Abstract
Background and Objectives
The similarities between the melatonin and oxytocin signaling could lead to increased contractility of myometrium. We designed this randomized double-blind, placebo-controlled trial to evaluate the efficacy of melatonin in reduction of blood loss during and after the lower segment cesarean section.
Methods
One hundred and twenty patients who had been scheduled for cesarean section under spinal anesthesia were enrolled in the study. We randomly allocated them to one of the three following groups to receive either melatonin 3 mg (M3), melatonin 6 mg (M6), or placebo (P) sublingually 20 min before the surgery. The hemoglobin levels before and 12 h after surgery, the mean weight of the materials used in the operation time, the need for additional oxytocic therapy, and the incidence of adverse effects were probed and recorded.
Results
There was a significant difference between the group M6 and both M3 and P in the mean weight of the materials (p = .024 and .041, respectively) and between M6 and P groups in terms of mean decrease in hemoglobin during 12 h after cesarean section (p = .029).
Conclusion
Using 6 mg melatonin, sublingually, as a premedication in patients undergoing cesarean section with spinal anesthesia could statistically reduce the amount of blood loss after the lower segment cesarean section, although it may not be clinically meaningful.
Registration number: ACTRN12612000117819 and ClinicalTrials.gov Identifier: NCT01572805
Keywords: Melatonin, Blood loss, Cesarean, Spinal anesthesia
Introduction
Postpartum hemorrhage (PPH) is responsible for between one-third and one-fourth of obstetric deaths. PPH is commonly defined as blood loss of ≥ 500 ml after vaginal delivery of a baby or ≥ 1000 ml after cesarean section [1]. However, these thresholds are considered for normal health status, and blood loss of as little as 200 ml can be life-threatening for a woman with severe anemia or cardiac disease [1]. Therefore, a decrease in blood loss at cesarean section reduces the risks of associated complications with blood transfusion and is beneficial to the patients. Although oxytocin is the first-line agent to prevent uterine atony during cesarean section, it may not be an effective agent for prevention of PPH especially in compromised patients [2]. Apart from anaphylactic reactions, oxytocin, especially in high doses, has negative inotropic, antiplatelet and antidiuretic effects [2]. Additional medications such as ergometrine, misoprostol and even surgical interventions have been investigated as the alternative and adjuvant therapeutic options for postpartum bleeding [3]. Melatonin or N-acetyl-methoxytryptamine plays a direct and integral role in uterine physiology in addition to its centrally mediated effects on reproduction [4]. This idea is supported by the recent detection of the transcripts of melatonin MT1 and MT2 receptors in human myometrium [4]. Moreover, it is reported that melatonin levels increase in maternal blood, amniotic fluid and urine of pregnant women throughout pregnancy, reaching a peak at term [5]. The beneficial effect of melatonin in placental and fetal well-being is frequently reported [6, 7]. Melatonin specially causes the circadian rhythms and promotes fetal growth and neurogenesis, while no adverse fetal or neonatal outcomes have been recorded by its use [7]. It is hypothesized that any change in the circulating melatonin level, which is synchronized with the light/dark cycle, is likely to be a major determinant of parturition time in pregnant women. Moreover, child birth (labor) occurs primarily in the night/morning hours and therefore it is suggested that the similarities between the melatonin and oxytocin signaling could lead to the increased contractility of myometrium [8–10].This favorable effect of melatonin may be valuable when it is used as an anxiolytic and uterotonic agent in the obstetric settings during spinal anesthesia. The use of melatonin as an anxiolytic agent has been extensively studied in humans and animals [11–13], but to the best of our knowledge, this study is the first in which the effect of melatonin has been evaluated on the amount of blood loss. Considering the high prevalence of anemia among pregnant women, even a small reduction of postpartum blood loss might be clinically significant and reduce distress in the patients. We designed this randomized double-blind, placebo-controlled study to figure out if melatonin, apart from the anxiolytic effects during cesarean section under spinal anesthesia, may decrease the blood loss after cesarean section without causing serious side effects.
Methods
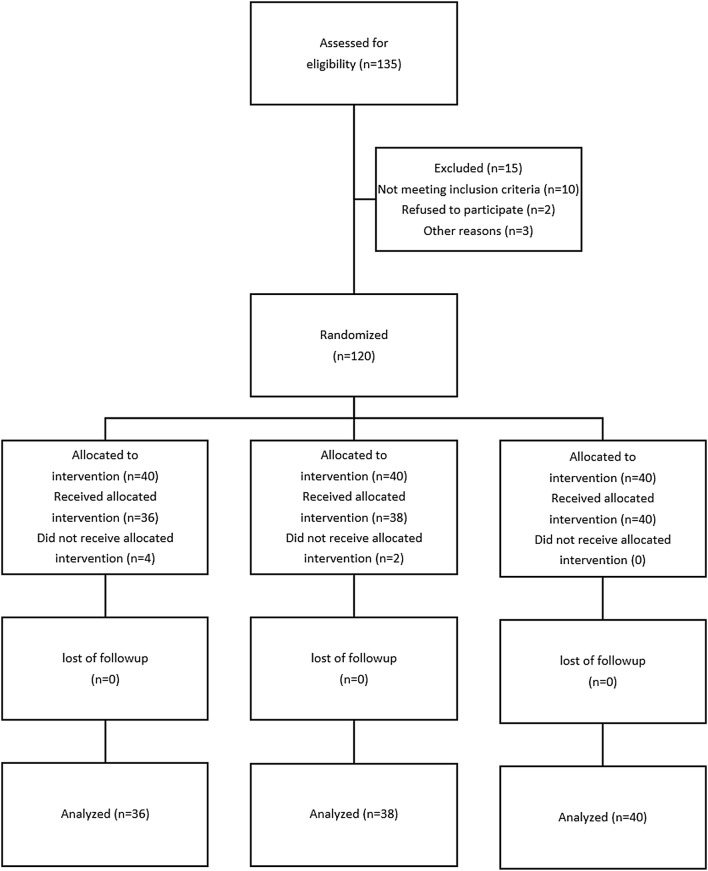
This clinical trial was registered at the United States National Institutes of Health (www.clinicaltrials.gov), with the number NCT01572805. Following Ethics Committee approval and informed patients’ consent, 135 patients whose age ranged between 18–40 years with ASA physical status of I or II who had been electively scheduled for cesarean section under spinal anesthesia were recruited in a prospective, double-blinded randomized controlled trial. Exclusion criteria were significant coexisting conditions such as hepatorenal disease, psychotic disorders, chronic anemia [hemoglobin (Hb) < 8 g %], cardiovascular disease, any contraindication to regional anesthesia such as local infection or bleeding disorders, allergy to melatonin, long-term antidepressant or analgesic drug use, any risk factor associated with an increased risk of postpartum hemorrhage such as multiple gestation, antepartum hemorrhage, polyhydramnios, severe preeclampsia and a history of previous ruptured uterus. The patients were randomly allocated to one of three groups of 40 each to receive either melatonin 3 mg (group M3), melatonin 6 mg (group M6) or placebo (group P) sublingually, 20 min before the spinal anesthesia. The Consolidated Standards of Reporting Trials (CONSORT) recommendations for reporting randomized, controlled clinical trials were followed (Fig. 1). The randomization was undertaken using computer-generated random numbers in the sealed opaque envelopes. The allocation was managed by a resident physician who was out of the project and the drugs given by a nurse non-involved in the study. The anesthetist was unaware to the patient’s group allocation, and a blinded observer recorded the study data. No premedication was given except for the drugs pre-determined by the study protocol. All patients received an intravenous preload of 5–7 ml/kg lactated Ringer’s solution before a subarachnoid block. After using an aseptic technique, a 25-gauge Quincke needle was inserted intrathecally via a midline approach into the L4-5 interspace with the patient in the sitting position by the same resident who was unaware of the assignment. Immediately after delivery of the newborn, intravenous infusion of oxytocin (20 IU syntocinon dissolved in 500 ml of lactated Ringer’s solution) was administered over a 15-min period. Additional bolus oxytocin (2.5 IU) was injected if the surgeon considered uterine tone as inadequate. Hemoglobin values were determined both before and 12 h following the surgery. For determination of blood loss, apart from the change in hemoglobin levels before and 12 h after surgery, the quantity of blood loss [milliliter (ml)] was measured as both a) the difference between the weight of the used materials before and after the surgery and b) the volume sucked in the suction bottle after placental delivery. The sedation level of patients during the surgery was assessed as sedation scores obtained on a 3-point scale with 1 = anxious and agitated, 2 = cooperative, oriented and calm, 3 = calm and drowsy. The primary outcome measures were any change in hemoglobin levels after delivery and determination of the quantity of blood loss in ml. The other outcomes of this study included assessment of (a) hemodynamic variables, (b) sedation scores, (c) ephedrine requirements, (d) need for additional oxytocin therapy, (e) hypoxemia [saturation of peripheral oxygen (SpO2) < 90] and (f) adverse events such as dizziness, pruritus, hypotension, bradycardia and postoperative nausea and vomiting.
Fig. 1.
Consort flow diagram
Sample size was calculated based on the change in hemoglobin level before (Hb1) and 12 h after surgery (Hb2) in a pilot sample of 15 patients. Considering the difference between Hb1 and Hb2 as 0.45, and ∂ = 0.71, we needed 40 patients in each arm to maintain the power at least as 80% and type I error at the maximum level of 0.05 (PS: Power and Sample Size Calculation version 3.1.2, 2014; Department of Biostatistics, Vanderbilt University). Data were analyzed using SPSS (SPSS 15.0, SPSS Inc. Chicago, IL, USA). Continuous variables were tested for normal distribution by the Kolmogorov–Smirnov test. Normally distributed data were expressed as the mean and standard deviation (SD) and analyzed by independent samples T test, analysis of variance (ANOVA) and Tukey post hoc test where it was needed. The effect of time on the hemodynamic parameters was analyzed using repeated measurements and analysis of variance and Scheffe’s post hoc test. The Chi-square test was used to analyze the incidence of side effects and other categorical variables. A p value < 0.05 was considered statistically significant.
Results
One hundred and thirty-five patients were recruited in the study of whom 15 were excluded due to logistic reasons or other factors violating the study protocol (Fig. 1).
There was no significant difference between the three groups regarding the demographic characteristics (age, body weight and height) and duration of surgery (Table 1). As shown in Table 2, the mean weight of the materials used in the operation periods (gauze and long gauze) was significantly lower in M6 group compared to P (95% CI − 6.39 to − 112.88; p = 0.024), and M3 (95% CI − 1.92 to − 109.92; p = 0.041). Table 2 shows that the difference in blood volume in the suction bottle after placental delivery between three groups was not significant (p = 0.312). As shown in Table 2, initial hemoglobin level in group P was slightly higher than other groups, while hemoglobin levels showed no significant difference between all groups after surgery. In other words, although hemoglobin levels decreased slightly after delivery in all three groups, the mean decrease in hemoglobin in M6 (1.02 ± 0.67 mg/dl) and M3 (1.22 ± 0.64 mg/dl) groups were smaller than and P (1.43 ± 0.76 mg/dl) and resulted in no difference between all three groups. The difference of the mean decline of hemoglobin between M6 and P groups was statistically significant (p = 0.029; 95% CI − 0.785 to − 0.0332), while there was no significant difference between M3 and P (p = 0.387) or M6 (p = 0.449). We adjusted the levels of Hb before C/S to compare three groups in terms of blood loss variables. After adjustment, the mean weight of the materials used in the operation was still significantly lower in M6 group (p = 0.017) while there was no significant difference between groups for Hb and blood in suction. The amount of additional oxytocin requirement was not different among all three groups (p = 0.739).
Table 1.
Demographic data associated with the study
| Groups | P (N = 38) |
M6 (N = 40) |
M3 (N = 36) |
p value |
|---|---|---|---|---|
| Weight (kg) | 75.21 ± 7.15 | 72.45 ± 6.59 | 74.75 ± 6.88 | 0.169 |
| Height (cm) | 161.6 ± 3.78 | 160.98± 3.17 | 160.56 ± 3.18 | 0.409 |
| Age (years) | 28.63 ± 5.31 | 28.38 ± 5.67 | 28.19 ± 6.21 | 0.947 |
| Duration of surgery (min) | 85.63 ± 15.70 | 79.16 ± 20.11 | 81.70 ± 18.76 | 0.840 |
Values are presented as mean ± SD
P placebo, M3 melatonin 3 mg, M6 melatonin 6 mg
Table 2.
Main outcome measures
| Groups | P N = 38 |
M6 N = 40 |
M3 N = 36 |
p value |
|---|---|---|---|---|
| Hemoglobin BCS (g/dl) | 13.2 ± 0.8 | 12.68 ± 0.75 | 12.83 ± 0.94 | 0.022 |
| Hemoglobin ACS (g/dl) | 11.76 ± 0.81 | 11.65 ± 0.83 | 11.61 ± 0.94 | 0.736 |
| Hemoglobin difference (g/dl) | 1.43 ± 0.76 | 1.02 ± 0.67 | 1.22 ± 0.64 | 0.039 |
| The mean weight of the gauze and long gauze (g) | 229.18 ± 102.9 | 169.55 ± 87.22 | 225.47 ± 106.65 | 0.014 |
| volume sucked in the suction bottle | 241.68 ± 163.52 | 297.5 ± 165.64 | 258.19 ± 166.77 | 0.312 |
Values are presented as mean ± SD
P placebo, M3 melatonin 3 mg, M6 melatonin 6 mg
BCS before cesarean section, ACS after cesarean section
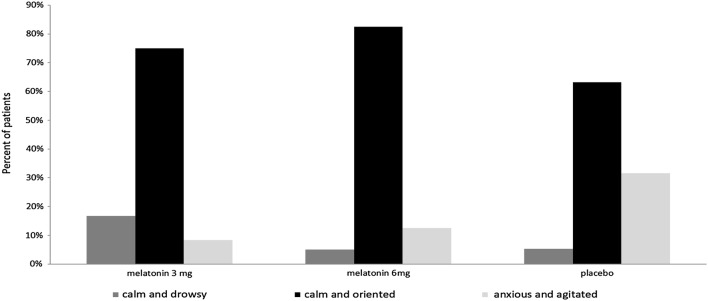
As shown in Fig. 2, three groups showed a significant difference in sedation score during surgery (p =0.023). The frequency of calm and oriented patients was the most in M6 group (82.5%), while anxiety and agitation were much more common in P group (31.6%).
Fig. 2.
Comparison of sedation scores in three study groups. Data are presented as percent % of patients. P placebo, M3 melatonin 3 mg, M6 melatonin 6 mg
Despite pre-block volume loading, transient hypotension occurred at different time points in three groups. These patients were treated by 5 mg of intravenous (IV) ephedrine boluses to maintain systolic blood pressure (SBP) within 20% of baseline values or 90 mmHg. As shown Fig. 3, comparison of mean arterial pressure (MAP) and heart rate (HR) changes during spinal anesthesia and surgery failed to reveal any statistically significant difference between all groups through repeated measure analysis (p = 0.547 and 0.227, respectively). In addition, the overall difference in ephedrine requirement between the three groups was not statistically significant (p = 0.239).
Fig. 3.
Hemodynamic changes in three study groups. Value are presented as mean ± SD, MAP = mean arterial blood pressure (mmHg); HR heart rate (bpm), SA spinal anesthesia. P placebo, M3 melatonin 3 mg, M6 melatonin 6 mg
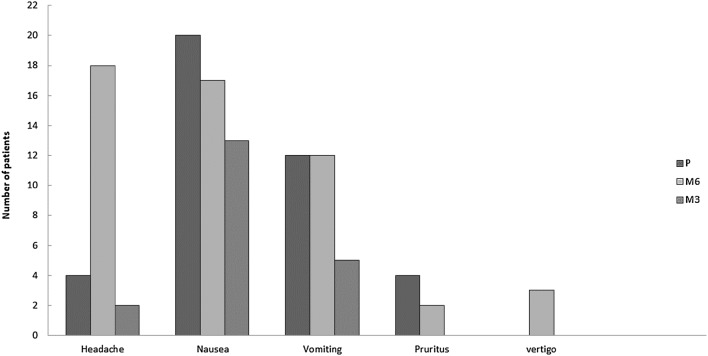
Figure 4 shows apart from headache there was no significant difference in terms of other intraoperative and postoperative side effects among the three groups of the study (i.e, pruritus, nausea and vomiting, shivering, and respiratory depression). The incidence of headache in group M6 was significantly higher than other groups (p < 0.001). All newborns in our study were free of any adverse effect. The Apgar scores did not reveal any significant difference among all groups at one (p=0.212) and five (p=0.367) minutes after delivery.
Fig. 4.
Side effects observed in three study groups. Data are presented as number of patients. P placebo, M3 melatonin 3 mg, M6 melatonin 6 mg
Discussion
This study demonstrated that premedication of patients with 6 mg sublingual melatonin reduced the amount of blood loss during and after the lower segment cesarean section, and its use was not associated with any serious side effects. Moreover, the sedation scores in patients who received melatonin were significantly higher than the placebo group.
According to the best of our knowledge, this study is the first in which the effect of melatonin on the amount of blood loss has been evaluated. However, our findings are indirectly supported by the two other studies [8, 9]. Sharkey et al. [8, 9] reported that the similarities between the melatonin and oxytocin signaling could lead to increased contractility of myometrium. They declared that melatonin sensitizes myometrial cells to subsequent pro-contractile signals in vitro through activation of the phospholipase signaling pathway, resulting in increasing actin availability for myosin binding and cross-bridging. It is also suggested that the sensitization would provide an in vivo mechanism for the increased nocturnal uterine contractility and labor that has been observed in late-term human pregnancy. Data from the study by Olces et al. demonstrated that acute inhibition of endogenous melatonin levels with light reversibly suppresses uterine contractions. Their results point to a significant role for circulating melatonin in the timing and degree of uterine contractions in late-term pregnancy. In contrary, Simşek et al. [14] suggested that oxytocin-induced uterine electrical activity of non-pregnant rat uterus was suppressed by melatonin.
However, discrepancy of this result shows that more studies are necessary to be performed in this topic. The authors of the present study consider at least two possible explanations for the effect of premedication with 6 mg of sublingual melatonin on reduced amount of blood loss after the lower segment cesarean section: First, melatonin augments oxytocin activity and enhances myometrial cell contractions leading to reduce blood loss [8, 9]. The second possible reason is that melatonin prevents high blood pressure and excessive hemorrhage by reducing anxiety and fear [15].
The other notable result of the present study is the insignificant difference in dose of oxytocin used between the groups. The possible explanation for this finding could be the gradual effect of melatonin which may not be clinically apparent.
We chose to administer melatonin sublingually 20 min before the surgery because the onset of melatonin-induced sedation has been reported to appear approximately 20–30 min after administration, and the melatonin concentration remains stable for approximately 1.5 h at its peak concentration [16]. The sublingual route allows quick absorption of drug leading to an immediate and more sustained therapeutic effect than oral administration as it avoids first pass effect. Since the effective dose of melatonin on blood loss has not yet been defined, we chose to administer 3 and 6 mg of melatonin because these are the most commonly used doses of melatonin as an anxiolytic during surgical procedures [11–13].
Despite the beneficial effect of melatonin in treating migraine and cluster headache [17], we observed a higher incidence of headache in patients who received 6 mg melatonin in this study. It is suggested that different mechanisms including free radical scavenging, anti-inflammatory effects, inhibition of nitric oxide activity and dopamine release, GABA potentiation, and neurovascular regulation have been responsible for the beneficial effects of melatonin in the treatment of migraine attacks and cluster headaches [17, 18]. However, the postpartum headache is a common troublesome complaint that can be worsened or caused by some factors including hormonal shifts, physiological changes and peri-partum procedures. In the present study, we excluded patients who had history of primary chronic headache. Since occurrence of postdural puncture headache (PDPH) following spinal anesthesia is partly influenced by a traumatic (pencil-point) and smaller-sized needles and anesthetic technique, [17, 18] these factors were controlled along with the treatment groups in the present study. Spinal anesthesia was performed by the same resident of anesthesiology and same technique which was mentioned in the methods section in current study. The authors of the present study assume that the high dose of melatonin may exaggerate the intracranial hypotension induced by the dural puncture. Dehghan et al. [19] reported that daily administration of melatonin for 72 h after TBI (traumatic brain injury) is effective on ICP and brain edema reduction. According to other studies, oral administration of melatonin 1 h prior and 1 h after ischemia induction in rats decreases brain edema [20, 21].
Conclusion
Based on this study, premedication of patients with 6 mg sublingual melatonin statistically reduced the amount of blood loss after lower segment cesarean section, which may not be clinically meaningful. Future studies are necessary to evaluate the effect of higher doses of melatonin on blood loss in patients undergoing cesarean section under general anesthesia.
Acknowledgments
This work was partially supported by the Vice Chancellor of Research of Qazvin University of Medical Sciences. The results described in this study are part of the thesis of Dr. Morteza Delkhosh Reihany. The authors would also like to especially thank the Kosar Hospital Research Center.
Dr. Marzieh Beigom Khezri
is working as associate professor of anesthesia in a tertiary teaching hospital, specialized for obstetrics and gynecology affiliated to Qazvin University of medical science. Her areas of interest in research are mostly in the fields of post-operative pain and management of obstetric hemorrhage management.
Funding
There is no conflict of interest between the authors themselves, the Research Department of Qazvin University of Medical Sciences as the sole financial supporting body, or any pharmaceutical company regarding the materials used in our study.
Compliance with Ethical Standards
Conflicts of interest
The authors of this paper declare no conflicts of interest.
Ethical Approval
We acknowledge that our work as clinical research was performed according to the ethical standards of the institution and national Iranian codes of research ethics as well as 1964 Helsinki declaration and its later amendments.
Footnotes
Marzieh Beigom Khezri is working as associate professor of anesthesia in a tertiary teaching hospital, specialized for obstetrics and gynecology affiliated to Qazvin University of medical science. Her areas of interest in research are mostly in the fields of post-operative pain and obstetric hemorrhage management; Morteza Delkhosh Reihany has worked as an anesthesiologist since 2013. He completed his fellowship in palliative medicine last year. Furthermore, he works as an anesthesiologist and also in pain clinic for relieving chronic pains now. Dr. Delkhosh is interested in clinical research in the field of pain and advanced methods of anesthesia; Talaat Dabbaghi Ghaleh is an expert gynecologist with 24 years of educational and academic experience. She has also completed a fellowship in infertility and works in an infertility clinic too. Dr. Dabbaghi is a faculty in Qazvin University of Medical Sciences with the rank of the associate professor now; Navid Mohammadi is a practiced physician and associate professor in community and preventive medicine at Qazvin University of Medical Sciences, Iran. Dr. Mohammadi is also a director member of the preventive medicine and public health research center in Iran University of Medical Sciences, Tehran, Iran. He has completed fellowship in “simulation in medical education” from the University of Ottawa, Canada in 2015. Dr. Mohammadi is interested in clinical and epidemiological research to improve patient care as well as the level of knowledge about health problems in the community.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McLintock C, James AH. Obstetric hemorrhage. J Thromb Haemost. 2011;9:1441–1451. doi: 10.1111/j.1538-7836.2011.04398.x. [DOI] [PubMed] [Google Scholar]
- 2.Pant D, Vohra VK, Pandey SS, et al. Pulseless electrical activity during caesarean delivery under spinal anaesthesia: a case report of severe anaphylactic reaction to syntocinon. Int J Obstet. 2009;18(1):85–8. doi: 10.1016/j.ijoa.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Pakniat H, Khezri MB. The effect of combined oxytocin–misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery: a prospective randomized double-blind study. J Obstet Gynecol India. 2015;65(6):376–381. doi: 10.1007/s13224-014-0607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura H, Nakamura Y, Terron M, et al. Melatonin and pregnancy in the human (review) Reprod Toxicol. 2008;25(3):291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Tenorio FD, Simões MDJ, Teixeira VW, et al. Effects of melatonin and prolactin in prolactin in reproduction: review of literature. Rev Assoc Med Bras. 2015;61(3):269–274. doi: 10.1590/1806-9282.61.03.269. [DOI] [PubMed] [Google Scholar]
- 6.Seron-Ferre M, Mendez N, Abarzua-Catalan L, et al. Circadian rhythms in the fetus. Mol Cell Endocrinol. 2012;349:68–75. doi: 10.1016/j.mce.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Nagai R, Watanabe K, Wakatsuki A, et al. Melatonin preserves fetal growth in rats by protecting against ischemia reperfusion-induced oxidative/nitrosative mitochondrial damage in the placenta. J Pineal Res. 2008;45:271–276. doi: 10.1111/j.1600-079X.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharkey JT, Cable C, Olcese J, et al. Melatonin sensitizes human myometrial cells to oxytocin in a protein kinase C[α]/extracellular-signal regulated kinase-dependent manner. J Clin Endocrinol Metab. 2010;95(6):2902–2908. doi: 10.1210/jc.2009-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharkey JT, Puttaramu R, Word RA. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab. 2009;94(2):421–427. doi: 10.1210/jc.2008-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olcese J, Beesley S. Clinical significance of melatonin receptors in the human myometrium. Fertil Steril. 2014;102(2):329–335. doi: 10.1016/j.fertnstert.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Khezri MB, Oladi MR, Atlasbaf A. Effect of melatonin and gabapentin on anxiety and pain associated with retrobulbar eye block for cataract surgery: a randomized double-blind study. Indian J Pharmacol. 2013;45(6):581–586. doi: 10.4103/0253-7613.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismail SA, Mowafi HA. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth Analg. 2009;108:1146–1151. doi: 10.1213/ane.0b013e3181907ebe. [DOI] [PubMed] [Google Scholar]
- 13.Yousaf F, Seet E, Venkatraghavan L, et al. Efficacy and safety of melatonin as an anxiolytic and analgesic in the perioperative period. Anesthesiology. 2010;113:968–976. doi: 10.1097/ALN.0b013e3181e7d626. [DOI] [PubMed] [Google Scholar]
- 14.Simşek Y, Parlakpınar H, Turhan U, et al. Dual effects of melatonin on uterine myoelectrical activity of non-pregnant rats. J Turk Ger Gynecol Assoc. 2014;15(2):86–91. doi: 10.5152/jtgga.2014.26932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdeshahi SK, Hashemipour MA, Mesgarzadeh V, et al. Effect of hypnosis on induction of local anaesthesia, pain perception, control of haemorrhage and anxiety during extraction of third molars: a case-control study. J Craniomaxillofac Surg. 2013;41(4):310–315. doi: 10.1016/j.jcms.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Markantonis SL, Tsakalozou E, Paraskeva A, Staikou C, Fassoulaki A, et al. Melatonin pharmacokinetics in premenopausal and postmenopausal healthy female volunteers. J Clin Pharmacol. 2008;48:240–245. doi: 10.1177/0091270007311112. [DOI] [PubMed] [Google Scholar]
- 17.Francis GJ, Becker WJ, Pringsheim TM. Acute and preventive pharmacologic treatment of cluster headache. Neurology. 2010;75(5):463–7326. doi: 10.1212/WNL.0b013e3181eb58c8. [DOI] [PubMed] [Google Scholar]
- 18.Klein AM, Loder E. Postpartum headache. Int J Obstet Anesth. 2010;19(4):422–430. doi: 10.1016/j.ijoa.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Dehghan F, Khaksari Hadad M, Asadikram G, et al. Effect of melatonin on intracranial pressure and brain edema following traumatic brain injury: role of oxidative stresses. Arch Med Res. 2013;44(4):251–258. doi: 10.1016/j.arcmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Bayir A, Kireşi DA, Kara H, et al. The effects of mannitol and melatonin on MRI findings in an animal model of traumatic brain edema. Acta Neurol Belg. 2008;108(4):149–154. [PubMed] [Google Scholar]
- 21.Li ZQ, Liang GB, Xue YX, et al. Effects of combination treatment of dexamethasone and melatonin on brain injury in intracerebral hemorrhage model in rats. Brain Res. 2009;1264:98–103. doi: 10.1016/j.brainres.2009.01.055. [DOI] [PubMed] [Google Scholar]