Abstract
From quorum sensing in bacteria to pheromone signaling in social insects, chemical communication mediates interactions among individuals in a local population. In Caenorhabditis elegans, ascaroside pheromones can dictate local population density, in which high levels of pheromones inhibit the reproductive maturation of individuals. Little is known about how natural genetic diversity affects the pheromone responses of individuals from diverse habitats. Here, we show that a niche-associated variation in pheromone receptor genes contributes to natural differences in pheromone responses. We identified putative loss-of-function deletions that impair duplicated pheromone receptor genes (srg-36 and srg-37), which were shown previously to be lost in population-dense laboratory cultures. A common natural deletion in srg-37 arose recently from a single ancestral population that spread throughout the world and underlies reduced pheromone sensitivity across the global C. elegans population. We found that many local populations harbor individuals with wild-type or a deletion allele of srg-37, suggesting that balancing selection has maintained the recent variation in this pheromone receptor gene. The two srg-37 genotypes are associated with niche diversity underlying boom-and-bust population dynamics. We hypothesize that human activities likely contributed to the gene flow and balancing selection of srg-37 variation through facilitating migration of species and providing favorable niche for recently arose srg-37 deletion.
Introduction
To maximize reproductive success, organisms must respond to changing environmental conditions. In a fluctuating environment, each response will likely have a fitness trade-off with reproductive success now or in the future. Caenorhabditis elegans can either grow to a reproductive adult in three days or delay maturity for months by entering the dauer diapause stage1. Food supply and pheromone signals act oppositely to promote either further reproductive growth or the development of a stress-resistant and long-lived dauer stage2,3. C. elegans secretes sugar-based pheromone compounds called ascarosides4, and animals must measure the amount of remaining food and the ascaroside pheromones to determine if it is advantageous to continue reproductive growth or enter the dauer stage to disperse and hopefully encounter a new food source. Therefore, dauer formation decreases reproductive success in the short-term in favor of future survival success. Decades of research have provided insights into the chemical and genetic basis of the dauer-pheromone response5. However, most studies used a single laboratory-adapted strain (N2), which has limited our understanding of the natural processes that have shaped the dauer-pheromone response.
After decades of focused laboratory research on C. elegans as a model organism, the natural history of this species has only recently been described from extensive field research6. These field studies have revealed that the dauer stage is important for the population dynamics in their natural habitat7. These dynamics are typified by a “boom” phase after initial colonization of a nutrient-rich habitat, followed by a “bust” phase when resources are depleted. At the end of the boom phase when the local population size is large and nutrients are limited, individual animals enter the dauer stage. Dauers exhibit a stage-specific behavior called nictation, which facilitates interspecific interactions between dauer larvae and more mobile animals to disperse to favorable environments8,9. Because dauer larvae are presumed to play a crucial role in the survival and dispersal of the species, it is likely that the genetic controls of dauer formation are under natural selection. Although differences in dauer development among a small number of wild C. elegans strains have been described previously10–15, no underlying natural genetic variant has been identified. Here, we integrate laboratory experiments, computational genomic analyses, and field research to further our understanding of the genetic basis underlying intraspecific variation in pheromone-mediated developmental plasticity. We identify natural genetic variation in responses to dauer pheromone and characterize a pheromone receptor allele that has spread around the globe.
RESULTS
Natural variation of the dauer-pheromone response was measured using a high-throughput dauer assay
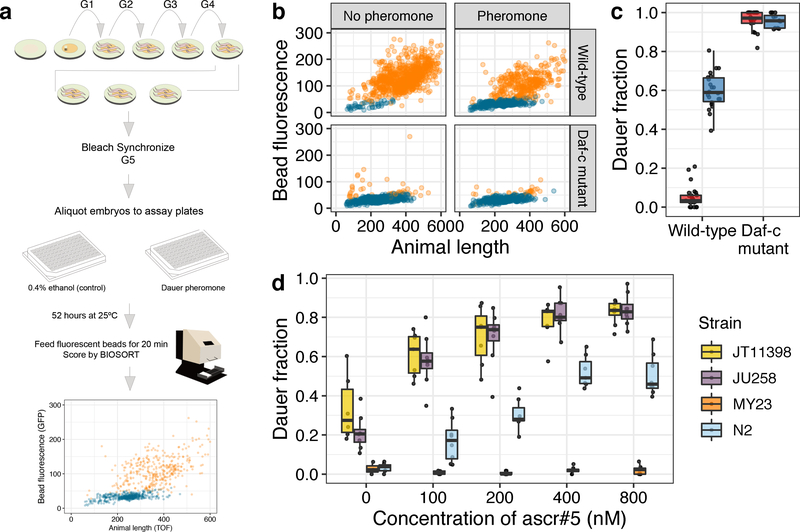
To explore the effects of natural genetic variation on the ability to enter the dauer stage, we developed a high-throughput dauer assay (HTDA) to quantify the dauer-pheromone responses of wild C. elegans strains. The HTDA takes advantage of the observation that dauer larvae have no pharyngeal pumping16. We treated animals with fluorescent microspheres that can be ingested and then quantified both fluorescence and size of individual animals using a large-particle flow cytometer (COPAS BIOSORT, Union Biometrica). These data facilitated computational classification of dauers (Fig. 1a, b; Materials and methods) and recapitulated the known differences in the dauer-pheromone responses between N2 and a constitutive dauer mutant daf-2(e1370), as well as the dauer-inducing effect of synthetic pheromone (Fig. 1b, c). To determine if genetic variation within the C. elegans species causes differential dauer-pheromone responses, we applied the HTDA to four genetically divergent C. elegans strains after treatment with various concentrations of three known dauer-inducing synthetic ascarosides (ascr#2, ascr#3, and ascr#5). We found significant variation in the dauer-pheromone responses among the strains tested, as measured by the fraction of individuals that enter the dauer stage (Fig. 1d, Supplementary Fig. 1). Among the conditions we tested, we found that 800 nM ascr#5 maximizes the among-strain variance and minimizes the within-strain variance in dauer-pheromone response. These results enabled us to survey the effects of genetic variation on the dauer-pheromone response across the C. elegans species.
Fig. 1: A high-throughput dauer assay measures natural variation of dauer-pheromone response.
(a) The workflow for the high-throughput dauer assay (HTDA) using a COPAS BIOSORT is shown (see Materials and methods for further description). (b) Optical measurements of the laboratory wild-type strain (N2) (Top) and a Daf-c mutant daf-2(e1370) (bottom) are shown under control (left) and pheromone-treated (ascr#5 800 nM) conditions (right) at 25°C using the HTDA. Animal size and fluorescence-intensity traits are used as variables to build a model that differentiates dauer (blue) and non-dauer populations (orange). Relative animal length measured by time-of-flight (TOF) is shown on the x-axis, and bead-derived fluorescent intensity is shown on the y-axis. (c) Tukey box plots of the dauer fraction quantification from (b) are shown with data points plotted behind. Box plots are colored by assay conditions (control (red) and ascr#5 800 nM treatment (blue)). The genotypes are shown on the x-axis, and fractions of dauer larvae are shown on the y-axis. (d) Tukey box plots of the ascr#5 dose response at 25°C for four divergent strains are shown with data points plotted behind. Concentrations of ascr#5 are shown on the x-axis, and fractions of dauer larvae are shown on the y-axis. (c, d) The horizontal line in the middle of the box is the median, and the box denotes the 25th to 75th quantiles of the data. The vertical line represents the 1.5 interquartile range.
Genome-wide association (GWA) mapping reveals multiple loci underlying natural variation of the ascr#5 response
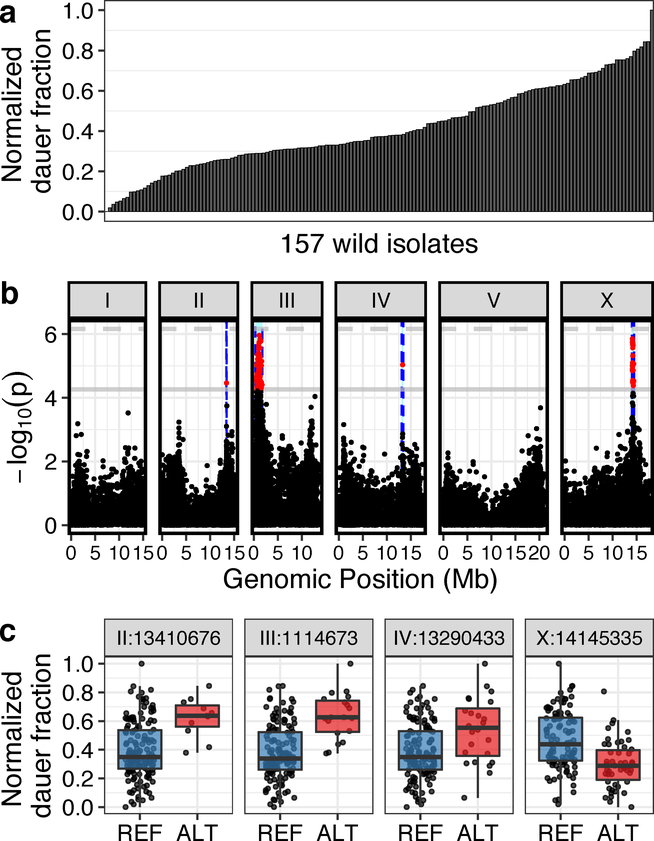
Next, we quantified dauer induction of 157 wild strains that have been isolated from diverse habitats across six continents (Supplementary Fig. 2)17,18. We found significant variation in the ascr#5 response with a broad-sense heritability estimate of 0.29 (H2, SE=0.14) and a narrow-sense heritability estimate of 0.18 (h2, SE=0.12) (Fig. 2a; Materials and methods). The two strains that represent the phenotypic extremes of the ascr#5 response are EG4349 and JU2576, where EG4349 did not enter dauer and was completely insensitive to ascr#5 treatment, and a large fraction of the JU2576 individuals entered the dauer stage in the same condition. Overall, we observed a continuous distribution of dauer-pheromone responses among these wild strains (mean = 0.41, standard deviation = 0.20), indicating that natural variation in this trait is likely not explained by a single gene.
Fig. 2: Genome-wide association (GWA) mapping reveals four major loci underlying natural variation in dauer-pheromone response.
(a) A bar plot for the natural variation of ascr#5-induced dauer formation at 25°C across 157 C. elegans wild isolates (one-way analysis of variance (ANOVA), log10p = −49.6598) is shown. Each bar represents the phenotypic response of a single wild isolate to 800 nM ascr#5. (b) A manhattan plot for single-marker based GWA mapping of the ascr#5-induced dauer formation trait from (a) is shown. Each dot represents a single-nucleotide variant (SNV) that is present in at least 5% of the 157 wild strains. The genomic position in Mb, separated by chromosome, is plotted on the x-axis, and the statistical significance of the correlation between genotype and phenotype is plotted on the y-axis. Two significance thresholds are shown. The horizontal dashed line denotes the Bonferroni-corrected p-value threshold using all markers, and the gray horizontal line denotes the Bonferroni-corrected p-value threshold using independent markers correcting for linkage disequilibrium (genome-wide eigen-decomposition significance threshold). SNVs are colored red if they pass the second threshold. The region of interest for each QTL is represented by vertical blue dotted lines. (c) Tukey box plots of phenotypes split by peak marker position of the four QTL (chrII:13410676, chrIII:1114673, chrIV:13290433, chrX:14145335) are shown. Each dot corresponds to the phenotype of an individual strain, which is plotted on the y-axis as the normalized dauer fraction phenotype. Strains are grouped by their genotype at each peak QTL position, where REF (blue) corresponds to the reference allele from the laboratory N2 strain and ALT (red) corresponds to the alternative allele. The horizontal line in the middle of the box is the median, and the box denotes the 25th to 75th quantiles of the data. The vertical line represents the 1.5 interquartile range.
To characterize the quantitative trait loci (QTL) associated with variation in the ascr#5 response, we performed genome-wide association (GWA) mappings and identified four QTL (Fig. 2b, c). The QTL that explained the most variation in pheromone-induced dauer induction (15.9%) is on the right arm of the X chromosome. Strains that have the non-reference (ALT) allele at the peak marker (X:14,145,335) of this QTL were less responsive to ascr#5 treatment than strains that have the reference (REF) allele (REF mean: 0.46; ALT mean: 0.30, log10p = −5.851505). The remaining QTL on chromosomes II, III, and IV, explain 8.4%, 15.1%, and 5.4% of the variation in the ascr#5 response, respectively. Because population structure can drive mapping of loci that are in interchromosomal linkage disequilibrium (LD) with causal QTL, we checked the LD among four QTL. We did not detect any obvious LD among these QTL (Supplementary Fig. 3), suggesting that multiple independent genomic loci underlie natural variation in the ascr#5 response.
A putative loss-of-function allele in an ascr#5 receptor gene is associated with reduced dauer formation
We focused our efforts on the largest effect QTL, which we named dauf-1 (dauer-formation QTL #1). The 469-kb surrounding the dauf-1 peak marker contains 82 protein-coding genes (Supplementary Fig. 4), including the duplicated genes srg-36 and srg-37, which encode ascr#5 receptors19. Both genes are expressed in the same pair of chemosensory neurons (ASI), which play an essential role in the dauer-pheromone response20,21. Notably, previous studies reported that both srg-36 and srg-37 are repeatedly deleted during long-term propagation of two independent laboratory-domesticated C. elegans lineages in high-density liquid cultures19.
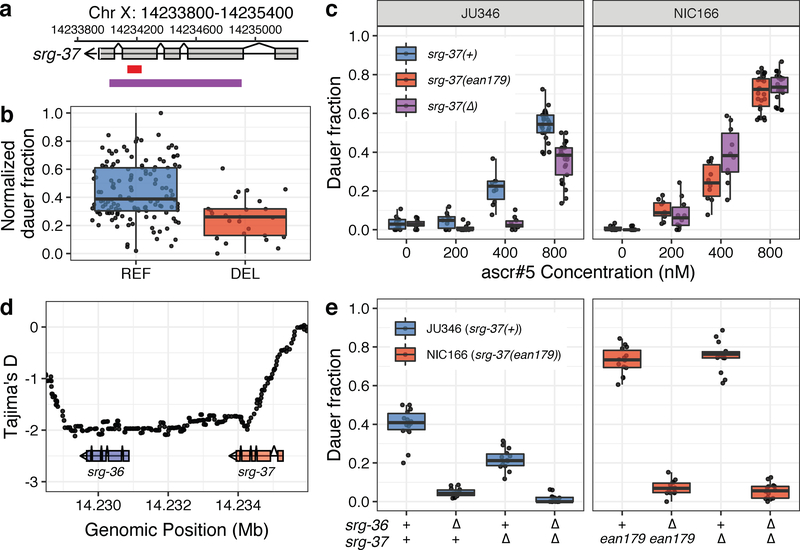
To evaluate whether similar mutations in these two genes underlie the dauf-1 QTL, we investigated the genome sequences of 249 wild strains available through the C. elegans Natural Diversity Resource (CeNDR)22,23. Although we could not find a large deletion that removes both srg-36 and srg-37, we found only one strain with a 411-bp deletion in srg-36 and many other strains with an identical 94-bp deletion in srg-37 (Fig. 3a, Supplementary Fig. 5). We named these deletions srg-36(ean178) and srg-37(ean179). To test whether these deletions can explain the dauf-1 QTL effect, we analyzed the association between the ascr#5 response and the two deletions. First, we found that srg-36(ean178), which is a deletion found only in the PB303 strain and removes the fourth and fifth exons, is associated with an insensitivity to a high dose of ascr#5 (2 μM) (Supplementary Fig. 6). Because this deletion allele was not found in any other wild strains, srg-36(ean178) cannot explain the population-wide differences in dauer formation. By contrast, we found that all wild strains with the srg-37(ean179) deletion belong to dauf-1(ALT) group and had reduced ascr#5 sensitivity (Fig. 3b, Welch’s t-test, p = 3.152e-06), suggesting that this deletion allele might cause a reduction in the acr#5 response.
Fig. 3: Natural variant in the ascr#5 receptor gene, srg-37, underlies natural differences in dauer formation.
(a) A schematic plot for the srg-37 gene structure (grey), 94-bp natural deletion allele ean179 (red), and CRISPR-Cas9 genome-editing target sequences for the putative loss-of-function deletion (purple) are shown. (b) Tukey box plots of dauer formation split by srg-37 genotype are shown. Each dot corresponds to the phenotype of an individual strain, which is plotted on the y-axis by the normalized dauer fraction. Strains are grouped by their srg-37 genotype, where REF (blue) corresponds to the wild-type reference allele from the laboratory N2 strain and DEL (red) corresponds to the natural 94-bp deletion allele (ean179). (c) Tukey box plots of the ascr#5 dose-response differences at 25°C among two wild isolates and srg-37(lf) mutants in both backgrounds are shown with data points plotted behind. A dose response comparison is shown between (Left) JU346 srg-37(+) (blue) and JU346 srg-37(lf) (purple) and (Right) NIC166 srg-37(ean179) (red) and NIC166 srg-37(lf) (purple). The concentration of ascr#5 is shown on the x-axis, and the fraction of dauer formation is shown on the y-axis. (d) Tajima’s D statistics across the srg-36 srg-37 locus are shown. Each dot corresponds to a Tajima’s D statistic calculated from the allele frequency spectrum of 50 SNVs across 249 wild isolates. The gene structures of srg-36 (blue) and srg-37 (pink) are shown below the plot. The genomic position in Mb is plotted on the x-axis, and Tajima’s D statistics are plotted on the y-axis. (e) Tukey box plots of srg-36 and srg-37 loss-of-function experiments under control (red, 0.4% ethanol) and ascr#5 pheromone conditions (blue, 2 μM of ascr#5) at 25°C are shown with data points plotted behind. Genotypes of srg-36 and srg-37 are shown on the x-axis, where triangles represent the CRISPR-Cas9-mediated deletions. Fractions of dauer formation are shown on the y-axis. (b, c, e) The horizontal line in the middle of the box is the median, and the box denotes the 25th to 75th quantiles of the data. The vertical line represents the 1.5 interquartile range.
The srg-37(ean179) deletion removes 31 amino acids surrounding the pocket structure of the G protein-coupled receptor and causes a frameshift mutation for the 46 C-terminal amino acids, together removing 23% (77/324) of the predicted SRG-37 amino acid sequence. Thus, this deletion likely impairs SRG-37 function, which could cause lower ascr#5 sensitivity. We hypothesized that, if srg-37(ean179) causes loss of gene function, removal of additional srg-37 coding sequence would not further reduce the ascr#5 sensitivity of srg-37(ean179) wild strains. Using CRISPR-Cas9 genome-editing24,25, we removed most of the coding sequences of srg-37 from wild strains with both wild-type (reference-like) srg-37 and the natural srg-37 deletion (Fig. 3a). Indeed, we observed that a large deletion in srg-37 did not change the ascr#5 sensitivities of two wild isolates with the natural deletion, but reduced the ascr#5 sensitivities of five wild isolates with reference-like srg-37 (Fig. 3c, Supplementary Fig. 7), indicating that the natural deletion is likely a loss-of-function allele. Taken together, these results show that deletion of an ascr#5 receptor gene underlies natural variation in the dauer-pheromone response across the C. elegans population.
Selection has shaped the genetic variation of the two duplicated C. elegans ascr#5-receptor genes
We performed population genetic analysis across the srg-36 and srg-37 region by analyzing the genome sequences of 249 wild strains. Natural selection and demographic change can shift the allele frequency spectrum from neutrality, as measured by Tajima’s D26. Purifying selection, a selective sweep, or a recent population expansion can cause accumulation of rare alleles at a given locus, indicated by a negative Tajima’s D value. We found that the Tajima’s D values were lowest across the promoter and coding regions of srg-36 and increase back to background neutrality rates in the promoter region of srg-37 (Fig. 3d, Supplementary Fig. 8). Differences in deletion allele frequencies between srg-36 and srg-37 suggest stronger purifying selection at srg-36. The 411-bp deletion allele, srg-36(ean178), is only found in a single wild isolate (PB303), whereas 18.4% (46/249) of wild isotypes (genome-wide genotypes) carry the 94-bp deletion allele, srg-37(ean179).
Although srg-36 and srg-37 are duplicated genes that are activated by the same ligand and are expressed in the same cells, differences in non-coding and coding sequences between the two genes can cause differences in gene expression levels and receptor activities. Notably, previous studies report that transgene expression of srg-36 showed a stronger effect than srg-37 on the ascr#5 response19. To test whether srg-36, which is likely under stronger purifying selection than srg-37, plays a larger role in the ascr#5 response, we performed loss-of-function experiments. We removed the entire srg-36 coding region in two wild strains – JU346 with wild-type (reference-like) srg-37 and NIC166 with the natural srg-37 deletion (Supplementary Fig. 5). First, we found that srg-36(lf) reduced ascr#5 sensitivity of both strains, indicating that srg-36 is functional in both genetic backgrounds (Fig. 3e). Second, we observed that loss of srg-36 reduced ascr#5 sensitivity more than loss of srg-37, supporting the conclusion that srg-36 plays a larger role than srg-37 in the ascr#5 response.
The higher activity of srg-36 could be explained by differences in gene expression levels. We investigated the relative levels of srg-36 and srg-37 at the L1 stage, when these genes play critical roles in the dauer-pheromone response, and found that the expression levels of both genes are not significantly different (Supplementary Fig. 9, Paired t-test, p = 0.1981; Materials and methods). It is more likely that differences in protein-coding sequences cause the functional differences in the ascr#5 response. Although SRG-36 and SRG-37 show similarities in size and transmembrane structures (Supplementary Fig. 10)27, only 46.4% of the amino acid residues are conserved between both receptors. The molecular differences between the two ascr#5 receptors could cause quantitative differences in ascr#5-receptor activities. Taken together, we hypothesized that srg-36 is the primary ascr#5-receptor gene and is maintained across the C. elegans species through purifying selection. By contrast, the redundancy of these two genes might allow srg-37 variation and a loss-of-function allele can arise and spread across the population.
The srg-37 deletion has spread globally and outcrossed with diverse genotypes
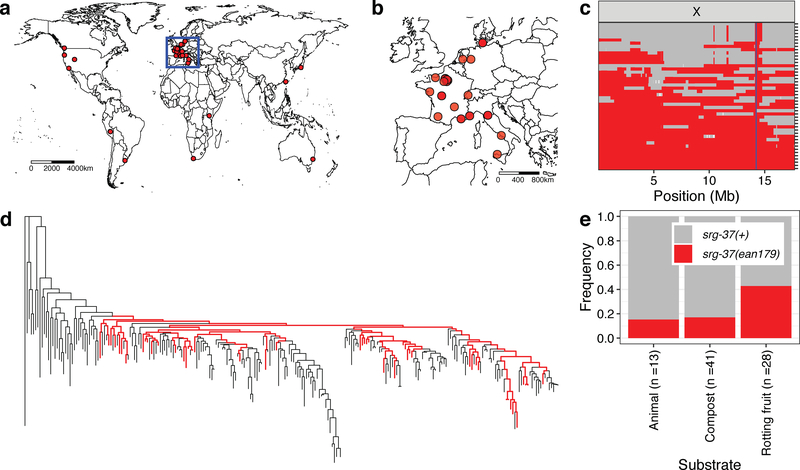
We investigated the locations where wild strains with the natural srg-37 deletion were isolated and found 46 wild isotypes with this allele were isolated from all six continents (Fig. 4a, b). Given the low probability of acquiring the same 94-bp deletion, we hypothesized that this allele did not independently arise across multiple global locations but originated from a single ancestral population and spread throughout the world. To test this hypothesis, we analyzed the haplotype composition of C. elegans wild isolates across the X chromosome. We reproduced previous studies that showed a recent global selective sweep on the X chromosome (Supplementary Fig. 11, 12)28. Notably, we found that all 46 isotypes with the srg-37 deletion exclusively share the swept haplotype at the srg-37 locus (Fig. 4c). By contrast, none of 203 isotypes with wild-type srg-37 carries the swept haplotype at the srg-37 locus. This result not only demonstrates that this allele arose at a single location, but also implies that it has spread throughout the world along with the recent selective sweep. Because the srg-37 locus is far from the most swept part of the X chromosome, many strains must have outcrossed, suggesting that srg-37 is unlikely the driver of the X chromosome sweep. Specifically, we found that 34.1% (85/249) of wild isotypes have an X chromosome that is swept more than 50% of its length but have diverse non-swept haplotypes at the srg-37 locus (Supplementary Fig. 13). Additionally, the genome-wide tree of 249 wild C. elegans isotypes shows that the srg-37 deletion is not present in many subpopulations (Fig. 4d). These results suggest that the srg-37 deletion spread globally with the selective sweeps but has been purged after more recent outcrossing.
Fig. 4: Worldwide and niche-associated gene flow shape the ascaroside (ascr#5) pheromone receptor locus.
(a) The global distribution of wild strains that contain the srg-37(ean179) deletion allele (red circle) is shown. (b) The geographic distribution of wild strains that are sampled from Europe (inset). Wild strains that contain the srg-37 deletion (red circle) are shown. (a, b) Scale bars are shown in the map. (c) Sharing of the swept haplotype on the X chromosome among 46 wild isotypes with srg-37(ean179) is shown. Each row is one of the 46 isotypes, ordered roughly by the extent of swept-haplotype sharing (red). Other haplotypes are colored grey. Genomic position of X chromosome is shown on the x-axis. The blue line shows the position of the srg-37 locus. (d) The genome-wide tree of 249 C. elegans wild isolates with those strains that have the srg-37 deletion shown as red. (e) Stacked bar plots of srg-37(+) (grey) and srg-37(ean179) (red) allele frequencies among three subpopulations that were sampled from different substrates across hybrid zone in Europe (see Materials and methods). Substrate types and sample sizes are shown on the x-axis, and allele frequencies of each allele are shown on the y-axis.
Two different srg-37 genotypes coexist in local habitat and associate with different niches
These signatures of multiple outcrossing events imply the co-occurance of wild strains with and without the srg-37 deletion in the same habitats. Indeed, we found that many local populations across the world harbor distinct individuals with either the wild-type srg-37 or the deletion allele (Supplementary Fig. 14, see Materials and methods). Because each genotype can be adaptive to different environmental conditions, we analyzed the allele frequencies of the srg-37 deletion among three subpopulations sampled from animals, compost, and rotting fruits across geographic locations where both srg-37 alleles were isolated. Because reduction of the dauer-pheromone response can promote reproductive growth, we investigated whether wild strains with the srg-37 deletion were sampled more often from substrates with proliferating populations. These populations are often found in nutritious habitats, such as rotting vegetation29. By contrast, C. elegans were sampled predominantly in the dauer stage from animal and compost substrates6,30. We found that wild strains with the srg-37 deletion were 67% enriched in rotting fruits (Fig. 4e, Supplementary Data 1, hypergeometric test, p = 0.0026). Thus, this allele is not only associated with lower dauer-pheromone responses but also with natural substrates that are known to support reproductive growth. Additionally, we analyzed FST statistics of the entire X chromosome for subpopulations from different substrates across shared geographic regions. Consistent with the niche association pattern of srg-37 genotypes, we found the highest genetic divergence between the subpopulation from rotting fruit and the subpopulation from animal substrates at a genomic locus around the srg-37 gene (Supplementary Fig. 15).
Discussion
Dauer pheromones are chemical signals that are perceived by sensory neurons using chemoreceptors and cGMP-mediated signaling5,31. In the absence of dauer-pheromone signaling, the insulin/IGF-1 and TGF-β signaling pathways promote reproductive growth through the production of steroid hormones (dafachronic acid)32. Genetic variation in the genes that mediate pheromone perception or downstream signaling likely alter an individual’s dauer-pheromone response. However, because the signaling pathways that act downstream of pheromone perception are involved in various biological processes33,34, mutations in these pathways might cause deleterious pleiotropic effects. Previous studies have shown that the ascr#5-receptors SRG-36 and SRG-37 were lost in two independent laboratory lineages of C. elegans19, suggesting that selection more readily acts at the pheromone perception step of this developmental pathway. In this study, we provide further support for this hypothesis by showing that 18% of wild C. elegans strains harbor a putative loss-of-function deletion in only the ascr#5-receptor SRG-37, and that these individuals are more likely to be found in nutrient-rich habitats. Thus, modification of pheromone-receptor activity might be favored in both laboratory and natural conditions to fine-tune dauer-pheromone responses with few pleiotropic effects19,35. However, we identified additional dauer-pheromone response QTL, suggesting that multiple loci are involved in ascr#5 responses. Interestingly, SRG-36 and SRG-37 are the only two known ascr#5 receptors involved in dauer-pheromone signaling. The presence of three additional ascr#5-response QTL suggest that natural genetic variants could affect uncharacterized ascr#5 receptors, novel or known factors that regulate receptor activity, or downstream signaling components.
Insights into the redundant functions of srg-36 and srg-37 were first gained from the observation that both genes were deleted from two independent laboratory-domesticated C. elegans lineages19. We did not find a single wild strain in the C. elegans population that carries a deletion of both srg-36 and srg-37. Investigations of neutrality statistics (Tajima’s D) suggest that selection acts on these two genes differently. Our results indicate that the srg-36 and srg-37 genes might not be functionally equivalent in the wild population. The loss-of-function experiments suggest that srg-36 plays a larger role in the ascr#5 response than srg-37. Substantial differences in amino acid sequences between SRG-36 and SRG-37 suggest that the SRG-37 protein is likely to have less ascr#5 binding affinity or weaker signal transduction activity than SRG-36. It is also possible that redundancy between SRG-36 and SRG-37 has been reduced since the time of gene duplication, and SRG-37 could gain sensitivities to other ascarosides while SRG-36 has maintained its ascr#5 specificity. Given the important role of the dauer stage in the long-term survival and dispersal of the species, purifying selection might act to conserve the primary ascr#5 receptor (SRG-36) in the C. elegans population to maintain the responsiveness to the dauer-inducing pheromone ascr#5.
In contrast to the rare deletion of srg-36, we identified a common deletion allele (18% allele frequency) of srg-37 in the global C. elegans population. Notably, we discovered that strains harboring different srg-37 genotypes (wild-type and deletion) have been found often in close proximity at various locations across the world, suggesting that balancing selection might have maintained both genotypes in local habitats. Previously, features of balancing selection were also reported for a locus with other pheromone receptor genes (srx-43 and srx-44) that underlie differences in C. elegans density-dependent foraging behavior36,37. Differences in food distribution can exert bidirectional fitness effects on foraging behavior. Similar to these effects, dauer formation can be disadvantageous during the population growth phase (boom phase) but beneficial during the dispersal phase (bust phase). Therefore, we hypothesize that the loss of srg-37, which reduces dauer formation, has trade-off effects between the boom and bust phases. Niche association patterns of srg-37 genotypes support this hypothesis. We found that wild strains with the srg-37 deletion are enriched in a rotting fruit niche, where ample bacterial food can support population growth during the boom phase. By contrast, the srg-37 deletion is not enriched in wild strains isolated from animal carriers, which is consistent with known behavioral ecology during the bust phase when dauer larvae can readily hitchhike on other animals for their dispersal6,8,9. Our FST analysis also demonstrated significant genetic divergence at the srg-37 locus between wild strains isolated from rotting fruit and animal carrier substrates. These observations suggest that the boom-and-bust population dynamics in wild habitats likely drive balancing selection of srg-37.
Population genomic analyses of the srg-37 locus imply that the srg-37 deletion arose recently and balancing selection might only have occurred for a short period of time. First, we found that strains with the srg-37 deletion all share the same swept haplotype at the srg-37 locus, which is estimated to have spread worldwide in the last few centuries28. Because mutation and recombination decrease linkage disequilibrium between a selected allele and the surrounding variants over time, this haplotype homogeneity suggests that the deletion allele arose recently. Second, we found no genomic signatures of long-term balancing selection. Tajima’s D statistics for the srg-37 locus did not show typical features of long-term balancing selection (D>>0). We also found that genetic diversity (π) is reduced at the srg-37 locus in strains that carry the srg-37 deletion versus strains that carry the srg-37(+) wild-type allele (Supplementary Fig. 16). This result is a signature of a recently established balanced situation38. We hypothesize that this recent balancing selection is related to human activities, which were also suggested to be drivers of the recent global selective sweeps28. Agriculture could have provided nutritious niches and therefore expanded boom phases spatiotemporally, which is likely to cause an increase in selective pressures to maintain the srg-37 deletion. Furthermore, human migration could facilitate the worldwide gene flow of the srg-37 deletion allele. Our studies imply that human civilization might exert a large impact on the natural selection and evolution of wild species.
Materials and methods
C. elegans strains
Animals were cultured at 20°C on modified nematode growth medium (NGMA) seeded with the E. coli strain OP5039. Prior to each assay, strains were passaged for at least four generations without entering starvation or encountering dauer-inducing conditions. For the genome-wide association (GWA) studies, 157 wild isolates from CeNDR (version 20170531) were used22,23. All strain information can be found in Supplementary Data 2.
High-throughput dauer assay
Strains were propagated for four generations on agar plates, followed by bleach synchronization. Approximately 50 embryos were titered and placed into each well of a 96-well microtiter plate filled with 50 μL of K medium40 with modified salt concentrations (10.2 mM NaCl, 32 mM KCl, 3 mM CaCl2, 3 mM MgSO4), 50 μM kanamycin, 5 mg/mL HB101 bacterial lysate (Pennsylvania State University Shared Fermentation Facility, State College, PA), and synthetic ascaroside41 dissolved in 0.4% ethanol or 0.4% ethanol alone. Animals were cultured for 52 hours at 25°C until they reached the young adult stage or arrested at the dauer stage. Animals were exposed to 0.5 μm fluorescent microspheres (Polysciences, cat. # 19507–5) at a final concentration of 7.28 × 108 particles/mL and 5 μL of 1 mg/mL HB101 bacterial lysate to promote feeding for 20 minutes. After this exposure, 200 μL of 50 mM sodium azide was added to each well to kill the animals, stop feeding, and straighten the animals. Using the COPAS BIOSORT large particle flow cytometer (Union Biometrica, Holliston MA), optical parameters of animals, including fluorescence intensity, time-of-flight (TOF, animal length), and extinction (optical density) were measured. Measured parameters were used to build a model that can differentiate dauer and adult stages of the population in each well through the R package EMCluster42. One cluster with lower fluorescence and smaller body size was assigned to the dauer population and the other to the non-dauer population. The dauer fraction was calculated per well as a fraction of dauer animals among total animals, which is shown as a single data point in each plot. From the control experiments, both the false positive ratio (false dauer detection in a wild-type sample without pheromone treatment) and the false negative ratio (false non-dauer detection in Daf-c mutant sample) were 5%, indicating 95% accuracy of the assay (Fig. 1b,c).
Genome-wide association mapping
A genome-wide association (GWA) mapping was performed using phenotype data from 157 wild C. elegans strains. The dauer fraction of 157 wild strains in ascr#5-treated (800 nM) conditions were measured from four batches of experiments with three independent high-throughput dauer assays each. Contaminated, over-crowded (n > 80), or uncrowded (n < 20) samples were filtered out from the dataset. Normalized dauer fraction was calculated using a linear model, dauer fraction ~ batch. Genotype data were acquired from the latest VCF release (Release 20180527) from CeNDR that was imputed as described previously22. We used BCFtools43 to filter variants that had any missing genotype calls and variants that were below 5% minor allele frequency. We used PLINK v1.944,45 to LD-prune the genotypes at a threshold of r2 < 0.8, using --indep-pairwise 50 10 0.8. The pruned genotype set comprised 72,568 markers that were used to generate the realized additive kinship matrix using the A.mat function in the rrBLUP R package46. These markers were also used for genome-wide mapping. However, because these markers still have substantial LD within this genotype set, we performed eigen decomposition of the correlation matrix of the genotype matrix using eigs_sym function in Rspectra package47. The correlation matrix was generated using the cor function in the correlateR R package48. We set any eigenvalue greater than one from this analysis to one and summed all of the resulting eigenvalues49. This number was 915.621, which corresponds to the number of independent tests within the genotype matrix. We used the GWAS function in the rrBLUP package to perform genome-wide mapping with the following command: rrBLUP::GWAS(pheno = dauer, geno = Pruned_Markers, K = KINSHIP, min.MAF = 0.05, n.core = 1, P3D = FALSE, plot = FALSE). Regions of interest are defined as +/− 100 SNVs from the rightmost and leftmost markers above the eigen-decomposition significance threshold. If regions of interest for separate QTL are within 1000 SNVs, they become grouped as a single region of interest.
Heritability calculations
Broad-sense heritability (H2) and narrow-sense heritability (h2) estimates were calculated using the phenotype data of 157 wild strains from the GWA mapping (ascr#5 800 nM). The A.mat and E.mat functions in the sommer R package were used to generate an additive genotype matrix and an epistatic genotype matrix, respectively, from the genotype matrix used for the GWA mapping50. These matrices were used to calculate the additive and epistatic variance components using the sommer mmer function. Variance components were used to estimate heritability and standard error through the pin function (H2 ~ V1 + V2 / V1 + V2 + V3, h2 ~ V1 / V1 + V2 + V3) in the sommer package.
Identification of natural deletion variants of srg-36 and srg-37
Whole-genome sequence data were aligned to WS245 using bwa (version 0.7.8-r455) with the following default parameters (t=1, k=19, w=100, d=100, r=1.5, c=10000, A=1, B=4, O=6, E=1, L=5, U=9, T=30, v=3). Optical/PCR duplicates were marked with PICARD (version 1.111)22,51–53. Alignments with greater than 100X coverage were subsampled to 100X using sambamba54. We called large deletions using the Manta structural variant caller (v1.4.0) using the default caller and filter settings (MinQUAL = 20, MinGQ = 15, MinSomaticScore = 30, MaxMQ0Frac = 0.4)55.
Generation of srg-36 and srg-37 deletion strains
srg-36 and srg-37 loss-of-function mutant strains were generated by CRISPR-Cas9-mediated genome editing, using a co-CRISPR approach and Cas9 ribonucleoprotein (RNP) delivery24,25. crRNAs synthesized by IDT (Skokie, IL) targeting srg-36 (exon 1 and the 3’ UTR) and srg-37 (exon 2 and exon 5) were used to generate deletions. The injection mixture (10 μL) was prepared with 0.88 μL of 200 μM tracrRNA (IDT, Product #1072532), 0.88 μL of 100 μM crRNA1 (5’ targeting) and crRNA2 (3’ targeting), and 0.12 μL of 100 μM dpy-10 crRNA (IDT) were mixed and incubated at 95°C for five minutes. After cooling to room temperature, 2.87 μL of 60 μM Cas9 protein (IDT Product #1074181) was added and incubated at room temperature for five minutes. Finally, 0.5 μL of 10 μM dpy-10 ssODN (IDT) repair template and 3.99 μL of nuclease-free water were added. RNP injection mixtures were microinjected into the germline of young adult hermaphrodites (P0), and injected animals were singled to fresh 6 cm NGM plates 18 hours after injection. Two days later, F1 progeny were screened, and animals expressing a Rol phenotype were transferred to new plates and allowed to generate progeny (F2). Then, F1 animals were genotyped by PCR. Deletion of srg-36 was detected with primers oECA1460–1463 and deletion of srg-37 was detected with primers oECA1429, oECA1430, and oECA1435. Non-Rol progeny (F2) of F1 animals positive for the desired deletion were propagated on separate plates to generate homozygous progeny. F2 animals were genotyped afterwards with same primer sets, and PCR products were Sanger sequenced for verification. All crRNA and oligonucleotide sequences are listed in the Supplementary Table 1.
Gene expression analysis of srg-36 and srg-37
Gene expression levels of srg-36 and srg-37 at the L1 larval stage (WBls:0000024) in the N2 strain were analyzed from published whole-animal (WBbt:0007833) RNA-seq datasets (ERP003471, SRP000253, SRP000401, SRP003492, SRP003783, SRP008969, SRP010374, SRP034522, SRP040623, SRP058023)56–65. To equally weight each dataset with different number of replicates, mean values of FPKM for each dataset were used for gene expression comparisons.
Population genetics
Sliding window analysis of population genetic statistics (Tajima’s D, FST, and π) was performed using the PopGenome package in R66. All sliding window analyses were performed using the imputed SNV VCF available on the CeNDR website with the most diverged strains XZ1516 set as the outgroup22,67,68. Linkage disequilibrium (LD) of QTL markers, which can be measured as the square of the correlation coefficient (r2), was calculated using the genetics package in R69. The formula for the correlation coefficient is r = -D / sqrt (p(A) * p(a) * p(B) * p(b)). Haplotype composition of each wild isolate was inferred by applying IBDseq70 with variants called by BCFtools71 and the following filters: Depth (DP) > 10; Mapping Quality (MQ) > 40; Variant quality (QUAL) > 10; (Alternate-allelic Depth (AD) / Total Depth (DP)) ratio > 0.5; < 10% missing genotypes; < 10% heterozygosity. To generate genome-wide tree, whole-population relatedness analysis was performed using RAxML-ng with the GTR+FO substitution model(DOI:10.5281/zenodo.593079). SNVs were LD-pruned using PLINK (v1.9) with the --indep-pairwise command `--indep-pairwise 50 1 0.95’. We used the vcf2phylip.py script (DOI:10.5281/zenodo.1257058) to convert the pruned VCF files to the PHYLIP format72 required to run RAxML-ng. To construct the tree that included 249 strains, we used the GTR evolutionary model available in RAxML-ng73,74. Trees were visualized using the ggtree (v1.10.5) R package75.
Substrate specificity analysis in the co-sampling zone
The co-sampling zone was defined as a location where both srg-37(+) and srg-37(ean179) were isolated (Supplementary Fig. 11). Collection information available on the CeNDR website were used to analyze correlations between isolated substrate and the srg-37 genotype of each isolate. Isolation of wild strains that share the same genome-wide genotypes (isotype) were counted as independent isolations if they were sampled from different locations or from different substrate types. We found that 95 isotypes were isolated in co-sampling zone from at least 119 independent isolations. Three substrates (animals, compost, rotting fruit) with more than ten independent isolated strains were selected for substrate enrichment test. In total, 82 wild strains (66 isotypes) were grouped into three subpopulations by the substrate where they were isolated, and allele frequencies of each subpopulation were calculated. Significant enrichment of srg-37(ean179) in each subpopulation was determined by hypergeometric tests using stats R package76.
Data and code availability
All data sets, including HTDA raw data, and code for generating figures are available on GitHub (https://github.com/AndersenLab/DauerSRG3637).
Supplementary Material
Acknowledgments
This work was supported by an NSF CAREER Award to E.C.A. S.Z was supported by the Cell and Molecular Basis of Disease training grant (T32GM008061) and The Bernard and Martha Rappaport Fellowship. D.E.C received the National Science Foundation Graduate Research Fellowship (DGE-1324585). Members of the Andersen Lab helped manuscript editing and Ying K. Zhang for assistance with the synthesis of ascarosides. Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We want to thank WormBase for providing genome data of Caenorhabditis species.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Cassada RC & Russell RL The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol 46, 326–342 (1975). [DOI] [PubMed] [Google Scholar]
- 2.Golden JW & Riddle DL The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol 102, 368–378 (1984). [DOI] [PubMed] [Google Scholar]
- 3.Schaedel ON, Gerisch B, Antebi A & Sternberg PW Hormonal signal amplification mediates environmental conditions during development and controls an irreversible commitment to adulthood. PLoS Biol. 10, e1001306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder FC Modular assembly of primary metabolic building blocks: a chemical language in C. elegans. Chem. Biol 22, 7–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludewig AH & Schroeder FC Ascaroside signaling in C. elegans. WormBook 1–22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Félix M-A & Braendle C The natural history of Caenorhabditis elegans. Curr. Biol 20, R965–9 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Frézal L & Félix M-AC elegans outside the Petri dish. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H et al. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat. Neurosci 15, 107–112 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Lee D et al. The genetic basis of natural variation in a phoretic behavior. Nat. Commun 8, 273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viney ME, Gardner MP & Jackson JA Variation in Caenorhabditis elegans dauer larva formation. Dev. Growth Differ 45, 389–396 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Harvey SC, Shorto A & Viney ME Quantitative genetic analysis of life-history traits of Caenorhabditis elegans in stressful environments. BMC Evol. Biol 8, 15 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green JWM, Snoek LB, Kammenga JE & Harvey SC Genetic mapping of variation in dauer larvae development in growing populations of Caenorhabditis elegans. Heredity 111, 306–313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green JWM, Stastna JJ, Orbidans HE & Harvey SC Highly polygenic variation in environmental perception determines dauer larvae formation in growing populations of Caenorhabditis elegans. PLoS One 9, e112830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz SA et al. Diverse and potentially manipulative signalling with ascarosides in the model nematode C. elegans. BMC Evol. Biol 14, 46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell MP, Chao P-H, Kammenga JE & Sengupta P Rictor/TORC2 mediates gut-to-brain signaling in the regulation of phenotypic plasticity in C. elegans. PLoS Genet. 14, e1007213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nika L, Gibson T, Konkus R & Karp X Fluorescent Beads Are a Versatile Tool for Staging Caenorhabditis elegans in Different Life Histories. G3 6, 1923–1933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans KS et al. Correlations of Genotype with Climate Parameters Suggest Caenorhabditis elegans Niche Adaptations. G3 7, 289–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulenburg H & Félix M-A The Natural Biotic Environment of Caenorhabditis elegans. Genetics 206, 55–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath PT et al. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477, 321–325 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bargmann CI & Horvitz HR Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251, 1243–1246 (1991). [DOI] [PubMed] [Google Scholar]
- 21.Schackwitz WS, Inoue T & Thomas JH Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17, 719–728 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Cook DE, Zdraljevic S, Roberts JP & Andersen EC CeNDR, the Caenorhabditis elegans natural diversity resource. Nucleic Acids Res. 45, D650–D657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahnel SR et al. Extreme allelic heterogeneity at a Caenorhabditis elegans beta-tubulin locus explains natural resistance to benzimidazoles. PLoS Pathog. 14, e1007226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H et al. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics 197, 1069–1080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paix A, Folkmann A, Rasoloson D & Seydoux G High Efficiency, Homology-Directed Genome Editing in Caenorhabditis elegans Using CRISPR-Cas9 Ribonucleoprotein Complexes. Genetics 201, 47–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas S & Akey JM Genomic insights into positive selection. Trends Genet. 22, 437–446 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Chang J-M, Di Tommaso P, Taly J-F & Notredame C Accurate multiple sequence alignment of transmembrane proteins with PSI-Coffee. BMC Bioinformatics 13 Suppl 4, S1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen EC et al. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat. Genet 44, 285–290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Félix M-A & Duveau F Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 10, 59 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrière A & Félix M-A High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr. Biol 15, 1176–1184 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Hu PJ Dauer. WormBook 1–19 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SS & Schroeder FC Steroids as central regulators of organismal development and lifespan. PLoS Biol. 10, e1001307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gumienny TL & Savage-Dunn C TGF-β signaling in C. elegans. WormBook 1–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy CT & Hu PJ Insulin/insulin-like growth factor signaling in C. elegans. WormBook 1–43 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gompel N & Prud’homme B The causes of repeated genetic evolution. Dev. Biol 332, 36–47 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Greene JS et al. Balancing selection shapes density-dependent foraging behaviour. Nature 539, 254–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene JS, Dobosiewicz M, Butcher RA, McGrath PT & Bargmann CI Regulatory changes in two chemoreceptor genes contribute to a Caenorhabditis elegans QTL for foraging behavior. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlesworth D Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet. 2, e64 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen EC, Bloom JS, Gerke JP & Kruglyak L A variant in the neuropeptide receptor npr-1 is a major determinant of Caenorhabditis elegans growth and physiology. PLoS Genet. 10, e1004156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyd WA, Smith MV & Freedman JH Caenorhabditis elegans as a model in developmental toxicology. Methods Mol. Biol 889, 15–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang YK, Sanchez-Ayala MA, Sternberg PW, Srinivasan J & Schroeder FC Improved Synthesis for Modular Ascarosides Uncovers Biological Activity. Org. Lett 19, 2837–2840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W-C, Maitra R & Melnykov V A Quick Guide for the EMCluster Package. R Vignette, URL http://cran.r-project.org/package=EMCluster (2012). [Google Scholar]
- 43.Li H A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell S et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Endelman JB Ridge Regression and Other Kernels for Genomic Selection with R Package rrBLUP. Plant Genome 4, 250–255 (2011). [Google Scholar]
- 47.Qiu Y RSpectra. (Github).
- 48.Bilgrau AE correlateR. (Github, 2018).
- 49.Li J & Ji L Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95, 221–227 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Covarrubias-Pazaran G Quantitative genetics using the Sommer package. R Found. Stat. Comput., Vienna https://cran.r-project.org/web/packages/sommer/vignettes/sommer.pdf (accessed 22 Mar. 2018) (2018). [Google Scholar]
- 51.Li H & Durbin R Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picard Tools - By Broad Institute. Available at: http://broadinstitute.github.io/picard/.
- 53.Cook DE et al. The Genetic Basis of Natural Variation in Caenorhabditis elegans Telomere Length. Genetics 204, 371–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ & Prins P Sambamba: fast processing of NGS alignment formats. Bioinformatics 31, 2032–2034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Shin H et al. Transcriptome analysis for Caenorhabditis elegans based on novel expressed sequence tags. BMC Biol. 6, 30 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerstein MB et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330, 1775–1787 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mortazavi A et al. Scaffolding a Caenorhabditis nematode genome with RNA-seq. Genome Res. 20, 1740–1747 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stadler M & Fire A Wobble base-pairing slows in vivo translation elongation in metazoans. RNA 17, 2063–2073 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamm AT, Stadler MR, Zhang H, Gent JI & Fire AZ Multimodal RNA-seq using single-strand, double-strand, and CircLigase-based capture yields a refined and extended description of the C. elegans transcriptome. Genome Res. 21, 265–275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maxwell CS, Antoshechkin I, Kurhanewicz N, Belsky JA & Baugh LR Nutritional control of mRNA isoform expression during developmental arrest and recovery in C. elegans. Genome Res. 22, 1920–1929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steijger T et al. Assessment of transcript reconstruction methods for RNA-seq. Nat. Methods 10, 1177–1184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grün D et al. Conservation of mRNA and protein expression during development of C. elegans. Cell Rep. 6, 565–577 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Wang J-J et al. The influences of PRG-1 on the expression of small RNAs and mRNAs. BMC Genomics 15, 321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dillman AR et al. Comparative genomics of Steinernema reveals deeply conserved gene regulatory networks. Genome Biol. 16, 200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfeifer B, Wittelsbürger U, Ramos-Onsins SE & Lercher MJ PopGenome: an efficient Swiss army knife for population genomic analyses in R. Mol. Biol. Evol 31, 1929–1936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Browning BL & Browning SR Genotype Imputation with Millions of Reference Samples. Am. J. Hum. Genet 98, 116–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danecek P et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warnes G, Gorjanc G, Leisch F, Man M. Genetics: Population genetics, R package version 1.3.6 (2012).
- 70.Browning BL & Browning SR Detecting identity by descent and estimating genotype error rates in sequence data. Am. J. Hum. Genet 93, 840–851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danecek P, Schiffels S & Durbin R Multiallelic calling model in bcftools (-m).
- 72.Felsenstein J PHYLIP - Phylogeny Inference Package (Version 3.2). Cladistics 5, 164–166 (1989). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sets, including HTDA raw data, and code for generating figures are available on GitHub (https://github.com/AndersenLab/DauerSRG3637).