Abstract
This study examined the relationship between cognitive change and instrumental activities of daily living (IADL) in a large, national, population-based sample. Cognitive change was assessed via verbal fluency, word list learning (WLL), and word list delayed recall (WLD). Incident cognitive impairment was defined by change in Six-Item Screener (SIS) status over a period of 10 years. Impaired IADL was defined as self-reported difficulty or needing assistance performing any IADL at Year 10. A one-word decrease in WLL over a 10-year span increased the odds of impaired IADL by 16% (95% CI 1.08–1.24) and incident cognitive impairment increased the odds of impaired IADL by 59% (95% CI 1.36–1.85) when adjusting for demographic factors, health-related behaviors, vascular risk factors and disease, and depressive symptoms. Cognitive change most strongly predicted impairment in managing finances (OR 2.47, 95% CI 2.04–3.00) and driving (OR 2.06, 95% CI 1.73–2.44).
Keywords: Cognitive Aging, Depression, Instrumental Activities of Daily Living, Longitudinal Studies, Stroke
INTRODUCTION
Instrumental activities of daily living (IADL) include complex daily tasks necessary for independent living (e.g., doing household chores, shopping, preparing meals, using transportation, managing finances and medications). Decline in IADL is significantly related to cognitive impairment and has been shown to predict both MCI and dementia-level cognitive impairment (Bangen et al., 2010; Barberger-Gateau et al., 1992; Peres et al., 2008; Perneczky et al., 2006; Rajan, Hebert, Scherr, Mendes de Leon, & Evans, 2013; Reppermund et al., 2013; Reppermund et al., 2011). This relationship is bidirectional; cognitive decline predicts the onset and rate of progression of functional deficits and disability (Cahn-Weiner et al., 2007; Rajan et al., 2012; Tomaszewski Farias et al., 2009). However, most of the research in this area has focused on IADL decline as a predictor of cognitive impairment; there is a relative lack of research prospectively examining cognition over time as a predictor of IADL impairment, especially in population-based samples.
The studies that have examined cognition as a predictor of IADL impairment have notable limitations: they utilized smaller samples of convenience (e.g., patients attending memory disorders clinics oversampling Alzheimer disease pathology, retirement communities oversampling affluent residents and healthy aging, or those comprised only of older adults) resulting in selection bias and limiting generalizability of results (Cahn-Weiner et al., 2007; Royall, Palmer, Chiodo, & Polk, 2004; Tomaszewski Farias et al., 2009); some of these studies did not control for relevant health variables, including depression, which have a significant impact on functional ability (Cahn-Weiner et al., 2007; Tomaszewski Farias et al., 2009); two of the published studies examined informant/clinical-rated functioning via the Blessed-Roth Dementia Rating Scale, which covers memory and orientation and is comprised of only two items relating to actual IADL as typically conceptualized (i.e., “perform household tasks”; “cope with small amounts of money”) (Cahn-Weiner et al., 2007; Tomaszewski Farias et al., 2009).
In the present study, we examined the relationship between longitudinally-assessed cognitive functioning and subsequent self-reported IADL functioning in a national, population-based sample. IADL are commonly assessed via self-report measures and have been shown to be valid in an MCI sample and less biased than informant-ratings in a non-demented sample (Dorevitch et al., 1992; Farias, Mungas, & Jagust, 2005), although informant-reported functional ability may better predict progression to dementia (McAlister, Schmitter-Edgecombe, & Lamb, 2016; Tabert et al., 2002). Furthermore, informant report is limited by the availability of a reliable informant; in one study up to 40% of informants either underreported or overreported the cognitive ability of older adults with memory difficulties (Kemp, Brodaty, Pond, & Luscombe, 2002). We hypothesized that incident cognitive impairment and cognitive decline over a 10-year period would result in significantly increased odds of IADL impairment (i.e., self-reported difficulty and/or assistance performing IADL) at follow-up, after controlling for variables that are potential confounders.
METHODS
Sample.
The REasons for Geographic and Racial Differences in Stroke (REGARDS) study is an ongoing national prospective cohort study of 30,239 adults aged 45 and above at baseline, enrolled between January 2003 and October 2007 (Howard et al., 2005). The cohort is 42% non-Hispanic African Americans (AA), 58% non-Hispanic Caucasians, 56% residents of eight southeastern “Stroke Belt” states, and 44% residents of the other 40 contiguous United States. A commercially available list was used to identify individuals who were contacted by mail, followed by telephone calls. Eligibility criteria for REGARDS included non-Hispanic AA or Caucasian race, age of 45 or greater (no upper age limit), absence of conditions associated with a life-expectancy of less than 5 years, not being in or on a waiting list for nursing home care, and ability to participate in interviews.
Trained interviewers obtained demographic information, medical history, and lifestyle factors through a computer-assisted telephone interview (CATI). Three to four weeks after the CATI, a home visit was conducted including written informed consent, a physical exam with blood pressure measurements, collection of blood and urine samples, and an electrocardiogram (ECG). Follow-up is by CATI every 6 months. Starting in April of 2013, using a similar protocol, active participants were invited to undergo a second risk factor assessment approximately 10 years after the baseline. Through December 2016, 15,434 participants completed the second in-person assessment. All participants provided informed consent for this follow-up visit. The study methods were approved by institutional review boards of all participating institutions.
In addition to participation in the Year 10 post-enrollment assessment, participants were required to have more than one Six-Item Screener (SIS) assessment for classification of incident cognitive impairment. Those with cognitive impairment at baseline (SIS ≤4) were excluded from analyses of incident cognitive impairment but not from analyses of cognitive change trajectories based on the short battery measures
Measures.
IADL.
The Minimum Data Set (MDS) is part of the United States federally mandated clinical assessment for those in Medicare or Medicaid certified nursing homes and reliably captures functional status (Hawes et al., 1995). Performance for IADL, as adapted from the MDS, was collected as a telephone-administered self-report measure during the Year 10 CATI assessment. Participants were queried to select one of three responses for each of seven IADL (i.e., doing household chores, purchasing items at a store, planning and preparing meals, managing money, using a telephone or cell phone, taking medications on time and as prescribed, and traveling by vehicle): ‘I could do it by myself with no difficulty’(0), ‘I could do it by myself with some difficulty’(1), or ‘I would need someone to help me do it’ (2). Total scores could range from 0 to 14. Impaired IADL was defined as any response other than ‘I could do it by myself with no difficulty’ (i.e., IADL ≥ 1).
Cognitive function assessments.
Cognitive measures, including the Six-Item Screener (SIS), Letter ‘F’ Fluency (LF) from the Montreal Cognitive Assessment (MoCA) (Kennedy et al., 2014) and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Animal Fluency (AF), Word List Learning (WLL), and Word List Delayed Recall (WLD) were telephone-administered longitudinally in REGARDS via standardized scripts by formally trained and certified interviewers. Telephone administration of these or similar measures is reliable and valid in older adults, with scores practically identical to in-person administration (Rapp et al., 2012; Unverzagt et al., 2007; R. S. Wilson et al., 2010).
The SIS was collected annually starting in December 2003. It consists of 3-item recall and 3-item temporal orientation (score range, 0–6). The SIS has been validated in both community and clinical samples and among both AA and Caucasian adults with strong positive and negative predictive values for the gold standard of clinically evaluated cognitive impairment inclusive of dementia (SIS score ≤4) (Callahan, Unverzagt, Hui, Perkins, & Hendrie, 2002). All other cognitive measures were collected biennially starting in 2006. The CERAD WLL measures list-learning ability across three trials (score range, 0–30) and WLD measures verbal memory (score range, 0–10) (Morris et al., 1989). AF and LF measure word generation with scores consisting of the number of valid responses generated in 60 seconds. For all measures, higher scores indicate better performance.
Covariates.
Baseline demographics included age in years, sex, race, education (‘less than high school’, ‘high school graduate’, ‘some college’, or ‘college graduate and above’), income (<$20k, $20k-$34k, $35k-$74k, ≥$75k per year, or refuse to answer), and region of residence (stroke belt or other). Baseline health covariates included self-reported physical activity (any bouts per week of intense physical activity sufficient to work up a sweat, or none), diabetes (fasting glucose >126 mL/dL, nonfasting glucose >200 mL/dL, or self-reported use of diabetes medications), hypertension (systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or self-reported use of hypertension medications), coronary artery disease (self-reported physician diagnosis of myocardial infarction, self-reported coronary revascularization, or evidence of myocardial infarction on ECG), and self-reported history of stroke (yes or no). Incident stroke during follow-up was adjudicated by study physicians using medical and hospital records (yes or no). Depressive symptoms were measured concurrently with the IADL assessment at the Year 10 post-enrollment visit using the 10-item Center for Epidemiological Studies Depression Scale (CES-D). A CES-D score ≥10 was used to indicate high depression risk (score range 0–30) (Andresen, Malmgren, Carter, & Patrick, 1994).
Statistical analyses.
Missingness was examined for covariates: 11% of included participants had a least one missing value; with 0.63% of data points missing overall. Multiple imputation was conducted and regression analyses were run on each imputed data set (m=5) and pooled to compute parameter estimates (Rubin, 1996, 2004).
For analyses that included the short battery of continuous cognitive measures as the predictor of interest, a two-stage model was used with the first stage being the estimation of slopes using separate linear mixed-effect models for each continuous cognitive measure (AF, LF, WLL, WLD) while controlling for age at baseline (Diggle, 2002). Time was computed as years from individual baseline date to date of respective assessments. The second stage used the individual estimated slopes from the mixed models (multiplied by −1 to code for decline in performance) in regression models (Diggle, 2002). For analyses that included incident cognitive impairment as the predictor of interest, incident cognitive impairment was defined as a shift from a SIS score ≥5 at the first assessment to a SIS score ≤4 at the latest available assessment. Time between the first and last SIS assessment was controlled for in respective models. Analyses were also performed for a stratified sample of those ≥65 years of age, of interest due increased risk of neurodegenerative dementia. Incrementally-adjusted logistic regression models were conducted for the cognitive predictors of interest.
Several sensitivity analyses were conducted. For analyses with the SIS as a predictor variable, we examined more rigorous definitions of incident cognitive impairment (i.e., a shift to ≤4 at the last two available assessments) as well as incident dementia-level impairment (i.e., a shift to ≤3 at the latest available assessment). Analyses were conducted using IBM SPSS Statistics Version 24.0 (Armonk, NY: IBM Corp.) and SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
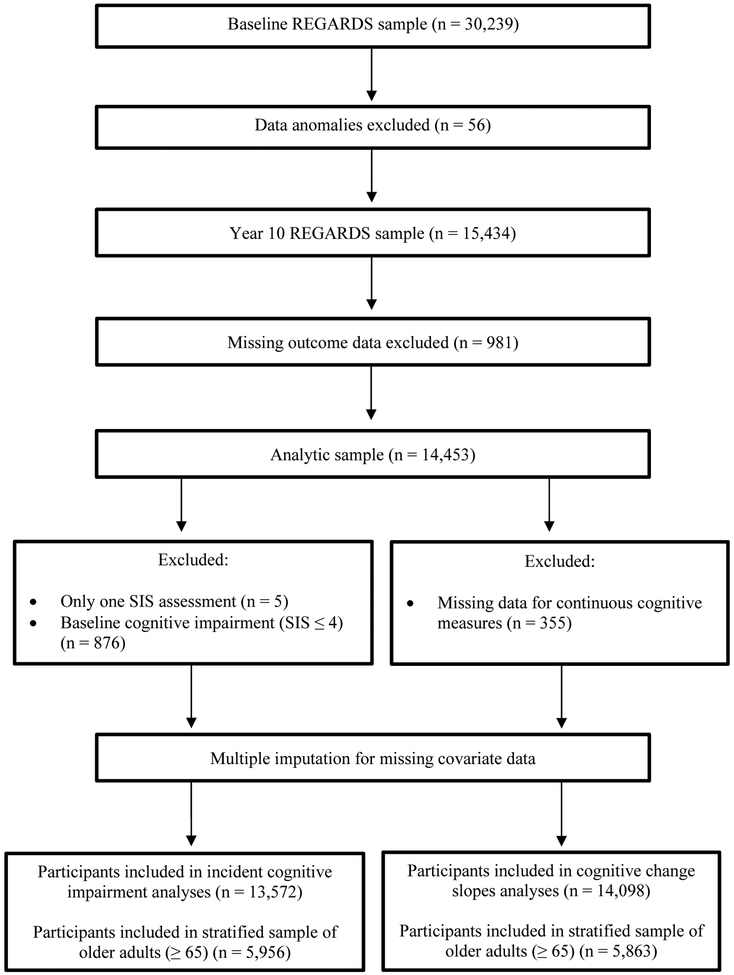
A total of 14,453 participants with complete outcome data at the Year 10 visit post-enrollment comprised the analysis cohort. Figure 1 displays a graphical representation of the participants. Table 1 presents the sample’s descriptive characteristics by IADL status; 33.6% endorsed IADL impairment. Those with impaired IADL were older, more likely to be women and AA, have lower education and income, less likely to engage in physical activity, have higher prevalence of cardiovascular risk factors/disease, and endorsed higher depressive symptom. Table 2 presents individual IADL item frequencies, including item frequencies by incident cognitive impairment status. The most endorsed impaired IADL item was “doing household chores” (21.5%) and the least endorsed item was “using a telephone or cell phone” (3.9%).
Figure 1 -. Participant Cohorts.
A visualization of participant cohorts for the present study analysis, as derived from the total REasons for Geographic and Racial Differences in Stroke (REAGRDS) sample. Sample sizes are displayed by exclusion criteria for respective predictors of interest (i.e., incident cognitive impairment and cognitive change slopes).
Table 1 -.
Baseline Participant Characteristics by IADL Status
| Variables | IADL = 0 (n = 9,602) | IADL ≥ 1 (n = 4,851) | p-value |
|---|---|---|---|
| Age (at baseline), mean (SD) | 62.68 (7.89) | 65.46 (8.72) | < .001 |
| Female | 5,161 (53.7%) | 3,031 (62.5%) | < .001 |
| African American | 3,340 (34.8%) | 2,067 (42.6%) | < .001 |
| Education | < .001 | ||
| Less than high school | 536 (5.6%) | 580 (12%) | |
| High school graduate | 2,097 (21.8%) | 1,321 (27.2%) | |
| Some college | 2,534 (26.4%) | 1,304 (26.9%) | |
| College graduate and above | 4,435 (46.2%) | 1,646 (33.9%) | |
| Income | < .001 | ||
| < $20,000 | 884 (9.2%) | 962 (19.8%) | |
| $20,000 - $34,000 | 1,886 (19.6%) | 1,285 (26.5%) | |
| $35,000 - $74,000 | 3,436 (35.8%) | 1,407 (29%) | |
| $75,000 + | 2,439 (25.4%) | 591 (12.2%) | |
| Refused | 957 (10%) | 606 (12.5%) | |
| Residing in stroke belt | 5,278 (55%) | 2,679 (55.2%) | .51 |
| Physical activity (any) | 7,120 (74.2%) | 3,052 (62.9%) | < .001 |
| Diabetes | 1,323 (13.8%) | 1,170 (24.1%) | < .001 |
| Hypertension | 4,723 (49.2%) | 3,129 (64.5%) | < .001 |
| Coronary artery disease | 1,017 (10.6%) | 873 (18%) | < .001 |
| Self-reported baseline stroke | 237 (2.5%) | 306 (6.3%) | < .001 |
| Incident adjudicated stroke | 207 (2.2%) | 266 (5.5%) | < .001 |
| Incident cognitive impairment | 508 (5.3%) | 624 (12.9%) | < .001 |
| High depression risk (CES-D ≥10 at Year 10) | 487 (5.1) | 1,174 (24.2) | < .001 |
CES-D = Center for Epidemiological Studies Depression Scale
IADL = 0 means no difficulty or assistance needed for any IADL
IADL ≥ 1 means difficulty or assistance needed for one or more IADL
Table 2 -.
Frequency of Specific IADL Items Endorsed by Incident Cognitive Impairment
| Variables | Incident cognitive impairment n = 1,132 |
No incident cognitive impairment n = 13,321 |
||
|---|---|---|---|---|
| Instrumental Activities of Daily Living | Difficulty | Assistance | ||
| Doing household chores | 2,007 (13.9%) | 1,100 (7.6%) | 329 (29%) | 2,778 (20.8%) |
| Purchasing items at the store | 804 (5.6%) | 785 (5.4%) | 242 (21.4%) | 1,347 (10.1%) |
| Planning and preparing meals | 1000 (6.9%) | 967 (6.7%) | 283 (25%) | 1,684 (12.7%) |
| Managing your money, such as paying bills | 477 (3.3%) | 650 (4.5%) | 248 (21.9%) | 879 (6.6%) |
| Using a telephone or cell phone | 458 (3.2%) | 107 (0.7%) | 97 (8.5%) | 468 (3.5%) |
| Taking medications on time and as prescribed by the doctor | 630 (4.4%) | 255 (1.8%) | 150 (13.2%) | 735 (5.6%) |
| Traveling by vehicle to places beyond walking distance | 668 (4.6%) | 1,217 (8.4%) | 354 (31.2%) | 1,531 (11.5%) |
| 1–2 IADL difficulty or assistance needed | 358 (31.6%) | 2,868 (21.5%) | ||
| 3–4 IADL difficulty or assistance needed | 141 (12.5%) | 904 (6.8%) | ||
| 5+ IADL difficulty or assistance needed | 125 (11%) | 455 (3.4%) | ||
Change slopes for the effect of each decade of time on cognitive measures revealed approximately 2 less AF words generated per decade of follow up (b= −0.23), 1 less LF word generated (b= −0.08), 0.4 additional words learned for WLL (b=0.04), and 0.2 additional words recalled for WLD (b=0.02) (p<.001 for all models). Table 3 presents the logistic regression summaries for models including estimated cognitive change slopes as the predictors of interest for impaired IADL; only WLL decline significantly predicted impaired IADL in the fully adjusted model. In the fully adjusted model for the full sample, a one-word decrease in WLL over a 10-year span increased the odds of impaired IADL by 19% (95% CI 1.11–1.27). The association of a one-word decrease in WLL over a 10-year span with impairment of specific IADL items, when accounting for control variables, was: ‘using a telephone or cell phone’ (OR 1.33, 95% CI 1.14–1.54); ‘managing your money, such as paying bills’ (OR 1.32, 95% CI 1.18–1.48); ‘purchasing items at a store’ (OR 1.28, 95% CI 1.16–1.41); ‘traveling by vehicle to places beyond walking distance’ (OR 1.21, 95% CI 1.10–1.33); ‘doing household chores’ (OR 1.13, 95% CI 1.05–1.22); and ‘planning and preparing meals’ (OR 1.13, 95% CI 1.04–1.24). ‘Taking medications on time and as prescribed by the doctor’ was not significantly related to a one-word decrease in WLL score.
Table 3 -.
Odds Ratios for Associations Between Cognitive Change Slopes and IADL Impairment †
| Unadjusted | Model 1 | Model 2 | Model 3 | Model 3 (age ≥ 65) | |
|---|---|---|---|---|---|
| Letter “F” Fluency | 1.02 (0.95–1.09) | 1.02 (0.95–1.10) | 1.02 (0.95–1.10) | 1.03 (0.96–1.11) | 1.02 (0.92–1.14) |
| Animal Fluency | 0.95 (0.92–0.98)b | 0.99 (0.95–1.02) | 0.99 (0.96–1.03) | 1.00 (0.96–1.04) | 1.03 (0.98–1.10) |
| Word List Learning Sum | 1.35 (1.27–1.43)c | 1.26 (1.18–1.34)c | 1.22 (1.14–1.30)c | 1.19 (1.11–1.27)c | 1.23 (1.12–1.35)c |
| Word List Learning Delayed Recall | 0.90 (0.81–1.01) | 0.86 (0.76–0.96)a | 0.89 (0.79–1.00) | 0.92 (0.81–1.04) | 0.93 (0.78–1.10) |
| Age (years at baseline) | 1.05 (1.04–1.05)c | 1.05 (1.04–1.05)c | 1.05 (1.05–1.06)c | 1.09 (1.08–1.10)c | |
| African American | 1.17 (1.09–1.27)c | 1.04 (0.96–1.13) | 1.06 (0.97–1.15) | 1.11 (0.97–1.26) | |
| Female | 1.29 (1.20–1.40)c | 1.34 (1.23–1.45)c | 1.30 (1.20–1.41)c | 1.36 (1.21–1.54)c | |
| Education | - | - | - | - | |
| Less than high school vs college graduate | 1.51 (1.30–1.76)c | 1.38 (1.18–1.60)c | 1.19 (1.02–1.40)a | 1.20 (0.96–1.49) | |
| High school graduate vs college graduate | 1.18 (1.06–1.30)b | 1.09 (0.99–1.21) | 1.05 (0.95–1.17) | 1.03 (0.88–1.20) | |
| Some college vs college graduate | 1.14 (1.04–1.25)b | 1.09 (0.99–1.20) | 1.05 (0.95–1.16) | 1.04 (0.90–1.21) | |
| Income | - | - | - | - | |
| $20,000–34,000 vs < $20,000 | 0.70 (0.61–0.79)c | 0.72 (0.63–0.81)c | 0.76 (0.67–0.87)c | 0.79 (0.66–0.95)a | |
| $35,000-$75,000 vs < $20,000 | 0.51 (0.45–0.58)c | 0.55 (0.48–0.62)c | 0.60 (0.53–0.69)c | 0.70 (0.58–0.85)b | |
| $75,000 or more vs < $20,000 | 0.38 (0.33–0.44)c | 0.42 (0.36–0.49)c | 0.49 (0.42–0.57)c | 0.74 (0.58–0.95)a | |
| Residing in stroke belt | 0.99 (0.92–1.06) | 0.97 (0.90–1.05) | 0.96 (0.89–1.04) | 0.94 (0.84–1.06) | |
| Physical Activity (any vs none) | 0.66 (0.61–0.72)c | 0.70 (0.65–0.76)c | 0.68 (0.60–0.77)c | ||
| Diabetes (yes or no) | 1.55 (1.41–1.72)c | 1.50 (1.36–1.67)c | 1.39 (1.20–1.61)c | ||
| Hypertension (yes or no) | 1.31 (1.21–1.41)c | 1.30 (1.20–1.42)c | 1.28 (1.13–1.44)c | ||
| Coronary artery disease (yes or no) | 1.45 (1.30–1.62)c | 1.40 (1.26–1.57)c | 1.39 (1.20–1.62)c | ||
| Self-reported baseline stroke (yes or no) | 1.74 (1.44–2.10)c | 1.61 (1.32–1.95)c | 1.72 (1.31–2.27)c | ||
| Incident adjudicated stroke (yes or no) | 1.92 (1.57–2.34)c | 1.86 (1.51–2.28)c | 1.72 (1.32–2.25)c | ||
| High depression risk (CES-D ≥10 at Year 10) | 5.33 (4.72–6.02)c N = 14,098 |
5.07 (4.16–6.18)c N = 5,863 |
Estimates are odds ratios (95% confidence intervals); CES-D = Center for Epidemiological Studies Depression Scale
p < .05;
p < .01;
< .001
Table 4 presents the model summaries for incident cognitive impairment as the predictor of interest. In the fully adjusted model, incident cognitive impairment increased the odds of impaired IADL by 64% (95% CI 1.41–1.90). In fully-adjusted sensitivity analyses, the association was larger for the more rigorous definition of persisting incident cognitive impairment on the SIS at the last two assessments (OR 2.14, 95% CI 1.65–2.78) and for incident dementia-level impairment (OR 1.91, 95% CI 1.53–2.39) (supplemental table). The association of incident cognitive impairment with impairment of specific IADL items, when accounting for control variables, was as follows: ‘managing your money, such as paying bills’ (OR 2.47, 95% CI 2.04–3.00); ‘traveling by vehicle to places beyond walking distance’ (OR 2.06, 95% CI 1.73–2.44); ‘purchasing items at a store’ (OR 1.51, 95% CI 1.25–1.83); ‘taking medications on time and as prescribed by the doctor’ (OR 1.50, 95% CI 1.19–1.89); ‘using a telephone or cell phone’ (OR 1.44, 95% CI 1.10–1.88); and ‘planning and preparing meals’ (OR 1.36, 95% CI 1.14–1.62). ‘Doing household chores’ was not significantly related to incident impairment.
Table 4 -.
Odds Ratios for Associations Between Incident Cognitive Impairment and IADL Impairment †
| Unadjusted | Model 1 | Model 2 | Model 3 | Model 3 (age ≥ 65) | |
|---|---|---|---|---|---|
| Cognitive impairment (SIS ≤ 4) | 2.62 (2.29–2.99)c | 1.76 (1.52–2.02)c | 1.75 (1.52–2.03)c | 1.64 (1.41–1.90)c | 1.48 (1.23–1.77)c |
| Assessment interval (days) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Age (years at baseline) | 1.05 (1.04–1.05)c | 1.04 (1.04–1.05)c | 1.05 (1.05–1.06)c | 1.09 (1.07–1.10)c | |
| African American | 1.14 (1.05–1.24)b | 1.01 (0.92–1.09) | 1.02 (0.93–1.11) | 1.07 (0.93–1.22) | |
| Female | 1.27 (1.17–1.37)c | 1.32 (1.21–1.43)c | 1.28 (1.18–1.40)c | 1.32 (1.16–1.50)c | |
| Education | - | - | - | - | |
| Less than high school vs college graduate | 1.55 (1.33–1.82)c | 1.40 (1.19–1.64)c | 1.21 (1.03–1.43)a | 1.20 (0.95–1.51) | |
| High school graduate vs college graduate | 1.15 (1.03–1.27)b | 1.05 (0.95–1.17) | 1.01 (0.91–1.13) | 0.98 (0.83–1.15) | |
| Some college vs college graduate | 1.13 (1.03–1.24)a | 1.08 (0.98–1.19) | 1.03 (0.93–1.14) | 1.03 (0.88–1.19) | |
| Income | - | - | - | - | |
| $20,000–34,000 vs < $20,000 | 0.70 (0.61–0.78)c | 0.71 (0.62–0.81)c | 0.76 (0.66–0.87)c | 0.83 (0.68–1.00) | |
| $35,000-$75,000 vs < $20,000 | 0.50 (0.44–0.57)c | 0.54 (0.47–0.61)c | 0.59 (0.51–0.67)c | 0.70 (0.57–0.85)c | |
| $75,000 or more vs < $20,000 | 0.36 (0.31–0.42)c | 0.40 (0.34–0.47)c | 0.47 (0.40–0.55)c | 0.70 (0.55–0.91)b | |
| Residing in stroke belt | 0.96 (0.89–1.04) | 0.94 (0.87–1.02) | 0.94 (0.87–1.02) | 0.90 (0.80–1.01) | |
| Physical Activity (any vs none) | 0.66 (0.61–0.72)c | 0.71 (0.65–0.77)c | 0.69 (0.61–0.77)c | ||
| Diabetes (yes or no) | 1.57 (1.42–1.74)c | 1.51 (1.36–1.68)c | 1.39 (1.19–1.62)c | ||
| Hypertension (yes or no) | 1.33 (1.22–1.44)c | 1.33 (1.22–1.45)c | 1.31 (1.16–1.48)c | ||
| Coronary artery disease (yes or no) | 1.45 (1.29–1.62)c | 1.40 (1.24–1.57)c | 1.36 (1.17–1.59)b | ||
| Self-reported baseline stroke (yes or no) | 1.72 (1.42–2.10)c | 1.61 (1.31–1.97)c | 1.77 (1.33–2.36)c | ||
| Incident adjudicated stroke (yes or no) | 2.02 (1.64–2.48)c | 1.99 (1.61–2.46)c | 1.82 (1.38–2.40)c | ||
| High depression risk (CES-D ≥10 at Year 10) | 5.55 (4.89–6.29)c N = 13,572 |
5.25 (4.26–6.48)c N = 5,956 |
Estimates are odds ratios (95% confidence intervals); SIS = Six-Item Screener; CES-D = Center for Epidemiological Studies Depression Scale
p < .05;
p < .01;
p < .001
DISCUSSION
We examined the association of cognitive change with subsequent self-reported IADL functioning in a national, population-based sample and demonstrated that incident cognitive impairment and decline in word list learning lead to self-reported IADL impairment (difficulty and/or assistance) in a national sample of adults from the 48 contiguous United States. This is also the first study we are aware of to delineate longitudinal cognitive change to impairments on specific IADL items in a national, population-based sample.
Managing finances and driving showed the largest discrepancies between those with incident cognitive impairment vs. those without, suggesting that these self-reported IADL may be particularly indicative of impaired cognition in this sample, which is concordant with the research showing that financial ability is the earliest IADL impacted by cognitive decline, likely due to its complex and multifaceted nature. It is also the IADL that most strongly predicts conversion to dementia (Gold, 2012). In fact, impairments for more cognitively demanding IADL, such as managing finances, has been shown to precede MCI diagnosis (Reppermund et al., 2013). This underlines the importance of examining specific IADL impairments in addition to broad functional ability in both healthy older adults and those with cognitive impairment, which is a strength of the present study.
Among the variables that were significantly related to IADL impairment in the models, high depression risk, health conditions (especially history of stroke and incident stroke), incident cognitive impairment, income level (i.e., high income was a protective factor), and being female showed the largest effect sizes. Interestingly, having lower education and being African American were not strongly associated with IADL impairment in the REGARDS sample despite a consistent relationship in the literature, suggesting that other variables (e.g., income, health status) may be in part responsible for this finding or that the magnitude of effect of education may be decreasing as the rate of functional impairment over time decreases (Freedman, Martin, & Schoeni, 2002). Being female is a well-established risk factor for functional impairment, which has been explained by the higher prevalence of chronic but nonfatal diseases and the higher prevalence of Alzheimer’s disease in females (Alzheimer’s, 2015; Murtagh & Hubert, 2004).
Despite variability across studies, deficits in executive functioning (namely, executive components of attention and working memory as opposed to word generation) and memory domains are the most implicated in functional impairment (Gold, 2012; McAlister et al., 2016). This is consistent with our findings, which showed that a decline in word list learning ability, an index of attention and working memory, was the only significant predictor of functional impairment among the four short battery measures. Long-delay and more contextually-based memory measures (e.g., narratives) seem to better predict functional ability (McAlister et al., 2016), which may explain in part why a modest decline in episodic memory (CERAD word list recall following a 5 minute delay) was not strongly associated with functional impairment in the current study. Although change slopes for continuous cognitive measures were coded for decline for the purposes of predictive models, the sample showed a very slight increase in WLL and WLD scores over time (i.e., less than 1 word increase over a 10-year time span). This was also true in a separate study utilizing the REGARDS sample (Levine et al., 2015), which is suggestive of a small practice effect that is characteristic of healthy survivors and not unexpected for a cohort study.
Although there is an established relationship between depression and IADL (Freedman et al., 2002; Rajan et al., 2013), this study highlighted the importance of depressive symptom burden and self-reported IADL impairment. The strong relationship between these variables is likely due in part to the fact that depressive symptoms and IADL were collected at the same time, depressive symptoms (e.g., amotivation) impact participation in day-to-day activities, and that depression as a state produces a strong tendency towards negative appraisals and symptom endorsement. This is important as there also is strong association between depression and dementia in older adults (Byers & Yaffe, 2011), such as an increased risk of developing Alzheimer’s disease (Robert S Wilson et al., 2002). A proposed mechanism for this association conceives of depression as contributing to both the unmasking of clinical symptoms and hippocampal damage (Jorm, 2001).
Primary strengths of this study are the large population-based sample and the long duration of follow-up. While etiology of cognitive impairment was not clinically determined, the inclusion of multiple medical comorbidities enabled us to control for common conditions such as hypertension and diabetes, and relatively rare conditions such as stroke. Therefore, the relationship of cognitive change over time to self-reported IADL status as elucidated in this study is highly generalizable to the population, both across the continuum of severity of cognitive decrements and encompassing both medical and all-cause cognitive change. This is important as poor cognitive functioning has been shown to be related to sharp functional decline, followed by death, even in the absence of dementia (Dodge, Du, Saxton, & Ganguli, 2006).
A limitation of this study was the lack of informant-reported IADL status, which would have helped assess the validity of the results. The preservation of insight is a complex and heterogeneous construct related to multiple variables (Howorth & Saper, 2003). Etiology of cognitive decline is important when considering level of insight, especially given that clinic studies tend to oversample amnestic MCI populations. Generally speaking, the validity of self-reported status decreases as the severity of cognitive impairment increases. However, evaluation of self-reported functional ability is important and widely used as a reliable informant may not always be available (particularly in the context of cohort studies) and the validity of informant-report may vary depending on the relationship to the individual in question. As in any longitudinal study examining health and older adults, participants are lost to follow-up due to increasing age, declining health, cognitive impairment, or death (Chatfield, Brayne, & Matthews, 2005). However, in a separate REGARDS study, longitudinal change was not different among returning participants and those lost to follow-up (Levine et al., 2015). Overall, attrition does not appear to affect relationships between variables and therefore does not necessarily limit generalizability (Goodman & Blum, 1996; Gustavson, von Soest, Karevold, & Roysamb, 2012; Salthouse, 2014).
IADL was measured at one time point in the present study. Research is lacking regarding the validity of self-reported severity and duration of impairment, but it is likely to be less accurate and more subject to recall bias as compared to self-report of current status. Duration of symptoms may be better ascertained by longitudinal serial assessments of functional status, which is an important future direction of this research. While the literature suggests that the association between functional and cognitive ability is comparable for questionnaires and performance-based measures, it is important to note that these methods are not highly correlated and are not equivalent to one another (McAlister et al., 2016). Incorporating a multimodal approach of assessing IADL status in future studies (including both self and informant-report questionnaires and performance-based measures) would increase the validity of findings and further elaborate on the relationship of cognition and IADL. Controversy remains as to how well any measure maps on to everyday, real-world functioning (McAlister et al., 2016). Given that ecological validity is essential to assessing functional ability, an additional important future direction would be to assess functional ability in-vivo when possible (e.g., on-the-road driving assessment, naturalistic in-home assessment).
Incident cognitive impairment significantly and strongly resulted in increased odds of self-reported IADL impairment at 10-year follow-up after controlling for multiple demographic and health variables in this large, population-based sample. Our results inform the relationship of cognitive change to IADL and imply that screening for impaired IADL is useful in population-based samples. In addition, it is not cognitive decline per se that is most feared by older adults. Rather, it is the loss of independence in performing cognitively demanding activities of daily living that is the greater fear and more clinically meaningful loss.
Supplementary Material
ACKNOWLEDGEMENTS
The REGARDS research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
This work was supported in part by National Institute on Aging (NIA) grant P30AG022838 (UAB Roybal Center) and National Center for Advancing Translational Sciences (NCATS) award number UL1TR00165 (UAB Center for Clinical and Translational Science).
Footnotes
Conflicts of Interest: The authors report no financial, personal, or potential conflicts of interest.
REFERENCES
- Alzheimer’s A (2015). 2015 Alzheimer’s disease facts and figures. Alzheimers Dement, 11(3), 332–384. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, & Patrick DL (1994). Screening for Depression in Well Older Adults: Evaluation of. Prev Med, 10, 77–84. [PubMed] [Google Scholar]
- Bangen KJ, Jak AJ, Schiehser DM, Delano-Wood L, Tuminello E, Han SD, … Bondi MW (2010). Complex activities of daily living vary by mild cognitive impairment subtype. J Int Neuropsychol Soc, 16(4), 630–639. doi: 10.1017/S1355617710000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberger-Gateau P, Commenges D, Gagnon M, Letenneur L, Sauvel C, & Dartigues JF (1992). Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc, 40(11), 1129–1134. [DOI] [PubMed] [Google Scholar]
- Byers AL, & Yaffe K (2011). Depression and risk of developing dementia. Nat Rev Neurol, 7(6), 323–331. doi: 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Farias ST, Julian L, Harvey DJ, Kramer JH, Reed BR, … Chui H (2007). Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc, 13(5), 747–757. doi: 10.1017/S1355617707070853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, & Hendrie HC (2002). Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care, 40(9), 771–781. doi: 10.1097/01.MLR.0000024610.33213.C8 [DOI] [PubMed] [Google Scholar]
- Chatfield MD, Brayne CE, & Matthews FE (2005). A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol, 58(1), 13–19. doi: 10.1016/j.jclinepi.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Diggle P (2002). Analysis of longitudinal data: Oxford University Press. [Google Scholar]
- Dodge HH, Du Y, Saxton JA, & Ganguli M (2006). Cognitive domains and trajectories of functional independence in nondemented elderly persons. J Gerontol A Biol Sci Med Sci, 61(12), 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorevitch MI, Cossar RM, Bailey FJ, Bisset T, Lewis SJ, Wise LA, & MacLennan WJ (1992). The accuracy of self and informant ratings of physical functional capacity in the elderly. J Clin Epidemiol, 45(7), 791–798. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, & Jagust W (2005). Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry, 20(9), 827–834. doi: 10.1002/gps.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman VA, Martin LG, & Schoeni RF (2002). Recent trends in disability and functioning among older adults in the United States: a systematic review. JAMA, 288(24), 3137–3146. [DOI] [PubMed] [Google Scholar]
- Gold DA (2012). An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J Clin Exp Neuropsychol, 34(1), 11–34. doi: 10.1080/13803395.2011.614598 [DOI] [PubMed] [Google Scholar]
- Goodman JS, & Blum TC (1996). Assessing the non-random sampling effects of subject attrition in longitudinal research. Journal of Management, 22(4), 627–652. doi: 10.1016/S0149-2063(96)90027-6 [DOI] [Google Scholar]
- Gustavson K, von Soest T, Karevold E, & Roysamb E (2012). Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health, 12, 918. doi: 10.1186/1471-2458-12-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes C, Morris JN, Phillips CD, Mor V, Fries BE, & Nonemaker S (1995). Reliability estimates for the Minimum Data Set for nursing home resident assessment and care screening (MDS). Gerontologist, 35(2), 172–178. [DOI] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, … Howard G (2005). The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology, 25(3), 135–143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- Howorth P, & Saper J (2003). The dimensions of insight in people with dementia. Aging Ment Health, 7(2), 113–122. doi: 10.1080/1360786031000072286 [DOI] [PubMed] [Google Scholar]
- Jorm AF (2001). History of depression as a risk factor for dementia: an updated review. Australian and New Zealand Journal of Psychiatry, 35(6), 776–781. [DOI] [PubMed] [Google Scholar]
- Kemp NM, Brodaty H, Pond D, & Luscombe G (2002). Diagnosing dementia in primary care: the accuracy of informant reports. Alzheimer Dis Assoc Disord, 16(3), 171–176. [DOI] [PubMed] [Google Scholar]
- Kennedy RE, Wadley VG, McClure LA, Letter AJ, Unverzagt FW, Crowe M, … Howard G (2014). Performance of the NINDS-CSN 5-minute protocol in a national population-based sample. J Int Neuropsychol Soc, 20(8), 856–867. doi: 10.1017/S1355617714000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, & Wadley VG (2015). Trajectory of Cognitive Decline After Incident Stroke. JAMA, 314(1), 41–51. doi: 10.1001/jama.2015.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister C, Schmitter-Edgecombe M, & Lamb R (2016). Examination of Variables That May Affect the Relationship Between Cognition and Functional Status in Individuals with Mild Cognitive Impairment: A Meta-Analysis. Arch Clin Neuropsychol, 31(2), 123–147. doi: 10.1093/arclin/acv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, … Clark C (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39(9), 1159–1165. [DOI] [PubMed] [Google Scholar]
- Murtagh KN, & Hubert HB (2004). Gender differences in physical disability among an elderly cohort. Am J Public Health, 94(8), 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres K, Helmer C, Amieva H, Orgogozo JM, Rouch I, Dartigues JF, & Barberger-Gateau P (2008). Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. J Am Geriatr Soc, 56(1), 37–44. doi: 10.1111/j.1532-5415.2007.01499.x [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Sorg C, Hartmann J, Komossa K, Alexopoulos P, … Kurz A (2006). Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing, 35(3), 240–245. doi: 10.1093/ageing/afj054 [DOI] [PubMed] [Google Scholar]
- Rajan KB, Hebert LE, Scherr P, Dong X, Wilson RS, Evans DA, & Mendes de Leon CF (2012). Cognitive and physical functions as determinants of delayed age at onset and progression of disability. J Gerontol A Biol Sci Med Sci, 67(12), 1419–1426. doi: 10.1093/gerona/gls098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan KB, Hebert LE, Scherr PA, Mendes de Leon CF, & Evans DA (2013). Disability in basic and instrumental activities of daily living is associated with faster rate of decline in cognitive function of older adults. J Gerontol A Biol Sci Med Sci, 68(5), 624–630. doi: 10.1093/gerona/gls208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Legault C, Espeland MA, Resnick SM, Hogan PE, Coker LH, … Group, C. A. T. S. (2012). Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc, 60(9), 1616–1623. doi: 10.1111/j.1532-5415.2012.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppermund S, Brodaty H, Crawford JD, Kochan NA, Draper B, Slavin MJ, … Sachdev PS (2013). Impairment in instrumental activities of daily living with high cognitive demand is an early marker of mild cognitive impairment: the Sydney memory and ageing study. Psychol Med, 43(11), 2437–2445. doi: 10.1017/S003329171200308X [DOI] [PubMed] [Google Scholar]
- Reppermund S, Sachdev PS, Crawford J, Kochan NA, Slavin MJ, Kang K, … Brodaty H (2011). The relationship of neuropsychological function to instrumental activities of daily living in mild cognitive impairment. Int J Geriatr Psychiatry, 26(8), 843–852. doi: 10.1002/gps.2612 [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, & Polk MJ (2004). Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc, 52(3), 346–352. [DOI] [PubMed] [Google Scholar]
- Rubin DB (1996). Multiple imputation after 18+ years. Journal of the American statistical Association, 91(434), 473–489. [Google Scholar]
- Rubin DB (2004). Multiple imputation for nonresponse in surveys (Vol. 81): John Wiley & Sons. [Google Scholar]
- Salthouse TA (2014). Selectivity of attrition in longitudinal studies of cognitive functioning. J Gerontol B Psychol Sci Soc Sci, 69(4), 567–574. doi: 10.1093/geronb/gbt046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, … Devanand DP (2002). Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology, 58(5), 758–764. [DOI] [PubMed] [Google Scholar]
- Tomaszewski Farias S, Cahn-Weiner DA, Harvey DJ, Reed BR, Mungas D, Kramer JH, & Chui H (2009). Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. Clin Neuropsychol, 23(3), 446–461. doi: 10.1080/13854040802360558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, Monahan PO, Moser LR, Zhao Q, Carpenter JS, Sledge GW Jr., & Champion VL (2007). The Indiana University telephone-based assessment of neuropsychological status: a new method for large scale neuropsychological assessment. J Int Neuropsychol Soc, 13(5), 799–806. doi: 10.1017/S1355617707071020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes L, De Leon CM, Aggarwal N, Schneider J, Bach J, … Evans D (2002). Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology, 59(3), 364–370. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Foroud TM, Sweet RA, Graff-Radford N, Mayeux R, … National Institute on Aging Late-Onset Alzheimer’s Disease Family Study, G. (2010). Telephone assessment of cognitive function in the late-onset Alzheimer’s disease family study. Arch Neurol, 67(7), 855–861. doi: 10.1001/archneurol.2010.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.