Abstract
Introduction:
The aim of this pilot study was to assess young adult dual e-cigarette (EC) and combustible cigarette (CC) users’ anticipated responses to hypothetical market restrictions regarding key e-cigarette characteristics.
Methods:
Data came from 240 young adult dual EC and CC users recruited via Amazon Mechanical Turk in June 2017. Descriptive statistics were used to report sociodemographic, CC smoking, and EC use characteristics. McNemar’s chi-square tests and chi-square tests were used to assess differences between groups in terms of anticipated responses to hypothetical EC market restrictions.
Results:
Hypothetical regulations resulted in reported intentions to reduce EC use and increase CC use; the greatest impact was found for restrictions regarding e-liquid nicotine content, followed by flavor and ability to modify EC devices. Moreover, individuals reporting use of flavored e-liquid, high nicotine content e-liquid, and customizable EC were most likely to report intentions to reduce EC use and increase CC use.
Conclusions:
This work provides preliminary evidence that restrictive regulations regarding key EC characteristics may increase intentions to increase CC use among young adult dual EC and CC users.
Keywords: ENDS, e-cigarette, cigarette, smoking, policy, tobacco regulatory science
INTRODUCTION
Between 2011 and 2017, the prevalence of past 30 day e-cigarette (EC) use rose among adults (1.3% to 2.8%) in the United States (U.S.) (Glasser et al., 2017; Mirbolouk et al., 2018; T. W. Wang et al., 2018). During this time, the EC market has become increasingly diverse, with estimates of 460 EC brands and more than 7,700 different e-liquids available in 2014 (Zhu et al., 2014). Consumers can choose from a variety of devices, ranging from 1st generation (1G) “cigalike” devices to more advanced 3rd generation (3G) devices that users can configure (e.g., change the wattage) or modify (e.g., change atomizer) (Farsalinos, Gillman, Hecht, Polosa, & Thornburg, 2017), to self-administer a variety of e-liquids. The ability to select and modify e-liquid nicotine content (Bullen et al., 2010; M. W. Johnson, Johnson, Rass, & Pacek, 2017; Lopez et al., 2016; Perkins, Karelitz, & Michael, 2017; Ramôa et al., 2016; Talih et al., 2015; Tseng et al., 2016), flavor (Baggett, Campbell, Chang, & Rigotti, 2016; Cheney, Gowin, & Wann, 2016; Cooper, Harrell, & Perry, 2016; Farsalinos et al., 2013; Kong, Morean, Cavallo, Camenga, & Krishnan-Sarin, 2015; McDonald & Ling, 2015; Patel et al., 2016; Rutten et al., 2015; Sussman et al., 2014; Villanti et al., 2017), and device type (Baweja et al., 2016; Cooper et al., 2016; Kistler et al., 2017; McKeganey & Dickson, 2017; Simmons et al., 2016; Vandrevala et al., 2017) may impact EC reinforcing effects, including nicotine pharmacokinetics and product satisfaction (i.e., ease of use, taste, throat hit, craving reduction, liking), and contribute to initiation, continued use, and substitutability for combusted cigarettes (CCs).
In 2016, the Food and Drug Administration (FDA) extended its regulatory authority over the manufacture, marketing, and distribution of all tobacco products, including ECs (Food and Drug Administration, 2016; United States Congress, 2009). The potential impact of regulation of this market is noteworthy: regulations that would reduce the diversity and subsequent appeal of these EC characteristics have the potential to benefit segments of the population, but may have unintended consequences for others. For instance, restricting the nicotine content of ECs to low levels may reduce their reinforcing effects, thus reducing uptake and continued use. Given that some research indicates that EC use is associated with reductions in cigarettes smoked per day (CPD) (Adriaens, Van Gucht, & Baeyens, 2017; Brose, Hitchman, Brown, West, & McNeill, 2015; Farsalinos, Romagna, & Voudris, 2015; Lechner et al., 2015; Rass, Pacek, Johnson, & Johnson, 2015; Rutten et al., 2015) (though also see (Goniewicz et al., 2018) who found no difference in CPD between dual EC/CC users and CC-only smokers), decreasing the nicotine delivery of ECs may also render them a less effective substitute for CCs. Notably, substantial CC use reduction (i.e., short of cessation/complete switching to EC) may still be insufficient to reduce significant health risks (e.g., coronary artery disease and stroke) (Hackshaw, Morris, Boniface, Tang, & Milenković, 2018). Additionally, given that EC use is often initiated with non-tobacco flavored e-liquids (Cheney et al., 2016; Farsalinos et al., 2015; Harrell et al., 2017; Villanti et al., 2017), limiting flavors may reduce the appeal and subsequent initiation of EC use among youth and non-users, while potentially reducing continued use of ECs among established users in general, as well as among those trying to reduce (Cheney et al., 2016; Rutten et al., 2015) or quit smoking (Farsalinos et al., 2013). Indeed, prior research among exclusive CC smokers utilizing a discrete choice paradigm indicates that EC flavor restrictions reduced the likelihood of selecting ECs from among ECs, CCs, and nicotine replacement therapy (Pesko, Kenkel, Wang, & Hughes, 2016). Lastly, limiting modifiability/customizability of EC devices may decrease the likelihood of battery malfunction (Rudy & Durmowicz, 2016), but may have a detrimental effect on the improved nicotine delivery that 3G devices confer over non-modifiable devices (Farsalinos, Romagna, Tsiapras, Kyrzopoulos, & Voudris, 2014; Talih et al., 2015; Wagener et al., 2017). In sum, while some EC product regulations may have benefits, they may also reduce palatability and user satisfaction.
It is important to consider the potential impact of regulations on the EC market to mitigate unintended negative consequences, particularly among at-risk populations such as young adult dual tobacco product users. Dual tobacco product users are a particularly high risk group: dual tobacco product use is associated with greater nicotine exposure (Bombard, Pederson, Nelson, & Malarcher, 2007; Bombard, Rock, Pederson, & Asman, 2008), nicotine dependence (Soule, Pomeranz, Moorhouse, & Barnett, 2015), and greater difficulties when attempting to quit as compared to single product users (Bombard et al., 2007; Wetter et al., 2002). When making a quit attempt, dual and multiple product use is associated with shorter time to relapse (Messer et al., 2015) and a decreased likelihood of cessation (Hamari, Toljamo, Kinnula, & Nieminen, 2013; Kasza et al., 2014; Tomar, Alpert, & Connolly, 2010; Wetter et al., 2002). It is worth noting that approximately 38% of current tobacco users are users of more than one tobacco product (Kasza et al., 2017). Moreover, dual use of EC and CC is the most prevalent two-product use combination among adult dual and multiple tobacco product users in the U.S. (Kasza et al., 2017), suggesting that this is a population warranting examination.
Moreover, young adulthood (i.e., age 18-29) represents a pivotal developmental period for the acquisition and escalation of tobacco product use and dependence (U.S. Department of Health and Human Services, 2014). Approximately 29% of young adults report CC smoking within the past 30 days (Kasza et al., 2017) and 13% are estimated to be current EC users, versus 6% of adults aged ≥25 (U.S. Department of Health and Human Services, 2016). It would be useful to anticipate how hypothetical restrictions on the EC market may impact EC and CC use in this population. We aimed to assess young adult dual EC/CC users’ intended responses to hypothetical market restrictions regarding key EC characteristics.
METHODS
Data source
Methods for this research have been reported previously (Pacek, Oliver, Sweitzer, & McClernon, 2019), but briefly: data were collected on Amazon Mechanical Turk (MTurk), which provides a cost-effective, rapid method for conducting studies that span multiple disciplines (Carter, DiFeo, Bogie, Zhang, & Sun, 2014; P. S. Johnson, Herrmann, & Johnson, 2015; Pacek, Rass, & Johnson, 2017; Rass et al., 2015). Inclusion criteria were: reside in the U.S.; having a ≥95% approval rating from previous MTurk tasks; age 18-29; smoking CCs for ≥3 months AND ≥ one day in the past week; and using ECs for ≥3 months AND ≥ one day in the past week. Eligible participants were given a code to access the survey, hosted by Qualtrics (Provo, UT). Participants were paid $2 upon completion. The survey was active from June 20-22, 2017. Participation was voluntary and anonymous. The Institutional Review Board at Duke University School of Medicine approved this study.
Measures
Sociodemographic, CC and EC history characteristics
Participants reported sociodemographic information and detailed CC and EC use history. The Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) (FTND) assessed CC dependence and a modified version of the FTND (eFTND) was used to assess EC dependence (Rass et al., 2015).
EC use characteristics
Participants reported the concentration/strength of nicotine that they used most often; Nicotine content was dichotomized (low [≤6 mg/mL] versus high [>6 mg/mL]). Participants also reported the “specific flavor that they use most often (e.g., cherry)” in their ECs; flavors were categorized as “flavored” versus “tobacco/menthol.” Participants uploaded a photo of their usual brand EC. Two coders categorized photos independently as 1G/2G/3G devices; disagreement was resolved by discussion. Given the relative lack of possibility for customizability in 1G and 2G devices as compared to 3G, device type was dichotomized (1G/2G versus 3G). A total of 20 participants’ devices could not be classified as 1G/2G/3G (e.g., uploaded photo contained multiple devices of various generations or were of a more advanced device type such as JUUL/pod devices).
Hypothetical EC market restrictions
Participants reported anticipated responses to three hypothetical EC regulations. Hypothetical scenarios were described to participants as follows: “Imagine that e-cigarettes available in the United States are like they are today BUT: 1) they are only available in nicotine-free (0 nicotine) e-liquid; 2) they are only available in tobacco/menthol flavors; and 3) they do not allow the user to modify or customize the device (e.g., wattage, air flow).” Under each scenario, participants indicated—separately for ECs and CCs—whether they would stop using ECs/CCs completely, use ECs/CCs a lot less often, use ECs/CCs a little less often, use ECs/CCs the same amount, use ECs/CCs a little more often, or use ECs/CCs a lot more often.
Statistical analysis
Descriptive statistics depicted the sociodemographic and CC/EC use characteristics of the sample. McNemar’s chi-square tests were used to assess differences between groups in terms of anticipated responses to hypothetical EC market restrictions (e.g., comparing anticipated EC vs. CC quitting, among the entire sample). Chi-square tests were also used when comparing anticipated use behaviors between subgroups (e.g., comparing anticipated quitting of EC use between high/low nicotine content users). Given the small number of participants (n=5) who used nicotine-free e-liquid, we could not evaluate whether a hypothetical regulation differentially impacts users of nicotine-free versus nicotine-containing e-liquids. Post hoc multinomial logistic regression analyses were run to assess whether EC and CC use frequency (i.e., days of use per week; EC bouts per day/CPD) or intentions to quit EC or CC use were associated with anticipated responses to hypothetical EC market restrictions.
RESULTS
Sociodemographic characteristics
Sample sociodemographic characteristics are presented in Supplemental Table 1. In total, 314 individuals initiated the survey, while 252 individuals completed the task in its entirety. Twelve were excluded for: not meeting EC use inclusion criteria (n=3); indicating that their data should not be used (n=8); and unreliable data (e.g., submitting photos of images that did not contain CC or EC products; n=4). These numbers do not sum to 12 due to overlap between categories. Analyses are based on a sample size of n=240. The sample was half male (49.2%), predominantly White (72.5%), had greater than a high school diploma/GED (87.5%), and was unmarried (76.3%).
Product use characteristics
CC and EC use characteristics are presented in Table 1. Approximately one-third (36.3%) were non-daily users of ECs and CCs, 25.4% used CCs daily but ECs non-daily, 17.1% used ECs daily but CCs non-daily, and 21.3% used ECs and CCs daily.
Table 1.
E-cigarette and combustible cigarette use characteristics of dual users, age 18–29 (n=240)
| Characteristic | EC | CC |
|---|---|---|
| Years used | 1.7 (1.9) | 5.8 (3.8) |
| Bouts per day/CPD | 16.9 (29.5) | 5.9 (5.4) |
| Days used per week | 4.8 (2.1) | 5.3 (2.1) |
| Daily use – n (%) | 92 (38.3) | 112 (46.7) |
| eFTND/FTND Dependence | 2.7 (2.3) | 3.0 (2.4) |
| Plans to quit in next month – n (%) | 67 (27.9) | 155 (64.6) |
| Menthol – n (%) | -- | 126 (52.5) |
| E-liquid flavor – n (%)a | -- | |
| Flavored | 139 (58.4) | -- |
| Tobacco/menthol | 99 (41.6) | -- |
| Nicotine concentration – n (%)b | -- | |
| Low (≤6 mg/mL) | 72 (36.2) | -- |
| High (>6 mg/mL) | 127 (63.8) | -- |
| Device type – n (%)c | -- | |
| 1G/2G | 145 (65.9) | -- |
| 3G | 75 (34.1) | |
Based on n=238; 2 participants’ self-reported flavor of choice unable to be classified
Based on n=238; 2 participants’ self-reported flavor of choice unable to be classified
Based on n=220; 20 participants’ photos unable to be classified as 1G/2G/3G devices
Responses to hypothetical EC market restrictions
EC nicotine content restrictions
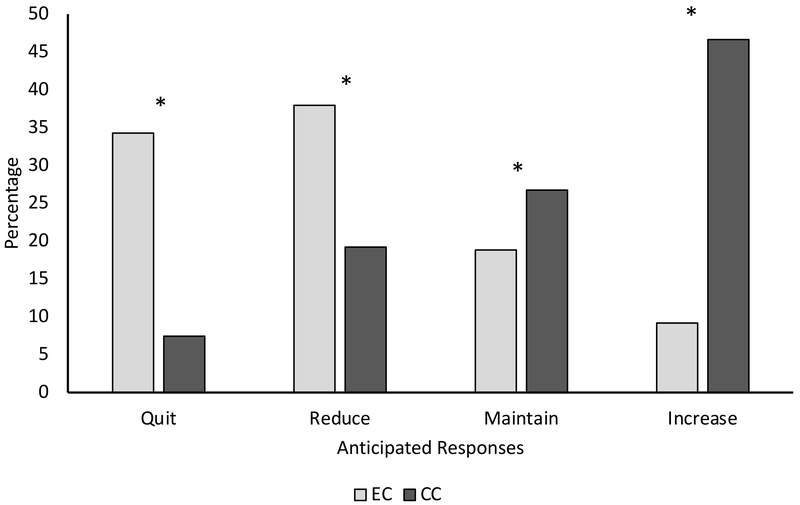
In response to nicotine content restrictions (Figure 1), participants were more likely to report intentions to quit or reduce EC versus CC use (McNemar’s χ2 (1, N=240)=46.6, p<0.001; McNemar’s χ2 (1, N=240)=22.3, p<0.001), and were more likely to report intentions to maintain or increase CC use versus EC use (McNemar’s χ2 (1, N=240)=6.6, p=0.010; McNemar’s χ2 (1, N=240)=60.5, p<0.001, respectively). We observed differential responses based on use of high versus low nicotine e-liquids. Among individuals using high nicotine content e-liquids (Supplemental Figure 1A), participants were more likely to indicate that they would quit or reduce EC versus CC use (47.2% versus 5.6%: χ2 (1, N=72)=26.5, p<0.001; 37.5% versus 15.3%: χ2 (1, N=72)=12,8, p<0.001, respectively), and were more likely to report intentions to maintain or increase use of CC versus EC (31.9% versus 12.5%: χ2 (1, N=72)=10.9, p=0.001; 47.2% versus 2.8%: χ2 (1, N=72)=28.4, p<0.001, respectively). Among persons using low nicotine content e-liquids, participants were more likely to indicate that they would quit or reduce use of EC versus CC (27.6% versus 5.6%: χ2 (1, N=127)=15.2, p<0.001; 37.5% versus 15.3%: χ2 (1, N=127)=7.7, p=0.006, respectively) and increase use of CC versus EC (48.8% versus 11.8%: χ2 (1, N=127)=28.7, p<0.001) (Supplemental Figure 1B).
Figure 1.
Anticipated responses to hypothetical regulation of nicotine content in EC
Note: Asterisks indicate statistically significant differences between product use categories
Moreover, participants using high nicotine e-liquids were significantly more likely than those using low nicotine e-liquids to report that they would quit EC use (47.2% versus 27.5%: χ2 (1, N=199)=.78, p=0.005). Additionally, users of high nicotine e-liquid were also less likely to indicate intentions to maintain or increase EC use (12.5% versus 25.2%: χ2 (1, N=199)=4.5, p=0.033; 2.8% versus 11.8%: χ2 (1, N=199)=4.8, p=0.028) (Supplemental Figure 2A).
EC flavor restrictions
In response to restrictions on e-liquid flavors (Figure 2), participants were more likely to report intentions to quit or reduce EC versus CC use (McNemar’s χ2 (1, N=240)=8.8, p=0.003; McNemar’s χ2 (1, N=240)=14.5, p<0.001) and more likely to report intentions to maintain or increase CC use versus EC use (McNemar’s χ2 (1, N=240)=13.1, p<0.001; McNemar’s χ2 (1, N=240)=13.5, p<0.001). Moreover, we observed differential responses based on use of flavored e-liquids. Among users of flavored e-liquids, participants were more likely to indicate that they would quit or reduce their use of EC versus CC (18.7% versus 8.6%: χ2 [(1, N=139)=6.1, p=0.013; 52.5% versus 28.1%: χ2 (1, N=139)=15.2, p<0.001), and maintain or increase CC versus EC use (44.6% versus 23.0%: χ2 (1, N=139)=15.5, p<0.001; 18.7% versus 5.8%: χ2 (1, N=139)=9.5, p=0.002) (Supplemental Figure 3). No differences regarding anticipated EC versus CC use were reported among users of tobacco/menthol e-liquid.
Figure 2.
Anticipated responses to hypothetical regulation of e-liquid flavor
Note: Asterisks indicate statistically significant differences between product use categories
Participants who used flavored e-liquids were significantly more likely than those using tobacco/menthol e-liquids to report that they would quit or reduce EC use (18.7% versus 7.1%: χ2 (1, N=238)=6.6, p=0.010; 52.5% versus 25.3%: χ2 (1, N=238)=17.7, p<0.001), while tobacco/menthol flavor users were more likely to report that they would maintain their EC use (61.6% vs. 23.0%: χ2 (1, N=238)=36.2, p<0.001) (Supplemental Table 4A). Users of flavored e-liquids were also more likely to report that they would quit CC use (8.6% versus 2.0%: χ2 (1, N=238)=4.6, p=0.033) and were less likely to report that they would maintain CC use (44.6% versus 63.6%: χ2 (1, N=238)=8.4, p=0.004) (Supplemental Figure 4B).
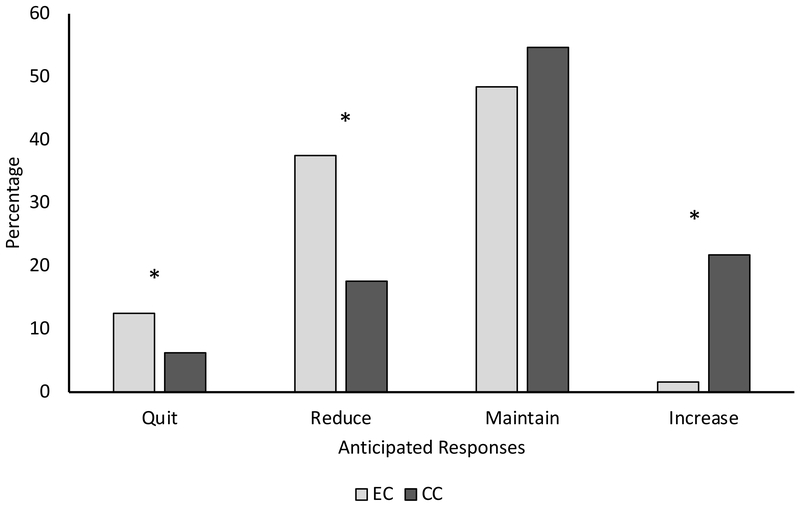
EC device type restrictions
In response to hypothetical restrictions on EC device type (Figure 3), participants were more likely to report intentions to quit or reduce EC use versus CC use (McNemar’s χ2 (1, N=240)=6.1, p=0.014; McNemar’s χ2 (1, N=240)=26.2, p<0.001), and more likely to report intentions to increase CC use versus EC use (McNemar’s χ2 (1, N=240)=42.7, p<0.001). Among users of 1G/2G devices, participants were more likely to report that they would quit or reduce EC versus CC use (10.3% versus 4.8%: χ2 (1, N=145)=4.0, p=0.046; 33.8% versus 16.6%: χ2 (1, N=145)=12.3, p<0.001), and more likely to report that they would increase CC versus EC use (15.9% versus 1.4%: χ2 (1, N=145)=17.6, p<0.001) (Supplemental Figure 5). Among users of 3G devices, participants were more likely to report that they would reduce EC versus CC use (44.0% versus 18.7%: χ2 (1, N=75)=13.4, p<0.001) and increase CC versus EC use (33.3% versus 0%; χ2 (1, N=75)=25.0, p<0.001) (Supplemental Figure 5).
Figure 3.
Anticipated responses to hypothetical regulation of EC device customizability
Note: Asterisks indicate statistically significant differences between product use categories
Additionally, 1G/2G device users were more likely than 3G users to report intentions to maintain EC use and CC use (54.5% versus 38.7%: χ2 (3, N=220)=4.9, p=0.026; 62.8% versus 40.0%: χ2 (3, N=220)=10.3, p=0.001) (Supplemental Figure 6A). Conversely, 3G EC device users were more likely than 1G/2G users to report intentions to increase CC use (33.3% versus 15.9%: χ2 (3, N=220)=8.9, p=0.003) (Supplemental Figure 6B).
Associations between responses to hypothetical EC market restrictions and EC/CC use frequency and intentions to quit using EC/CC
In the hypothetical nicotine content restriction scenario, participants reporting a greater number of EC bouts per day were more likely to report anticipating an increase in CC use (RRR=1.02, 95% CI=1.01, 1.03). Regarding the hypothetical restriction of EC flavors, participants reporting a greater number of EC bouts per day were more likely to report anticipating that they would reduce EC use (RRR=1.01, 95% CI=1.01, 1.02). In the flavor restriction scenario, participants reporting greater days of CC use per week were less likely to anticipate quitting CC use (RRR=0.66, 95% CI=0.51, 0.85). In response to hypothetical restrictions on EC device type, participants reporting greater CPD were more likely to report anticipating that they would quit EC (RRR=1.07, 95% CI=1.01, 1.14). Participants reporting greater EC bouts per day were also more likely to report anticipating that they would increase CC use (RRR=1.02, 95% CI=1.01, 1.03) if EC device type was restricted. Overall intentions to quit using EC and CC were not associated with anticipated responses to hypothetical EC market restrictions.
DISCUSSION
This work assessed young adult dual EC/CC users’ anticipated responses to hypothetical regulations of key EC characteristics. Preliminary findings suggest that young adult dual users self-report their intention to reduce EC use and increase CC use. These findings are consistent with research wherein participants indicated that the restriction of e-liquid flavors would reduce the appeal of ECs by making them less enjoyable (69%) and more boring (46%), and lower their likelihood of reducing or quitting smoking (40%) (Farsalinos et al., 2013). In addition, flavor restrictions decreased the expected likelihood of selecting ECs versus CCs (Pesko et al., 2016). Though additional studies will examine the effect of these restrictions, limited research indicates that ECs—while conferring greater harm than nicotine replacement therapy or complete cessation of tobacco product use—generally offer a more favorable toxicant profile than CCs (D’Ruiz, Graff, & Robinson, 2016; Hecht et al., 2015). It is possible that regulations that deter complete switching to potentially lower-harm tobacco products, such as ECs and result in increases in the proportion of CCs used among dual EC/CC users may have a negative effect on public health. For example, a recent study found that, among dual EC/CC users in the U.S. general population, the frequency of CC use (i.e., daily use vs. use on some days) is positively correlated with tobacco toxicant concentration (Goniewicz et al., 2018).
Moreover, hypothetical restrictions regarding EC characteristics were most relevant for individuals who reported utilizing those characteristics in their typical EC use. For example, participants who typically use flavored e-liquids (other than tobacco/menthol flavor) were more likely to indicate intention to quit or reduce EC use and simultaneously indicate intentions to increase CC use in response to restricting available flavors to only tobacco or menthol. These findings are particularly noteworthy given that most EC users use flavored e-liquids (69.3%-97.9%) and modifiable EC devices (53.6%-73.5%) (Farsalinos et al., 2013, 2014). In some instances, responses to hypothetical EC market restrictions also varied based on participants’ frequency of EC and CC use—both in terms of the number of days of use per week as well as CPD and EC bouts per day.
This work should be considered in light of several limitations. First, the results of this study have limited generalizability. Although recent work indicates that data from substance-using samples gathered via MTurk are valid, this study was limited to dual EC/CC users available in this database (Kim et al., 2016; Mortensen & Hughes, 2018); future work should investigate the impact of hypothetical regulations on product use intentions in more diverse groups of tobacco users, including exclusive EC and exclusive CC users. Relatedly, data were collected only among young adult dual users whose intended and actual behaviors may differ from those of older adults or single product users. Second, the hypothetical regulations considered in the present analyses were negative in tone and would result in reductions in product diversity, which may have contributed to participants’ intentions to decrease EC use following such regulations. To reduce response bias, future work should evaluate anticipated responses to hypothetical regulations of a more positive nature (e.g., new requirements for child-safety packaging; ensured ingredient purity) alongside those having a more negative focus. Third, data were collected via self-report; behavioral and biochemical verification of EC and CC use was not conducted. Fourth, participants’ responses regarding EC and CC use were based on hypothetical scenarios concerning EC market regulation and may not reflect actual behavior. Future work may evaluate dual users’ behavioral responses to regulations in the context of laboratory and/or clinical trials methodology. Fifth, though within this paper we discuss both cessation and reduction of CC use as being potentially desirable outcomes, it merits mentioning that reducing CC consumption to even very low levels confers significant health risks (Hackshaw et al., 2018). To date, though the overall health impact of dual use is not yet definitively known, accumulating research indicates that dual EC/CC use may confer greater negative health risks than CC use alone (Osei et al., 2019; J. B. Wang et al., 2018). In order to maximize health benefits, efforts to promote complete switching from CC to EC or total cessation from tobacco products should be prioritized. Nonetheless, reductions in CC smoking have been associated with increased quit attempts and cessation (Broms, Korhonen, & Kaprio, 2008; J. Hughes & Carpenter, 2006; Hyland et al., 2005) and may present an opportunity to re-engage smokers in supported cessation efforts (e.g., counseling, medication), which are associated with increased cessation success (Centers for Disease Control and Prevention, 2011; J. R. Hughes, 2003).
Lastly, the hypothetical scenarios were the most stringent possibilities (i.e., eliminating nearly all flavor categories, all nicotine, and all modifications) and were presented in isolation of each other. Although FDA has not proposed these regulations for EC, two recent advanced notices of proposed rulemaking have asked for public comment on the implications of 1) limiting nicotine levels in CC (Gottlieb & Zeller, 2017); and 2) limiting flavors in tobacco products. It is difficult to prospectively hypothesize if and how ECs may be regulated in these domains. Additionally, zero-nicotine is not a legal possibility (per the FSPTCA, the product standard cannot eliminate nicotine (United States Congress, 2009)). Future work may also evaluate the impact of differing levels of hypothetical regulation, independently and together, on EC and CC use. Limitations notwithstanding, to our knowledge, this paper represents one of the first to explore young adult dual EC/CC users’ anticipated responses to potential regulations regarding the diversity of the EC market.
Findings from our study suggest that eliminating the availability of flavored e-liquid, nicotine content, and customizable EC devices may lead to intentions to reduce EC use and simultaneous intentions to increase CC use among young adult dual EC/CC users. Given that 38% of tobacco users are dual or multiple tobacco product users—and that 23% of this group specifically use CC and EC (Kasza et al., 2017)—these findings serve as a useful baseline indicator of what a significant proportion of tobacco product users believe they would do in response to regulations. These findings and additional studies—including those that assess actual EC and CC use behavior—can provide useful information about the potential impact of EC regulatory actions on intended and actual tobacco use behaviors among dual EC/CC users in the U.S. In addition, determining the correlation between anticipated use behaviors and actual use behaviors, in response to product regulation, will be important for interpreting results of hypothetical measures such as this one.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge and thank Ms. Erin Locey for her assistance with coding photos of e-cigarette devices. This publication represents the views of the author(s) and does not represent Food and Drug Administration/Center for Tobacco Products position or policy.
Funding: This work was supported by the National Institutes of Health (K01DA043413, K23DA039294, and K23DA042898). The funding source had no other role other than financial support.
Footnotes
Disclosure of Interests: The authors have no conflicts of interest to declare.
REFERENCES
- Adriaens K, Van Gucht D, & Baeyens F (2017). Differences between Dual Users and Switchers Center around Vaping Behavior and Its Experiences Rather than Beliefs and Attitudes. International Journal of Environmental Research and Public Health, 15(1). 10.3390/ijerph15010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggett TP, Campbell EG, Chang Y, & Rigotti NA (2016). Other tobacco product and electronic cigarette use among homeless cigarette smokers. Addictive Behaviors, 60, 124–130. 10.1016/j.addbeh.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baweja R, Curci KM, Yingst J, Veldheer S, Hrabovsky S, Wilson SJ, … Foulds J (2016). Views of Experienced Electronic Cigarette Users. Addiction Research & Theory, 24(1), 80–88. 10.3109/16066359.2015.1077947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombard JM, Pederson LL, Nelson DE, & Malarcher AM (2007). Are smokers only using cigarettes? Exploring current polytobacco use among an adult population. Addictive Behaviors, 32(10), 2411–2419. 10.1016/j.addbeh.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Bombard JM, Rock VJ, Pederson LL, & Asman KJ (2008). Monitoring polytobacco use among adolescents: do cigarette smokers use other forms of tobacco? Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 10(11), 1581–1589. 10.1080/14622200802412887 [DOI] [PubMed] [Google Scholar]
- Broms U, Korhonen T, & Kaprio J (2008). Smoking reduction predicts cessation: Longitudinal evidence from the Finnish adult twin cohort. Nicotine & Tobacco Research, 10(3), 423–427. 10.1080/14622200801888988 [DOI] [PubMed] [Google Scholar]
- Brose LS, Hitchman SC, Brown J, West R, & McNeill A (2015). Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction (Abingdon, England), 110(7), 1160–1168. 10.1111/add.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, & Laugesen M (2010). Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tobacco Control, 19(2), 98–103. 10.1136/tc.2009.031567 [DOI] [PubMed] [Google Scholar]
- Carter RR, DiFeo A, Bogie K, Zhang G-Q, & Sun J (2014). Crowdsourcing awareness: exploration of the ovarian cancer knowledge gap through Amazon Mechanical Turk. PloS One, 9(1), e85508 10.1371/journal.pone.0085508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2011). Quitting smoking among adults: United States, 2001–2010. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6044a2.htm
- Cheney MK, Gowin M, & Wann TF (2016). Electronic Cigarette Use in Straight-to-Work Young Adults. American Journal of Health Behavior, 40(2), 268–279. 10.5993/AJHB.40.2.12 [DOI] [PubMed] [Google Scholar]
- Cooper M, Harrell MB, & Perry CL (2016). Comparing young adults to older adults in e-cigarette perceptions and motivations for use: implications for health communication. Health Education Research, 31(4), 429–438. 10.1093/her/cyw030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ruiz CD, Graff DW, & Robinson E (2016). Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health, 16, 543 10.1186/s12889-016-3236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Gillman G, Hecht SS, Polosa R, & Thornburg J (2017). Analytical Assessment of e-Cigarettes: From contents to chemical and partical exposure profiles. Amsterdam, Netherlands: Elsevier. [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, & Voudris V (2013). Impact of flavour variability on electronic cigarette use experience: an internet survey. International Journal of Environmental Research and Public Health, 10(12), 7272–7282. 10.3390/ijerph10127272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, & Voudris V (2014). Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. International Journal of Environmental Research and Public Health, 11(4), 4356–4373. 10.3390/ijerph110404356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, & Voudris V (2015). Factors associated with dual use of tobacco and electronic cigarettes: A case control study. The International Journal on Drug Policy, 26(6), 595–600. 10.1016/j.drugpo.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. , (2016). [PubMed] [Google Scholar]
- Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, & Villanti AC (2017). Overview of Electronic Nicotine Delivery Systems: A Systematic Review. American Journal of Preventive Medicine, 52(2), e33–e66. 10.1016/j.amepre.2016.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, … Hyland AJ (2018). Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Network Open, 1(8), e185937 10.1001/jamanetworkopen.2018.5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, & Zeller M (2017). A Nicotine-Focused Framework for Public Health. The New England Journal of Medicine, 377(12), 1111–1114. 10.1056/NEJMp1707409 [DOI] [PubMed] [Google Scholar]
- Hackshaw A, Morris JK, Boniface S, Tang J-L, & Milenković D (2018). Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ, j5855. 10.1136/bmj.j5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamari AK, Toljamo TI, Kinnula VL, & Nieminen PA (2013). Dual use of cigarettes and Swedish snuff (snus) among young adults in Northern Finland. European Journal of Public Health, 23(5), 768–771. 10.1093/eurpub/cks131 [DOI] [PubMed] [Google Scholar]
- Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, … Eriksen MP (2017). Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Preventive Medicine Reports, 5, 33–40. 10.1016/j.pmedr.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BWS, … Hatsukami DK (2015). Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(6), 704–709. 10.1093/ntr/ntu218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, & Carpenter M (2006). Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine & Tobacco Research, 8(6), 739–749. 10.1080/14622200600789726 [DOI] [PubMed] [Google Scholar]
- Hughes JR (2003). Motivating and helping smokers to stop smoking. Journal of General Internal Medicine, 18(12), 1053–1057. 10.1111/j.1525-1497.2003.20640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Levy DT, Rezaishiraz H, Hughes JR, Bauer JE, Giovino GA, & Cummings KM (2005). Reduction in Amount Smoked Predicts Future Cessation. Psychology of Addictive Behaviors, 19(2), 221–225. 10.1037/0893-164X.19.2.221 [DOI] [PubMed] [Google Scholar]
- Johnson MW, Johnson PS, Rass O, & Pacek LR (2017). Behavioral economic substitutability of e-cigarettes, tobacco cigarettes, and nicotine gum. Journal of Psychopharmacology (Oxford, England), 31(7), 851–860. 10.1177/0269881117711921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Herrmann ES, & Johnson MW (2015). Opportunity costs of reward delays and the discounting of hypothetical money and cigarettes. Journal of the Experimental Analysis of Behavior, 103(1), 87–107. 10.1002/jeab.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, … Hyland AJ (2017). Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. The New England Journal of Medicine, 376(4), 342–353. 10.1056/NEJMsa1607538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Bansal-Travers M, O’Connor RJ, Compton WM, Kettermann A, Borek N, … Hyland AJ (2014). Cigarette smokers’ use of unconventional tobacco products and associations with quitting activity: findings from the ITC-4 U.S. cohort. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 16(6), 672–681. 10.1093/ntr/ntt212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lim J, Buehler SS, Brinkman MC, Johnson NM, Wilson L, … Clark PI (2016). Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tobacco Control, 25(Suppl 2), ii55–ii61. 10.1136/tobaccocontrol-2016-053221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler CE, Crutchfield TM, Sutfin EL, Ranney LM, Berman ML, Zarkin GA, & Goldstein AO (2017). Consumers’ Preferences for Electronic Nicotine Delivery System Product Features: A Structured Content Analysis. International Journal of Environmental Research and Public Health, 14(6). 10.3390/ijerph14060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, & Krishnan-Sarin S (2015). Reasons for Electronic Cigarette Experimentation and Discontinuation Among Adolescents and Young Adults. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(7), 847–854. 10.1093/ntr/ntu257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner WV, Meier E, Wiener JL, Grant DM, Gilmore J, Judah MR, … Wagener TL (2015). The comparative efficacy of first-versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction (Abingdon, England), 110(5), 862–867. 10.1111/add.12870 [DOI] [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramôa CP, Karaoghlanian NV, Lipato T, … Eissenberg T (2016). Effects of Electronic Cigarette Liquid Nicotine Concentration on Plasma Nicotine and Puff Topography in Tobacco Cigarette Smokers: A Preliminary Report. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 18(5), 720–723. 10.1093/ntr/ntv182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald EA, & Ling PM (2015). One of several “toys” for smoking: young adult experiences with electronic cigarettes in New York City. Tobacco Control, 24(6), 588–593. 10.1136/tobaccocontrol-2014-051743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeganey N, & Dickson T (2017). Why Don’t More Smokers Switch to Using E-Cigarettes: The Views of Confirmed Smokers. International Journal of Environmental Research and Public Health, 14(6). 10.3390/ijerph14060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer K, Vijayaraghavan M, White MM, Shi Y, Chang C, Conway KP, … Pierce JP (2015). Cigarette smoking cessation attempts among current US smokers who also use smokeless tobacco. Addictive Behaviors, 51, 113–119. 10.1016/j.addbeh.2015.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbolouk M, Charkhchi P, Kianoush S, Uddin SMI, Orimoloye OA, Jaber R, … Blaha MJ (2018). Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Annals of Internal Medicine, 169(7), 429 10.7326/M17-3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K, & Hughes TL (2018). Comparing Amazon’s Mechanical Turk Platform to Conventional Data Collection Methods in the Health and Medical Research Literature. Journal of General Internal Medicine. 10.1007/s11606-017-4246-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Benjamin EJ, … Blaha MJ (2019). The association between e-cigarette use and cardiovascular disease among never and current combustible cigarette smokers: BRFSS 2016 & 2017. The American Journal of Medicine. 10.1016/j.amjmed.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Pacek LR, Oliver JA, Sweitzer MM, & McClernon FJ (2019). Young adult dual combusted cigarette and e-cigarette users’ anticipated responses to a nicotine reduction policy and menthol ban in combusted cigarettes. Drug and Alcohol Dependence, 194, 40–44. 10.1016/j.drugalcdep.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Rass O, & Johnson MW (2017). Knowledge about nicotine among HIV-positive smokers: Implications for tobacco regulatory science policy. Addictive Behaviors, 65, 81–86. 10.1016/j.addbeh.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Davis KC, Cox S, Bradfield B, King BA, Shafer P, … Bunnell R (2016). Reasons for current E-cigarette use among U.S. adults. Preventive Medicine, 93, 14–20. 10.1016/j.ypmed.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Michael VC (2017). Effects of nicotine versus placebo e-cigarette use on symptom relief during initial tobacco abstinence. Experimental and Clinical Psychopharmacology, 25(4), 249–254. 10.1037/pha0000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko MF, Kenkel DS, Wang H, & Hughes JM (2016). The effect of potential electronic nicotine delivery system regulations on nicotine product selection. Addiction (Abingdon, England), 111(4), 734–744. 10.1111/add.13257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramôa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, … Eissenberg T (2016). Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tobacco Control, 25(e1), e6–9. 10.1136/tobaccocontrol-2015-052447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Pacek LR, Johnson PS, & Johnson MW (2015). Characterizing use patterns and perceptions of relative harm in dual users of electronic and tobacco cigarettes. Experimental and Clinical Psychopharmacology, 23(6), 494–503. 10.1037/pha0000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy SF, & Durmowicz EL (2016). Electronic nicotine delivery systems: overheating, fires and explosions. Tobacco Control. 10.1136/tobaccocontrol-2015-052626 [DOI] [PubMed] [Google Scholar]
- Rutten LJF, Blake KD, Agunwamba AA, Grana RA, Wilson PM, Ebbert JO, … Leischow SJ (2015). Use of E-Cigarettes Among Current Smokers: Associations Among Reasons for Use, Quit Intentions, and Current Tobacco Use. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(10), 1228–1234. 10.1093/ntr/ntv003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons VN, Quinn GP, Harrell PT, Meltzer LR, Correa JB, Unrod M, & Brandon TH (2016). E-cigarette use in adults: a qualitative study of users’ perceptions and future use intentions. Addiction Research & Theory, 24(4), 313–321. 10.3109/16066359.2016.1139700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule EK, Pomeranz JL, Moorhouse MD, & Barnett TE (2015). Multiple tobacco use and increased nicotine dependence among people with disabilities. Disability and Health Journal, 8(2), 258–263. 10.1016/j.dhjo.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Sussman S, Garcia R, Cruz TB, Baezconde-Garbanati L, Pentz MA, & Unger JB (2014). Consumers’ perceptions of vape shops in Southern California: an analysis of online Yelp reviews. Tobacco Induced Diseases, 12(1), 22 10.1186/s12971-014-0022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Shihadeh A (2015). Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 17(2), 150–157. 10.1093/ntr/ntu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL, Alpert HR, & Connolly GN (2010). Patterns of dual use of cigarettes and smokeless tobacco among US males: findings from national surveys. Tobacco Control, 19(2), 104–109. 10.1136/tc.2009.031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T-Y, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, & Shelley D (2016). A Randomized Trial Comparing the Effect of Nicotine Versus Placebo Electronic Cigarettes on Smoking Reduction Among Young Adult Smokers. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 18(10), 1937–1943. 10.1093/ntr/ntw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Congress. Family smoking prevention and tobacco control federal reform act. , Pub. L. No. Pub. L. No. 111–312009 (2009).
- U.S. Department of Health and Human Services. (2014). The health consequences of smoking: 50 years of progress. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- U.S. Department of Health and Human Services. (2016). E-cigarette use among youth and young adults. A report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Vandrevala T, Coyle A, Walker V, Cabrera Torres J, Ordoña I, & Rahman P (2017). “A good method of quitting smoking” or “just an alternative to smoking”? Comparative evaluations of e-cigarette and traditional cigarette usage by dual users. Health Psychology Open, 4(1), 2055102916684648. 10.1177/2055102916684648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, Hyland A (2017). Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013-2014). American Journal of Preventive Medicine, 53(2), 139–151. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Queimado L (2017). Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control, 26(e1), e23–e28. 10.1136/tobaccocontrol-2016-053041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Olgin JE, Nah G, Vittinghoff E, Cataldo JK, Pletcher MJ, & Marcus GM (2018). Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PloS One, 13(7), e0198681 10.1371/journal.pone.0198681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, King BA (2018). Tobacco Product Use Among Adults — United States, 2017. MMWR. Morbidity and Mortality Weekly Report, 67(44), 1225–1232. 10.15585/mmwr.mm6744a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, McClure JB, de Moor C, Cofta-Gunn L, Cummings S, Cinciripini PM, & Gritz ER (2002). Concomitant use of cigarettes and smokeless tobacco: prevalence, correlates, and predictors of tobacco cessation. Preventive Medicine, 34(6), 638–648. 10.1006/pmed.2002.1032 [DOI] [PubMed] [Google Scholar]
- Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, & Lee M (2014). Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tobacco Control, 23 Suppl 3, iii3–9. 10.1136/tobaccocontrol-2014-051670 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.