Abstract
Purpose
Lung-MAP (SWOG S1400) is a master platform trial assessing targeted therapies in squamous non-small cell lung cancer (sqNSCLC). The objective of study C (S1400C) was to evaluate the response rate to palbociclib, a CDK 4/6 inhibitor, in patients with cell cycle gene abnormalities.
Methods
Patients with sqNSCLC, Performance status (PS) 0–2, normal organ function, who had progressed after at least one prior platinum-based chemotherapy with CDK 4 or CCND1/2/3 amplifications on tumor specimens were eligible. The study was originally designed as a phase II/III trial comparing palbociclib to docetaxel, but was modified to a single arm phase II trial with primary endpoint of response when immunotherapy was approved. If two or fewer responses were seen in the first 20 pts, then the study would cease enrollment.
Results
Eighty-eight patients (9% of patients screened) were assigned to S1400C, and 53 patients enrolled (including 17 to docetaxel). One patient registered to docetaxel was re-registered to receive palbociclib after progression on docetaxel. The frequency of cell cycle gene alterations in the eligible palbociclib patients (N=32) were: CCND1 (n=26, 81%), CCND2 (n=3, 9%), CCND3 (n=2, 6%), CDK4 (n=1, 3%). Thirty-two eligible patients received palbociclib. There were two partial responses (6% RR, 95% CI: 0%−15%), both with CCND1 amplification. Twelve patients had stable disease (38%, 95% CI: 21%−54%). Median progression-free survival was 1.7 months (95% CI: 1.6–2.9 months) and median overall survival was 7.1 months (95% CI: 4.2–12.5).
Conclusions
Palbociclib as monotherapy failed to demonstrate the pre-specified criteria for advancement to phase III testing.
Introduction
Despite substantial progress in identifying targeted agents with activity in advanced non-squamous non-small cell lung cancer (NSCLC), there have been few advances in developing such agents in squamous cell NSCLC (sqNSCLC). Several studies have identified potentially actionable mutations in this subset of disease.1 The Lung Master Protocol (Lung-MAP, SWOG 1400) was developed with the goal of establishing a mechanism for genomically screening a large, homogeneous, population of sqNSCLC and subsequently assigning and accruing simultaneously to sub-studies evaluating agents targeting specific molecular abnormalities.2 When originally designed, biomarker-driven sub-studies in the protocol compared new targeted therapy or targeted therapy combinations to standard of care therapy (i.e., docetaxel) based on designated therapeutic biomarker-drug combinations, with the ultimate goal of obtaining FDA approval of new targeted therapies in this setting. In December 2015, due to the rapid development and approval of immunotherapy in NSCLC, the protocol was re-designed to evaluate biomarker-driven therapies using a single arm screening design to be followed by randomized assessments if specified levels of activity were met.3–6
Sub-study C (S1400C) of Lung-MAP tested the concept of targeting cyclin dependent kinase (CDK) abnormalities with the selective CDK4/6 inhibitor palbociclib. Unrestricted proliferation is a hallmark of cancer and frequently results from abnormality in the retinoblastoma (Rb) pathway. Rb in its active, unphosphorylated state, inhibits progression through the cell cycle. For the cell to enter mitosis, Rb is phosphorylated by the cyclin D-CDK 4/6 complex. Inactivation of Rb, by deletion, mutation or increased activation of the cyclin D-CDK 4/6 complex results in unrestricted proliferation. In sqNSCLC, Rb itself is rarely mutated. However, abnormalities of CDKN2, which encodes p16 (INK4), the primary inhibitor of the cyclin D-CDK 4/6 complex are common, as is amplification of D-type cyclins (encoded by CCND1,2,3) or CDK-4/6. In squamous cell carcinoma, CCND1 was found to be amplified in a number of tumors evaluated, including sqNSCLC. The Cancer Genome Atlas (TCGA) demonstrated CCND1 amplification in 12% of squamous cell carcinoma, although these often occur in association with other abnormalities of the CDK4/Rb pathway. In addition, many of these amplifications were associated with loss of p16, contributing to further disruption of the cyclin D-CDK4/6-Rb-p16 axis. Although Rb loss was detected in some of the analyzed specimens, they did not overlap with CCND1 amplifications.1, 7
Palbociclib (PD0332991) is an oral, selective CDK4/6 inhibitor that has been tested in multiple phase I, II and III trials and approved in combination with letrozole for advanced breast cancer. In prior studies, a schedule of 3 weeks on treatment/1 week off treatment was found to be the best tolerated and active and was therefore chosen for this trial.8, 9
Patients and Methods
When the trial opened in June 2014, the eligibility criteria specified that patients were allowed to have only a single line of prior therapy for stage III or IV recurrent disease and have a performance status (PS) = 0–2. In April 2015, the study was amended to allow any number of lines of prior therapy for stage IV NSCLC or for lower stage disease within one year. In December 2015, the study was amended to only allow PS 0–1 and redesigned to be a single arm study. In addition to these criteria, patients were required to have normal hematologic, hepatic and renal function. Patients on strong CYP3A4 inhibitors and/or inducers were not allowed to enroll. Due to the known cardiac effects of the drug, patients with a known personal or family history of long or short QT syndrome, Brugada syndrome or torsade de pointes were excluded. Measurable disease (by RECIST 1.1) was required. Patients with treated brain metastases were allowed as long as (1) metastases had been locally treated and remained clinically controlled and asymptomatic for at least 14 days following treatment, AND (2) patient had no residual neurological dysfunction and was off corticosteroids for at least one day prior to sub-study registration. Following the modification to a single arm design, patients previously registered to the docetaxel arm were allowed to re-register to the palbociclib arm. No patient could be enrolled at an institution prior to review and approval by either the local Institutional Review Board or by the National Cancer Institute Central IRB. Written informed consent was required from all patients prior to enrollment in the master protocol and a separate consent was required for the specific sub-study.
The cell cycle gene alterations required for eligibility were amplifications of CDK4, CCND1, CCND2, or CCND3. Disease characterized by substitutions or fusion alterations were not eligible. Amplification was defined as ≥ 6 estimated copies (or ≥ 7 for triploid or ≥8 for tetraploid samples). Mutational analysis was performed on archival formalin-fixed paraffin-embedded tumor specimens using the Foundation One testing platform.10
Palbociclib was administered orally at a dose of 125 mg daily and taken with food. A cycle of treatment was 28 days with treatment given continuously for 21 days and 7 days off treatment. Disease assessment occurred every six weeks, and treatment could continue until progression. Dose reductions and adjustments were specified in the protocol (supplemental materials) and were to be discussed with the study chair.
Statistical Considerations
S1400C originally was a phase II/III trial with randomization between palbociclib and docetaxel. The primary endpoint of the phase II component was progression free survival (PFS) and the study included co-primary endpoints, PFS and overall survival (OS), in the phase III component. In December 2015, the design was amended to a single arm phase II trial and the docetaxel arm permanently closed to accrual. Patients on the docetaxel arm were not included in the analyses presented in this paper. The primary objective for the modified design was to evaluate the objective response rate (ORR) (confirmed and unconfirmed, complete and partial) in patients treated with palbociclib with stage IV refractory sqNSCLC. The sample size was based on a design with 91% power to rule out an ORR of 15% at the 5% level, if the true rate were 35%. The observation of 10/40 (25% ORR) would be considered evidence to rule out the null ORR and evidence to pursue an independent randomized phase III. The design included an interim analysis at 20 patients evaluable for response and would continue accrual if at least three responses were observed. A key secondary objective was an investigator assessment of median PFS (mPFS). If the ORR rate was less than 25% but the mPFS was at least 4.5 months, this would be considered sufficient evidence to continue to the follow-on Phase III. With 40 patients, this design had 90% power to rule out a median PFS of 3 months or less, if the true mPFS was 6 months, at the 0.05 1-sided level. The observation of an mPFS of at least 4.5 months would be considered evidence to rule out a mPFS of 3 months or less.
Binary proportions and associated 95% confidence intervals were estimated. Survival distributions were estimated using the method of Kaplan-Meier and the Brookmeyer-Crowley method was used to estimate confidence intervals.
Results
The study was open to accrual from June 15, 2014 to September 1, 2016. In this time, 973 patients were screened for the overall study. Eighty-eight patients (9% of those screened while the study was actively accruing) were assigned to S1400C. Fifty-three patients were enrolled (including 17 to docetaxel before the study redesign). One patient registered to docetaxel re-registered to palbociclib after progression on docetaxel. The study did not meet the criterion to continue past the interim analysis and was closed to accrual on September 1, 2016. Of the 37 patients enrolled to the palbociclib arm, five were ineligible (four inadequate baseline labs, one did not progress on prior therapy). The demographics for the 32 eligible and evaluable patients are shown in Table 1. The frequency of cell cycle gene alterations in the eligible palbociclib patients (N=32) were as follows: CCND1 (n=26, 81%), CCND2 (n=3, 9%), CCND3 (n=2, 6%), CDK4 (n=1, 3%), Table 2.
Table 1:
Patient Demographics and Characteristics
| N (%) (N = 32) | |
|---|---|
| Age Median (range) | 67.3 (53–80.7) |
| Male Gender | 21 (66%) |
| Performance status | |
| 0 | 13 (41%) |
| 1 | 18 (56%) |
| 2 | 1 (3%) |
| Race/Ethnicity | |
| White | 28 (88%) |
| Black | 3 (9%) |
| Asian | 1 (3%) |
| Hispanic | 0 (0%) |
| Number of Prior Lines of Therapy for Stage IV Disease | |
| 0 | 8 (25%)* |
| 1 | 23 (72%) |
| 2 or more | 1 (3%) |
| Smoking Status | |
| Current Smoker | 13 (41%) |
| Former Smoker | 18 (56%) |
| Never Smoker | 1 (3%) |
| Brain Metastases | |
| Present | 2(6%) |
| Absent | 30 (94%) |
per protocol, patients were eligible if they received chemotherapy in the adjuvant or as part of combined modality therapy within one year of enrollment.
Table 2:
Gene Alterations Detected on FMI NGS Screening
| Palbociclib (n=32) | |
|---|---|
| CCGA Study Gene Alterations | |
| CCND1 | 26(81%) |
| CCND2 | 3(9%) |
| CCND3 | 2(6%) |
| CDK4 | 1(3%) |
| Number of CCGA Study Gene Alterations | |
| 1 | 32(100%) |
| Other Concomitant Gene Alterations | |
| Short Variants | |
| TP53 | 30(94%) |
| CDKN2A, MLL2 | 8(25%) |
| NFE2L2 | 6(19%) |
| PTEN | 4(13%) |
| ARID1A, LRP1B, PIK3CA | 3(9%) |
| APC, BAP1, KRAS | 2(6%) |
| ATRX, BRCA2, BRIP1, CREBBP, DAXX, DNMT3A, EZH2, FANCA, FBXW7, FLT4, GRIN2A, IGF1, IKZF1, KLHL6, MET, NCOR1, NF1, NF2, NOTCH1, NOTCH3, NOTCH4, SF3B1, STAT4, STK11, TET2, TRRAP, TSC2, WT1 | 1(3%) |
| Copy Number Alterations | |
| FGF19, FGF3, FGF4 | 24(75%) |
| SOX2 | 16(50%) |
| PIK3CA | 11(34%) |
| CDKN2A | 10(31%) |
| CDKN2B | 9(28%) |
| FGF12, MYC | 6(19%) |
| KRAS | 5(16%) |
| EPHB1, FGFR1, MYST3, REL, ZNF703 | 3(9%) |
| AKT2, EGFR, EMSY, ERBB2, FGF23, FGF6, JAK2, KDM5A, KDR, PTEN | 2(6%) |
| AKT3, BCL2L2, CCNE1, CDK4, IGF1R, IKBKE, KDM6A, KIT, LRP1B, MDM2, MDM4, NFKBIA, NKX2–1, RICTOR, STK11, TP53 | 1(3%) |
| Rearrangements | |
| LRP1B | 3(9%) |
| ABL1, MLH1, MLL2, PIK3R2 | 1(3%) |
Patients received a median of two cycles (range = 1–17) of palbociclib. The AEs were as expected for palbociclib (Table 3). Three patients discontinued therapy due to AEs. Five patients experienced Grade 4 AEs including lymphopenia (3), neoplasms (1) and thrombocytopenia (1). Thirteen others experienced Grade 3 treatment-related AEs.
Table 3:
Adverse Events Attributed to Treatment
| Adverse event | Grade of AE N = 32 | ||
|---|---|---|---|
| 3 | 4 | 5 | |
| AST increased | 1(3%) | ||
| Anemia | 4(13%) | ||
| Anorexia | 1(3%) | ||
| Chronic Kidney Disease | 1(3%) | ||
| Dyspnea | 1(3%) | ||
| Fatigue | 5(16%) | ||
| Generalized muscle weakness | 1(3%) | ||
| Hyperglycemia | 2(6%) | ||
| Hyponatremia | 1(3%) | ||
| Hypotension | 1(3%) | ||
| Lung infection | 1(3%) | ||
| Lymphopenia | 6(19%) | 3(9%) | |
| Neoplasms, all | 1(3%) | ||
| Neutropenia | 5(16%) | ||
| Thrombocytopenia | 1(3%) | 1(3 %) | |
| Leucopenia | 6(19%) | ||
| Maximum grade of any AE | 13(41%) | 5(16%) | 0 |
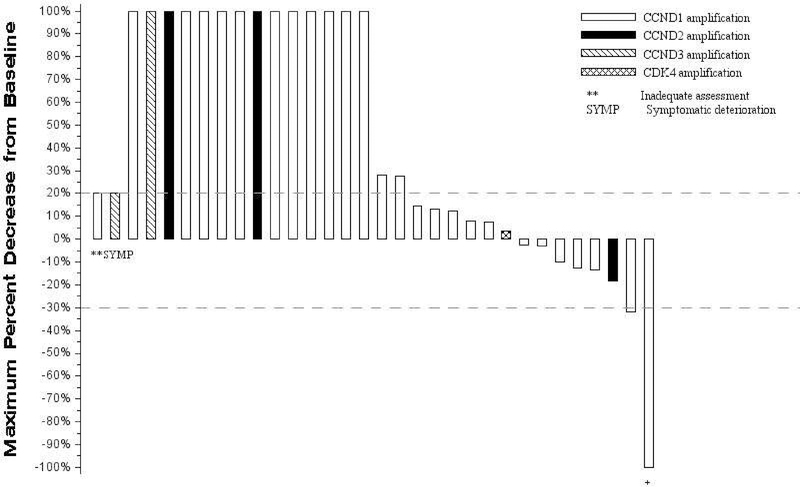
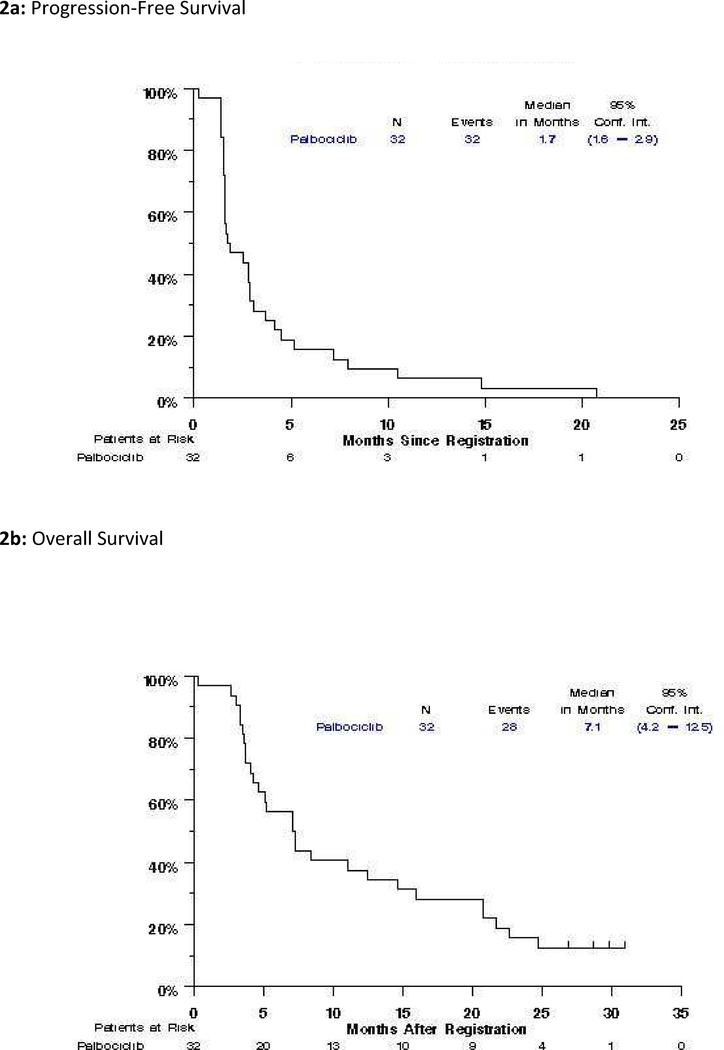
There were two confirmed partial responses observed for a response rate of 6% (95% CI: 0%−15%). A waterfall plot depicting tumor response is presented in Figure 1. Twelve patients demonstrated stable disease (38%, 95% CI: 21%−54%) for a disease control rate of 44% (95% CI: 27%−61%), response was not assessable in one patient, and one patient had symptomatic deterioration as their best objective response with no follow-up tumor measurements. The median progression free survival was 1.7 months (95% CI: 1.6–2.9) and overall survival was 7.1 months (95% CI: 4.2–12.5) (Figure 2). The one- and two-year estimates of survival are 37.5% and 12.1%, respectively. Of the two partial responses, one has progressed (duration of response, 7.7 months), and one died without evidence of progression (duration of response 12 months). Of note, both of these patients demonstrating partial response had CCND1 amplification.
Figure 1.
Waterfall plot of response to palbociclib
Each vertical bar represents a patient’s best percent decrease in tumor burden when compared to baseline as defined by RECIST 1.1. Only patients with measurable disease at baseline are presented in the plot. Patients who did not have follow up tumor disease assessment are presented at the very left of the plot marked with ‘**’. Patients who had new lesions appear at their first follow-up assessment or who expired prior to the first scheduled the disease assessment and the death can reasonably be assumed to be due to disease progression are represented graphically as a 100% increase in tumor burden. Patients who had symptomatic deterioration at first disease assessment are coded as “Symptomatic deterioration”. Negative numbers represent decrease in tumor burden from baseline while positive numbers represent increase in tumor burden from baseline.
+ this patient had complete disappearance of disease and would have been considered a CR, however, the patient’s non-target brain lesion was removed surgically and per RECIST 1.1 guidelines is coded as having a partial response.
Figure 2:
Progression-free Survival and Overall Survival
Discussion
Palbociclib did not demonstrate antitumor activity in this genomically selected patient population with sqNSCLC. In contrast, well-differentiated or dedifferentiated liposarcoma (WDLD/DDLS) is the paradigm malignancy characterized by CDK4 amplification and palbociclib has demonstrated growth inhibition in WDLS/DDLS cells in vitro and in xenograft models. Proof of principle that targeting CDK4 amplified cancer with palbociclib can be therapeutically important was established in a phase II trial of palbociclib in WDLD/DDLS. In 60 evaluable patients, the 12-week PFS was 57.2% and median PFS 17.9 weeks. One patient had a complete response.11
Despite this proof of principle demonstration that CDK/cyclin abnormalities might predict for benefit of this type of agent, it is not clear that such abnormalities are necessary for activity. All three currently approved CDK 4/6 inhibitors (palbociclib, abemaciclib, ribociclib) have primarily demonstrated benefit in hormone responsive breast cancer when combined with anti-estrogens or other hormonally active agents regardless of demonstration of genetic abnormalities.12 Abemaciclib has demonstrated single agent activity in hormone receptor positive breast cancer as well.13 This likely occurs because the CDK4/6-cyclin D1 complex is a direct target of estrogen receptor signaling.14
It is possible that the wrong subgroup of lung cancer patients was targeted in this trial. Preclinical and early clinical evidence demonstrates that the CDK4/6 inhibitor abemaciclib is particularly active in KRAS mutant lung cancers. These are almost exclusively seen in non-squamous carcinoma.15 A study evaluating abemaciclib versus erlotinib in the second line treatment of NSCLC with K-Ras mutations (and therefore likely adenocarcinoma) was negative for the primary endpoint of OS, however demonstrated superior PFS (3.6 months vs. 1.9 months, p <.001) and higher response rate (8.9% vs. 2.7%, p =.01).15
An additional issue is that the postulated mechanism of action for CDK/cyclin agents, inhibition of progression through the cell cycle, is hypothetically more likely to demonstrate cytostatic activity and stable disease as opposed to cytocidal activity and tumor response. For this reason, the secondary endpoint of mPFS was incorporated into the trial. Unfortunately, this was also negative.
Despite the results of this study, there was some evidence of activity with two partial responses. It should be noted that palbociclib has demonstrated little activity as a single agent in breast cancer, but is clearly beneficial when combined with other agents.12 Additionally, evidence from other trials that indicates that additional evaluation of this pathway, possibly in combination with other agents, may still be beneficial in some patients with sqNSCLC.
Acknowledgments
Funding: this research supported in part by NIH/NCI grants CA180888, CA180819, CA180820, CA180821, CA180868, CA189821, CA189812, CA180801, CA189830, CA189872, CA180846, CA189873, CA 189822, CA 189809, CA180828, CA180834, CA190002, CA189954, CA180818, CA180826, CA46368, CA46282, CA11083; and by Amgen, AstraZeneca, Bristol-Myers Squibb Company, Genentech and Pfizer through the Foundation for the National Institutes of Health, in partnership with Friends of Cancer Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest: MJE reports grants, Apexigen, Merck, and Bristol Myers Squibb, personal fees from Armo, BerGen Bio, Syndax, WindMil Therapeutics, and Bristol Myers Squibb, other from Biomarker Strategies, outside the submitted work; KAB reports personal fees from Novartis, Pfizer, Genomic Health Inc, Myriad, Genentech/Roche, and other from Puma, outside of the submitted work; SNW reports grants from 1 UM1 CA186704-01, other from F. Hoffmann-La Roche Ltd, Ariad, Pfizer Pharmaceuticals, Inc., Hengrui Therapeutics, Xcovery, EMD Serono Research & Development Institute, Inc., Checkpoint Therapeutics, Inc., Genentech, Inc., Lilly, Stemcentrx, Inc., Ignyta, Inc Bristol-Myers Squibb Pharmaceutical, Synermore Biologics Co., Ltd., Novartis Pharmaceuticals Corporation, Merck & Company, Inc., NewLink Genetics Corporation, Celegene, outside the submitted work; VAP reports advisory Boards for Nektar Therapeutics, Astra Zeneca Pharmaceuticals, Arrys Therapeutics, Merck&Co, LOXO Oncology, Araxes Pharma, F.Hoffman-LaRoche Ltd, Janssen Research Foundation, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly &Co, Novartis Pharmaceuticals Corp. Takeda Pharmaceuticals, Abbvie, TRM Oncology, Exelixis, Abbvie, Tesaro Research/Grants from: Eli Lilly &Co, Novartis, Merck, Astra Zeneca Pharmaceuticals, F Hoffman-La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte; KK reports: Dr. Kelly reports personal fees from AstraZeneca, Bristol-Myers Squibb, grants and personal fees from Genentech and Pfizer, outside the submitted work; DRG reports consulting for Pfizer during the study; RSH reports personal fees from Abbvie Pharmaceuticals, grants and personal fees from AstraZeneca, personal fees from Biodesix, personal fees from Bristol-Myers Squibb, grants and personal fees from Eli Lilly and Company, personal fees from EMD Serrano, personal fees from Genentech/Roche, personal fees from Heat Biologics, personal fees from Jun Shi Pharmaceuticals, personal fees from Loxo Oncology, grants and personal fees from Merck and Company, personal fees from Nektar, personal fees from NextCure, personal fees from Novartis, personal fees from Pfizer, personal fees from Sanofi, personal fees from Seattle Genetics, personal fees from Shire PLC, personal fees from Spectrum Pharmaceuticals, personal fees from Symphogen, personal fees from TESARO, personal fees from Neon Therapeutics, personal fees from Infinity Pharmaceuticals, outside the submitted work;.
Footnotes
No conflicts of interest: MWR, ECM, NMR, DP, KM, JM
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Gandara DR, Hirsch FR, et al. Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for Accelerating Development of Therapies for Squamous Cell Lung Cancer: SWOG S1400. Clin Cancer Res 2015;21:1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 6.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 2006;24:1770–1783. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 2012;18:568–576. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer 2011;104:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson MA, Schwartz GK, Keohan ML, et al. Progression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated With CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol 2016;2:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 2015;21:995–1001. [DOI] [PubMed] [Google Scholar]
- 13.Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–753. [DOI] [PubMed] [Google Scholar]
- 14.Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006;9:23–32. [DOI] [PubMed] [Google Scholar]
- 15.Goldman JW, Mazieres J, Barlesi F, et al. A randomized phase 3 study of abemaciclib versus erlotinib in previously treated patients with stage IV NSCLC with KRAS mutation: JUNIPER. J Clin Oncol 2018;36:9025–9025. [Google Scholar]