Abstract
Background:
Transcutaneous electrical nerve stimulation (TENS) and transcutaneous neuromuscular electrical stimulation (t-NMES) are commonly used therapies in the treatment of chronic hemiplegic shoulder pain. These treatments are often utilized during physical or occupational therapy sessions, yet research into the acute analgesic effects of TENS and t-NMES on hemiplegic shoulder pain and use during therapy is limited.
Objective:
To compare the acute effects of transcutaneous electrical nerve stimulation (TENS), transcutaneous neuromuscular electrical stimulation (t-NMES), and no stimulation on pain-free passive range of motion of the shoulder in subjects with hemiplegic shoulder pain.
Methods:
Prospective cohort study of ten subjects randomly treated with t-NMES, TENS, and one non-stimulation experimental condition. Pain-free passive external rotation and abduction range of motion of the affected shoulder were measured during stimulation.
Results:
There was not a significant within-subject difference in pain-free range of motion for external rotation or abduction. Subject to subject differences explained the majority of the variability in pain-free range of motion.
Conclusion:
This pilot study is the first to measure pain-free passive range of motion during electrical stimulation. Our findings demonstrate the lack of an acute effect of TENS and t-NMES on pain reduction.
Keywords: TENS, NMES, electrical stimulation therapy, Stroke, Hemiplegic Shoulder Pain, Upper extremity
Introduction
Hemiplegic shoulder pain is a common and debilitating complication of stroke with incidence reported as high as 72%1–3. The etiology of this condition is believed to originate from a complex interplay of factors, including spasticity3, capsular constriction4, rotator cuff injury5, somatosensory abnormalities6, traction of the brachial plexus7,8, and central sensitization9–11. The multifactorial etiology and lack of confirmed mechanism has made treatment of post-stroke shoulder pain difficult.
Transcutaneous electrical nerve stimulation (TENS) and transcutaneous neuromuscular electrical stimulation (t-NMES) are commonly used therapies in the treatment of hemiplegic shoulder pain and both have been shown to decrease shoulder pain when applied over several weeks12–14. While best studied for chronic usage, these treatments are also utilized during physical or occupational therapy sessions to allow improved participation in therapeutic exercise through immediate reduction of shoulder pain15. Research into the acute analgesic effects of TENS and t-NMES on hemiplegic shoulder pain and use during therapy is limited. Studies of acute, short-term and single application use of electrical stimulation for analgesia in a variety of other painful disorders have shown mixed results16–18. Two studies assessing improvement in movement-associated pain with use of single or short-term TENS application noted reduction in post-surgical pain18 and fibromyalgia pain17. Whether single use application of electrical stimulation also improves movement-associated hemiplegic shoulder pain is unknown.
The objective of this study was to compare the effects of TENS, t-NMES, and no stimulation on pain-free passive range of motion measured during stimulation of a painful hemiplegic shoulder. In this report we address the following questions: (1) is pain-free passive shoulder range of motion greater during stimulation with TENS and t-NMES, as compared to without stimulation; and (2) is pain-free passive shoulder range of motion greater during t-NMES than TENS? We hypothesized that due to acute analgesia from simultaneous electrical stimulation, pain-free passive shoulder range of motion would be greater during stimulation with either TENS or t-NMES, as compared to without simultaneous stimulation. We expected simultaneous stimulation with t-NMES to result in greater pain-free passive range of motion than with TENS due to the addition of muscle contraction obtained with t-NMES but not TENS.
Methods
This protocol was approved by the local institutional review board. This manuscript conforms to the STROBE Guidelines.
Participants
Ten subjects were recruited from a stroke rehabilitation center at an urban, academic hospital. To qualify for study inclusion, subjects had to be 21 years or older and at least 3 months post-stroke, have their worst shoulder pain in the last week at least 4 on the 0–10 numerical rating scale, and adequate cognitive ability to be able to rate their pain in the past week. Diagnosis of hemiplegic shoulder pain was made according to these inclusion criteria. In accordance with prior studies and reports noting that hemiplegic shoulder pain often has multiple underlying pathologies the presumed etiology of shoulder pain was not collected as part of this study19,20. Subjects were excluded if they had a history of tachyarrhythmia with decreased blood pressure, uncontrolled seizures (defined as more than one per month), an implanted electrical device, or uncompensated hemi-neglect.
Stimulation System and Stimulation Parameters
The electrical stimulation was delivered via an EMPI 300PV™ (Empi, St. Paul, MN), a commercially available electrical stimulation device that can provide both TENS and t-NMES. Two inch by two inch electrodes were placed by an occupational therapist on the skin overlying the motor points of the middle deltoid and upper trapezius muscles of the affected shoulder. The TENS stimulation parameters were of a symmetric waveform, a frequency of 100 Hz, and a pulse duration of 300 microseconds (EMPI 300PV™ program PPR #7). The TENS current intensity was set by adjusting the amplitude to the highest level tolerable without initiation of pain, but below motor threshold (program range 0–100mA). The t-NMES parameters were a symmetric waveform with 2 second ramp-up and 2 second ramp-down, a frequency of 35Hz, and a pulse duration of 300 microseconds (EMPI 300PV™ program PPR #3). The t-NMES current intensity was set by adjusting the amplitude to yield the strongest contraction of the underlying muscles without initiating pain. The stimulation was delivered by a trained occupational therapist under three conditions: 10 seconds of TENS, 10 seconds of t-NMES, and 10 seconds of no stimulation. The minimum duration of stimulation to achieve treatment effect from TENS or t-NMES is unknown. A 10-second stimulation duration was chosen to specifically assess the immediate analgesic effects of the treatment with minimal risk for prolonged effect crossing into subsequent stimulation trials. Each subject was exposed to each of the three stimulation conditions three times in a computer-generated random sequence for each outcome measure with a 5-minute wash-out period between each stimulation.
Assessment Protocol
Pain-free passive range of motion of the shoulder in external rotation and abduction was assessed by a trained, blinded assessor. The angles of external rotation and abduction were measured using a hand-held goniometer. The same individual performed the measurement for every trial. External rotation and abduction were selected due to their common post-stroke impairment.
The subject was supine for both assessments, with the shoulder covered in a way that allowed for measurement and movement, but so that no muscle contraction could be seen. External rotation was measured from the start position of the shoulder with elbow flexed to 90 degrees and shoulder adducted and fully internally rotated such that the forearm was resting on the subject’s abdomen. The start position of the shoulder for the abduction measurement was with the elbow extended, shoulder adducted and forearm neutral. The occupational therapist turned on the stimulation and signaled when to initiate the movement. The shoulder was slowly externally rotated or abducted with the scapula stabilized until the subject reported the initiation or worsening of shoulder pain, at which point the angle measurement was recorded. The movement and measurement were completed within the ten seconds that the stimulation was delivered.
Data Analysis
Differences in range of motion associated with stimulation type were analyzed with repeated measures ANOVA. To evaluate whether the results were influenced by order of presentation, data were also analyzed using general linear mixed models with external rotation and abduction as dependent variables. The independent variable was stimulation with a random coefficient for each subject. The initial models also included fixed effects variables indicating trial order and an interaction term for subject and stimulation. Parsimony was sought in the models and variables were removed when not significant at α=0.05. Statistical analysis was completed with SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
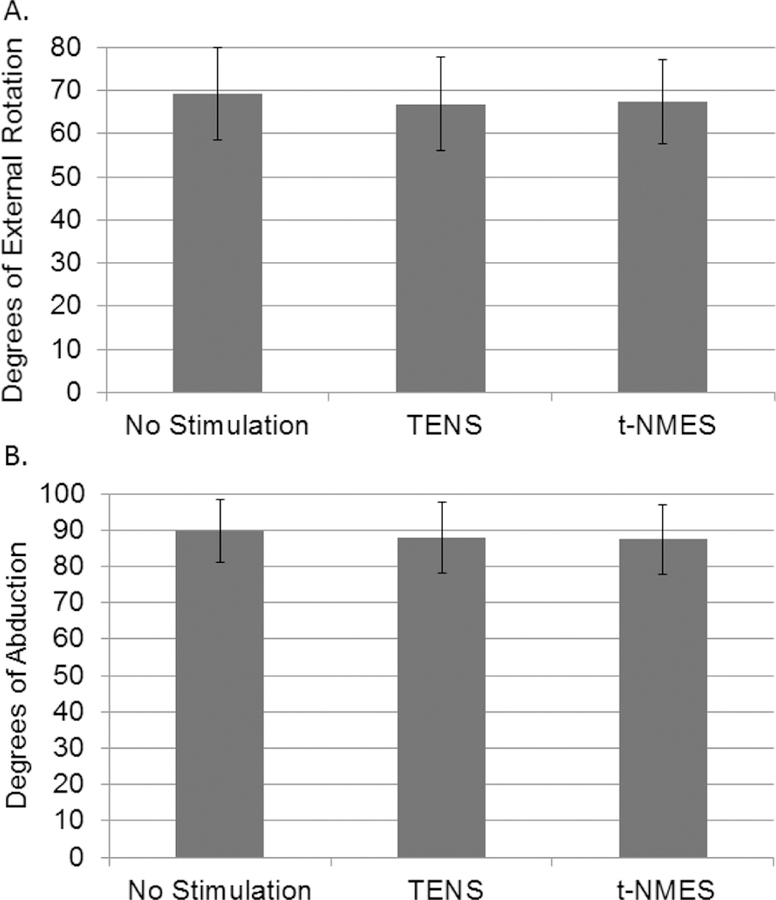
All 10 subjects completed the study. No complications or deviations from the protocol occurred. The subjects’ characteristics and demographics are shown in Table 1. The mean values for TENS, t-NMES, and no stimulation are shown in Figure 1. The mean external rotation was 67.4 degrees (95% CI 49.4 – 85.4 degrees) with t-NMES, 66.9 degrees (95% CI 46.9 – 86.8 degrees) with TENS, and 69.1 degrees (95% CI 49.6 – 88.7 degrees) with no stimulation. The mean abduction was 87.5 degrees (95% CI 69.8 – 105.1 degrees) with t-NMES, 87.8 degrees (95% CI 70.0 – 105.7 degrees) with TENS, and 89.6 degrees (95% CI 73.9 – 105.4 degrees) with no stimulation. There was no significant difference in pain-free passive range of motion within subjects due to stimulation pattern for external rotation range of motion (F(2,18)=0.4, p=0.7) or abduction (F(2,18)=0.8, p=0.4). The only independent variable in the initial model with significant impact on pain-free range of motion was subject, with the final subject-only model explaining 93.1% of the variability in external range of motion (p<0.0001) and 94.8% of the variability in abduction range of motion (p<0.0001).
Table 1:
Baseline characteristics of ten subjects with hemiplegic shoulder pain. SD: standard deviation.
| Subject characteristics | |
|---|---|
| n | 10 |
| Mean Age (SD) | 59.1 (11.3) |
| 25th-75th percentile | 50–67.5 |
| Female, % | 60 |
| Months since stroke, mean (SD) | 46.8 (42.1) |
| 25th-75th percentile | 17.5–66 |
| Pain Score, mean (SD) | 7.8 (2.5) |
| Right hemisphere stroke, % | 60 |
Figure 1.
There are no significant differences between (A) the degree of pain-free passive external rotation, or (B) the degree of pain-free passive shoulder abduction among the different stimulation settings. Abbreviations: TENS: transcutaneous electrical nerve stimulation; t-NMES: transcutaneous neuromuscular electrical stimulation.
Discussion
This is the first study to assess hemiplegic shoulder pain reduction concurrent with the application of electrical stimulation, a practice commonly utilized by therapists during sessions of treatment. Our study measured pain perception through assessment of pain-free passive range of motion during TENS and t-NMES. Contrary to expectations, our research found that there was no difference in pain-free passive shoulder range of motion during electrical stimulation with TENS versus t-NMES, or when either was compared to no stimulation. These results were consistent across shoulder external rotation and shoulder abduction. No increase or decrease in range of motion was seen with progression of the session, suggesting an adequate wash-out period between treatments and no effect of repeated ranging on pain.
TENS and t-NMES are two electrical stimulation techniques commonly used to treat hemiplegic shoulder pain. TENS stimulates sensory nerves without producing muscle contraction, while t-NMES stimulates both the sensory and motor nerves with the goal of producing muscle contraction21. The pain reduction associated with TENS is believed to be due to stimulation of large-diameter afferent fibers in the deep tissue, leading to opioid and GABA-mediated analgesia22,23. The mechanisms through which t-NMES promotes analgesia have not been as well studied. Studies have shown that continuous t-NMES increases the sensory threshold24. Similarly, the improvement in hemiplegic shoulder pain noted in recent studies of single lead intramuscular electrical stimulation also suggests a sensitization-reduction mechanism for improvement with NMES regardless of biomechanical etiology factors25. When used long-term, the muscle contraction induced by t-NMES may improve joint positioning26 and reduction in abnormal painful strain on joint components. It is not known whether t-NMES induced muscle contraction contributes to pain reduction after short-term use.
The use of electrical stimulation for reduction of hemiplegic shoulder pain has mostly been assessed for prolonged usage. Short-term use of electrical stimulation to improve therapy tolerance is used clinically, but mostly relies on the theoretical mechanisms described above22,27, animal studies22, and studies of other pain disorders17,18. These studies suggest that there may be an acute analgesic effect of electrical stimulation in addition to the known long-term effects, though the acute effect on hemiplegic shoulder pain had not been tested prior to this study. Participation limitations from hemiplegic shoulder pain have been shown to lead to worse functional outcomes28; thus, acute pain relieving treatments have the potential to increase therapy tolerance and participation and promote recovery.
Unfortunately, our study did not find improvement in pain-free passive shoulder range of motion with concurrent stimulation with TENS or t-NMES as compared to no stimulation. There are multiple potential explanations for our results. First, it is possible that the immediate pain-reducing effects of TENS and t-NMES that were expected based on neuromodulation theories may not be applicable to those with chronic shoulder pain. Rather, a longer duration of stimulation may be necessary to obtain pain reduction through endogenous analgesia, as has been shown in animal studies29. Second, location of stimulation may also have played a role in the findings. Placement of the electrodes was carefully determined to provide the best possible response for the two movements and stimulation types to maintain blinding; however, this limitation may have led to a sub-optimal location for either or both types of stimulation. The small sample size is another limitation that may have contributed to these findings. This was a small pilot study to guide and power future research. While the power may affect statistical significance, it is notable that we could not identify a clinically significant difference between stimulation protocols that would suggest a treatment effect.
Conclusion
Our findings demonstrate the lack of an immediate effect from TENS and t-NMES on pain reduction. Future studies should be directed toward determining whether any benefit may exist for acute application of stimulation for a longer duration.
Acknowledgments
We would like to thank Steven M. Sidik1,2 for his assistance with the statistical analyses. (1) Cleveland FES Center, (2) Department of Statistics, Case Western Reserve University The Effect of Electrical Stimulation on Impairment of the Painful Post-Stroke Shoulder
This work was supported by Grant Number K24HD054600 from the National Institutes of Health (NIH)/ National Institute of Child Health and Human Development (NICHD)
Footnotes
Disclosures:
No conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
No author received financial benefit from this study.
ClinicalTrials.gov identification number
References
- 1.Gamble GE, Barberan E, Laasch HU, Bowsher D, Tyrrell PJ, Jones AK. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. European journal of pain (London, England) 2002;6(6):467–474. [DOI] [PubMed] [Google Scholar]
- 2.Roy CW, Sands MR, Hill LD. Shoulder pain in acutely admitted hemiplegics 1994;8(4):334–340. [Google Scholar]
- 3.Van Ouwenaller C, Laplace PM, Chantraine A. Painful shoulder in hemiplegia. Archives of physical medicine and rehabilitation 1986;67(1):23–26. [PubMed] [Google Scholar]
- 4.Rizk TE, Christopher RP, Pinals RS, Salazar JE, Higgins C. Arthrographic studies in painful hemiplegic shoulders. Archives of physical medicine and rehabilitation 1984;65(5):254–256. [PubMed] [Google Scholar]
- 5.Najenson T, Yacubovich E, Pikielni SS. Rotator cuff injury in shoulder joints of hemiplegic patients. Scandinavian journal of rehabilitation medicine 1971;3(3):131–137. [PubMed] [Google Scholar]
- 6.Yu DT, Chae J, Walker ME, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Archives of physical medicine and rehabilitation 2004;85(5):695–704. [DOI] [PubMed] [Google Scholar]
- 7.Estape R, Ferel D, Barth R. Brachial plexus lesions in hemiplegics. Acta neurologica Belgica 1979;79(6):444–449. [PubMed] [Google Scholar]
- 8.Kaplan PE, Meridith J, Taft G, Betts HB. Stroke and brachial plexus injury: a difficult problem. Archives of physical medicine and rehabilitation 1977;58(9):415–418. [PubMed] [Google Scholar]
- 9.Roosink M, Renzenbrink GJ, Buitenweg JR, van Dongen RT, Geurts AC, Ijzerman MJ. Somatosensory symptoms and signs and conditioned pain modulation in chronic post-stroke shoulder pain. The journal of pain : official journal of the American Pain Society 2011;12(4):476–485. [DOI] [PubMed] [Google Scholar]
- 10.Hoo J Soo, Paul T, Chae J, Wilson RD. Central hypersensitivity in chronic hemiplegic shoulder pain. American journal of physical medicine & rehabilitation 2013;92(1):1–9; quiz 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson R An etiological paradigm shift for chronic hemiplegic shoulder pain. Am J Phys Med Rehabil 2014;93(10):929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chantraine A, Baribeault A, Uebelhart D, Gremion G. Shoulder pain and dysfunction in hemiplegia: effects of functional electrical stimulation. Archives of physical medicine and rehabilitation 1999;80(3):328–331. [DOI] [PubMed] [Google Scholar]
- 13.Faghri PD, Rodgers MM, Glaser RM, Bors JG, Ho C, Akuthota P. The effects of functional electrical stimulation on shoulder subluxation, arm function recovery, and shoulder pain in hemiplegic stroke patients. Archives of physical medicine and rehabilitation 1994;75(1):73–79. [PubMed] [Google Scholar]
- 14.Leandri M, Parodi CI, Corrieri N, Rigardo S. Comparison of TENS treatments in hemiplegic shoulder pain. Scandinavian journal of rehabilitation medicine 1990;22(2):69–71. [PubMed] [Google Scholar]
- 15.Fedorczyk JM. The Use of Physical Agents in Hand Rehabilitation. In: Mosby E, ed. Rehabilitation of the Hand and Upper Extremity 2 ed. Philadelphia, PA: 2011:1495–1511. [Google Scholar]
- 16.Chesterton LS, Lewis AM, Sim J, et al. Transcutaneous electrical nerve stimulation as adjunct to primary care management for tennis elbow: pragmatic randomised controlled trial (TATE trial). BMJ (Clinical research ed) 2013;347:f5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dailey DL, Rakel BA, Vance CGT, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 2013;154:2554–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. The journal of pain : official journal of the American Pain Society 2003;4(8):455–464. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RD, Chae J. Hemiplegic Shoulder Pain. Physical medicine and rehabilitation clinics of North America 2015;26(4):641–655. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RD. An etiological paradigm shift for chronic hemiplegic shoulder pain. American journal of physical medicine & rehabilitation 2014;93(10):929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergquist AJ, Clair JM, Lagerquist O, Mang CS, Okuma Y, Collins DF. Neuromuscular electrical stimulation: implications of the electrically evoked sensory volley. European journal of applied physiology 2011;111(10):2409–2426. [DOI] [PubMed] [Google Scholar]
- 22.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. The Journal of pharmacology and experimental therapeutics 1999;289(2):840–846. [PubMed] [Google Scholar]
- 23.Maeda Y, Lisi TL, Vance CG, Sluka KA. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain research 2007;1136(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SF, Chen CC, Liao WS, Shyu BC. Different types of variant muscle nociception after intermittent and continuous neuromuscular stimulation in rats. Journal of biomedical science 2005;12(3):467–479. [DOI] [PubMed] [Google Scholar]
- 25.Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain practice : the official journal of World Institute of Pain 2013;13(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker VM, Wade DT, Hewer R Langton. Loss of arm function after stroke: measurement, frequency, and recovery. International rehabilitation medicine 1986;8(2):69–73. [DOI] [PubMed] [Google Scholar]
- 27.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. The journal of pain : official journal of the American Pain Society 2005;6(10):673–680. [DOI] [PubMed] [Google Scholar]
- 28.Roy C, Sands MR, Hill LD, Harrison A, Marshall S. The effect of shoulder pain on outcome of acute hemiplegia 1995;9(1):21–27. [Google Scholar]
- 29.Fukazawa Y, Maeda T, Hamabe W, et al. Activation of spinal anti-analgesic system following electroacupuncture stimulation in rats. Journal of pharmacological sciences 2005;99(4):408–414. [DOI] [PubMed] [Google Scholar]