Abstract
BACKGROUND:
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) share common genetic factors but seem to have specific patterns of psychiatric comorbidities. There are few systematic studies on adults; therefore, we compared psychiatric comorbidities in adults with these two neurodevelopmental disorders using population-based data and analyzed their genetic correlations to evaluate underlying factors.
METHODS:
Using data from Norwegian registries, we assessed patterns of psychiatric disorders in adults with ADHD (n = 38,636; 2.3%), ASD (n = 7528; 0.4%), and both diagnoses (n = 1467; 0.1%) compared with the remaining adult population (n = 1,653,575). We calculated their prevalence ratios (PRs) and differences using Poisson regression, also examining sex-specific relations. Genetic correlations (rg) among ADHD, ASD, and the examined psychiatric disorders were calculated by linkage disequilibrium score regression, exploiting summary statistics from relevant genome-wide association studies.
RESULTS:
For all psychiatric comorbidities, PRs differed between ADHD and ASD. Associations were strongest in individuals with ADHD and ADHD+ASD for most comorbidities, in both men and women. The relative prevalence increase of substance use disorder was three times larger in ADHD than in ASD (PRADHD, 6.2; 95% confidence interval [CI], 6.1–6.4; PRASD, 1.9; 95% CI, 1.7–2.2; p<.001); however, the opposite was true for schizophrenia (PRASD,13.9; 95% CI, 12.7–15.2; PRADHD, 4.4; 95% CI, 4.1–4.7; p<.001). Genetic correlations supported these patterns but were significantly different between ADHD and ASD only for the substance use disorder proxies and personality traits (p<.006 for all).
CONCLUSIONS:
Adults with ADHD, ASD, or both ADHD and ASD have specific patterns of psychiatric comorbidities. This may partly be explained by differences in underlying genetic factors.
Keywords: ADHD, ASD, Genetics, Psychiatric comorbidity, Schizophrenia, SUD
Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are highly heritable neurodevelopmental conditions and major contributors to human suffering worldwide (1,2). There is emerging evidence of polygenicity and environmental factors contributing to both disorders There is emerging evidence of polygenicity and environmental factors contributing to both disorders (3,4). Genetic, epidemiological, and twin studies show that ADHD and ASD often co-occur and share common underlying genetic factors (5–8), but with different phenotypic characteristics. The shared genetic factors are believed to affect the structure and function of molecular networks in the brain, possibly involved in the etiology of ADHD (9) and ASD (10).
Individuals with either ADHD or ASD have a 65% to 90% risk of developing concomitant psychiatric disorders (11–13), but with seemingly different patterns of comorbidity. Adults with ASD present high rates of co-occurring anxiety, depression (13), bipolar disorder (BD), and schizophrenia spectrum disorder (SCZ) (14–16), while adult ADHD is reported to co-occur with anxiety disorder and major depressive disorder (MDD) (17–19), BD (11,19–22), personality disorders (PDs) (18,19,23), SCZ, (19,22,24,25), and substance use disorder (SUD) (19,20,26).
ADHD and ASD share genetic factors with the abovementioned psychiatric disorders (27), and significant genetic correlations between different phenotype-specific traits for ADHD and ASD have been demonstrated (28).
Nonetheless, except for a couple of small clinical studies, patterns of psychiatric comorbidities have not been systematically compared between adults with ASD or ADHD (29,30). Further, previous studies have reported that children with both ADHD and ASD may have more severe impairments than those with ASD alone; however, comparable studies in adults are lacking (31,32). Only one single population-based study has directly compared individuals with ADHD alone, ASD alone, or both ADHD and ASD with unaffected individuals, but this was in a population too young to be diagnosed with adult-onset psychiatric disorders (33).
We aimed at evaluating similarities and differences in psychiatric comorbidity between ADHD and ASD in adulthood, both clinically and genetically. Therefore, we compared the prevalence of psychiatric disorders in adults with ADHD alone, ASD alone, and both ADHD and ASD with adults without ADHD or ASD and supplemented these analyses with the estimation of genetic correlations between the studied disorders.
METHODS AND MATERIALS
Registries
We conducted cross-sectional analyses in a cohort of adults by linking information from four nationwide, population-based registries, all with compulsory notification: the Medical Birth Registry of Norway, established in 1967 (34); the Norwegian Prescription Database (NorPD) (35), established in 2004; the Norwegian Patient Registry (NPR) (36), with data since 2008; and the National Educational Database from Statistics Norway (37,38) (see Supplement for more details).
Individual records from the registries were linked by means of the unique national identification numbers, given to all individuals residing in the country. The Regional Ethics Committee in Norway approved the study (2011/2272). No informed consent was required for the analyses, as the records were anonymized. To guide the reporting of this study, we used the Strengthening the Reporting of Observational Studies in Epidemiology statement (39).
Study Population and Exposure Groups
The study included 1,701,206 individuals born in Norway between 1967 and 1997 who were alive and living in Norway in 2015, the year of data linkage. This population consists of individuals mainly of European descent, with 8.2% of births registered to mothers from non-European countries by 2015. We defined adults with ADHD only (ADHD) as those who were dispensed ADHD medication at any time between 2004 and 2015 (NorPD) or had an ADHD diagnosis (ICD-10 code F90), but no ASD diagnosis, registered in the NPR during 2008 to 2015, and who were 18 years of age or older. The ADHD medications were methylphenidate, racemic amphetamine, dexamphetamine, and atomoxetine. Individuals prescribed central stimulants for narcolepsy were excluded (see Supplement for details).
Adults with ASD only (ASD) were defined as individuals with an ASD diagnosis (ICD-10 codes F84.0–1+F84.5+F84.8–9) (40,41) who were 18 years of age or older, were registered in the NPR during 2008 to 2015, and had no ADHD diagnosis. Adults (18 years of age or older) with both ADHD and ASD as defined above comprised the combined group (ADHD+ASD).
The remaining population included all adults who were neither dispensed ADHD medication registered in the NorPD nor had an ADHD or ASD diagnosis registered in the NPR. Parents of adults with and without ADHD/ASD were also identified through the Medical Birth Registry of Norway and included in the analyses to account for parental factors associated with ADHD, ASD, and other psychiatric disorders (e.g., sociodemographic factors, pregnancy-related factors, parental psychiatric disorders) and relatedness.
Outcome Diagnoses
We studied the following major comorbid psychiatric disorders typically diagnosed in late adolescence and adulthood (42), all registered in the NPR, at 18 years of age or older: anxiety disorders (ICD-10 codes F40–F42); MDD (F32–F33); BD (F30– F31); PDs (F60–F61), with a separate analysis on antisocial personality disorder (F60.2) (only included in the main analyses because of a small number of cases); SCZ (F20–F29); and SUD (F10–F19). For BD, we also included those individuals who were dispensed lithium during 2004 to 2015, according to NorPD.
Summary Statistics From Large-Scale Genome-wide Association Studies
Summary statistics from the large-scale genome-wide association studies (GWASs) for the psychiatric disorders examined (10,43–51) were downloaded from the LD Hub GWAS share center (http://ldsc.broadinstitute.org/gwashare/) (52). To date, no GWAS has examined the genetics of individuals diagnosed with both ADHD and ASD. Combining the existing data from individual GWASs on ADHD and ASD, respectively, would result in associations heavily biased toward the larger study. Thus, we analyzed ADHD and ASD separately.
As not all data are publicly available, and no large-scale, well-powered GWASs were performed on all disorders examined, we used some proxy traits. Owing to the lack of adequate genetic data on PDs, we combined the data from the five GWASs on the five traits in the NEO Personality Inventory (NEO) (neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness) (48,53) using the inverse variance method in METAL software (54). As all five NEO personality traits were analyzed in same-sized samples, there was no bias toward any of the traits. We also used the trait “antisocial behavior” as a proxy for antisocial personality disorder (49). As proxies for SUD, we used smoking behavior (ever smoked vs. never smoked) (51) and alcohol dependence (50).
In all analyses, we restricted the examined data to single nucleotide polymorphisms of good imputation quality (INFO ≥0.8), minor allele frequency ≥1% (common variation), and the data were derived from individuals of European descent only.
Statistical Analyses
We estimated prevalence ratios (PRs) using Poisson regression with robust standard errors (55) to examine the associations of ADHD, ASD, and ADHD+ASD with other psychiatric disorders, using the remaining adult population as reference. To adjust for potential confounders, we performed two regression models, which included the following covariates: 1) model I, which included birth year, maternal marital status, maternal age and paternal age in years at delivery, parents’ highest attained level of education at record linkage, the individual’s gestational age in weeks, and the individual’s gestational age- and sex-specific birth weight Z scores (56); and 2) model II, which included covariates of model I and mothers’ and fathers’ psychiatric diagnoses, including ADHD or any other psychiatric diagnosis from the NPR from 2008 to 2015 (for details about the covariates, see footnotes beneath the tables and figures and in the Supplement). To account for correlations between siblings, we used mother’s identification number as a cluster variable in the analyses.
Analyses were performed on the total sample and stratified by sex. Our main analyses were based on the multiplicative scale using relative effect measures; however, when assessing sex differences, we supplemented the analyses with absolute effect measures. For this, we estimated prevalence differences of psychiatric disorders between men and women with and without ADHD, ASD, or ADHD+ASD using predicted prevalence from a Poisson regression model with adjustment for birth year (5-year periods). Significance of interaction by sex on the multiplicative scale was evaluated by comparing the Poisson regression models with and without the interaction term (sex × ADHD) included, tested by likelihood ratio tests, and significance of interaction by sex on the additive scale was evaluated using relative excess risk due to interaction (57).
Two-sided tests with a significance threshold of p <.05 were employed in all analyses.
Analyses were carried out with SPSS version 22.0 (IBM Corp., Armonk, NY) and STATA intercooled version 14 (StataCorp, College Station, TX) from November 3, 2017, to March 13, 2019.
Genetic correlations (rg) were calculated using linkage disequilibrium score regression, which quantifies the similarities in genetic architecture between two traits by evaluating the relationship between single nucleotide polymorphism association strengths and genetic linkage disequilibrium (8). Owing to sample overlap between the examined datasets, the correlations were calculated without constraining the intercept. To calculate if the genetic correlations in the ADHD group were significantly different from those in the ASD group, we applied the following formula: , where rg1 refers to the genetic correlation between a comorbidity and ADHD, rg2 refers to the genetic correlation between the same comorbid disorder and ASD, a refers to SE of the rg1 estimate, and b refers to the standard error of the rg2 estimate. The significance was calculated using a twotailed Z test. To account for multiple testing, Bonferroni correction was applied to the significant threshold of .05, bringing the adjusted significance threshold to .00625.
Sensitivity Analyses
We conducted several sensitivity analyses to evaluate the robustness of our results. We reran all the analyses 1) excluding individuals with a diagnosis of intellectual disability,2) excluding all with comorbid diagnoses of SCZ when analyzing BD and SUD, 3) excluding all individuals with SUD when analyzing SCZ, and 4) including only individuals who had their psychiatric diagnosis registered at least twice in the NPR. Missing values in covariates (6% for gestational age and birth weight Z scores, <,1% for other variables) were handled by listwise deletion in the main analyses. In the sensitivity analyses, we also used multiple imputation with chained equations (58) to evaluate possible bias due to missing information in gestational age when adjusting for covariates. The outcome variables, all specified covariates, and also birth weight, maternal pre-eclampsia, and mother’s chronic diseases (yes/no) were used for this information.
RESULTS
Study Population
Among the 1,701,206 individuals included in the study, we identified 38,636 adults (2.3% of the population; 45% women) with ADHD, 7528 adults (0.4%; 27.9% women) with ASD, 1467 adults (0.1%; 28.8% women) with ADHD+ASD, and 1,653,575 adults (97.2%; 49% women) in the remaining population. In 2015, the mean ages of individuals in the ADHD, ASD, and ADHD+ASD groups were 31, 26, and 27 years of age, respectively, compared with 33 years of age in the remaining population (Table 1). Among parents, significantly more mothers of individuals with ASD and ADHD+ASD had the highest level of education compared with the ADHD group and the remaining population, likely explained by the mothers of individuals with ASD/ADHD+ASD having been born later in our study period, in a time during which higher education was more common. In addition, being diagnosed with at least one psychiatric disorder was more prevalent among parents of individuals in all exposure groups, compared with the remaining population (Table 1).
Table 1.
Sample Characteristics of the 1,701,206 Adults in the Study Population, All Born From 1967 to 1997 and Followed Until 2015
| Variable | ADHD | ASD | ADHD+ASD | Remaining Population |
|---|---|---|---|---|
| Population | 38,636 (2.3) | 7528 (0.4) | 1467 (0.1) | 1,653,575 (97.2) |
| Women | 17,393(45.0), p <.001a | 2099 (27.9), p =.491b | 422 (28.8), p <.001c | 809,962 (49.0), p <.001d |
| Male/Female Ratio | 1.22 | 2.58 | 2.48 | 1.04 |
| Age in 2015, Years | 31.3 ± 8.3, p <.001a | 26.2 ± 7.9, p =.99b | 26.8 ± 7.1, p <.001c | 33.2 ± 9.3, p <.001d |
| Maternal Age at Birth | p <.001a | p <.001b | p <.001c | p <.001d |
| <20 years | 4417(11.4) | 372 (4.9) | 107(7.3) | 112,674(6.8) |
| 20–24 years | 13,587 (35.2) | 1936(25.7) | 435 (29.7) | 498,264 (30.1) |
| 25–29 years | 11,865 (30.7) | 2539 (33.7) | 525 (35.8) | 575,743 (34.8) |
| 30–34 years | 6144(15.9) | 1747(23.2) | 279 (19.0) | 327,637(19.8) |
| 35–39 years | 2215(5.7) | 774 (10.3) | 102 (7.0) | 116,139 (7.0) |
| 40+ years | 408(1.1) | 160(2.1) | 19(1.3) | 23,118(1.4) |
| Paternal Age at Birth | p <.001a | p <.001b | p <.001c | p <.001d |
| <20 years | 1054(2.7) | 75 (1.0) | 21 (1.4) | 24,546 (1.5) |
| 20–24 years | 9013 (23.3) | 1093 (14.5) | 267 (18.2) | 302,939(18.3) |
| 25–29 years | 12,941 (33.5) | 2242 (29.8) | 484 (33.0) | 563,896 (34.1) |
| 30–34 years | 8728 (22.6) | 2120(28.2) | 392 (26.7) | 432,678 (26.2) |
| 35–39 years | 4086(10.6) | 1154 (15.3) | 182 (12.4) | 207,833(12.6) |
| 40–44 years | 1621 (4.2) | 519 (6.9) | 78 (5.3) | 76,947 (4.7) |
| 45–49 years | 489(1.3) | 171 (2.3) | 25(1.7) | 24,796 (1.5) |
| 50+ years | 183(0.5) | 70 (0.9) | 3 (0.2) | 8741 (0.5) |
| Missing | 521 (1.4) | 84(1.1) | 15(1.0) | 11,199(0.7) |
| Maternal Marital Statusd | p <.001a | p <.007b | p =.016c | p <.001d |
| Single | 6483(16.8) | 909 (12.1) | 225 (15.3) | 151,975(9.2) |
| Married/cohabiting | 31,122 (80.6) | 6508 (86.5) | 1220 (83.2) | 1,482,685 (89.7) |
| Other | 924 (2.4) | 96(1.3) | 20 (1.4) | 16,154(1.0) |
| Missing | 107(0.3) | 15(0.2) | 2 (0.1) | 2761 (0.2) |
| Maternal Education Status | p <.001a | p =.169b | p <.001c | p <.001d |
| Low | 13,498(34.9) | 1904 (25.3) | 394 (26.9) | 430,874 (26.1) |
| Middle | 16,478(42.7) | 3022 (40.1) | 583 (39.7) | 768,773 (46.5) |
| High | 8415(21.8) | 2565 (34.1) | 488 (33.3) | 447,380 (27.1) |
| Missing | 245 (0.6) | 37 (0.5) | 2 (0.1) | 6548 (0.4) |
| Paternal Education Status | p <.001a | p <.001b | p <.001c | p <.001d |
| Low | 12,501 (32.4) | 1639(21.8) | 388 (26.5) | 381,154(23.1) |
| Middle | 18,626(48.2) | 3486 (46.3) | 694 (47.3) | 834,541 (50.5) |
| High | 6475 (16.8) | 2237 (29.7) | 352 (24.0) | 414,399 (25.1) |
| Missing | 1034(2.7) | 166(2.2) | 33 (2.3) | 23,481 (1.4) |
| Maternal Psychiatric Disorder | p =.073a | p <.001b | p =.001c | p <.001d |
| None | 28,140(72.8) | 5557 (73.8) | 1009 (68.8) | 1,432,294(86.6) |
| Paternal Psychiatric Disorder | p <.001a | p =.010b | p =.492c | p <.001d |
| None | 31,478 (82.7) | 6289 (84.7) | 1189 (82.0) | 1,479,367 (90.2) |
Values are n (%) or mean 6 SD.
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder.
Difference between the ADHD and ASD cases (Pearson chi-square test and t test for equality of means).
Difference between the ASD and ADHD1ASD cases (Pearson chi-square test and t test for equality of means).
Difference between the ADHD and ADHD1ASD cases (Pearson chi-square test and t test for equality of means).
Difference between cases relative to the comparison population (Pearson chi-square test and t test for equality of means).
Psychiatric Comorbidity in Adults With ADHD, ASD, or ADHD+ASD
Comorbid psychiatric disorders were 2 to 14 times more common in adults with ADHD, ASD, or ADHD+ASD than in the remaining population (Table 2). Overall, the PRs differed significantly between adults with ADHD and ASD for all psychiatric disorders studied. Relative to the remaining population, the association with BD was the strongest and the association with MDD was the weakest in adults with ADHD (PRBD, 7.1; 95% confidence interval [CI], 6.8–7.4; PRMDD, 3.7;95% CI, 3.6–3.8), while the association with SCZ was the strongest and the association with SUD was the weakest in adults with ASD (PRSUD, 13.9; 95% CI, 12.7–15.2; PRASD, 1.9;95% CI, 1.7–2.2) (Table 2, model II). The associations with anxiety disorders, BD, MDD, PDs, and SUD were significantly stronger in adults with ADHD than in adults with ASD (p <.001 for all), with SUD revealing a particularly pronounced difference (PRADHD, 6.2 vs. PRASD, 1.9). The association with SCZ, however, was stronger in adults with ASD than in adults with ADHD (PRASD, 13.9 vs. PRADHD, 4.4; p <.001).
Table 2.
Prevalence Ratios of Psychiatric Disorders Comparing Adults With and Without ADHD, ASD or ADHD1ASD, Based on 1.7 Million Individuals Born in Norway (1967–1997) and Followed Until 2015
| Psychiatric Disorders (ICD-10 Codes) | Crude PR (95% Cl)a | PR Model I (95% Cl)b | PR Model II (95% Cl)c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Remaining Population | ADHD | ASD | ADHD+ASD | ADHD | ASD | ADHD+ASD | ADHD | ASD | ADHD+ASD | ||
| Anxiety Disorders (F40-F42) | 1 (ref) | 4.2 (4.2–4.3), p <.001d | 3.5 (3.3–3.7), p <.001e | 4.7 (4.3–5.2), p =.001f | 4.1 (4.0–4.2), p =.020d | 3.6 (3.4–3.8), p <.001e | 4.7 (4.2–5.2), p <.001f | 3.8 (3.7–3.9), p =.023d | 3.4 (3.2–3.6), p <.001e | 4.3 (3.9–4.7), p <.001f | |
| Bipolar Disorder (F30-F31 or Medicationg) | 1 (ref) | 7.8 (7.5–8.1), p <.001 | 5.3 (4.7–6.0), p <.001 | 9.4 (7.7–11.4), p =.006 | 7.8 (7.4–8.1), p <.001 | 5.4 (4.7–6.2), p <.001 | 9.3 (7.6–11.5), p =.020 | 7.1 (6.8–7.4), p <.001 | 5.0 (4.4–5.7), p <.001 | 8.4 (6.8–10.3), p =.021 | |
| Major Depressive Disorder (F32-F33) | 1 (ref) | 4.2 (4.1–4.2), p <.001 | 3.1 (2.9–3.3), p <.001 | 4.0 (3.6–4.4), p =.801 | 4.0 (3.9–4.1), p <.001 | 3.2 (3.0–3.3), p =.001 | 3.9 (3.5–4.3), p =.886 | 3.7 (3.6–3.8), p <.001 | 3.0 (2.8–3.1), p =.001 | 3.6 (3.2–4.0), p =.898 | |
| Personality Disorders (F60-F61) | 1 (ref) | 8.1 (7.9–8.4), p <.001 | 5.9 (5.4–6.5), p <.001 | 8.1 (7.9–10.6), p =.009 | 7.4 (7.2–7.7), p <.001 | 6.0 (5.5–6.6), p <.001 | 8.6 (7.3–10.1), p =.014 | 6.8 (6.5–7.0), p =.001 | 5.6 (5.1–6.2), p <.001 | 7.7 (6.5–9.1), p =.015 | |
| Antisocial Personality Disorder (F60.2) | 1 (ref) | 20.7(18.4–23.2) p <.001 | 4.1 (2.4–7.2), p <.001 | 24.8 (15.4–40.0), p =.215 | 17.2 (15.1–19.7), p <.001 | 4.1 (2.3–7.5), p <.001 | 24.0 (14.4–40.1), p =.070 | 15.4(13.5–17.7), p <.001 | 3.8 (2.1–6.9), p <.001 | 21.1 (12.6–35.2), p =.070 | |
| Schizophrenia Spectrum Disorder (F20-F29) | 1 (ref) | 4.8 (4.6–5.1), p <.001 | 15.1 (13.9–16.4), p =.194 | 13.3 (11.1–16.0), p <.001 | 4.8 (4.5–5.1), p <.001 | 14.9(13.6–16.2) p =.470 | , 13.8(11.4–16.8), p <.001 | 4.4 (4.1–4.7), p <.001 | 13.9(12.7–15.2) p =.424 |
12.5(10.3–15.1), p <.001 | |
| Substance Use Disorder (F10-F19) | 1 (ref) | 7.8 (7.6–7.9), p <.001 | 2.1 (1.9–2.3), p <.001 | 4.2 (3.7–4.9), p <.001 | 6.8 (6.6–7.0), p <.001 | 2.1 (1.9–2.3), p <.001 | 4.1 (3.5–4.8), p <.001 | 6.2 (6.1–6.4), p <.001 | 1.9 (1.7–2.2), p <.001 | 3.7 (3.2–4.3), p <.001 | |
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CI, confidence interval; ICD-10, International Classification of Diseases of the World Health Organization, 10th ed.; PR, prevalence ratio; ref, reference population.
Adjusted for birth year (5-year groups, from 1967 to 1997, with 1967–1973 as the reference period).
Adjusted for birth year, maternal marital status (single, married/cohabiting [reference], other), maternal and paternal education (low [<10 years], middle [10–12 years], and high [>12 years] [reference]), maternal age (<20, 20–24, 25–29 [reference], 30–34, 35–39, 40+ years of age) and paternal age (<20, 20–24, 25–29, 30–34 [reference], 35–39, 40–44, 45–49, 50+ years of age) at delivery, gestational age (<28, 28–31, 32–34, 35–36, 37–41 [reference], 42+ weeks of age), and gestational age- and sex-specific birth weight Z scores (< ‒2.0; ‒2.0 to ‒0.51; ‒0.5 to 0.5 [reference]; 0.51 to 2.0; 2.01+).
Adjusted for covariates as in model I and additionally adjusted for maternal and paternal psychiatric disorders (yes/no).
Difference between the ADHD and the ASD cases (likelihood ratio test).
Difference between the ASD and the ADHD1ASD cases (likelihood ratio test).
Difference between the ADHD and the ADHD1ASD cases (likelihood ratio test).
Lithium, from the Norwegian Prescription Database (2004–2015).
In the ADHD+ASD group, the PRs ranged from 3.6 for MDD (95% CI, 3.2–4.0) to 12.5 for SCZ (95% CI, 10.3–15.1) (Table 2, model II). For all disorders, except MDD and SUD, associations with ADHD+ASD were significantly stronger than for those with ADHD. For SCZ, associations with ADHD+ASD and ASD were similar, and both were significantly stronger than associations with ADHD (PRADHD1ASD, 12.5; 95% CI, 10.3–15.1;PRASD, 13.9; 95% CI, 12.7–15.2; PRADHD, 4.4; 95% CI,4.1–4.7; p <.001).
Sex-Specific Results
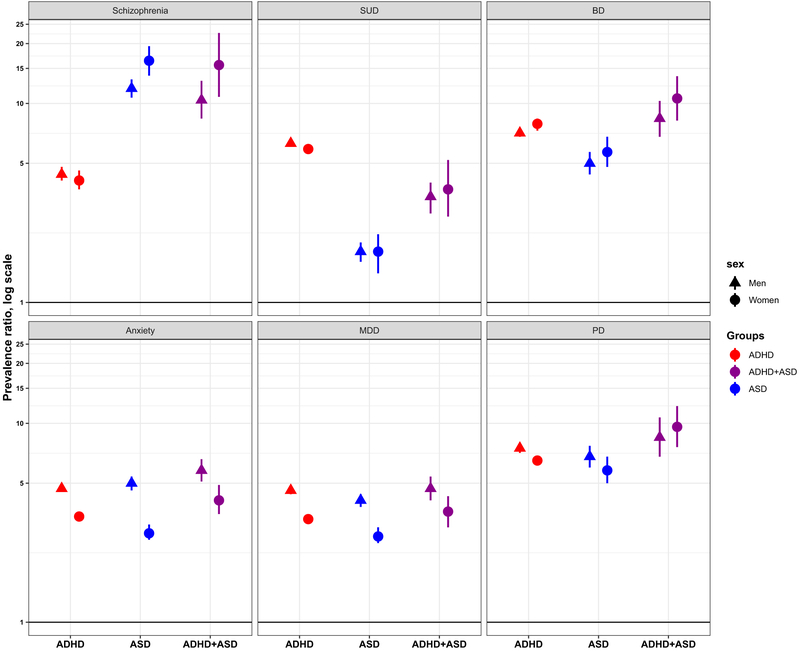
When stratifying on sex, patterns of psychiatric comorbidity corresponded with those in the total sample (Figure 1; Supplemental Tables S2, S3). In both men and women, PR estimates for SCZ were significantly larger in ASD or ADHD+ASD than in ADHD, while for SUD, estimates were significantly larger for ADHD or ADHD+ASD than for ASD. For anxiety disorders, the PR estimates were significantly larger in men than in women for all exposure groups, and for SCZ, the PR was significantly larger in women with ASD than in men with ASD.
Figure 1.
Prevalence ratios of psychiatric disorders in adults with attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), or ADHD+ASD relative to the remaining population, by sex. Log scale, 95% confidence interval, error bars. Adjusted for birth year (5-year groups, from 1967 to 1997, with 1967–1973 as the reference), maternal marital status (single, married/cohabiting [reference], other), maternal and paternal education (low [,10 years], middle [10–12 years] and high [.12 years] [reference]), maternal age (<20, 20–24, 25–29 [reference], 30–34, 35–39, 40+ years of age) and paternal age (<20, 20–24, 25–29, 30–34 [reference], 35–39, 40–44, 45–49, 50+ years of age) at delivery, gestational age (<28, 28–31, 32–34, 35–36, 37–41 [reference], 42+ weeks of age), gestational age- and sex-specific birth weight Z scores (< ‒2.0;‒2.0 to ‒0.51; ‒0.5 to 0.5 [reference]; 0.51 to 2.0; 2.01+), and maternal and paternal psychiatric disorders (yes/no). BD, bipolar disorder; MDD, major depressive disorder; PD, personality disorders; schizophrenia, schizophrenia spectrum disorder; SUD, substance use disorder.
When evaluating associations and interactions on the additive scale, sex differences were more pronounced, and the prevalence difference estimates were significantly different for all the disorders in women and men with ADHD but for only three disorders in men and women with ASD (Figure 2; Supplemental Table S4). Further, the associations were reversed, and prevalence of most psychiatric disorders increased more in women than in men in all exposure groups except SUD and SCZ, in which men in all exposure groups showed the highest prevalence increase.
Figure 2.
Prevalence difference of psychiatric disorders in adults with attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), ADHD+ASD relative to the remaining population, by sex. Prevalence difference, 95% confidence interval, error bars, analog scale. Adjusted for birth year (5-year groups, from 1967 to 1997). BD, bipolar disorder; MDD, major depressive disorder; PD, personality disorders; schizophrenia, schizophrenia spectrum disorder; SUD, substance use disorder.
Genetic Correlations
As shown in Figure 3, the patterns of genetic correlations (rg) were similar to those of the PRs for psychiatric comorbidities based on the epidemiological data. The two proxies for SUD revealed significantly stronger correlations with ADHD than with ASD (Figure 3, right panel; Supplemental Table S5). For SCZ, the rg estimate for ASD was almost twice as large as that for ADHD (rg(ASD+SCZ): 0.211 [SE: 0.048, p <.0001]; rg(ADHD1SCZ): 0.127 [SE: 0.036, p =.0004]), but this difference was not statistically significant (p =.16). The differences in genetic correlations between ADHD and ASD with the examined comorbid disorders were statistically significant only for the SUD proxies and for the NEO personality dimensional traits (Supplemental Table S5).
Figure 3.
(Left panel) The pattern of prevalence ratios of psychiatric comorbidity in adults with attention-deficit/hyperactivity disorder (ADHD) (n = 38,636) or autism spectrum disorder (ASD) (n = 7528) observed in this study and (right panel) genetic correlations (rg) calculated from genome-wide association studies.(Left panel) Prevalence ratio, model II, log-scale, 95% confidence interval, error bars. Adjusted for birth year (5-year groups, from 1967 to 1997, with 1967–1973 as the reference), maternal marital status (single, married/cohabiting [reference], other), maternal and paternal education (low [< 10 years], middle [10–12 years] and high [> 12 years] [reference]), maternal age (,20, 20–24, 25–29 [reference], 30–34, 35–39, 401 years of age) and paternal age (< 20, 20–24, 25–29, 30–34 [reference], 35–39, 40–44, 45–49, 50+ years of age) at delivery, gestational age (< 28, 28–31, 32–34, 35–36, 37–41 [reference], 42+ weeks of age), gestational age- and sex-specific birth weight Z scores (<‒2.0; ‒2.0 to ‒0.51; ‒0.5 to 0.5 [reference]; 0.51 to 2.0; 2.01+), and maternal and paternal psychiatric disorders (yes/no). (Right panel) Genetic correlations, rg, linear scale, SE bars, with “Ever vs Never Smoked” and “Alcohol Dependence” as proxies for substance use disorder, and “NEO–5–Personality Traits” as proxy for personality disorder.
Sensitivity Analyses
When we excluded individuals with intellectual disability, the results changed only for ASD, in which the PR for SCZ increased slightly (PRcrude, 15.1; 95% CI, 13.9–16.4; PRsensitivity,16.5; 95% CI, 15.1–18.1). For the ADHD and the ADHD+ASD groups, there were no substantial changes. All the other sensitivity analyses yielded results that were very similar to those of the main analyses (Supplemental Tables S6, S7).
DISCUSSION
In this first nationwide, population-based study combining epidemiological data on adults with ADHD only, ASD only, or both ADHD and ASD, together with corresponding genetic data, we found different patterns of psychiatric comorbidities between the groups, overall and also when stratifying by sex. These patterns were also reflected in the genetic correlations; however, only proxies for two of the six traits showed a significant difference between ADHD and ASD. We also found that adults with both ADHD and ASD have severe additional psychiatric morbidity relative to adults with either ADHD or ASD alone.
When comparing ADHD and ASD in our epidemiological data, we observed significant differences in the associations with all psychiatric comorbidities examined, with individual estimates being consistent with previous studies (11–13,15,24,30–32). The most marked differences were found for SCZ and SUD, in which SCZ was more common in adults with ASD and SUD was more common in adults with ADHD. Associations with anxiety disorders, BD, and PDs were strongest in adults with both ADHD and ASD, indicating that this group of adults has more severe impairments than those with ADHD or ASD only (59). This is supported by previous studies in children (32,60). Further, we found that adults with both ADHD and ASD had a similar increase in risk for SCZ as that for adults with ASD only, which, as far as we know, has not been shown before. To our knowledge, only one other population-based study reported the prevalence of psychiatric disorders among individuals with ADHD, ASD, or ADHD+ASD compared with unaffected individuals in the same population(33). However, this population was young (mean age ranging from 13.6 to 18.3 years) and had not reached the typical age of onset for most psychiatric disorders (42). The reported estimates were, therefore, likely to be biased.
With regard to the genetic correlations, the patterns were similar to those we observed in the epidemiological data, and two—the SUD proxies and NEO personality traits—revealed significant differences in their correlations with ADHD and ASD. The genetic correlation (rg) between ADHD and ASD has been estimated to be 0.36 (10), indicating shared genetic etiology between them. Nonetheless, it is conceivable that their polygenic architecture is still different, as supported by our epidemiological observation of significantly different patterns of comorbidities between these two disorders. It has also been shown that individuals with various clinical manifestations of ASD reveal distinct loads of genetic variants associated with this disorder (10,61).
Etiological similarities, as well as differences between ADHD and ASD, can be further and more specifically evaluated by examining their symptom dimensions, each of which may have independent and different explanatory values for the clinical diagnoses of ADHD, ASD, and their comorbidities. Ghirardi et al. (28), for example, have reported that the symptoms of hyperactivity/impulsivity (ADHD) show different levels of genetic correlation with symptoms of ASD (e.g., a strong genetic correlation with repetitive and restricted behaviors [ASD] and a weak correlation with social interaction and communication). Our current diagnostic criteria are based on clinically observed aggregates of symptoms but may not relate to distinct underlying biological pathways. Hence, well-powered GWASs on clearly defined specific psychiatric phenotypes and narrower symptom domains are needed to uncover the biological mechanisms underlying the multifaceted etiologies of ADHD and ASD.
Apart from genetics, the observed differences in patterns of comorbidities between ADHD and ASD may also be explained by diagnostic factors. Diagnosing psychiatric comorbidities in adults with ASD is difficult, as many diagnostic tools are not customized for these individuals (31,62). In addition, clinicians may not look for additional psychiatric disorders in ASD patients and explain their symptoms as part of the underlying ASD (32), i.e., diagnostic overshadowing (63). Further, on a more psychological level, even if individuals with ADHD or ASD often experience peer rejection and relational problems (64,65), individuals with ASD are diagnostically defined by their struggle to communicate and hence are less able to communicate their problems because of their large impairments (66,67). This may contribute to a lower level of diagnosed psychiatric comorbidities in individuals with ASD.
The sex differences in risk of psychiatric comorbidities were different among adults with ADHD and ASD, on both the relative and absolute scales. The present findings for adults with ADHD are also presented in our previous publication (19) and were confirmed by a recent Swedish study (68). Sex differences are strongly dependent on the scale used for analyses, with stronger associations for most psychiatric comorbidities in men than in women on the relative scale and stronger associations in women than in men on the absolute scale. This may be explained by the lower prevalence in psychiatric disorders among men than women in the reference group, which has a profound influence on the relative effect measures but not on the risk differences. We suggest that the smaller sex differences observed in adults with ASD than ADHD may partly be explained by the larger male/female ratio in adults with ASD and partly by women with ASD struggling more to communicate internalizing symptoms than women with ADHD (69,70).
The behavioral patterns in the individuals of the two phenotypically different disorders also lead to different interactions with their environments. The differences in the associations with SUD between ADHD and ASD may partly be due to the ADHD-associated novelty seeking and impulsive behavior, increasing the risk of developing SUD to a larger degree in ADHD than in ASD, in which a rigid and norm-abiding behavior, with limited social contact, may be relatively protective (30,71,72). Thus, both genetic and environmental risk factors, as well as their interactions, may alter the expression of genes and affect the structure and function of molecular networks in the brain, thereby modifying the risk of ADHD and ASD (32).
Strengths and Limitations
To our knowledge, this is the largest population-based study comparing psychiatric comorbidity in adults with ADHD, ASD, or both ADHD and ASD conducted so far. The analyses were also stratified according to sex and included genetic correlations in the interpretation. We used data from nationwide, population-based registries with compulsory notification, thus reducing selection bias to a minimum. As our patient groups were large enough to allow us to study individuals with either ADHD alone or ASD alone, or both ADHD and ASD, we were able to study differences between more homogeneous groups.
We designed our study specifically to examine an adult population, allowing all participants to reach the typical age of diagnosis of the conditions investigated (42). Notably, this study covers the first birth cohort for which ASD became prevalent enough in adulthood to enable such a study to be performed.
In Norway, formal diagnoses of ASD and ADHD, and pharmacological treatment of ADHD, are always based on clinical evaluation by specialists. Thus, identification of ADHD and ASD cases was not based solely on symptom scores or self-reports. We chose to identify the ADHD cases either by a dispensed prescription of ADHD medication from the NorPD or by an ADHD diagnosis from the NPR, similar to other Scandinavian studies (22,73–75). ASD, BD, and SCZ diagnoses in the NPR have been validated with good results (36,76), suggesting acceptable validity for other NPR-registered psychiatric diagnoses as well. However, we cannot exclude a possible misdiagnosis of SCZ and ASD. Our definition of BD was also based on dispensed lithium, a medication mainly used for treating BD.
Limitations include the fact that our analyses were crosssectional and based on data registered from 2004 to 2015 (NorPD) and from 2008 to 2015 (NPR), preventing the examination of temporal relations. However, as ADHD and ASD are defined as neurodevelopmental disorders with an onset in childhood, we assume that they were present before the comorbid psychiatric disorders, all typically diagnosed in late adolescence and adulthood (42).
Up to 2013, an ASD diagnosis would preclude a diagnosis ADHD according to the DSM-IV and ICD-10 (77,78). This may have affected the diagnostic procedures and hindered clinicians from diagnosing both disorders if the criteria for ASD were fulfilled. However, clinical practice has not adhered strictly to these criteria, as growing evidence supported the importance of diagnosing both conditions when present to provide the best treatment (31).
Further, it may be argued that adults with ADHD or ASD could more easily get other psychiatric diagnoses because they are already in touch with the health care system (79). However, all adults with severe psychiatric disorders are likely to be in touch with secondary health care throughout life, independent of underlying neurodevelopmental disorders (80).
With regard to the genetic correlations, it is important to note that their estimations are highly dependent on the sample size of the utilized GWAS. In addition, because there are no large-scale GWASs with freely available summary statistics for some of the examined comorbidities, proxy phenotypes were examined. Furthermore, patients’ comorbidities are not always taken into account in the genetic studies, although individuals with known combined ADHD and ASD were excluded from some studies (43). Currently, psychiatric genetics are lacking GWASs on patients with comorbidities or with specific symptoms of psychiatric disorders, which limits our ability to examine the genetic variability that may be responsible for the diverse clinical landscape of psychiatric disorders.
Conclusions
In conclusion, our study provides robust and representative estimates of differences in psychiatric comorbidities among adults diagnosed with ADHD, ASD, or ADHD+ASD. With the results from analyses of genetic correlations, this finding contributes to our understanding of these disorders as being distinct neurodevelopmental disorders with partly shared common genetic factors. Clinicians should be aware of the overall high level of comorbidity in adults with ADHD, ASD, or ADHD+ASD and of the distinct patterns of psychiatric comorbidities to detect these conditions and offer early treatment. It is also important to take into account the observed sex differences. The distinct comorbidity patterns may provide further information regarding etiologic research on biological mechanisms underlying the pathophysiology of these neurodevelopmental disorders.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by Stiftelsen Kristian Gerhard Jebsen Grant No. SKGJ-MED-002 (to JH, KK, TZ); the University of Bergen; EU Horizon 2020 Grant No. 667302 (Comorbid Conditions of ADHD); and the U.S. Department of Health and Human Services National Institute of Mental Health Grant No. 5U01MH109539–03 (Psychiatric Genomics Consortium) (to TZ). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
JH has served as a speaker for Eli Lilly, HB Pharma, and Shire. The other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2019.04.021.
REFERENCES
- 1.Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG (2015): The epidemiology and global burden of autism spectrum disorders. Psychol Med 45:601–613. [DOI] [PubMed] [Google Scholar]
- 2.Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, et al. (2018): Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur Neuropsychopharmacol 28:1059–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tick B, Colvert E, McEwen F, Stewart C, Woodhouse E, Gillan N, et al. (2016): Autism spectrum disorders and other mental health problems: Exploring etiological overlaps and phenotypic causal associations. J Am Acad Child Adolesc Psychiatry 55:106–113 e104. [DOI] [PubMed] [Google Scholar]
- 4.Faraone SV, Larsson H (2018): Genetics of attention deficit hyperactivity disorder. Mol Psychiatry 24:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stergiakouli E, Davey Smith G, Martin J, Skuse DH, Viechtbauer W, Ring SM, et al. (2017): Shared genetic influences between dimensional ASD and ADHD symptoms during child and adolescent development. Mol Autism 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polderman TJC, Hoekstra RA, Posthuma D, Larsson H (2014): The cooccurrence of autistic and ADHD dimensions in adults: An etiological study in 17 770 twins. Transl Psychiatry 4:e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghirardi L, Brikell I, Kuja-Halkola R, Freitag CM, Franke B, Asherson P, et al. (2018): The familial co-aggregation of ASD and ADHD: A registerbased cohort study. Mol Psychiatry 23:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium, et al. (2015): LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet 47:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. (2015): Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1:15020. [DOI] [PubMed] [Google Scholar]
- 10.Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. (2019): Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 51:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobanski E (2006): Psychiatric comorbidity in adults with attentiondeficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 256(suppl 1):i26–i31. [DOI] [PubMed] [Google Scholar]
- 12.Rai D, Heuvelman H, Dalman C, et al. (2018): Association between autism spectrum disorders with or without intellectual disability and depression in young adulthood. JAMA Network Open 1:e181465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lever AG, Geurts HM (2016): Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J Autism Dev Disord 46:1916–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkmar FR, Cohen DJ (1991): Comorbid association of autism and schizophrenia. Am J Psychiatry 148:1705–1707. [DOI] [PubMed] [Google Scholar]
- 15.Selten JP, Lundberg M, Rai D, Magnusson C (2015): Risks for nonaffective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: A population-based study. JAMA Psychiatry 72:483–489. [DOI] [PubMed] [Google Scholar]
- 16.Vannucchi G, Masi G, Toni C, Dell’Osso L, Erfurth A, Perugi G (2014): Bipolar disorder in adults with Aspergers Syndrome: A systematic review. J Affect Disord 168:151–160. [DOI] [PubMed] [Google Scholar]
- 17.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. (2006): The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry 163:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. (2006): Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychol Med 36:167–179. [DOI] [PubMed] [Google Scholar]
- 19.Solberg BS, Halmoy A, Engeland A, Igland J, Haavik J, Klungsoyr K (2018): Gender differences in psychiatric comorbidity: A populationbased study of 40 000 adults with attention deficit hyperactivity disorder. Acta Psychiatr Scand 137:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biederman J, Faraone SV, Monuteaux MC, Bober M, Cadogen E (2004): Gender effects on attention-deficit/hyperactivity disorder in adults, revisited. Biol Psychiatry 55:692–700. [DOI] [PubMed] [Google Scholar]
- 21.Halmoy A, Halleland H, Dramsdahl M, Bergsholm P, Fasmer OB, Haavik J (2010): Bipolar symptoms in adult attention-deficit/hyperactivity disorder: A cross-sectional study of 510 clinically diagnosed patients and 417 population-based controls. J Clin Psychiatry 71:48–57. [DOI] [PubMed] [Google Scholar]
- 22.Larsson H, Ryden E, Boman M, Langstrom N, Lichtenstein P, Landen M (2013): Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. Br J Psychiatry 203:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthies S, Philipsen A (2016): Comorbidity of personality disorders and adult attention deficit hyperactivity disorder (ADHD)-Review of recent findings. Curr Psychiatry Rep 18:33. [DOI] [PubMed] [Google Scholar]
- 24.Dalsgaard S, Mortensen PB, Frydenberg M, Maibing CM, Nordentoft M, Thomsen PH (2014): Association between attentiondeficit hyperactivity disorder in childhood and schizophrenia later in adulthood. Eur Psychiatry 29:259–263. [DOI] [PubMed] [Google Scholar]
- 25.Shyu YC, Yuan SS, Lee SY, Yang CJ, Yang KC, Lee TL, et al. (2015): Attention-deficit/hyperactivity disorder, methylphenidate use and the risk of developing schizophrenia spectrum disorders: A nationwide population-based study in Taiwan. Schizophr Res 168:161–167. [DOI] [PubMed] [Google Scholar]
- 26.Capusan AJ, Bendtsen P, Marteinsdottir I, Larsson H (2016): Comorbidity of adult ADHD and its subtypes with substance use disorder in a large population-based epidemiological study [published online ahead of print Feb 2]. J Atten Disord. [DOI] [PubMed] [Google Scholar]
- 27.Consortium Brainstorm, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. (2018): Analysis of shared heritability in common disorders of the brain. Science 360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghirardi L, Pettersson E, Taylor MJ, Freitag CM, Franke B, Asherson P, et al. (2018): Genetic and environmental contribution to the overlap between ADHD and ASD trait dimensions in young adults: A twin study [published online ahead of print Sep 7]. Psychol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahlberg O, Soderstrom H, Rastam M, Gillberg C (2004): Bipolar disorder, schizophrenia, and other psychotic disorders in adults with childhood onset AD/HD and/or autism spectrum disorders. J Neural Transm (Vienna) 111:891–902. [DOI] [PubMed] [Google Scholar]
- 30.Sizoo B, van den Brink W, Koeter M, Gorissen van Eenige M, van Wijngaarden-Cremers P, van der Gaag RJ (2010): Treatment seeking adults with autism or ADHD and co-morbid substance use disorder: Prevalence, risk factors and functional disability. Drug Alcohol Depend 107:44–50. [DOI] [PubMed] [Google Scholar]
- 31.Antshel KM, Zhang-James Y, Faraone SV (2013): The comorbidity of ADHD and autism spectrum disorder. Expert Rev Neurother 13:1117– 1128. [DOI] [PubMed] [Google Scholar]
- 32.Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, Faraone SV (2016): An update on the comorbidity of ADHD and ASD: A focus on clinical management. Expert Rev Neurother 16:279–293. [DOI] [PubMed] [Google Scholar]
- 33.Chen MH, Wei HT, Chen LC, Su TP, Bai YM, Hsu JW, et al. (2015): Autistic spectrum disorder, attention deficit hyperactivity disorder, and psychiatric comorbidities: A nationwide study. Res Autism Spect Dis 10:1–6. [Google Scholar]
- 34.Irgens LM (2000): The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 79:435–439. [PubMed] [Google Scholar]
- 35.Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT (2010): The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol 106:86–94. [DOI] [PubMed] [Google Scholar]
- 36.Nesvag R, Jonsson EG, Bakken IJ, Knudsen GP, Bjella TD, Reichborn-Kjennerud T, et al. (2017): The quality of severe mental disorder diagnoses in a national health registry as compared to research diagnoses based on structured interview. BMC Psychiatry 17:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steingrimsdottir OA, Naess O, Moe JO, Groholt EK, Thelle DS, Strand BH, et al. (2012): Trends in life expectancy by education in Norway 1961–2009. Eur J Epidemiol 27:163–171. [DOI] [PubMed] [Google Scholar]
- 38.Statistics Norway (2019): Educational attainment of the population. Available at: https://www.ssb.no/en/utdanning/statistikker/utniv. Accessed February 1, 2019.
- 39.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. (2007): The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 40.Ottosen C, Petersen L, Larsen JT, Dalsgaard S (2016): Gender differences in associations between attention-deficit/hyperactivity disorder and substance use disorder. J Am Acad Child Adolesc Psychiatry 55:227–234 e224. [DOI] [PubMed] [Google Scholar]
- 41.Kohler-Forsberg O, Petersen L, Gasse C, Mortensen PB, Dalsgaard S, Yolken RH, et al. (2018): A nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents [published online ahead of print Dec 5]. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- 43.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. (2019): Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 51:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Psychiatric GWAS Consortium Bipolar Disorder Working Group (2011): Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 43:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. (2018): Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, et al. (2016): Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry 21:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Moor MH, Costa PT, Terracciano A, Krueger RF, de Geus EJ, Toshiko T, et al. (2012): Meta-analysis of genome-wide association studies for personality. Mol Psychiatry 17:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tielbeek JJ, Johansson A, Polderman TJC, Rautiainen MR, Jansen P, Taylor M, et al. (2017): Genome-wide association studies of a broad spectrum of antisocial behavior. JAMA Psychiatry 74:1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. (2018): Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci 21:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobacco, Genetics C (2010): Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 42:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. (2017): LD Hub: A centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costa PT, Mccrae RR (1992): The 5-factor model of personality and its relevance to personality-disorders. J Pers Disord 6:343–359. [Google Scholar]
- 54.Willer CJ, Li Y, Abecasis GR (2010): METAL: Fast and efficient metaanalysis of genomewide association scans. Bioinformatics 26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou G (2004): A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159:702–706. [DOI] [PubMed] [Google Scholar]
- 56.Skjaerven R, Gjessing HK, Bakketeig LS (2000): Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand 79:440–449. [PubMed] [Google Scholar]
- 57.VanderWeele TJ, Knol MJ (2014): A tutorial on interaction. Epidemiol Methods 3:33–72. [Google Scholar]
- 58.Azur MJ, Stuart EA, Frangakis C, Leaf PJ (2011): Multiple imputation by chained equations: What is it and how does it work? Int J Meth Psych Res 20:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattard-Labrecque C, Ben Amor L, Couture MM (2013): Children with autism and attention difficulties: A pilot study of the association between sensory, motor, and adaptive behaviors. J Can Acad Child Adolesc Psychiatry 22:139–146. [PMC free article] [PubMed] [Google Scholar]
- 60.Tye C, Asherson P, Ashwood KL, Azadi B, Bolton P, McLoughlin G (2014): Attention and inhibition in children with ASD, ADHD and comorbid ASD 1 ADHD: An event-related potential study. Psychol Med 44:1101–1116. [DOI] [PubMed] [Google Scholar]
- 61.Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, et al. (2014): Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci U S A 111:15161–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnuson KM, Constantino JN (2011): Characterization of depression in children with autism spectrum disorders. J Dev Behav Pediatr 32:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jopp DA, Keys CB (2001): Diagnostic overshadowing reviewed and reconsidered. Am J Ment Retard 106:416–433. [DOI] [PubMed] [Google Scholar]
- 64.Hoza B, Gerdes AC, Mrug S, Hinshaw SP, Bukowski WM, Gold JA, et al. (2005): Peer-assessed outcomes in the multimodal treatment study of children with attention deficit hyperactivity disorder. J Clin Child Adolesc Psychol 34:74–86. [DOI] [PubMed] [Google Scholar]
- 65.Sasson NJ, Morrison KE (2017): First impressions of adults with autism improve with diagnostic disclosure and increased autism knowledge of peers [published online ahead of print Oct 1]. Autism. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Itzchak E, Kirzon M, Peled N, Zachor DA (2018): Coherence and content of relating emotions to life events in autism spectrum disorder and typical development: A cross-sectional age study. J Abnorm Child Psychol 46:415–422. [DOI] [PubMed] [Google Scholar]
- 67.Hickey A, Crabtree J, Stott J (2018): ‘Suddenly the first fifty years of my life made sense’: Experiences of older people with autism. Autism 22:357–367. [DOI] [PubMed] [Google Scholar]
- 68.Chen Q, Hartman CA, Haavik J, Harro J, Klungsoyr K, Hegvik TA, et al. (2018): Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: A population-based crosssectional study. PLoS One 13:e0204516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ottosen C, Larsen JT, Faraone SV, Chen Q, Hartman C, Larsson H, et al. (2019): Sex differences in comorbidity patterns of attentiondeficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 58:412–422.e3. [DOI] [PubMed] [Google Scholar]
- 70.Larson FV, Lai MC, Wagner AP, Consortium MA, Baron-Cohen S, Holland AJ (2015): Testing the ‘extreme female brain’ theory of psychosis in adults with autism spectrum disorder with or without comorbid psychosis. PLoS One 10:e0128102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramos M, Boada L, Moreno C, Llorente C, Romo J, Parellada M (2013): Attitude and risk of substance use in adolescents diagnosed with Asperger syndrome. Drug Alcohol Depend 133:535–540. [DOI] [PubMed] [Google Scholar]
- 72.Santosh PJ, Mijovic A (2006): Does pervasive developmental disorder protect children and adolescents against drug and alcohol use? Eur Child Adolesc Psychiatry 15:183–188. [DOI] [PubMed] [Google Scholar]
- 73.Larsson H, Chang Z, D’Onofrio BM, Lichtenstein P (2014): The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med 44:2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skoglund C, Chen Q, Franck J, Lichtenstein P, Larsson H (2015): Attention-deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biol Psychiatry 77:880–886. [DOI] [PubMed] [Google Scholar]
- 75.Kendler KS, Ohlsson H, Sundquist K, Sundquist J (2016): Crossgenerational transmission from drug abuse in parents to attention-deficit/hyperactivity disorder in children. Psychol Med 46:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suren P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, et al. (2012): Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics 130:e152–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Orginazation (1993): The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva, Switzerland: WHO Press. [Google Scholar]
- 78.American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IVTR). Washington, DC: American Psychiatric Press. [Google Scholar]
- 79.Woodfine JD, Redelmeier DA (2015): Berkson’s paradox in medical care. J Intern Med 278:424–426. [DOI] [PubMed] [Google Scholar]
- 80.Weiser M, Werbeloff N, Dohrenwend BP, Levav I, Yoffe R, Davidson M (2012): Do psychiatric registries include all persons with schizophrenia in the general population? A population-based longitudinal study. Schizophr Res 135:187–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.