Abstract
Tissue-resident memory CD8+ T (Trm) cells mediate potent local innate and adaptive immune responses and play a central role against solid tumors. However, whether Trm cells cross-talk with dendritic cells (DCs) to support anti-tumor immunity remains unclear. Here we show that antigen-specific activation of skin Trm cells leads to maturation and migration to draining lymph nodes of cross-presenting dermal DCs. Tumor rejection mediated by Trm cells triggers the spread of cytotoxic CD8+ T cell responses against tumor-derived neo- and self-antigens via dermal DCs. These responses suppress the growth of intradermal tumors and disseminated melanoma lacking the Trm cell-targeted epitope. Moreover, analysis of RNA sequencing data from human melanoma tumors reveals that enrichment of a Trm cell gene signature associates with DC activation and improved survival. This work unveils the ability of Trm cells to amplify the breath of cytotoxic CD8+ T cell responses through DCs, thereby strengthening anti-tumor immunity.
Subject terms: Melanoma, Tumour immunology, Immunological memory, Immunization, Dendritic cells
Immunotherapy can induce antigen spreading of antitumor T cell response, which correlates with better outcomes. Here the authors show that tissue-resident memory CD8 T cells promote antigen spreading via lysing tumor cells and promoting their uptake and cross-presentation by dendritic cells, thereby eliciting de novo T cell responses.
Introduction
Cytotoxic CD8+ T lymphocytes (CTL) play a pivotal role in providing effective antigen-specific immunity against tumors. Tumor-specific CTL responses are initiated at secondary lymphoid organs when naïve CD8+ T cells are activated by mature migratory dendritic cells (DCs) presenting tumor-derived antigens on MHC class I molecules1,2. Antigen-specific CD8+ T cells massively proliferate and differentiate into cytotoxic effector T (Teff) cells, which can then migrate to peripheral tissues and recognize cancer cells through their T-cell receptor (TCR). CTL destroy target tumor cells through mechanisms including release of granules containing perforin and granzymes and inducing FasL-mediated apoptosis. To achieve long-lasting anti-tumor immunity, it is necessary to establish memory CD8+ T-cell responses3,4. Classically, the circulating memory compartment consists of central-memory (Tcm) and effector-memory (Tem) CD8+ T cells5. Tcm cells circulate between secondary lymphoid organs and blood, whereas Tem cells circulate between blood and non-lymphoid tissues5. In contrast to these circulating subsets, tissue-resident memory CD8+ T (Trm) cells stably reside in lymphoid and non-lymphoid tissues where they provide potent local innate and adaptive immune responses6. The remarkable ability of Trm cells to mediate protective immunity has prompted the development of more potent vaccination strategies by eliciting Trm cells against infectious diseases7,8. Evidence supporting a central role of Trm cells in tumor immunosurveillance has recently emerged from animal models9,10. We and others have demonstrated that antigen-specific Trm cell responses mediate strong tissue-restricted immunity against cutaneous melanoma and other tumor models9,11–13. However, the precise mechanisms by which Trm cells mediate enhanced anti-tumor immunity are poorly understood.
In human cancers, infiltration of CD103+ CD8+ T cells in solid tumors has been associated with longer survival in patients with breast, lung, endometrial, ovarian, cervical, urothelial and melanoma tumors14–23. Moreover, tumor-infiltration of Trm cells was recently associated with improved survival in melanoma patients that received an antibody blocking the inhibitory receptor PD-123. T-cell residency across different tissues, including tumors, is defined by a distinctive gene expression program commanded by transcription factors, including Runx3, Blimp1, Hobit and Nur7724–26. Tissue adaptation involves constitutive upregulation of CD69, CD49a and CD103, which sustain enhanced ability of Trm cells to become established in the tumor niche and better suited to fight tumors. CD69 is a C-type lectin expressed by Trm cells from most tissues that render these cells unresponsive to tissue egress signals such as sphingosine-1-phosphate (S1P) by reducing the levels of S1P receptor27. CD49a, or α1β1 integrin, is an adhesion molecule that binds to the extracellular matrix proteins collagen and laminin28 and distinguish Trm cells with higher cytotoxic potential in skin and melanoma tumors29,30. Trm cells confined to epithelial barriers also express CD103 (integrin αEβ7), which binds to E-cadherin and facilitates their interaction with epithelial cells31. In CD8+ T cells isolated from lung tumors, CD103 molecules have been shown to distribute preferentially near the immune synapse formed with the target tumor cell and to facilitate cytotoxic vesicle degranulation in an E-cadherin-dependent fashion32. Thus, tissue adaptation-related features and enhanced cytotoxicity may contribute to the superior protective potential of Trm cells in human solid cancers.
Complementary to their cytotoxic activity, Trm cells secrete large amounts of effector molecules, most prominently IFN-γ and TNF-α, which can activate other immune cells with anti-tumor potential. In the context of viral infections, IFN-γ produced by Trm cells triggers an innate-like alarm state characterized by the production of chemokines and antimicrobial molecules in the tissue and the recruitment of circulating memory CD8+ T cells33,34. In cancer, the presence of a tissue-resident gene signature is associated with higher density and enhanced cytotoxicity of CTLs infiltrating lung and breast tumors16,35. In viral models, Trm cell-derived IL-2 has been shown to promote upregulation of granzyme B in NK cells36. Additionally, antigen-specific activation of Trm cells leads to the production of TNF-α, which promotes rapid DC maturation and up-regulation of the lymph node homing chemokine receptor CCR736,37. However, whether Trm cell activation in the tissue causes DC migration to draining lymph nodes and the subsequent initiation of protective CTL immune responses remains unknown. Particularly in the context of the tumor microenvironment, the innate–like capabilities of Trm cells and their potential cross-talk with DCs remain largely unexplored. We hypothesized that Trm cells cooperate with DCs to support anti-tumor immunity by initiating secondary T-cell responses against tumor-derived antigens.
Here, we demonstrate that skin Trm cell activation promotes maturation and trafficking to draining lymph nodes of migratory dermal DCs. Trm cell-mediated melanoma rejection leads to the spreading of circulating CTL responses against tumor-derived neo- and self-antigens that protects against intradermal tumors and disseminated melanoma lacking the Trm cell-targeted antigen. Transcriptional analysis of human melanoma tumors reveals that a Trm cell gene signature associates with DC activation and improved survival. This work highlights the ability of Trm cells to cross-talk with DCs and orchestrate the broadening of anti-tumor CTL immunity.
Results
Trm cells trigger maturation and migration of dermal DCs
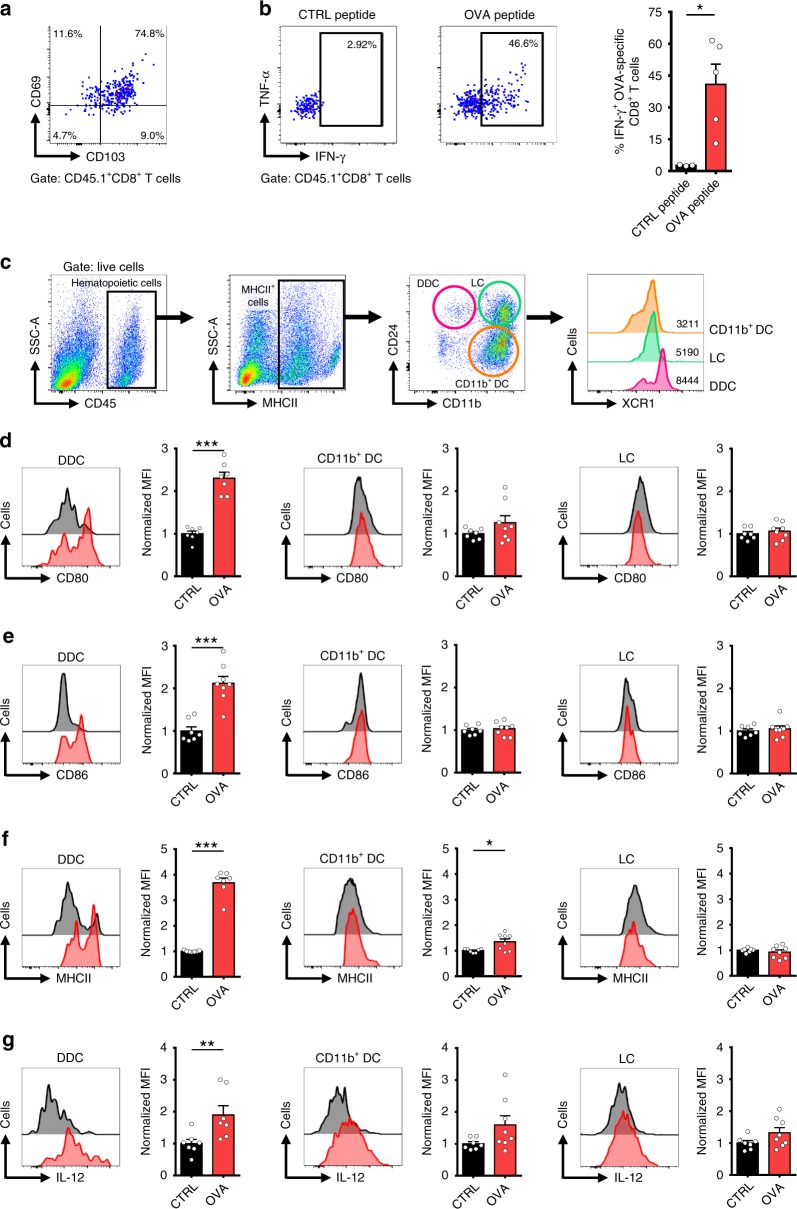
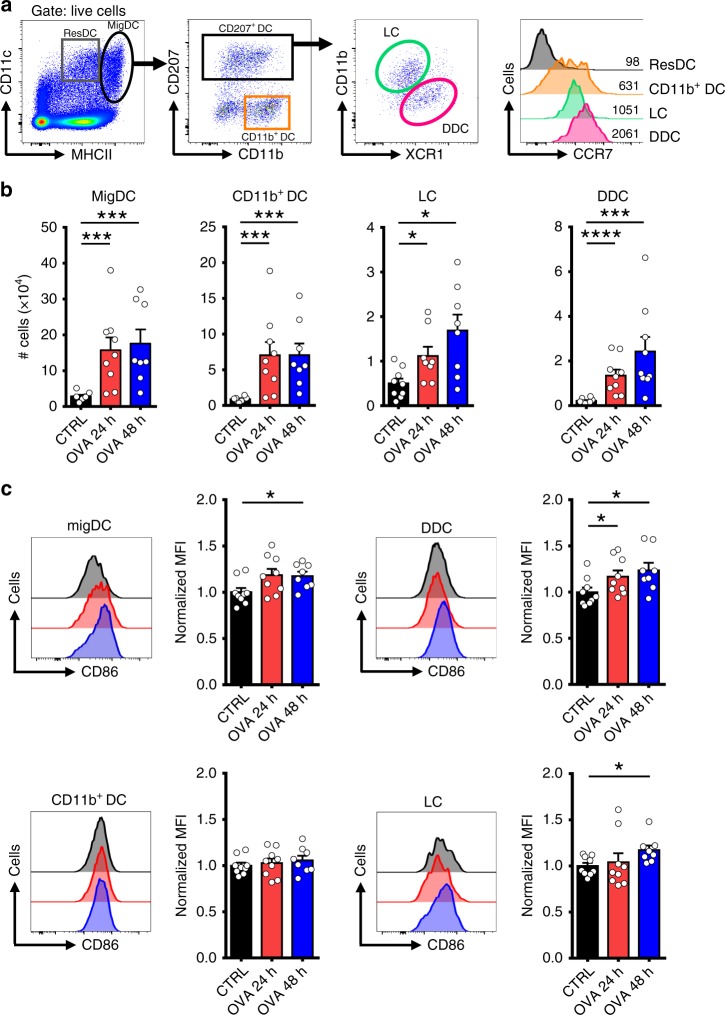
We first aimed to study the potential interplay between Trm cells and migratory DCs in the skin. To this end, we generated ovalbumin (OVA)-specific skin Trm cells in mice using intradermal (i.d.) vaccination. This was followed by administration of an anti-CD8 antibody during the memory phase of the response (>4 weeks post vaccination), which efficiently deplete circulating CD8+ T cells (Supplementary Fig. 1a, b), including circulating memory and effector OVA-specific CD8+ T cells in lymphoid and non-lymphoid tissues, as previously shown11. Then, depletion-resistant Trm cells (Fig. 1a) were specifically activated by i.d. injection of the immunodominant OVA(257-264) peptide, readily producing IFN-γ and TNF-α within the first 6 h (Fig. 1a, b). Interestingly, we observed that skin XCR1+ conventional type 1 DCs (cDC1), also known as dermal DCs or DDCs (Fig. 1c) upregulated CD80, CD86, MHC class II and IL-12 molecules 24 h after Trm cell activation (Fig. 1d–g). These data indicate that Trm cells induce maturation of dermal DCs, which are specialized in antigen cross-presentation and priming of CD8+ T cells38. Then, we analyzed the presence of skin migratory DCs in draining lymph nodes based on the expression of high levels of MHC class II, CD207 (langerin) and CCR7 (Fig. 2a). We observed a marked accumulation of different migratory DC subsets at 24 and 48 h after Trm cell activation, including dermal DCs, Langerhans cells (LCs) and CD11b+ DCs (Fig. 2b). Among these subsets, dermal DCs displayed upregulated expression of the maturation marker CD86 (Fig. 2c). These results indicate that antigen-specific activation of Trm cells triggers maturation and migration to draining lymph nodes of skin-derived dermal DCs, revealing a cross-talk among these cells.
Fig. 1.
Skin Trm cell activation induces maturation of dermal DCs. C57BL/6 mice bearing OVA-specific skin Trm cells and depleted of circulating CD8+ T cells by administration of an anti-CD8 antibody were intradermally inoculated with control (CTRL) or OVA(257-264) (OVA) peptides to activate Trm cells. a, b OVA-specific CD45.1+ CD8+ T cells in the skin were analyzed 6 h later by intracellular cytokine staining and flow cytometry. a Representative pseudocolor dot-plot showing CD69 and CD103 expression. b Representative pseudocolor dot-plots and graph showing IFN-γ and TNF-α production by skin Trm cells. c–g DCs in the skin were analyzed 24 h after peptide stimulation. c Gating strategy used to identify skin DC subpopulations, including CD11b+ DCs, Langerhans cells (LC) and dermal DCs (DDC) and representative histograms showing XCR1 expression in each subset. d–g Representative histograms (black: CTRL; red: OVA) and graphs showing of the expression of CD80, CD86, MHCII and IL-12 of each skin DC subset. For quantification, the geometric mean fluorescence intensity (MFI) was normalized relative to the average of the control group. Pooled data from two independent experiments, n = 7 mice in the group treated with control peptide and n = 8 mice in the group stimulated with OVA(257-264) peptide. Bars are the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 by Mann-Whitney unpaired test
Fig. 2.
Trm cell activation promotes migration of dermal DCs. C57BL/6 mice bearing OVA-specific skin Trm cells and depleted of circulating CD8+ T cells by administration of an anti-CD8 antibody were intradermally inoculated with control (CTRL) or OVA(257-264) peptides followed by analysis of inguinal draining lymph nodes after 24 h (OVA 24 h) and 48 h (OVA 48 h). a Gating strategy used to identify the different DC subsets in skin draining lymph nodes, including: lymph node-resident DCs (ResDC), migratory DCs (MigDC), CD11b+ DCs, LCs and DDCs. Representative histograms showing CCR7 expression on relevant subpopulations. b Quantification of total numbers of the different DC subsets. c Representative histograms (black: CTRL; red: OVA 24 h; blue: OVA 48 h) and graphs showing CD86 expression of each DC population. For quantification, the geometric mean fluorescence intensity (MFI) was normalized relative to the average of the control group. Pooled data from two independent experiments n = 9 mice per group. Bars are the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.01 by Mann-Whitney unpaired test
Trm cells spread CTL responses against tumor neo-antigens
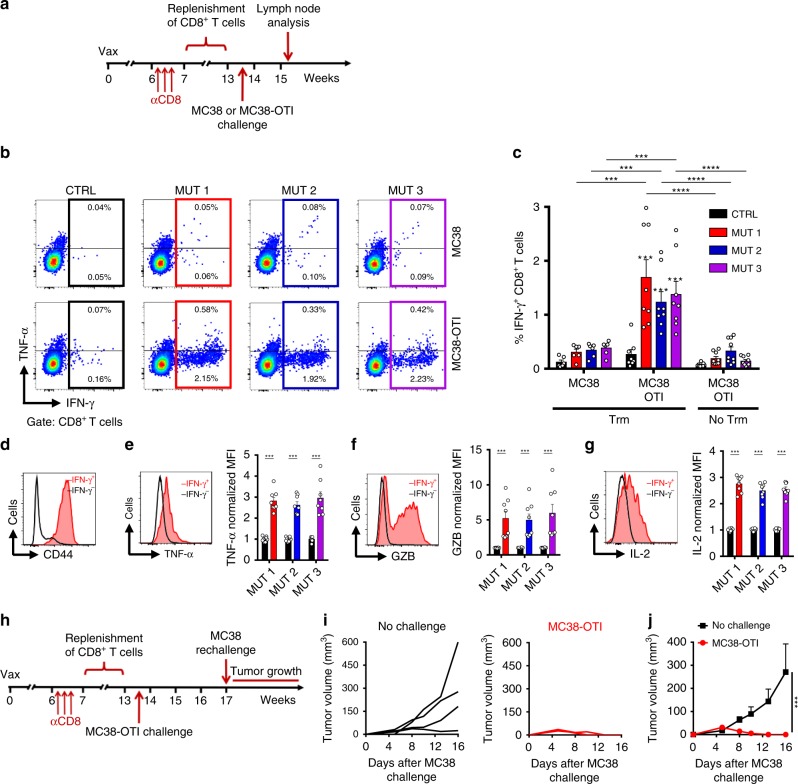
Given the ability of Trm cells to activate migratory skin DCs and also mediate tumor-cell killing11,36, we hypothesized that antigen-specific Trm cell-mediated tumor rejection would lead to the generation of secondary responses against other tumor-derived antigens, a phenomenon known as antigen spreading. To test this, mice bearing OVA-specific Trm cells were depleted from circulating CD8+ T cells and let during 8 weeks to replenish this compartment (Supplementary Fig. 1c). This allows resetting the endogenous repertoire in terms of specificity while maintaining OVA-specific Trm cells in the skin. Then, mice were challenged i.d. with MC38 cells expressing the OVA(257-264) peptide (MC38-OTI), which were rejected by OVA-specific Trm cells. As controls, unvaccinated mice (no Trm) challenged with MC38-OTI and Trm cell-bearing mice challenged with parental MC38 were used. Secondary CD8+ T-cell responses raised against highly relevant neo-epitopes present in MC38 cell line39 were analyzed 12 days later in tumor-draining lymph nodes (Fig. 3a). To this end, lymph node cells were ex vivo stimulated with neo-epitopes carrying missense mutations MUT 1 (SIIVFNLL from Dpagt1 gene), MUT 2 (AQLANDVVL from Reps1 gene) and MUT 3 (ASMTNMELM from Adpgk gene) and the production of effector molecules was analyzed by intracellular staining and flow cytometry. In contrast to control groups, rejection of MC38-OTI mediated by OVA-specific Trm cells resulted in the expansion of CD8+ T cells specific to all neo-epitopes tested (Fig. 3b, c), detected as IFN-γ-producing CD8+ T cells, which also expressed high levels of CD44 (Fig. 3d). These results indicate that spreading of CD8+ T-cell responses to multiple antigens is triggered by Trm cell-mediated tumor rejection. Neo-epitope-specific CD8+ T cells displayed high expression of other effector molecules, such as TNF-α, granzyme B and IL-2 (Fig. 3e, g), which is consistent with anti-tumor cytotoxic activity. Indeed, these mice were able to reject a re-challenge with MC38 cells, which express the neo-epitopes but cannot be recognized by OVA-specific Trm cells (Fig. 3h-k).
Fig. 3.
Trm cell-mediated tumor rejection spread CTL responses against neo-antigens. a–g C57BL/6 mice bearing OVA-specific skin Trm cells were depleted of circulating CD8+ T cells by administration of an anti-CD8 antibody. Eight weeks later, mice were challenged intradermally with MC38 (n = 6) or MC38-OTI (n = 8) cells. A group of unvaccinated mice (No Trm) was challenged with MC38-OTI cells (n = 9) as additional control. b–g Expression of IFN-γ, CD44, TNF-α, granzyme B and IL-2 was analyzed 12 days after tumor challenge in CD8+ T cells present in draining lymph nodes after ex vivo stimulation with control (CTRL) or neo-epitope peptides (MUT 1, MUT 2 and MUT 3) followed by intracellular cytokine staining. a Experimental timeline scheme. b Representative pseudocolor dot-plots showing IFN-γ and TNF-α production from a mouse challenged with MC38 (upper panels) and MC38-OTI (lower panels). c Graph showing the frequencies of IFN-γ-producing CD8+ T cells. d–g Representative histograms (black histograms: IFN-γ- CD8+ T cells; red histograms: IFN-γ+ CD8+ T cells) and graphs (black bars: IFN-γ- CD8+ T cells; colored bars: IFN-γ+ CD8+ T cells) showing the expression of CD44 (d), TNF-α (e), granzyme B (f), and IL-2 (g). For quantification, the geometric mean fluorescence intensity (MFI) was normalized relative to the average of IFN-γ- CD8+ T cells. h-j Mice bearing OVA-specific Trm cells that rejected MC38-OTI cells, were re-challenged with MC38 cells (MC38-OTI, n = 4) and tumor growth was monitored. Mice that did not received initial challenge (No challenge, n = 4) were used as controls. h Experimental timeline scheme. i Individual curves showing tumor growth. j Graphs showing the mean of tumor volume. Bars are the mean ± SEM. ***p ≤ 0.001, ****p ≤ 0.0001 by Mann-Whitney unpaired test for c, e–g and two-way ANOVA Bonferroni post-hoc test for j
Trm cells promote melanoma-antigen spreading through DCs
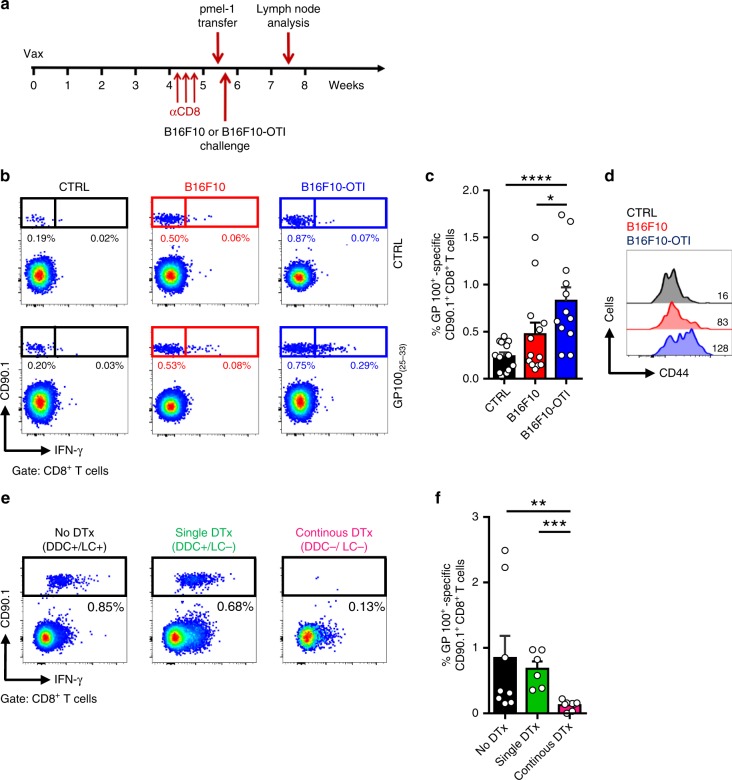
To confirm these results in a relevant metastatic melanoma model, we used B16F10 cells, which are less immunogenic and express melanocyte-associated self-antigens, such as gp100. Favorably, responses against H-2 Kb-restricted gp100(25-33) peptide can be tracked by transferring congenic TCR-transgenic CD8+ T cells from pmel-1 mice without the need to wait for the replenishment of the endogenous repertoire. Mice bearing OVA-specific Trm cells and devoid of circulating CD8+ T cells received i.v. transfer of pmel-1 CD8+ T cells (Fig. 4a). The following day, mice were challenged i.d. with B16F10 cells expressing the OVA(257-264) peptide (B16F10-OTI), which are rejected by OVA-specific Trm cells, as previously shown by us11. After 12 days, the generation of gp100-specific CTL responses was analyzed in the draining lymph nodes. Control groups were either left unchallenged (CTRL) or challenged with B16F10 parental cell line that do not activate OVA-specific Trm cells. As compared to control groups, only mice challenged with B16F10-OTI presented a significant expansion of gp100-specific CD8+ T cells (Fig. 4b, c), which produced IFN-γ after ex vivo peptide stimulation and displayed high expression of CD44 (Fig. 4d), indicating that they were efficiently primed. Since both B16F10-OTI and B16F10 cells express gp100 but only B16F10-OTI can be recognized by OVA-specific Trm cells, these results suggest that melanoma recognition by Trm cells triggers the spreading of CD8+ T-cell responses to tumor-derived antigens.
Fig. 4.
Trm cells promote melanoma-antigen spreading through dermal DCs. C57BL/6 and Langerin-DTR mice bearing OVA-specific skin Trm cells and depleted of circulating CD8+ T cells were intravenously transferred with gp100-specific CD90.1+ pmel-1 CD8+ T cells and one day later challenged intradermally with B16F10-OTI melanoma cells. Control groups were either unchallenged (CTRL) or challenged with or B16F10 melanoma cells that do not activate Trm cells. Analysis of gp100-specific pmel-1 CD8+ T cells was performed 12 days after tumor challenge in inguinal draining lymph nodes stimulated ex vivo with cognate gp100(25-33) peptide to analyze IFN-γ production by intracellular cytokine staining. a Experimental scheme. b Representative pseudocolor dot-plots showing the frequency of gp100-specific pmel-1 CD8+ T cells and IFN-γ production. c Graph showing frequencies of gp100-specific pmel-1 CD8+ T cells. d Representative histograms (CTRL: black histogram, upper panel; B16F10: red histogram, middle panel; B16F10-OTI: blue histogram, lower panel) and geometric mean fluorescence intensity values of CD44 expression in CD90.1+ pmel-1 CD8+ T cells. e, f Representative pseudocolor dot-plots (e) and graph (f) showing the frequency of gp100-specific pmel-1 CD8+ T cells and IFN-γ production in Langerin-DTR mice that were either: non-depleted of LCs and DDCs (No DTx; DDC+/LC+ ); depleted of only LCs by administrating a single dose of diphtheria toxin (DTx) 14 days before the tumor challenge (Single DTx; DDC+/LC-); or depleted of both DDCs and LCs through the continuous administration of DTx (Continous DTx; DDC-/LC-). Pooled data from three independent experiments in b–d, n = 13 mice per group, and two independent experiments in e–f, n = 7-8 mice per group. Bars are the mean ± SEM. *p < 0.05, **p ˂0.01, ***p < 0.001, ****p < 0.0001, by Mann-Whitney unpaired test
To explore whether cross-presenting dermal DCs mediate antigen spreading, we carried out similar experiments using Langerin-DTR mice, which allow the selective depletion of CD207/langerin+ dermal DCs and LCs from the skin after diphtheria toxin (DTx) administration40–42. Taking advantage of the relatively faster repopulation of dermal DCs (~2 weeks) derived from bone marrow precursors, in comparison to LCs (>4 weeks) that arise from slowly proliferating skin precursors, we performed the B16F10-OTI challenge in mice devoid of only LCs (single DTx administration two weeks before challenge) or depleted of both LCs and dermal DCs (continuous DTx administration starting one day before challenge) (Supplementary Fig. 2)43,44. Similar to wild-type mice, pmel-1 CD8+ T cells were clonally expanded following B16F10-OTI challenge in Langerin-DTR mice that were not treated with DTx (No DTx; DDC+/LC+) or received a single DTx dose (Single DTx; DDC+/LC-) (Fig. 4e, f). Interestingly, the expansion of pmel-1 CD8+ T cells was severely reduced in the case of mice depleted of both dermal DCs and LCs by continuous DTx administration (Continuous DTx; DDC- LC-), indicating that dermal DCs are necessary for antigen spreading induced by Trm cells. If dermal DCs directly present tumor-derived antigens to naïve CD8 + T cells in the lymph nodes remain to be determined.
Trm cell-induced CTL spreading suppresses melanoma growth
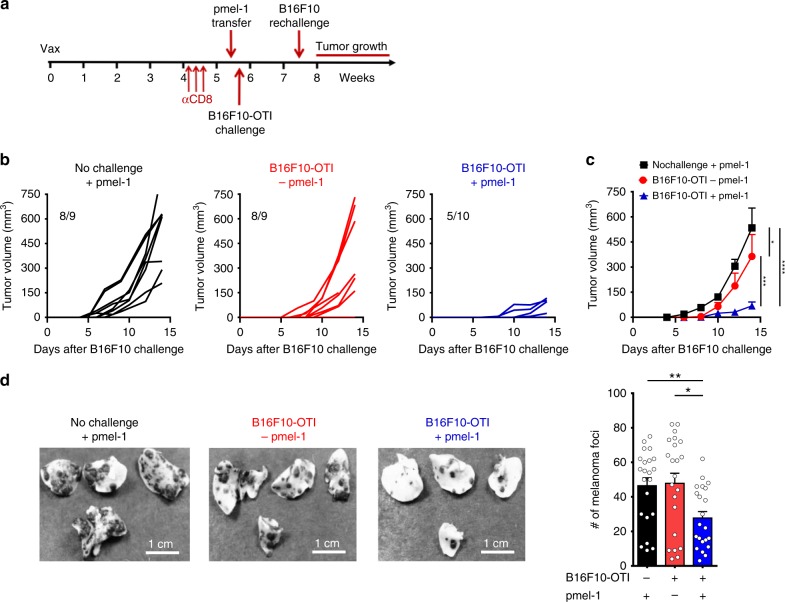
We next determined whether secondary CD8+ T-cell responses triggered by Trm cells were able to protect against B16F10 melanoma cells lacking OVA antigen and cannot be recognized by vaccination-induced Trm cells11. To this end, mice that rejected B16F10-OTI cells were injected 2–3 weeks later in the opposite flank with B16F10 melanoma cells (re-challenge) (Fig. 5a). Interestingly, these mice suppressed the growth of cutaneous tumors as compared to control mice that did not receive initial B16F10-OTI challenge (Fig. 5b,c), and therefore did not prime gp100-specific pmel-1 CD8+ T cells. In addition, no protection against B16F10 re-challenge was observed in mice that rejected B16F10-OTI melanoma but that did not receive transfer of pmel-1 CD8+ T cells (Fig. 5b, c), directly implicating the participation of primed pmel-1 CD8+ T cells in the anti-tumor effects observed. These results imply that Trm cell-mediated melanoma rejection triggers the spreading of CD8+ T-cell responses against melanoma-associated antigens, providing cross-protection against melanoma lacking Trm cell-targeted antigen. This can potentially be important to control highly heterogeneous tumors containing antigen-loss escape mutants.
Fig. 5.
Trm cell-induced antigen spreading suppress melanoma growth. C57BL/6 mice bearing OVA-specific skin Trm cells and depleted of circulating CD8+ T cells were intravenously transferred with gp100-specific CD90.1+ pmel-1 CD8+ T cells and one day later challenged intradermally with B16F10-OTI cells (B16F10-OTI + pmel-1). Control groups were not challenged but received pmel-1 CD8+ T cells (no challenge + pmel-1) or challenged but did not received pmel-1 CD8+ T cells (B16F10-OTI - pmel-1). Two to three weeks later, mice were re-challenged intradermally in the contralateral flank (b-c) or intravenously (d) with B16F10 melanoma cells. a Experimental scheme. b Individual curves showing tumor growth. c Graph showing the mean of tumor volume in all groups d Representative picture of lungs and graph showing the number of melanoma foci. Pooled data from two independent experiments in b, c n = 10, and three independent experiments in d n = 22 mice. Bars are the mean ± SEM. *p ˂0.05, **p ˂0.01, ***p ≤ 0.001, and ****p ≤ 0.0001 by two-way ANOVA Bonferroni post-hoc test for c, and Mann-Whitney unpaired test for d
To address the potential of Trm cell-induced gp100-specific CTL responses to protect against tumors disseminated in distant tissues, we repeated the previous experiment but substituted i.d. for i.v. B16F10 re-challenge to form disseminated pulmonary melanoma foci. Similar to i.d. re-challenge experiments, we observed that gp100-specific CTLs educated upon Trm cell-mediated melanoma rejection suppressed the formation of melanoma foci, as compared to unchallenged or non-transferred controls (Fig. 5d). These data suggest that Trm cells can orchestrate the generation of systemic CTL responses, which have the potential to protect against metastatic tumors.
Trm cell-DC cross-talk in human melanoma
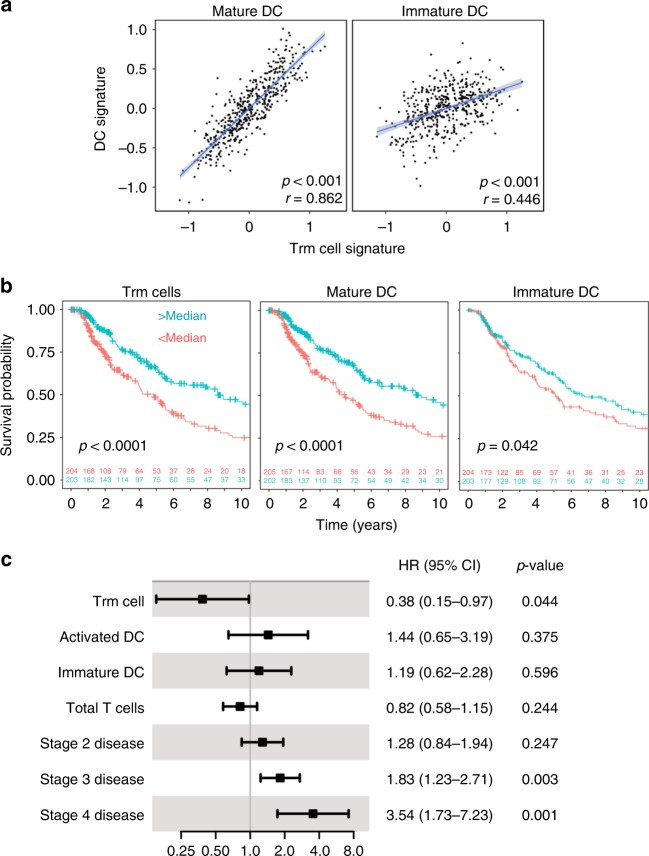
Finally, we set out to determine whether there is evidence of cross-talk between Trm cells and DCs in human cancer. Using previously described gene signatures for Trm cells, activated and immature DCs35,45, we analyzed tumor transcriptomic data of patients with cutaneous melanoma available within The Cancer Genome Atlas46,47. We found a striking correlation between Trm cell and activated DC signatures (r = 0.862), with a weaker correlation between Trm cells and immature DCs (r = 0.446; Fig. 6a). This is in keeping with our finding that Trm cells promote DC maturation in mice and suggests a similar process may occur in human melanoma. Whilst both Trm and activated DC enrichment correlated with better overall survival, this association was weaker for immature DCs (Fig. 6b). As these signatures are correlated, we carried out multivariable Cox regression analysis to estimate their individual contributions to patient survival, additionally correcting for total T-cell infiltrate using a previously published signature48 and stage, showing Trm cell enrichment to remain a strong predictor of survival (Fig. 6c). These results suggest that that a similar Trm cell-DC cross-talk may occur in human melanoma.
Fig. 6.
Trm cell signature correlates with DC maturation and improved survival. The relationship between previously described gene transcription signatures of Trm cells, DCs and survival was investigated in the TCGA melanoma cohort. a Correlation between Trm cell gene signature and either mature or immature DC gene signatures, summarized as enrichment z-scores (n = 468). Pearson correlation coefficients and associated p-values are shown. b Kaplan-Meier plots showing the overall survival of patients (n = 407 with available data) grouped according the median value of signatures (light blue curves: upper half; red curves: lower half) corresponding to Trm cells, mature DC and immature DCs. Log-rank p-values are shown. c Multivariable Cox survival regression carried out on the same cases represented in b. The forest plot shows the hazard ratio, 95% confidence interval and associated p-values for each variable in the model
Discussion
There is compelling evidence supporting a central role for Trm cells in anti-tumor immunity. However, the precise mechanisms by which Trm cells can control solid tumors are just starting to be deciphered. Here we show that, in parallel to their well-documented direct tumor killing capability49, Trm cells can activate cross-presenting dermal DCs, resulting in the subsequent priming and expansion of new CD8+ T cells specific to tumor-derived neo- and self-antigens. Importantly, this secondary response confers protection against re-challenge with tumor cells lacking the antigen initially recognized by Trm cells. To our knowledge, this is the first study to show that Trm cells can orchestrate the broadening of circulating CTL responses via DCs to strengthen protective immunity. Interestingly, these secondary CTL responses were able to suppress the growth of cutaneous tumors and also disseminated melanoma in the lungs. The ability of Trm cells to trigger the generation of broader and systemic CTL responses to control disseminated tumors implies that they can overcome their tissue-restricted nature, spreading their protective potential to other organs. This notion is supported by our findings in human data indicating that a Trm cell gene signature positively correlates with overall survival.
This reverse flow of information from adaptive to innate immune responses was initially described during anti-viral immune responses. A few reports have demonstrated that following Trm cell activation, a strong innate-like alarm state is induced in the tissue through the production of a plethora of effector molecules33. Among these, TNF-α has been shown to promote maturation of DCs36. However, whether Trm cell-induced DC maturation results in the generation of new CD8+ T-cell responses had not previously been addressed. The present study reveals that such cross-talk between Trm cells and DCs occurs in the context of anti-tumor immunity and, more importantly, that it results in the propagation of circulating anti-tumor CTL responses.
The results obtained in mouse models are supported by human data showing a strong correlation between Trm cell and activated DC gene signatures in tumors from melanoma patients. The broader anti-tumor CTL responses triggered by Trm cells can eventually underlie the association between Trm cell infiltration and higher density of CTLs observed in some human solid tumors16,35, as well as the superior predictive potential and better response to immunotherapy that Trm cells have in comparison to total CD8+ T-cell infiltration16. This mechanism may have broader implications because Trm cell-infiltration has been shown to predict better clinical outcome in other types of solid tumors14–23. On the other hand, cross-presenting migratory DCs are key players in the generation of anti-tumor T-cell immunity and their absence abolishes the rejection of immunogenic tumors and decreases the response to immune checkpoint blockade and adoptive T-cell therapy1,50–52.
Emerging evidence indicates that effective anti-tumor immunity requires the coordinated action of tissue-resident and circulating T-cell compartments12,53,54. However, how these two compartments team-up to control tumors is poorly understood. It has been previously demonstrated that virus-specific Trm cells can recruit circulating bystander memory CD8+ T cells to the infection site after antigen recognition through the production of IFN-γ33. In tumor models, circulating Tcm cells have been shown to differentiate into Trm cells12. Here we show that Trm cells can increase the breadth of the circulating CD8+ T-cell repertoire. Our findings suggest a novel mechanism by which resident and circulating T cells can collaborate to fight tumors.
The mechanism described in this manuscript could be of particular relevance to control highly heterogeneous tumors, which represents a major challenge to oncological treatments and, in particular, immunotherapies. Indeed, tumor-cell exon sequencing has revealed that multiple regions inside the same tumor or different lesions in the same patient have divergent mutation patterns55,56 and probably a differential expression of antigens. Consequently, Trm cells derived from different metastasis of the same patient have a high interlesional TCR diversity57. On the other hand, the adaptive immune system exerts a selective pressure on tumor cells, driving the survival of more resistant cancer cell subpopulations, a phenomenon known as immune editing58. As a result, cancer cell clones that do not express immune targeted antigens can escape immune control and form new tumors58. In this regard, Trm cell responses can drive the control of resistant clones, such as antigen-loss escape mutants, by broadening anti-tumor CTL responses against multiple tumor-derived antigens, as shown here.
In summary, we propose that Trm cells represent a new orchestrator of anti-tumor immunity. Interestingly, it has been suggested that Trm cells are major targets of checkpoint blockade57 and that checkpoint blockade promotes Trm cell formation in tumors12. Hence, we envision that the ability of Trm cells to increase the breath of anti-tumor T-cell immunity via DCs may play an important role in cancer immunotherapy. Accordingly, recent studies have evidenced the importance of the cross-talk between DCs and CD8+ T cells for effective cancer immunotherapy59. Moreover, a recent study has revealed that PD-1 blockade leads to the expansion of new tumor-reactive T-cell clones in patients with advanced skin cancer60. In consequence, the development of therapeutic approaches, such as vaccines, T-cell-based therapies and monoclonal antibodies, that boost the ability of Trm cells to broaden anti-tumor CTL immunity are expected to have a greater protective potential in cancer, particularly to control highly heterogeneous tumors and metastatic disease.
Methods
Animals
C57BL/6/J wild-type (CD45.2), B6.Cg-Thy1a/Cy Tg(TcraTcrb)8Rest/J (pmel-1), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I), CBy.SJL(B6)-Ptprca/J (CD45.1), B6.129S2-Cd207tm3(DTR/GFP)Mal/J (Langerin-DTR) mice were purchased from Jackson Laboratories, kept at the animal facility of Fundación Ciencia & Vida and maintained according to the “Guide to Care and Use of Experimental Animals, Canadian Council on Animal Care”. This study was carried out in accordance with the recommendations of the “Guidelines for the welfare and use of animals in cancer research, Committee of the National Cancer Research Institute”. All procedures complied with all relevant ethical regulations for animal research and were approved by the “Committee of Bioethics and Biosafety” of Fundación Ciencia & Vida. Blinding or randomization strategies were done whenever it was possible, no animals were excluded from the analysis and male and female mice were used indistinctly. Mice were allocated randomly in the different experimental procedures.
Intravenous transfer of CD8+ T cells
Naïve CD45.1+ OTI and CD90.1+ pmel-1 TCR-transgenic CD8+ T cells were purified from secondary lymphoid organs of transgenic mice using the EasySep Mouse CD8+ T-Cell Enrichment Kit (StemCell Technologies, ref 19853). Mice were intravenously injected with 2.0-2.5 × 105 cells in 100 µL of sterile PBS (ThermoFisher Scienfic, ref 10010023).
Immunizations
One day after intravenous transfer of OTI CD8+ T cells, mice were intradermally immunized in the lower back skin with 40 µg of pVAX plasmid encoding a membrane bound form of chicken OVA (pOVA). Immediately, DNA electroporation was performed by placing a parallel needle array electrode (two rows of four 2 mm pins, 1.5 × 4 mm gaps; 47-0040, BTX electroporation systems) over the injected blebs to deliver the electric pulses (two 1125 V/cm, 0.05 ms pulses followed by eight 275 V/cm, 10 ms pulses) using the AgilePulse In Vivo System (BTX Molecular Delivery Systems, ref 47–0400N). Plasmids were purified using the NucleoBond PC10000 EF (Macherey-Nagel, ref 740548) or the NucleoBond XtraMidi EF (Macherey-Nagel, ref 740420.50).
CD8+ T-cell depletion
At least 4 weeks after vaccination, mice were intraperitoneally injected with three doses of 20 µg of rat monoclonal anti-CD8 antibody (BioXCell, clone YTS169.4, ref BE0117) in consecutive days.
Preparation of tissue cell suspensions
Inguinal lymph nodes were mechanically disaggregated and digested in 1 mL non-supplemented RPMI 1640 medium (ThermoFisher Scientific, ref 61870-036) containing 5 mg/mL of collagenase type IV (Gibco, ref 17104019) and 5 µg/mL of DNAse I (AppliChem, ref A3778,0010) for 30 min at 37 °C. Single-cell suspension was obtained using a 70 μm cell strainer (BD Falcon, ref 352350). For skin preparations, vaccinated skin was excised, cut in small fragments and digested in 1 mL non-supplemented RPMI 1640 medium (ThermoFisher Scientific, ref 61870-036) containing 5 mg/mL of collagenase type IV (Gibco, ref 17104019) and 5 µg/mL of DNAse I (AppliChem, ref A3778,0010) for 30 min at 37 °C, skin pieces were then disaggregated mechanically using microscope slides with ground edges (Sail Brand, ref 7105) and single-cell suspension was obtained using a 70 μm cell strainer (BD Falcon, ref 352350) followed by a second digestion with 1 mL of supplemented of RPMI 1640 medium containing 5 µg/mL of DNAse I (AppliChem, ref A3778,0010) during 5 min on ice.
Flow cytometry staining
Cells were incubated 10 min with the TruStain fcX (clone 93) washed and incubated with the antibodies during 20 minutes followed by two washes with PBS. Monoclonal antibodies specific for mouse molecules were purchased from Biolegend: CD3-FITC (clone 17A2), CD3-APC (clone 17A2), CD3-PerCp/Cy5.5 (clone 17A2), CD8-Brillant Violet 421 (clone 53-6.7), CD45-PE (clone 30-F11), CD45-PerCP(clone 30-F11), CD45.1-PE/Cy7 (clone A20), CD45.1-FITC (clone A20), CD103-APC (clone 2E7), CD103-PerCP (clone 2E7), CD69-APC/Cy7 (clone H1.2F3), CD69-APC (clone H1.2F3), CD44-PerCP (clone IM7), IFN-γ-PE (clone XMG1.2), IFN-γ-APC (clone XMG1.2), TNF-α-APC/Cy7 (clone MP6-XT22), CD11b-FITC (clone M1/70), CD207-PE (clone 4C7), XCR1-APC (clone ZET), XCR1-PerCP-Cy5.5 (clone ZET), CD11c-PE/Cy7 (clone N418), MHCII-APC/Cy7 (clone M5/114.15.2), CD24-PerCP-Cy5.5 (clone M1/69), CD80-APC (clone 16-10A1), CD80-PE/Cy7 (clone 16-10A1), CD86 Brilliant Violet 421 (clone GL-1), CCR7-PE/Cy7 (clone 4B12), IL-2-PE/Cy7 (clone JES6-5H4) IL-12/23-APC (clone C15.6), granzyme B-APC (clone GB11) and viability dye Zombie Aqua (ref 423101). Samples were acquired in a FACSCanto II cytometer (BD Bioscience) and data were analyzed using FlowJo version X.0.7 (Tree Star, Inc.). Gating strategies for all flow cytometry experiments are shown in Supplementary Fig. 4.
Ex vivo intracellular cytokine staining
Inguinal lymph nodes were obtained 12 days after the tumor challenge and CD8+ T cells were stimulated ex vivo with the gp100(25-33) peptide (KVPRNQDWL, synthesized at Genscript) for 8 h. Brefeldin A (1 µg/mL, Sigma–Aldrich, ref B6542) was added during the last 6 h. In the case of neo-epitopes MUT1 (SIIVFNLL), MUT2 (AQLANDVVL) and MUT3 (ASMTNELM), stimulation was carried out during 20 h. Brefeldin A (1 µg/mL, Sigma–Aldrich, ref B6542) was added during the last 4 h. Intracellular staining was performed using the Cytofix/Cytoperm Fixation/Permeabilization solution set (BD Biosciences, ref 554714) according to the manufacturer’s instructions.
In vivo Trm cell stimulation and intracellular cytokine staining
OVA-specific Trm cells were stimulated by intradermal injection with 20 µg of OVA(257-264) peptide (SIINFEKL) or control SURV20-28 peptide (ATFKNWPFL) diluted in PBS near to the vaccination site. Mice were sacrificed 6, 24 or 48 h later and lymph nodes and skin were analyzed as described above. In the case of intracellular cytokine staining of skin Trm cells, 20 µg of brefeldin A was co-injected with the peptides. In the case of intracellular IL-12 staining of skin DCs, brefeldin A was injected after 24 h and analysis was performed 4 h later.
Diphteria toxin administration
To deplete langerin-expressing DCs, Langerin-DTR mice received 1 µg of diphtheria toxin (Sigma–Aldrich, ref D0564 1MG) by intravenous injection in the tail vein. In the experiments where DC depletion was continuously maintained, mice received 0.35 µg of diphtheria toxin intraperitoneally every 3 days.
Cell lines
Mouse melanoma cell line B16F10 (ATCC CLR-6475) was obtained from American Type Culture Collection. MC38 tumor cells were kindly provided by Dr. Burkhard Becher (University of Zurich, Switzerland) to Dr. Sergio A. Quezada. B16F10-OTIx5-ZsGreen (B16F10-OTI) and MC38-OTIx5-ZsGreen (MC38-OTI) cells were generated by lentiviral transduction of B16F10 cell line with the pLVX-OTIx5-ZsGreen vector encoding the OTI epitope minigene fused to ZsGreen11. B16F10 and MC-38 cell lines were cultured in complete RPMI 1640 (ThermoFisher Scientific, ref 61870-036) and DMEM (HyClone, ref SH30081.02) media, respectively, supplemented with penicillin, streptomycin (ThermoFisher Scientific, ref 15140122), non-essential amino acids (ThermoFisher Scientific, ref 11140050), sodium pyruvate (ThermoFisher Scientific, ref 11360070) and 10% of heat-inactivated fetal bovine serum (ThermoFisher Scientific, ref 10437010) in a humidified incubator at 37 °C with 5% CO2. All cell lines were routinely tested for mycoplasm contamination.
Tumor challenge
Mice were injected intradermally in the lower back skin close to the vaccination site with 50 μL of PBS containing 1 × 106 of tumor cells. Tumor growth was monitored by measuring perpendicular tumor diameters with calipers. Tumor volume was calculated using the following formula: V = (D x d2)/2 where V is the volume (mm3), D is larger diameter (mm) and d is smaller diameter (mm). Mice were sacrificed when moribund or when the mean tumor diameter was ≥15 mm,) according to the approved ethical protocol. When indicated, mice were re-challenged with 1 × 106 B16F10 or MC38 cells in the contralateral site. For intravenous re-challenge 1 × 106 of B16F10 melanoma cells in 200 ul of PBS were inoculated through the tail vein. Mice were sacrificed two weeks later and lungs were obtained, washed in PBS and stored in 3 mL of Fekete´s solution. Lung foci quantification was performed taking pictures of the lungs on both sides (Canon EOS rebel T5) followed by quantification of dark melanoma foci.
RNA sequencing analysis
Upper quartile normalized RSEM expected RNA transcript counts and clinical data47 from The Cancer Genome Atlas (TCGA) project were downloaded from the National Cancer Institute GDC PanCanAtlas project website (https://gdc.cancer.gov/about-data/publications/pancanatlas) and cutaneous melanoma cases (SKCM) filtered. Trm cell and DC gene signatures were previously described by Charoetntong et al. and Savas et al.35,45. A tumor-infiltrating T-cell signature was used as previously described by Danaher et al.48. Non-protein coding genes were removed from these signatures for consistency with TCGA data. For each signature, enrichment scores were calculated by taking the mean log10 + 1 normalized expression of each gene, followed by z-score transformation. The correlation between Trm cell and DC gene signatures was evaluated by Pearson correlation.
Statistical analysis
Statistical analysis was performed using Graphpad Prism software (Graphpad Software Inc.). RNA sequencing and survival analyses were carried out in the R statistical programming environment. Mann-Whitney unpaired tests were performed between relevant groups. Statistical analyses for tumor growth was performed using two-way ANOVA Bonferroni post-hoc test. Error bars in figures indicate the mean plus SEM. Survival analysis by Cox regression was carried out with the “survival” package and Kaplan-Meier survival curves were drawn using the “survminer” package with patients grouped on the median value of each variable tested and with log-rank p values reported. Overall p value <0.05 was considered statistically significant; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was funded by the Programa de Apoyo a Centros con Financiamiento Basal AFB 170004 to Fundacion Ciencia & Vida from CONICYT - Comisión Nacional de Investigación Científica y Tecnológica de Chile; and FONDECYT 1171703 from Fondo Nacional de Desarrollo Científico y Tecnológico de Chile. F.G.C., E.L., D.A.F. were supported by CONICYT scholarships.
Author contributions
A.L. conceived and designed the project. E.M., F.G.C. and P.C.M. developed methodology. E.M., F.G.C., P.C.M., E.L., X.D., J.S.A., D.A.F. and E.R. acquired the data. A.L., E.M., F.G.C., P.C.M. and E.G. analyzed and interpreted the data. S.A.Q. contributed with technical and material support. A.L. wrote and reviewed the manuscript. All the authors discussed the results and the manuscript.
Data availability
The data that support the findings of this study are available from the authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Eric Tartour and other, anonymous, reviewer(s) for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Evelyn Menares, Felipe Gálvez-Cancino.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-12319-x.
References
- 1.Broz ML, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmon H, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reading JL, et al. The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol. Rev. 2018;283:194–212. doi: 10.1111/imr.12657. [DOI] [PubMed] [Google Scholar]
- 4.Gebhardt T, Palendira U, Tscharke DC, Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 2018;283:54–76. doi: 10.1111/imr.12650. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zens, K. D., Chen, J. K. & Farber, D. L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI insight1, pii: 85832 (2016). [DOI] [PMC free article] [PubMed]
- 8.Davies B, et al. Cutting edge: tissue-resident memory T cells generated by multiple immunizations or localized deposition provide enhanced immunity. J. Immunol. 2017;198:2233–2237. doi: 10.4049/jimmunol.1601367. [DOI] [PubMed] [Google Scholar]
- 9.Malik, B. T. et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol.2, pii: eaam6346 (2017). [DOI] [PMC free article] [PubMed]
- 10.Park SL, et al. Tissue-resident memory CD8(+) T cells promote melanoma-immune equilibrium in skin. Nature. 2019;565:366–371. doi: 10.1038/s41586-018-0812-9. [DOI] [PubMed] [Google Scholar]
- 11.Galvez-Cancino F, et al. Vaccination-induced skin-resident memory CD8(+) T cells mediate strong protection against cutaneous melanoma. Oncoimmunology. 2018;7:e1442163. doi: 10.1080/2162402X.2018.1442163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enamorado M, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat. Commun. 2017;8:16073. doi: 10.1038/ncomms16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nizard M, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 2017;8:15221. doi: 10.1038/ncomms15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZQ, et al. CD103 and intratumoral immune response in breast cancer. Clin. Cancer Res. 2016;22:6290–6297. doi: 10.1158/1078-0432.CCR-16-0732. [DOI] [PubMed] [Google Scholar]
- 15.Djenidi F, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 16.Ganesan AP, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh J, et al. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 2017;8:13762–13769. doi: 10.18632/oncotarget.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workel HH, et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur. J. Cancer. 2016;60:1–11. doi: 10.1016/j.ejca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 20.Komdeur FL, et al. CD103+ intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRalphabeta+ CD8alphabeta+ T cells that can be targeted for cancer immunotherapy. Oncotarget. 2016;7:75130–75144. doi: 10.18632/oncotarget.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komdeur FL, et al. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology. 2017;6:e1338230. doi: 10.1080/2162402X.2017.1338230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, et al. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J. Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 23.Edwards J, et al. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during Anti-PD-1 treatment. Clin. Cancer Res. 2018;24:3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 24.Milner JJ, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 26.Boddupalli CS, et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J. Clin. Invest. 2016;126:3905–3916. doi: 10.1172/JCI85329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay LK, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 28.Belkin VM, Belkin AM, Koteliansky VE. Human smooth muscle VLA-1 integrin: purification, substrate specificity, localization in aorta, and expression during development. J. Cell Biol. 1990;111:2159–2170. doi: 10.1083/jcb.111.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheuk S, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray T, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front. Immunol. 2016;7:573. doi: 10.3389/fimmu.2016.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cepek KL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 32.Gauthier Ludiane, Corgnac Stéphanie, Boutet Marie, Gros Gwendoline, Validire Pierre, Bismuth Georges, Mami-Chouaib Fathia. Paxillin Binding to the Cytoplasmic Domain of CD103 Promotes Cell Adhesion and Effector Functions for CD8+Resident Memory T Cells in Tumors. Cancer Research. 2017;77(24):7072–7082. doi: 10.1158/0008-5472.CAN-17-1487. [DOI] [PubMed] [Google Scholar]
- 33.Ariotti S, et al. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 34.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savas P, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 36.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berthier-Vergnes O, et al. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 2005;579:3660–3668. doi: 10.1016/j.febslet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 38.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 39.Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 40.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Nagao K, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl Acad. Sci. USA. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seneschal J, Jiang X, Kupper TS. Langerin+ dermal DC, but not Langerhans cells, are required for effective CD8-mediated immune responses after skin scarification with vaccinia virus. J. Invest. Dermatol. 2014;134:686–694. doi: 10.1038/jid.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charoentong P, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 46.Shalek AK, et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature. 2014;510:363–369. doi: 10.1038/nature13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–416 e411. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danaher P, et al. Gene expression markers of tumor infiltrating leukocytes. J. Immunother. Cancer. 2017;5:18. doi: 10.1186/s40425-017-0215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amsen D, van Gisbergen K, Hombrink P, van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 2018;19:538–546. doi: 10.1038/s41590-018-0114-2. [DOI] [PubMed] [Google Scholar]
- 50.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez-Paulete AR, et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 2016;6:71–79. doi: 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 53.Kadoki M, et al. Organism-level analysis of vaccination reveals networks of protection across tissues. Cell. 2017;171:398–413. doi: 10.1016/j.cell.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spitzer MH, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487–502. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boddupalli CS, et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI insight. 2016;1:e88955. doi: 10.1172/jci.insight.88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 59.Garris CS, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148–1161. doi: 10.1016/j.immuni.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yost KE, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors on reasonable request.