Abstract
Objective
Possible regenerative treatments for lumbar intervertebral disc degeneration (DD) are rapidly emerging. There is consensus that the patient that would benefit most has early‐stage DD, with a predicted deterioration in the near future. To identify this patient, the aim of this study was to identify prognostic factors for progression of DD.
Study design
Systematic review.
Methods
A systematic search was performed on studies evaluating one or more prognostic factor(s) in the progression of DD. The criteria for inclusion were (a) patients diagnosed with DD on MRI, (b) progression of DD at follow‐up, and (c) reporting of one or more prognostic factor(s) in progression of DD. Two authors independently assessed the methodological quality of the included studies. Due to heterogeneity in DD determinants and outcomes, only a best‐evidence synthesis could be conducted.
Results
The search generated 3165 references, of which 16 studies met our inclusion criteria, involving 2.423 patients. Within these, a total of 23 clinical and environmental and 12 imaging factors were identified. There was strong evidence that disc herniation at baseline is associated with progression of DD at follow‐up. There is limited evidence that IL6 rs1800795 genotype G/C male was associated with no progression of DD. Some clinical or environmental factors such as BMI, occupation and smoking were not associated with progression.
Conclusions
Disc herniation is strongly associated with the progression of DD. Surprisingly, there was strong evidence that smoking, occupation, and several other factors were not associated with the progression of DD. Only one genetic variant may have a protective effect on progression, otherwise there was conflicting or only limited evidence for most prognostic factors. Future research into these prognostic factors with conflicting and limited evidence is not only needed to determine which patients should be targeted by regenerative therapies, but will also contribute to spinal phenotyping.
Keywords: degenerative disease, disc herniation, environmental factor, imaging, low back pain, review
1. INTRODUCTION
Intervertebral disc degeneration (DD) is a complex disease involving structural degradation of the normal, healthy matrix of the intervertebral disc. This matrix disruption is radiologically visualized by loss of disc height, an inhomogeneous structure of the disc, and the loss of distinction between nucleus pulposus and annulus fibrosus,1, 2, 3, 4 often resulting in physical complaints like low back pain and morning stiffness.5, 6, 7 The exact pathophysiology of DD is not yet completely understood, but it often starts at a quite young age with an imbalance in the interplay of biomechanics, cell behavior, and extracellular matrix, ending up in a cascade of degeneration.8
Many initiating factors have been identified that can push the intervertebral disc into the vicious cycle of degeneration, such as mechanical overloading by heavy physical workload or systemic inflammatory disorders like diabetes,9, 10, 11, 12, 13, 14 but the factors that encourage this downward spiral are less addressed, nor is there an overview of these prognostic factors present in literature. A prognostic factor is a clinical or biological aspect that is objectively measurable and that provides information on the possible course of the condition in an untreated patient.15 Insight in these prognostic factors in the progression of DD will help us to differentiate between the different spinal phenotypes: the multiple appearances of DD in which the genotype and environment of the patient have a different interaction. It will also provide patients with more targeted information about their prognosis, and will also streamline the crucial process of shared‐decision making, as no clear clinical algorithm is present for diagnosing or treating DD.
Currently, most patients with symptomatic DD (ie, degenerative disc disease) are put to pain medication and physical therapy, with spinal fusion as final option in end‐stage degeneration.16, 17, 18 More recently, preclinical studies on intervertebral disc regeneration show promising results for restoring cell homeostasis in moderate DD,19, 20, 21, 22, 23 with expected increase of in vivo testing of these therapies in patients.24, 25 It seems trivial that end‐stage degeneration is too late to interfere with regenerative therapies. Therefore, the patient most suited for regenerative therapies has early‐stage DD, with a high chance of deterioration in the near future. Therefore, the aim of this study was to identify and evaluate prognostic clinical, environmental and imaging factors that are associated with outcome, relative to baseline (ie, progression of DD), by a systematic review of the literature.
2. METHODS
A review protocol was developed based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA)‐statement (www.prisma-statement.org).26
2.1. Literature search and selection of studies
The search strategy was developed with the consultation of an experienced health sciences librarian. PubMed, Embase.com, and Clarivate Analytics/Web of Science Core Collection were searched from inception up to 4 June 2018 (by C.M.E.R. and J.C.F.K.). The following terms were used (including synonyms and closely related words) as index terms or free‐text words: “intervertebral disc degeneration” and “epidemiologic studies” and “disease progression” or “prognosis”. The full search strategies for all databases can be found in the File S1. Duplicate articles were excluded. Manuscripts in English or German were accepted. Two investigators (C.M.E.R. and S.S.A.F.) screened all titles and abstracts for relevance independently from each other, using Covidence, an online screening tool for reviews by the Cochrane Collaboration (www.covidence.org). The in‐ and exclusion criteria were set prior to screening. Full‐text articles were included when (a) patients were diagnosed with DD on MRI at baseline, (b) progression of DD was measured at follow‐up ≥1 year after baseline, and (c) one or more prognostic factors in progression of DD was evaluated. Reviews, meta‐analyses, congress abstracts, animal studies, and case series were excluded. Then the references of included articles were checked for any possible additional articles. When there was no consensus on the inclusion of an article by both investigators, the full‐text was screened again and debated until consensus was reached. A third person (KSE) was available in case no consensus could be reached.
2.2. Data extraction
A data‐extraction form was developed to obtain the following information: authors, year of publication, number of patients, gender, age, months of follow‐up, imaging modality, definition of DD, definition of progression, and researched prognostic factors, including their measurement methods, and statistical analyses method(s).
2.3. Quality assessment
The included studies were independently subjected to a quality assessment by two authors (C.R. and S.F.), based on criteria described by Hayden et al.27 These criteria were developed to assess the methodological quality of prediction studies and include 13 items, distributed over six categories: (a) study participation, (b) study attrition, (c) measurement of prognostic factors, (d) adjustment for confounding, (e) measurement of outcomes, and (f) appropriateness of statistical analyses. When an item was sufficiently addressed, the category was scored as 1. Otherwise, 0 points were scored, so there was a maximum score of 13 points. A score of ≥9 was regarded as a high‐quality study, and studies with a score of <9 were considered as low‐quality studies. The same approach of quality assessment has been applied in studies evaluating prognostic factors in knee and hip arthritis.28, 29 In case of disagreement, points were discussed until consensus was reached. Cohen's kappa statistic for inter‐observer agreement30 was calculated using IBM spss Statistics for Macintosh, Version 25.0. Armonk, New York: IBM Corp.
2.4. Best‐evidence‐synthesis
Correlation coefficients of prognostic factors were statistically pooled if there was sufficient clinical and statistical homogeneity regarding the definition of DD, progression of DD, study population, and measurement methods of outcomes. In the absence of statistical analysis (correlation or beta coefficients) and heterogeneity in definition, measurement methods, and study design, the strength of evidence for prognostic factors was assessed according to a best‐evidence synthesis. This method was introduced by Bastick, based on recommendations by of the Cochrane back review group,28, 31 and is considered to be the golden standard for conducting analyses in heterogenic studies. Prognostic factors were categorized as follows:
Strong evidence: consistent [>75%] findings in multiple (≥2) high‐quality studies
Moderate evidence: findings in one high‐quality study and consistent [>75%] findings in multiple (≥2) low‐quality studies
Limited evidence: findings in one high‐quality study or consistent [>75%] findings in ≥3 low‐quality studies
Inconclusive evidence: findings found in <3 low‐quality studies
Conflicting evidence: <75% of the studies reported consistent findings
3. RESULTS
3.1. Studies included
The literature search generated a total of 4261 studies. After the removal of duplicates, we screened the titles and abstracts of 3165 studies. Of these, we identified a total of 16 studies that met our inclusion criteria (Figure 1). There was a large variation in sample size (range: 19‐617), with a total of 2434 patients, and the mean age varied between 13.1 and 65.4 years old. There was a large variety in the determination of DD and progression, but all included studies used MRI as imaging modality both at baseline and during follow‐up. Some studies assessed DD and progression based on Pfirrmann's method,32, 33, 34, 35, 36, 37 others used the Schneiderman's classification38 or the Pearce classification,39 or they developed their own method of DD and progression determination.40, 41, 42, 43, 44, 45, 46, 47 Study characteristics can be found in Table 1.
Figure 1.
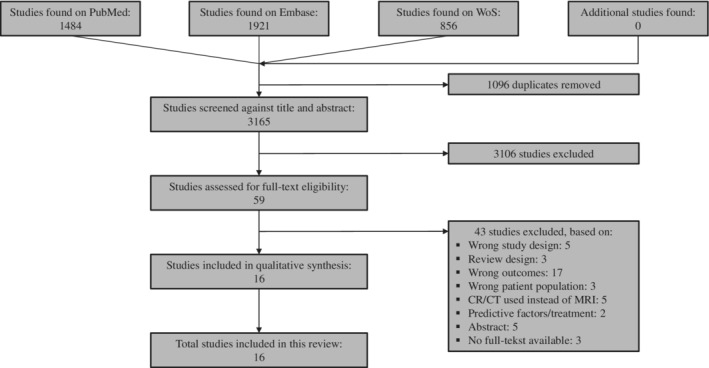
PRISMA flowchart
Table 1.
Study characteristics
| Author, year | Study design | No. of patients (n men) | Age of total group in years, mean (range) | Months of follow‐up, mean (range) | Imaging modality at baseline | Imaging modality at follow‐up | Definition of DD | Definition of progression |
|---|---|---|---|---|---|---|---|---|
| Burnett et al, 199645 | Cohort study | 19 (19) | 13.6, SD 0.6 at baseline; 16.3, SD 0.6 at FU | 32.4 | MRI | MRI | Loss of disc space with any evidence of collapse, no smooth borders of both AF and NP, any evidence of disc herniation, no clear white signal of the disc | Evidence of progression from baseline to follow‐up |
| Elfering et al, 200239 | Prospective cohort study | 41 (30) | 35.90 (20‐50) | 62 (54‐72) | MRI | MRI | Pearce classificationa. Grade III to V are attributed to degeneration |
Changes with regard to disc abnormalities (ie, same, better, worse) |
| Eskola et al, 201246 | Cohort study | 166 (74) | 13.1, SD 0.4 at baseline; 15.7, SD 0.3 at FU | 32.4 (26.4‐37.2) | MRI | MRI | Signal intensity changes (0‐3; 2 or 3 for DD), or change in disc contour (0‐4; 1‐4 for DD) at one or more levels | Worsened or new decrease in disc signal intensity, new disc bulge or herniation, new endplate change, or new Modic change at ≥1 lumbar levels, compared to baseline. Significant new annular tears (AT) and significant high intensity zone lesions (HIZ) |
| Farshad‐Amacker et al, 201432 | Case‐control study | 90b | 59.4 | 59.4; SD 10.2 | MRI | MRI | Pfirrmann; ≥ grade 3 | Increase in Pfirrmann grade |
| Farshad‐Amacker et al, 201433 | Retrospective cohort study | 90 (27) | 61.3 | Median 60, range 45.6‐80.4c | MRI | MRI | Pfirrmann | Increase in Pfirrmann grade in any level for DD |
| Farshad‐Amacker et al, 201734 | Case‐control study | 90 (27) | 61.1 | 60; SD 0.8 | MRI | MRI | Pfirrmann | Increase in Pfirrmann grade from 1 towards 5 on the same level during the period of observation |
| Kerttula et al, 201247 | Cohort study | 54 (9) | 43.6 (24‐65) | 12 (11‐18) | MRI | MRI | Endplate lesions, loss of disc height, and decrease in signal intensity, posterior bulge | Increase of endplate lesions, decrease of disc height and change in disc signal intensity, increase in posterior bulge |
| Liuke et al, 200540 | Retrospective longitudinal study | 129 (129) | 44 (41‐46) | 48 | MRI | MRI | Decreased signal intensity of NP compared to signal intensity of the cerebrospinal fluid | 4‐y changes in the number of discs with decreased signal intensity of the NP |
| Makino et al, 201738 | Prospective cohort study | 84 (0)d | First MRI: 20.9 (20‐22); Second MRI 30.6 (28‐35) | 117.6 (84‐168) | MRI | MRI | Schneiderman's four‐grade classification; summation of the degeneration grades of all disc levels, with five at the minimum | Worsening of Schneiderman's grade |
| Nagashima et al, 201341 | Cohort study | 192 (192) | 15 at baseline, 17 at FU | 24 | MRI | MRI | Decreased signal intensity of NP compared to signal intensity of the cerebrospinal fluid; mean signal intensity of six discs from T12L1 to L5S1 | Decrease in mean signal intensity of the NP at the 2‐y follow‐up |
| Sharma, 200936 | Retrospective longitudinal study | 46 (13) | 53.6 (20‐88) | 31.8 (4‐69)e | MRI | MRI | Loss of signal intensity, Pfirrmann | Increase in signal‐intensity grade, increase in Pfirrmann grade |
| Sharma et al, 201137 | Retrospective longitudinal study | 63 (23) | 30; SD 6.7 | 30 (3‐85)f | MRI | MRI | Pfirrmann (>2), conspicuity of AF | Increase in Pfirrmann, increase in conspicuity of AF |
| Teraguchi et al, 201735 | Cohort study | 617 (178) | 65.4 at FU; SD 12 | 48 | MRI | MRI | Pfirrmann; ≥ 4 |
|
| Videman et al, 200643 | Cohort study | 140 (140) | 49 (35‐69) | 57.6 (48‐68.4) | MRI | MRI | Signs of disc height narrowing, disc bulging, disc herniations, high intensity zones, osteophytes, upper endplate irregularities and fatty degeneration of vertebrae, annular tears, disc herniations. Each sign was rated from 0 (normal) to 3 (most abnormal) | Progression in degenerative signs |
| Videman et al, 200842 | Longitudinal study | 134 (134) | 49 (35‐69) | 57.6 (48‐68.4) | MRI | MRI | Quantitative measures of disc height and bulging | Changes in percentage of the baseline value for each quantitative measure |
| Williams et al, 201144 | Cohort study | 468 (24) | 53.6 (40.1‐68.7) at baseline | 128.4 (91.2‐164.4) | MRI | MRI | Progressive scale of 0‐3 for disc height measured in the middle of the disc, disc signal intensity within the NP, lumbar disc extension posteriorly into the spinal canal and anterior osteophytes. 0 = normal; 3 = highly degenerate disc | Subtraction of the baseline score from the FU score, adjusted for the time interval between the two MRI scans |
Abbreviations: AF, annulus fibrosus; DD, disc degeneration; FU, follow‐up; NP, nucleus pulposus.
Classification based on the structure of the disc, the distinction between NP and AF, signal intensity, and disc height.
Seventy‐two discs in total, of which 34 discs were male.
Of those with progression, the control group is described separately.
Disc progression in 44 subjects.
There was a follow‐up of >1 year in 40 subjects.
There was a follow‐up of >6 months in 90%, and a follow‐up of >12 months in 76.2%.
3.2. Methodological quality
The Cohen's kappa statistic for inter‐observer agreement was 0.75, representing good agreement.30 Of the 16 studies included, 12 studies had a score of 9 points or more and were classified as high quality. Most methodological shortcomings concern lack of adequate blinding (item H and J). An overview is presented in Table 2.
Table 2.
Quality assessment
| Author, year | Total score | A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burnett et al, 199645 | 9 | 1 | 0a | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Elfering et al, 200239 | 10 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Eskola et al, 201246 | 12 | 1 | 1 | 1 | 1 | 1a | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Farshad‐Amacker et al, 2014 (AT)32 | 8 | 1 | 0a | 1 | 1 | 0a | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Farshad‐Amacker et al, 201433 | 7 | 1 | 0a | 0a | 1 | 0a | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Farshad‐Amacker et al, 201734 | 8 | 1 | 0a | 1 | 1 | 0a | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Kerttula et al, 201247 | 9 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| Liuke et al, 200540 | 12 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Makino et al, 201738 | 9 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 |
| Nagashima et al, 201341 | 7 | 1 | 1 | 0a | 1 | 0a | 0 | 0a | 0 | 1 | 0 | 1 | 1 | 1 |
| Sharma et al, 200936 | 9 | 1 | 1 | 1 | 1 | 0a | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 |
| Sharma et al, 201137 | 10 | 1 | 1 | 1 | 1 | 0a | 1 | 0a | 1 | 1 | 0 | 1 | 1 | 1 |
| Teraguchi et al, 201735 | 12 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Videman et al, 200643 | 10 | 1 | 1 | 1 | 1 | 1 | 0a | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| Videman et al, 200842 | 10 | 1 | 1 | 1 | 1 | 1 | 0a | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| Williams et al, 201144 | 10 | 1 | 0a | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
The item for which there was interobserver disagreement. Kappa = 0.75. The criteria were as follows, with 1 point for “yes” and 0 points for “no”; A: Clear description of study population; B: Valid in‐ and exclusion criteria; C: Sufficient description of baseline characteristics; D: Follow‐up of ≥4 years; E: Prospective data collection; F: Loss to follow‐up ≤15%; G: Information provided about loss of follow‐up; H: Exposure assessment blinded for the outcome; I: Exposure measured identically at baseline and follow‐up; J: Outcome assessment blinded for exposure; K: Outcome measured identically at baseline and follow‐up; L: Measure of association or variance given; M: Adjustment for confounding variables.
3.3. Identified prognostic factors
We identified 12 imaging and 23 clinical and environmental prognostic factors. A full overview of the prognostic factors, their measurement methods and association with DD progression is presented in Tables 3 and 4. Only 12 studies include or report a correlation analysis of the prognostic factor's association with DD progression. Due to the lack of studies with sufficient statistical analysis and due to large heterogeneity between studies in measurement methods, including a high variation in DD and progression definitions, statistical pooling of the results was not possible. Consequently, it was necessary to summarize each prognostic factor according to a best‐evidence synthesis to determine the strength of association with DD progression.
Table 3.
Clinical/environmental determinants as prognostic factors for progression in DD
| Clinical or environmental determinant | Author, year | Study quality | Measurement method | Study population | Reported effect sizes | Statistics | Association with DD progression |
|---|---|---|---|---|---|---|---|
| Age | Elfering et al, 200239 | High | Continuous (in years) | Asymptomatic individuals at baseline and ≥5 y FU | Mean age 36.71, SD 7.82 with progression compared to mean age 35.08, SD 7.83 without progression | P > .05 | a |
| Farshad‐Amacker et al, 2014 32 | Low | Continuous (in years) | Patients with an MRI at baseline and 4 y FU | Mean age 60.3, SD 14.1 with progression compared to mean age 62.2, SD 17.3 without progression | P = .56 | a | |
| Makino et al, 201738 | High | Continuous (in years) | Nursing students with an MRI at baseline and 9.8 y FU when working as a nurse | Mean age 30.3 with progression compared to mean age 30.8 without progression | P > .05 | a | |
| Teraguchi et al 201735 | High | Continuous (in years) | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | Not reported | P > .05; OR 1.01; CI 0.99‐1.02 | a | |
| Sharma et al, 201137 | High | Continuous (in years) | Patients with a MRI of the lumbar spine at baseline and FU | Sum of squares 0.068933; F ratio 0.3246 | P = .5692 | a | |
| Williams et al, 201144 | High | Continuous (in years) | Twin pairs from the UK and Australia at baseline and 10 y FU | Disc degeneration summary score of 71 in age at baseline <50 compared to disc degeneration summary score of 50 in age at baseline >60 | Not provided | a | |
| Gender | Elfering et al, 200239 | High | Male vs female | Asymptomatic individuals at baseline and ≥ 5 y FU | Male 26.8%, female 14.6% with progression compared to male 46.3%, female 12.2% without progression | P > .05 | a |
| Farshad‐Amacker et al, 201433 | Low | Male vs female | Patients with an MRI at baseline and 4 y FU | Thirty‐nine women and 17 men with progression compared to 24 women and 10 men without progression | P = .92 | a | |
| Sharma et al, 201137 | High | Male vs female | Patients with a MRI of the lumbar spine at baseline and FU | Sum of squares 0.000717; F ratio 0.0034 | P = .9537 | a | |
| Teraguchi et al, 201735 | High | Male vs female | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | Two hundred and sixty‐five women compared to 92 men | P < .05; OR 1.68; CI 1.18‐2.42 | b | |
| Body weight | Videman et al, 200842 | High | Continuous (kg) | Male monozygotic Finnish twins at baseline and 5 y FU | Mean weight 79.4 kg at follow‐up compared to mean weight 79.0 at baseline | Not provided | a |
| Videman et al, 200643 | High | Continuous (kg) | Male monozygotic Finnish twins at baseline and 5 y FU | Mean weight 79.4 kg at follow‐up compared to mean weight 79.0 at baseline | Not provided | a | |
| BMI | Elfering et al, 200239 | High | Continuous (mean kg/m2) | Asymptomatic individuals at baseline and ≥ 5 y FU | Mean 23.61, SD 2.89 with progression compared to mean 23.19, SD 3.50 without progression | P > .05 | a |
| Farshad‐Amacker et al, 201432 | Low | Continuous (mean kg/m2) | Patients with an MRI at baseline and 4 y FU | Mean 27.1, SD 4.9 with progression compared to mean 26.7, SD 5.3 without progression | P = .78 | a | |
| Makino et al, 201738 | High | Continuous (mean kg/m2) | Nursing students with an MRI at baseline and 9.8 y FU when working as a nurse | Mean BMI 20.5 for subjects with progression compared to mean BMI 20.0 without progression | P > .05 | a | |
| Williams et al, 201144 | High | Continuous (mean kg/m2) | Twin pairs from the UK and Australia at baseline and 10 y FU | Not reported | Not provided | a | |
| Overweight | Liuke et al, 200540 | High | BMI ≥ 25 kg/m2 | Working middle‐aged men repressing three occupations: machine drivers, construction carpenters, and office workers | Not reported | OR 4.3; CI 1.3‐14.3 | b |
| Nagashima et al, 201341 | Low | BMI ≥ 25 kg/m2 | High school American Football players with an MRI at baseline and 2 y of FU | Not reported | P = .449; PRC 1.12; CI −1.79‐4.02 | a | |
| Obesity | Teraguchi et al, 201735 | High | BMI ≥ 25 kg/m2 | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | One hundred and eighteen subjects with BMI ≥ 25 kg compared to 239 subjects with BMI < 25 | P > .05; OR 1.31; CI 0.92‐1.86 | a |
| Heritability | Williams et al, 201144 | High | Monozygotic vs Dizygotic same‐sex twin pairs | Twin pairs from the UK and Australia at baseline and 10 y FU | Mean summary lumbar score of 13.84 in monozygotic twins compared to 12.41 in dizygotic twins | P = .02 | b |
| Genetic risk factors | Eskola et al, 201246 | High | Previously identified candidate SNPs in adolescents 1: T‐allele IL1A rs1800587 female 2: IL6 rs1800795 genotype G/C male |
Danish adolescent population at baseline and 3 y FU | Not reported | 1. P = .037; OR 2.45; CI 1.03‐5.82 2. P = .024; OR 0.32; CI 0.12‐0.88 |
1. b
2. c |
| Pregnancy | Makino et al, 201738 | High | Experience of pregnancy | Nursing students with an MRI at baseline and 9.8 y FU when working as a nurse | 21.7% with progression compared to 25% without progression | P = .86; RR 1.06; CI 0.58‐1.92 | a |
| Diabetes mellitus | Teraguchi et al, 201735 | High | Serum HbA1c level ≥ 6.1% | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | Thirty‐four subjects with DM compared to 323 subjects without DM | P > .05; OR 1.59; CI 0.87‐3.01 | a |
| Hypertension | Teraguchi et al, 201735 | High | SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | Two hundred and forty‐six subjects with hypertension compared to 100 subjects without hypertension | P > .05; OR 0.98; CI 0.68‐1.82 | a |
| Back injuries | Liuke et al, 200540 | High | History of accidental back injuries before baseline | Working middle‐aged men represting three occupations: machine drivers, construction carpenters, and office workers | Not reported | OR 1.2; CI 0.5‐2.8 | a |
| Smoking | Liuke et al, 200540 | High | Smoking status at baseline | Working middle‐aged men represting three occupations: machine drivers, construction carpenters, and office workers | Not reported | OR 0.6; CI 0.8‐1.4 | a |
| Makino et al, 201738 | High | Smoking ≥1 year | Nursing students with an MRI at baseline and 9.8 y FU when working as a nurse | About 4.3% with progression compared to 3.8% without progression | P = .74; RR 0.77; CI 0.15‐3.92 | a | |
| Teraguchi et al, 201735 | High | Regularly smoking >1 per month | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | Thirty‐three smoking subjects compared to 323 non‐smoking subjects | P > .05; OR 0.65; CI 0.39‐1.08 | a | |
| Videman et al, 200842 | High | Current and lifetime cigarette smoking | Male monozygotic Finnish twins at baseline and 5 y FU | Mean packs per day of 0.3 at follow‐up compared to 0.2 at baseline; 16.1 packs per year at baseline | P = .018 | b | |
| Videman et al, 200643 | High | Current and lifetime cigarette smoking | Male monozygotic Finnish twins at baseline and 5 y FU | Mean packs per day of 0.3 at follow‐up compared to 0.2 at baseline; 16.1 packs per year at baseline | Not provided | a | |
| Car driving | Liuke et al, 200540 | High | >15 000 km/y car driving before baseline | Working middle‐aged men represting three occupations: machine drivers, construction carpenters, and office workers | Not reported | OR 1.2; CI 0.6‐2.2 | a |
| Videman et al, 200643 | High | Occupational driving (hours/day) | Male monozygotic Finnish twins at baseline and 5 y FU | Mean 1.2 at follow‐up compared to 1.2 at baseline | Not provided | a | |
| Teraguchi et al, 201735 | High | Driving for ≥ 6 h/d | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | Forty‐five driving subjects compared to 305 non‐driving subjects | P > .05; OR 1.10; CI 0.68‐1.82 | a | |
| Occupation | Liuke et al, 200540 | High | 1. Construction carpenter, 2. Machine operator, or 3. Office worker |
Working middle‐aged men represting three occupations: machine drivers, construction carpenters, and office workers | Not reported | 1. OR 1.8; CI 0.7‐4.9; 2. OR 1.3; CI 0.5‐3.2 3. ‐ |
a |
| Makino et al, 201738 | High | Years of working as a nurse | Nursing students with an MRI at baseline and 9.8 years FU when working as a nurse | Mean 5.8 years in subjects with progression compared to mean 5.9 years in subjects without progression | P = .36; RR 1.30; CI 0.73‐2.30 | a | |
| Work schedule | Elfering et al, 200239 | High | Working evening/night shifts | Asymptomatic individuals at baseline and ≥5 y FU | Yes 29.3%, No 12.2% with progression compared to Yes 56.1%, No 2.4% without progression | P < .05; OR 23.01; CI 1.26‐421.31 | b |
| Working style | Makino et al, 201738 | High | Major working style for >half of their carrier | Nursing students with an MRI at baseline and 9.8 y FU when working as a nurse | About 65% ward, operation room or intensive care unit and 35% clinic or others for subjects with progression compared to 59.2% ward, operation room or intensive care and 40.8% clinic or others for subjects without progression | P = .41; RR 0.79; CI 0.46‐1.37 | a |
| Weight lifted at work | Videman et al, 200842 | High | Maximum weight lifted at work (kg) | Male monozygotic Finnish twins at baseline and 5 y FU | AR2 = 4.9% | P = .0082 | b |
| Sports activities | Elfering et al, 200239 | High | Frequency of sports activities, from 1 (ie, no sports) to 4 (ie, regular competitive sports) | Asymptomatic individuals at baseline and ≥ 5 y FU | Mean 2.12, SD 1.05 with progression compared to mean 2.71, SD 0.99 without progression | P < .05; OR 2.71; CI 1.04‐7.07 | c |
| Recreational activities at leisure time | Videman et al, 200842 | High | Leisure time activities with heavy physical loading (years of >1 time/wk) | Male monozygotic Finnish twins at baseline and 5 y FU | Not reported | Not provided | a |
| Videman et al, 200643 | High | Leisure time activities with heavy physical loading (years of >1 time/wk) | Male monozygotic Finnish twins at baseline and 5 y FU | Mean 0.3 at follow‐up compared to 0.6 at baseline | Not provided | a | |
| Resistance training | Videman et al, 200842 | High | Resistance training frequency | Male monozygotic Finnish twins at baseline and 5 years FU | Not reported | Not provided | a |
| Videman et al, 200643 | High | Resistance training frequency (frequency/week) | Male monozygotic Finnish twins at baseline and 5 y FU | Mean 0.1 at follow‐up compared to 0.1 at baseline | P = .039 | b | |
| Lifting weight | Teraguchi et al, 201735 | High | Lifting loads weighting ≥10 kg > 1/wk | Volunteers from the coastal region of Wakayama with an MRI at baseline and 4 y FU | 162 subjects lifting weight compared to 189 subjects not lifting weight | P > .05; OR 0.91; CI 0.66‐1.26 | a |
| Videman et al, 200842 | High | Occupational lifting | Male monozygotic Finnish twins at baseline and 5 years FU | Not reported | Not provided | a | |
| Videman et al, 200643 | High | Occupational lifting (1‐4 code) | Male monozygotic Finnish twins at baseline and 5 y FU | Mean 2.5 at follow‐up compared to 2.4 at baseline | P = .021 | b | |
| American Football position | Nagashima et al, 201341 | Low | Position played during career | High school American Football players with an MRI at baseline and 2 y of FU | Not reported | P = .006; PRC 3.47; CI 1.01‐5.93 | b |
| American Football playing career | Nagashima et al, 201341 | Low | Length of playing career | High school American Football players with an MRI at baseline and 2 y of FU | Mean decrease in signal intensity of 4.30% in continuing players compared to 1.41% in noncontinuing players | P = .12 | a |
| Fast bowling | Burnett et al, 199645 | High | Different techniques | Fast bowlers using the mixed bowling technique at baseline and FU compared to those who used this technique during baseline or follow‐up only | Eight fast bowlers compared to one fast bowler | P = .015 | b |
Abbreviations: AF, annulus fibrosus; BMI, body mass index; CI, confidence interval; FU, follow‐up; OR, odds ratio; PRC, partial regression coefficient; RR, relative risk.
No association/no relationship found between prognostic factor and disk degeneration progression.
Positive association between prognostic factor and increased disk progression.
Negative association between prognostic factor and increased disk progression.
Table 4.
Imaging determinants as prognostic factors for progression in DD
| Imaging determinant | Author, year | Study quality | Measurement method | Study population | Reported effect sizes | Statistical analysis | Association with DD progression |
|---|---|---|---|---|---|---|---|
| Lumbar lordosis | Farshad‐Amacker et al, 201432 | Low | Mean degree of lordosis L1‐S1 | Patients with an MRI at baseline and 4 y FU | Mean 43, SD 12 with progression compared to mean 49, SD 11 without progression | P = .017 | a |
| Sacral slope | Farshad‐Amacker et al, 201433 | Low | Mean degree of sacral slope | Patients with an MRI at baseline and 4 y FU | Mean 39, SD 7 with progression compared to mean 41, SD 8 without progression | P = .11 | b |
| Disc level | Sharma et al, 201137 | High | Segmental disc level | Patients with a MRI of the lumbar spine at baseline and FU | Sum of squares 1.58; F ratio 1.49 | P = .1921 | b |
| Degenerated discs | Elfering et al, 200239 | High | Number of degenerated discs | Asymptomatic individuals at baseline and ≥ 5 y FU | Mean 1.00, SD 0.79 with progression compared to mean 0.38, SD 0.71 without progression | P < .01 | a |
| Disc herniation | Elfering et al, 200239 | High | Initial extent of disc herniation; from 1 (ie, normal) to 4 (ie, sequestration) | Asymptomatic individuals at baseline and ≥ 5 y FU | Mean 2.06, SD 0.56 with progression compared to mean 1.63, SD 0.49 without progression | P < .05; OR 12.63; CI 1.24‐128.49 | a |
| Nagashima et al, 201341 | Low | Presence at baseline | High school American Football players with an MRI at baseline and 2 y of FU | Not reported | P = .018; PRC 4.09; CI 0.72‐7.46 | a | |
| Sharma, 201137 | High | Presence at baseline | Patients with a MRI of the lumbar spine at baseline and FU | Sum of squares 10.108357; F ratio 47.5933 | P < 0.0001 | a | |
| Modic type I | Kerttula et al, 201247 | High | Presence and change of M1 | M1 type change in the upper endplate in relation to 1. the change of disc height and 2. change of disc signal intensity |
1. 59 discs of total 270 discs 2. 61 of total 270 discs |
1. P < .001 2. P < .001 |
a |
| Scoliosis | Farshad‐Amacker et al, 201734 | Low | Apex of the scoliosis | Apex of scoliosis at same level | 11% in progression compared to 4% without progression | P = .07; OR 2.97; CI 0.91‐9.58 | b |
| Farshad‐Amacker et al, 201432 | Low | Mean degree of scoliosis | Patients with an MRI at baseline and 4 y FU | Mean 7, SD 9 with progression compared to mean 9, SD 10 without progression | P = .26 | b | |
| Listhesis | Farshad‐Amacker et al, 201734 | Low | Presence and level | Listhesis at the same level | 6% in progression compared to 3% without progression | P = .27; OR 2.06; CI 0.92‐9.58 | b |
| Farshad‐Amacker et al, 201432 | Low | Yes or no | Patients with an MRI at baseline and 4 y FU | Thirty‐three subjects with progression compared to 14 subjects without progression | P = .99 | b | |
| Endplate degeneration | Farshad‐Amacker et al, 201734 | Low | Endplate score for each endplate | Endplate score of ≥4 | About 29% in progression compared to 15% without progression | P = .03; OR 2.32; CI 1.07‐5.01 | a |
| Annulus tear | Farshad‐Amacker et al, 201432 | Low | Presence of hyperintense zone within the AF | Subjects who had a lumbar spine MRI with a previous MRI > 4 y apart | About 25% of the case group compared to 22% of the control group | P = 1.00; OR 0.86 | b |
| Sharma et al, 200936 | High | Presence of hyperintense signal intensity within the peripheral annulus | Patients with low back pain with an MRI at baseline and ± 2.5 y FU | Increase of 0.42 in signal‐intensity grade for discs with annular tears compared to a change of 0.15 for discs without annular tears | P < .0001 | a | |
| Radial tear | Sharma et al, 201137 | High | Annular tears that appeared contiguous with the hyperintensity of the nucleus | Patients with a MRI of the lumbar spine at baseline and FU | Sum of squares 1.188153; F ratio 5.5942 | P = .0185 | a |
| Schmorl nodes | Nagashima et al, 201341 | Low | Presence at baseline | High school American Football players with an MRI at baseline and 2 y of FU | Not reported | P = .017; PRC* 3.58; CI 0.66‐6.50 | a |
Abbreviations: AF, annulus fibrosus; BMI, body mass index; CI, 95% confidence interval; FU, follow‐up; OR, odds ratio; PRC, partial regression coefficient; RR, relative risk.
Positive association between prognostic factor and increased disk progression.
No association/no relationship found between prognostic factor and disk degeneration progression.
Negative association between prognostic factor and increased disk progression.
3.4. Best‐evidence synthesis
There was only strong evidence (consistent [>75%] findings in multiple (≥ 2) high‐quality studies) found that disc herniation at baseline is associated with progression of DD at follow‐up (Table 5). Both the heterogeneity between and the limited amount of the included studies resulted at best in limited evidence for most prognostic factors, thereby limiting the informative value of the best‐evidence synthesis. Limited evidence (findings in one high‐quality study or consistent [>75%] findings in ≥3 low‐quality studies) was found that that heritability, genetic risk factors (ie, T‐allele IL1A rs1800587 female), fast bowling, weight lifted at work, work schedule, lack of sports activities, number of degenerated discs, presence and change of Modic type I and radial tears were, to some extent, associated with progression. There was also some inconclusive evidence (findings found in <3 low‐quality studies) due to the low‐quality of the corresponding studies that lumbar lordosis, endplate degeneration, Schmorl nodes and the field position played in American football during high school are associated with progression. Conflicting evidence (<75% of the studies reported consistent findings) for progression was found for overweight, resistance training, lifting weight, and annulus tears.
Table 5.
Best‐evidence synthesis of prognostic factors in the progression of DD
| Associated with progression | Associated with no progression | Not‐associated with progression | |
|---|---|---|---|
| Strong evidence (Consistent (>75%) findings in multiple (≥2) high‐quality studies) |
Disc herniation | Age, gender, body weight, BMI, smoking, car driving, occupation, recreational activities at leisure time | |
| Moderate evidence (Findings in one high‐quality study and consistent (>75%) findings in ≥2 low‐quality studies) |
| Insufficient evidence for association with progression | Insufficient evidence for association with no progression | Insufficient evidence for no association with progression | |
|---|---|---|---|
| Limited evidence (Findings in one high‐quality study or consistent (>75%) findings in ≥ 3 low‐quality studies) |
Heritability, genetic risk factors (ie, T‐allele IL1A rs1800587 female), fast bowling, weight lifted at work, work schedule, lack of sports activities, number of degenerated discs, presence and change of Modic type I, radial tears | Genetic risk factor (ie, IL6 rs1800795 genotype G/C male) | Obesity, pregnancy, DM, hypertension, back injuries, working style, disc level |
| Inconclusive evidence (Findings found in < 3 low‐quality studies) |
American Football position played during career, lumbar lordosis, endplate degeneration, Schmorl nodes | American Football playing career, sacral slope, scoliosis, listhesis | |
| Conflicting evidence (<75% of the studies reported consistent findings) |
Overweight, resistance training, lifting weight, annulus tear |
Strong evidence (consistent (>75%) findings in multiple (≥ 2) high‐quality studies) was found that age, gender, body weight, BMI, smoking, car driving, occupation, and recreational activities at leisure time are not associated with progression, and there was limited evidence (findings in one high‐quality study or consistent (>75%) findings in ≥3 low‐quality studies) that obesity, pregnancy, DM, hypertension, back injuries, working style, and disc level were not associated with progression. Inconclusive evidence (findings found in <3 low‐quality studies) was found that American football playing career, sacral slope, scoliosis, and listhesis are not associated with progression. IL6 rs1800795 genotype G/C male was the only factor that was associated with no progression, but this can only be qualified as limited evidence as this factor was only studied in one high‐quality study.
4. DISCUSSION
Intervertebral DD progresses over time and is hard to stop or reverse, as treatments that successfully interfere with progression are still not available. However, recent in vitro and in vivo studies on regenerative therapies for DD show some promising results,19, 20, 23, 24, 25, 48, 49, 50, 51 and more insight in the factors that encourage the progression of DD may therefore provide valuable stepping stones towards personalized treatments, as we then know which patients should be targeted with these therapies. The aim of this systematic review was to identify the prognostic factors associated with progression of DD. Despite the differences between definitions and the heterogeneity in measured determinants between the studies, we provided an overview of 12 imaging and 23 clinical and environmental prognostic factors.
Strong evidence was found that the presence of disc herniation is associated with progression of DD at the same level. Disc herniation increases the mechanical stresses to the intervertebral disc as the main shock absorber, the nucleus pulposus, is pushed through the annulus, making the disc prone for the cascade of degeneration. Three studies showed that disc herniation at baseline was associated with progression of DD at follow‐up. In those studies, disc herniation was determined on MRI. Elfering et al (a high‐quality study) report that the initial extent of disc herniation (ie, protrusion or extrusion) was a significant risk factor for progression.39 In this study, patients with symptomatic disc herniation that required surgery were included, although it is unclear whether these patients were operated during the follow‐up period. Nagashima et al. (ie, low‐quality study) found that a disc herniation (ie, protrusion or extrusion) evaluated at baseline on MRI significantly related to decrease in signal intensity of the nucleus pulposus 2 years later in 29 high school American Football players (P = .018).41 Although the authors do not mention it explicitly, it seems that their study population did not suffer from any symptoms at baseline, as the study subjects were recruited from high school American Football players. It is unknown whether the American Football players diagnosed with disc herniation were put to any therapy. The study of Sharma et al found that disc herniation at the time of the initial MRI study was significantly related to nuclear degeneration at follow‐up.37 Due to the retrospective design of the study, in which they searched their radiology report database without consulting the corresponding patients, it is unclear whether the patients with disc herniation on MRI were put to any medical treatment. The indication for the MRI was the only information provided, with low back pain as the most common indication (53 out of 63 patients).37 They defined disc herniation as radial tears with associated contour abnormality, seen on MRI. This differs from the other two studies, just like their definition of DD. Sharma et al defined DD as grade 2 or more on the Pfirrmann classification,37 whereas Elfering et al use the Pearce classification and Nagashima et al describe it as a decreased signal intensity of the nucleus pulposus compared to the signal intensity of the cerebrospinal fluid.39, 41 Despite these differences in definition, the results of these studies indicate that both DD and disc herniation have a synergistic effect to the cascade of DD. This might seem trivial, but the underlying pathomechanism is not cleared up yet, nor is it clear whether disc herniation is truly a causal factor of progression and even the initiation of DD, or that it is a consequence of the native, in this case presumably inferior, quality of the discs.52, 53 Since none of the three studies reported whether patients with disc herniation received any medical treatment, it is also unknown whether this affected the course of degeneration.
The most surprising outcome of this study is probably that strong evidence was found that age, gender, body weight, BMI, smoking, car driving, the type of occupation (ie, working as a nurse or construction carpenter) nor recreational activities at leisure time were associated with the progression of DD. This was unexpected, as several studies show that heavy physical activity or work and smoking are key factors in the onset of DD.11, 54, 55, 56 For smoking, however, there is one high‐quality study (out of five) that finds that smoking during follow‐up to a greater reduction in disc height.42 This is in contrast to a study by the same authors 2 years earlier, in which they found that smoking did not have any effect on the change in degeneration.43 Liuke et al found that there were no statistically significant differences in the number of discs with decreased signal intensity at baseline and follow‐up between construction carpenters, machine operators, and office workers.40 These results seem to indicate that some clinical and environmental factors (eg, age, gender, body weight, and smoking) are not associated with progression of DD.
Limited evidence (findings in one high‐quality study or consistent (>75%) findings in ≥3 low‐quality studies) was found for one genetic marker (specifically: IL6 rs1800795 genotype G/C male) to have a protective effect on progression, although the authors note in their study that correction for multiple testing weakened the associations for IL6 polymorphisms in their study.46 IL6 is involved as an important cytokine in inflammatory reactions and seems to be produced at the site of lumbar disc herniation.57, 58 A polymorphism to this gene might therefore have a preventive effect on damage to the extracellular matrix. Since modern techniques to evaluate genetic risk factors are becoming more accessible, more of these protective factors are expected to be discovered.
The results of the present study should be interpreted with some caution and may not be directly applicable to the individual patient, as we did not include any symptoms into our inclusion criteria, resulting in an asymptomatic study population in many of the included studies. We also only included manuscripts published in English or German, and therefore might have missed some other prognostic factors. In addition, no studies were found that studied molecular biomarkers in relation to progression of DD. This is surprising, as biomarkers are subject of many studies in relation to DD,59, 60, 61, 62 but are presumably related to the onset of DD and not progression, which is beyond the scope of this review. Second, there was a high heterogeneity between studies regarding the definition of DD and its progression, which made it impossible to pool data. We were also unable to perform a quantitative analysis of the included studies, and therefore it was not possible to study the interplay and relative contribution of each prognostic factors in the progression of DD. Third, the causal relation between DD and low back pain remains disputed. We did not study the relationship of DD to the clinical presentation of patients. Although previous studies have demonstrated that degenerative disc disease is associated with low back pain,63, 64 more research needs to be conducted to identify how progression of DD is related to clinical course. The identification of prognostic factors for progression is crucial to establish optimal follow‐up strategies and timing for regenerative medicine. Fourth, in some studies, it appeared that the same patient population was used within different studies, such as the three papers of Farshad‐Amacker et al.32, 33, 34 Since we present an overview of all studies that describe prognostic factors in the progression of DD, we presented these studies as three separate studies, since the outcomes would not have been affected if the results were presented in just a single study. Finally, there was conflicting evidence for overweight, resistance training, lifting weight and annulus tears, and many factors have been addressed by only one study, resulting in a high number of prognostic factors with limited evidence.
This high number of conflicting and limited evidence and the heterogeneity between the studies indicates that the current definition of DD is not on‐point and that the natural history of DD is unclear. This is also reflected by the mostly unclear definitions of progression. In most studies, it was defined as an increase in the grade of the specific grading system that was used compared to baseline, without any further description. A clear description of the studied subjects often lacked and it was sometimes unknown whether the study population suffered from symptoms or was asymptomatic. In addition, the inter‐observer reliability scores are usually reliability scores by the designers of the grading systems and not by independent and representative observers for grading DD. The reported statistic values are often concise and the effect sizes small. Subsequently, it is hard to draw firm conclusions or recommendations for clinical practice based on the outcomes of this study as it is difficult to predict which patients will reach the final stages of DD earlier than others patients, and thus, which patients should be targeted with regenerative therapies. Future research using identical determinants and outcome parameters on these factors may give a better insight in their role in the cascade of degeneration. These future studies should include a high number of patients, such as a population screening, as rapid progression of DD is probably the result of a synergistic effect between several prognostic factors, and not just one. The outcome of those studies would not only add to the understanding of the pathophysiology of DD, but also sharpen the definition of DD and its natural history, and provide valuable information for spinal phenotyping and clinical decision making. We would then know what combination of patients' specific factors will encourage progression of DD, which will enable physicians to predict which patients with DD will most likely progress to severe degeneration. This directly contributes to personalized medicine and thus, will clarify which patients should be targeted with regenerative therapies.
5. CONCLUSION
This review shows strong evidence that disc herniation is associated with progression in DD, while most clinical and environmental risk factors (eg, age, gender, body weight, and smoking) are not associated with progression. However, limited or conflicting evidence was found for most of the prognostic factors, due to diversity in determinants and outcome parameters between the included studies. This makes it difficult to predict any risk factors for the progression of DD and shows that the current definition of DD is not on‐point and that the natural history of DD is unclear. Future studies on these factors are recommended in order to identify the target group of patients for regenerative therapies and to sharpen the definition and natural history of DD. Future studies should use uniform definitions and well‐described and universal determinants, in order to avoid confusion and to facilitate clearer comparisons.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHORS CONTRIBUTIONS
All authors have contributed to the manuscript and approved the final version.
Supporting information
File S1. Supporting Information.
ACKNOWLEDGMENTS
No financial support, grant or other benefits from commercial sources were received for this article.
Rustenburg CME, Faraj SSA, Ket JCF, Emanuel KS, Smit TH. Prognostic factors in the progression of intervertebral disc degeneration: Which patient should be targeted with regenerative therapies? JOR Spine. 2019;2:e1063 10.1002/jsp2.1063
REFERENCES
- 1. Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(4):652‐655. [DOI] [PubMed] [Google Scholar]
- 2. Detiger SEL, Holewijn RM, Hoogendoorn RJW, et al. MRI T2* mapping correlates with biochemistry and histology in intervertebral disc degeneration in a large animal model. Eur Spine J. 2015;24:1935‐1943. 10.1007/s00586-014-3498-1. [DOI] [PubMed] [Google Scholar]
- 3. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151‐2161. [DOI] [PubMed] [Google Scholar]
- 4. Emanuel KS, Vergroesen P‐PA, Peeters M, Holewijn RM, Kingma I, Smit TH. Poroelastic behaviour of the degenerating human intervertebral disc: a ten‐day study in a loaded disc culture system. Eur Cell Mater. 2015;29:330‐341. [DOI] [PubMed] [Google Scholar]
- 5. Siepe CJ, Hitzl W, Meschede P, Sharma AK, Khattab MF, Mayer MH. Interdependence between disc space height, range of motion and clinical outcome in total lumbar disc replacement. Spine. 2009;34(9):904‐916. [DOI] [PubMed] [Google Scholar]
- 6. Kalichman L, Kim D, Li L, Guermazi A, Hunter D. Computed tomography‐evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self‐reported low back pain. Spine J. 2010;10(3):200‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheele J, De Schepper EIT, Van Meurs JBJ, et al. Association between spinal morning stiffness and lumbar disc degeneration: the Rotterdam study. Osteoarthr Cartil. 2012;20(9):982‐987. [DOI] [PubMed] [Google Scholar]
- 8. Vergroesen P‐PA, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23(7):1057‐1070. [DOI] [PubMed] [Google Scholar]
- 9. Paul C, Schoorl T, Zuiderbaan H, et al. Dynamic and static overloading induce early degenerative processes in Caprine lumbar intervertebral discs. PLoS One. 2013;8(4):e62411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25(12):1477‐1483. [DOI] [PubMed] [Google Scholar]
- 11. Hung Y, Shih T, Chen B, et al. The dose‐response relationship between cumulative lifting load and lumbar disk degeneration based on magnetic resonance imaging findings. Phys Ther. 2014;94(11):1582‐1593. [DOI] [PubMed] [Google Scholar]
- 12. Metz L, Lovell A, Graham J, Liebenberg E, Havel P, Lotz J. Does diabetes cause the intervertebral disc to degenerate? Spine J. 2012;12(9):74S‐75S. [Google Scholar]
- 13. Silberberg R, Adler JH, Meier‐Ruge W. Effects of hyperinsulinism and of diabetes on proteoglycans of the intervertebral disc in weanling sand rats. Exp Cell Biol. 1986;54(3):121‐127. [DOI] [PubMed] [Google Scholar]
- 14. Robinson D, Mirovsky Y, Halperin N, Evron Z, Nevo Z. Changes in proteoglycans of intervertebral disc in diabetic patients: a possible cause of increased back pain. Spine. 1998;23(8):849‐855. [DOI] [PubMed] [Google Scholar]
- 15. Italiano A. Prognostic or predictive? It's time to get back to definitions! J Clin Oncol. 2011;29(35):4718. [DOI] [PubMed] [Google Scholar]
- 16. Tsahtsarlis A, Wood M. Minimally invasive transforaminal lumber interbody fusion and degenerative lumbar spine disease. Eur Spine J. 2012;21(11):2300‐2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J. 2010;19(8):1262‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tschugg A, Michnacs F, Strowitzki M, Meisel HJ, Thome C. A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: study protocol. Trials. 2016;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiraishi S, Schol J, Sakai D, et al. Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model. JOR Spine. 2018;1:e1013 10.1002/jsp2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith LJ, Silverman L, Sakai D, et al. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: recommendations of the ORS spine section. JOR Spine. 2018;1:e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buckley CT, Hoyland JA, Fujii K, Pandit A, Iatridis JC, Grad S. Critical aspects and challenges for intervertebral disc repair and regeneration‐harnessing advances in tissue engineering. JOR Spine. 2018;1:e1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thorpe AA, Bach FC, Tryfonidou MA, et al. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine. 2018;1:e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakai D, Andersson GBJ. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243‐256. [DOI] [PubMed] [Google Scholar]
- 24. Noriega DC, Ardura F, Hernández‐Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101:1945‐1951. [DOI] [PubMed] [Google Scholar]
- 25. Oehme D, Goldschlager T, Ghosh P, Rosenfeld JV, Jenkin G. Cell‐based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cells Int. 2015;2015:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses : the PRISMA statement. BMJ. 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- 27. Hayden JA, Côté P, Bombardier C, Cote P. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427‐437. [DOI] [PubMed] [Google Scholar]
- 28. Bastick A, Runhaar J, Belo J, Bierma‐Zeinstra S. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther. 2015;17(152):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lievense A, Bierma‐Zeinstra S, Verhagen A, Verhaar J, Koes B. Prognostic factors of progress of hip osteoarthritis: a systematic review. Arthritis Rheum. 2002;47(5):556‐562. [DOI] [PubMed] [Google Scholar]
- 30. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360‐363. [PubMed] [Google Scholar]
- 31. Furlan AD, Pennick V, Bombardier C, Van Tulder M. 2009 updated method guidelines for systematic reviews in the cochrane back review group. Spine (Phila Pa 1976). 2009;34(18):1929‐1941. [DOI] [PubMed] [Google Scholar]
- 32. Farshad‐Amacker NA, Hughes AP, Aichmair A, Herzog RJ, Farshad M. Is an annular tear a predictor for accelerated disc degeneration? Eur Spine J. 2014;23(9):1825‐1829. [DOI] [PubMed] [Google Scholar]
- 33. Farshad‐Amacker NA, Hughes AP, Aichmair A, Herzog RJ, Farshad M. Determinants of evolution of endplate and disc degeneration in the lumbar spine: a multifactorial perspective. Eur Spine J. 2014;23(9):1863‐1868. [DOI] [PubMed] [Google Scholar]
- 34. Farshad‐Amacker NA, Hughes A, Herzog RJ, Seifert B, Farshad M. The intervertebral disc, the endplates and the vertebral bone marrow as a unit in the process of degeneration. Eur Radiol. 2017;27(6):2507‐2520. [DOI] [PubMed] [Google Scholar]
- 35. Teraguchi M, Yoshimura N, Hashizume H, et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population‐based cohort: the Wakayama spine study. Osteoarthr Cartil. 2017;25(7):1122‐1131. [DOI] [PubMed] [Google Scholar]
- 36. Sharma A, Pilgram T, Wippold FJ. Association between annular tears and disk degeneration: a longitudinal study. Am J Neuroradiol. 2009;30(3):500‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma A, Parsons M, Pilgram T. Temporal interactions of degenerative changes in individual components of the lumbar intervertebral discs: a sequential magnetic resonance imaging study in patients less than 40 years of age. Spine. 2011;36(21):1794‐1800. [DOI] [PubMed] [Google Scholar]
- 38. Makino H, Kawaguchi Y, Seki S, et al. Lumbar disc degeneration progression in young women in their 20's: a prospective ten‐year follow up. J Orthop Sci. 2017;22(4):635‐640. [DOI] [PubMed] [Google Scholar]
- 39. Elfering A, Semmer N, Birkhofer D, Zanetti M, Hodler J, Boos N. Young investigator award 2001 winner: risk factors for lumbar disc degeneration: a 5‐year prospective MRI study in asymptomatic individuals. Spine. 2002;27(2):125‐134. [DOI] [PubMed] [Google Scholar]
- 40. Liuke M, Solovieva S, Lamminen A, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes. 2005;29:903‐908. [DOI] [PubMed] [Google Scholar]
- 41. Nagashima M, Abe H, Amaya K, et al. Risk factors for lumbar disc degeneration in high school American football players: a prospective 2‐year follow‐up study. Am J Sports Med. 2013;41(9):2059‐2064. [DOI] [PubMed] [Google Scholar]
- 42. Videman T, Battie MC, Parent E, Gibbons LE, Vainio P, Kaprio J. Progression and determinants of quantitative magnetic resonance imaging measures of lumbar disc degeneration: a five‐year follow‐up of adult male monozygotic twins. Spine. 2008;33(13):1484‐1490. [DOI] [PubMed] [Google Scholar]
- 43. Videman T, Battie MC, Ripatti S, Gill K, Manninen H, Kaprio J. Determinants of the progression in lumbar degeneration: a 5‐year follow‐up study of adult male monozygotic twins. Spine. 2006;31(6):671‐678. [DOI] [PubMed] [Google Scholar]
- 44. Williams FMK, Popham M, Sambrook PN, Jones AF, Spector TD, MacGregor AJ. Progression of lumbar disc degeneration over a decade: a heritability study. Ann Rheum Dis. 2011;70(7):1203‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burnett AF, Khangure MS, Elliott BC, Foster DH, Marshall RN, Hardcastle PH. Thoracolumbar disc degeneration in young fast bowlers in cricket: a follow‐up study. Clin Biomech. 1996;11(6):305‐310. [DOI] [PubMed] [Google Scholar]
- 46. Eskola PJ, Kjaer P, Sorensen JS, et al. Gender difference in genetic association between IL1A variant and early lumbar disc degeneration: a three‐year follow‐up. Int J Mol Epidemiol Genet. 2012;3(3):195‐204. [PMC free article] [PubMed] [Google Scholar]
- 47. Kerttula L, Luoma K, Vehmas T, Grönblad M, Kääpä E. Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1‐year follow‐up study. Eur Spine J. 2012;21:1135‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frauchiger DA, Tekari A, Wöltje M, Fortunato G, Benneker LM, Gantenbein B. A review of the application of reinforced hydrogels and silk as biomaterials for intervertebral disc repair. Eur Cell Mater. 2017;34:271‐290. [DOI] [PubMed] [Google Scholar]
- 50. Iatridis JC, Kang J, Kandel R, Risbud MV. New horizons in spine research: intervertebral disc repair and regeneration. J Orthop Res. 2017;35:5‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bachmeier BE, Nerlich A, Mittermaier N, et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18:1573‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schroeder JE, Dettori JR, Brodt ED, Kaplan L. Disc degeneration after disc herniation: are we accelerating the process? Evid Based Spine Care J. 2012;3(4):33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seidler A, Euler U, Bolm‐Audorff U, et al. Physical workload and accelerated occurrence of lumbar spine diseases: risk and rate advancement periods in a German multicenter case‐control study. Scand J Work Environ Health. 2011;37(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 55. Nemoto Y, Matsuzaki H, Tokuhasi Y, et al. Histological changes in intervertebral discs after smoking and cessation: experimental study using a rat passive smoking model. J Orthop Sci. 2006;11:191‐197. [DOI] [PubMed] [Google Scholar]
- 56. Elmasry S, Asfour S, De Rivero Vaccari JP, Travascio F. Effects of tobacco smoking on the degeneration of the intervertebral disc: a finite element study. PLoS One. 2015;10(8):e0136137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith AJP, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43‐59. [DOI] [PubMed] [Google Scholar]
- 58. Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Jt Surg Br. 2002;84:196‐201. [DOI] [PubMed] [Google Scholar]
- 59. Zhou X, Chen L, Grad S, et al. The roles and perspectives of microRNAs as biomarkers for intervertebral disc degeneration. J Tissue Eng Regen Med. 2017;11:3481‐3487. [DOI] [PubMed] [Google Scholar]
- 60. Tang X, Jing L, Richardson WJ, et al. Identifying molecular phenotype of nucleus pulposus cells in human intervertebral disc with aging and degeneration. J Orthop Res. 2016;34:1316‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu F, Gao F, Liu Y, et al. Bioinformatics analysis of molecular mechanisms involved in intervertebral disc degeneration induced by TNF‐α and IL‐1β. Mol Med Rep. 2016;13:2925‐2931. [DOI] [PubMed] [Google Scholar]
- 62. Weber KT, Jacobsen TD, Maidhof R, et al. Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics. Curr Rev Musculoskelet Med. 2015;8:18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brinjikji W, Diehn FE, Jarvik JG, et al. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta‐analysis. Am J Neuroradiol. 2015;36(12):2394‐2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427‐434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Supporting Information.


