Abstract
Mesenchymal stem cells (MSCs) are a promising cell source for tissue repair and regeneration due to their multilineage capacity, potential for autologous use, and secretion of potent bioactive factors to catalyze the endogenous repair program. However, a major limitation to current cell-based tissue engineering approaches is the drastic loss of cells upon transplantation. The causation of this loss, whether due to apoptosis following a dramatic change in the microenvironment or migration away from the defect site, has yet to be determined. MSCs formed into aggregates, known as spheroids, possess a strong therapeutic advantage compared to the more commonly used dissociated cells due to their improved resistance to apoptosis and increased secretion of endogenous trophic factors. Furthermore, the use of biomaterials such as alginate hydrogels to transplant cells in situ improves cell survival, localizes payloads at the defect site, and facilitates continued instruction of cells by manipulating the biophysical properties of the biomaterial. Transplantation of MSC spheroids without a vehicle into tissue defects comprises the majority of studies to date, ceding control of spheroid function due to the cell’s interaction with the native tissue extracellular matrix and abrogating the established benefits of spheroid formation. Thus, there is a significant need to consider the role of biomaterials in transplanting MSC spheroids using an appropriate carrier. In this chapter, we describe high-throughput formation of spheroids, steps for further characterization, and encapsulation in alginate hydrogels with an eye toward localizing MSC spheroids at the target site.
Keywords: Mesenchymal stem cell, Encapsulation, Transplantation, Spheroid, Alginate
1. Introduction
Personalized medicine remains at the forefront of clinical practice, aiming to provide treatment for individual patients based on their unique chemistries and biological makeup. The use of mesenchymal stem cells (MSCs) in cell-based approaches for tissue engineering holds great promise for improved tissue development in light of their multilineage capacity, ability to achieve large numbers of cells from culture expansion, and potential for autologous use [1]. When given the appropriate cues, MSCs can readily differentiate toward osteoblasts, chondrocytes, and adipocytes [2], yet clinically, these cells are more commonly studied for their potential to indirectly contribute to tissue repair through secretion of endogenous trophic factors and immunomodulatory potential [3]. However, current shortcomings include rapid loss in viability upon cell transplantation and low cell persistence at the transplant site. Forming MSCs into spheroids promotes cell-cell interaction by forced adhesion while also enabling cell-matrix interactions with endogenous cell-secreted matrix within the spheroid [4], resulting in enhanced cell survival, improved resistance to apoptosis, and increased secretion of potent trophic factors such as vascular endothelial growth factor (VEGF) [5, 6]. Spheroids have been utilized in a multitude of applications including promoting angiogenesis as a potential treatment for peripheral vascular disease [7] and chemotherapeutic screening for cancer treatment [8]. It is evident that 3D culture has great potential to mimic biological environments for therapeutic purposes.
Numerous techniques are successfully used for spheroid fabrication such as the hanging-drop method [9] and methylcellulose encapsulation [10]. Although effective at producing spherical aggregates, the hanging-drop procedure requires multiple iterations to achieve adequate cell numbers for transplantation purposes and is labor intensive. These challenges increase variability in the characteristics of resulting spheroids and limit the number of spheroids that can be produced in a given time. Methylcellulose requires additional materials and steps that yield similar results to the method described below, thereby extending the time required to form spheroids.
Here, we demonstrate that spheroids can be successfully fabricated in a high-throughput manner by rapid centrifugation in agarose molds, creating an efficient, standardized platform. This elegant design enables tailoring of spheroid size by changing the concentration of cells added to each well. The size of the spheroid is proportional to the cell concentration, based on the average number of cells per microwell. Spheroid morphology and size can be easily analyzed and quantified, further bolstering its reproducibility [9].
The therapeutic efficacy of MSC spheroids has been demonstrated in preclinical models of peripheral artery disease and wound repair [11, 12]. Compared to an equal number of dissociated cells, spheroids survived longer and were more efficient at promoting neovascularization due to increased trophic factor secretion. However, these studies are commonly performed by transplanting spheroids directly into the tissue, thereby ceding control of cell function achieved through cohesion to the surrounding extracellular matrix following adhesion to the tissue and subsequent migration [13, 14]. Substantial evidence demonstrates the efficacy of biomaterials to localize dissociated cells at the target site [15]. Therefore, one approach for sustaining the therapeutic advantages of MSC spheroids is to transplant these aggregates using hydrogel carriers that can localize and instruct cells at the implant site. There are a host of synthetic and naturally derived biomaterials that are relevant for this purpose including polyethylene glycol diacrylate (PEGDA), hyaluronic acid (HA), collagen, and fibrin [16]. As another example, alginate is a well-characterized polymer in drug delivery and tissue engineering due to its non-fouling, bioinert, and injectable properties [17, 18]. Additionally, it can be easily tailored through peptide conjugation using various chemistries to promote cell adhesion [19], permitting an understanding of signals presented independently and in combination. Compared to unmodified alginate, spheroids entrapped in arginine-glycine-aspartic acid (RGD)-modified alginate hydrogels exhibited increased viability and function [5]. Thus, encapsulation of spheroids in alginate hydrogels offers a quick, reliable mode for increased stability of spheroid function and efficacy.
A plethora of applications can be pursued with the ability to reproducibly and efficiently encapsulate spheroids of varying cell numbers and sizes in an established biomaterial. This extends beyond MSCs and could be used with varying cell types. The method described here allows for robust cell culture and will enhance research procedures for improved cell-based applications.
2. Materials
Prepare and store all reagents at room temperature unless otherwise indicated. Follow all chemical and biological waste regulations when disposing waste materials.
100 mL graduated cylinder.
Magnetic stir plate.
Magnetic stir bar.
100 mL glass bottle.
Autoclave.
Autoclave pouch.
Biological safety cabinet.
Weighing scale (10 mg to 220 g range).
1.5% Agarose gel solution: UltraPure Agarose (Invitrogen, Carlsbad, CA).
Silicone rubber, 10 mm thickness (Hydrosil 1:1, SILADENT Dr. Böhme & Schöps GmbH, Goslar, Germany).
100–1000 μL pipettes.
Sterile pipet tips.
Sterile tissue culture-treated culture dish (24-well plate, Falcon, Corning, NY).
Spatula, autoclaved at 250°C for 1 h.
Centrifuge with attachments that accommodate well plates.
Cell culture incubator.
Sterile tissue culture-treated culture flasks (225 cm2).
Cell counting device.
MSCs: Bone marrow-derived MSCs can be harvested and isolated as previously described [2–20]. Commercial suppliers are also available as reliable sources. Culture under standard conditions until sufficient cell numbers are achieved [2].
Mesenchymal stem cell culture medium: α-Modified Eagle’s medium (α-MEM) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 mg/mL streptomycin.
Microplate shaker.
2.1% RGD-conjugated alginate solution (see Note 1). Store at 4°C for up to 6 months.
200 mM CaCl2: Store at room temperature for up to 6 months.
Silicone rubber, 1.5 mm thickness (3M, St. Paul, MN, USA).
8 mm biopsy punch.
Forceps.
Flat dialysis membrane sheet: 3.5 kDa MWCO (Spectrum Labs, Rancho Dominguez, CA, USA). Cut as needed to specifications. See Note 2.
10 cm × 10 cm glass plate: dimensions of plate can be variable. This is to provide a sterile, flat surface for the silicon mold.
70% ethyl alcohol/ethanol.
Phosphate-buffered saline (PBS) solution: Dulbecco’s phosphate-buffered saline.
Sterile, 50 mL reagent reservoirs.
Sterile 24.5 cm × 24.5 cm square culture dish (Corning, Corning, New York, USA): Dimensions of dish can be variable. This will provide a sterile surface for the glass plate.
3. Methods
Carry out all procedures in an aseptic biological safety cabinet unless otherwise specified. The following steps (Subheading 3.1, steps 1–10) yield one well of spheroids. Personal protective equipment (PPE) must be worn at all times.
3.1. Spheroid Formation
Prepare the 1.5% agarose gel solution outside of the biological safety cabinet: Measure 90 mL deionized water in a graduated cylinder and transfer to a glass bottle. Add a stir bar and place on a magnetic stir plate. While stirring, weigh 1.355 mg of agarose and transfer to the glass bottle and cap. Stir for 3 additional minutes. The agarose will not fully dissolve until heat is applied. Autoclave at 250°C for 1 h, at which the agarose should be fully dissolved. See Note 3.
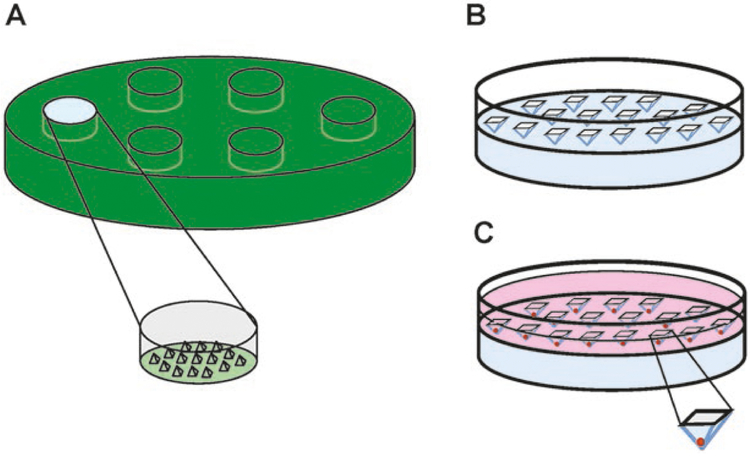
Prepare the master mold outside of the biological safety cabinet: Using 10 mm thick silicone, create a cast 6 mm in depth and 15.5 mm in diameter, with 300 microwells protruding the cast surface, each 800 μm in diameter at the base (Fig. 1a) as previously described [21]. Seal in an autoclave pouch and autoclave at 250°C for 2 h. See Note 4.
Remove the master mold from the autoclaved pouch and place on a sterile flat surface in the biological safety cabinet.
Pipet 850 μL of 1.5% agarose into one well of the master mold. Allow to cool to room temperature for 10 min. See Note 5.
Remove the agarose gel from the master mold and gently place it into a 24-well plate (Fig. 1b). Use a spatula to push down the agarose gel as far as possible without disturbing the microwells. The microwells should be facing up from the plate surface. See Note 6.
Pipet 1 mL of media on top of each agarose mold. This will add weight to ensure that the agarose gel is properly secured to the bottom of the plate.
Secure the lid onto the 24-well plate. Outside of the biological safety cabinet, centrifuge the plate at 290 × g for 3 min. Inside the biological safety cabinet, aspirate excess media from the well. See Note 7.
Harvest the MSCs from tissue culture plates and quantify the number of available cells using any appropriate counting device (i.e., cell counter, hemacytometer).
Suspend MSCs in serum-containing complete α-MEM culture medium at the concentration for the desired number of cells for 1 mL per well. See Note 8.
Pipet 1 mL cell suspension into one well of the 24-well plate containing the agarose mold. Pipet up and down gently several times to evenly disperse cells (Fig. 1c). Secure the lid onto the plate. See Note 9.
Outside of the biological safety cabinet, centrifuge the 24-well plate at 163 × g for 8 min.
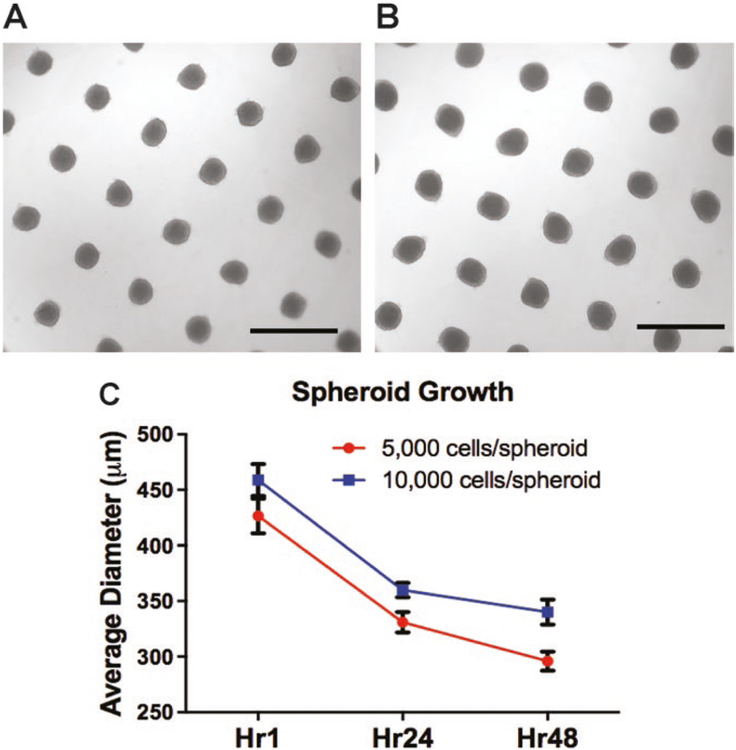
Incubate the 24-well plate in 37°C for up to 48 h. See Note 10. Fig. 2a, b illustrates the result of spheroid formation after 48 h with 5,000 or 10,000 cells/spheroid. Spheroids with 10,000 cells are visibly larger in the microwell, also indicated by a greater diameter in Fig. 2c.
Fig. 1.
Spheroid formation method. (a) Outline of the master mold framework. A section of PDMS is cast to produce wells with 800 μm microwell protrusions. (b) Acellular agarose gel in a well of a 24-well plate after centrifugation. (c) Agarose gel after centrifugation with cell solution. As agarose is largely non-adherent, cells aggregate at the bottom of microwells. Diagrams not drawn to scale
Fig. 2.
Spheroid size and diameter measurement. Spheroid formation in agarose well after 48 h with (a) 5,000 cells/spheroid or (b) 10,000 cells/spheroid. (c) Spheroid diameter measurements for 5,000 and 10,000 cells/spheroid over time
3.2. Spheroid Entrapment in Alginate Hydrogels
Prepare the 2.1% RGD-conjugated alginate solution: In a 50 mL conical tube, add the necessary volume of sterile α-MEM to RGD-modified alginate to obtain an alginate solution of 2.1% by weight. See Note 11. Tape conical tube to a microplate shaker, and set speed to 18 × g and temperature to 37°C to allow alginate to mix overnight.
Prepare the CaCl2 solution: Weigh 14.701 g of solid calcium chloride dehydrate (molecular weight 147.01 g/mol). Mix and dissolve in 500 mL deionized H2O. Sterile filter into a sterile 500 mL glass bottle.
Prepare the silicone mold: Place 1.5 mm thick silicone rubber on sterile surface and use an 8 mm biopsy punch to punch out desired number of holes approximately 1 cm apart.
After spheroid formation, typically 1–2 days, collect the spheroids from the well plate by gently pipetting up and down to displace spheroids from the microwells. If needed, wash the well with extra media to collect remaining spheroids. Pipet the spheroids into a 15 mL conical tube.
Allow spheroids to settle to the bottom of the conical tube. Alternatively, gently spin down tube at 163 × g for 1 min. Carefully aspirate the media. See Note 12.
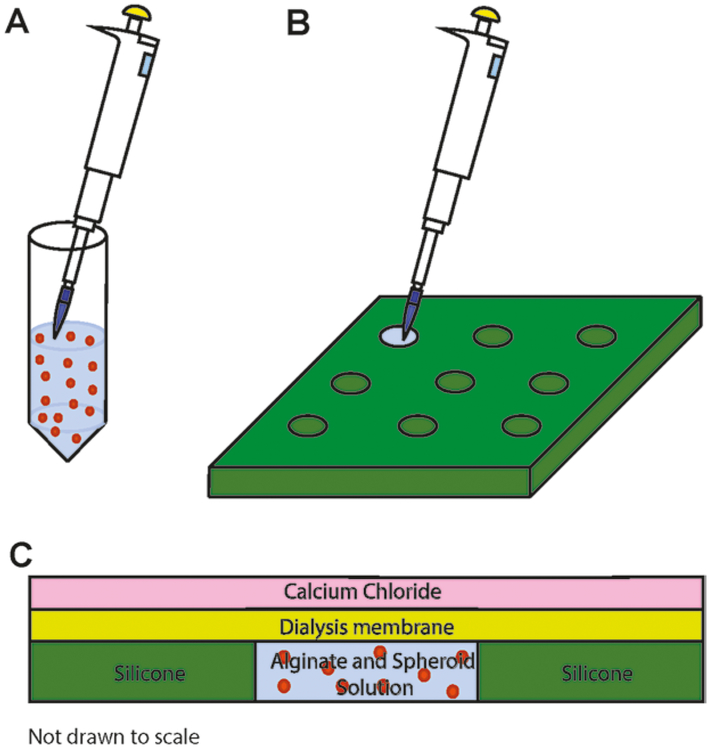
Resuspend spheroids in 2.1% alginate solution (Fig. 3a). The concentration of spheroids depends on the cell concentration desired in alginate hydrogels. We suggest at least 5 × 106 cells per mL to ensure adequate cellular response for necessary assays.
Within the biosafety cabinet, set up gelling area. Place the glass plate in a sterile, square culture dish. Place silicon mold on top of the glass plate and make sure that it is firmly adhered. See Note 13.
Pipet 90 μL of alginate/spheroid solution into each well in the silicone mold (Fig. 3b). See Note 14.
Using sterile forceps, pick up the dialysis membrane from PBS solution and dab corners on sterile gauze to remove excess liquid. Carefully place dialysis membrane over the wells of the silicone mold and use forceps to flatten completely. See Note 15.
Pipet 200 mM CaCl2, 3–4 mL, over the membrane to cover the wells (Fig. 3). Let the solution sit for 5 min, which allows calcium ions to diffuse into the hydrogel discs. Carefully slide the membrane off laterally and pipet more CaCl2 solution over the wells for direct contact and immersion. Alginate cross-linking proceeds for an additional 5 min. See Note 16.
After 10 min of alginate cross-linking is complete, tilt the glass plate to remove CaCl2 solution. Use a sterile spatula to carefully remove excess alginate on top of the silicone mold.
Hydrogels can now be lifted and transferred to a 24-well plate for culture in the desired culture medium. These can be transplanted in vivo or maintained in culture for in vitro studies. See Note 17.
Fig. 3.
Spheroid entrapment in alginate hydrogels. (a) Spheroids suspended in 2.1% alginate solution. (b) Layout of silicone mold for incorporation of spheroids in alginate suspension. (c) Cross-sectional view of alginate hydrogel assembly for cross-linking initiation
4. Notes
A variety of peptides can be conjugated using various chemistries. We illustrate the use of RGD due to its widespread use and ease of conjugation via carbodiimide chemistry [15–22].
The dialysis membrane must be cut and hydrated in deionized water for 10 min prior to use. After hydration, membranes should be placed into a plastic container containing 70% ethanol to be sterilized for 30 min. After sterilization, place membranes in a sterile reagent reservoir containing sterile PBS to rinse off residual 70% ethanol.
Agarose solution will solidify at room temperature. For repeated use, store at room temperature and reheat on a hot plate, using the stir bar to create a homogeneous solution. Alternatively, a microwave can be used with subsequent stirring.
Ensure that the thickness of the mold will allow enough room for media when the agarose gel is placed into a 24-well plate (Subheading 3.1, step 5). The geometry and dimensions can be customized to achieve molds that yield aggregates of other shapes.
The agarose gel volume should be filled enough to form a concave meniscus over the top of the well. This is to make sure that the well is not underfilled, which can allow for media to rush under the agarose and disturb spheroids during the formation process.
Be careful to avoid damaging the mold. Tilt the spatula and press the agarose along the side of the gel, making sure not to directly touch the microwells.
If the mold is still not set at the bottom of the well, repeat this step at a higher revolution speed. Avoid speeds of 3220 × g because these forces can deform the agarose. Centrifuge accessories that can accommodate well plates are necessary.
It is important that the desired number of cells is suspended in 1 mL of media for each well. This volume allows for adequate oxygen supply and prevents the well from overflowing.
Avoid creating bubbles, as this will affect cell viability. Additionally, ensure that the cell density is low enough that the cells do not overflow and create poorly formed spheroids. The maximum total cell number for a microwell with 800 μm base diameter is 20,000 cells.
Place the well plate in a location with minimal movement. Movement of the plate during formation can disrupt the integrity of the aggregates and result in either poorly formed aggregates or inconsistent spheroid sizes.
A 2.1% (w/v) solution is used to account for the small amount of volume occupied by cells. When combined, the two components will form a 2% (w/v) solution of alginate and cells. Varying percentages of alginate may also be used, as well as different types of alginate.
Use a micropipette to gently pipet media out of the conical tube. This allows for more controlled suction. If using a vacuum line with Pasteur pipette, adjust vacuum line to allow for gentle aspiration.
Complete adherence of the silicone mold to the glass plate is critical. Make visual checks beneath the plate to see if there are any air pockets and from a lateral view to make sure that there are no areas where the mold is lifted off the plate. This ensures that no alginate will leak under the mold. Mold will lift off if contact is too dry. To address this, a small amount of PBS can be applied to allow for proper adherence.
Alginate volume should overflow and form a concave meniscus over the top of the well. This is to make sure that the well is not underfilled, which can create bubbles that displace the alginate. Excess alginate will be spread off to the side when the dialysis membrane is placed over the mold.
Place the dialysis membrane on top of the mold by flattening it from the bottom up. Be careful not to lift up the membrane once it has made contact with the mold. This will cause bubbles to form in the well, as the alginate sticks to the membrane.
Membrane must be slid off laterally. If removed vertically, it will also remove alginate from the well. Sliding to the side will retain alginate in the wells. Do not reuse membrane, as alginate may obstruct pores and prevent efficient dialysis on subsequent gels.
It is prudent to remove excess alginate from the mold and encircle the wells with a spatula by tracing the gel circumference before removal so as not to lift up residual material or cause rips in the hydrogel.
Acknowledgment
This work was supported by NIH Grant R01-DE025475 to JKL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript. CEV was supported by the T32 Training Program in Basic and Translational Cardiovascular Science (T32 HL086350). JW was supported by the National Science Foundation Graduate Research Fellowship (1650042). SH was supported by the T32 Animal Models of Infectious Disease Training Program Kirschstein-NRSA (T32 AI060555).
References
- 1.Caplan AI (2005) Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 11(7–8):1198–1211 [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147 [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI (2016) MSCs: the sentinel and safe-guards of injury. J Cell Physiol 231(7):1413–1416 [DOI] [PubMed] [Google Scholar]
- 4.Murphy KC et al. (2016) Mesenchymal stem cell spheroids retain osteogenic phenotype through alpha2beta1 signaling. Stem Cells Transl Med 5(9):1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho SS et al. (2016) Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med 5(6):773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhang SH et al. (2012) Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. J Tissue Eng Regen Med 6:295–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenger A et al. (2004) Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng 10(9–10):1536–1547 [DOI] [PubMed] [Google Scholar]
- 8.Thoma CR et al. (2014) 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 69:29–41 [DOI] [PubMed] [Google Scholar]
- 9.Murphy KC, Fang SY, Leach JK (2014) Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res 357(1):91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattermann K, Held-Feindt J, Mentlein R (2011) Spheroid confrontation assay: a simple method to monitor the three-dimensional migration of different cell types in vitro. Ann Anat 193(3):181–184 [DOI] [PubMed] [Google Scholar]
- 11.Bhang SH et al. (2011) Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32(11):2734–2747 [DOI] [PubMed] [Google Scholar]
- 12.Ylostalo JH et al. (2012) Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30(10):2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Han YS, Lee SH (2016) Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells. Biomol Ther 24(3):260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sart S et al. (2014) Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev 20(5):365–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi CX et al. (2015) Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell 6(9):638–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caliari SR, Burdick JA (2016) A practical guide to hydrogels for cell culture. Nat Methods 13(5):405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augst AD, Kong HJ, Mooney DJ (2006) Alginate hydrogels as biomaterials. Macromol Biosci 6(8):623–633 [DOI] [PubMed] [Google Scholar]
- 18.Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersel U, Dahmen C, Kessler H (2003) RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24(24):4385–4415 [DOI] [PubMed] [Google Scholar]
- 20.Soleimani M, Nadri S (2009) A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4(1):102–106 [DOI] [PubMed] [Google Scholar]
- 21.Dahlmann J et al. (2013) The use of agarose microwells for scalable embryoid body formation and cardiac differentiation of human and murine pluripotent stem cells. Biomaterials 34(10):2463–2471 [DOI] [PubMed] [Google Scholar]
- 22.Rowley JA, Madlambayan G, Mooney DJ (1999) Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20(1):45–53 [DOI] [PubMed] [Google Scholar]