Abstract
Background:
Animal studies on cardiac arrest found that a combination of epinephrine with esmolol attenuates post-resuscitation myocardial dysfunction. Based on these findings, we hypothesized that esmolol-epinephrine combination therapy would be superior to a reported cardioprotective esmolol therapy alone in a mouse model of myocardial ischemia and reperfusion (IR) injury.
Methods:
C57BL/6J mice were subjected to 60 min of myocardial ischemia and 120 min of reperfusion. Mice received either saline, esmolol (0.4 mg/kg/h), epinephrine (0.05 mg/kg/h), or esmolol combined with epinephrine (esmolol: 0.4 mg/kg/h or 0.8 mg/kg/h and epinephrine: 0.05 mg/kg/h) during reperfusion. After reperfusion, infarct sizes in the area-at-risk and serum cardiac troponin-I levels were determined. Hemodynamic effects of drugs infused were determined by measurements of heart rate (HR) and mean arterial blood pressure (MAP) via a carotid artery catheter.
Results:
Esmolol during reperfusion resulted in robust cardioprotection (esmolol vs. saline: 24.3±8% vs. 40.6±3% infarct size), which was abolished by epinephrine co-administration (38.1±15% infarct size). Increasing the esmolol dose, however, was able to restore esmolol-cardioprotection in the epinephrine-esmolol (18.6±8% infarct size) co-treatment group with improved hemodynamics compared to the esmolol group (epinephrine-esmolol vs. esmolol: MAP 80 vs. 75 mmHg, HR 452 vs. 402 beats/min).
Conclusion:
These results confirm earlier studies on esmolol-cardioprotection from myocardial IR-injury and demonstrate that a dose optimized epinephrine-esmolol co-treatment maintains esmolol-cardioprotection with improved hemodynamics compared to esmolol treatment alone. These findings might have implications for current clinical practice in hemodynamically unstable patients suffering from myocardial ischemia.
Keywords: epinephrine, esmolol, myocardial ischemia and reperfusion injury, troponin, TTC, blood pressure, heart rate
Introduction
Epinephrine is a particularly potent β1 adrenergic receptor agonist with moderate β2 and α1 adrenergic receptor activity. Clinically, low doses of epinephrine increase cardiac output (CO) because of the β1 adrenergic receptor inotropic and chronotropic effects, while the β2 adrenergic receptor vasodilation often offsets the α adrenergic receptor-induced vasoconstriction. Therefore low dose epinephrine results in an increased CO, with decreased systemic vascular resistance [1]. However, at higher epinephrine doses the alpha-adrenergic receptor effect causes increased peripheral vasoconstriction, in addition to an increased CO. Epinephrine is most often used for the treatment of anaphylaxis and management of hypotension following coronary artery bypass grafting.
Furthermore, epinephrine is currently the only treatment for cardiac arrest. In cardiac arrest, epinephrine shows the most efficacy in the return of spontaneous circulation [2]. However, recent studies have raised doubts about the benefit of epinephrine regarding neurological outcomes in cardiac arrest [3–5]. Also, epinephrine can increase post-resuscitation myocardial dysfunction [6]. Moreover, epinephrine use in the stabilization of a cardiogenic shock in post-myocardial infarction patients has been found to increase the incidence of refractory shock [7]. In fact, beta-adrenergic receptor stimulation has been suggested to have deleterious effects as stimulation of this pathway increases oxygen consumption and reduces sub-endocardial perfusion [6].
In contrast, esmolol, a cardio-selective β1-blocker, has been shown to provide cardioprotection after myocardial ischemia in animal [8] and human studies [9]. Therefore, esmolol co-administration to epinephrine may help to reduce epinephrine-reperfusion injury but maintain esmolol-cardioprotection and epinephrine mediated increases in chronotropy and inotropy. Indeed, recent studies in animals found beneficial effects of epinephrine and esmolol co-administration in a cardiac arrest model [10]. Based on these findings, we investigated esmolol-epinephrine combination therapy in a mouse model of myocardial ischemia and reperfusion injury. Using an in-situ mouse model for myocardial ischemia and reperfusion injury in combination with measurement of hemodynamics, we demonstrated that esmolol-epinephrine co-administration during reperfusion maintain an esmolol mediated reduction in infarct sizes and troponin I levels but at the same time keep some β1 adrenergic effects with improved hemodynamics.
Methods
Mouse Experiments.
Experimental protocols were approved by the Institutional Review Board (Institutional Animal Care and Use Committee [IACUC]) at the University of Colorado Denver, USA. They were following the NIH guidelines for the use of live animals. To eliminate gender- and age-related variations, we routinely used 12- to 16-week-old male C57BL/6J mice [11–13]. C57BL/6J is the most widely used inbred strain and the first to have its genome sequenced. To guarantee reproducibility and to be able to compare these data to previous and future studies, this strain was chosen.
Experimental groups:
C57BL/6J wildtype mice [n=6 per group; 5 groups, total of 30 mice] received either saline, esmolol (0.4mg/kg/h), epinephrine (0.05 mg/kg/h), or esmolol combined with epinephrine (esmolol: 0.4 mg/kg/h or 0.8 mg/kg/h and epinephrine: 0.05 mg/kg/h) during 120 min of reperfusion following 60 min of myocardial ischemia via intraarterial continuous infusion. The initial dose of esmolol (0.4 mg/kg/h) was chosen as it was below the inotropic blocking threshold effect in the mouse [14]. The epinephrine dose was chosen as the average dose used in cardiogenic shock after myocardial infarction [7].
Pharmacological compounds.
Epinephrine (Merit Pharmaceutical, USA) and esmolol (Baxter, USA) infusion rates during 120 min of reperfusion were 0.05 mg/kg/h (~0.83 μg/kg/min) and 0.4 mg/kg/h or 0.8 mg/kg/h (~6.7 or l3.3 μg/kg/min), respectively.
Murine Model for myocardial ischemia and reperfusion injury [11, 12, 15–21].
C57BL/6J were obtained from Jackson Laboratories. Anesthesia was induced with 70mg/kg i.p. and maintained with 10 mg/kg/h i.p. of pentobarbital as necessary. Myocardial ischemia and reperfusion injury in mice was performed as described previously [11, 12, 15–21]. Infarct sizes were determined by calculating the percentage of infarcted myocardium to the area at risk (AAR) using a double staining technique with Evan’s blue and triphenyltetrazolium chloride (TTC). Using planimetry via the NIH software Image 1.0 (National Institutes of Health, Bethesda, MA), the AAR and the infarct size were determined. All animals were under deep anesthesia while performing surgical procedures. After study completion, animals were euthanized with an overdose of pentobarbital and exsanguination.
Heart Enzyme Measurement.
Blood was collected by central venous puncture for troponin I (cTnI) measurements using a quantitative rapid cTnI assay (Life Diagnostics, Inc., West Chester, PA, USA). cTnI is highly specific for myocardial ischemia and has a well-documented correlation with the infarct size in mice [15, 17, 18, 20] and humans [22].
Blood pressure and heart rate measurements.
A polyethylene catheter was inserted in the right carotid artery as described previously [23]. The catheter was connected to a Deltran® pressure transducer (Utah Medical Products Inc., Salt Lake City, UT, USA) located at the same hydrostatic level as the mouse, which was connected to the CyQ BMP02 system (CyberSense, Inc., Nicholasville, KY, USA) designed to measure invasively systolic, diastolic, pulse pressure, mean arterial blood pressure (MAP) and heart rate (HR). Due to a sampling rate of 1,000 Hz, the device automatically calculates HR from the amplitude of the pressure signal.
Data analysis.
Data were compared by one-way ANOVA with Tukey’s post-hoc test, by Student’s t-test or linear regression where appropriate. Values are expressed as mean (±SD). P<0.05 was considered statistically significant. The Kolmogorov Smirnov test was used to confirm normal distribution. For all statistical analysis, GraphPad Prism 7.0 software was used. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Epinephrine during myocardial ischemia and reperfusion does not increase infarct sizes.
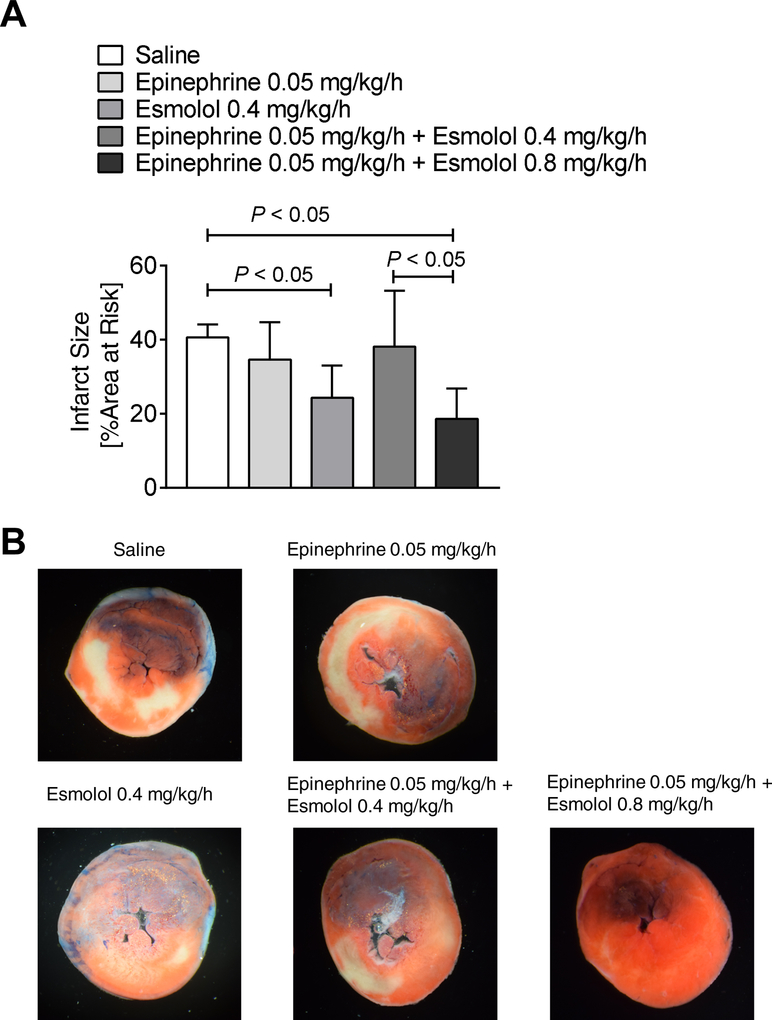
As epinephrine increases heart rate and contractility, thereby leading to an increase in oxygen consumption [24] and based on earlier studies on cardiac arrest that found that epinephrine significantly increased the severity of post-resuscitation myocardial dysfunction and decreased the survival rate [6], we first evaluated the effects of epinephrine on infarct sizes during acute myocardial ischemia and reperfusion (IR) injury. After 60 minutes of myocardial ischemia, epinephrine infusion was started at a rate of 0.05 mg/kg/h intravenously for 120 min of reperfusion. Following analysis of infarct sizes, however, revealed no differences in infarct sizes when compared to saline-treated control mice (epinephrine vs. saline: 34.6±10% vs. 40.6±3%, mean±SD, n=6, Figure 1 A, B). Taken together, epinephrine does not increase infarct sizes during myocardial IR-injury.
Figure 1. Increased esmolol dose restores esmolol-cardioprotection in epinephrine-esmolol co-administration during myocardial ischemia and reperfusion injury.
C57BL/6J wildtype mice underwent 60 min of myocardial ischemia followed by 120 minutes of reperfusion. Wildtype mice received either saline, esmolol (0.4mg/kg/h), epinephrine (0.05 mg/kg/h), or esmolol combined with epinephrine (esmolol: 0.4 mg/kg/h or 0.8 mg/kg/h and epinephrine: 0.05 mg/kg/h) during 120 min of reperfusion. Infarct sizes were determined by using standard double staining with Evan’s blue and TTC (triphenyl-tetrazolium chloride). The percent of the area at risk (AAR) that underwent infarction indicates the infarct size. (A) Infarct sizes as the percent of AAR. (B) Representative infarct staining (n=6; mean±SD; p<0.05).
Esmolol is cardioprotective in myocardial ischemia and reperfusion injury.
As multiple studies demonstrated a cardioprotective effect of esmolol [8–10], we next evaluated esmolol infusion during reperfusion injury. Indeed, esmolol at a rate of 0.4 mg/kg/h during reperfusion revealed significantly smaller infarcts sizes when compared to saline-treated control mice (esmolol vs. saline: 24.3±8.6% vs. 40.6±3%, mean±SD, n=6, Figure 1 A, B). Taken together, selective β1-blockade during reperfusion provides robust cardioprotection from IR-injury which is in line with previous studies.
Co-administration of epinephrine-esmolol abolishes esmolol cardioprotection.
Based on findings on the beneficial effects of epinephrine-esmolol co-administration in a cardiac arrest model [10], we next evaluated the effects of epinephrine plus esmolol infusion during reperfusion on infarcts sizes. However, epinephrine and esmolol co-administration resulted in similar infarct size as in the saline-treated group (epinephrine+esmolol vs. saline: 38.1±15% vs. 40.6±3%, mean±SD, n=6, Figure 1 A, B.). Taken together, epinephrine-esmolol co-administration during myocardial ischemia and reperfusion injury can compromise the cardioprotective effects of esmolol.
Increased esmolol dose restores esmolol-cardioprotection in epinephrine-esmolol co-administration during myocardial ischemia and reperfusion injury.
Based on findings on the beneficial effects of epinephrine-esmolol co-administration in a cardiac arrest model [10], we adapted the esmolol dose to achieve a similar ratio of esmolol: epinephrine of 15:1 as used in the aforementioned cardiac arrest model. Indeed, using 0.8 mg/kg/h esmolol with 0.05 mg/kg/h epinephrine infusion during reperfusion revealed the same cardioprotective effects as esmolol therapy alone (epinephrine+esmolol-high vs. saline: 18.6±8% vs. 40.6±3%, mean±SD, n=6, Figure 1 A, B.). Taken together, increasing the esmolol dose can antagonize the adverse β1 adrenergic effects of epinephrine.
Troponin I values correlate with infarct size measurements.
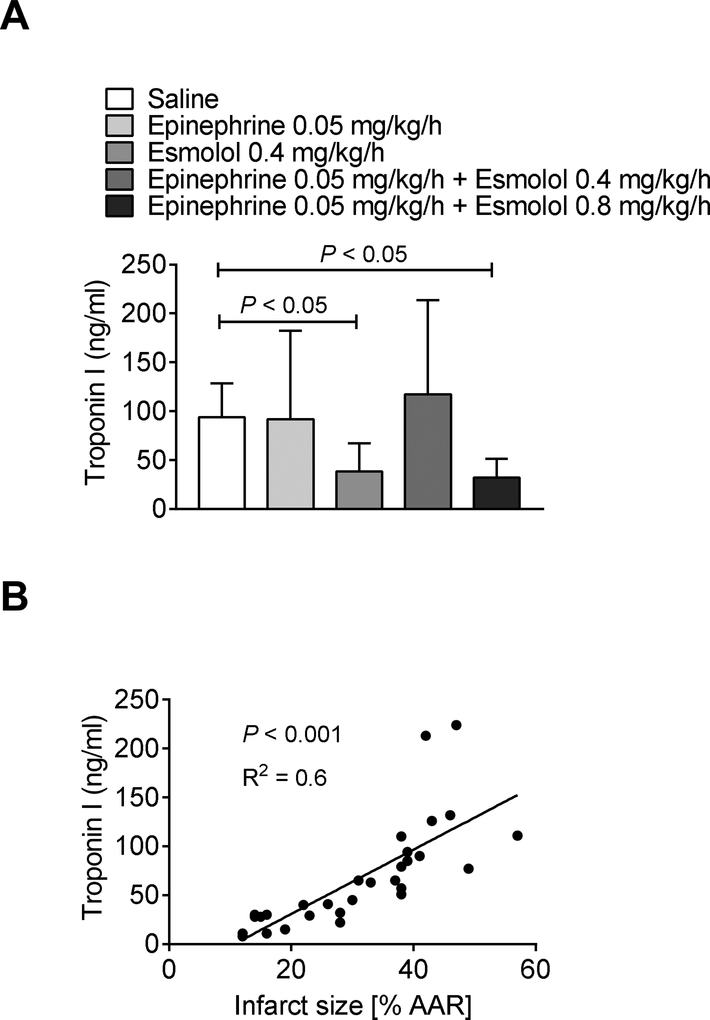
As the infarct size determination via TTC staining can result in falsely small infarct sizes if the reperfusion is compromised due to a no-reflow phenomenon [25], we also determined troponin-I serum levels which have been shown to correlate with infarct sizes [15]. Troponin-I measurements in all groups revealed similar myocardial injury as found with our infarct size measurements (Figure 2 A), validating our TTC based infarct size staining. Also, we confirmed a significant correlation between infarct size and serum troponin I levels (Figure 2 B). Taken together, epinephrine infusion in combination with a higher esmolol dose during myocardial ischemia and reperfusion injury significantly reduces infarct sizes and troponin I serum levels.
Figure 2. Troponin I values correlate with infarct size measurements.
C57BL/6J wildtype mice underwent 60 min of myocardial ischemia followed by 120 minutes of reperfusion. Wildtype mice received either saline, esmolol (0.4mg/kg/h), epinephrine (0.05 mg/kg/h), or esmolol combined with epinephrine (esmolol: 0.4 mg/kg/h or 0.8 mg/kg/h and epinephrine: 0.05 mg/kg/h) during 120 min of reperfusion. Serum troponin-I levels were measured by enzyme-linked immunosorbent assay (ELISA). (A) Serum troponin-I levels. (B) The linear correlation between serum troponin-I levels and infarct sizes as the percent of the area at risk (AAR; n=6; mean±SD; p<0.05).
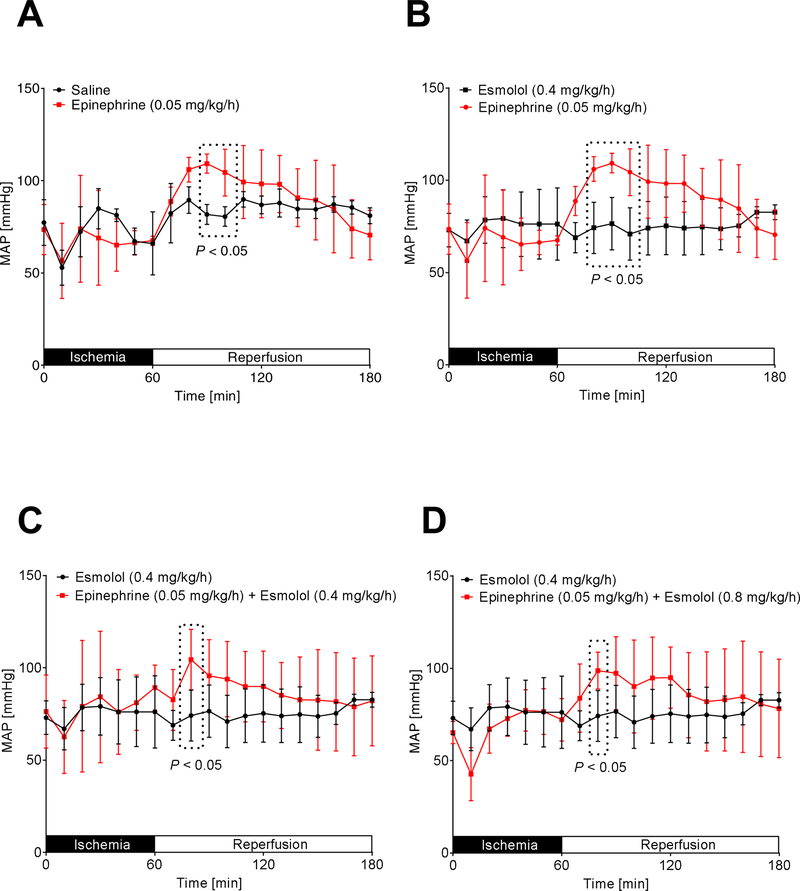
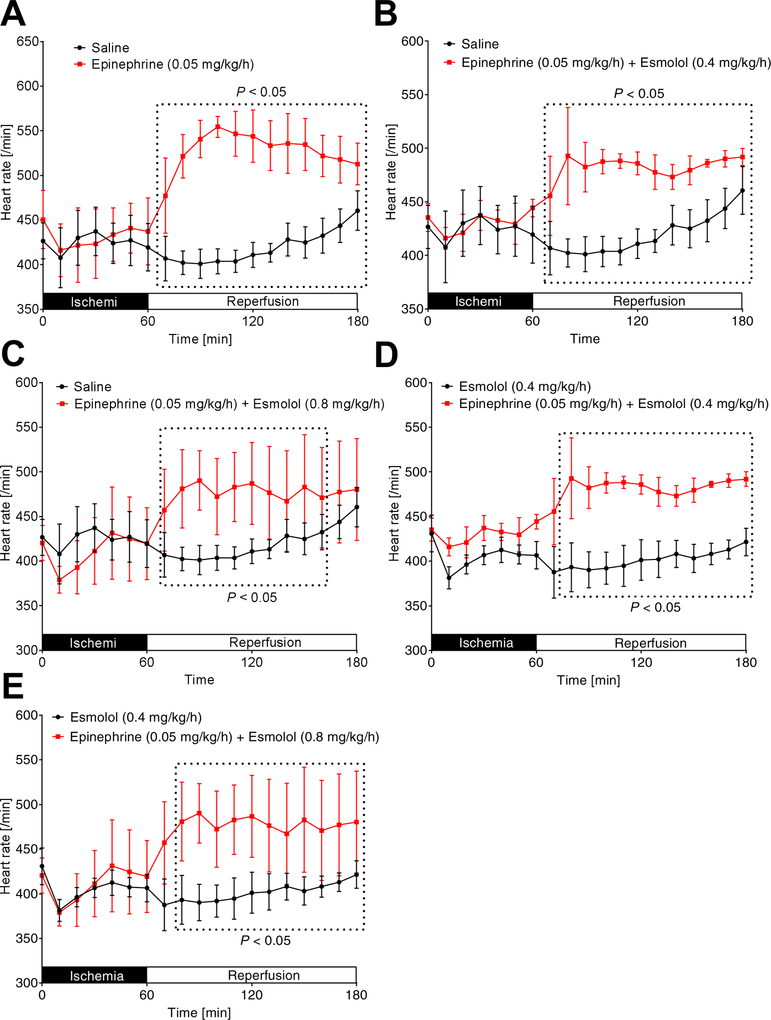
Epinephrine increases blood pressure and heart rate even in combination with high dose esmolol.
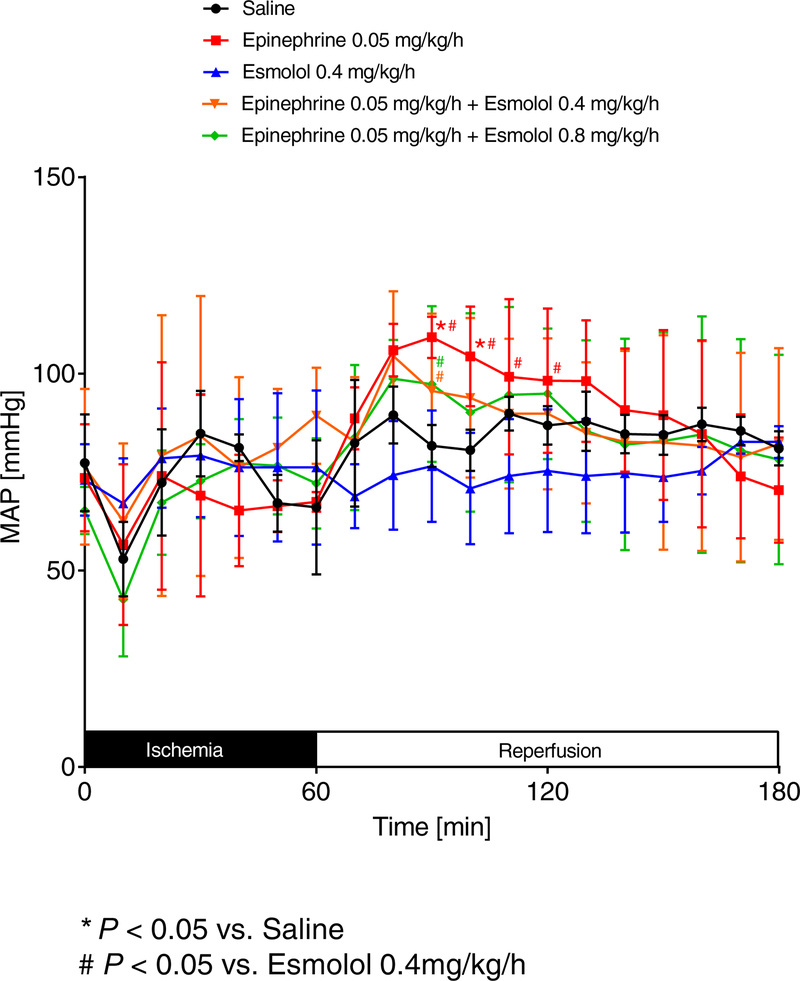
To understand if our cardioprotective epinephrine co-treatment with high esmolol would also result in better hemodynamic parameters than esmolol alone, we next evaluated the effects of our treatment protocols on mean arterial blood pressure (MAP) and heart rate (HR) using invasive monitoring. As shown in Figure 3 and 4, epinephrine most consistently increased the HR during reperfusion in all groups, while MAP increases were less pronounced. We next compared individual groups to analyze significant HR and MAP changes during reperfusion. We found that epinephrine [0.05 mg/kg/h] vs. saline, epinephrine [0.05 mg/kg/h] vs. esmolol [0.4 mg/kg/h], epinephrine [0.05 mg/kg/h] + esmolol [0.4 mg/kg/h] vs. esmolol [0.4 mg/kg/h] or epinephrine [0.05 mg/kg/h] + esmolol [0.8 mg/kg/h] vs. esmolol [0.4 mg/kg/h] significantly increased the MAP during a short period in the early reperfusion period (Figure 5A–D). However, a statistical comparison of saline vs. esmolol [0.4 mg/kg/h] revealed no significant differences in MAP.
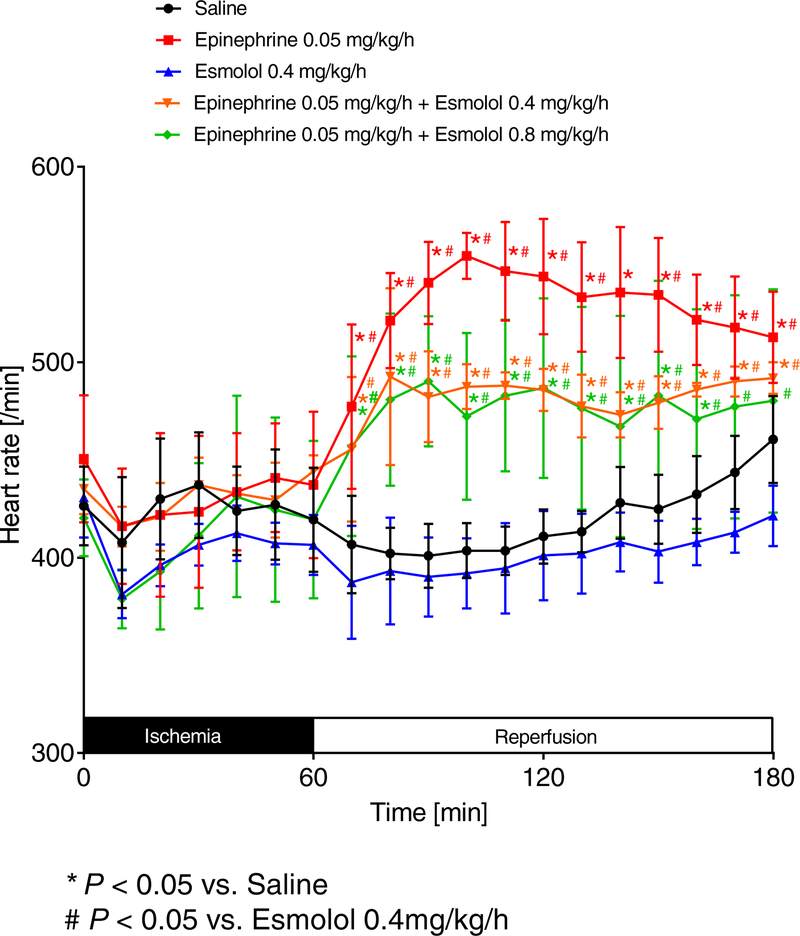
Figure 3. Comparison of heart rates.
C57BL/6J mice were anesthetized with pentobarbital and mechanically ventilated. The common carotid artery was cannulated with a polyethylene tube. The arterial catheter was then connected to a pressure transducer which automatically calculated the heart rate (HR) by analyzing the amplitude of the pressure signal. Values were recorded to computer hard drive for further analysis. Mice underwent 60 min of myocardial ischemia followed by 120 minutes of reperfusion. During reperfusion, mice received either saline, esmolol (0.4mg/kg/h), epinephrine (0.05 mg/kg/h), or esmolol combined with epinephrine (esmolol: 0.4 mg/kg/h or 0.8 mg/kg/h and epinephrine: 0.05 mg/kg/h). HR was recorded during the whole procedure; (n=6; mean±SD; p<0.05).
Figure 4. Comparison of mean arterial blood pressures.
Following insertion of a catheter into the common carotid artery, mean arterial blood pressure (MAP) was measured while C57BL/6J wildtype mice underwent 60 min of myocardial ischemia followed by 120 minutes of reperfusion. During reperfusion, mice received either saline, esmolol (0.4mg/kg/h), epinephrine (0.05 mg/kg/h), or esmolol combined with epinephrine (esmolol: 0.4 mg/kg/h or 0.8 mg/kg/h and epinephrine: 0.05 mg/kg/h). MAP was recorded during the whole procedure (n=6; mean±SD; p<0.05).
Figure 5. Epinephrine plus high dose esmolol leads to higher blood pressures than low dose esmolol treatment.
Mean arterial blood pressure (MAP) was measured while C57BL/6J wildtype mice underwent 60 min of myocardial ischemia followed by 120 minutes of reperfusion. Only significant comparisons are shown: epinephrine [0.05 mg/kg/h] vs. saline (A), epinephrine [0.05 mg/kg/h] vs. esmolol [0.4 mg/kg/h] (B), epinephrine [0.05 mg/kg/h] + esmolol [0.4 mg/kg/h] vs. esmolol [0.4 mg/kg/h] (C) or epinephrine [0.05 mg/kg/h] + esmolol [0.8 mg/kg/h] vs. esmolol [0.4 mg/kg/h] (D) significantly increased the MAP during the reperfusion period (n=6; mean±SD; p<0.05).
As epinephrine is a very potent β1 agonist, we further evaluated the heart rate for significant changes during the reperfusion period. We demonstrated that epinephrine [0.05 mg/kg/h] vs. saline, epinephrine [0.05 mg/kg/h] + esmolol [0.4 mg/kg/h] vs. saline, epinephrine [0.05 mg/kg/h] + esmolol [0.8 mg/kg/h] vs. saline, epinephrine [0.05 mg/kg/h] + esmolol [0.4 mg/kg/h] vs. esmolol [0.4 mg/kg/h] or epinephrine [0.05 mg/kg/h] + esmolol [0.8 mg/kg/h] vs. esmolol [0.4 mg/kg/h] had significantly higher HRs during the full period of 120 min of reperfusion (Figure 6A–E). However, a statistical comparison of saline vs. esmolol [0.4 mg/kg/h] revealed no significant differences in HR. Together, these data indicate that our cardioprotective epinephrine co-treatment with an increased esmolol dose significantly activates β1 receptors which ultimately results in improved hemodynamics when compared to esmolol treatment alone. In addition, these data suggest that esmolol cardioprotection only requires to block of a small portion of β1 receptors.
Figure 6. Epinephrine plus high dose esmolol leads to higher heart rates than low dose esmolol treatment.
Heart rates (HRs) were measured while C57BL/6J wildtype mice underwent 60 min of myocardial ischemia followed by 120 minutes of reperfusion. Only significant comparisons are shown: epinephrine [0.05 mg/kg/h] vs. saline (A), epinephrine [0.05 mg/kg/h] + esmolol [0.4 mg/kg/h] vs. saline (B), epinephrine [0.05 mg/kg/h] + esmolol [0.8 mg/kg/h] vs. saline (C), epinephrine [0.05 mg/kg/h] + esmolol [0.4 mg/kg/h] vs. esmolol [0.4 mg/kg/h] (D) or epinephrine [0.05 mg/kg/h] + esmolol [0.8 mg/kg/h] vs. esmolol [0.4 mg/kg/h] (E) had significantly higher HRs during the full period of 120 min of reperfusion (n=6; mean±SD; p<0.05).
Discussion
In the present study, we pursued the role of an epinephrine-esmolol co-therapy after myocardial infarction, based on data from a cardiac arrest animal model [10]. Comparing different esmolol doses in combination with epinephrine, we demonstrate that at a specific esmolol-epinephrine ratio (15:1), esmolol-cardioprotection and epinephrine β1 mediated hemodynamic activity can both simultaneously exist during myocardial ischemia and reperfusion injury. These findings might have implications for current clinical practice in the treatment of patients with cardiogenic shock [26].
Already in the early 1970s, studies have shown that in open-chest anesthetized dogs, early intravenous administration of beta-blockers reduced myocardial infarct sizes [27]. Animal models further revealed that when administered either before or during coronary artery occlusion, beta blockers decreased structural damage to mitochondria and microvasculature injury [28]. Subsequently, studies on the ultrashort-acting esmolol after the occlusion but before reperfusion found reduced myocardial infarct sizes in experimental models [29]. A more recent clinical trial on beta-blockers after STEMI (METOCARD-CNIC) observed that early intravenous metoprolol before reperfusion decreased infarct size and increased left ventricular ejection fraction in patients after STEMI [30].
In contrast, a cardiogenic shock after myocardial ischemia disallows the use of esmolol due to hemodynamic instability. Interestingly, a definite recommendation for a specific catecholamine regimen in cardiogenic shock is lacking [31]. The therapeutic goal of a cardiogenic shock is to maintain arterial pressure adequate for tissue perfusion. Dopamine, however, which was once considered the drug of choice, should be used with caution, as dopamine can increase the rate of adverse events in cardiogenic shock [32]. As such, clinicians often use norepinephrine with low doses of dobutamine, epinephrine [31] or norepinephrine [7].
While studies have shown that epinephrine is as effective as norepinephrine-dobutamine co-administration or norepinephrine alone, epinephrine can increase the heart rate, cause arrhythmias, and compromise gastric mucosa perfusion. Moreover, epinephrine use in the stabilization of a cardiogenic shock in post-myocardial infarction patients has been found to increase the incidence of a refractory shock [7]. Also, beta-adrenergic receptor stimulation has been suggested to have harmful effects as stimulation of this pathway increases oxygen consumption and reduces sub-endocardial perfusion [6]. Thus, the combination of norepinephrine-dobutamine or norepinephrine alone appears to be a safer strategy [7, 31].
Combination therapy of epinephrine with esmolol seems less intuitive in cardiogenic shock after myocardial ischemia: higher esmolol doses could compromise epinephrine mediated increases of cardiac output via β1 adrenergic receptor inotropic and chronotropic effects, or higher epinephrine doses could compromise esmolol mediated cardioprotection via β1 adrenergic receptor blockade. Indeed, our studies demonstrate that combining a standard dose of epinephrine - as used in cardiogenic shock - with a low dose of esmolol, that does not compromise the inotropic effects but is cardioprotective, ultimately abolished esmolol mediated infarct size reduction in myocardial ischemia and reperfusion injury.
Surprisingly, by increasing the esmolol dose, we were able to restore esmolol-cardioprotection while heart rate and some blood pressures in the early reperfusion phase were significantly increased compared to an esmolol treatment alone. This finding is novel and highlights that esmolol cardioprotection is not fully understood. Having increased heart rates, which is β1 mediated, and at the same time seeing cardioprotection via esmolol β1 blockade, indicates that only a part or short-term blockade of β1 receptors is necessary for the salutary effects of esmolol in myocardial ischemia and reperfusion injury.
While we did not directly measure cardiac output in our animals studies, the heart rate is a significant determinant of the cardiac output [33]. Because cardiac output is the product of heart rate and stroke volume, humans have a much greater ability than rodents to increase their cardiac output during exercise by both heart rate and stroke volume mediated mechanisms. In other words, the cardiac reserve of humans is much greater than rodents. Unlike humans, mice possess a limited ability to increase cardiac output by increasing stroke volume. Instead, mice cardiac output is significantly dependent on heart rate, indicating they have less preload reserve [34, 35]. Moreover, the heart rate increase, as seen in our cardioprotective epinephrine-high-esmolol co-therapy is β1 receptor mediated. As β1 adrenergic receptor activation has chronotropic and inotropic effects, increased cardiac output seems very likely in our setting. Also, the combination of increased heart rate with moderate increases of blood pressure further indicates increases in the cardiac output instead of increases in peripheral vasoconstriction.
While some clinicians occasionally use esmolol in patients on epinephrine infusion due to cardiogenic shock going off cardiac bypass to treat epinephrine-induced arrhythmias, no study to date has evaluated potential cardioprotective effects of esmolol-epinephrine co-administration during cardiac bypass surgery or a cardiogenic shock. As this is the first animal study on epinephrine-esmolol co-administration during myocardial ischemia and reperfusion injury, further studies in larger animals using multiple dosing protocols are indicated. In fact, one of our biggest limitation is that we only used two different esmolol+epinephrine combination therapies. Thus, further studies will be required to evaluate 1) multiple dose-effect combination therapies and 2) the underlying mechanism of how esmolol can be still cardioprotective even when the epinephrine β1 activating action is dominating the esmolol β1 blocking effects. However, if our findings are reproducible, human studies might be indicated to understand if patients in cardiogenic shock could benefit from an epinephrine-esmolol co-therapy.
Summary
Our studies confirm earlier studies on esmolol-cardioprotection from myocardial ischemia and reperfusion injury and demonstrate that a dose optimized epinephrine-esmolol co-treatment maintains esmolol-cardioprotection with improved hemodynamics compared to esmolol treatment alone. These findings might have implications for current clinical practice in hemodynamically unstable patients suffering from myocardial ischemia or undergoing cardiac bypass surgery.
Source of financial support for the work:
National Heart, Lung, and Blood Institute (NIH-NHLBI) 5R01HL122472 Grant to T.E.; American Heart Association (AHA) Postdoctoral Fellowship 19POST34380105 Grant to Y.O.
Footnotes
Conflict of Interest
The authors declare there are no conflicts of interest.
References
- [1].Allwood MJ, Cobbold AF, Ginsburg J. Peripheral vascular effects of noradrenaline, isopropylnoradrenaline and dopamine. Br Med Bull, 1963; 19: 132–6. [DOI] [PubMed] [Google Scholar]
- [2].Soar J, Donnino MW, Maconochie I, Aickin R, Atkins DL, Andersen LW, Berg KM, Bingham R, Bottiger BW, Callaway CW, Couper K, Couto TB, de Caen AR, Deakin CD, Drennan IR, Guerguerian AM, Lavonas EJ, Meaney PA, Nadkarni VM, Neumar RW, Ng KC, Nicholson TC, Nuthall GA, Ohshimo S, O’Neil BJ, Ong GY, Paiva EF, Parr MJ, Reis AG, Reynolds JC, Ristagno G, Sandroni C, Schexnayder SM, Scholefield BR, Shimizu N, Tijssen JA, Van de Voorde P, Wang TL, Welsford M, Hazinski MF, Nolan JP, Morley PT. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations Summary. Resuscitation, 2018; 133: 194–206. [DOI] [PubMed] [Google Scholar]
- [3].Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. Jama, 2012; 307: 1161–8. [DOI] [PubMed] [Google Scholar]
- [4].Lin S, Callaway CW, Shah PS, Wagner JD, Beyene J, Ziegler CP, Morrison LJ. Adrenaline for out-of-hospital cardiac arrest resuscitation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation, 2014; 85: 732–40. [DOI] [PubMed] [Google Scholar]
- [5].Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, Regan S, Long J, Slowther A, Pocock H, Black JJM, Moore F, Fothergill RT, Rees N, O’Shea L, Docherty M, Gunson I, Han K, Charlton K, Finn J, Petrou S, Stallard N, Gates S, Lall R. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N Engl J Med, 2018; 379: 711–721. [DOI] [PubMed] [Google Scholar]
- [6].Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation, 1995; 92: 3089–93. [DOI] [PubMed] [Google Scholar]
- [7].Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G, Quenot JP, Kimmoun A, Cariou A, Lassus J, Harjola VP, Meziani F, Louis G, Rossignol P, Duarte K, Girerd N, Mebazaa A, Vignon P. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol, 2018; 72: 173–182. [DOI] [PubMed] [Google Scholar]
- [8].Geissler HJ. Reduction of myocardial reperfusion injury by high-dose beta-blockade with esmolol. Thorac Cardiovasc Surg, 2002; 50: 367–72. [DOI] [PubMed] [Google Scholar]
- [9].Er F, Dahlem KM, Nia AM, Erdmann E, Waltenberger J, Hellmich M, Kuhr K, Le MT, Herrfurth T, Taghiyev Z, Biesenbach E, Yuksel D, Eran-Ergoknil A, Vanezi M, Caglayan E, Gassanov N. Randomized Control of Sympathetic Drive With Continuous Intravenous Esmolol in Patients With Acute ST-Segment Elevation Myocardial Infarction: The BEtA-Blocker Therapy in Acute Myocardial Infarction (BEAT-AMI) Trial. JACC Cardiovasc Interv, 2016; 9: 231–240. [DOI] [PubMed] [Google Scholar]
- [10].Zhang Q, Li C. Combination of epinephrine with esmolol attenuates post-resuscitation myocardial dysfunction in a porcine model of cardiac arrest. PLoS One, 2013; 8: e82677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One, 2013; 8: e71493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med, 2012; 18: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bartman CM, Oyama Y, Brodsky K, Khailova L, Walker L, Koeppen M, Eckle T. Intense light-elicited upregulation of miR-21 facilitates glycolysis and cardioprotection through Per2-dependent mechanisms. PLoS One, 2017; 12: e0176243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ibrahim-Zada I, Rhee P, Gomez CT, Weller J, Friese RS. Inhibition of sepsis-induced inflammatory response by beta1-adrenergic antagonists. J Trauma Acute Care Surg, 2014; 76: 320–7; discussion 327–8. [DOI] [PubMed] [Google Scholar]
- [15].Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol, 2006; 291: H2533–40. [DOI] [PubMed] [Google Scholar]
- [16].Eckle T, Koeppen M, Eltzschig H. Use of a hanging weight system for coronary artery occlusion in mice. J Vis Exp, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation, 2008; 118: 166–75. [DOI] [PubMed] [Google Scholar]
- [18].Warth A, Eckle T, Kohler D, Faigle M, Zug S, Klingel K, Eltzschig HK, Wolburg H. Upregulation of the water channel aquaporin-4 as a potential cause of postischemic cell swelling in a murine model of myocardial infarction. Cardiology, 2007; 107: 402–10. [DOI] [PubMed] [Google Scholar]
- [19].Koeppen M, Harter PN, Bonney S, Bonney M, Reithel S, Zachskorn C, Mittelbronn M, Eckle T. Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology, 2012; 116: 1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation, 2007; 116: 1784–94. [DOI] [PubMed] [Google Scholar]
- [21].Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, Harter PN, Ravid K, Eltzschig HK, Mittelbronn M, Walker L, Eckle T. Differential Tissue-Specific Function of Adora2b in Cardioprotection. J Immunol, 2015; 195: 1732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem, 2008; 54: 617–9. [DOI] [PubMed] [Google Scholar]
- [23].Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One, 2009; 4: e6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Revelly JP, Gardaz JP, Nussberger J, Schutz Y, Chiolero R. Effect of epinephrine on oxygen consumption and delivery during progressive hemorrhage. Crit Care Med, 1995; 23: 1272–8. [DOI] [PubMed] [Google Scholar]
- [25].Schaper J, Kostin S. Cell death and adenosine triphosphate: the paradox. Circulation, 2005; 112: 6–8. [DOI] [PubMed] [Google Scholar]
- [26].van Diepen S Norepinephrine as a First-Line Inopressor in Cardiogenic Shock: Oversimplification or Best Practice? J Am Coll Cardiol, 2018; 72: 183–186. [DOI] [PubMed] [Google Scholar]
- [27].Reimer KA, Rasmussen MM, Jennings RB. Reduction by propranolol of myocardial necrosis following temporary coronary artery occlusion in dogs. Circ Res, 1973; 33: 353–63. [DOI] [PubMed] [Google Scholar]
- [28].Rasmussen MM, Reimer KA, Kloner RA, Jennings RB. Infarct size reduction by propranolol before and after coronary ligation in dogs. Circulation, 1977; 56: 794–8. [DOI] [PubMed] [Google Scholar]
- [29].Lange R, Kloner RA, Braunwald E. First ultra-short-acting beta-adrenergic blocking agent: its effect on size and segmental wall dynamics of reperfused myocardial infarcts in dogs. Am J Cardiol, 1983; 51: 1759–67. [DOI] [PubMed] [Google Scholar]
- [30].Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, Fernandez-Ortiz A, Garcia-Ruiz JM, Garcia-Alvarez A, Iniguez A, Jimenez-Borreguero J, Lopez-Romero P, Fernandez-Jimenez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vazquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Perez de Prado A, Fernandez-Campos MJ, Casado I, Garcia-Rubira JC, Garcia-Prieto J, Sanz-Rosa D, Cuellas C, Hernandez-Antolin R, Albarran A, Fernandez-Vazquez F, de la Torre-Hernandez JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation, 2013; 128: 1495–503. [DOI] [PubMed] [Google Scholar]
- [31].Levy B, Perez P, Perny J, Thivilier C, Gerard A. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit Care Med, 2011; 39: 450–5. [DOI] [PubMed] [Google Scholar]
- [32].De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med, 2010; 362: 779–89. [DOI] [PubMed] [Google Scholar]
- [33].Vincent JL. Understanding cardiac output. Crit Care, 2008; 12: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther, 2014; 141: 235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol, 2001; 534: 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]