Abstract
Background:
Inflammation contributes to neonatal hypoxic-ischemic brain injury pathogenesis. We evaluated the neuroprotective efficacy of azithromycin, a safe, widely available antibiotic with anti-inflammatory properties, in a neonatal rodent hypoxic-ischemic brain injury model.
Methods:
Seven day old rats underwent right carotid artery ligation followed by 90 min 8% oxygen exposure; this procedure elicits quantifiable left forepaw functional impairment and right cerebral hemisphere damage. Sensorimotor function (vibrissae-stimulated forepaw placing, grip strength) and brain damage were compared in azithromycin- and saline-treated littermates two to four weeks later. Multiple treatment protocols were evaluated (variables included doses ranging from 15-45 mg/kg; treatment onset 15 min to 4h post-hypoxia, and comparison of 1 vs. 3 injections).
Results:
All azithromycin doses improved function and reduced brain damage; efficacy was dose-dependent, and declined with increasing treatment delay. Three azithromycin injections, administered over 48 hours, improved performance on both function measures and reduced brain damage more than a single dose.
Conclusion:
In this neonatal rodent model, azithromycin improved functional and neuropathology outcomes. If supported by confirmatory studies in complementary neonatal brain injury models, azithromycin could be an attractive candidate drug for repurposing and evaluation for neonatal neuroprotection in clinical trials.
Introduction
Discovering new uses for approved drugs i.e. repurposing, provides the quickest possible route for transition from “bench to bedside” for new therapeutic applications. Azithromycin has been widely used as an antibiotic for over 25 years. Its antibiotic effects are mediated by interfering with bacterial protein synthesis. Of interest, independent of its antibiotic activity, azithromycin has well-defined anti-inflammatory properties, mediated at least in part by modulating the phenotype of myeloid lineage cells. Recent reports showed that azithromycin treatment decreased injury severity in adult rodent stroke and spinal cord injury models (1,2). These reports, coupled with robust evidence that inflammatory mechanisms contribute to injury and recovery after neonatal hypoxic-ischemic brain injury, prompted us to hypothesize that azithromycin could be an attractive candidate drug to repurpose for neonatal neuroprotection.
Neonates are already being exposed to azithromycin in two distinct, yet complementary contexts. In some obstetric protocols, azithromycin is administered to women at risk for infection during labor; it crosses the placenta to a limited extent, and accumulates in the fetus (3, 4). Little systematic data have been collected regarding the impact of pre-natal azithromycin treatment in their newborns; however, no deleterious effects have been discerned (5). In a proof-of-concept trial in Gambia, a single dose of oral azithromycin, administered to women in labor, reduced the rate of clinical infections in their newborns (6). Based on these findings, investigators recently implemented a clinical trial (NCT03199547) that plans to enroll 12,500 women in labor to determine if oral administration of a single dose of azithromycin pre-delivery reduces neonatal sepsis or death. In addition, azithromycin pharmacokinetics and safety have been evaluated in premature newborns in phase I-II studies of its efficacy to eradicate respiratory tract Ureaplasma infection and decrease the incidence of chronic lung disease of prematurity (7, 8).
In this study, we evaluated the neuroprotective efficacy of azithromycin in a well-characterized neonatal rodent brain hypoxic-ischemic injury model. Outcome measures included performance on two independent tests of sensorimotor function (illustrated in supplemental online videos) and severity of brain damage. After initial evaluation of the dose-response and therapeutic window for drug administration using single-dose treatment, we refined protocols to incorporate multiple injections, which might better model dosing regimens that would be feasible and could be implemented in clinical trials and clinical practice. To facilitate interpretation of our results in the context of available human data, we also evaluated azithromycin pharmacokinetics.
Methods
Overview of experimental design.
Animals from a single litter were lesioned concurrently, and allocated to drug-treatment and saline-control groups. Since sex may influence susceptibility to brain injury and endogenous recovery mechanisms (9, 10), males and females were distributed equally between the groups. In initial proof-of-principle experiments, animals underwent sensorimotor testing on P14 and P21, were euthanized on P21, and brain injury was evaluated by comparison of bilateral cerebral hemisphere weights (11). In subsequent confirmatory experiments, sensorimotor testing was extended to include P28 and P35 evaluations, animals were then euthanized, and brain injury was evaluated in histopathology sections. Euthanasia was by pentobarbital intraperitoneal (i.p.) injection (Fatal Plus ®, Vortech Pharmaceuticals, Dearborn, MI). All protocols were approved by the University of Michigan Animal Care and Use Committee.
Animal lesioning.
Isofluorane-anesthesized P7 Wistar rats underwent right carotid artery ligation (12), recovered in incubators (90 min, at 36.5°C, Hovabator, GQF, Savannah, GA), were placed in acrylic containers partially submerged in a water-bath (36.5 °C), and exposed to 8% oxygen/balance nitrogen for 90 min. They recovered in incubators (15 min, 36.5°C), and then returned to dams.
In one experiment, the treatment protocol was replicated in P10 animals (13). Based on prior experience (unpublished observation) that P10 Wistar rats have higher acute mortality rates with this lesioning protocol, and published reports of relatively high mortality (13%) with 80-85 minutes hypoxia-ischemia in P10 Wistar rats (13, 14), after carotid ligation, animals were exposed to 8% oxygen/balance nitrogen for 60 (rather than 90) min.
Physiology measurements.
Animals were weighed on P7, P8, and then weekly. Rectal temperatures were measured (YSI thermometer 43T with probe 554; YSI, Yellow Springs, OH) before surgery, at the end of hypoxia, and 15 min, 60 min,120 min and 24h later.
Drug treatment.
Sterile lyophilized azithromycin for injection, USP (Fresenius Kabi USA, Lake Zurich IL) was dissolved in sterile 0.9% NaCl solution, as required to achieve a uniform i.p. injection volume of 100 microliter per 10 gm body weight; intravenous injection is not feasible in rats at this age. Controls were injected with 0.1 ml/10 gm body weight of saline.
To collect samples for pharmacokinetic analysis, additional P7 rats underwent hypoxic-ischemic (HI) lesioning, as described above, and received i.p. injections of azithromycin 10 or 40 mg/kg 15 min after HI. Animals were euthanized (pentobarbital i.p. injection) 2, 4, 7, 24, 48 or 72 h later (n=3-4/dose/time) and atrial blood samples (0.5 ml) were collected prior to tissue perfusion with saline (10 ml); then left and right cerebral hemisphere samples were collected. All samples were frozen until assayed.
Treatment allocation.
Animals received either one or three injections of azithromycin or saline. The dose range for single injection regimens stemmed from preliminary experiments in which we tested the highest dose reported in adult murine studies (1), 150 mg/kg, in a single injection immediately after HI (data not shown). Although that dose was effective, because it was much higher than the clinical range, it was not incorporated in the studies reported here. The 3 injection dosing incorporated both results of the preceding single-dose experiments, and clinical practice that often includes 50% azithromycin dose reductions on subsequent days of treatment. Single dose treatment regimens with outcomes to P21:
injection 15 min post-HI; doses: 15, 30, or 45 mg/kg (n = 20/group).
injection 1, 2, or 4 h post-HI; dose 45 mg/kg (n = 8-9group).
Three dose treatment regimens with outcomes to P35:
first injection (45 mg/kg) 2h post-HI, second (22.5 mg/kg) at 24h post-HI, and third (22.5 mg/kg) at 48h post-HI [comparator groups received single dose (45 mg/kg) 2h post-HI, or saline 3 doses (2h, 24h, 48h); n=14-15/group].
replication in P10 rats (HI duration = 60 min); injections at 2h, 24h and 48h post-HI, with drug doses 45, 22.5, 22.5 mg/kg respectively (compared to saline, 3 doses, same timing, n=12/group).
Sensorimotor function testing.
Performance on two independent tests that consistently demonstrate asymmetric sensorimotor deficits in this model was evaluated; a supplemental online video illustrates these methods.
Vibrissae-stimulated forepaw placing (10 trials/side) was tested weekly from P14, and up to P35; one point was assigned for full and 0.5 points for partial extension (normal= 10/10 bilaterally) (12, 15). Scoring was off-line using video recordings, and the observer had no knowledge of treatment identity. Right-forepaw placement was consistently normal, and only left forepaw scores are reported in the results.
Forepaw grip strength was measured (3 trials/side, Digital Force Gauge DFIS-2, Chatillon Force Measurement Products, Largo, FL, USA) on P21 (youngest age at which reliable measures could be obtained), and weekly up to P35 (12) by an observer that did not know treatment identity at the time of measurement. In normal rats, grip strength increases from P21-P35 and remains symmetric bilaterally. To take into account age-related increases in grip strength, results are expressed as left/right (L/R) forepaw grip strength ratios.
Brain injury measures:
Animals were euthanized on P21 or P35 (pentobarbital i.p. injection), brains were rapidly removed, placed on ice, and photographed.
In experiments that compared outcomes at P21, right and left cerebral hemispheres were separated and weighed (11). Right hemisphere % damage, reflecting both initial tissue injury and impaired subsequent growth, was expressed as %difference in hemisphere weight [100*(left-right)/left].
In experiments that evaluated outcomes at P35, brains were rapidly frozen, coronal brain sections (20 microns) were prepared and cresyl-violet stained. Bilateral cross-sectional areas of striatum, neocortex, hippocampus, and cerebral hemisphere were measured (using NIH Image J) by an observer unaware of treatment identity on regularly spaced sections from the level of the anterior genu to the posterior genu of the corpus callosum, bilateral regional volumes were calculated, and right hemisphere damage values were defined and expressed as above (12).
Azithromycin Pharmacokinetics:
To precipitate proteins in blood samples, 180 μL of acetonitrile containing 50 ng/ml of an internal standard was added to 30 μL of blood and vortexed (10 min). Extracts were centrifuged at 15000 rpm, and supernatant was transferred to autosampler vials for liquid chromatograph-tandem mass spectrometry (LC–MS/MS). Tissue samples were homogenized (Precellys tissue homogenizer, Bertin Technologies, Montigny-le-Bretonneux, France) with the addition of diluent solution, at a 5:1 volume (ml):weight (g) ratio of 20% acetonitrile in water to tissue.
Homogenized tissue solution was processed using the same extraction procedure as for blood samples, to prepare for LC–MS/MS analysis. The azithromycin analytical curve was constructed with 10 nonzero standards by plotting the peak area ratio of azithromycin to the internal standard versus the sample concentration, using samples from un-treated P7 controls. The concentration ranges evaluated were from 1 to 1000 ng/g (brain) and 1 to 2500 ng/ml (blood). A blank sample (matrix sample processed without internal standard) was used to exclude contamination or interference.
Blood (ng/ml) and brain tissue (ng/g) azithromycin concentrations were determined by the LC–MS/MS method developed and validated for this study. The LC–MS/MS method consisted of a Shimadzu LC-20AD HPLC system (Kyoto, Japan), and chromatographic separation of the tested compound was achieved using a Waters XBridage reverse phase C18 column (5 cm × 2.1 mm I.D., packed with 3.5 μm) at 25 °C. Five microliters of the supernatant was injected. The flow rate of gradient elution was 0.4 ml/min with mobile phase A (0.1% formic acid in purified deionized water) and mobile phase B (0.1% formic acid in acetonitrile). An AB Sciex QTrap 4500 mass spectrometer equipped with an electrospray ionization source (ABI-Sciex, Toronto, Canada) in the positive-ion multiple reaction monitoring (MRM) mode was used for detection. Protonated molecular ions and the respective ion products were monitored at the transitions of m/z 749.5 > 591.4 for azithromycin and 455.2 > 425.2 for the internal standard. We adjusted the instrument settings to maximize analytical sensitivity and specificity of detection. Data were processed with software Analyst (version 1.6).
Data analysis:
Rectal temperatures and body weights over time were compared among groups by repeated measures ANOVA (Prism 7.00, GraphPad Software, San Diego, CA). ANOVA or repeated measure ANOVA, factoring treatment and sex, was applied to evaluate sensorimotor testing measures. ANOVA, factoring region and treatment, was applied to compare cerebral hemisphere and regional volumes. Hemisphere weights, and total percentage right forebrain damage calculated from regional volumes were compared with ANOVA factoring treatment and sex. A non-parametric population pharmacokinetic approach was used to model the azithromycin concentration-time data (PMetrics® library, Laboratory of Applied Pharmacokinetics and Bioinformatics, University of Southern California, Los Angeles, CA) (16).
Results
No adverse effects of azithromycin treatment were identified. Azithromycin had no impact on body temperature for up to 24 h after HI (see Supplemental Data Table S1 (online)). Mortality was low (<5%) in all azithromycin-treated groups and in saline controls, and weight gain did not differ between controls and any azithromycin group (see Supplemental Data Table S2 (online)).
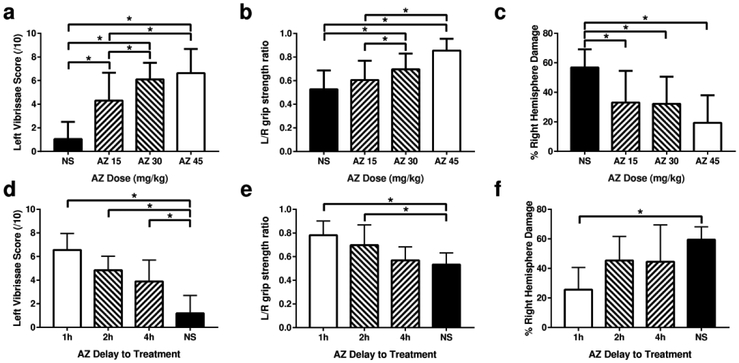
In single-injection studies, we found dose- and time-dependent attenuation of functional deficits and severity of brain damage (Figure 1). At the earliest administration time point examined (15 min after HI), we found dose-dependent attenuation of contralateral (left forepaw) functional deficits and right cerebral hemisphere tissue damage over the dose range tested (15-45 mg/kg; Figure 1, p<0.0001 ANOVA with post hoc test for trend). When a single azithromycin dose (45 mg/kg) was administered at times varying from 15 min to 4h after HI, neuroprotective efficacy waned as the time-delay increased (Figure 1, p<0.001 ANOVA with post hoc test for trend). Forepaw placing scores differed from controls even up to a 4-hour delay, whereas improvements in grip strength ratios waned earlier (remaining significant, p <0.05 in the 2h delay group), as did hemisphere weight differences (differing from controls only in the 1h delay group, p<0.0005). There was no effect of animal sex on any outcome (see Supplemental Data Table S3 (online)).
Figure 1: Azithromycin (AZ) Neuroprotection is Dose- and Onset-time-dependent.
Postnatal day 7 (P7) rats underwent right carotid ligation, followed by 90 min in 8% O2 (hypoxia-ischemia, HI, see Methods) (n=6/group). To evaluate the AZ dose-response, AZ 15 mg/kg (AZ15), 30 mg/kg (AZ30) or 45 mg/kg (AZ45), or saline (NS) was injected intraperitoneally (i.p.) 15 min after the end of HI (a-c). To evaluate the impact of delayed-onset treatment, a single AZ dose (45 mg/kg) was injected i.p. 1 h (AZ 1h), 2 h (AZ 2h), or 4 h (AZ 4h) after HI; controls received saline injections (NS) at 1 h (d-f). In both groups of experiments, sensorimotor function was evaluated on P21 with lateral vibrissae-stimulated forepaw placing (10 trials/side, normal score = 10,a, d) and bilateral forepaw grip strength [3 trials/side, expressed as left/right (L/R) forepaw ratio, normal = 1,b, e). Animals were euthanized on P21. Cerebral hemispheres were weighed; % right hemisphere damage (weight loss, reflecting both initial tissue injury and impaired subsequent growth) was expressed as: 100*(left-right)/left,c, f). All 3 AZ doses attenuated sensorimotor deficits (a, b) and decreased the magnitude of right hemisphere tissue loss (c); protection was greater with higher doses (p<0.0001 ANOVA, with post-test for linear trend). Improvements in sensorimotor function declined with increasing duration delays of AZ treatment (d, e, p<0.0001 ANOVA, with post-test for linear trend); in the AZ 4h delay group, vibrissae scores were higher than in controls (d, *p<0.01 ANOVA with Dunnet’s multiple comparison test), but grip strength ratios were not (e). Significant attenuation of right hemisphere damage was limited to the AZ 1h delay group (f, *p<0.05 ANOVA with Dunnet’s multiple comparison test).
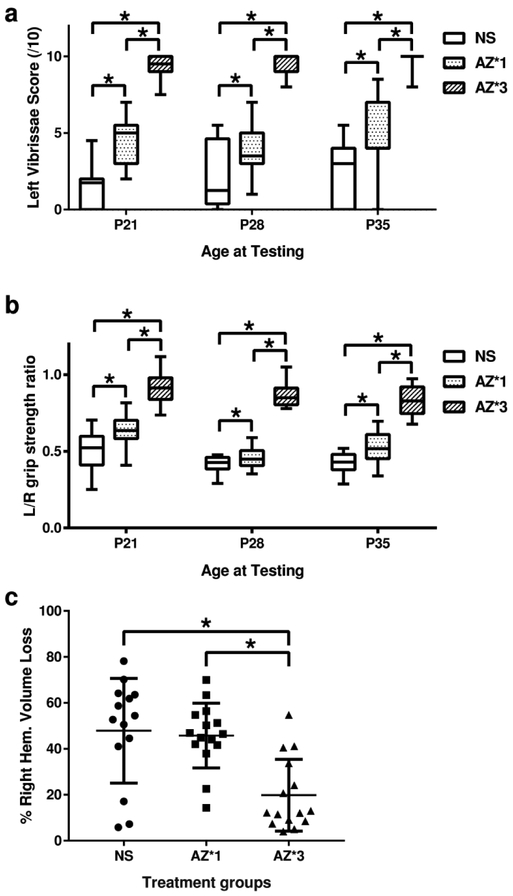
We next demonstrated that a three-dose azithromycin regimen that included 45 mg/kg delayed until 2h after the end of HI, and 22.5 mg/kg/dose at 24 and 48h, provided greater neuroprotection than 45 mg/kg, 2h post-HI, alone (which conferred intermediate benefit in preceding experiments). Contralateral sensorimotor function was superior (p<0.0001, RM-ANOVA Tukey multiple comparisons test; Figure 2) and right-hemisphere regional and total volume losses were attenuated in the 3-dose group relative to both saline controls and the single-dose regimen, (p<0.001 ANOVA with Tukey multiple comparisons test; Figure 2, Figure 3, Table 1). The 3-dose regimen attenuated ipsilateral damage in all 4 regions analyzed (p<0.01, Tukey post-hoc tests). There was no difference in treatment effects between sexes (see Supplemental Data Table S3 (online)).
Figure 2: Repeated Dosing Improves Azithromycin (AZ) Neuroprotection.
To determine if repeated AZ administration augments neuroprotection, sensorimotor and pathology outcomes were compared in P7 rats that underwent right carotid artery ligation, followed by 90 min in 8% O2(hypoxia-ischemia, HI) (n=15/group), and then received either one AZ injection 2 h after the end of HI (45 mg/kg; AZ*1), or a 3-injection regimen (45 mg/kg 2 h after HI; 22.5 mg/kg at 24 h and 48 h after HI; AZ*3); controls received saline (NS) injections 2 h, 24 h and 48 h after HI. Sensorimotor function was evaluated weekly (seeMethods) with lateral vibrissae-stimulated forepaw placing (10 trials/side, normal score = 10,a) and bilateral forepaw grip strength [3 trials/side, expressed as left/right (L/R) forepaw ratio, normal = 1,b). Animals were euthanized on P35; frozen, coronal brain sections were stained for digital morphometry and calculation of hemisphere (c) and regional volumes (seeMethods). Contralateral deficits were attenuated with both AZ regimens (a, b; * p<0.01, ANOVA with post-hoc Tukey test vs. NS); performance was superior with AZ*3 compared to AZ*1 (a, b; p<0.01, ANOVA with post-hoc Tukey test). Only the 3-injection AZ regimen (AZ*3) conferred significant attenuation of right hemisphere damage (c; * p<0.0001 ANOVA with post-hoc Tukey test).
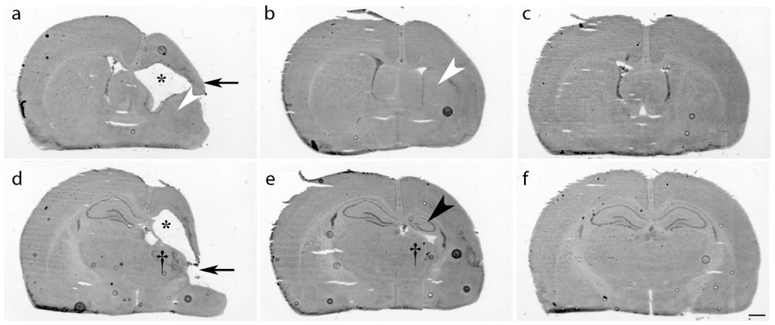
Figure 3: Dose-Dependent Azithromycin(AZ) Neuroprotection.
Postnatal day 7 (P7) rats underwent right carotid artery ligation followed by 90 min in 8% O2. Two hours later, treatment was initiated with one of three regimens: saline (2h, 24h and 48h after HI; a, d), AZ 45 mg/kg i.p. once (b, e), or a 3-dose AZ regimen [45 mg/kg i.p. 2 h after HI, then 22.5 mg/kg at 24 h and 48 h (c, f)]. Coronal cresyl violet-stained sections from P35, at the level of striatum (a-c), and dorsal hippocampus (d-f) illustrate typical features of right-hemisphere damage in each treatment group. In the control (a, d), note widespread injury with marked cortical thinning (arrow, a) and infarction (arrow, d), striatal tissue loss (a, white arrowhead), thalamic tissue loss (†, d) right dorsal hippocampus destruction (d), thinning of white matter tracts, and resultant ventriculomegaly (a, d, asterisks). In the single AZ dose treatment group, typical histopathology features (b, e) included cortical thinning, with atrophy of striatum (b, white arrowhead), thalamus (†, e) and dorsal hippocampus (e, black arrowhead), but substantially greater tissue preservation than in controls. In contrast, in the 3-AZ dose treatment group (c, f), typical abnormal findings were limited to subtle reductions in right hemisphere cross-sectional areas at both sectioning levels, demonstrating widespread preservation of grey and white-matter structures. Scale bar = 1 mm.
Table 1.
: Three-dose Azithromycin (AZ) regimen is superior to a single-dose regimen, after neonatal cerebral hypoxia-ischemia on P7: Regional volumes and % damage on P 35.
| Treatment | Number of Injections |
Time(s) of injection (h after HI) |
n | death | Side | Regional Volume a (mean ±SD, mm3) and LR % diff. a (mean ±SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | Striatum | Hippocampus | Other b | ||||||||||
| Volume | % Diff. | Volume | % Diff. | Volume | % Diff. | Volume | % Diff. | ||||||
| Saline | 3 | 2, 24, 48 | 15 | 1 | Left | 208 ±16 | 86 ±8 | 27 ±7 | 259 ±22 | ||||
| Right | 100±60 | 52±27 | 30 ±18 | 65 ±21 | 8 ±5 | 69 ±25 | 164 ±56 | 37 ±21 | |||||
| AZ | 1 | 2 | 15 | 0 | Left | 204 ±18 | 83 ±8 | 27±3 | 252 ±27 | ||||
| Right | 107±37 | 48 ±16 | 30±10 | 64±14 | 10 ±6 | 64 ±24 | 160 ±36 | 36 ±13 | |||||
| AZ * | 3 | 2, 24, 48 | 15 | 0 | Left | 214±18 | 86 ±11 | 28±4 | 260 ±20 | ||||
| Right | 170 ±36† | 20±17* | 56 ±13 | 34±18* | 20±8 | 29 ±26* | 226±47† | 14 ±14* | |||||
P7 rats underwent right carotid artery ligation, and were exposed to 8% oxygen (90 min) (see Methods). Abbreviations: P: post-natal day; HI: hypoxia-ischemia; AZ: Azithromycin.
Bilateral regional brain volumes were estimated by regional morphometry from coronal sections (13). Volumes, expressed as means ± SD, were compared by RM ANOVA, factoring region and treatment, with region as repeated measure. Left(L)-Right(R) %Difference (% Diff.), a measure of % right hemisphere tissue volume loss, i.e. of damage severity, was calculated for each region from bilateral volumes as 100*(L-R)/L.
“Other” represents all other intact hemisphere tissue that was not designated as either cortex, striatum or hippocampus, and includes grey matter regions (e.g. thalamus, globus pallidus, septum) and white matter tracts.
Percent left-right difference in regional volumes was lower overall in the Azithromycin 3-injection group than in controls, or in the Azithromycin singleinjection group, and for all 4 regions by post-hoc testing (p<0.01, RM ANOVA factoring treatment and region; with Tukey multiple comparison test).
In additional post-hoc testing to evaluate regional variation in treatment effects, right-sided regional volumes were greater in the Azithromycin 3-injection group than in controls, or in the Azithromycin single-injection group (p<0.0001, RM ANOVA, with Tukey multiple comparison test), for Cortex and “Other” b.
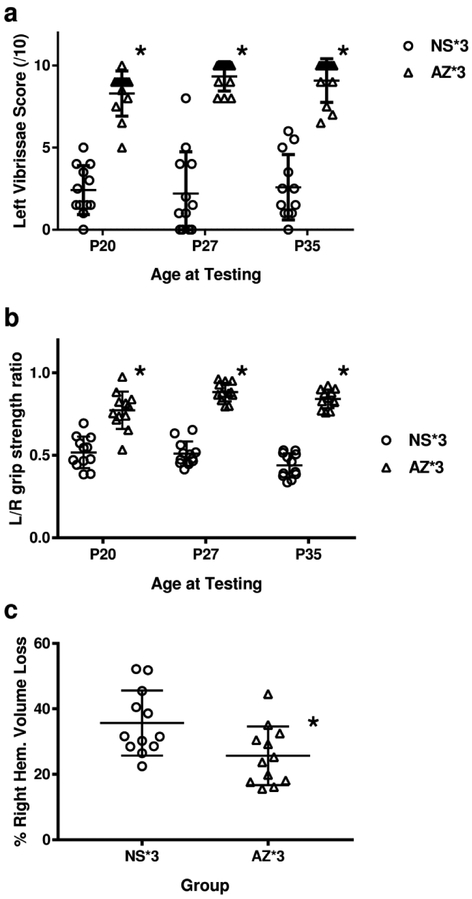
The same delayed-initiation 3-dose regimen was re-evaluated in P10 rats after moderate HI (60 min) (13). Contralateral forepaw placing and grip-strength deficits were attenuated (p< 0.0001 repeated-measured ANOVA; Figure 4 A., B.), and both right hemisphere volume loss (p<0.05, t-test; Figure 4C.) and regional volume losses (p< 0.05, RM-ANOVA; Table 2) were reduced in the 3-dose azithromycin group (vs. saline controls). There were no differences in treatment effects between sexes.
Figure 4: Azithromycin (AZ) improves outcomes after hypoxia-ischemia (HI) in P10 rats.
P10 rats underwent right carotid artery ligation, followed by 60 min in 8% O2 (n=12/group). AZ was administered in a 3-injection regimen (45 mg/kg 2 h after HI; 22.5 mg/kg at 24 h and 48 h after HI; AZ*3); controls received saline (NS) injections at the same time-points. Sensorimotor function was evaluated (on P20, P27, P35) with lateral vibrissae-stimulated forepaw placing (10 trials/side, normal score = 10, a) and bilateral forepaw grip strength (3 trials/side, expressed as left/right (L/R) forepaw ratio, normal = 1, b). Animals were euthanized on P35. Right hemisphere damage was evaluated in coronal brain sections by digital morphometry (see Methods). AZ*3 attenuated contralateral deficits (p<0.0001, Repeated-Measures ANOVA; a, b), and right cerebral hemisphere damage (c; *p<0.025, t-test).
Table 2:
Azithromycin (AZ) treatment is neuroprotective after neonatal cerebral hypoxia-ischemic on postnatal day 10: Regional volumes and percent damage on postnatal day 35.
| Treatment | Number of Injections |
Time(s) of injection (hours after HI) |
n | death | Side | Regional Volume a (mean ±SD, mm3) and LR % diff. a (mean ±SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortex | Striatum | Hippo campus |
Other b | ||||||||||
| Volume | % diff. | Volume | % diff. | Volume | % diff. | Volume | % diff. | ||||||
| Saline | 3 | 2, 24, 48 | 12 | 1 | Left | 210±10 | 82±7 | 31±5 | 260±19 | ||||
| Right | 132±34 | 36 ±16 | 34±10 | 58±12 | 11±5 | 64±14 | 197±21 | 24±7 | |||||
| AZ * | 3 | 2, 24, 48 | 12 | 0 | Left | 216±12 | 85±6 | 30±3 | 264±13 | ||||
| Right | 170±27† | 22±12† | 41±6 | 52±8 | 13±4 | 56 ±11 | 219±2† | 17±8 | |||||
P10 rats underwent right carotid artery ligation, and were exposed to 8% oxygen (60 min) (see Methods).
Bilateral regional volumes were estimated by regional morphometry from coronal brain sections (13). Volumes, expressed as means ± SD, were compared by RM ANOVA, factoring region and treatment, with region as repeated measure. Left(L)-Right(R) % Difference (% Diff.), a measure of right hemisphere tissue volume loss, i.e. of damage severity, was calculated for each region from left and right-side volumes as 100*(L-R)/L.
“Other” represents all other intact hemisphere tissue that was not designated as either cortex, striatum or hippocampus. This includes grey matter regions (e.g. thalamus, globus pallidus, septum) and white matter tracts.
Abbreviations: HI, hypoxia-ischemia; AZ, Azithromycin
Percent left-right difference in regional volumes was lower in the Azithromycin 3-injection group than in controls (p<0.05, ANOVA, factoring treatment and region, with region as repeated measure).
In additional post-hoc testing for regional variation in treatment effects, right hemisphere regional volumes were greater in the Azithromycin 3-injection group than in controls, for Cortex and “Other”† (p<0.01, Tukey multiple comparison test); similarly, regional percent left-right difference was lower in the Azithromycin 3-injection group, for cortex† (p<0.01, Tukey multiple comparison test).
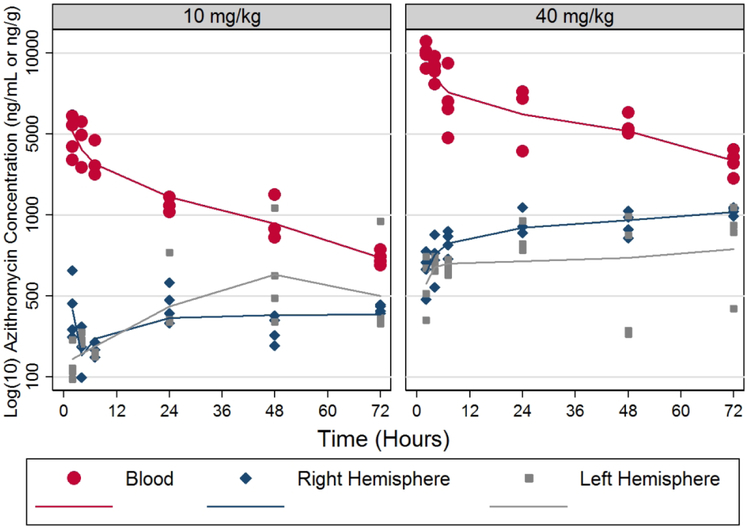
Figure 5 illustrates the concentration-time profile of azithromycin in whole blood and brain tissue from animals that had undergone HI lesioning. Values were approximately two-fold higher in blood and brain at 40 mg/kg compared to 10 mg/kg doses. The terminal elimination half-life in blood was similar between doses (44-47 hours). Brain concentrations were similar in both brain hemispheres and reached near maximal concentration approximately 24 hours after dosing.The two-compartment base model provided the best fit to the whole blood data, compared to models of higher complexity. The estimated volume of distribution was lower for the right compared to the left hemisphere. The ratios of transfer rate constants from central compartment to brain tissue were 0.91 (right hemisphere) and 1.25 (left hemisphere). The areas under the concentration-time curves were similar for both brain hemispheres by dose. These findings suggest a small but measurable shift in the rate but not extent of azithromycin distribution between the right and left hemispheres, perhaps attributable to acute right hemisphere injury.
Figure 5: Azithromycin (AZ) Pharmacokinetics.
AZ levels were measured in blood and brain samples from postnatal day 7 (P7) rats that underwent right carotid ligation, followed by 90 min in 8% O2 (hypoxia-ischemia, HI, see Methods) and received AZ injections (10 or 40 mg/kg i.p.) 15 min after HI. Blood and brain (right hemisphere) samples were collected at 2, 4, 7, and 24 h after injections. Blood (ng/mL) and tissue (ng/g) AZ concentrations were measured by LC–MS/MS (see Methods). Samples from non-lesioned, non-injected animals were used to generate standard curves and for negative controls. Blood concentrations (ng/mL) best fit a 2-compartment linear model, with terminal phase t ½ ~10 hours.
Discussion
In this neonatal rat model of hypoxic-ischemic brain injury, rescue (i.e. post-hypoxia-ischemia) treatment with azithromycin improved functional outcomes and reduced the extent of brain damage. We found no treatment-related adverse effects or any outcome differences between males and females. These results are congruent with reports of azithromycin-mediated neuroprotection in adult rodent stroke (1) and spinal-cord (2) injury models.
Azithromycin’s efficacy was dose-dependent and diminished with delay to initiation of treatment; in a single-dose protocol, improvement in sensorimotor function was retained with treatment administered up to 4 hours after HI. More sustained drug treatment, with a three-dose regimen, beginning 2h after HI, and two subsequent doses 24 and 48 h later, was more effective than the initial dose, alone.
Evaluation of safety outcomes was limited to comparisons of body temperature, weight gain, and survival among groups; no adverse effects were detected. Similarly, no treatment regimen unexpectedly amplified functional deficits or brain damage.
We focused on delayed initiation treatment protocols in view of their potential clinical relevance for applications in the of setting of neonatal resuscitation and post-resuscitation stabilization after birth asphyxia. Of potential translational relevance for treatment of HI brain injury in low-resource settings, azithromycin was beneficial in the absence of concurrent or subsequent hypothermia treatment. The original description of the neonatal HI brain injury model included 7-day-old (P7) rats. The precise equivalence between rodent and human brain maturational stage is challenging to ascertain, and some investigators suggest that P10 rat brain development more closely approximates the term human stage (13). To address possible maturational stage related factors, we replicated an effective repeated-dose protocol in P10 rats, and outcomes were similar.
The sensorimotor outcome measures that were studied are pragmatic but do not replicate the complex repertoire of human sensorimotor deficits after hypoxic-ischemic encephalopathy (HIE). Both vibrissae-stimulated forepaw placement and grip strength measures provide reproducible quantifiable measures of lateralized deficits, contralateral to the injured cerebral hemisphere. In controls, deficits of similar magnitude persisted over a one month recovery period, and in the 3-dose azithromycin groups, functional improvements were sustained over the same testing period. The precise neuroanatomical correlates that underlie improved sensorimotor function in azithromycin-treated groups are uncertain, and we could not distinguish whether better performance primarily reflected prevention of initial tissue damage and/or enhanced functional recovery.
Azithromycin is a particularly attractive candidate drug for repurposing for neonatal neuroprotection since its safety and efficacy have already been evaluated in human neonatal studies. Phase 1 and 2 trials established pharmacokinetics and examined whether azithromycin treatment eradicated ureaplasma infection and decreased the incidence of chronic lung disease of prematurity (7, 8). In these studies, as in the rodent data, a 3-dose regimen was more effective than a single dose, and no adverse effects were associated with either regimen.
Complementary clinical evidence regarding neonatal azithromycin exposure stems from its increasing utilization in obstetric practice. Azithromycin, which crosses the placenta to a limited extent, may be administered intravenously to pregnant women prior to Cesarean section, or to initiate treatment of preterm prolonged rupture of membranes. It accumulates in the fetus and is excreted in breast milk for an extended period after peripartum administration (3-5, 17, 18). Although systematic evaluation of neonatal outcomes in these settings have been limited, no adverse effects have been discerned. A randomized trial of oral azithromycin during labor in a low-resource setting reported decreased colonization of newborns with typical neonatal pathogens (19), and no deleterious treatment-attributable effects.
Body-weight based drug doses may differ substantially between rodents and humans; yet, neonatal rat azithromycin blood levels with doses of 10 or 40 mg/kg were in a similar range as azithromycin blood concentrations reported in phase 1 and 2 studies in very low birthweight human premature neonates who received clinical-range azithromycin doses of 10 or 20 mg/kg intravenously (8). No adverse effects were reported in those relatively small human neonatal studies.
With the 40 mg/kg neonatal rat azithromycin dose, that yielded blood levels similar to human regimens, brain azithromycin brain concentrations were similar to those reported to decrease brain inflammation in a murine Toxoplasma encephalitis model (20). Evidence that azithromycin persists in neonatal rat brain for several days after a single systemic injection, and long after blood levels have declined, is consistent with reports that azithromycin accumulates in many tissues, including brain, and is eliminated slowly (21-24).
This study did not address mechanisms of neuroprotection. Based on evidence from other models (1, 25, 26) we speculate that the primary sites of azithromycin action include microglia and/or monocyte-derived macrophages. Initial studies of microglia in neonatal brain injury models characterized morphological changes, e.g. enlarged cell bodies, interpreted as evidence of injury-induced activation (25). Since activated microglia-macrophages secrete a variety of neurotoxic substances (26), these observations implicated activated microglia as cellular mediators of ischemic brain injury. Yet considerable evidence has also emerged indicating that microglia may contribute to injury repair and recovery, both in neonatal (27) and adult brain (28-30). Understanding of distinct roles of pro-inflammatory, injury-amplifying (classified as M1) vs. injury resolving (M2) phenotypes of microglia/macrophage in the pathogenesis of CNS injury vs. recovery is incomplete (31). Evidence that azithromycin promotes macrophage antiinflammatory, M2, phenotype expression (1, 32, 33) supports the hypothesis that its neuroprotective properties in the neonatal hypoxia-ischemia brain injury model are mediated by promotion of microglial and/or monocyte M2 properties.
This study has several additional limitations. In this neonatal rodent model of hypoxic-ischemic brain injury, metabolic measures (e.g. blood glucose) and physiologic parameters (e.g. blood pressure) are not rigorously controlled, seizures are not monitored or treated, drugs are injected p., rather than intravenously, and, animals are otherwise healthy, whereas neonates with HIE often have multi-organ injury and receive multiple concurrent medications. Relevant to azithromycin’s potential to cause prolongation of the QT interval, our animals did not undergo electrocardiographic monitoring; mortality rates were very low, even at highest doses tested.
Our findings lay the groundwork for a broad range of future studies. It would be of interest to evaluate pre-HI azithromycin administration, a scenario that could be clinically feasible with intra-partum drug administration. It would similarly be of interest to examine the efficacy of azithromycin in a model of inflammation-sensitized hypoxic-ischemic injury, particularly since intrauterine infection (e.g. chorioamnionitis, funisitis) is a common co-morbidity of fetal hypoxia-ischemia (34, 35). The safety and neuroprotective efficacy of azithromycin in combination with post hypoxic-ischemic hypothermia would be most effectively evaluated in a larger animal model, where prolonged hypothermia, replicating typical clinical intervention, would be feasible; this would be warranted prior to clinical translation, as both azithromycin and therapeutic hypothermia can prolong the QT interval and increase risk of cardiac dysrhythmia (36-38). An additional rationale for replication in another species is confirmation of neuroprotective efficacy; there have been discrepant trends with respect to efficacy reported in neonatal HI models in different species, for example in rats vs. mice treated with minocycline (39, 40).
In conclusion, azithromycin shows promise as a rescue treatment for neonatal hypoxic-ischemic encephalopathy. Important directions for future research that could support translation to clinical trials include further evaluation, in combination with therapeutic hypothermia, assessment of safety and efficacy in complementary larger mammalian models of perinatal cerebral hypoxia-ischemia, and assessment of efficacy in models of inflammation-sensitized hypoxic-ischemic brain injury.
Supplementary Material
Acknowledgments
Statement of Financial Support:
Research reported in this publication was supported by the Michigan Institute for Clinical & Health Research (MICHR) that is supported by the National Institutes of Health under award number UL1TR002240. Research was also supported by the National Institutes of Health under award number R21 HD096251 (to J.D.E.B. and F.S.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the Neonatal Therapeutic Hypothermia Gift Fund, and The Reiter HIE Research Fund.
Footnotes
Disclosure:
J.D.E.B., Y.L., M.P., L.W. and F.S.S. declare no conflict of interest.
References
- 1.Amantea D, Certo M, Petrelli F, et al. 2016. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp Neurol 275 Pt 1:116–125. [DOI] [PubMed] [Google Scholar]
- 2.Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM 2017. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep 7:40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton AL, Acosta EP, Larson KB, Kerstner-Wood CD, Tita AT, Biggio JR 2015. Perinatal pharmacokinetics of azithromycin for cesarean prophylaxis. Am J Obstet Gynecol 212:812.e811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemp MW, Miura Y, Payne MS, et al. JP 2014. Maternal intravenous administration of azithromycin results in significant fetal uptake in a sheep model of second trimester pregnancy. Antimicrob Agents Chemother 58:6581–6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tita AT, Szychowski JM, Boggess K, et al. 2016. Adjunctive Azithromycin Prophylaxis for Cesarean Delivery. N Engl J Med 375:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oluwalana C, Camara B, Bottomley C, et al. 2017. Azithromycin in Labor Lowers Clinical Infections in Mothers and Newborns: A Double-Blind Trial. Pediatrics 139:e20162281. [DOI] [PubMed] [Google Scholar]
- 7.Viscardi RM, Othman AA, Hassan HE, et al. 2013. Azithromycin to prevent bronchopulmonary dysplasia in ureaplasma-infected preterm infants: pharmacokinetics, safety, microbial response, and clinical outcomes with a 20-milligram-per-kilogram single intravenous dose. Antimicrob Agents Chemother 57:2127–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchan LM, Hassan HE, Terrin ML, et al. 2015. Pharmacokinetics, microbial response, and pulmonary outcomes of multidose intravenous azithromycin in preterm infants at risk for Ureaplasma respiratory colonization. Antimicrob Agents Chemother 59:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AL, Alexander M, Rosenkrantz TS, Sadek ML, Fitch RH 2014. Sex differences in behavioral outcome following neonatal hypoxia ischemia: insights from a clinical metaanalysis and a rodent model of induced hypoxic ischemic brain injury. Exp Neurol 254:54–67. [DOI] [PubMed] [Google Scholar]
- 10.Chavez-Valdez R, Martin LJ, Razdan S, Gauda EB, Northington FJ 2014. Sexual dimorphism in BDNF signaling after neonatal hypoxia-ischemia and treatment with necrostatin-1. Neuroscience 260:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andine P, Thordstein M, Kjellmer I, et al. 1990. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods 35:253–260. [DOI] [PubMed] [Google Scholar]
- 12.Barks JD, Liu Y, Shangguan Y, Djuric Z, Ren J, Silverstein FS 2017. Maternal high-fat diet influences outcomes after neonatal hypoxic-ischemic brain injury in rodents. J Cereb Blood Flow Metab 37:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SD, Pierce L, Ciardiello A, et al. 2015. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: characterization of a translational preclinical model. Pediatr Res 78:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishna S, Hutton A, Aronowitz E, Moore H, Vannucci SJ 2018. The effects of adding prophylactic phenobarbital to therapeutic hypothermia in the term-equivalent hypoxic-ischemic rat. Pediatr Res 83:506–513. [DOI] [PubMed] [Google Scholar]
- 15.Felt BT, Schallert T, Shao J, Liu Y, Li X, Barks JDE 2002. Early appearance of functional deficits after neonatal excitotoxic and hypoxic-ischemic injury: fragile recovery after development and role of the NMDA receptor. Dev Neurosci 24:418–425. [DOI] [PubMed] [Google Scholar]
- 16.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD 2003. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol 188:714–718. [DOI] [PubMed] [Google Scholar]
- 18.Salman S, Davis TM, Page-Sharp M, et al. 2015. Pharmacokinetics of Transfer of Azithromycin into the Breast Milk of African Mothers. Antimicrob Agents Chemother 60:1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roca A, Oluwalana C, Bojang A, et al. 2016. Oral azithromycin given during labour decreases bacterial carriage in the mothers and their offspring: a double-blind randomized trial. Clin Microbiol Infect 22:565 e561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumas JL, Chang R, Mermillod B, Piguet PF, Comte R, Pechere JC 1994. Evaluation of the efficacy of prolonged administration of azithromycin in a murine model of chronic toxoplasmosis. J Antimicrob Chemother 34:111–118. [DOI] [PubMed] [Google Scholar]
- 21.Foulds G, Shepard RM, Johnson RB 1990. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 25 Suppl A:73–82. [DOI] [PubMed] [Google Scholar]
- 22.Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, Phuenpathom N, Tussanasunthornwong S 1996. Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother 40:825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shepard RM, Falkner FC 1990. Pharmacokinetics of azithromycin in rats and dogs. J Antimicrob Chemother 25 Suppl A:49–60. [DOI] [PubMed] [Google Scholar]
- 24.Davila D, Kolacny-Babic L, Plavsic F 1991. Pharmacokinetics of azithromycin after single oral dosing of experimental animals. Biopharm Drug Dispos 12:505–514. [DOI] [PubMed] [Google Scholar]
- 25.Ivacko JA, Sun R, Silverstein FS 1996. Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatr Res 39:39–47. [DOI] [PubMed] [Google Scholar]
- 26.Giulian D, Li J, Leara B, Keenen C 1994. Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int 25:227–233. [DOI] [PubMed] [Google Scholar]
- 27.Faustino JV, Wang X, Johnson CE, et al. 2011. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci 31:12992–13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sica A, Mantovani A 2012. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szalay G, Martinecz B, Lenart N, et al. 2016. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 7:11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin WN, Shi SX, Li Z, et al. 2017. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab 37:2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellstrom Erkenstam N, Smith PL, Fleiss B, et al. 2016. Temporal Characterization of Microglia/Macrophage Phenotypes in a Mouse Model of Neonatal Hypoxic-Ischemic Brain Injury. Front Cell Neurosci 10:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy BS, Sundareshan V, Cory TJ, Hayes D Jr., Anstead MI, Feola DJ 2008. Azithromycin alters macrophage phenotype. J Antimicrob Chemother 61:554–560. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC 2015. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation 12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tann CJ, Nakakeeto M, Willey BA, et al. 2018. Perinatal risk factors for neonatal encephalopathy: an unmatched case-control study. Arch Dis Child Fetal Neonatal Ed 103:F250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker SJ, Kuzniewicz M, Niki H, Wu YW 2018. Antenatal and Intrapartum Risk Factors for Hypoxic-Ischemic Encephalopathy in a US Birth Cohort. J Pediatr 203:163–169. [DOI] [PubMed] [Google Scholar]
- 36.2013. FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. U.S. Food & Drug Administration, www.fda.gov. [Google Scholar]
- 37.Battin MR, Penrice J, Gunn TR, Gunn AJ 2003. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics 111:244–251. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Lu M, Zhang C, et al. 2017. Therapeutic hypothermia increases the risk of cardiac arrhythmia for perinatal hypoxic ischaemic encephalopathy: A meta-analysis. PLoS One 12:e0173006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM 2002. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol 52:54–61. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji M, Wilson MA, Lange MS, Johnston MV 2004. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol 189:58–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.