Abstract
Child maltreatment is associated with a variety of risk behaviors in young adulthood; however, the underlying cognitive and neural mechanisms of this relation are not well understood. The primary aim of the present study was to examine the direct and indirect effects between maltreatment in childhood and downstream impulsivity via neural activity during a cognitive task. In a sample of emerging adult women from the rural southeastern United States, childhood abuse and neglect were assessed using the childhood trauma questionnaire. Outcome measures of neural activity during a functional magnetic resonance imaging N-back verbal working memory (WM) task and trait impulsivity on the Impulsive Behavior Scale were assessed approximately 1 year later. Results indicate that adults with higher levels of reported childhood maltreatment demonstrate worse behavioral performance and lower neural response during a difficult verbal WM task. Furthermore, neural activity significantly mediated the relation between abuse and neglect in childhood and trait impulsivity. These new findings demonstrate an association between neurocognitive functioning and reported childhood abuse and neglect, and indicate that such changes may underlie the relation between maltreatment and trait-level impulsivity.
Keywords: child maltreatment, neurocognition, cognitive development, memory, child abuse
Neural development is the result of complex interactions including the influence of environmental experiences. Childhood maltreatment (CM), defined as physical, emotional, or sexual abuse or neglect prior to the age of 18, can have a profound impact on neural plasticity such as experience-dependent modification of structure and function through the strengthening of synaptic connections and pruning (Twardosz & Lutzker, 2010). Ultimately, these changes in neurodevelopment result in a cascade of effects on cognitive and behavioral functioning (e.g., self-regulation deficits, trait impulsivity) that are associated with poorer adolescent and young adult outcomes (Brems, Johnson, Neal, & Freemon, 2004; Gilbert et al., 2009; Heim, Shugart, Craighead, & Nemeroff, 2010; Oshri, Rogoach, & Cicchetti, 2013; Shin, Edwards, & Heeren, 2009). Higher level cognitive and executive processes that develop throughout adolescence and early adulthood appear to be uniquely vulnerable to the impact of CM given the longer duration of neural development in underlying brain regions (Blakemore & Choudhury, 2006; Glaser, 2000). Deficits in these domains secondary to altered neural functioning may account for some of the established relationship between CM and impulsivity (Romer, 2010); however, this indirect path has not been tested. To expand existing understanding of the complex relationship between cognitive and behavioral associates of CM, the present study aims to determine the extent to which neural activity during an executive task statistically mediates the relationship between CM and impulsivity.
Existing evidence demonstrates that environmental factors such as CM impact brain structure and function (De Bellis, 2005; Heim & Nemeroff, 2001; Teicher, Samson, Anderson, & Ohashi, 2016). Structurally, CM has been associated with reduced hippocampal, corpus callosal, orbitofrontal, and prefrontal volumes as well as reduced neuronal density in the anterior cingulate and altered cortical symmetry in the superior temporal gyrus and frontal lobes (Cohen et al., 2006; De Bellis et al., 2002; De Bellis et al., 2015; De Brito et al., 2013; Teicher et al., 2003; Teicher et al., 2004; Vythilingam et al., 2002). Demonstrated functional consequences of CM include altered pituitary–adrenal and autonomic stress responses (i.e., hypothalamic–pituitary–adrenal axis; Heim, Newport, Mletko, Miller, & Nemeroff, 2008; Heim et al., 2010). CM has further been associated with aberrant amygdalar and anterior cingulate cortex reactivity and frontal lobe dysfunction (Clark et al., 2017; Dannlowski et al., 2012; Hart & Rubia, 2012; McCrory, De Brito, & Viding, 2010). While there has been a recent emergence in the literature, relatively few studies have examined the direct and indirect effects of CM on neural activity and associated cognitive functions and behavior using functional magnetic resonance imaging (fMRI).
Cognition is closely tied to neural function and appears to be susceptible to the effects of CM. This is particularly true of executive functions (EFs), a group of top-down cognitive processes that are instrumental in reasoning, problem-solving, and planning (Diamond, 2013), which continue to develop in tandem with neuroanatomical correlates well into adolescence and emerging adulthood (Bos, Fox, Zeanah, & Nelson, 2009; Chugani et al., 2001; Pechtel & Pizzagalli, 2011; Pollak et al., 2010). One such EF, working memory (WM), the ability to mentally hold and manipulate modality-specific information, is a critical cognitive process that allows one to keep representations of the world active to support goal-oriented behavior (Baddeley & Hitch, 1974; Finn, 2002). WM facilitates other cognitive operations including decision-making, self-control and regulation, inhibition of impulsive behavior, and engagement in socially adaptive behavior (Barkley, 2001; Bechara, Damasio, & Damasio, 2000; Bickel, Yi, Landes, Hill, & Baxter, 2011). This cognitive construct is divided into verbal and nonverbal WM, each with distinct yet overlapping neuroanatomical correlates (Brahmbhatt, McAuley, & Barch, 2008). Neuroimaging studies find that both verbal and nonverbal WMs are associated with neural reactivity in the bilateral dorsolateral prefrontal cortices (DLPFCs, surrounding the rostral middle frontal gyri), the posterior parietal cortices (PPCs, superior and inferior parietal lobules), and the supplementary motor areas (SMAs, the caudal medial frontal gyri) during verbal WM tasks (Aloia et al., 2009; Brahmbatt et al., 2008; Smith & Jonides, 1997; Sweet et al., 2008). As several of these regions continue to develop across childhood and adolescence, it is likely that exposure to CM would affect both behavioral performance and neural response during WM tasks (Coswell, Ciccetti, Rogosch, & Toth, 2015; DePrince, Weinzierl, & Combs, 2009; Hanson et al., 2010; Majer, Nater, Lin, Capuron, & Reeves, 2010).
While there has been a recent emergence in the literature, relatively few studies have examined the associations between CM and neural activity associated with the EFs using fMRI. Existing evidence suggests that CM is associated with altered neural activity during WM performance (Philip et al., 2013, 2016). Specifically, Philip and colleagues (2016) demonstrated that exposure to childhood trauma was associated with altered activation and behavioral performance on a complex WM task. However, this study included predominately Caucasian and urban-residing participants, thus limiting generalizability of findings to people from other backgrounds. More generally, no studies to date have attempted to demonstrate an association between the altered neural functioning during WM performance and related behavioral difficulties (e.g., impulsivity) that may account for poorer outcomes among those exposed to CM.
The detrimental effects of CM on WM are of particular interest, given the important role that WM plays in impulsivity, a multifaceted construct that includes sensation seeking, poor behavioral inhibition, and decision-making without premeditation (Whiteside & Lynam, 2003). Both exposure to CM and deficits in WM have been associated with increased impulsivity (Hinson, Jameson, & Whitney, 2003; Lovallo et al., 2013; Thush et al., 2008). Additionally, it has been suggested that individuals with WM deficits may struggle to inhibit impulses in the pursuit of goal-driven decisions when faced with novel or exciting opportunities (Braquehais, Oquendo, Baca-Garcia, & Sher, 2010; Wanklyn, Day, Hart, & Girard, 2012). This, in combination with the known effect of CM on WM, suggests that WM may mediate the observed relationship between CM and impulsivity.
The Present Study
The present study aims to deepen the scientific understanding of the neurobiological impacts of CM that may lead to subsequent impulsivity. Specifically, we examine the direct and indirect effects of CM on impulsive traits via neural activity during a WM task. We sought to extend prior evidence that CM negatively impacts behavioral and neural WM functioning (Philip et al., 2013, 2016) by examining whether altered WM is associated with variation in trait impulsivity in a high-risk sample of low-socioeconomic status, rural, young adult women. This sample has been at particular risk for CM compared to their urban counterparts, in part, because rural youth have less access to services that are known to reduce rates of maltreatment such as child welfare and mental health-care facilities (Belanger & Stone, 2008; Smith, Kay, &Pressley, 2018). Furthermore, while exposure to abuse is detrimental to all individuals, women affected by CM may be disproportionately susceptible to its effects, as female victims of child abuse appear to be at a greater risk for both physical and mental health problems than their male counterparts (MacMillan et al., 2001; Thompson, Kingree, & Desai, 2004). While recent evidence indicates that the neurobiological pathways by which CM affects individuals also vary by gender (Doom, Cicchetti, Rogosh, & Dackis, 2013; Teicher et al., 2003), specific neural and cognitive consequences of CM on young women remain poorly understood.
The specific hypotheses and aims of the present study are as follows. First, we examine the relationships between CM and behavioral performance on a difficult WM task, neural correlates of WM performance, and self-reported trait impulsivity. Based on prior literature, we hypothesize that exposure to higher levels of CM will be significantly negatively associated with behavioral performance on the WM task. In addition, we predict that CM will be associated with altered brain activation in WM networks and significantly positively related to self-reported traits associated with impulsive behavior. Second, the study aims to identify direct and indirect linkages between CM and impulsive behaviors via neural reactivity in WM networks. We hypothesize a significant direct link between CM and impulsivity, as well as a significant indirect link between CM and impulsivity via neural activity, such that individuals exposed to higher levels of CM will demonstrate lower average neural response within WM networks, which drive associations with impulsivity.
Method
Participants
Thirty women from the rural southeastern United States, aged between 18 and 25, were recruited to participate in the present study. Participants were a randomly selected subsample of female participants currently enrolled in a larger community-based longitudinal study (N = 225). The study was conducted across three waves approximately 1 year apart (for additional information regarding recruitment of the larger sample, see Oshri, Liu, Duprey, MacKillop, 2018). Participants recruited for the present study (mean age at first wave = 20.63, SD = 2.20) were racially and ethnically diverse (63.3% Caucasian, 16.7% African American, 13.3% Hispanic/Latino, and 6% multiethnic), native-English speakers, and noncollege educated. Average reported family income ranged from US $10,000 to US $120,000 (mean = US $43,970 and SD = US $27,883). Neuroimaging data were collected as a part of the second wave, approximately 1 year after initial recruitment. To participate in the present study, participants needed to be proficient in English, 18 years of age or older, and have normal or corrected vision and hearing at the time of testing. Exclusion criteria included medical contraindications for magnetic resonance imaging (MRI; e.g., some metal implants, claustrophobia), left-handedness, a history of diagnosed neurological disorder (e.g., traumatic brain injury, epilepsy), and current (i.e., within the last 12 months) diagnosis of or treatment for psychiatric illness (e.g., depression, anxiety). The present study was approved and monitored by the University of Georgia Institutional Review Board. All participants provided written informed consent prior to study participation and were monetarily compensated.
Childhood Trauma Questionnaire (CTQ)
The CTQ (Bernstein & Fink, 1998) is a psychometrically validated 28-item self-report inventory that assessed participants’ accounts of their own experiences of maltreatment in childhood (Scher, Stein, Asmundson, McCreary, & Forde, 2001). Participants were asked to make ratings on a 5-point Likert-type scale ranging from never true (1) to very often true (5) regarding their childhood experiences across five domains of maltreatment (i.e., physical neglect, physical abuse, emotional neglect, emotional abuse, and sexual abuse). The CTQ was completed at the first wave of data collection. The total score from this scale was used in subsequent analyses as a continuous measure of CM. Internal consistency among items in the current investigation was high (α = .899).
Impulsive Behavior Scale (UPPS-P)
The Impulsive Behavior Scale (Lynam, Smith, Whiteside, & Cyders, 2006) is a 59-item self-report inventory of five traits associated with impulsive behavior including negative urgency, positive urgency, sensation seeking, lack of perseverance, and lack of premeditation (i.e., UPPS-P). Ratings are made on a 4-point Likert-type scale ranging from strongly agree (1) to strongly disagree (4). The UPPS-P has been well validated with evidence of measurement invariance across sex (Cyders, 2013). The UPPS-P was completed during the second wave of data collection, at the same time point as the neuroimaging data. Global scores of overall endorsement of impulsive behaviors across domains were used for the present analyses. Internal consistency among items was high (α = .934).
Cognitive Task: N-back
The verbal N-back is an assessment of WM that has been linked to individual variability in higher cognitive and executive functions including fluid intelligence and attentional control (Jaeggi, Buschkuel, Perrig, & Meier, 2010). The task requires participants to monitor a series of verbal stimuli (i.e., consonants) and indicate whether new stimuli are the same as that presented n trials previously. The N-back is widely used in functional neuroimaging studies as it demonstrates reliable patterns of neural activation (Owen, McMillan Laird, & Bullmore, 2005; Sweet et al., 2008). The present study included three components: task-free baseline, 0-back, and 2-back. Stimulus presentation parameters were designed based upon the paradigms of previous functional neuroimaging studies of the N-back (Braver et al., 1997; Smith & Jonides, 1997; Sweet et al., 2008). During the task, a series of individual consonants were presented visually every 3 s. Consonants were arranged in a pseudorandom order from a list of all consonants except “L” due to ambiguity in a lower case form. Subjects completed two imaging runs of the paradigm; each run included three 2-back blocks (15 stimuli) and three 0-back blocks (9 stimuli), with a 27-s rest periods following each 2-back/0-back cycle (i.e., 2 per imaging run). Responses were made and recorded using the first two fingers on their right hand on a MR compatible two-button response box. Response accuracy was used for subsequent analysis of behavioral performance on the N-back task.
0-Back control task.
The 0-back (“Letter H Task”) condition required participants to respond “yes” each time a target stimulus (“H” or “h”) was presented and “no” when any other consonants appeared. Consonants were presented for 500 ms with an interstimulus interval (ISI) of 2,500 ms (33% targets).
2-Back task.
The 2-back condition asked participants to respond “yes” if a consonant was the same as the consonant presented two earlier (e.g., f, H, F, r, x, R, responses warranting a yes response are underlined) and “no” if it was not. Consonants were presented for 500 ms with an ISI of 2,500 ms (33% targets).
MRI Acquisition
MRI data were collected during the second wave of data collection using a GE 16-channel Signa HDx 3.0 Tesla scanner at the University of Georgia Bio-Imaging Research Center. Whole-brain high-resolution T1-weighted fast-spoiled gradient echo scans were acquired for anatomical reference (TR = 7.8 s; TE = 3.1 ms; flip angle, 20°; FOV = 256 × 256 mm; matrix = 256 × 256; 160 contiguous 1-mm axial slices; voxel size = 1 mm3). Whole-brain functional images were acquired using T2* echo-planar imaging with a single-shot gradient echo pulse sequence (TR = 2,000 ms; TE = 25 ms; flip angle, 90°; FOV = 225 × 225 mm; matrix = 64 × 64; 40 contiguous 3.5-mm axial slices; voxel size = 3.5 mm3). The N-back paradigm consisted of two 4-min and 48-s imaging runs of 144 brain volumes each.
Apparatus
The N-back task was presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Computer video signal was projected through MR-compatible goggles that were placed in a stationary position on the participants face within the coil, and responses were collected using a two-button response box.
Image Processing and N-back Analyses
FMRI data processing and analysis were conducted using Analysis of Functional Neuroimages software (AFNI; Cox, 1996). Functional data sets were aligned to T1 data sets and volume registered. The first four volumes of each run were removed to allow the scanner to reach steady state. Volumes exhibiting intervolume movement greater than 0.3 mm along any axis were censored. Two participants were excluded due to greater than 25% of volumes censored. Additionally, three participants were removed due to chance-level performance accuracy on the N-back test (50% accuracy or below). Following motion correction (i.e., censoring of TRs with excessive motion in accordance with the AFNI standard preprocessing pipeline), data sets were transformed into standard stereotaxic space (Talairach & Tournoux, 1988) and individual volumes from each run were registered to a base volume. Data were spatially smoothed using a 6-mm full width half maximum Gaussian filter. Raw blood oxygenation-level-dependent (BOLD) signal was scaled to percent signal change from mean signal intensity.
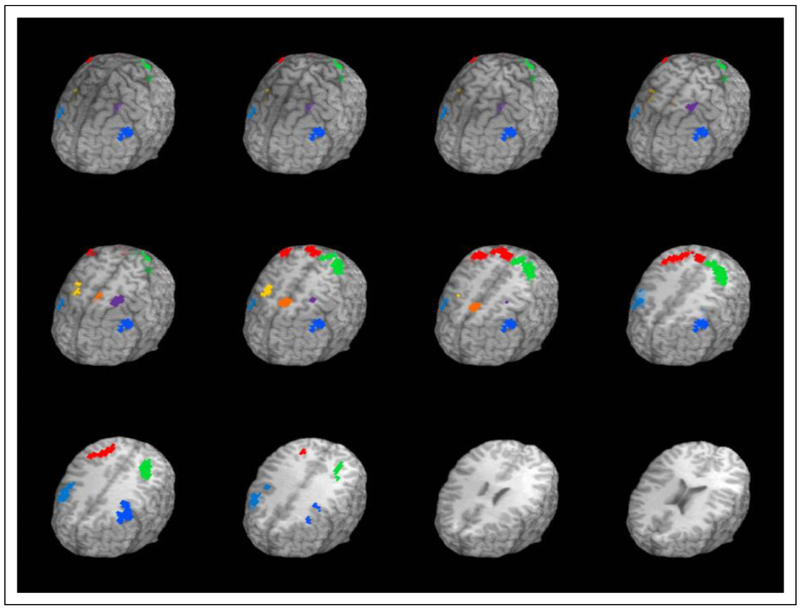
A voxelwise, general linear model (GLM) procedure was conducted to quantify the relationship between observed brain activity and the time course of the 2-back paradigm. For each voxel of each individual, a GLM of the temporal pattern of 2-back presentation (including hemodynamic transitions modeled as a gamma function), 0-back control task, and covariates (observed movement and linear drift) was performed using the BOLD signal over time as the dependent variable. Resulting individual maps of β coefficients for each voxel represent the 2-back effects versus 0-back effects. Individual activation maps were used to create group summary maps of 2-back effects, which were then compared to a hypothetical mean of zero for each voxel using pooled-variance one-sample Student’s t tests. A two-tailed threshold of p < .05 with false discovery rate protection was used to reduce Type I error (Benjamini & Hochberg, 1995). Thus, this group summary activation map represented the voxels in which there was statistically significant average neural activity when completing the 2-back WM task above and beyond the 0-back control task across the sample (see Table 1 and Figure 1). Six regions of interest (ROIs) were defined using a family-wise error rate of q = .05 with a minimum cluster size of 10 adjacent voxels. The functionally defined ROI approach was chosen to yield valid task-related ROIs and avoid Type 2 error risk inherent in a priori ROI approaches. For each participant, a single value that represented the average activity during the 2-back task above and beyond the 0-back task within each identified ROI was calculated. Subsequently, an average of 2-back-associated neural activity across all regions, weighted by the number of voxels within each ROI, was calculated for each participant for use in subsequent analyses.
Table 1.
Functionally Defined Regions of Interest (i.e., Significant 2-back Response) and Correlations With CM and Impulsivity.
| Center of Mass Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region of Interest | # Voxels | X | Y | Z | CTQ r | UPPS-P r |
| L. Posterior parietal lobule | 212 | +22.9 | +58.0 | +42.3 | −.570** | −.300 |
| R. Posterior parietal lobule | 167 | −29.3 | +56.0 | +42.1 | −.571** | −.356 |
| R. Rostral middle frontal gyrus | 87 | −40.9 | −19.9 | +33.9 | −.190 | −.574** |
| L. Rostral middle frontal gyrus | 64 | +41.0 | −13.7 | +35.5 | −.223 | −.380* |
| L. Caudal middle frontal gyrus | 42 | +27.8 | −1.5 | +54.6 | −.356* | −.256 |
| R. Caudal middle frontal gyrus | 31 | −29.0 | −9.6 | +51.2 | −.151 | −.533** |
Note. L = left; R = right; CTQ = childhood trauma questionnaire; UPPS-P = impulse Behavior Scale. Posterior parietal lobule includes both inferior and superior portions. Coordinates are in Talairach space (RAI). Correlations describe the relation of CTQ and UPPS-P scores, respectively, to the average activity in each brain region (one-tailed).
p < .05.
p < .01.
Figure 1.
Group-level N-back activation: 2-back versus the 0-back control task. Functionally defined regions of interest (ROIs) used in the present analyses. Axial slices show three-dimensional brain regions that exhibited significant activity during the 2-back task above and beyond the activity exhibited during the 0-back control task. Talairach Z-plane coordinates = +80 to 25 in 5-mm slices; q = .05.
Statistical Analyses Plan
Data were analyzed using the Statistical Package for Social Sciences (SPSS; Version 22) and Mplus Version 7.4 (Muthén & Muthén, 2010). Missing data were tested and determined to be missing at random (MAR; Little & Rubin, 2002). Estimation of missing data was conducted using full information maximum likelihood (FIML). FIML utilizes all available data to estimate parameters and has been shown to produce unbiased estimates with MAR data (Enders & Bandalos, 2001). Pearson’s correlations were used to examine associations between CTQ, UPPS-P, neural activity, and cognitive performance. Path analyses were performed using the robust maximum likelihood (MLR) estimator to account for data non-normality identified in the health risk behavior indicators. Competing indirect associations were evaluated using the distribution of the product approach using bias-corrected bootstrap (5,000 replications) confidence intervals (Preacher & Hayes, 2008).
Results
Descriptive analyses were conducted to verify normality of distributions; skewness and kurtosis were examined for all variable distributions. All variables met normality criteria. Successful performance of the 2-back task was associated with significantly increased bilateral brain activity in expected WM network regions relative to recruitment during the 0-back active control task. Clusters of recruitment centered on the bilateral posterior parietal lobules, bilateral caudal middle frontal gyri, and bilateral rostral middle frontal gyri (see Figure 1).
Pearson’s correlation coefficients indicated that CM was negatively associated with both WM task performance, r(23) = −.401, p < .05, and overall neural response during the N-back task, r(23) = −.498, p < .05 (see Table 2; correlations with individual ROIs are presented in Table 1). In addition, higher rates of CM were significantly positively associated with self-reported impulsive traits, r(23) = .421, p < .05. Finally, WM neural activity and impulsive traits were significantly negatively correlated, r(23) = −.498, p < .05.
Table 2.
Correlations Testing Study Hypotheses.
| Study Variables | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Proportion 2-back Accuracy | — | |||
| 2. Childhood Trauma Questionnaire | −.401* | — | ||
| 3. N-back Activity | .196 | −.498* | — | |
| 4. Impulsive Behavior Scale (UPPS-P) | −.171 | .421* | −.498* | — |
| Mean | .740 | 60 | .280 | 10.741 |
| SD | .089 | 20.86 | .167 | 2.226 |
| Range | .60 to .89 | 36 to 112 | −.04 to 16.45 | 7.52 to 16.35 |
Note. Childhood trauma questionnaire = total score on 28-item CTQ; N-back Activity = weighted average of neural activity across six clusters when completing the 2-back task above and beyond the 0-back control task; Impulsive Behavior Scale = total score on the UPPS-P.
p < .05.
To investigate the indirect effect between CM and impulsive traits via neural activity during the N-back task, a path model was tested (see Table 3). Results indicated that CM significantly negatively predicted 2-back activity, B = −.666, SE = .180, β = −.533, p < .001, and 2-back response significantly negatively predicted impulsivity, B =−6.060, SE = 2.966, β = −.491, p < .05, after controlling the variance associated with CM. The product coefficient method to test the indirect effect was employed using a maximum likelihood estimator revealing a significant indirect effect, B = 4.038, 95% CI [0.183, 7.893], p < .05. These findings support the hypothesized mediational model from CM to impulsive traits via neural activity through WM networks.
Table 3.
Path Analyses Parameters of the Associations Between CM, Impulsivity, and N-back Activity.
| Paths | B (SE) | β | 95% CI of B |
|---|---|---|---|
| Direct effects | |||
| CM → UPPS-P | 1.730 (3.270) | .112 | [−4.679, 8.139] |
| CM → N-back Activity | −.666 (.180) | −.533 | [−1.019, −.313]*** |
| N-back Activity → UPPS-P | −6.060 (2.966) | −.491 | [−11.873, −.247]* |
| Indirect effect | |||
| CM → UPPS-P | 4.038 (1.967) | [.183, 7.893]* |
Note. N = 30. The model is just identified. SE = standard error; comparative fit index (CI) = confidence interval; CM = CTQ total score; UPPS-P = measure total score; N-back Activity = average neural activity during N-back task. CFI = 1.0, Standardized Root Mean Residual (SRMR) = .000.
p < .05.
p < .01.
p < .001.
Discussion
The present study examined the role of verbal WM-associated brain activity in the association between CM and self-reported tendencies toward impulsive behavior. Overall, our results revealed significant associations between CM, neurocognitive functioning, and impulsivity. Findings from the present sample are consistent with those of previous studies that show a link between CM and impulsivity among young adults, such that those with greater exposure to CM report more impulsive traits and behaviors (Oshri et al., 2018). Similarly, behavioral performance on the WM task indicated that those with higher levels of endorsed CM demonstrated poorer performance accuracy on a difficult WM task. This finding is corroborated by prior studies reporting poorer WM and other executive skills among individuals who have been exposed to CM (Clark, Arce Rentería, Hegde, & Morgello, 2018; Cowell, Cicchetti, Rogosch, & Toth, 2015; Pechtel & Pizzagalli, 2011; Philip et al., 2016). Consistent with the pattern of poorer performance accuracy, individuals who reported greater levels of maltreatment during childhood had reduced neural activity in a network of task-related brain regions including the bilateral posterior parietal lobule, bilateral caudal middle frontal gyrus, and bilateral rostral middle frontal gyrus. Finally, results indicated that the relation of CM to impulsivity was statistically mediated by neural response to the WM task. These findings highlight the importance of considering neurocognitive mechanisms that may underlie the relation between maltreatment and trait impulsivity.
CM and Neural Response
Neural activity across several regions regularly identified as a part of a verbal WM network was significantly related to CM, such that those exposed to more CM demonstrated less neural activity when successfully completing this difficult task. When examined by region (Table 1), it appears that this relationship was primarily driven by reduced neural activity in the left caudal middle frontal gyrus (MFG) and bilateral posterior parietal regions. The left caudal MFG is a premotor region traditionally related to coordinated motor sequencing and language function. Thus, differential activity in this area may be indicative of variability in subvocal articulatory rehearsal demands inherent in the verbal N-back (Smith & Jonides, 1997). The posterior parietal lobe is a central hub of the frontoparietal attention network and thought to mediate short-term memory buffering during WM (Smith & Jonides, 1997). It is also important for integration of sensory information across modalities, which is consistent with conversion of consonant stimuli to phonemes available for subvocal articulatory rehearsal during the verbal N-back (Sweet et al., 2008). While it is widely recognized that the frontal cortex is the last to myelinate and that cognitive functions associated with it are the last to develop, larger networks including the posterior parietal cortex also demonstrate later developmental maturation and thus may also be vulnerable to the impacts of CM (Barber, Caffo, Pekar, & Mostofsky, 2013). These results complement a previous between-group study that demonstrated that exposure to higher levels of CM was associated with recruitment of additional brain regions during an identical WM task (Philip et al., 2016), indicating a possible neural inefficiency. Taken together, these results suggest that CM may attenuate the effectiveness of the frontoparietal networks, which may be the most vulnerable to CM because they are among the last to develop.
In the context of the present study, it appears that frontal and parietal portions of the brain that continued to develop during childhood may have been susceptible to the influence of CM. Several theories may explain these alterations, including the substantial evidence that suggests that neuroendocrine and neurobiological regulator systems are changed by exposure to CM and subsequently impact the development of brain regions (Lupien, McEwen, Gunnar, & Heim, 2009; Gunnar & Quevedo, 2007; Teitcher et al., 2003; Teitcher 2005). A second theory that may explain the present changes in neural functioning implicates extrinsic or environmental factors in brain development via neural plasticity: one’s experiences shape the pruning, strengthening, or formation of synaptic connections (Grassi-Oliveira, Ashy & Stein, 2008; Singer, 1995). Components of development that are experience-expectant, or those that develop in the presence of a particular experience during a critical period, may be limited for individuals raised in abusive or neglectful environments (Rutter, O’Connor, & English and Romanian Adoptees Study Tea, 2004). As a result of a dearth of experiential learning cues (e.g., parents reading to their children during early childhood) or presentation of new stimuli across the child’s development in a “safe, predictable, nurturing” environment (Perry & Pollard, 1998), neural development may be disrupted, resulting in abnormal neural activation in later life (Perry, Pollard, Blaicley, Baker, & Vigilante, 1995).
CM, WM, and Impulsivity
Support of our final hypothesis, the indirect path from CM to impulsive traits via neural activity during a WM task, is consistent with the notion that the environment impacts neural development, cognition, and subsequent attributes. Specifically, given the lack of stability and enrichment that may have been present in the environments of the children who were raised with CM, it is possible that there were fewer opportunities for these individuals to develop their executive and specifically WM skills, thus resulting in attenuated network development, poorer performance, and reduced activity in network nodes during completion of the task. When these limited WM resources are overextended, the dynamic process of decision-making is negatively affected, which may result in difficulty weighing one’s choices and considering consequences, thus leading to a tendency toward impulsive behaviors (Finn, 2002). While these results must be further confirmed by longitudinal investigations, the present findings support the notion that the neurobiological consequences of CM may bring about changes in neural and cognitive function that ultimately place individuals at a disadvantage when it comes to impulse control and engaging in behavior that is consistent with long-term goals.
These findings may also allude to a possible cognitive adaptation to adverse and dangerous environments. Accordingly, maltreated youth who are exposed to scarce, adverse, and unreliable environments may have adapted to be more resourceful by acting with more immediacy and planning less for the future, which may appear more uncertain and unrealizable for them. This idea is supported by findings that show that exposure to scarcity and child maltreatment affects how youth and young adults think about the future (Shah, Shafir, & Mullainathan, 2015; Oshri, Duprey, Carlson, Kogan, & Liu, 2018, in press). Thus, impulsive decision-making may make survival sense for individuals who grew up in enduring scarcity (Pepper & Nettle, 2017); however, during emerging adulthood as individuals strive to be self-sufficient, a tendency toward impulsivity might be less adaptive, placing these youths at an increased risk of engaging in harmful behaviors (e.g., risky driving and financial investing) or developing psychiatric difficulties (Mahalik et al., 2013; Willoughby, Good, Adachi, Hamza, & Tavernier, 2013).
Limitations and implications.
The present study has important limitations. Results must be interpreted within the context of the sample size, which, although common within the field of neuroimaging (Carrion, Haas, Garrett, Song, & Reiss, 2009; Philip et al., 2016), nevertheless, limits the chance of detecting a true effect. Secondly, data used in this study are cross sectional in nature, with reliance on retrospective self-report measures of the historical early CM variable. Thus, inferences on causal pathways are limited. While the CTQ is a commonly used instrument within the child maltreatment literature, it is important to consider that responses on self-report may be susceptible to recall bias. Thus, the present findings highlight the need for longitudinal studies to investigate the development of WM and its role in the mechanisms that mediate the link between diverse types of CM and impulsive traits among young adults. Finally, the present study examined two variables, CM and trait impulsivity, as total-score global estimates in order to address study hypotheses. As the CTQ and UPPS-P both contain subdomain classifications, future studies may wish to examine whether there may be differential impacts of CM as a function of the domains of reported maltreatment or impulsivity.
It was the intention of the present study to fill a gap within the existing literature that has examined the impact of CM on the neural mechanism of WM and impulsivity by extending it to a previously unstudied and historically underrepresented sample. To do so, the study sample comprised young women from diverse racial and ethnic backgrounds living in the rural southeastern United States. In fulfilling this goal; however, it is important to note that generalizability of the results may be limited. Specific additional predisposing factors unique to this rural and low-income population (e.g., less access to consistent medical care, financial instability, and fewer occupational opportunities) may contribute to the present findings (Hartley, 2004; Vernon-Feagans & Cox, 2013) in a manner that should be considered. Future studies may wish to replicate these findings in other environments.
Despite these limitations, the implications of the present study are notable. While prior research has demonstrated associations between CM, WM, and impulsivity, the current study offers unique insight into the potential direct and indirect relations among these variables. Specifically, less efficient neural processing of WM demands may serve as a neurocognitive mechanism underlying the existing relationship between maltreatment and impulsive behavior. As efficiency in WM processing serves as a foundation for other higher order cognitive abilities such as decision-making and problem-solving, these deficits may become more impactful in real-world settings when EF demands are greater (Finn, 2002; Hinson et al., 2003). As greater impulsivity is associated with higher rates of risk-taking behaviors (e.g., substance abuse; Romer, 2010), this possible neural underpinning may represent an area for prevention and intervention. WM training interventions have demonstrated improvements in not only WM behaviorally, but in neural efficiency of a frontoparietal neural network across numerous fMRI studies (for more information, see review by Klingberg, 2010). This is notable as efficiency of the frontoparietal network has been linked to improved performance of not only WM tasks but also tasks of attention, cognitive control, and impulsivity (Corbetta & Shulman, 2002; Li et al., 2013; Somerville & Casey, 2010). While cognitive interventions that target the frontoparietal network as a whole appear promising, it is important to note findings for transfer of effects have been mixed, and further research is needed to support the efficacy of interventions in other settings and participant samples (Karbach & Verhaeghen, 2014).
Conclusions.
The current findings support existing literature that suggests that exposure to maltreatment during childhood is associated with altered patterns of performance accuracy and task-associated activation during a 2-back WM task. Furthermore, both CM and WM neural activities were significantly related to self-reported impulsivity, with the latter partially mediating the relation between CM and proclivity toward impulsive behaviors. These findings are important as they suggest that neurocognitive consequences of CM extend to impulse control, which may result in deficits of decisionmaking and subsequently engagement in risky behavior. Given the present findings, it appears that prevention and intervention strategies aimed to minimize the neurocognitive effects of CM in young adults may ameliorate CM-related elevations in impulsivity and subsequent problematic outcomes.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Mental Health (grant number K23 MH096628) and National Institute on Drug Abuse (grant number P30 DA027827).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aloia MS, Sweet LH, Jerskey BA, Zimmerman M, Arnedt JT, & Millman RP (2009). Treatment effects on brain activity during a working memory task in obstructive sleep apnea. Journal of Sleep Research, 4, 404–410. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, & Hitch G (1974). Working memory. Psychology of Learning and Motivation, 8, 47–89. [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, & Mostofsky SH (2013). Developmental changes in within-and between-network connectivity between late childhood and adulthood. Neuropsychologia, 51, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (2001). The executive functions and self-regulation: An evolutionary neuropsychological perspective. Neuropsychology Review, 11, 1–29. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, & Damasio AR (2000). Emotion, decision-making and the orbitofrontal cortex. Cerebral Cortex, 10, 295–307. [DOI] [PubMed] [Google Scholar]
- Belanger K, & Stone W (2008). The social service divide: Service availability and accessibility in rural versus urban counties and impact on child welfare outcomes. Child Welfare, 87, 101. [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistics Society, 57, 289–300. [Google Scholar]
- Bernstein DP, & Fink L (1998). Childhood trauma questionnaire: A retrospective self-report: Manual. New York: Psychological Corporation. [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, & Baxter C (2011). Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry, 69, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, & Choudhury S (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47, 296–312. [DOI] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, & Nelson CA (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience, 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt SB, McAuley T, & Barch DM (2008). Functional developmental similarities and differences in the neural correlates of verbal and nonverbal working memory tasks. Neuropsychologia, 46, 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquehais MD, Oquendo MA, Baca-García E, & Sher L (2010). Is impulsivity a link between childhood abuse and suicide? Comprehensive Psychiatry, 51, 121–129. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, & Noll DC (1997). A parametric study of prefrontal cortex involvement in human working memory. Neuroimage, 5, 49–62. [DOI] [PubMed] [Google Scholar]
- Brems C, Johnson ME, Neal D, & Freemon M (2004). Childhood abuse history and substance use among men and women receiving detoxification services. American Journal of Drug and Alcohol Abuse, 30, 799–821. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Haas BW, Garrett A, Song S, & Reiss AL (2009). Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. Journal of pediatric psychology, 35, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, & Chugani DC (2001). Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. Neuroimage, 14, 1290–1301. [DOI] [PubMed] [Google Scholar]
- Clark US, Sweet LH, Morgello S, Philip NS, & Cohen RA (2017). High early life stress and aberrant amygdala activity: Risk factors for elevated neuropsychiatric symptoms in HIV adults. Brain Imaging and Behavior, 11, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Arce Rentería M, Hegde RR, & Morgello S (2018). Early life stress-related elevations in reaction time variability are associated with brain volume reductions in HIV + adults. Frontiers in Behavioral Neuroscience, 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, … Niaura R (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological psychiatry, 59, 975–982. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, & Toth SL (2015). Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology, 27, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R (1996). AFNI: Software for analysis and visualization of functional magnetic resonance images. Computer Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cyders MA (2013). Impulsivity and the sexes: Measurement and structural invariance of the UPPS-P Impulsive Behavior Scale. Assessment, 20, 86–97. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … Lindner C (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry, 71, 286–293. [DOI] [PubMed] [Google Scholar]
- DePrince AP, Weinzierl KM, & Combs MD (2009). Executive function performance and trauma exposure in a community sample of children. Child Abuse and Neglect, 33, 353–361. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, … Hall J (2002). Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biological psychiatry, 51, 544–552. [DOI] [PubMed] [Google Scholar]
- De Bellis M (2005). The psychobiology of neglect. Child Maltreatment : Journal of the American Professional Society on the Abuse of Children, 10, 150. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, … Woolley DP (2015). Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Development and Psychopathology, 27, 1555–1576. Retrieved from 10.1017/S0954579415000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, & Maris H McCrory EJ (2013). Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry, 54, 105–112. [DOI] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, & Dackis MN (2013). Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology, 38, 1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural equation modeling, 8, 430–457. [PubMed] [Google Scholar]
- Finn PR (2002). Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behavioral and Cognitive Neuroscience Reviews, 1, 183–205. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, & Janson S (2009). Burden and consequences of child maltreatment in high-income countries. The Lancet, 373, 68–81. [DOI] [PubMed] [Google Scholar]
- Glaser D (2000). Child abuse and neglect and the brain—A review. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 41, 97–116. [PubMed] [Google Scholar]
- Grassi-Oliveira R, Ashy M, & Stein LM (2008). Psychobiology of childhood maltreatment: Effects of allostatic load? Revista brasileira de psiquiatria, 30, 60–68. [DOI] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, … Pollak SD (2010). Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30, 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, & Rubia K (2012). Neuroimaging of child abuse: a critical review. Frontiers in human neuroscience, 6, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D (2004). Rural health disparities, population health, and rural culture. American Journal of Public Health, 94, 1675–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, & Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological psychiatry, 49, 1023–1039. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, & Nemeroff CB (2008). The link between childhoodtrauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology, 33, 693–710. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, & Nemeroff CB (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology, 52, 671–690. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, & Whitney P (2003). Impulsive decision making and working memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29, 298. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, & Meier B (2010). The concurrent validity of the N-back task as a working memory measure. Memory, 18, 394–412. doi: 10.1080/09658211003702171 [DOI] [PubMed] [Google Scholar]
- Karbach J, & Verhaeghen P (2014). Making working memory work: A meta-analysis of executive-control and working memory training in older adults. Psychological Science, 25, 2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T (2010). Training and plasticity of working memory. Trends in Cognitive Sciences, 14, 317–324. [DOI] [PubMed] [Google Scholar]
- Li N, Ma N, Liu Y, He XS, Sun DL, Fu XM, … Zhang DR (2013). Resting-state functional connectivity predicts impulsivity in economic decision-making. Journal of Neuroscience, 33, 4886–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, & Rubin DB (2002). Statistical analysis with missing data. Hoboken, NJ: Wiley; c2002 Retrieved from http://proxy-remote.galib.uga.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cat00002a&AN=gua2855584&site=eds-live [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, & Vincent AS (2013). Early life adversity contributes to impaired cognition and impulsive behavior: Studies from the Oklahoma family health patterns project. Alcoholism: Clinical and Experimental Research, 37, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews. Neuroscience, 10, 434. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, & Cyders MA (2006). The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University. [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, … Beardslee WR (2001). Childhood abuse and lifetime psychopathology in a community sample. American Journal of Psychiatry, 158, 1878–1883. [DOI] [PubMed] [Google Scholar]
- Mahalik JR, Coley RL, Lombardi CM, Lynch AD, Markowitz AJ, & Jaffee SR (2013). Changes in health risk behaviors for males and females from early adolescence through early adulthood. Health Psychology. doi: 10.1037/a0031658 [DOI] [PubMed] [Google Scholar]
- Majer M, Nater UM, Lin JM, Capuron L, & Reeves WC (2010). Association of childhood trauma with cognitive function in healthy adults: A pilot study. BMC Neurology, 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, & Viding E (2010). Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry, 51, 1079–1095. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998-2017). Mplus User’s Guide. 8th Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Oshri A, Duprey E, Carlson M, Kogan S, & Liu S (2018). Growth patterns of future orientation among maltreated youth: A prospective examination of the emergence of resilience. Developmental Psychology, 54, 1456–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Liu S, Duprey EB, & MacKillop J (2018). Child maltreatment, delayed reward discounting, and alcohol and other drug use problems: The moderating role of heart rate variability. Alcoholism: Clinical and Experimental Research, 42, 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Rogosch FA, & Cicchetti D (2013). Child maltreatment and mediating influences of childhood personality types on the development of adolescent psychopathology. Journal of Clinical Child & Adolescent Psychology, 42, 287–301. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, & Bullmore E (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25, 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology, 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper GV, & Nettle D (2017). The behavioural constellation of deprivation: Causes and consequences. Behavioral and Brain Sciences, 40, e314. [DOI] [PubMed] [Google Scholar]
- Perry B, & Pollard R (1998). Homeostasis, stress, trauma, and adaptation: A neurodevelopmental view of childhood trauma. Child and Adolescent Clinics of North America, 7, 33–51. [PubMed] [Google Scholar]
- Perry BD, Pollard RA, Blakley TL, Baker WL, & Vigilante D (1995). Childhood trauma, the neurobiology of adaptation, and use dependent development of the brain: How states become traits. Infant Mental Health Journal, 16, 271–291. [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Carpenter SL, Albright SE, Price LH, … Carpenter LL (2016). Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: Implications for a commonly used working memory task. Brain Imaging Behavior, 10, 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Carpenter LL, Yuliya IK, … Niaura RS (2013). Early life stress is associated with greater default network deactivation during working memory in healthy controls: A preliminary report. Brain Imaging and Behavior, 7, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, … Gunnar MR (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development, 81, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. doi: 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Romer D (2010). Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 52, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, & Forde DR (2001). The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress, 14, 843–857. [DOI] [PubMed] [Google Scholar]
- Shah AK, Shafir E, & Mullainathan S (2015). Scarcity frames value. Psychological Science, 26, 402–412. [DOI] [PubMed] [Google Scholar]
- Shin SH, Edwards EM, & Heeren T (2009). Child abuse and neglect: Relations to adolescent binge drinking in the national longitudinal study of Adolescent Health (AddHealth) Study. Addictive Behaviors, 34, 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1995). Development and plasticity of cortical processing architectures. Science, 270, 758–764. [DOI] [PubMed] [Google Scholar]
- Smith BD, Kay ES, & Pressley TD (2018). Child maltreatment in rural southern counties: Another perspective on race, poverty and child welfare. Child Abuse & Neglect, 80, 52–61. [DOI] [PubMed] [Google Scholar]
- Smith EE, & Jonides J (1997). Working memory: A view from neuroimaging. Cognitive Psychology, 33, 5–42. [DOI] [PubMed] [Google Scholar]
- Somerville LH, & Casey BJ (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20, 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Paskavitz JF, Haley AP, Gunstad JJ, Mulligan RC, Nyalakanti PK, … Cohen RA (2008). Imaging phonological similarity effects on verbal working memory. Neuropsychologia, 46, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988) Co-planar stereotactic atlas of the hu-man brain: 3-dimensional proportional system, an approach to cerebralimaging. Stuttgard, Germany: Thieme. [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, & Kim DM (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews, 27, 33–44. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, & Andersen SL (2004). Childhood neglect is associated with reduced corpus callosum area. Biological psychiatry, 56, 80–85. [DOI] [PubMed] [Google Scholar]
- Teicher MH (2005). Childhood abuse and regional brain development: evidence for sensitive periods. American Academy of Child Adolesc. Psychiatry, 25, 78. [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17, 652–666. [DOI] [PubMed] [Google Scholar]
- Thompson MP, Kingree JB, & Desai S (2004). Gender differences in long-term health consequences of physical abuse of children: Data from a nationally representative survey. American Journal of Public Health, 94, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenard JL, Sussman S, & Stacy AW (2008). Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug and Alcohol Dependence, 94, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardosz S, & Lutzker JR (2010). Child maltreatment and the developing brain: A review of neuroscience perspectives. Aggression and Violent Behavior, 15, 59–68. [Google Scholar]
- Vernon - Feagans L, & Cox M (2013). The family life project: An epidemiological and developmental study of young children living in poor rural communities: I. Poverty, rurality, parenting, and risk: An introduction. Monographs of the Society for Research in Child Development, 78, 1–23. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, … Nemeroff CB (2002). Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry, 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanklyn SG, Day DM, Hart TA, & Girard TA (2012). Cumulative childhood maltreatment and depression among incarcerated youth: Impulsivity and hopelessness as potential intervening variables. Child Maltreatment, 17, 306–317. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, & Lynam DR (2003). Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: Application of the UPPS impulsive behavior scale. Experimental and Clinical Psychopharmacology, 11, 210. [DOI] [PubMed] [Google Scholar]
- Willoughby T, Good M, Adachi PJ, Hamza C, & Tavernier R (2013). Examining the link between adolescent brain development and risk taking from a social–developmental perspective. Brain and Cognition, 83, 315–323. [DOI] [PubMed] [Google Scholar]