Abstract
A hybrid transition metal/radical process is described that results in the addition of organozinc reagents and alkyl halides across alkenylboron reagents in an enantioselective catalytic fashion. The reaction can be accomplished both intermolecularly and intramolecularly, providing useful product yields and high enantioselectivities in both manifolds.
Keywords: Boron, Cross-Coupling, Nickel, Catalysis
Graphical Abstract
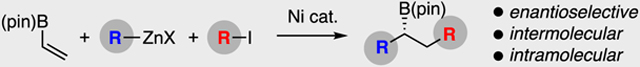
A Radical Hydrid: Dicarbofunctionalization of vinylB(pin) is accomplished through the use of a Ni-based chiral catalyst. The reaction employs alkyl halide and organozinc reagents and can be accomplished with good levels of enantioselectivity in either an intra- or intermolecular fashion.
A number of catalytic reactions can provide access to stereochemically-defined, synthetically useful organoboron compounds.1 To this end, recent efforts in our laboratory have led to the development of a catalytic conjunctive cross-coupling reaction (Scheme 1a).2 This transformation adds both a nucleophile and an electrophile across an alkenylboron reagent by a mechanism that includes a 1,2-metalate-shift involving a four-coordinate borate intermediate. Within the conjunctive cross-coupling manifold, processes have been developed that employ alkyl, aryl, and alkenyllithium or Grignard reagent nucleophiles (RM) to generate the four-coordinate borate, and the process has been extended to aryl, alkenyl, and alkyl electrophiles.3 A current limitation to the broad use of C(sp3) electrophiles in conjunctive coupling is that they only operate with aryl migrating groups. Additionally, highly hindered tertiary electrophiles could not be engaged successfully in this reaction. In this report, we address these shortcomings through an alternate process that involves a transition metal/radical hybrid reaction, and show that these reactions can be accomplished in an enantioselective catalytic fashion.
Scheme 1.
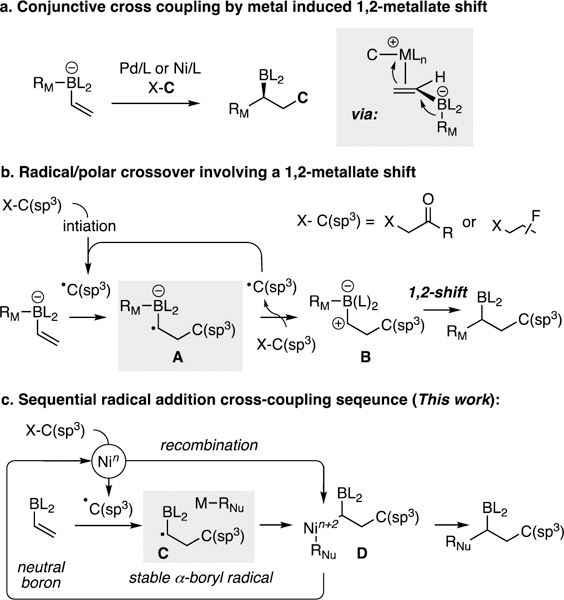
Catalytic Dialkylation of Vinylboron Compounds.
In addition to the metal-catalyzed process in Scheme 1a, the addition of two alkyl components across an alkenyl boron compound can be accomplished by a radical/polar crossover process (Scheme 1b) described by Studer,4 Aggarwal,5 and Renaud6 employing chemical and photochemical initiators, and by our group employing Ni complexes as initiators.3d These studies revealed that stabilized/electron-poor alkyl halides (haloperfluoroalkanes and α-halocarbonyls) could, upon homolytic cleavage, generate carbon-centered radicals that combine directly with electron-rich four-coordinate alkenylboron ‘ate’ complexes and generate the corresponding α-boryl radical (A). In turn, this species could undergo single electron transfer to the alkyl halide substrate, resulting in regeneration of the alkyl radical with concomitant formation of an α-boryl carbocation (B); the latter species participates in a 1,2-metallate shift providing the organoboron product.
While the radical/polar crossover mode of reactivity constitutes a highly efficient approach to generating alkyl boron compounds, it does not provide a means to readily control product stereochemistry. In the context of Ni catalysis of the radical/polar crossover reaction, a critical impediment to catalyst-based stereocontrol is that SET from the species A to the alkyl halide outcompetes recombination of A with the Ni complex, and thus the Ni complex only operates as an initiator for the radical reaction. To address this obstacle, we targeted formation of a neutral α-boryl radical (C, Scheme 1c) that would be more stable and less prone to engage in an SET-based radical propagation reaction.7,8 If the intermediate radical might instead react with the Ni complex furnishing D, then catalyst-based stereocontrol might be realized even though the process involves radical intermediates. The underlying principles that would enable such a process are aligned with other metal catalyzed intermolecular dicarbofunctionalizations9 of alkenes, which take place either through carbometallation/cross-coupling10 pathways or through radical addition/cross-coupling cascades.11 The latter examples provide compelling support that the process in Scheme 1c might operate effectively. That only a few examples of enantioselective intermolecular dicarbofunctionalizations have been reported perhaps highlights the challenge in identifying effective systems for this type of transformation.12
To begin our investigation of the transition metal/radical process proposed in Scheme 1c, we first sought a stoichiometric organometallic reagent that would react with Ni-based intermediates, but would not react directly with three-coordinate vinylboron reagents. While organolithium, and to some extent Grignard reagents, will covert vinylB(pin) to the derived “ate” complex, it was found that neither aryl or alkylzinc reagents would react with vinylB(pin). With regards to the electrophilic component, it was considered that an electron-rich alkyl radical would most effectively add to an electron-deficient alkenyl boronic ester and thus tert-butyl iodide was selected for initial studies.8 Lastly, in regards to a starting point for reaction conditions, we considered that a system developed by Fu13 for the enantioconvergent cross-coupling of organozinc reagents to α-haloboronic esters would be appropriate since this process appears to involve selective recombination of a Ni complex with an α-boryl radical similar to the transformation of C to D described above.
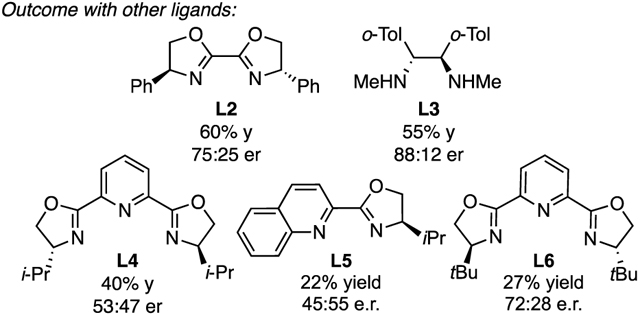
As depicted in entry 1 of Table 1, treatment of t-BuI, alkylzinc bromide 1, and vinylB(pin) with 13 mol% L1 and 10 mol% NiBr2•glyme in THF/DMA at 0 °C for 18 h provided the three-component coupling product 2 in good yield and modest enantioselectivity (87:13 er). Subsequent experiments demonstrated that both the Ni salt and the ligand are required for the reaction (entries 2, 3) and that the three-component coupling product is not generated from reactions with a tertiary bromide (entry 4) or primary iodide (entry 5). Additionally, a survey of other vinylboronic esters revealed vinylB(pin) to be the most effective coupling partner (entries 6–8), whereas four-coordinate vinylB(mida) did not furnish any coupling product (entry 8) perhaps revealing the importance of an electron-deficient alkenylboron reagent for these reactions. It was also found that bidentate and tridentate nitrogen-based ligands L2-L6 were also effective in this reaction, however, diamine L1 provided the best overall results in terms both of yield and selectivity. Finally, the nature of the alkyl zinc reagents was found to have a significant effect on the outcome of the reaction (entry 9–11). In particular, alkyl zinc chloride reagents derived from addition of the corresponding organolithium reagent to zinc chloride provided the product with slightly diminished yield but an improved level of enantioselectivity (95:5 er). Of note, this effect could be recapitulated by adding LiCl to reactions of alkylzinc bromide reagents (entry 11).14
Table 1.
Three-Component Cross-Coupling of Zinc Reagent 1, VinylB(pin) and t-BuI.[a]
 | |||
|---|---|---|---|
| entry | modification | yield 2 (%) | er |
| 1 | none | 71 | 87:13 |
| 2 | no NiBr2•glyme | <5 | n/a |
| 3 | no ligand | <5 | n/a |
| 4 | t-BuBr | <5 | n/a |
| 5 | n-BuI | <5 | n/a |
| 6 | vinylB(neo) | 43 | 81:19 |
| 7 | vinylB(dan) | 80 | 60:40 |
| 8 | vinylB(mida) | <5 | n/a |
| 9 | PhCH2CH2CH2Znl | 75 | 86:14 |
| 10 | PhCH2CH2CH2ZnCl•LiCl | 54 | 95:5 |
| 11 | PhCH2CH2CH2ZnBr•2 LiCl | 55 | 95:5 |
 | |||
See text and Supporting Information for details. Yields refer to isolated yield of purified material and are an average of two experiments. Er determined by chiral SFC analysis. Entry 7 product isolated as the B(dan) derivative.
Analysis of different substrates in the Ni-catalyzed three-component coupling reaction showed that a range of functional groups could be included in the organozinc reagent, with ether, protected alcohols, and amino groups being tolerated (Table 2). That methylzinc chloride could be employed (product 4) is noteworthy considering that methyl migration in conjunctive coupling has proven to be a challenge. It is also of note that arylzinc reagents could be used in place of alkylzinc reagents under the same reaction conditions, providing enantiomerically enriched benzylic boronate derivatives. In terms of the electrophilic partner, carbon scaffolds with oxygen- and nitrogen-based functional groups were accommodated (products 6 and 8). Product 9 was generated with moderate diastereoselectivity (the major diastereomer was determined by 1H NMR analysis) from the corresponding iodide, highlighting the possibility for using substrate control in the case where a quaternary carbon stereocenter is formed in the first radical addition step.15 Lastly, it should be noted that reactions of secondary, benzylic, allylic, α-oxygenated and α-silyl iodides provided <10% of the three-component coupling product, and it was also found that added substitution at the α carbon of the vinyl boronic ester inhibited the coupling reaction.
Table 2.
Ni-Catalyzed Three-Component Coupling of Organozinc Reagents and Alkyl Halides.[a]
 |
See text and Supporting Information for experimental details. Yields refer to isolated yield of purified material and are an average of two experiments.
RZnCl•LiCl obtained from addition of RLi to ZnCl2.
A series of experiments was conducted to learn more about mechanistic features that underlie this three-component coupling reaction. As shown in eq. 1, addition of TEMPO ( 1 equiv.) completely suppressed formation of the three-component coupling product thereby suggesting the intermediacy of radical species. Assuming radicals are involved, the inability to employ primary iodides in the reaction could be due to a less facile C–I oxidative addition (halogen abstraction), or it could be due to competitive side reactions with this substrate class. An experiment conducted in the absence of vinylB(pin) (eq. 2) suggested that primary alkyl iodides do indeed undergo reaction with the Ni complex: with primary alkyl iodide 16, cross-coupling with organozinc reagent 17 furnished 18 in addition to homo-coupling of the organozinc reagent (19).16 Of note, when the same reaction was conducted with t-butyl iodide, the homo-coupling product was formed exclusively. Additional experiments suggest the formation of carbon-centered radicals, even from primary alkyl iodides, in these coupling reactions. As shown in eq. 4, reaction of 20 and PhZnCl in the presence of Ni/L1 results in efficient cyclization/coupling to give product 21 with excellent levels of enantiomeric purity.17 That the cyclization of the primary iodide is likely a radical process is suggested by the reaction of 22, which furnished a 1:1 diastereomer mixture of 23: were this reaction to occur by oxidative addition/stereospecific migratory insertion, stereocontrol in the formation of both new C–C bonds would be likely. Collectively, the experiments in Scheme 2 suggest that the three-component coupling occurs by a radical addition.
Scheme 2.

Mechanistic Experiments in Three-Component Coupling.
While it is difficult to rigorously exclude mechanisms from the many that might operate in this catalytic process, one plausible cycle that accounts for the observations described above is presented in Scheme 3. This proposal involves halide abstraction from the alkyl halide by a Ni(I) alkyl complex (A) to furnish radical C along with nickel species B. Subsequent to addition of radical C to vinylB(pin), Ni(II) complex B combines with D to generate a Ni(III) complex E. Complex E then undergoes reductive elimination and delivers the product along with a Ni(I) iodide (F). Transmetalation between the organozinc reagent and F regenerates the starting Ni(I) alkyl complex A. This cycle accounts for the homo-coupling and cross-coupling products that are observed in the absence of vinylB(pin) as well as the observation that enantioselectivity in the formation of product is dependent upon the nature of the R group. Lastly, this cycle and the experiments above suggest a requirement for effective intermolecular reactions: subsequent to formation of a carbon-centered radical by action of the Ni catalyst on the alkyl iodide, capture of the radical by the vinylboronic ester must outcompete direct cross-coupling that arises from recombination of the Ni-complex with the radical center.
Scheme 3.

Prospective Catalytic Cycle for Three-Component Ni/Radical Coupling Reaction.
The intramolecular cyclization reaction uncovered through the studies above promises to expand the utility of this radical-based coupling reaction. The intramolecular variant offers the distinctive feature of forging two C–C bonds while generating an exocyclic boron-containing stereocenter in an enantioselective fashion. In addition to finding that this process could apply to the construction of both five-membered and six-membered ring systems (Table 3, products 21, 24–28), it was reasoned that by employing a combination of substrate-based stereocontrol18,15b in the ring closure, along with catalyst-based control of the boron-containing center, the overall transformation may be used to obtain complex cyclic structures efficiently. With these considerations, a number of additional substrates were explored in the intramolecular cyclization/cross-coupling sequence. It was found that cross-coupling could be carried out to construct substituted monocyclic and bicyclic products and that a number of sensitive functional groups can be accommodated (products 29–34).
Table 3.
Intramolecular Radical Cyclization/Cross-Coupling Reaction.[a]
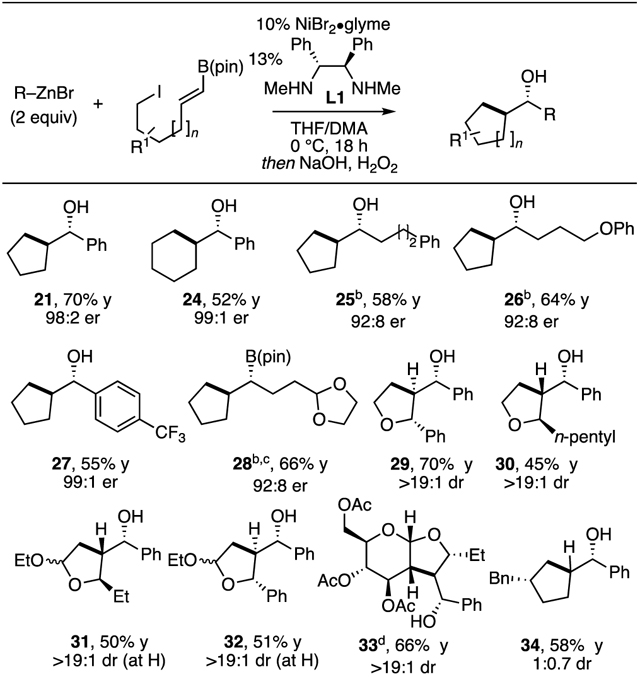 |
See text and Supporting Information for experimental details. Yields refer to isolated yield of purified material and are an average of two experiments. Unless otherwise noted, RZnCl•LiCl obtained from addition of RLi to ZnCl2.
RZnBr employed; reaction in dimethylacetamide solvent at room temperature.
Because the alcohol derivative was not stable, this compound was isolated as the organoboron.
This product was prepared using (R,R)-L1 ligand.
In conclusion, we have described a method for the enantioselective nickel-catalyzed coupling of organozinc reagents, alkyl iodides and alkenyl boron compounds. The reaction appears to proceed by a tandem radical addition/cross-coupling cascade and may be useful for constructing complex, densely-functionalized motifs.
Supplementary Material
Acknowledgements
This work was supported by a grant from the US National Institutes of Health (NIGMS GM-R35–127140).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) For reviews: Collins BSL, Wilson CM, Myers EL, Aggarwal VK, Angew. Chem. Int. Ed. 2017, 56, 11700. [DOI] [PubMed] [Google Scholar]; b) Fernandez E, Whiting A Synthesis and Application of Organoboron Compounds, Topics in Organometallic Chemistry 49, Springer, Switzerland, 2015. [Google Scholar]
- [2].Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP, Morken JP, Science 2016, 351, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Lovinger GJ, Aparece MD, and Morken JP, J. Am. Chem. Soc. 2017, 139, 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Edelstein EK, Namirembe S, and J. Morken P, J. Am. Chem. Soc. 2017, 139, 5027. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chierchia M, Law C, and Morken JP, Angew. Chem. Int. Ed. 2017, 56, 11870. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lovinger GJ, and Morken JP, J. Am. Chem. Soc. 2017, 139, 17293. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Myhill JA, Zhang L, Lovinger GJ, and Morken JP, Angew. Chem. Int. Ed. 2018, 57, 12799. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Myhill JA, Wilhelmsen CA, Zhang L, Morken JP, J. Am. Chem. Soc. 2018, 140, 15181. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Law C, Meng Y, Koo SM, and Morken JP, Angew. Chem. Int. Ed. 2019, 58, 6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Kischkewitz M, Okamoto K, Mück-Lichtenfeld C, Studer A, Science 2017, 355, 936. [DOI] [PubMed] [Google Scholar]; b) Gerleve C, Kischkewitz M, Studer A, Angew. Chem. Int. Ed. 2018, 57, 2441. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kischkewitz M, Gerleve C, Studer A, Org. Lett. 2018, 20, 3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Silvi M, Sandford C, Aggarwal VK, J. Am. Chem. Soc. 2017, 139, 5736. [DOI] [PubMed] [Google Scholar]
- [6].Tappin NDC, Gnägi-Lux M, Renaud P, Chem. Eur. J. 2018, 24, 11498. [DOI] [PubMed] [Google Scholar]
- [7].Walton JC, McCarroll AJ, Chen O, Carboni B, Nziengui R, J. Am. Chem. Soc. 2000, 122, 5455. [Google Scholar]
- [8].a) Guennouni N, Lhermitte F, Cochard S, Carboni B, Tetrahedron 1995, 51, 6999. [Google Scholar]; b) Lo JC, Gui J, Yabe Y, Pan CM, Baran PS, Nature 2014, 516, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Quiclet-Sireand B, Zard SZ, J. Am. Chem. Soc. 2015, 137, 6762. [DOI] [PubMed] [Google Scholar]; d) Noble A, Mega RS, Pflästerer D, Myers EL, Aggarwal VK, Angew. Chem. 2018, 130, 2177. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Shu C, Mega RS, Andreassen BJ, Noble A, Aggarwal VK, Angew. Chem. Int. Ed, 2018, 57, 15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Reviews: Dhungana RK, KC S, Basnet P, Giri PR, Chem. Rec. 2018, 18, 1314; [DOI] [PubMed] [Google Scholar]; b) Zhang J-S, Liu L, Chen T, Han L-B, Chem. Asian J. 2018, 13, 227; [DOI] [PubMed] [Google Scholar]; c) Jensen KH, Sigman MS, Org. Biomol. Chem, 2008, 6, 4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Selected examples of intermolecular olefin dicarbofunctionalization by carbometallation/cross-coupling: Derosa J, van der Puyl VA, Tran VT, Liu M, Engle KM, Chem. Sci. 2018, 9, 5278; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Derosa J, Tran VT, Boulous MN, Chen JS, Engle KM J. Am. Chem. Soc. 2017, 139, 10657; [DOI] [PubMed] [Google Scholar]; c) Shrestha B, Basnet P, Dhungana RK, KC S, Thapa S, Sears JM, Giri R, J. Am. Chem. Soc. 2017, 139, 10653; [DOI] [PubMed] [Google Scholar]; d) Derosa J, Kleinmans R, Tran VT, Karunananda MK, Wisniewski SR, Eastgate MD, Engle KM, J. Am. Chem. Soc, 2018, 140, 17878; [DOI] [PubMed] [Google Scholar]; e) Basnet P, KC S, Dhungana RK, Shrestha B, Boyle TJ, Giri R, J. Am. Chem. Soc. 2018, 140, 15586; [DOI] [PubMed] [Google Scholar]; f) Thapa S, Dhungana RK, Thapa-Magar R, Shrestha B, KC S, Giri R, Chem. Sci. 2018, 9, 904; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Li W, Boon JK, Zhao Y, Chem. Sci. 2018, 9, 600; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Gao p., Chen L-A, Brown MK J. Am. Chem. Soc. 2018, 140, 10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Selected examples of intermolecular dicarbofunctionalization by radical cascade/cross-coupling: Guo L, Tu H, Zhu S, Chu L, Org. Lett. 2019, ASAP DOI: 10.1021/acs.orglett.9b01658; [DOI] [PubMed] [Google Scholar]; b) Klauck FJR, Yoon H, James MJ, Lautens M, Glorius F ACS Catal. 2019, 9, 236; [Google Scholar]; c) KC S, Dhungana R, Shrestha B, Thapa S, Khanal N, Basnet P, Lebrun R, Giri R J. Am. Chem. Soc. 2018, 140, 9801; [DOI] [PubMed] [Google Scholar]; d) García-Domínguez A, Li Z, Nevado C J. Am. Chem. Soc. 2017, 139, 6835; [DOI] [PubMed] [Google Scholar]; e) Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS Science 2016, 352, 801; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Gu J-W, Min Q-Q, Yu L-C, Zhang X Angew. Chem. Int. Ed. 2016, 55, 1227; [DOI] [PubMed] [Google Scholar]; q) Ma Y, Zhang D, Yan Z, Wang M, Bian C, Gao X, Bunel EE, Lei A, Org. Lett. 2015, 17, 2174; [DOI] [PubMed] [Google Scholar]; g) Fusano A, Sumino S, Fukuyama T, Ryu I Org. Lett. 2011, 13, 2114; [DOI] [PubMed] [Google Scholar]; h) Terao J, Nii S, Chowdhury FA, Nakamura A, Kambe N, Adv. Synth. Catal. 2004, 346, 905; [Google Scholar]; i) Mizutani K, Shinokubo H, Oshima K Org. Lett. 2003, 5, 3959; [DOI] [PubMed] [Google Scholar]; j) Terao J, Saito K, Nii S, Kambe N, Sonoda N, J. Am. Chem. Soc. 1998, 120, 11822. [Google Scholar]
- [12].a) Enantioselective intermolecular dicarbofunctionalization by carbometallation cross coupling: Stokes BJ, Liao L, de Andrade AM, Wang Q, Sigman MS Org. Lett 2014, 16, 4666; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wu X, Lin H-C, Li M-L, Li L-L, Han Z-Y, Gong L-Z J. Am. Chem. Soc. 2015, 137, 13476; [DOI] [PubMed] [Google Scholar]; c) Anthony D, Lin Q, Baudet J, Diao T Angew. Chem. Int. Ed. 2019, 58, 3198; Enantioselective intermolecular dicarbofunctionalization by radical cascade/cross-coupling: [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang F, Wang D, Mu X, Chen P, Liu G J. Am. Chem. Soc. 2014, 136, 10202; [DOI] [PubMed] [Google Scholar]; e) Wang F, Wang D, Wan X, Wu L, Chen P, Liu G J. Am. Chem. Soc. 2016, 138, 15547; [DOI] [PubMed] [Google Scholar]; f) Wu L, Wang F, Wan X, Wang D, Chen P, Liu G J. Am. Chem. Soc. 2017, 139, 2904; [DOI] [PubMed] [Google Scholar]; g) Xiong Y, Zhang G J. Am. Chem. Soc. 2018, 140, 2735; [DOI] [PubMed] [Google Scholar]; h) Lin J, Li T, Liu J-R, Jiao G, Gu Q, Cheng J, Guo Y, Hong X, Liu X J. Am. Chem. Soc. 2019, 141, 1074; [DOI] [PubMed] [Google Scholar]; i) Wu L, Wang F, Chen P, Liu G J. Am. Chem. Soc. 2019, 141, 1887. [DOI] [PubMed] [Google Scholar]
- [13].Schmidt J, Choi J, Liu AT, Slusarczyk M, Fu Science GC 2016, 354, 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Zhang G, Li J, Deng Y, Miller JT, Kropf AJ, Bunel EE, Lei A Chem. Commun. 2014, 50, 8709; [DOI] [PubMed] [Google Scholar]; b) Fleckenstein JE, Koszinowski K, Organometallics 2011, 30, 5018; [Google Scholar]; c) Hevia E, Mulvey E Angew. Chem. Int. Ed. 2011, 50, 6448; [DOI] [PubMed] [Google Scholar]; d) Krasovskiy A, Malakhov V, Gavryushin A, Knochel P Angew. Chem. Int. Ed. 2006, 45, 6040; [DOI] [PubMed] [Google Scholar]; e) Boymond L, Rottländer M, Cahiez G, Knochel P Angew. Chem. Int. Ed. 1998, 37, 1701; [DOI] [PubMed] [Google Scholar]; f) Hong L, Sun W, Yang D, Li G, Wang R Chem. Rev. 2016, 116, 4006. [DOI] [PubMed] [Google Scholar]
- [15].a) Jamison CR, Overman LE Acc. Chem. Res. 2016, 49, 1578; [DOI] [PubMed] [Google Scholar]; b) Curran D,P, Porter NA, Giese B Stereochemistry of Radical reactions: Concepts, Guidelines, and Synthetic Applications, Wiley-VCH, 1996. [Google Scholar]
- [16].a) For related catalytic homocoupling of organometallic reagents, see: Jin L, Zhang H, Li P, Sowa JR Jr, Lei A J. Am. Chem. Soc. 2009, 131, 9892; [DOI] [PubMed] [Google Scholar]; b) Lei A, Zhang X Org. Lett. 2002, 4, 2285; [DOI] [PubMed] [Google Scholar]; c) Lei A, Zhang X Tetrahedron Lett. 2002, 43, 2525; Review: [Google Scholar]; d) Liu C, Zhang H, Shi W, Lei A Chem. Rev. 2011, 111, 1780. [DOI] [PubMed] [Google Scholar]
- [17].a) For aligned radical cyclization/cross-coupling reactions, see: Wakabayashi K, Yorimitsu H and Oshima K J. Am. Chem. Soc. 2001, 123, 5374; [DOI] [PubMed] [Google Scholar]; b) Phapale VB, Buñuel E, García-Iglesias M, Cárdenas DJ Angew. Chem. Int. Ed. 2007, 46, 8790; [DOI] [PubMed] [Google Scholar]; c) Thapa S, Basnet P, Giri R J. Am. Chem. Soc. 2017, 139, 5700; [DOI] [PubMed] [Google Scholar]; d) KC S, Basnet P, Thapa S, Shrestha B, Giri R J. Org. Chem. 2018, 83, 2920; [DOI] [PubMed] [Google Scholar]; e) Kuang Y, Wang X, Anthony D, Diao T Chem. Commun. 2018, 54, 2558; [DOI] [PubMed] [Google Scholar]; f) Jin Y, Wang C Chem. Sci. 2019, 10, 1780; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Yan CS, Peng Y, Xu XB, Wang YW Chem. Eur. J. 2012, 18, 6039; [DOI] [PubMed] [Google Scholar]; h) Yu Xi., Yang T, Wang S, Xu H, Gong H Org. Lett. 2011, 13, 2138; [DOI] [PubMed] [Google Scholar]; i) Jiang B, Liu JX, Wei Y, Shi M Org. Lett. 2018, 20, 6229; [DOI] [PubMed] [Google Scholar]; j) For selected enantioselctive cyclization/cross-coupling reactions, see: Wang K, Ding Z, Zhou Z, Kong W J. Am. Chem. Soc. 2018, 140, 12364; [DOI] [PubMed] [Google Scholar]; k) Yasui H Kamisaki Y Takemoto Org. Lett. 2008, 10, 3303; [DOI] [PubMed] [Google Scholar]; l) Tian Z-X, Qiao J-B, Xu G-L, Pang X, Qi L, Ma WY, Zhao Z-Z, Duan J, Du Y-F, Su P, Liu X-Y, Shu X-Z J. Am. Chem. Soc. 2019, 141, 7637; [DOI] [PubMed] [Google Scholar]; m) You W, Brown MK J. Am. Chem. Soc. 2015, 137, 14578; [DOI] [PubMed] [Google Scholar]; n) Cong H, Fu GC J. Am. Chem. Soc. 2014, 136, 3788; [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Jin Y, Wang C Angew. Chem. Int. Ed. 2019, 58, 6722; [DOI] [PubMed] [Google Scholar]; p) Souillart L, Parker E, Cramer N Angew. Chem. Int. Ed. 2014, 53, 3001; [DOI] [PubMed] [Google Scholar]; q) Xu T, Ko HM, Savage NA, Dong G J. Am. Chem. Soc. 2012, 134, 20005; [DOI] [PubMed] [Google Scholar]; r) Liu L, Ishida N, Murakami M Angew. Chem. Int. Ed. 2012, 51, 2485. [DOI] [PubMed] [Google Scholar]
- [18].a) RajanBabu TV Acc. Chem. Res. 1991, 24, 139; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Spellmeyer DC, Houk KN J. Org. Chem. 1987, 52, 959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


