival. Journal of Thoracic Oncology, 10(7), 1014–1019. doi: 10.1097/JTO.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SA, Louzon SA, & Gritz ER (2012). Why do cancer patients smoke and what can providers do about it? Community Oncology, 9(11), 344–352. doi: 10.1016/j.cmonc.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. (2008). Clinical practice guideline: Treating tobacco use and dependence 2008 update. Rockville, MD: U.S. Department of Health and Human Services: Public Health Service. [Google Scholar]
- Gritz ER (2000). Facilitating smoking cessation in cancer patients. Tobacco Control, 9(Suppl. 1), I50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Dresler C, & Sarna L (2005). Smoking, the missing drug interaction in clinical trials: Ignoring the obvious. Cancer Epidemiology, Biomarkers & Prevention, 14(10), 2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, & Reece GP (2006). Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer, 106(1), 17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- Hanna N, Mulshine J, Wollins DS, Tyne C, & Dresler C (2013). Tobacco cessation and control a decade later: American society of clinical oncology policy statement update. Journal of Clinical Oncology, 31(25), 3147–3157. doi: 10.1200/JCO.2013.48.8932. [DOI] [PubMed] [Google Scholar]
- Morales NA, Romano MA, Michael Cummings K, Marshall JR, Hyland AJ, Hutson A, et al. (2013). Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control, 24(6), 1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYS Smokers’ Quitline (2009). New York state smokers’ quitline: 2009 annual report. Retrieved from http://www.nysmokefree.com/download/Quitline2009Report.pdf.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. (1982). Toxicity and response criteria of the eastern cooperative oncology group. American Journal of Clinical Oncology, 5(6), 649–655. [PubMed] [Google Scholar]
- Park ER, Japuntich S, Temel J, Lanuti M, Pandiscio J, Hilgenberg J, et al. (2011). A smoking cessation intervention for thoracic surgery and oncology clinics: A pilot trial. Journal of Thoracic Oncology, 6(6), 1059–1065. doi: 10.1097/JTO.0b013e318215a4dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, & Ellerbeck EF (2015). It’s time to change the default for tobacco treatment. Addiction, 110(3), 381–386. doi: 10.1111/add.12734. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Rothman RL, Newman H, Lerman C, Miller SM, Movsas B, et al. (2004). Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: Implications for the development of tobacco control programs for cancer patients. Psychooncology, 13(5), 346–358. doi: 10.1002/pon.756. [DOI] [PubMed] [Google Scholar]
- Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS, AACR Subcommittee on Tobacco and Cancer (2013). Assessing tobacco use by cancer patients and facilitating cessation: An American association for cancer research policy statement. Clinical Cancer Research, 19(8), 1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. (2013). Ask-Advise-connect: A new approach to smoking treatment delivery in health care settings. JAMA Internal Medicine, 173(6), 458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Shete S, Li Y, Cao Y, Alford MH, Galindo-Talton M, et al. (2013). The ask-advise-connect approach for smokers in a safety net healthcare system: A group-randomized trial. American Journal of Preventive Medicine, 45(6), 737–741. doi: 10.1016/j.amepre.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Alberg AJ, Kraft AS, & Cummings KM (2014). The 2014 surgeon general’s report: “The health consequences of smoking – 50 years of progress”: A paradigm shift in cancer care. Cancer, 120(13), 1914–1916. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Marshall JR, Cummings KM, Toll B, Gritz ER, Hutson A,… Dresler C (2013a). Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. Journal of Thoracic Oncology, 8(5), 543–548. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Marshall JR, Cummings KM, Toll BA, Gritz ER, Hutson A,… Dresler CA (2013b). Addressing tobacco use in patients with cancer: A survey of American Society of Clinical Oncology members. Journal of Oncology Practice, 9(5), 258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Marshall JR, Cummings KM, Zevon MA, Reed R, Hysert P, et al. (2014b). Automated tobacco assessment and cessation support for cancer patients. Cancer, 120(4), 562–569. doi: 10.1002/cncr.28440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Danhauer SC, Tooze JA, Blackstock AW, Spangler J, Thomas L, et al. (2012). Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist, 17(3), 455–462. doi: 10.1634/theoncologist.2011-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]