Abstract
How connectivity affects plant assemblages is a central issue in landscape ecology. So far, empirical studies have produced contradictory results, possibly because studies: (1) inaccurately assess connectivity by prioritizing the respective effect of the type of habitat on plant assemblages and (2) omit the range of possible plant responses to connectivity depending on dispersal vectors. We focused on three dominant habitat types in agricultural landscapes (woodland, grassland and cropland), and analysed the effect of connectivity on herbaceous plant assemblage similarity for three primary dispersal modes (animal-dispersed, wind-dispersed and unassisted). Using circuit theory, we measured connectivity provided by woodland, grassland and cropland habitats independently. The similarity of plant assemblages was evaluated relative to the random expectation based on the regional pool. Overall, plant assemblage similarity in woodlands and temporary grasslands was dependent on connectivity, but not in wheat croplands. Only animal-dispersed species responded to connectivity. The similarity of animal-dispersed assemblages in woodlands was increased by the connectivity provided by woodland habitats, but was reduced by cropland habitats, whereas in temporary grasslands, similarity was increased by the connectivity provided by cropland habitats. Our results suggest that animal-dispersed species supplement their dispersal pathways, thus improving our knowledge of plant assembly rules in fragmented landscapes.
Subject terms: Biogeography, Community ecology
Introduction
Habitat isolation is a major cause of loss of plant diversity in agricultural landscapes1,2. In such heterogeneous landscapes, plant species are distributed across a set of local communities linked through dispersal, which ensures their persistence as a metacommunity in the landscape3,4. Dispersal between communities is enhanced by landscape connectivity5. High connectivity enhances plant dispersal and increases genetic fluxes among local populations, thereby reducing the adverse effects of isolation. Therefore, high connectivity should enhance plant coexistence in multi-species assemblages. In experimental landscapes, connected patches have been shown to have higher species richness than unconnected patches6. This pattern is less clear in empirical studies where the relationship between connectivity and species richness and/or similarity in composition has been shown to be positive7–9, negative10, or absent11,12.
Inaccurate assessments of landscape connectivity may explain why empirical studies fail to demonstrate a common pattern. Assessing connectivity in situ is difficult and different approaches have been attempted. Some authors estimated connectivity through a binary landscape representation13,14 using the abundance and spatial configuration of patches of a given habitat (i.e. structural connectivity15). For example, the connectivity of a woodland fragment is calculated as a combination of the sizes of nearby woodland fragments and the Euclidean distance to those fragments [incidence function model index (IFM)16,17, e.g. Piessens et al.7, Lindborg & Eriksson11, Cousins et al.12]. Other studies improved these estimates of connectivity by using functional indices [e.g. integral index of connectivity (IIC)18 and probability of connectivity (PC)19]. Both IIC and PC indices incorporate dispersal and plant habitat specificities in addition to the abundance and spatial configuration of patches (i.e. potential functional connectivity15) and are based on graph theory (least-cost path distance20) or circuit theory (resistance distance21,22). Initially developed for animals, these indices are now increasingly used for plants although not yet widely at the plant community scale (but see Thiele et al.8,9,23 and Mony et al.24). These functional indices still consider that species which co-exist in plant communities may occupy different ecological niches and rely on different habitats to disperse25. Functional landscape connectivity is indeed calculated using resistance maps. These maps include all the landscape patches with associated resistance values per habitat type (i.e. the higher the type of habitat suitable to plant dispersal, the lower the habitat type resistance value), hence including the heterogeneity of the landscape matrix13,26,27. However, one limitation to using such indices is assigning resistance values, which is based on expert knowledge (i.e. subjective)28–31 and assumes a role hierarchy exerted by the various types of habitat on the connectivity among habitat patches. Understanding how the different habitat types between patches are independently involved in connectivity may thus improve our understanding of their effects on plant biodiversity. For example, a patch of a given habitat type can be connected to other patches of the same type by woodland, grassland and cropland habitat patches, even though their respective effect may differ. Improved understanding of the influence of landscape connectivity on species assemblages can be achieved by evaluating the additive influences of each type of habitat (the resulting effect, computed as the sum of the independent individual effect of the connectivity provided by each habitat type) on plant composition.
Considering each habitat type separately when assessing landscape connectivity is even more important in the case of plants. Plant seeds are dispersed in a landscape by a wide variety of vectors, whose response to connectivity is likely to differ depending on the type of habitat. In the case of animal-dispersed plant species, there is a broad consensus that animal movements are promoted by landscape connectivity in a wide range of habitats6,32–35. Therefore, all habitat types should facilitate dispersal of plants by animal species (e.g. by forest birds and mammals through woodland habitats36–38 and by amphibians through aquatic habitats39). However, for other modes of dispersal, plant dispersal is likely to depend on the type of habitat on which connectivity was based. For example, wind dispersal depends on prevailing winds. Open habitats (e.g. grasslands, grassy strips and croplands) affect wind dynamics (redirection and promotion of airflow) thereby increasing the likelihood of wind-dispersed seed uplift. In contrast, closed habitats (e.g. forests, woodlands and hedgerows) are known to impede wind dispersal40–42. Therefore, connectivity provided by open habitat patches should promote the dispersal of wind-dispersed species, while that provided by woodland habitats should impede their dispersal. In unassisted species (i.e. plant that are not dispersed by vectors), dispersal occurs step-by-step from the parent plant over successive generations because their seeds are not adapted for dispersal43. Because annual dispersal occurs over such short distances in unassisted species and because their progenies require a similar habitat to that of their parents, dispersal should be enhanced only by the connectivity provided by the focal habitat (habitat of interest) and inhibited by others. Accounting for the influence of connectivity provided by each habitat type according to the dispersal mode of plant species may provide insight into plant community assembly.
The aim of this study was to test the effect of landscape connectivity on herbaceous plant assemblages according to their dispersal mode. Our analysis focused on three habitat types typical of agricultural landscapes: woodland, grassland, and cropland. In these three habitat types, we assessed the response of plant assemblages to connectivity. Connectivity was measured as the addition of the independent effects of connectivity provided by the patches of each habitat type.
We calculated connectivity using the resistance distance for each habitat type. For a given habitat type, the effect of connectivity on plant assemblage similarity was tested with respect to each mode of dispersal (animal-dispersed, wind-dispersed and unassisted dispersal). Plant response was evaluated relative to the random similarity expected based on the regional species pool. In the three habitats, we specifically tested the following assumptions:
Similarity between plant assemblage pairs for a given focal habitat is better predicted by simultaneously accounting for the contribution of the various habitat types to connectivity rather than by only examining only the focal habitat type.
- The independent effect of the connectivity provided by the different habitat types depends on the dispersal mode considered.
- The similarity among animal-dispersed species assemblages is increased by connectivity provided by any habitat type.
- The similarity among wind-dispersed species assemblages is increased by connectivity provided by open habitats (e.g. grassland and cropland) and reduced by closed habitats (e.g. woodland), because of their barrier effect
- Due to the short dispersal distances of unassisted species, the similarity among such species assemblages is increased by connectivity provided by habitats of the same type and is reduced by others.
Results
Characteristics of the plant assemblages studied
The regional species pools of woodlands, temporary grasslands and wheat croplands contained 94, 103 and 78 herbaceous species, respectively. Similarity values between species pools using Sørensen indices were: 0.26 between woodlands and temporary grasslands, 0.23 between woodlands and wheat croplands and 0.53 between temporary grasslands and wheat croplands, revealing marked differences between plant assemblages in the three habitat types. Total herbaceous species richness according to habitat type and mode of dispersal were as follows: (1) woodland: 40 animal-dispersed, 34 wind-dispersed and 27 unassisted species, (2) temporary grassland: 17 animal-dispersed, 25 wind-dispersed and 17 unassisted species and (3) wheat cropland: 22 animal-dispersed, 40 wind-dispersed and 32 unassisted species. No exotic species were recorded in plant assemblages in woodlands, temporary grasslands or wheat croplands, based on the List of invasive vascular plants in Brittany44. Characteristics of the plant assemblages for woodlands, temporary grasslands and wheat croplands are summarized in Table 1 and Fig. 1.
Table 1.
Characteristics of herbaceous species assemblages (species richness and Sørensen similarity index) for woodland, grassland and cropland habitats.
| Woodlands | Temporary grasslands | Wheat croplands | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Min-Max | Mean (SD) | Min-Max | Mean (SD) | Min-Max | |
| Whole plant assemblages | ||||||
| Richness | 15.8 (8.3) | 2–36 | 26.9 (8.4) | 17–51 | 13.6 (6.9) | 3–36 |
| Similarity | 0.29 (0.15) | 0–0.67 | 0.59 (0.07) | 0.41–0.75 | 0.29 (0.14) | 0–0.67 |
| Animal-dispersed assemblages | ||||||
| Richness | 8.7 (3.6) | 2–17 | 8.80 (2.9) | 4–14 | 4.3 (2.2) | 0–10 |
| Similarity | 0.37 (0.17) | 0–0.80 | 0.62 (0.12) | 0.35–0.92 | 0.22 (0.21) | 0–0.89 |
| Wind-dispersed assemblages | ||||||
| Richness | 1.6 (1.4) | 0–5 | 6.6 (2.8) | 3–15 | 2.5 (2.0) | 0–8 |
| Similarity | 0.09 (0.20) | 0–1 | 0.57 (0.14) | 0.17–0.91 | 0.37 (0.17) | 0–0.80 |
| Unassisted assemblages | ||||||
| Richness | 3.7 (2.8) | 0–12 | 11.1 (3.7) | 6–22 | 6.6 (3.6) | 1–19 |
| Similarity | 0.20 (0.22) | 0–0.80 | 0.60 (0.11) | 0.35–0.90 | 0.36 (0.17) | 0–0.86 |
Plant assemblages were sampled 25 patches of woodlands [sampling area = 6 × (14 × 5 m) quadrats = 420 m²], temporary grasslands [sampling area = 10 × (5 × 5 m) quadrats = 250 m²] and wheat croplands [sampling area = 5 × (5 × 5 m) quadrats = 125 m²] selected for the study. SD = standard deviation.
Figure 1.
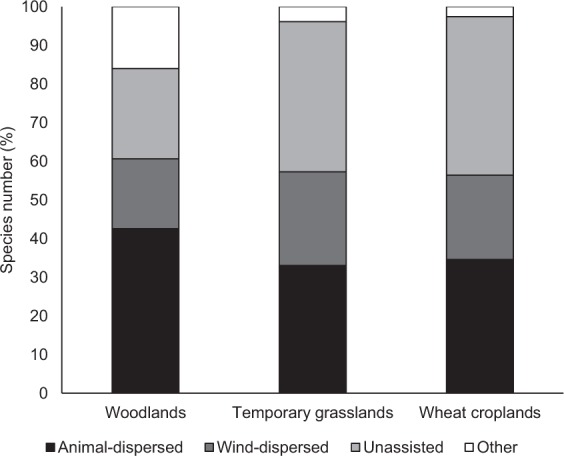
Number of herbaceous species (in percentage), by mode of dispersal and habitat type (woodland, temporary grassland, and wheat cropland). “Other” refers to water-dispersed species that were not included in this study.
Effect of connectivity on assemblage similarity
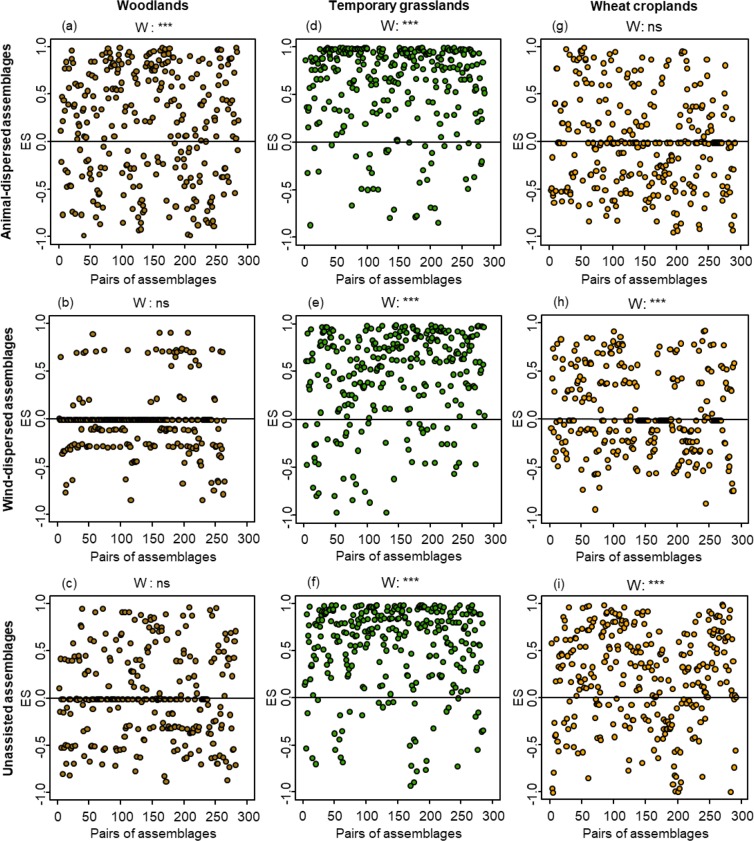
We demonstrated a non-random pattern of plant similarity between patches for all assemblages, except for three models (Fig. 2): wind-dispersed and unassisted plant assemblages in woodlands and animal-dispersed plant assemblages in wheat croplands. For the non-random patterns, effect size (ES) of similarity values were best explained by models that included resistance distance (distR) of the various habitat types than by models that included only distR of the same habitat type (Supplementary Table S1). In most cases, the probability of the distR of the other habitat types to appear in the best model explaining ES values was at least equal to the probability of the distR of the same habitat type, as indicated by their relative importance (Table 2).
Figure 2.
Effect size (ES) of similarity values for all pairs of animal-dispersed, wind-dispersed, and unassisted assemblages in habitats of woodland (brown dots), temporary grassland (green dots) and wheat cropland (yellow dots). P-values for the Wilcoxon tests (W) are on each graph. ***: p < 0.001; **: p < 0.01; *: p < 0.05; ns (not significant): p > 0.05.
Table 2.
Summary of the model-averaged estimates, relative importance (RI) and 95% confidence interval (CI) of the independent variable(s) according to the AICc framework.
| Models | Intercept | DistR woodlands | DistR grasslands | DistR croplands | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | R²m | R²c | Estimate | RI | CI | Estimate | RI | CI | Estimate | RI | CI | ||
| Woodlands | |||||||||||||
| Animal-dispersed assemblages | |||||||||||||
| ES | 283 | 0.09 | 0.48 | 0.20 | −0.32 | 1.00 | (−0.53, −0.10) | 0.06 | 0.44 | (−0.12, 0.23) | 0.21 | 1.00 | (0.07, 0.34) |
| Wind-dispersed assemblages | |||||||||||||
| ES | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Unassisted assemblages | |||||||||||||
| ES | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Temporary grasslands | |||||||||||||
| Animal-dispersed assemblages | |||||||||||||
| ES | 283 | 0.09 | 0.56 | 0.62 | 0.01 | 0.37 | (−0.06, 0.09) | 0.03 | 0.49 | (−0.05, 0.10) | −0.13 | 1.00 | (−0.22, −0.04) |
| Wind-dispersed assemblages | |||||||||||||
| ES | 283 | 0.00 | 0.62 | 0.50 | −0.01 | 0.32 | (−0.06, 0.05) | −0.01 | 0.36 | (−0.06, 0.04) | −0.00 | 0.26 | (−0.05, 0.04) |
| Unassisted assemblages | |||||||||||||
| ES | 283 | 0.04 | 0.45 | 0.59 | −0.07 | 0.74 | (−0.19, 0.05) | −0.02 | 0.45 | (−0.11, 0.07) | 0.03 | 0.45 | (−0.06, 0.11) |
| Wheat croplands | |||||||||||||
| Animal-dispersed assemblages | |||||||||||||
| ES | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Wind-dispersed assemblages | |||||||||||||
| ES | 287 | 0.02 | 0.20 | 0.07 | −0.01 | 0.38 | (−0.08, 0.06) | −0.05 | 0.73 | (−0.13, 0.03) | −0.01 | 0.28 | (−0.04, 0.06) |
| Unassisted assemblages | |||||||||||||
| ES | 290 | 0.00 | 0.34 | 0.22 | 0.00 | 0.21 | (−0.04, 0.04) | −0.01 | 0.28 | (−0.05, 0.04) | −0.00 | 0.21 | (−0.04, 0.04) |
Full models included three independent variables, resistance distance of woodlands (DistR woodlands), grasslands (DistR grasslands), and croplands (DistR crops), and one dependent variable, the effect size (ES) of similarity values. Models were done for animal-, wind-dispersed and unassisted assemblages for woodland, temporary grassland and wheat cropland habitats. Abbreviation: “−” [a random similarity pattern (ES not different from zero)]. Models were not done in this latter case. Confidence interval that did not encompass zero are in bold.
Woodland assemblages
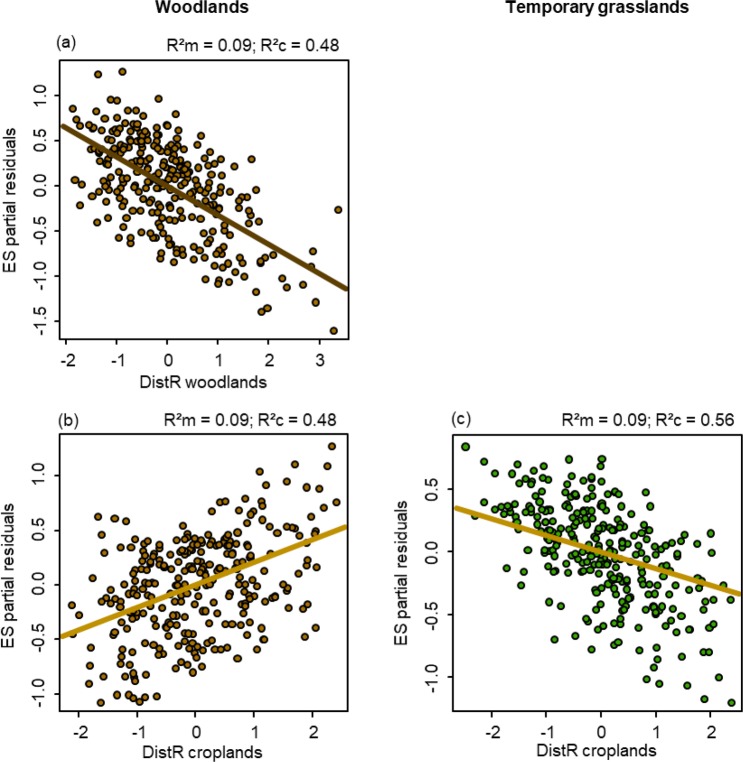
Animal-dispersed plant assemblages were more similar than expected when the connectivity provided by woodland habitats increased (dispersal enhancing effect) and when the connectivity provided by cropland habitats decreased (dispersal barrier effect) (Table 2; Fig. 3a,b). The observed similarity values between wind-dispersed and unassisted plant assemblages did not differ from random distributions.
Figure 3.
Partial residual plots denoting the significant impact of the resistance distance (distR) of woodlands (brown lines) and croplands (yellow lines) on the effect size (ES) of similarity values for animal-dispersed assemblages in woodlands (left, brown dots) and temporary grasslands (right, green dots). These plots show the effects of a given independent variable when all other independent variables are statistically fixed. The marginal (R²m) and conditional (R²c) R² of the model are on each plot.
Temporary grassland assemblages
Animal-dispersed plant assemblages were more similar than expected by chance when connectivity provided by cropland habitats increased (dispersal enhancing effect) (Fig. 3c), whereas wind-dispersed and unassisted assemblages were independent of connectivity regardless of habitat type on which connectivity was based on (Table 2).
Wheat cropland assemblages
Observed similarity values for animal-dispersed assemblages did not differ from random distributions. ES values for wind-dispersed and unassisted assemblages were independent of connectivity (Table 2).
Discussion
Regardless of habitat type and of the primary dispersal mode, most plant assemblages displayed non-random dispersal patterns, suggesting a dispersal limitation. The three assemblages showing a random dispersal pattern – wind-dispersed and unassisted assemblages of woodlands and animal-dispersed assemblages of wheat croplands – were characterised by low species richness and a restricted regional species pool. These characteristics may explain why observed similarity values were statistically similar to expected similarity values.
Plant assemblages in temporary grasslands and woodlands were dependent on landscape connectivity but wheat cropland assemblages were not. By taking the influences of woodland, grassland and cropland habitats into account simultaneously, we improved our ability to predict species response to changes in connectivity in accordance with our first hypothesis. This suggests that the dispersal of plant species is affected by the connectivity provided by various habitat types simultaneously. As a common pattern for woodland and temporary grasslands, the connectivity provided by grassland habitats had no effect on the similarity of plant assemblages between habitat patches. The absence of any effect has been demonstrated for grassland assemblages11,12,37,45 and is extended here to woodland assemblages. Plant dispersal in grassland habitats is mainly achieved by anthropogenic vectors, such as livestock and agricultural machinery46–48. Dispersal in grasslands might thus be more dependent on farm logistics than on connectivity. However, the absence of effect here could also be due to our inability to map narrow, linear, herbaceous-dominated habitats, such as grassy strips along the edges of croplands, road and railway verges, even though they are all likely to play an important role in the dispersal of grassland plant species8,49. The absence of a landscape connectivity effect in wheat cropland plant assemblages may be explained by two non-exclusive hypotheses: (1) the high proportion (>30%) of croplands in the landscape may be more important to seed dispersal than the role of connectivity, which is in agreement with the fragmentation threshold hypothesis50 and (2) the dominance of generalist species (which occupy broad ecological niches) in wheat cropland assemblages, due to the high disturbance rate and environmental variability of cropland habitat. Such generalist species display high dispersal abilities and are not restricted to any specific habitat type51,52, which implying that generally, they may not be affected by connectivity53,54.
In woodland and temporary grassland assemblages, we detected common patterns of response to the connectivity provided by grassland habitats. However, we did not find any general pattern for the connectivity provided by woodland and cropland habitats. The ability of woodland and cropland habitats to provide connectivity appeared to be dependent on the primary dispersal mode of plant species, in agreement with our second hypothesis.
We partially validated our first sub-hypothesis, since the dispersal of animal-dispersed plants (i.e. similarity between assemblages) was enhanced by the connectivity provided by certain types of habitat. However, their dispersal was also reduced or not affected by others. For example, the dispersal of animal-dispersed plant species inhabiting woodland habitats was enhanced by the connectivity provided by woodland habitats but inhibited by the connectivity provided by cropland habitats. This may be explained by two processes. Movement of animal species is indeed known to preferentially occur through woodland habitats55. In contrast, wood-dwelling animal species, such as mammals56,57 or birds58–60 may avoid open habitats due to the higher risk of predation. We found that in temporary grassland assemblages, dispersal of animal-dispersed plant species was enhanced by the connectivity provided by cropland habitats, but was not affected by other types. We hypothesise that seeds are transferred by animal vectors (i.e. granivorous animal species) from croplands to grasslands during periods of seed dispersal in grasslands. This may occur after cereals have been harvested, when seed resources in croplands are low and animals switch their foraging focus to wild grass seeds. A change from a cropland to a grassland habitat for foraging has already been shown to occur in carabid species61,62. Our results suggest an expansion of this concept to the landscape scale.
In contrast to our second sub-hypothesis, we found that wind dispersal was not affected by landscape connectivity even through wind can disperse seeds over long distances63,64. Specifically, for these species, we were unable to demonstrate either a dispersal barrier effect of woodland habitats or a dispersal enhancing effect of open habitats (i.e. grassland and cropland habitats), even though such effects have already been demonstrated in simplified experimental conditions40–42. One possible explanation for our results is that fine-scale habitat structure may be more important in enhancing or inhibiting seed dispersal than the simple presence or absence of woodland or open habitats. For example, hedgerows vary in height, width, vertical stratification and frequency of gaps, all of which affect small-scale wind patterns and hence, drive wind-dispersal patterns. Similarly, crops vary in height, density and cover depending on the type of management practices applied. Betbeder et al.65 used new remote sensing techniques to quantify fine-scale landscape structure to improve animal species distribution models at the landscape scale. A similar approach should be used to examine plant distribution. Such studies could be coupled with large-scale wind dynamics data, which could be used to determine the distance, direction, frequency and intensity of seed dispersal patterns63,64,66,67.
Unassisted dispersal was independent of landscape connectivity for all assemblage types (temporary grassland and wheat cropland assemblages), thus contradicting our third sub-hypothesis. Despite the fact unassisted species only disperse over a short distance (a minimum of 5 m y−1)68, these species did not depend on the connectivity provided by their own habitats. Our results suggests that the ploughing up of grassland (every 5 years) and cropland (every 1 year) habitats on average in our study area is probably too frequent for unassisted plant species to disperse from one habitat patch to another. In such intensively managed habitats, connectivity depends on time, which would require incorporating past land-cover maps11,12 in the assessment of connectivity effects69,70.
Taking into account the additive effects of the connectivity provided by the three types of habitats shed light on the mechanisms of plant dispersal.
We found opposite effects on dispersal of the connectivity provided by habitats of the same type (dispersal enhancing effect) and by the other habitat types (dispersal barrier effect) (e.g. woodland animal-dispersed assemblages). Both enhancement and inhibition may partly compensate for one another. We also found that the connectivity provided by habitat of the same type does not affect plant assemblages of the same habitat, but that provided by other habitat types enhance dispersal (e.g. grassland animal-dispersed assemblages). Such positive effects of other habitat types have been demonstrated at the local scale for animals of a given habitat type [e.g. for birds71 and for beetles72], leading to the concept of landscape supplementation73. Organisms supplement their resource intake by using resources in nearby patches of the same habitat or by using a substitutable resource in nearby patches of a different habitat type73. In agreement with Dunning et al., our results suggest that plant species could also supplement their “natural” dispersal pathway (i.e habitats of the same type) by using other types of habitats. The supplementation of dispersal pathways for animal-dispersed plant species may be indirectly linked to habitat supplementation by animal species. The validation of this concept in animal-dispersed plant species – and its possible extension to wind-dispersed and unassisted species – is an interesting perspective and could be achieved by analysing the genetic similarity of some representative species of each primary dispersal mode. Using a combination of dispersal pathways would increase a plant’s effective dispersal and improve population fitness, especially in intensified agricultural landscapes where habitats are extremely fragmented.
Methods
Study area and selected sites
This study was carried out in the LTSER site “Zone Atelier Armorique” (ZAAr, ca. 13,000 ha) located in Brittany, western France (48° 36′ N, 1° 32′ W). The study area is characterised by a bocage landscape dominated by multicrop-livestock farming systems, determined by similar physical constraints. The topology is flat, the soil is on granitic and sandstone bedrocks and the climate is temperate oceanic. The landscape in this study area has been changing since 1955 as a result of successive agricultural policies74. Changes included a decrease in the length and the connectivity of the hedgerow network75, abandonment of (i) permanent grasslands and (ii) of root and tuber croplands in favour of (i) temporary grasslands (i.e. regularly ploughed) and (ii) maize fields76 resulting in an increase in the size of grassland and cropland patches.
We analysed the effect of connectivity on plant assemblages in three habitat types that represent 90% of the landscape: cropland (44%), grassland (31%) and woodland (15%) (for more details about changes over time in the study area, see Supplementary Fig. S1). We assessed the effect of connectivity provided by cropland, grassland and woodland habitats on plant assemblage similarity for each habitat type. For each habitat type studied (wheat croplands, temporary grasslands and woodlands), we selected 25 patches scattered throughout the landscape with a distance of at least 150 m between any two patches to reduce spatial autocorrelation. Patch selection was based on land-cover maps in the ZAAr database. The maps were constructed using aerial imagery based on ten land-cover categories: woodland, grassland, grassy strip, fallow land, maize, cereal cropland, other cropland, built up area, road, and aquatic habitat. The hedgerow network was obtained from the 2016 Kermap (geographic raster) database for woodland habitats. We selected patches of similar size (about 2 ha for woodland, 1 ha for temporary grassland and 2.5 ha for wheat cropland), based on the average patch size of these three habitat types in the study area. We interviewed farmers to determine and standardize patch age, the management regime and previous land cover (i.e. the habitat type on which the patch was established) for woodland, temporary grassland and wheat cropland, and initial species sowing composition for temporary grassland and wheat cropland. Since the initial species composition was not available for planted woodlands, we selected woodland patches with similar dominant tree species composition, assuming they reflect the initial planting composition (Table 3). We checked that patches did not originate from the fragmentation of an initially single habitat patch (up to 1952, which is the oldest land-cover map available in the study area, for woodlands and to previous land cover for temporary grassland and wheat croplands).
Table 3.
Habitat patches characteristics of woodland, temporary grassland, and wheat cropland habitats.
| Woodlands | Temporary grasslands | Wheat croplands | ||
|---|---|---|---|---|
| Size (ha) | Median | 2 | 1 | 2.5 |
| Range | 1.06–8.00 | 0.44–2.48 | 1.95–3.12 | |
| Age (years) | >64 | 6–7 | 1 | |
| Initial sowing species composition | Trifolium repens and Lolium perenne | Triticum sp. | ||
| Dominant tree species composition | Fagus sylvatica, Quercus robur and Castanea sativa | |||
| Management regime | Very extensive or absence of management | Grazing with occasional mowing and an occasional use of a soil enrichment product | Intensive crop management | |
| Previous land cover | Cropland or grassland | Cropland | Cropland (Zea mays) | |
Assessment of similarity among plant assemblages
We performed floristic surveys in the 25 patches of woodland, temporary grassland and wheat cropland selected for the study. Floristic surveys were conducted in six 14 × 5 m quadrats in woodlands, ten 5 × 5 m quadrats in temporary grasslands and five 5 × 5 m quadrats in wheat croplands, i.e. the minimum recommended sampling area for each cover type77. These quadrats were located equidistantly from each other in the patch and at least 20 m from the edge of the patch. In each quadrat, we identified species composition and measured species abundance (in percentage cover). Only herbaceous plant species were considered, since ligneous species recorded were related to local management practices linked to tree plantations (i.e. dominant tree species composition of woodlands or dominant tree and shrub species composition of hedgerows surrounding habitat patches). We sampled plant assemblages in woodlands in 2013 (some of these data are already published in Mony et al.24) and in temporary grasslands and wheat croplands in 2016.
The effect of connectivity on similarity among species assemblages was analysed for each primary dispersal mode. We assessed the primary dispersal mode for each species using the Baseflor database78 with the following typology: animal-dispersed, wind-dispersed, unassisted (i.e. gravity-dispersed and autochorous species) and water-dispersed species (referred as “other” in Fig. 1, since they were not included in this study). The main animal species in our study area which could be involved in plant dispersal are mostly small species (e.g. insects, small mammals and granivorous birds), following the disappearance of most large wild species due to the low carrying capacities of the remaining natural areas79. We focused on the primary dispersal mode for each species, although most species have the ability to be dispersed by more than one vector (2.15 on average80), owing to the absence of data on these secondary (or other) dispersal modes in the existing databases. Data on the primary mode of dispersal were available for all the species identified. Plant species in a few genera (e.g. Carex, Epilobium, Leotodon, Taxaracum and Oxalis sp.) could not be determined to species level (5.3% of plants in woodlands, 7.8% in temporary grasslands and 6.4% in wheat croplands). In these particular cases, we assigned the primary dispersal mode of close relatives by assuming phylogenetic conservatism81.
For each habitat type, we assessed similarity in plant composition for each pair of habitat patches as a proxy of dispersal8,23. Thanks to the standardisation of habitat patches mentioned above, we assumed that similarity in plant composition could substitute the most reliable proxy of dispersal, i.e. genetic similarity, which is restricted to the population level82. For each pair of habitat patches, we separately calculated the Sørensen similarity index Ssør for assemblages of animal-dispersed, wind-dispersed and unassisted species. This index was calculated using the ade4 package83. The higher the similarity index (Ssør), the higher the similarity between a given pair of habitat patches.
Assessment of landscape connectivity between habitat patches
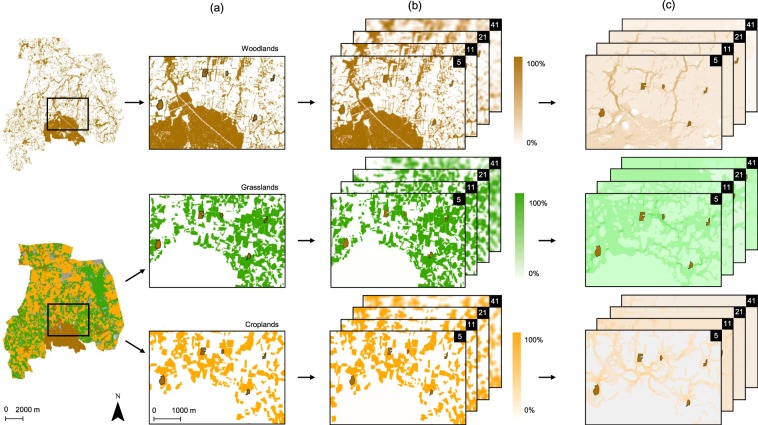
For each habitat type (see Fig. 4 for the example of sampled woodland habitats), we assessed the landscape connectivity provided by three types of habitat: woodland, grassland and cropland. We considered woodland habitats as forests, woodlots, and hedgerows; grassland habitats as grasslands, grassy strips and fallow lands and cropland habitats as maize, cereal cropland and other cropland types and grouped these land-cover types accordingly based on our land-cover maps (Fig. 4a). The landscape connectivity provided by each habitat type (woodland, grassland and cropland) was measured for all pairs of habitat patches in each habitat type (i.e. 25 habitat patches is equivalent to 300 pairs of habitat patches for each habitat type).
Figure 4.
Illustration of the method used to model connectivity as provided by woodland, grassland and cropland habitats. Example of sampled woodland habitats. Sampled woodlands are indicated in brown and surrounded with a black edge on all maps. (a) Extraction of woodland, (top), grassland (middle) and cropland (bottom) habitats derived from (i) the Kermap geographic raster database for woodland habitats and (ii) the land-cover map of the study area. (b) Calculation of the proportion of woodland, grassland and cropland habitat derived from the number of pixels in circular sliding windows of 5-, 11-, 21- and 41-diameter pixels. (c) Electric current fluxes based on circuit theory between woodlands, based on woodland, grassland and cropland habitats. For a given habitat type, a focal pixel for which the proportion of the habitat over the window (i.e. focal neighbourhood) is 100% was assigned a resistance value of 1 (i.e. highly suitable to dispersal), whereas a focal pixel for which the habitat proportion is 0% was assigned a resistance value of 100 (i.e. slightly suitable to dispersal).
We used circuit theory21,22 to model connectivity between patches. This modelling method depends on random walk theory and incorporates the contributions of all dispersal pathways to evaluate the degree of connectivity between patches. These contributions depend on the resistance to movement between habitats. The connectivity value that results from this modelling method is a measure of the degree of isolation, called resistance distance: the higher the resistance distance, the lower the connectivity.
Creating resistance maps
This modelling method requires the creation of resistance maps. Resistance maps were created using sliding (moving) window analysis, which makes it possible to assign a resistance value to each focal pixel of the landscape, the value being calculated and mediated by the neighbouring pixel values84. Based on the gradient (or continuum) model84,85, this method avoids assigning resistance values based on a discrete representation of the landscape matrix84–88. For each habitat type, resistance values were calculated as one hundred and one minus the proportion of the habitat type in a given landscape window, here simulated as a sliding circular window of a given diameter. A focal pixel for which the proportion of the habitat type over the window (i.e. focal neighbourhood) is 100% was assigned a resistance value of 1 (i.e. highly suitable to dispersal). Conversely, a focal pixel for which the proportion of the habitat type is 0% was assigned a resistance value of 101 (i.e. slightly suitable to dispersal) (see Supplementary Fig. S2). The creation of resistance maps based on the proportion of woodland, grassland and cropland habitat for each habitat type reduced subjectivity (i.e. expert knowledge) and avoided hierarchisation of habitat types when assigning resistance values. The proportion of woodland, grassland and cropland habitats was calculated from the number of pixels in the circular sliding window with a displacement and interpolation values of one pixel (i.e. 5 m). We tested sliding windows of different diameters of 5 (25 m), 11 (55 m), 21 (105 m) and 41 (205 m) pixels – closely linked to the range of local dispersal distances of plants41,89 – to test the sensitivity of the connectivity indices to continuous landscape representation (Fig. 4b). These resistance maps were produced using Chloe2012 software90.
Landscape connectivity assessment
We calculated landscape connectivity using resistance distance. For each pair of habitat patches, we calculated the resistance distance based on the resistance maps using Circuitscape software22 (Fig. 4c). Graphab 3.0.2 software91 was used to generate Euclidean links between each pair of habitat patches for each type of habitat. Pairs of habitat patches located at the boundary of the study area, for which Euclidean links were outside the study area, were excluded from subsequent analyses. We used Pearson correlations to examine relationships among the different resistance distances we calculated for the various diameters (5–205 m) of the sliding windows. Resistance distances obtained from habitats of the same habitat type for the different sliding window sizes were strongly correlated (r ≥ 0.95, p < 0.001). We then selected resistance distances obtained from the intermediate-diameter sliding window (11 pixels, i.e. 55 m).
Statistical analysis
Detection of non-random response patterns using a null model
To focus on non-stochastic processes, we assessed the effect of connectivity on the similarity between pairs of assemblages (observed Ssør similarity) according to the distribution of the similarity index based on the calculation of random assemblages (expected Ssør similarity) using a null model. This random similarity reflected random dispersal of seeds (i.e. a lack of a dispersal filter). The null distribution was calculated from data on assemblages derived from a random sampling of species from the regional species pool92. Random assemblages had similar species richness to the corresponding observed assemblage. The probability of each species being selected in the random assemblages was weighted by the occurrence of the species within the regional species pool considered (i.e. all recorded observed species). This process was repeated for each primary dispersal mode and for each type of habitat.
An effect size (ES) was calculated based on the probability that the observed value Ssør was lower than the value expected under the null hypothesis (i.e. the quantile of the null distribution for which the observed value was derived93,94, see Supplementary Methods for calculation). ES of similarity values vary between –1 and 1. In our study, when the ES was close to zero, the observed similarity value between each pair of assemblages was considered to be random. Due to the non-normality of ES values, we used a Wilcoxon test, which is routinely used to test whether ES values were significantly overall different from zero95–98, and thus for the significance of non-random plant assemblage similarities between pairs of habitat patches. When the Wilcoxon test was significant, negative ES values indicated that observed similarity values between each pair of assemblages were lower than expected by chance under the null hypothesis (i.e. dispersal barrier effect). In contrast, positive ES values revealed that the observed similarity values were higher than expected under the null hypothesis (i.e. dispersal enhancing effect).
Influence of landscape connectivity on similarity between plant assemblages
We used linear mixed models to assess the influence of landscape connectivity on the similarity of plant assemblages using the ES of similarity value for each pair of assemblages and primary dispersal mode as the dependent variable and the resistance distances obtained between each pair of habitat patches as independent variables for: (1) woodland, (2) grassland and (3) cropland. To account for the partial dependence of the data on matrices of ES values, the two habitat patches comprising each pair of assemblages analysed were considered as the two random effects23,99,100. In order to ensure unbiased (i) estimates of fixed effects and (ii) model selection procedures101–106, these linear mixed models were fitted using the maximum likelihood (ML) estimation of the variance components. Resistance distances were centered and scaled to ensure the regression coefficients were comparable among models. Each model was performed using a model-averaging method, which takes model selection uncertainty into account107. We built all possible models based on all additive combinations of explanatory variables and ranked them using the Akaike information criterion corrected for small sample sizes (AICc). We then generated full-model averaged estimates of each independent variable across the most parsimonious models107 (ΔAICc < 4, Supplementary Table S1). We considered independent variables to be significant if their 95% confidence intervals did not overlap with zero107. Finally, we calculated the proportion of variance explained by fixed effects (marginal R²) and by fixed and random effects (conditional R²) of the most parsimonious model108.
We also checked whether the effect of connectivity on plant assemblage similarity was not indirectly due to the patches having similar local environmental conditions. To this end, we used the similarity of Ellenberg’s indicator values between each pair of assemblages [moisture, nutrient availability and pH], in addition to using resistance distances to explain ES similarity values (see Supplementary Methods). The results of the averaged models obtained from these preliminary analyses were consistent with the averaged models, which included only the resistances distances as fixed effects (Supplementary Table S2). This confirmed that the relationships we demonstrated between resistance distance and plant assemblage similarity were robust.
All statistical analyses were performed using R.3.5.1 (R Core Team). Linear mixed models, model averaging and associated marginal and conditional R² were calculated using lmerTest109, MuMIn110 and piecewiseSEM111 packages.
Supplementary information
Acknowledgements
We are grateful to Jeremie Guy and Juliette Abadie for assistance in the field, Maxime Dahirel for help with the statistical analyses and Hugues Boussard for help with Chloe2012 software. This work was funded by the French Ministry of Agriculture (DIVA – Agriconnect project) and the Fondation de France (BISCO project) and was supported by landscape databases collected in the LTSER site “Zone Atelier Armorique”. Land-cover maps were established by Jean Nabucet (LETG-Rennes-Costel) and Kermap.
Author Contributions
C.M. and A.E. conceived the idea of the project and all authors contributed to the methodology of the study; all authors collected the data; L.U. analysed the data and wrote the manuscript. All authors contributed critically to interpretations of results and to the drafts and gave final approval for publication.
Data Availability
All data and scripts used to perform the main analyses are available on Figshare (10.6084/m9.figshare.7321268).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50184-2.
References
- 1.Luoto M, Rekolainen S, Aakkula J, Pykälä J. Loss of Plant Species Richness and Habitat Connectivity in Grasslands Associated with Agricultural Change in Finland. AMBIO A J Hum Environ. 2003;32:447–452. doi: 10.1579/0044-7447-32.7.447. [DOI] [PubMed] [Google Scholar]
- 2.Hooftman DAP, Edwards B, Bullock JM. Reductions in connectivity and habitat quality drive local extinctions in a plant diversity hotspot. Ecography. 2016;39:583–592. doi: 10.1111/ecog.01503. [DOI] [Google Scholar]
- 3.Wilson DS. Complex Interactions in Metacommunities, with Implications for Biodiversity and Higher Levels of Selection. Ecology. 1992;73:1984–2000. doi: 10.2307/1941449. [DOI] [Google Scholar]
- 4.Leibold MA, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett. 2004;7:601–613. doi: 10.1111/j.1461-0248.2004.00608.x. [DOI] [Google Scholar]
- 5.Fahrig L, Merriam G. Habitat Patch Connectivity and Population Survival. Ecology. 1985;66:1762–1768. doi: 10.2307/2937372. [DOI] [Google Scholar]
- 6.Haddad NM, et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances. 2015;1:e1500052. doi: 10.1126/sciadv.1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piessens K, Honnay O, Nackaerts K, Hermy M. Plant Species Richness and Composition of Heathland Relics in North-Western Belgium: Evidence for a Rescue-Effect? J Biogeogr. 2004;31:1683–1692. doi: 10.1111/j.1365-2699.2004.01056.x. [DOI] [Google Scholar]
- 8.Thiele Jan, Buchholz Sascha, Schirmel Jens. Using resistance distance from circuit theory to model dispersal through habitat corridors. Journal of Plant Ecology. 2017;11(3):385–393. doi: 10.1093/jpe/rtx004. [DOI] [Google Scholar]
- 9.Thiele Jan, Kellner Simon, Buchholz Sascha, Schirmel Jens. Connectivity or area: what drives plant species richness in habitat corridors? Landscape Ecology. 2018;33(2):173–181. doi: 10.1007/s10980-017-0606-8. [DOI] [Google Scholar]
- 10.Bornette G, Amoros C, Lamouroux N. Aquatic plant diversity in riverine wetlands: the role of connectivity. Freshwater Biol. 1998;39:267–283. doi: 10.1046/j.1365-2427.1998.00273.x. [DOI] [Google Scholar]
- 11.Lindborg R, Eriksson O. Historical Landscape Connectivity Affects Present Plant Species Diversity. Ecology. 2004;85:1840–1845. doi: 10.1890/04-0367. [DOI] [Google Scholar]
- 12.Cousins SAO, Ohlson H, Eriksson O. Effects of historical and present fragmentation on plant species diversity in semi-natural grasslands in Swedish rural landscapes. Landsc Ecol. 2007;22:723–730. doi: 10.1007/s10980-006-9067-1. [DOI] [Google Scholar]
- 13.Ricketts TH. The Matrix Matters: Effective Isolation in Fragmented Landscapes. The American Naturalist. 2001;158:87–99. doi: 10.1086/320863. [DOI] [PubMed] [Google Scholar]
- 14.Watling JI, Nowakowski AJ, Donnelly MA, Orrock JL. Meta-analysis reveals the importance of matrix composition for animals in fragmented habitat. Global Ecology and Biogeography. 2011;20:209–217. doi: 10.1111/j.1466-8238.2010.00586.x. [DOI] [Google Scholar]
- 15.Calabrese JM, Fagan WF. A comparison-shopper’s guide to connectivity metrics. Front Ecol Environ. 2004;2:529–536. doi: 10.1890/1540-9295(2004)002[0529:ACGTCM]2.0.CO;2. [DOI] [Google Scholar]
- 16.Hanski I. A Practical Model of Metapopulation Dynamics. J Anim Ecol. 1994;63:151–162. doi: 10.2307/5591. [DOI] [Google Scholar]
- 17.Moilanen A, Nieminen M. Simple Connectivity Measures in Spatial Ecology. Ecology. 2002;83:1131–1145. doi: 10.1890/0012-9658(2002)083[1131:SCMISE]2.0.CO;2. [DOI] [Google Scholar]
- 18.Pascual-Hortal L, Saura S. Comparison and development of new graph-based landscape connectivity indices: towards the priorization of habitat patches and corridors for conservation. Landsc Ecol. 2006;21:959–967. doi: 10.1007/s10980-006-0013-z. [DOI] [Google Scholar]
- 19.Saura S, Pascual-Hortal L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc Urban Plan. 2007;83:91–103. doi: 10.1016/j.landurbplan.2007.03.005. [DOI] [Google Scholar]
- 20.Adriaensen F, et al. The application of ‘least-cost’ modelling as a functional landscape model. Landscape and Urban Planning. 2003;64:233–247. doi: 10.1016/S0169-2046(02)00242-6. [DOI] [Google Scholar]
- 21.McRae BH. Isolation by Resistance. Evolution. 2006;60:1551–1561. doi: 10.1111/j.0014-3820.2006.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 22.McRae BH, Dickson BG, Keitt TH, Shah VB. Using Circuit Theory to Model Connectivity in Ecology, Evolution, and Conservation. Ecology. 2008;89:2712–2724. doi: 10.1890/07-1861.1. [DOI] [PubMed] [Google Scholar]
- 23.Thiele J, Schirmel J, Buchholz S. Effectiveness of corridors varies among phytosociological plant groups and dispersal syndromes. PLOS ONE. 2018;13:e0199980. doi: 10.1371/journal.pone.0199980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mony C, Abadie J, Gil‐Tena A, Burel F, Ernoult A. Effects of connectivity on animal-dispersed forest plant communities in agriculture-dominated landscapes. Journal of Vegetation Science. 2018;29:167–178. doi: 10.1111/jvs.12606. [DOI] [Google Scholar]
- 25.Chesson P. A need for niches? Trends Ecol. Evol. (Amst.) 1991;6:26–28. doi: 10.1016/0169-5347(91)90144-M. [DOI] [PubMed] [Google Scholar]
- 26.Ziółkowska E, Ostapowicz K, Radeloff VC, Kuemmerle T. Effects of different matrix representations and connectivity measures on habitat network assessments. Landscape Ecol. 2014;29:1551–1570. doi: 10.1007/s10980-014-0075-2. [DOI] [Google Scholar]
- 27.Bender DJ, Fahrig L. Matrix Structure Obscures the Relationship Between Interpatch Movement and Patch Size and Isolation. Ecology. 2005;86:1023–1033. doi: 10.1890/03-0769. [DOI] [Google Scholar]
- 28.Rayfield B, Fortin M-J, Fall A. The sensitivity of least-cost habitat graphs to relative cost surface values. Landscape Ecol. 2010;25:519–532. doi: 10.1007/s10980-009-9436-7. [DOI] [Google Scholar]
- 29.Spear SF, Balkenhol N, Fortin M-J, Mcrae BH, Scribner K. Use of resistance surfaces for landscape genetic studies: considerations for parameterization and analysis. Molecular Ecology. 2010;19:3576–3591. doi: 10.1111/j.1365-294X.2010.04657.x. [DOI] [PubMed] [Google Scholar]
- 30.Zeller KA, McGarigal K, Whiteley AR. Estimating landscape resistance to movement: a review. Landscape Ecology. 2012;27:777–797. doi: 10.1007/s10980-012-9737-0. [DOI] [Google Scholar]
- 31.Koen EL, Bowman J, Walpole AA. The effect of cost surface parameterization on landscape resistance estimates. Molecular Ecology Resources. 2012;12:686–696. doi: 10.1111/j.1755-0998.2012.03123.x. [DOI] [PubMed] [Google Scholar]
- 32.Haddad, N. M. & Tewksbury, J. J. Impacts of corridors on populations and communities. in Connectivity Conservation: maintaining connections for nature (eds. Crooks, K. R. & Sanjayan, M.) 390–415 (Cambridge University Press 2006).
- 33.Gilbert-Norton L, Wilson R, Stevens JR, Beard KH. A meta-analytic review of corridor effectiveness. Conserv Biol. 2010;24:660–668. doi: 10.1111/j.1523-1739.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 34.Haddad, N. M. et al. Assessing positive and negative ecological effects of corridors. in Sources, Sinks and Sustainability (eds. Liu, J., Hull, V., Morzillo, A. T. & Wiens, J. A.) 475–503, 10.1017/CBO9780511842399.024 (Cambridge University Press 2011).
- 35.Fletcher RJ, Burrell NS, Reichert BE, Vasudev D, Austin JD. Divergent Perspectives on Landscape Connectivity Reveal Consistent Effects from Genes to Communities. Curr Landscape Ecol Rep. 2016;1:67–79. doi: 10.1007/s40823-016-0009-6. [DOI] [Google Scholar]
- 36.Gelling M, Macdonald DW, Mathews F. Are hedgerows the route to increased farmland small mammal density? Use of hedgerows in British pastoral habitats. Landscape Ecol. 2007;22:1019–1032. doi: 10.1007/s10980-007-9088-4. [DOI] [Google Scholar]
- 37.Gil-Tena A, Lecerf R, Ernoult A. Disentangling community assemblages to depict an indicator of biological connectivity: A regional study of fragmented semi-natural grasslands. Ecological Indicators. 2013;24:48–55. doi: 10.1016/j.ecolind.2012.05.022. [DOI] [Google Scholar]
- 38.Martensen AC, Ribeiro MC, Banks‐Leite C, Prado PI, Metzger JP. Associations of Forest Cover, Fragment Area, and Connectivity with Neotropical Understory Bird Species Richness and Abundance. Conservation Biology. 2012;26:1100–1111. doi: 10.1111/j.1523-1739.2012.01940.x. [DOI] [PubMed] [Google Scholar]
- 39.Watts, A. G. et al. How spatio-temporal habitat connectivity affects amphibian genetic structure. Front Genet6 (2015). [DOI] [PMC free article] [PubMed]
- 40.Greene DF, Johnson EA. Wind Dispersal of Seeds from a Forest Into a Clearing. Ecology. 1996;77:595–609. doi: 10.2307/2265633. [DOI] [Google Scholar]
- 41.Schurr FM, Steinitz O, Nathan R. Plant fecundity and seed dispersal in spatially heterogeneous environments: models, mechanisms and estimation. Journal of Ecology. 2008;96:628–641. doi: 10.1111/j.1365-2745.2008.01371.x. [DOI] [Google Scholar]
- 42.Damschen EI, et al. How fragmentation and corridors affect wind dynamics and seed dispersal in open habitats. PNAS. 2014;111:3484–3489. doi: 10.1073/pnas.1308968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willson MF, Rice BL, Westoby M. Seed dispersal spectra: a comparison of temperate plant communities. Journal of Vegetation Science. 1990;1:547–562. doi: 10.2307/3235789. [DOI] [Google Scholar]
- 44.Quéré, E. & Geslin, J. Liste des plantes vasculaires invasives de Bretagne. (2016).
- 45.Helm A, Hanski I, Pärtel M. Slow response of plant species richness to habitat loss and fragmentation. Ecol Lett. 2006;9:72–77. doi: 10.1111/j.1461-0248.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 46.Strykstra RJ, Verweij Gl, Bakker Jp. Seed dispersal by mowing machinery in a Dutch brook valley system. Acta Botanica Neerlandica. 1997;46:387–401. doi: 10.1111/plb.1997.46.4.387. [DOI] [Google Scholar]
- 47.Couvreur M, Couvreur M, Vandenberghe B, Verheyen K, Hermy M. An experimental assessment of seed adhesivity on animal furs. Seed Science Research. 2004;14:147–159. doi: 10.1079/SSR2004164. [DOI] [Google Scholar]
- 48.Cosyns, E., Claerbout, S., Lamoot, I. & Hoffmann, M. Endozoochorous seed dispersal by cattle and horse in a spatially heterogeneous landscape. Plant ecology (2005).
- 49.Tikka PM, Högmander H, Koski PS. Road and railway verges serve as dispersal corridors for grassland plants. Landscape ecology. 2001;16:659–666. doi: 10.1023/A:1013120529382. [DOI] [Google Scholar]
- 50.Andrén H. Effects of Habitat Fragmentation on Birds and Mammals in Landscapes with Different Proportions of Suitable Habitat: A Review. Oikos. 1994;71:355. doi: 10.2307/3545823. [DOI] [Google Scholar]
- 51.Fischer M, Stöcklin J. Local Extinctions of Plants in Remnants of Extensively Used Calcareous Grasslands 1950 –1985. Conservation Biology. 1997;11:727–737. doi: 10.1046/j.1523-1739.1997.96082.x. [DOI] [Google Scholar]
- 52.Honnay O, Hermy M, Coppin P. Effects of area, age and diversity of forest patches in Belgium on plant species richness, and implications for conservation and reforestation. Biol Cons. 1999;87:73–84. doi: 10.1016/S0006-3207(98)00038-X. [DOI] [Google Scholar]
- 53.Adriaens D, Honnay O, Hermy M. No evidence of a plant extinction debt in highly fragmented calcareous grasslands in Belgium. Biol Cons. 2006;133:212–224. doi: 10.1016/j.biocon.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Brückmann SV, Krauss J, Steffan-Dewenter I. Butterfly and plant specialists suffer from reduced connectivity in fragmented landscapes. J Appl Ecol. 2010;47:799–809. doi: 10.1111/j.1365-2664.2010.01828.x. [DOI] [Google Scholar]
- 55.Pardini R, de Souza SM, Braga-Neto R, Metzger JP. The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biological Conservation. 2005;124:253–266. doi: 10.1016/j.biocon.2005.01.033. [DOI] [Google Scholar]
- 56.Rosalino LM, Ferreira D, Leitão I, Santos-Reis M. Usage patterns of Mediterranean agro-forest habitat components by wood mice Apodemus sylvaticus. Mammalian Biology - Zeitschrift für Säugetierkunde. 2011;76:268–273. doi: 10.1016/j.mambio.2010.08.004. [DOI] [Google Scholar]
- 57.Wereszczuk, A. & Zalewski, A. Spatial Niche Segregation of Sympatric Stone Marten and Pine Marten – Avoidance of Competition or Selection of Optimal Habitat? PLoS One10, (2015). [DOI] [PMC free article] [PubMed]
- 58.Lima SL, Dill LM. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. doi: 10.1139/z90-092. [DOI] [Google Scholar]
- 59.Rodríguez A, Andrén H, Jansson G. Habitat-mediated predation risk and decision making of small birds at forest edges. Oikos. 2001;95:383–396. doi: 10.1034/j.1600-0706.2001.950303.x. [DOI] [Google Scholar]
- 60.Turcotte Y, Desrochers A. Landscape-dependent response to predation risk by forest birds. Oikos. 2003;100:614–618. doi: 10.1034/j.1600-0706.2003.12234.x. [DOI] [Google Scholar]
- 61.French BW, Elliott NC, Berberet RC, Burd JD. Effects of Riparian and Grassland Habitats on Ground Beetle (Coleoptera: Carabidae) Assemblages in Adjacent Wheat Fields. Environ Entomol. 2001;30:225–234. doi: 10.1603/0046-225X-30.2.225. [DOI] [Google Scholar]
- 62.Madeira F, et al. Spillover of arthropods from cropland to protected calcareous grassland – the neighbouring habitat matters. Agriculture, Ecosystems & Environment. 2016;235:127–133. doi: 10.1016/j.agee.2016.10.012. [DOI] [Google Scholar]
- 63.Nathan R, et al. Mechanisms of long-distance dispersal of seeds by wind. Nature. 2002;418:409–413. doi: 10.1038/nature00844. [DOI] [PubMed] [Google Scholar]
- 64.Tackenberg O. Modeling Long-Distance Dispersal of Plant Diaspores by Wind. Ecol Monogr. 2003;73:173–189. doi: 10.1890/0012-9615(2003)073[0173:MLDOPD]2.0.CO;2. [DOI] [Google Scholar]
- 65.Betbeder J, Hubert-Moy L, Burel F, Corgne S, Baudry J. Assessing ecological habitat structure from local to landscape scales using synthetic aperture radar. Ecological Indicators. 2015;52:545–557. doi: 10.1016/j.ecolind.2014.11.009. [DOI] [Google Scholar]
- 66.Nathan R, et al. Mechanistic models of seed dispersal by wind. Theoretical Ecology. 2011;4:113–132. doi: 10.1007/s12080-011-0115-3. [DOI] [Google Scholar]
- 67.Tackenberg O, Poschlod P, Kahmen S. Dandelion Seed Dispersal: The Horizontal Wind Speed Does Not Matter for Long-Distance Dispersal - it is Updraft! Plant Biology. 2003;5:451–454. doi: 10.1055/s-2003-44789. [DOI] [Google Scholar]
- 68.van Dorp D, Schippers P, van Groenendael JM. Migration rates of grassland plants along corridors in fragmented landscapes assessed with a cellular automation model. Landscape ecology. 1997;12:39–50. doi: 10.1007/BF02698206. [DOI] [Google Scholar]
- 69.Williams PD, Hastings A, Křivan AEV, McPeek EMA. Stochastic Dispersal and Population Persistence in Marine Organisms. The American Naturalist. 2013;182:271–282. doi: 10.1086/671059. [DOI] [PubMed] [Google Scholar]
- 70.Zeigler, S. L. & Fagan, W. F. Transient windows for connectivity in a changing world. Mov Ecol2 (2014). [DOI] [PMC free article] [PubMed]
- 71.Whitcomb BL, Whitcomb RF, Bystrak D. Island biogeography and ‘Habitat Islands’ of eastern forest. III. Long-term turnover and effects of selective logging on the avifauna of forest fragments. American Birds. 1977;31:7. [Google Scholar]
- 72.Campbell LA, Meinke LJ. Seasonality and Adult Habitat Use by Four Diabrotica Species at Prairie-Corn Interfaces. Environmental Entomology. 2006;35:922–936. doi: 10.1603/0046-225X-35.4.922. [DOI] [Google Scholar]
- 73.Dunning JB, Danielson BJ, Pulliam HR. Ecological Processes That Affect Populations in Complex Landscapes. Oikos. 1992;65:169–175. doi: 10.2307/3544901. [DOI] [Google Scholar]
- 74.Meynier A, Deniel J. Les talus et l’aménagement de l’espace rural. Penn ar Bed. 1965;41:41–54. [Google Scholar]
- 75.Burel, F. & Baudry, J. Ecologie du paysage. Concepts, méthodes et applications. (Editions TEC et DOC 1999).
- 76.Huyghe C. Evolution des prairies et cultures fourragères et de leurs modalités culturales et d’utilisation en France au cours des cinquante dernières années. Fourrages. 2009;200:404–428. [Google Scholar]
- 77.Mueller-Dombois, D. & Ellenberg, H. Aims and Methods of Vegetation Ecology. (John Wiley & Sons 1974).
- 78.Julve, P. Baseflor. Index botanique, écologique et chorologique de la flore de France. (1998).
- 79.Groupe Mammalogique Breton. Atlas des mammifères de Bretagne. (Locus Solus 2015).
- 80.Ozinga WA, Bekker RM, Schaminée JHJ, Van Groenendael JM. Dispersal potential in plant communities depends on environmental conditions. Journal of Ecology. 2004;92:767–777. doi: 10.1111/j.0022-0477.2004.00916.x. [DOI] [Google Scholar]
- 81.Baeten L, Davies TJ, Verheyen K, Calster HV, Vellend M. Disentangling dispersal from phylogeny in the colonization capacity of forest understorey plants. J Ecol. 2015;103:175–183. doi: 10.1111/1365-2745.12333. [DOI] [Google Scholar]
- 82.Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C. Individual dispersal, landscape connectivity and ecological networks: Dispersal, connectivity and networks. Biol Rev. 2013;88:310–326. doi: 10.1111/brv.12000. [DOI] [PubMed] [Google Scholar]
- 83.Dray S, Dufour A-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. Journal of Statistical Software. 2007;22:1–20. doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 84.McGarigal, K. & Cushman, S. A. The Gradient Concept of Landscape Structure. in Issues and Perspectives in Landscape Ecology 112–119, 10.1017/CBO9780511614415.013 (2005).
- 85.Fischer J, Lindenmayer DB. Beyond fragmentation: the continuum model for fauna research and conservation in human-modified landscapes. Oikos. 2006;112:473–480. doi: 10.1111/j.0030-1299.2006.14148.x. [DOI] [Google Scholar]
- 86.Cushman Samuel A., Gutzweiler Kevin, Evans Jeffrey S., McGarigal Kevin. Spatial Complexity, Informatics, and Wildlife Conservation. Tokyo: Springer Japan; 2010. The Gradient Paradigm: A Conceptual and Analytical Framework for Landscape Ecology; pp. 83–108. [Google Scholar]
- 87.Lausch A, et al. Understanding and quantifying landscape structure – A review on relevant process characteristics, data models and landscape metrics. Ecological Modelling. 2015;295:31–41. doi: 10.1016/j.ecolmodel.2014.08.018. [DOI] [Google Scholar]
- 88.Brudvig LA, et al. Evaluating conceptual models of landscape change. Ecography. 2017;40:74–84. doi: 10.1111/ecog.02543. [DOI] [Google Scholar]
- 89.Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- 90.Boussard, H. & Baudry, J. Chloe2012: a software for landscape pattern analysis (2014).
- 91.Foltête J-C, Clauzel C, Vuidel G. A software tool dedicated to the modelling of landscape networks. Environmental Modelling & Software. 2012;38:316–327. doi: 10.1016/j.envsoft.2012.07.002. [DOI] [Google Scholar]
- 92.Mason NWH, Lanoiselée C, Mouillot D, Wilson JB, Argillier C. Does niche overlap control relative abundance in French lacustrine fish communities? A new method incorporating functional traits. Journal of Animal Ecology. 2008;77:661–669. doi: 10.1111/j.1365-2656.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- 93.Kelt DA, Taper ML, Meserve PL. Assessing the Impact of Competition on Community Assembly: A Case Study using Small Mammals. Ecology. 1995;76:1283–1296. doi: 10.2307/1940935. [DOI] [Google Scholar]
- 94.Chase JM, Kraft NJB, Smith KG, Vellend M, Inouye BD. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere. 2011;2:1–11. doi: 10.1890/ES10-00117.1. [DOI] [Google Scholar]
- 95.Kraft NJB, Valencia R, Ackerly DD. Functional Traits and Niche-Based Tree Community Assembly in an Amazonian. Forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- 96.Swenson NG, Enquist BJ. Opposing assembly mechanisms in a Neotropical dry forest: implications for phylogenetic and functional community ecology. Ecology. 2009;90:2161–2170. doi: 10.1890/08-1025.1. [DOI] [PubMed] [Google Scholar]
- 97.Bernard-Verdier M, et al. Community assembly along a soil depth gradient: contrasting patterns of plant trait convergence and divergence in a Mediterranean rangeland. J Ecol. 2012;100:1422–1433. doi: 10.1111/1365-2745.12003. [DOI] [Google Scholar]
- 98.Favre-Bac L, Mony C, Burel F, Seimandi-Corda G, Ernoult A. Connectivity drives the functional diversity of plant dispersal traits in agricultural landscapes: the example of ditch metacommunities. Landscape Ecol. 2017;32:2029–2040. doi: 10.1007/s10980-017-0564-1. [DOI] [Google Scholar]
- 99.Clarke RT, Rothery P, Raybould AF. Confidence limits for regression relationships between distance matrices: Estimating gene flow with distance. Journal of Agricultural, Biological, and Environmental Statistics. 2002;7:361–372. doi: 10.1198/108571102320. [DOI] [Google Scholar]
- 100.Van Strien MJ, Keller D, Holderegger R. A new analytical approach to landscape genetic modelling: least-cost transect analysis and linear mixed models. Mol. Ecol. 2012;21:4010–4023. doi: 10.1111/j.1365-294X.2012.05687.x. [DOI] [PubMed] [Google Scholar]
- 101.Faraway, J. J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models, Second Edition. (Chapman and Hall/CRC 2016).
- 102.Verbeke, G. & Molenberghs, G. Linear Mixed Models for Longitudinal Data (2000).
- 103.Orelien JG, Edwards LJ. Fixed-effect variable selection in linear mixed models using R2 statistics. Computational Statistics & Data Analysis. 2008;52:1896–1907. doi: 10.1016/j.csda.2007.06.006. [DOI] [Google Scholar]
- 104.Zuur, A., Ieno, E. N., Walker, N., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R. (Springer-Verlag 2009).
- 105.Bolker BM, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. (Amst.) 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 106.Row JR, Knick ST, Oyler-McCance SJ, Lougheed SC, Fedy BC. Developing approaches for linear mixed modeling in landscape genetics through landscape-directed dispersal simulations. Ecol Evol. 2017;7:3751–3761. doi: 10.1002/ece3.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. (Springer-Verlag 2002).
- 108.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- 109.Kuznetsova A, Brockhoff PB, Christensen RH. B. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 110.Barton, K. MuMIn: Multi-Model Inference. (2018).
- 111.Lefcheck J. S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods in Ecology and Evolution. 2016;7:573–579. doi: 10.1111/2041-210X.12512. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and scripts used to perform the main analyses are available on Figshare (10.6084/m9.figshare.7321268).