Abstract
Videofluoroscopic swallow studies are widely used in clinical and research settings to assess swallow function and to determine physiological impairments, diet recommendations, and treatment goals for people with dysphagia. Videofluoroscopy can be used to analyze biomechanical events of swallowing, including hyoid bone displacement, to differentiate between normal and disordered swallow function. Previous research has found significant associations between hyoid bone displacement and penetration/aspiration during swallowing, but the predictive value of hyoid bone displacement during swallowing has not been explored. The primary objective of this study was to build a model based on aspects of hyoid bone displacement during swallowing to predict the extent of airway penetration or aspiration during swallowing. Aspects of hyoid bone displacement from 1433 swallows from patients referred for videofluoroscopy were analyzed to determine which aspects predicted risk of penetration and aspiration according to the Penetration-Aspiration Scale. A generalized estimating equation incorporating components of hyoid bone displacement and variables shown to impact penetration and aspiration (such as age, bolus volume, and viscosity), was used to evaluate penetration and aspiration risk. Results indicated that anterior-horizontal hyoid bone displacement was the only aspect of hyoid bone displacement predictive of penetration and aspiration risk. Further research should focus on improving the model performance by identifying additional physiological swallowing events that predict penetration and aspiration risk. The model built for this study, and future modified models, will be beneficial for clinicians to use in the assessment and treatment of people with dysphagia, and potentially to track improvement in hyolaryngeal excursion resulting from dysphagia treatment, thus mitigating adverse outcomes that can occur secondary to dysphagia.
Keywords: hyoid bone, penetration/aspiration, deglutition, generalized estimation equation, deglutition disorders
1. Introduction
Dysphagia affects approximately one in twenty-five adults in the United States annually [1–5]. It can occur in patients secondary to a variety of etiologies such as stroke, Parkinsons disease [6], head and neck cancer, and brain injuries [7], as well as many other neurological, iatrogenic, and developmental conditions. Aspiration may occur because of dysphagia, which can lead to adverse outcomes including aspiration pneumonia, malnutrition, and dehydration [8–11]. Additionally, dysphagia and secondary medical complications often result in reduced quality of life for patients. Because of this, it is necessary to rapidly identify dysphagia and determine aspiration risk of patients through timely diagnosis and management.
Videofluoroscopy (VF) is one instrumental evaluation tool used to assess physiological impairments of swallowing and reduced airway protection[12,13]. Clinicians rely on subjective interpretation of the biomechanical events of swallowing observed during VF to determine patient risk of penetration and aspiration. Standardized tools exist for categorizing swallow events during VF, such as the Modified Barium Swallow Impairment Profile (MBSImP) and the Penetration-Aspiration Scale (PAS). The MBSImP is a widely used clinical tool that allows clinicians to evaluate 17 physiological components of swallowing using a subjective ordinal scale. The PAS is an 8-point interval rating scale used to determine the severity of penetration and aspiration. The PAS is used to determine how far material enters the airway and whether or not patients are able to clear penetrated or aspirated material from the airway. While these tools are useful in identifying gross impairments in swallowing function, they involve subjectivity to quantify the degree of swallowing impairment. Swallow kinematic analysis is an objective way to quantify biomechanical events of swallowing, however it requires standardized training and experience to perform with high levels of intra- and inter-rater reliability and is not used in most settings.
VF exposes patients to radiation, which forces clinicians to conduct swallowing evaluations in a short period of time. Because of these time constraints, swallowing evaluations may not fully capture a patients risk of penetration and aspiration. For this reason, clinicians would benefit from having a tool that objectively and automatically measures physiological events that occur during swallowing, such as hyoid bone displacement, to more accurately quantify patient risk of penetration or aspiration.
While differences in hyoid bone displacement are known to exist among healthy, especially aging, individuals [14–16], is it known to be associated with an increased risk of penetration and aspiration [17] and can be measured in both horizontal and vertical planes, as described in previous research [18]. However, the exact relationship between hyoid bone displacement and penetration and aspiration risk remains unknown due to conflicting research [19–23].
We previously investigated six aspects of hyoid bone displacement using coordinates based on anatomical landmarks of the vertebral column. Results revealed that reduced anterior-horizontal displacement was the only aspect of hyoid bone displacement associated with higher scores on the PAS [24]. The primary aim of the current study was to determine which aspects of hyoid bone displacement predict the risk of penetration and aspiration. We also investigated the effect of patient demography and clinical variables in the model. We hypothesized that a predictive model would reasonably predict penetration and aspiration risk using aspects of hyoid bone displacement and important clinical variables that are associated with penetration and aspiration risk. To test this hypothesis, we built a generalized estimating equation (GEE) model by extracting aspects of hyoid bone displacement data from VF images.
2. Methods
2.1. Data Acquisition
Two hundred and sixty-five patients with suspected dysphagia were enrolled in this prospective study and underwent VF at the University of Pittsburgh Medical Center Presbyterian Hospital. The protocol for the study was approved by the Institutional Review Board at the University of Pittsburgh and all participants provided informed consent. Patients with tracheostomies or anatomical abnormalities of the head and neck were excluded from the study.
The data for this study was collected in the course of standard clinical care rather than solely for research purposes. We intentionally did not interfere with clinical decision-making in the conduct of the VF examinations. Clinicians who conducted VF modified the protocol for the administration of boluses (e.g. number of swallows, bolus consistencies, head positions, etc.) based on clinical hypotheses and the patients clinical presentation of dysphagia. The following consistencies were used: E-Z-EM Canada, Inc. Varibar thin (Bracco Diagnostics, Inc.) (<5cPs viscosity), Varibar nectar (300 cPs viscosity), Varibar pudding (5000 cPs viscosity), and Keebler Sandies Mini Simply Shortbread Cookies (Kellogg Sales Company). Clinicians administered boluses by spoon (3–5mL) or had participants self-administer a comfortable volume by cup. Head positions included neutral and chin down. Participant characteristics and methods for VF data collection can be found in Table 1.
Table 1.
Statistics and characteristics of patients involved in the investigation
| Features | Frequency(%) | Features | Frequency(%) | ||
|---|---|---|---|---|---|
| PA | 1 | 687(47.94%) | Viscosity & Volume | thin liquid by teaspoon | 264(18.4%) |
| 2 | 442(30.84%) | thin liquid by cup | 614(42.8%) | ||
| 3 | 138(9.63%) | not recorded utensil with nectar | 1(0.007%) | ||
| 4 | 48(3.35%) | nectar by teaspoon | 195(13.6%) | ||
| 5 | 29(2.02%) | nectar by cup sip | 209(14.6%) | ||
| 6 | 33(2.30%) | pudding by spoon | 94(6.6%) | ||
| 7 | 23(1.61%) | cookie | 42(2.9%) | ||
| 8 | 33(2.30%) | Gender | male | 155(58.49%) | |
| Type | single | 498(34.73%) | female | 110(41.51%) | |
| multiple(1) | 360(25.10%) | Head Position | neutral | 1136(79.22%) | |
| multiple(2) | 534(37.24%) | chin down | 252(17.57%) | ||
| not record | 42(2.93%) | not record | 46(3.21%) | ||
VF was conducted in the lateral plane using a 30PPS pulse rate and recorded at 60FPS by a video card (AccuStream Express HD, Foresight Imaging, Chelms-ford, MA) and recorded to a hard drive with a LabVIEW program. Videos were converted into digital movie clips of 720 × 1080 resolution and then down-sampled to 30 frames per second to eliminate duplicate frames.
2.2. Image Analysis
Over 3000 video clips were obtained from VF swallow evaluations. The final data set used for analysis included 1434 video clips because over half of the original clips were unacceptable for tracking hyoid bone displacement due to poor image quality or obstruction of hyoid bone landmarks by the shoulder or other medical equipment such as cardiac monitor lines, pacemaker leads, etc. Videos were segmented into individual swallow events based on the frame in which the head of the bolus reached the ramus of the mandible (onset), and the frame in which the bolus tail passed the upper esophageal sphincter (UES) (offset) [25]. Swallows were categorized into single (one swallow per bolus), multiple 1 (two swallows per bolus), and multiple 2 (more than two swallows per bolus).
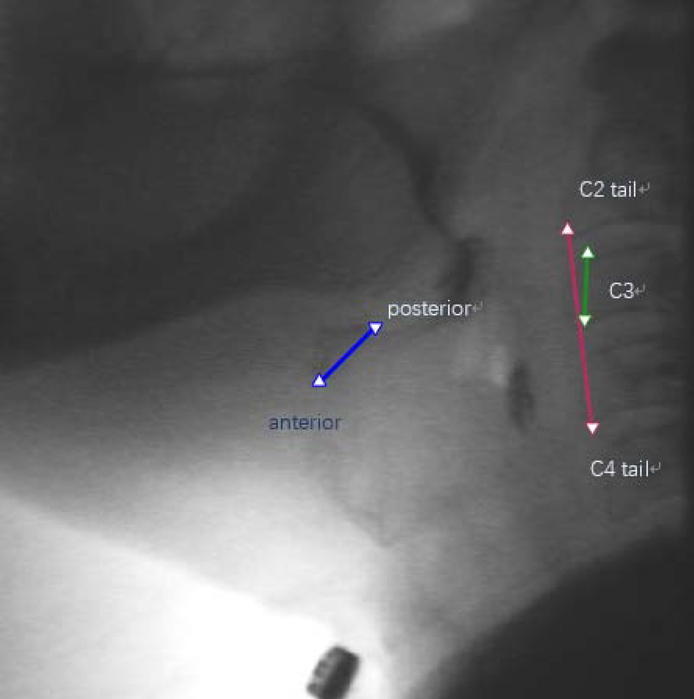
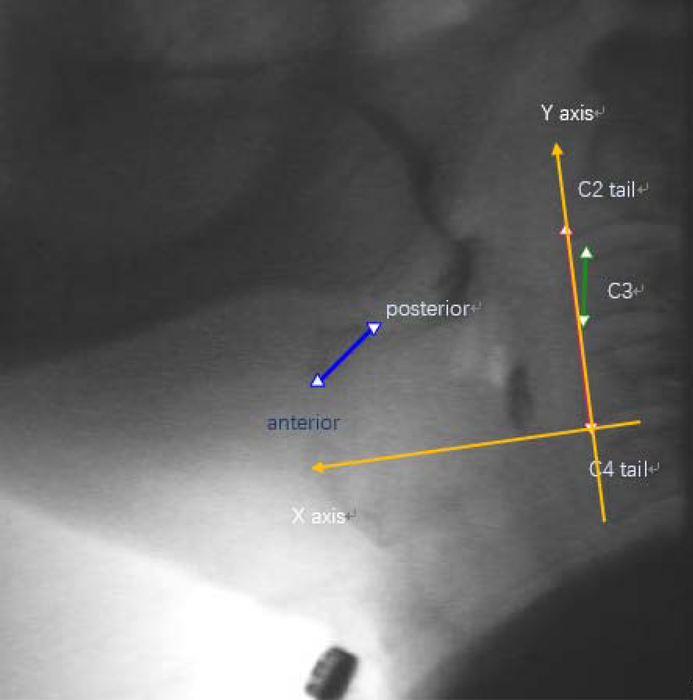
As shown in Fig. 1, an expert judge, trained in swallow kinematic rating, initially identified the following points of interest in each video frame: (1) anterior-inferior corner of C2 vertebral body; (2) anterior-inferior corner of the C4 vertebral body; (3) anterior-inferior corner of the body of the hyoid bone; (4) posterior-superior corner of the body of the hyoid bone; (5) anterior-inferior corner of C3 vertebral body; (6) anterior-superior corner of C3 vertebral body. The anterior-inferior corner of the C4 vertebral body (2) was defined as the origin. The straight line connecting (2) and (1) was defined as the y-axis. The x-axis was defined as the horizontal line perpendicular to the y-axis and intersecting with (2). To normalize patients with different heights to a common anatomical referent, the anatomical scaling factor for displacement measures was defined as the length between (5) and (6) (i.e. the height of the C3 vertebral body). Image pixels were used to measure distance.
Fig. 1.
The landmarks for hyoid bone, C2, C3, C4 and established coordinate.
Three raters trained in swallow kinematic analysis identified anatomical points of interest in each of the 1434 swallows, and tracked hyoid displacement using frame-by-frame analysis in MATLAB (R2015b, The MathWorks, Inc., Natick, MA, USA). Reliability was established on 10% of the videos with ICCs of over .99 and intra-rater reliability was maintained throughout testing to avoid judgment drift. Two clinicians trained in PAS analysis established a priori inter- and intra-rater reliability with ICCs of 0.99. All raters were blinded to participant age, sex and diagnosis.
2.3. Statistical Analysis
SAS ® version 9.3 (SAS Institute, Inc., Cary, North Carolina) was used for all statistical analyses with the GENMOD procedure for obtaining the main results. A dichotomous (normal; disordered) operational definition of PAS scores (1–2, and 3–8 respectively) was used for analyses, because there was a skewed distribution of PAS scores. Logistic regression models that are typically used with dichotomous data could not be used, because the independence criterion was not met due to having multiple swallows in the data set from each patient. Therefore, a GEE model [26] with a binomial distribution, a logit link function, and an exchangeable working correlation structure (which is an extension of a logistic regression model suitable for analyzing auto-correlated data) was used. Age, gender, swallow type (single/multiple 1/multiple 2), viscosity (thin/nectar/pudding/cookie), utensil (cup/spoon), head position (neutral/chin down), and swallow duration were used as forced-in independent variables based on face validity and prior knowledge of their dependence on PAS scores. In addition to these independent variables, we examined various aspects of hyoid bone displacement using a forward selection strategy with an entry criterion of p <0.05. The measurement of these landmarks (superior hyoid hone and anterior hyoid bone) includes maximal displacement, maximal peak position, velocity, acceleration and duration in horizontal and vertical direction. To assess the predicted and observed disordered PAS scores, we created a contingency table based on the predicted probability deciles. The deciles were formed by sorting and separating the predicted probabilities into ten subgroups based on each patients risk profile, from lowest to highest risk (1–10). We examined the observed percentage of disordered PAS swallows (3–8) within each decile compared to the predicted percentage according to the model. See Appendix A for the predictive model.
3. Results
Table 1 illustrates the descriptive statistics and participant characteristics. The swallow analysis data was presented in this study for 1433 swallows from 265 distinct patients. Ninety-one swallows were excluded from the analysis due to missing information or incorrect recording. The age range of the subjects was from 19 to 94 and the average ± standard variation age was 64.8 ± 13.6 years. 1129 swallows had PA scores of 1 or 2 and 304 swallows had PA scores greater or equal to 3.
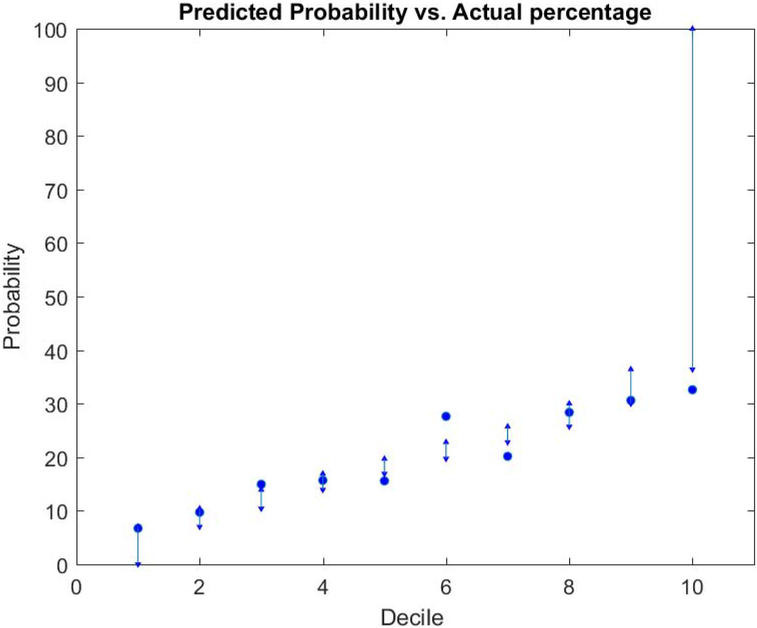
Table 2 illustrates the statistical results of focused-in clinical variables and aspects of hyoid bone displacement that met the 0.05 entry criterion for the model. Clinical variables shown in Table 2 were forced-in to the model with forward selection. Maximum anterior-horizontal hyoid bone displacement was the only aspect of hyoid bone displacement that was significantly predictive of normal versus disordered PAS scores and included in the model. Patient age was significantly predictive of normal versus disordered PAS scores, although the confidence interval included OR = 1.00. For each additional year of age, the odds of a disordered PAS score increased by 3% (OR=1.03, 95% C.I. = 1.00 1.05; p=0.0178). There was a trend toward a single swallow being less likely (36%) to have a disordered PAS score compared to multiple swallows, (OR=.64, 95% C.I. =.40–1.04; p=0.0708). Two swallows per bolus (multiple 1) was significantly more likely to have a disordered PAS score (OR=1.58, 95% C.I = 1.16 2.15; p=0.0040) than more than two swallows per bolus (multiple 2). There was strong evidence that swallows of thin liquid had a significantly greater odds of a disordered PAS score than a cookie swallow (OR=3.62, 95% C.I. = 1.37 9.58; p=0.0096). The model predicted the risk of penetration and aspiration for each patient based on the variables included in the model. Table 3 shows the predicted probability of having a disordered PAS score in each decile compared to the observed percentage of disordered PAS scores in each decile. For instance, as shown in the table, the predicted probability for decile 1 indicates that 0–7% of the swallows will be disordered. The predictive model effectively captured patient risk profiles for this decile because 6.72% of the swallows had a disordered PAS score. Similar observations can be made for deciles 2, 4, 8, and 9. Deciles 3, 5, 6, 7, and 10 captured the increasing probability trend of penetration and aspiration, although the observed percentage of swallows with disordered PAS scores were slightly outside of the predicted ranges.
Table 2.
Final model with forward selection with 0.05 entry criterion
| Parameter | Estimate | P value | Odds ratio | Odds 95% CI |
|---|---|---|---|---|
| type: multiple(1) | 0.4545 | 0.0040* | 1.58 | 1.16–2.15 |
| max. dis. of anterior in horizontal direction | −0.0583 | 0.0064* | 0.94 | 0.90–0.98 |
| viscosity: thin | 1.2862 | 0.0096* | 3.62 | 1.37–9.58 |
| age | 0.0265 | 0.0178* | 1.03 | 1.00–1.05 |
| type: single | −0.4435 | 0.0708 | 0.64 | 0.40–1.04 |
| viscosity: nectar | 0.7049 | 0.1664 | 2.02 | 0.75–5.49 |
| utensil: spoon | 0.1622 | 0.3538 | 1.18 | 0.83–1.66 |
| viscosity: pudding | −0.5334 | 0.3789 | 0.59 | 0.18–1.92 |
| sex: male | 0.1398 | 0.6998 | 1.15 | 0.57–2.34 |
| head position: neutral | 0.0994 | 0.7104 | 1.18 | 0.65–1.87 |
| swallow duration | −0.0004 | 0.9549 | 1.00 | 0.99–1.01 |
| type: multiple(2) | 0.0000 | . | 1.00 | 1.00 |
| sex: female | 0.0000 | . | 1.00 | 1.00 |
| viscosity: cookie | 0.0000 | . | 1.00 | 1.00 |
| utensil: cup | 0.0000 | . | 1.00 | 1.00 |
| head position: chin down | 0.0000 | . | 1.00 | 1.00 |
Table 3.
Predicted probability decile cut-off and observed percentage based on the model (* actual% of swallows with disordered PA scores was within the predicted probability range based on hyoid displacement features)
| Predicted Probability Decile | Predicted Percentage of High PA swallows | Number of Swallows | Actual Number (Percentage) of High PA Swallows |
|---|---|---|---|
| 1 | 0.0 – 7.0 | 134 | 9(6.72)* |
| 2 | 7.0 – 10.4 | 134 | 13(9.70) * |
| 3 | 10.4 – 13.9 | 134 | 20(14.93) |
| 4 | 13.9 – 16.9 | 135 | 21(15.67)* |
| 5 | 16.9 – 19.7 | 134 | 21(15.56) |
| 6 | 19.7 – 22.8 | 134 | 37(27.61) |
| 7 | 22.8 – 25.7 | 134 | 27(20.15) |
| 8 | 25.7 – 30.0 | 134 | 38(28.36)* |
| 9 | 30.0 – 36.4 | 134 | 41(30.60)* |
| 10 | 36.4 – 100 | 135 | 44(32.59) |
4. Discussion
This study found that a predictive model that included maximum anterior-horizontal hyoid bone displacement and other variables known to affect penetration and aspiration risk can reasonably predict the risk of penetration and aspiration in patients with dysphagia. While this predictive model accurately captured the increasing probability trend of penetration and aspiration risk of patients, the predicted and observed probabilities did not always match. Current clinical practice is for clinicians to assess physiological impairments of swallowing and reduced airway protection by subjectively interpreting VF images. However, one limitation of using VF as an assessment tool is that aspiration may not be observed during VF due to the time constraints of the examination to minimize radiation exposure. Creating a predictive model based on objective measurements of physiological swallowing events, such as the measurements of hyoid bone displacement that were used in this study, would allow clinicians to more accurately capture patient risk profiles of penetration and aspiration. This model could be used to improve assessment of swallow function, effectively track progress in therapy, and proactively and objectively identify physiologic markers of elevated risk of adverse events that occur secondary to dysphagia, such as aspiration pneumonia.
5. Limitation
The GEE model in this study used anterior-horizontal hyoid bone displacement and other independent variables to reasonably predict penetration and aspiration risk for patients with dysphagia. However, swallowing and airway protection are complex, multifactorial processes. It is probable that the variables included in this model are not the only predictors of aspiration. One limitation of the current predictive model is that it underestimates the risk of penetration and aspiration for patients with disordered PAS scores. The predictive model will likely be improved by including other swallow kinematic measurements.
6. Conclusion
This research work developed a preliminary GEE model that can reasonably predict penetration and aspiration risk for patients with dysphagia. This is an important and necessary first step toward developing a more sophisticated and accurate predictive model that can be used in clinical settings. In the future, clinicians could use a predictive model based on physiological aspects of swallow function to calculate penetration and aspiration risk profiles for patients by entering patient specific information into the equation. By objectively determining patient risk profiles, clinicians could develop individualized treatment plans to prevent adverse outcomes (i.e. dehydration, malnutrition, and aspiration pneumonia) based on risk severity level, and objectively track the effectiveness of dysphagia treatment on functional patient outcome measures. Future research should examine the predictive ability of additional swallow kinematic measures on penetration and aspiration risk in patients with dysphagia. Variables such as hyoid bone velocity, initiation of the pharyngeal swallow, laryngeal elevation, laryngeal vestibular closure, UES duration, and other physiological parameters related to swallow function should be investigated. Including these kinematic events in the predictive model may increase the models predictive value, which would further improve its clinical application.
Fig. 2.
The predicted probability and the actual observed probability. The dot represents the actual observed probability in each subgroup and predicted probability are presented as intervals.
Funding:
This study was funded by two grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD092239, while the data was collected under Award Number R01HD074819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A
Prediction Equation Steps:
Let XB = − 3.479−0.0583 × (x1min2maxdistance)+0.0265 × (age)− − 0.0004× (duration)
Subtract from XB 0.4435 if the swallow is single, add to XB 0.4545 if the swallow is multiple 1, or do nothing if multiple 2.
Subtract from XB 0.1398 if the sex=2(female?), or do nothing if sex=1(male?).
Add 1.2862 to XB if viscosity=thin, add 0.7049 if nectar, subtract 0.5334 if pudding, and do nothing if cookie.
Add to XB 0.1622 if spoon, or do nothing if cup.
Add to XB 0.0994 if chin down, or do nothing if head position is neutral.
Compute the probability of a high PA swallow as exp (XB)/(1 + exp(XB)).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: We have no conflict of interest to declare.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Zhenwei Zhang, Email: zhz87@pitt.edu, Department of Electrical and Computer Engineering, Swanson School of Engineering, University of Pittsburgh, Pittsburgh, PA, 15261, USA.
Subashan Perera, Email: ksp9@pitt.edu, Department of Medicine, Division of Geriatrics, University of Pittsburgh, Pittsburgh, PA, 15261, USA.
Cara Donohue, Email: cad191@pitt.edu, Department of Communication Science and Disorders, University of Pittsburgh, Pittsburgh, PA, 15260, USA.
Atsuko Kurosu, Email: atk22@pitt.edu, Department of Communication Science and Disorders, University of Pittsburgh, Pittsburgh, PA, 15260, USA.
Amanda S. Mahoney, Email: asm100@pitt.edu, Department of Communication Science and Disorders, University of Pittsburgh, Pittsburgh, PA, 15260, USA.
James L. Coyle, Email: jcoyle@pitt.edu, Department of Communication Science and Disorders, University of Pittsburgh, Pittsburgh, PA, 15260, USA.
Ervin Sejdić, Email: esejdic@ieee.org, Department of Electrical and Computer Engineering, Swanson School of Engineering, University of Pittsburgh, Pittsburgh, PA, 15261, USA, Tel.: +1-412-624-0508, Fax: +1-412-624-8003.
References
- 1.Clavé P and Shaker R, “Dysphagia: current reality and scope of the problem,” Nature Reviews Gastroenterology and Hepatology, vol. 12, no. 5, pp. 259–270, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Dudik JM, Coyle JL, El-Jaroudi A, Sun M, and Sejdić E, “A matched dual-tree wavelet denoising for tri-axial swallowing vibrations,” Biomedical Signal Processing and Control, vol. 27, pp. 112–121, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AJ, “The neurobiology of swallowing and dysphagia,” Developmental Disabilities Research Reviews, vol. 14, no. 2, pp. 77–86, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Movahedi F, Kurosu A, Coyle JL, Perera S, and Sejdić E, “Anatomical directional dissimilarities in tri-axial swallowing accelerometry signals,” IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 25, no. 5, pp. 447–458, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya N, “The prevalence of dysphagia among adults in the United States,” Otolaryngology–Head and Neck Surgery, vol. 151, no. 5, pp. 765–769, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Murray J, Manual of dysphagia assessment in adults. Cengage Learning, 1999. [Google Scholar]
- 7.Lazarus C and Logemann A, “Swallowing disorders in closed head trauma patients.” Archives of Physical Medicine and Rehabilitation, vol. 68, no. 2, pp. 79–84, 1987. [PubMed] [Google Scholar]
- 8.Cook IJ and Kahrilas PJ, “AGA technical review on management of oropharyngeal dysphagia,” Gastroenterology, vol. 116, no. 2, pp. 455–478, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Gordon C, Hewer RL, and Wade DT, “Dysphagia in acute stroke.” British Medical Journal, vol. 295, no. 6595, pp. 411–414, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbert IA and Robbins J, “Dysphagia in the elderly,” Physical Medicine and Rehabilitation Clinics of North America, vol. 19, no. 4, pp. 853–866, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorell JM, Johnson CC, and Rybicki BA, “Parkinson’s disease and its comorbid disorders An analysis of Michigan mortality data, 1970 to 1990,” Neurology, vol. 44, no. 10, pp. 1865–1865, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Coyle JL, Davis LA, Easterling C, Graner DE, Langmore S, Leder SB, Lefton-Greif MA, Leslie P, Logemann JA, Mackay L, Martin-Harris B, Murray JT, Sonies B, and Steele CM, “Oropharyngeal Dysphagia Assessment and Treatment Efficacy: Setting the Record Straight (Response to Campbell-Taylor),” Journal of the American Medical Directors Association, vol. 10, no. 1, pp. 62–66, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Rugiu M, “Role of videofluoroscopy in evaluation of neurologic dysphagia,” Acta Otorhinolaryngologica Italica, vol. 27, no. 6, p. 306, 2007. [PMC free article] [PubMed] [Google Scholar]
- 14.Molfenter SM and Steele CM, “Physiological variability in the deglutition literature: hyoid and laryngeal kinematics,” Dysphagia, vol. 26, no. 1, pp. 67–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y and McCullough GH, “Maximum hyoid displacement in normal swallowing,” Dysphagia, vol. 23, no. 3, pp. 274–279, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kang B-S, Oh B-M, Kim IS, Chung SG, Kim SJ, and Han TR, “Influence of aging on movement of the hyoid bone and epiglottis during normal swallowing: a motion analysis,” Gerontology, vol. 56, no. 5, pp. 474–482, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Harris B and Jones B, “The videofluorographic swallowing study,” Physical Medicine and Rehabilitation Clinics of North America, vol. 19, no. 4, pp. 769–785, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Kurosu A, Coyle J, Perera S, and Sejdić E, “Hyoid displacement during swallowing is related to penetration-aspiration scores,” Under Review. [Google Scholar]
- 19.Kendall KA and Leonard RJ, “Hyoid movement during swallowing in older patients with dysphagia,” Archives of Otolaryngology–Head & Neck Surgery, vol. 127, no. 10, pp. 1224–1229, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Perlman AL, Booth B, and Grayhack J, “Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia,” Dysphagia, vol. 9, no. 2, pp. 90–95, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Su J, Yuan C, and Shu K, “Hyoid bone displacement during swallowing have no association with penetration/aspiration severity in dysphagic stroke patients,” Archives of Physical Medicine and Rehabilitation, vol. 95, no. 10, e16, 2014. [Google Scholar]
- 22.Molfenter SM and Steele CM, “Kinematic and temporal factors associated with penetration–aspiration in swallowing liquids,” Dysphagia, vol. 29, no. 2, pp. 269–276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo HG, Oh B-M, and Han TR, “Swallowing kinematics and factors associated with laryngeal penetration and aspiration in stroke survivors with dysphagia,” Dysphagia, vol. 31, no. 2, pp. 160–168, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, and Wood JL, “A penetration-aspiration scale,” Dysphagia, vol. 11, no. 2, pp. 93–98, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Lof GL and Robbins J, “Test-retest variability in normal swallowing,” Dysphagia, vol. 4, no. 4, pp. 236–242, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, “Generalized estimating equations in longitudinal data analysis: A review and recent developments,” Advances in Statistics, vol. 2014, 2014. [Google Scholar]