Abstract
Pseudocapillaria tomentosa is an important pathogen in zebrafish facilities. We investigated heat, ultraviolet (UV) light, chlorine, iodine, and dessciation for killing the parasite's eggs. Eggs released with feces larvate in about 5–10 days, and treatments were evaluated by exposing fresh eggs and subsequently comparing larvation to untreated eggs as an indication of survival. Collectively, untreated eggs in all trials showed high levels of survival. Eggs were exposed to elevated temperatures (40°C, 45°C and 50°C) for 1, 8, or 24 h, which resulted in substantial reduction in viability of eggs. UV radiation was effective, with no larvation at 50–300 mWs/cm2 and <2% at 20 mWs/cm2. Three chlorine products (JT Baker, Clorox®, and Bi-Mart) were tested at 25, 50, 100, 500, and 3,000 ppm (pH 7.0–7.3) with 10 min exposure. All were effective at 500 or 1,000 ppm. There was variability between three products and trials at lower concentrations, but overall chlorine was not very effective at 25–100 ppm except for Bi-Mart brand at 100 ppm. Povidone-iodine was not effective at 25 or 50 ppm for 10 min, but was effective at 200 ppm for 1 h. Desiccation was effective, and no eggs larvated after 2 h drying.
Keywords: zebrafish, Pseudocapillaria tomentosa, heat, UV, iodine, chlorine, desiccation
Introduction
Pseudocapillaria tomentosa is a pathogenic nematode parasite in laboratory zebrafish, causing emaciation and severe inflammatory lesions in the intestine.1,2 The parasite has been observed in about over 10% of zebrafish research facilities based on data from diagnostic service of the NIH Zebrafish International Resource Center, Eugene, Oregon3 Approaches to control the infection first start with avoidance, that is, not introducing infected fish into research facilities. If the infection occurs, gastrointestinal helminths, including those infecting fish, can be controlled with anthelminthics added to feed. Several of these drugs have been used with considerable success with P. tomentosa in zebrafish.4–7
In addition to treating infected fish, it is important to reduce or eliminate eggs in the environment in which the fish are maintained. This is accomplished by various methods to kill the parasite eggs, including chemical disinfectants, heat, and ultraviolet (UV) irradiation. In aquaculture, including zebrafish, disinfectants are used for disinfecting fertilized eggs or embryos, as well as water and equipment that are not holding fish.8 Zebrafish embryos are typically disinfected with chlorine, but more recently iodine is being used with zebrafish9 as is commonly used with salmonid fishes.
Nematode eggs, such as Ascaris suum, are notoriously resistant to physical and chemical agents, and thus are often used a surrogates for successful sewage sludge disinfection.10–12 For example, A. suum eggs are highly resistant to chemical disinfectants such as 70% methanol, 10% povidone iodine (∼200 ppm of free iodine), or chlorine.13–15 Martins et al.16 evaluated the efficacy of chlorine and heat to kill P. tomentosa eggs and observed that lower doses of chlorine (i.e., 500 or 1,000 ppm) actually enhanced larvation compared with controls, and 3,000 to 6,000 ppm was required to kill the eggs. These authors also exposed eggs to either 40°C or 50°C for 1 h, and the higher temperature was effective for killing eggs. Recently, we have shown that eggs do not survive freezing, including those frozen in sperm cryopreservant.17 In this study, we further evaluated chlorine and heat to kill P. tomentosa eggs. Effects of UV irradiation, desiccation, and iodine on P. tomentosa egg survival were also evaluated as these are also commonly used to control pathogens in zebrafish research facilities.
Materials and Methods
Several trails were conducted to evaluate the efficacy of disinfectants commonly used in zebrafish facilities. Trials were initiated on separate days, and each subsequent trial was designed following results from previous trials.
Eggs source, purification, and assay conditions
Eggs were collected and purified using a protocol similar to which we previously described.16,17 The source of eggs was from a large stock of fish that were held in-house and maintained by continuous infection by periodically adding naive zebrafish. Infected fish were removed from the stock population to harvest fresh eggs in a static aquarium, in which with an insert with a 2 mm2 mesh screen at the bottom was included to allow for the parasite eggs to pass through and accumulate at the bottom of the tank. After 3 days, the fish were removed from the collection tanks, and eggs were collected from the bottom of the tank and concentrated as follows: 1) water in the tank was allowed to settle, 2) the bottom 25% of the tank water was collected, 3) this water was filtered through 300 μm and then 100 μm screens, 4) the filtrate was centrifuged at 1,500 g for 45 min in 300 mL plastic bottles, 5) the concentrate from these bottles was combined into a 50 tube, and then 6) spun at 1500 g for 15 min. The pellets were then resuspended in 10–15 mL sterile water from our zebrafish system (conductivity at 115–125 microsiemens, pH ∼7.5), and eggs were enumerated by placing 25 μL on a glass slide and covering with a 25 × 60 mm coverslip. The system water used in all experiments for egg preparation was sterilized by high heat in an autoclave. All trials were conducted the same day as egg collection, and therefore eggs ranged in time from release from fish from 6 to about 42 h.
Multiple trials were conducted over a period of 1 year, and hence, methods were slightly different between some trials. Following exposures, eggs were held for 6–15 days in depression slides with system water for the following trials; UV light, chlorine, iodine, and desiccation trial. A 40 μL sample was placed in the depression slide and covered with a 22 × 22 mm cover slip. Depression glasses were incubated at 27°C in a moist sealed chamber at ∼85% humidity. Samples from the heat trials following exposure were held in in 1.5 mL tubes at 27°C.
Viability of eggs were determined by comparing percent of eggs following exposure to a disinfectant that developed (larvated) compared to unexposed control eggs at the same time. Eggs were scored as larvated (live) when elongated larvae were visible after 6–14 days post exposure (DPE) as Martins et al.16 showed that larvation occurs over this time period. Eggs scored as “dead” included undeveloped or empty eggs. Eggs were evaluated when a high percentage of control eggs had larvated, and some trials included examination of eggs at two time points. The number of total eggs, which was the sum of live and dead eggs, and corresponding number of live eggs for each of three replicates were determined (Tables 1–5). The percentage of the total eggs that were alive was calculated for each replicate (live eggs/total eggs) and the mean percent for the two or three replicates was included.
Table 1.
Effects of Increased Temperature on Survival of Pseudocapillaria tomentosa Eggs Based on Larvation
| Temperature | DPE | Exposure time (h) | Total eggs | Live eggs (%) | Fold reduction |
|---|---|---|---|---|---|
| 40 | 9 | 0 | 157/128/96 | 89/88/59 (62.4) | |
| 9 | 1 | 73/112/67 | 9/23/21 (21) | 3 | |
| 9 | 8 | 70/35/21 | 7/3/5 (8.5) | 7 | |
| 9 | 24 | 87/113/92 | 0/0/1 (0.3) | 208 | |
| 45 | 9 | 0 | 30/38/68 | 30/34/64 (94) | |
| 9 | 1 | 64/14/15 | 0/0/0 (0) | 85 | |
| 9 | 8 | 15/50/11 | 0/0/1 (1.3) | 72 | |
| 9 | 24 | 54/28/12 | 1/0/0 (1.1) | 85 | |
| 45 | 9 | 0 | 30/38/68 | 30/34/64 (94.1) | |
| 9 | 1 | 64/14/15 | 0/0/0 (0) | 85 | |
| 9 | 8 | 15/50/11 | 0/0/1 (1.3) | 72 | |
| 9 | 24 | 54/28/12 | 1/0/0 (1.6) | 59 | |
| 45 | 11 | 0 | 14/70/67 | 13/68/66 (97.3) | |
| 11 | 1 | 64/61 | 0/1 (1) | 98 | |
| 11 | 8 | 25/41/23 | 0/0/0 (0) | 86 | |
| 11 | 24 | 11/28/13 | 1/0/0 (1.9) | 57 | |
| 45 | 8 | 0 | 20/24/44 | 19/23/35 (91.6) | |
| 8 | 1 | 24/49/40 | 0/2/1 (2.8) | 33 | |
| 8 | 8 | 26/25/57 | 0/0/0 (0) | 100 | |
| 8 | 24 | 24/20/17 | 0/0/1 (1.5) | 62 | |
| 50 | 8 | 0 | 20/24/44 | 19/23/35 (91.6) | |
| 8 | 1 | 20/35/61 | 0/0/0 (0) | 106 | |
| 8 | 8 | 32/28/14 | 1/0/0 (1.3) | 70 | |
| 8 | 24 | 18/15/21 | 0/0/0 | 50 | |
| 50 | 13 | 0 | 36/20/41 | 32/15/34 (84) | |
| 13 | 1 | 45/44/30 | 0/0/0 (0) | 100 | |
| 13 | 8 | 37/48/28 | 0/0/0 (0) | 96 | |
| 13 | 24 | 33/39/26 | 0/1/0 (1) | 84 |
Exposure time of 0 = no exposure to high temperature, held at 25°C. The number of total eggs, the sum of live and dead eggs, and corresponding number of live eggs for each replicate is included. The percentage of the total eggs that were alive was calculated for each replicate (live eggs/total eggs), and the mean percent for the replicates is included parenthetically. Fold reduction = 1/(percent treatment/percent control).
Table 2.
Effects of Ultraviolet Radiation on Development of Pseudocapillaria tomentosa Eggs in Two Trials
| Trial | Dose | DPE | Total eggs | Live eggs (%) | Fold reduction |
|---|---|---|---|---|---|
| 1 | 0 | 10 | 105/37 | 89/35 (87.3) | |
| 50 | 69/44/36 | 0/0/0 (0) | 149 | ||
| 100 | 91/43/49 | 0/0/0 (0) | 181 | ||
| 300 | 40/24/47 | 0/0/0 (0) | 111 | ||
| 0 | 14 | 89/35 | 82/34 (93.5) | ||
| 50 | 35/37/33 | 0/0/0 (0) | 98 | ||
| 100 | 80/48/33 | 0/0/0 (0) | 151 | ||
| 300 | 33/24/31 | 0/0/0 (0) | 83 | ||
| 2 | 0 | 8–10 | 21/35/34 | 13/12/17 (46.6) | |
| 20 | 95/43/34 | 2/1/0 (2) | 23 | ||
| 35 | 69/35/48 | 1/0/0 (0.7) | 67 | ||
| 50 | 45/23/34 | 0/0/0 | 48 | ||
| 0 | 10–12 | 23/29/44 | 14/23/24 (63) | ||
| 20 | 73/56/46 | 1/0/0 (0.6) | 105 | ||
| 35 | 76/60/53 | 0/0/0 (0) | 119 | ||
| 50 | 40/57/64 | 0/0/0 (0) | 102 |
Dose is expressed mWs/cm2. The number of total eggs, the sum of live and dead eggs, and corresponding number of live eggs for each of the replicates is included. The percentage of the total eggs that were alive was calculated for each replicate (live eggs/total eggs) and the mean percent for the replicates is included parenthetically. Fold reduction = 1/(percent treatment/percent control).
DPE, days postexposure.
Table 3.
Effects of Chlorine on Survival of Pseudocapillaria tomentosa Eggs Based on Larvation
| Trial | Brand | DPE | Level (ppm) | Total | Live (%) | Fold reduction |
|---|---|---|---|---|---|---|
| 1 | Control | 8 | 0 | 83/91/79 | 75/83/68 (89.2) | |
| Bi-Mart | 8 | 100 | 73/49/57 | 0/0/1 (0.57) | 156 | |
| Bi-Mart | 8 | 500 | 58/21/41 | 0/0/0 | 107 | |
| Bi-Mart | 8 | 3,000 | 62/51/85 | 0/0/0 (0) | 175 | |
| Bi-Mart | 14 | 0 | 68/80/85 | 61/69/47 (88.35) | ||
| Bi-Mart | 14 | 100 | 72/46/49 | 0/0/0 (0) | 152 | |
| Bi-Mart | 14 | 500 | 49/24/37 | 0/0/0 (0) | 98 | |
| Bi-Mart | 14 | 3,000 | 55/47/81 | 0/0/0 (0) | 162 | |
| 2 | Control | 8 | 0 | 41/38/55 | 32/32/42 (79) | |
| Clorox® | 8 | 100 | 32/30/30 | 2/0/6 (8.3) | 10 | |
| JT Baker | 8 | 100 | 35/36/38 | 2/24/21 (28.4) | 3 | |
| 3 | Control | 8 | 0 | 81/89/48 | 74/68/37 (82.0) | |
| Clorox | 8 | 50 | 70/63/31 | 16/17/5 (23.0) | 4 | |
| Bi-Mart | 8 | 50 | 68/31/40 | 2/1/0 (2.0) | 41 | |
| JT Baker | 8 | 50 | 35/35/36 | 3/6/8 (16.0) | 5 | |
| 4 | Control | 8 | 0 | 39/35/100 | 36/28/85 (85.6) | |
| ThioS | 8 | 25 | 46/47/54 | 40/34/39 (76.9) | 1 | |
| ThioS | 8 | 50 | 58/54/94 | 41/42/81 (79.1) | 1 | |
| ThioS | 8 | 100 | 48/49/36 | 41/35/27 (77.4) | 1 | |
| ThioS | 8 | 500 | 48/62/50 | 40/43/43 (78.7) | 1 | |
| ThioS | 8 | 3,000 | 57/36/54 | 37/24/44 (71.4) | 1 | |
| Clorox | 8 | 25 | 47/89/45 | 14/17/10 (23) | 4 | |
| Bi-Mart | 8 | 25 | 34/57/50 | 10/21/20 (29) | 3 | |
| JT Baker | 8 | 25 | 39/29/34 | 12/20/12 (43) | 2 | |
| Clorox | 8 | 50 | 56/43/95 | 6/6/17 (15) | 6 | |
| Bi-Mart | 8 | 50 | 38/51/66 | 10/14/15 (24) | 4 | |
| JT Baker | 8 | 50 | 44/52/58 | 9/7/15 (20) | 4 | |
| Clorox | 8 | 100 | 61/80/38 | 10/15/7 (15.6) | 5 | |
| Bi-Mart | 8 | 100 | 32/50/45 | 1/0/1 (1.6) | 53 | |
| JT Baker | 8 | 100 | 48/74/60 | 1/2/2 (2.7) | 36 | |
| Clorox | 8 | 500 | 36/42/35 | 0/0/0 (1) | 98 | |
| Bi-Mart | 8 | 500 | 33/22/38 | 0/2/0 (2) | 43 | |
| JT Baker | 8 | 500 | 63/26/40 | 1/0/0 (0.8) | 110 | |
| Clorox | 8 | 3,000 | 39/21/13 | 0/1/0 (1.4) | 53 | |
| Bi-Mart | 8 | 3,000 | 26/22/39 | 0/1/0 (1.2) | 87 | |
| JT Baker | 8 | 3,000 | 69/33/46 | 1/0/0 (0.67) | 150 |
All trials were conducted for 10 min, and then chlorine was inactivated with sodium thiosulfate. The number of total eggs, the sum of live and dead eggs, and corresponding number of live eggs for each of three replicates is included. The percentage of the total eggs that were alive was calculated for each replicate (live eggs/total eggs) and the mean percent for the three replicates is included parenthetically. Fold reduction = 1/(percent treatment/percent control). Fold reduction is based on comparing sodium thiosulfate with chlorine at corresponding concentrations for Trial 4.
Table 4.
Effects of Free Iodine the Survival of Pseudocapillaria tomentosa Eggs Based on Larvation
| Trial | DPE | Concentration and time | Total eggs | Live eggs (%) | Fold reduction |
|---|---|---|---|---|---|
| 1 | 8 | 0 | 83/91/79 | 75/83/68 (89.2) | |
| 8 | 25 ppm, 10 min | 26/57/57 | 23/53/48 (88.6) | 0 | |
| 8 | 200 ppm, 1 h | 51/36/51 | 3/0/0 (2.0) | 45 | |
| 14 | 0 | 68/80/55 | 61/69/47 (88.3) | ||
| 14 | 25 ppm, 10 min | 27/69/54 | 23/64/51 (91.0) | 0 | |
| 14 | 200 ppm, 1 h | 21/31/38 | 2/0/0 (3.2) | 27 | |
| 2 | 8 | 0 | 64/77/145 | 57/71/122 (88.4) | |
| 8 | 25 ppm, 10 min | 134/42/20 | 113/34/13 (77.0) | 1 | |
| 8 | 50 ppm, 10 min | 112/50/82 | 45/21/31 (40) | 2 | |
| 8 | 200 ppm, 1 h | 69/76/41 | 0/0/2 (1.6) | 55 | |
| 14 | 0 | 120/68/95 | 102/55/75 (82) | ||
| 14 | 25 ppm, 10 min | 67/63 | 35/35 (54) | 1 | |
| 14 | 50 ppm, 10 min | 34/50/38 | 11/30/18 (47) | 2 | |
| 14 | 200 ppm, 1 h | 36/6/47 | 0/0/0 (0) | 73 |
Iodine was delivered as povidone-iodine (Ovadine) and free iodine was calculated from the manufactures guidelines. Eggs were exposed for either 10 min or 1 h. The number of total eggs, the sum of live and dead eggs, and corresponding number of live eggs for each replicate is included. The percentage of the total eggs that were alive was calculated for each replicate (live eggs/total eggs) and the mean percent for the replicates is included parenthetically. Fold reduction = 1/(percent treatment/percent control).
Table 5.
Effects of Desiccation on the Survival of Pseudocapillaria tomentosa Eggs Based on Larvation
| DPE | Desiccation time | Total | Live (%) | Fold reduction |
|---|---|---|---|---|
| 6 | 0 | 21/43/30 | 18/38/22 (83) | |
| 6 | 2 h | 57/80/73 | 0/0/0 (0) | 177 |
| 7 | 20 | 61/80/89 | 0/1/0 (0.4) | 208 |
| 8 | 48 | 85/51/58 | 0/0/0 (0) | 162 |
Control eggs were evaluated at 6 days postinitiation of desiccation. The number of total eggs, the sum of live and dead eggs, and corresponding number of live eggs for each replicate is included. The percentage of the total eggs that were alive was calculated for each replicate (live eggs/total eggs) and the mean percent for the replicates is included parenthetically. Fold reduction = 1/(percent treatment/percent control).
Statistical evaluations and data presentation
All treatment regimens (e.g., control, 8 h at 45°C) within each trial were conducted in triplicate unless otherwise indicated (Tables 1–4). Fold reduction was determined by comparing the percent of live eggs in a group within a specific trial that was exposed to an agent to the control (untreated eggs) within the trial, which was examined at the same time: 1/(mean percent alive treatment/mean percent alive in the control untreated). Moreover, the eggs in each trial were from the same original population. To allow calculation of fold reduction, a small value (1 egg) was added to the treatment group when no larvated eggs were observed.
Before statistical analysis, trials with the same conditions but different examination times were combined as larvation rates from trials, in which eggs were examined at different DPE, were not significantly different (analysis of variance [ANOVA], F1,59 = 0.029, p = 0.87). For the UV, iodine, and desiccation experiments, one-way ANOVAs were used to determine if a treatment had a significant effect on percent larvation compared with controls. A two-way ANOVA was used to quantify the effect of temperature, length of exposure, and the interaction between temperature and length of exposure on larvation rates. The effect of brand of bleach, chlorine concentration, and the interaction between brand and concentration on larvation rates was quantified using a two-way ANOVA. Tukey's Honest Significant Differences were used to identify significant differences between treatment levels. Variations in evaluation of the data for specific agents are described below. Results presented were also analyzed using Excel 2016 (Microsoft, Redmond, CA) and SigmaPlot (version 13; Systat Software, Inc., San Jose, CA). The limit of quantification presented in the figures was between 2.0 and 2.75 log10 (Figs. 1–5).
FIG. 1.
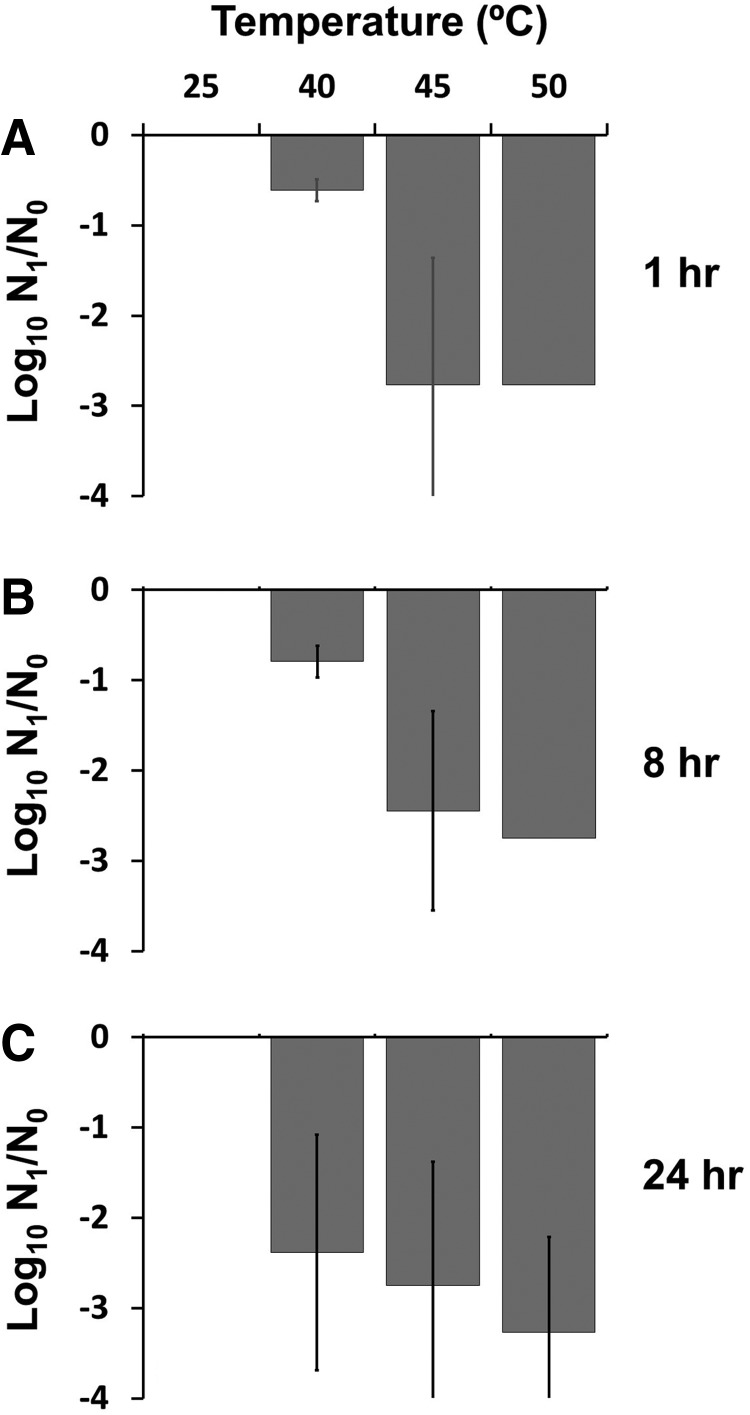
Egg survival rates following treatment with heat. Eggs were exposed to 25°C, 40°C, 45°C, or 50°C for 1 h (A), 8 h (B), or 24 h (C). Following treatment, the eggs were allowed to larvate for 9–13 days. Results presented are mean and standard deviations of three independent experiments each done in triplicate.
FIG. 2.
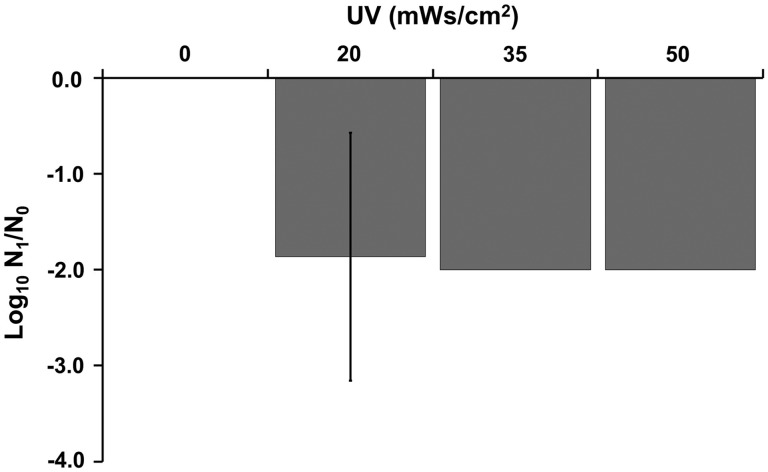
Effects of UV irradiation on egg viability. Eggs were exposed to varying doses of UV irradiation (0–50 mWs/cm2). Following UV irradiation, eggs were allowed to larvate for 9–14 days. Results represent the mean log10 reduction and standard deviation of triplicate samples. UV, ultraviolet.
FIG. 3.
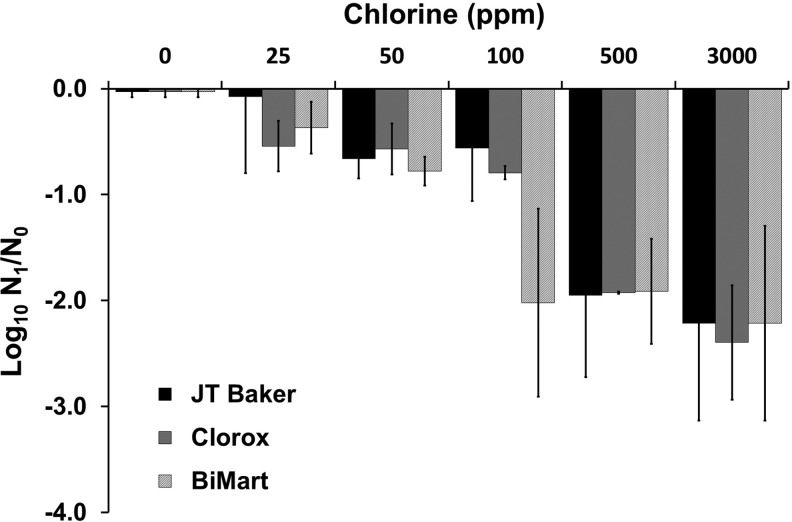
Effects of various commercially available sodium hypochlorite solution on egg viability. Eggs were treated with increasing concentration of sodium hypochlorite, from JT Baker, Clorox® or Bi-Mart as described in the Materials and Methods section. Eggs were treated with varying concentrations of sodium hypochlorite 0–3000 ppm and then allowed to larvate for up to 14 days postexposure. Results shown are mean log10 reduction and standard deviation of triplicate samples.
FIG. 4.
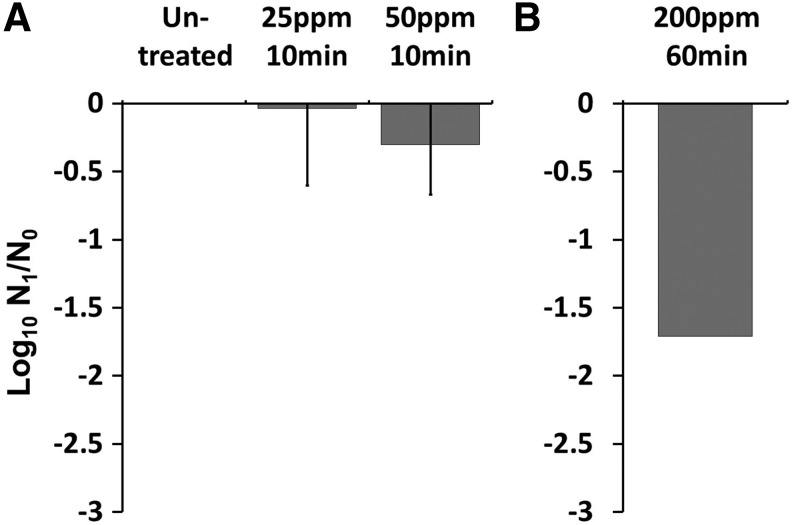
Effects of iodine treatment on egg viability. Untreated or treated eggs exposed to 25 or 50 ppm iodine solution 10 min are shown in (A). Eggs treated with 300 ppm of iodine solution for 60 min are shown in (B). Results shown are mean log10 reduction and standard deviation of triplicate samples.
FIG. 5.
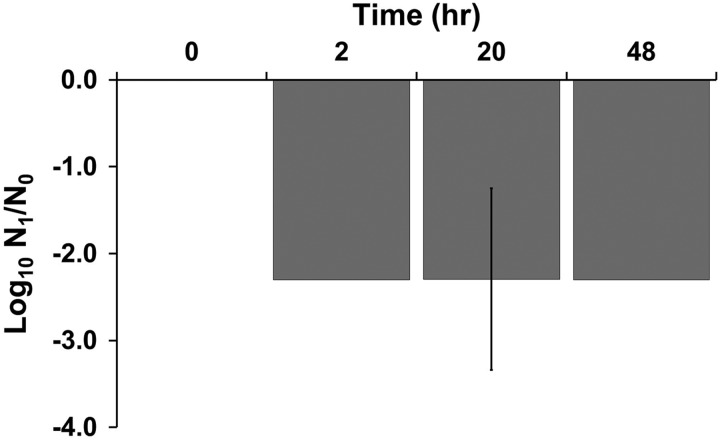
Effects of desiccation on egg viability. Eggs were desiccated between 2 and 48 h and then allowed to larvated for 6–8 days. Viable eggs were then enumerated in triplicate and shown as mean log10 reduction and standard deviation.
Heat
The effects of elevated temperature were conducted by placing the eggs in 1.5 mL Eppendorf vials in a water bath for 0, 1, 8, or 24 h (Table 1). The vials were then transferred to 27°C and held in these tubes until examined for larvation 8–13 days later. Extending from our earlier studies, we exposed eggs at 27°C (controls), 40°C, 45°C, or 50°C.
UV treatment
Eggs were collected as described above, but further purified using our sugar centrifugation method.16 This step was added to approximate conditions of postfiltered water that is treated by UV irradiation in zebrafish recirculating systems. Purified eggs were exposed to UV irradiation (0–300 mWs/cm2) (Table 2) by placing 40 μL of sample in the wells of a depression slide so that the maximum water overlying the eggs was ∼1 mm. Irradiation was performed using a Stratagene UV Stratalinker 1800 (La Jolla, CA). After the appropriate time to achieve the desired exposure (ranging from about 20 to 145 s) the slides were removed, topped off with system water, covered with 22 × 22 mm coverslips, and then incubated at 27°C as described above.
Chlorine
Most zebrafish research facilities disinfect embryos using chlorine at 25–50 ppm for 10 min7 or sometimes at higher levels with shorter periods18 using either laboratory grade or commercial bleach products. In this study, we evaluated one laboratory grade product (JT Baker 5%; Avantor Performance Materials, Central Valley, PA) and two commercial bleach products; Clorox Regular Liquid Bleach (5.25%) lot 36021CA3 and Bi-Mart Bleach 6% (lot 17199 F1 352). Four separate trials were conducted evaluating these different products at different concentrations as described in Table 3. Chlorine concentrations were calibrated with an Exactstick chlorine meter (Extech Instruments Corp., Waltham, MA) for trials 1 and 2 and a Hanna Instruments free and ultrahigh range portable photometer (Hanna Instruments, Woonsocket, RI) for trials 3 and 4. pH was then adjusted to each mixture by adding HCL or NaOH and read using a hand held pH meter (pHep; Hanna Instruments). pH ranged 7.0–7.3 for all four trials.
Exposures were conducted for 10 min in 1.5 mL Eppendorf tubes in 0.7 mL volume. After 10 min exposure at 27°C, a 2 × concentration of sodium thiosulfate based on bleach concentration was added in equal volume to the chlorine to create a 1 × concentration of sodium thiosulfate in the exposure solution to inactivate the bleach and terminate the exposure. The tubes were briefly agitated by vortex mixing every 2 min during exposure after thiosulfate was added. The tubes were then centrifuged, and supernatant was removed and replaced with sterile system water to remove sodium thiosulfate. Tubes were then incubated at 27°C for 8–14 days (Table 3).
Iodine
The efficacy of iodine was tested with two regimes, targeting protocols similar to those used for disinfecting zebrafish embryos.9 Eggs were exposed to 25 or 50 ppm free iodine for 10 min or 200 ppm for 1 h, the latter being a protocol similar to that routinely used in aquaculture for disinfection of surfaces, nets, and so on in the absence of fish. Two separate trials were conducted (Table 4). Eggs were exposed to povidone-iodine using a commercial aquaculture product, Ovadine® (Syndel Laboratories, Ferndale, WA). This is a buffered 1% Iodine solution (Iodophor) specifically formulated for use in disinfecting fish eggs. It contains a 10% Povidone-Iodine (PVP Iodine) complex, which provides 1% available iodine. The protocol described above for egg collections was followed, except that after the final centrifugation at 300 g for 5 min, the concentrate (pellet) was resuspended in the appropriate concentration of iodine and incubated for designated time. Tubes were then rinsed and centrifuged three times to remove iodine, and tank water was then added. Iodine was made by appropriate dilution of stock Ovadine using our system water and concentration confirmed by both LaMotte iodine test papers (La Motte, Chestertown, MD) and Waterworks Iodine Check test strips (Industrial Test Systems, Inc., Rock Hill, SC). The pH was 7.2–7.3 for trial 1 and 7.3–7.4 for trial 2.
Desiccation
Given that the P. tomentosa is strictly an aquatic parasite, we hypothesized that its eggs would be susceptible to desiccation. Desiccation was performed on isolated eggs as described above. First, an 80 μL of egg sample was placed in a depression slide well and allowed to dry at room temperature (approximately 23°C–25°C). This procedure required about 1 h. Samples were then incubated for additional 1, 19, and 47 h at 23°C–25°C (Table 5). Then, 40 μL of sterile system water was added to each well to rehydrate the samples. A coverslip was then overlaid, and slides were then incubated in the moist sealed chamber for 6–8 DPE at 27°C.
Results
Larvation in controls
All trials involved comparing treatments with corresponding controls (Tables 1–5 and Figs. 1–5). There were 17 control groups, 15 comprised 3 replicates and 2 with 2 replicates. A total of 84% of samples (combing the replicates within each group) showed ≥79% larvation, with the remaining three sample groups showing 46%, 62%, or 63% larvation (Tables 1–4). Mean percent larvation of controls was 83.1% (standard deviation 13.5). The mean percent larvation based on days posttreatment or exposure to an agent (DPE) with controls showed no statistical correlation with day of examination (ANOVA, F1,59 = 0.029, p = 0.87). For example, control eggs examined at 6 DPE showed 83% larvation, while those at 13 and 14 DPE showed a mean of 87% larvation.
Temperature
Eggs incubated at 27°C consistently exhibited over 60% larvation (Table 1). All treatment regimens evaluated resulted in a significant decrease in larvation rates compared with controls (p < 1.0 × 10−8), with at least a 2.5-log10 reduction in viability (Fig. 1). There was no >3% survival when eggs were exposed to 45°C or 50°C. Longer exposure times generally resulted in greater egg killing, and this trend was more pronounced at the lowest temperature (40°C; Fig. 1). Collectively, at 40°C, there was a time-dependent relationship, with 21% survival at 1 h (3-fold reduction) compared to 0.3% larvation (208-fold reduction) following 24 h exposure. At 45°C and 50°C, only six or one larvated egg was observed, respectively, with <2% larvation at these temperatures. In addition to undeveloped or degenerate material within eggs, about 10%–20% % of the eggs were empty; that is, they were void of internal contents. Longer exposure to this highest temperature resulted in more empty eggs. Both elevated temperature (F3,74 = 773.0, p < 2 × 10−16) and the interaction between temperature and exposure duration (F4,74 = 3.0, p = 0.02) resulted in significant reductions in larvation rates. All treatment regimens evaluated resulted in a significant decrease in larvation rates compared with controls (p < 1.0 × 10−8), with at least a 2.5-log10 reduction in viability (Fig. 1).
UV irradiation
We first conducted a trial in which eggs were exposed to relatively high levels, ranging from 50 to 300 mWs/cm2, and no larvation was observed after exposure of eggs when they were held for an additional 10 and 14 days (Table 2 and Fig. 2). The second trial included exposing eggs to a lower level (20 mWs/cm2). Control untreated eggs demonstrated a high percent of larvation, whereas <1% of the eggs exposed to 20 mWs/cm2 larvated. Hence, UV exposure resulted in a profound and significant reduction in egg larvation (F5,40 = 74.7, p < 2 × 10−16), with at least a 2-log10 reduction in viability (Fig. 2). While each dose significantly decreased larvation rate compared with controls, the magnitude of larvation suppression was similar and thus showed no significant differences between doses of UV.
Chlorine
Variable results in efficacy were observed, particularly at lower concentrations. Three different sources of chlorine were evaluated, one laboratory grade (JT Baker) and two from commercial (grocery store) bleach products, Bi-Mart and Clorox® brands (Fig. 3 and Table 3). A dose–response was observed with all three brands at lower levels, particularly within the range that would be used for treating embryos (i.e., 25, 50, or 100 ppm). Brand (F4,87 = 243.0, p < 2 × 10−16), concentration (F4,87 = 13.0, p = 2.3 × 10−8), and the interaction between brand and concentration (F12,87 = 2.291, p = 0.01) of chlorine significantly influenced egg larvation levels. For example, Bi-Mart was particularly more effective than the other two brands at 50 ppm (Fig. 3). Nevertheless, many eggs larvated following exposure of eggs at 25 or 50 ppm for 10 min (see Trial 4, Table 3).
Increasing the concentration to 100 ppm improved efficacy with the commercial brands, particularly Bi-Mart (Fig. 3). Trial 1 evaluated only Bi-Mart brand, and here, only one egg larvated at 100 ppm, and complete killing at the other doses. In trial 2, Clorox showed higher activity than the laboratory grade (JT Baker) product at 100 ppm, with about 8% versus 28% egg development. Exposing eggs to high levels that could be used for disinfecting tanks and laboratory surfaces was consistently effective with all three brands (Fig. 3), but even at these levels a few eggs showed larvation (Table 3). Nevertheless, a 2-log10 reduction of egg viability was observed at chlorine concentrations above 100 ppm for Bi-Mart and 500 ppm for JT Baker and Clorox bleach. Regarding controls, there was a slight decrease in larvation with controls treated with sodium thiosulfate compared with untreated controls (Table 3, Trial 4), but this was not statistically different (Tukey Honest Significant Difference; p = 0.53).
Iodine
Two trials were conducted using Ovadine, a commercial povidone product often used in aquaculture. Iodine treatment significantly altered egg larvation rates (F4,87 = 218.3, p < 2.0 × 10−16) in a dose-dependent manner. Many eggs larvated at 25 ppm, which would be at levels that zebrafish embryos could tolerate. Similar results were observed with Trial 2. An additional concentration (50 ppm for 10 min) was also evaluated, and at this concentration, about half of the eggs larvated, compared to >80% larvation in the controls. Iodine was very effective at preventing the development of eggs at 200 ppm for 1 h, with 1.7 log10 reduction in viability (Fig. 4 and Table 4). However, a few eggs survived at this concentration in both Trials 1 and 2. Across trials, iodine exposure significantly decreased egg larvation at all doses (p < 1.0 × 10−6), except 25 ppm (p = 0.27), when compared with untreated controls. Significant differences in larvation rate were also observed between all iodine treatment groups (p < 1.0 × 10−6).
Desiccation
Removing water was very effective for preventing larvation, and larvation was only detected in one egg (Table 5 and Fig. 5). This was following 20 h desiccation, whereas no eggs larvated following 2 or 48 h. Desiccation overall was significantly associated with reduced larvation rates (F3,8 = 310.8, p < 1.3 × 10−8) and rates of larvation were significantly decreased at all exposure times when compared with controls (p < 1.0 × 10−6). No significant differences were observed in larvation between treated groups.
Discussion
All methods for inactivating the eggs of P. tomentosa were effective, generally with a dose- and time-dependent pattern. The treatment protocols provide effective and practical approaches for inactivation of eggs in water and surfaces in aquatic facilities that do not contain live fish. However, concentrations of chlorine and iodine that are traditionally used for disinfection of embryos are not adequate to inactivate the nematode eggs.
We occasionally observed larvation of one or two eggs in some samples even at the highest concentrations of heat, chlorine, iodine, or desiccation. These larvated eggs probably represent those that survived treatment. Alternatively, we do rarely see larvated eggs in fresh feces, which probably represented larvated eggs that were previously ingested by a zebrafish, did not hatch, and then were passed in the feces. Hence, it is possible that a few eggs could have been already larvated at the time of exposure. However, this is unlikely because we assume that an effective agent would have killed these larvae at time 0 and that the dead larvae would degenerate over the 8 days or longer period before examination.
Heat
P. tomentosa is a member of the Order Trichinellida, and hence among terrestrial parasite nematodes comparisons with Trichuris spp. are particularly appropriate. Ghiglietti et al.18 showed that 40°C was detrimental to the survival of Trichuris muris eggs, which is consistent with our findings that 40°C, even for only 1 h, caused a significant reduction in larvation in our study. And at 45°C, almost all the eggs of P. tomentosa did not develop, including those exposed for only 1 h. These results provide the basis for a practical disinfection method in recirculating systems—that is, removal of all fish, and raising temperatures above 40°C–45°C, perhaps for 24 h.
Ultraviolet
UV light exposure is a central part of water disinfection in recirculating zebrafish systems in zebrafish facilities. For example, at the Zebrafish International Resource Center, large systems are treated at about 132 mWs/cm2.19 UV within the recirculating systems of smaller standalone systems is also routinely used. It is also becoming a more common form of disinfection in drinking water and sewage systems. Hence, there have been several studies evaluating UV effects on parasitic nematode eggs, and in general they are rather resistant. Brownell and Nelson20 reported inactivation of A. suum eggs with 100 mWs/cm2 for 19.5 min resulted in a one log10 reduction in egg viability, while at 50 mWs/cm2 for 10 min, only a 0.44 log10 reduction was observed. Toxoplasma gondii oocysts, the environmentally resistant form of the parasite, can also be effectively inactivated using UV, and a dose as low as 10 mWs/cm2 resulted in 3-log10 reduction in oocyst viability.21 Similarly, in our study, P. tomentosa eggs treated with 20 mWs/cm2 resulted in ∼2-log10 reduction in egg viability, suggesting that UV treatment can be an effective disinfection treatment for treating eggs of this parasite (Fig. 2).
Chlorine
High levels of chlorine with all three bleach products were consistently effective at killing the eggs, with only a few larvated eggs observed. A survey conducted by the Zebrafish Husbandry Association in 2018 found that 23 of 36 laboratories used various commercial (nonlaboratory grade) bleach products for embryo disinfections, ranging from 25 to 50 ppm sodium hypochlorite, and only six of these laboratories buffered the treated water. Some other laboratories occasionally use higher concentrations for shorter periods.22 We observed variable results between trials and with different products, but at lower concentrations, the Bi-Mart bleach was most effective. One explanation for the increased egg killing in commercial products designed for laundry may be that chemicals other than sodium hypochlorite are frequently added to bleach products to enhance whitening of clothes. For example, Clorox Regular Liquid Bleach (5.25%) also contains sodium carbonate, sodium chloride, sodium hydroxide, and sodium polyacrylate. As seen with the spores of Pseudoloma neurophilia,23 regardless of bleach brand, chlorine at concentrations used with embryo disinfections (25 or 50 ppm for 10 min) did not kill >90% of the nematode eggs. Concentrations of 100 ppm for 10 min was much more effective, but this regime is often toxic to zebrafish embryos.24
Iodine
In contrast to zebrafish facilities, most aquaculture facilities generally use iodine to disinfect fish eggs. However, iodine is increasingly being used as an alternative disinfectant for zebrafish embryos.9 Following toxicity studies, they recommended treating embryos at 12.5 ppm free iodine for only 2–5 min. Here, we found iodine at 25 ppm at 10 min was not effective, and thus would not be an effective agent for removing viable nematodes eggs associated with embryos. Likewise, iodine treatment of oocysts of T. gondii25 or eggs of A. suum eggs14 did not have any observable effects. More aggressive treatment, 200 ppm for 1 h, was effective and thus provides a useful method for treating water and surfaces without zebrafish.
Desiccation
While terrestrial nematodes generally show some resistance to desiccation,26 we found that the 2 h of desiccation (our shortest time point) killed all the eggs. This is likely because this aquatic nematode has not evolved to survive in dry conditions. Likewise, eggs of the monogene Benedinia seriolae are prevented from hatching following only 3 min desiccation.27
Recommendations for Control and Treatment P. tomentosa
The following are some general recommendations for control of this pathogenic nematode in zebrafish facilities based on results in the current and previous studies.
Live feeds
Oligochaete worms may be a source of the infection of capillarid nematodes of fishes,28 and thus should be avoided as food, particularly if their source is unknown.
Maternal transmission and spawning
There is a risk of maternal transmission as the parasite eggs could be released in feces and contaminate eggs or sperm, and eggs are resistant to chlorine and iodine at the levels used for disinfecting zebrafish embryos. Therefore, infected brood stock should not be used for generating F1 progeny for introduction to a main facility. Murray et al.19 provided a flowchart for responding to different biosecurity risks posed by cryopreserved sperm. The eggs of P. tomentosa do not survive freezing in cryopreservant.16 Therefore, cryopreserved sperm from infected males could be used for in vitro fertilization, but resultant progeny should be kept isolated and the tank debris screened for the presence of eggs multiple times.19 When introducing a new stock of fish into a facility, particularly from a suspect source, one may consider disinfecting embryos at higher concentrations (e.g., 100 ppm) with buffering the solution to pH 7. This could kill some of the embryos,24 but would reduce the risk of introducing this serious pathogen in the animal facility. Nevertheless, a more effective approach would be to use brood fish that were shown to be free of the infection. Also, it is important to instruct staff to place embryos in pathogen free water after disinfection. In one study,29 simple mechanical rinsing of embryos was shown to eliminate detectable pathogens in embryos from parents that were infected with a variety of zebrafish pathogens, including P. tomentosa. Hence, adding a step of simple flushing embryos with pathogen-free water should further reduce the risk of transmission.
Water and surface disinfection
With recirculation systems containing fish, the general practice of disinfecting water with UV at a minimum dose of about 35 mWs/cm2 should be effective for killing developing larvae in eggs, as long as the system is operated properly with prefiltration of water to maintain water clarity, routine cleaning of sleeves, and so on with timely changing of the UV bulb. Disinfection of a system without fish can be accomplished with high levels of bleach, iodine, or elevated temperatures. Heat has the advantage over chemical disinfectants as removal or inactivation of a chemical agent is not required following treatment. Higher temperatures (i.e., 45°C or 50°C) were most effective, but even 40°C should be effective if the temperature is maintained for at least 24 h. Nevertheless, a few eggs may survive even at 50°C. Desiccation would be an additional useful procedure as the parasite eggs are effectively killed within 2 h of drying.
Drug treatment
With an infected system, fish could be removed and euthanized or treated with drugs, the system disinfected, and then the parasite-free fish was used to repopulate the system. For oral treatment, we have found emamectin incorporated in the feed to be very effective,5 and we have recently found that the drug can be increased to 0.35 mg/kg/day for 2 weeks with increased efficacy and no side effects (unpublished data). Other anthelminthics that are routinely used in diets for treating parasitic nematodes have also been shown to be effective.6,7
Acknowledgment
This project was funded, in part, by the NIH ORIP R24OD010998 to M.L.K.
Disclosure Statement
No competing financial interests exist.
References
- 1. Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med 2002;52:362–366 [PubMed] [Google Scholar]
- 2. Murray KN, Peterson TS. Pathology in practice. J Am Vet Med Ass 2015;246:201–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zebrafish International Resource Center. Health services; https://zebrafish.org/wiki/health/submission/report (accessed April4, 2019) [Google Scholar]
- 4. Pack M, Belak J, Boggs C, Fishman M, Driever W. Intestinal capillariasis in zebrafish. Zebrafish Newslett. 1995;3:1–3 [Google Scholar]
- 5. Collymore C, Watral V, White JR, Colvin ME, Rasmussen S, Tolwani RJ, et al. Tolerance and efficacy of emamectin benzoate and ivermectin for the treatment of Pseudocapillaria tomentosa in laboratory zebrafish (Danio rerio). Zebrafish 2014;11:490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maley D, Laird AS, Rinkwitz S, Becker TS. A simple and efficient protocol for the treatment of zebrafish colonies infected with parasitic nematodes. Zebrafish 2013;10:447–450 [DOI] [PubMed] [Google Scholar]
- 7. Samaee S-Y. Experimental assessment of the efficacy of five veterinary broad-spectrum antihelminthics to control intestinal capillariasis in zebrafish (Danio rerio). Zebrafish 2015;12:255–267 [DOI] [PubMed] [Google Scholar]
- 8. Harper C, Lawrence C. The Laboratory Zebrafish. CRC Press, Boca Raton, FL, 2010 [Google Scholar]
- 9. Chang CT, Amack JD, Whipps CM. Zebrafish embryo disinfection with povidone–iodine: evaluating an alternative to chlorine bleach. Zebrafish 2016;13:S1–S96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osborn DW, Hattingh WHJ. Disinfection of sewage sludge: a review. Water SA 1978;4:170–178 [Google Scholar]
- 11. USEPA. Biosolids Generation, Use, and Disposal in the United States. EPA/832/R-93/003. USEPA, Office of Waste Management, Washington, DC, 1999 [Google Scholar]
- 12. USEPA. Control of Pathogens and Vector Attraction in Sewage Sludge. EPA/625/R-92/013. USEPA, Office of Research and Development, Washington, DC, 2003. www.epa.gov/sites/production/files/2015-04/documents/control_of_pathogens_and_vector_attraction_in_sewage_sludge_july_2003.pdf (accessed April4, 2019) [Google Scholar]
- 13. Asaolu SO, Ofoezie IE. Ascaris spp. Part 4. Helminths. In: Global Water Pathogen Project. Rose JB. and Jiménez-Cisneros B. (eds), Michigan State University UNESCO, East Lansing MI, 2018. www.waterpathogens.org/book/ascaris accessed June7, 2019 [Google Scholar]
- 14. Oh K-S, Kim G-T, Ahn K-S, Shin S-S. Effects of disinfectants on larval development of Asciaris suum eggs. Kor J Parastiol 2016;54:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naidoo A, Archer C, Louton B, Rodda N. Testing household disinfectants for the inactivation of helminth eggs on surfaces and in spills during pit latrine emptying. Water SA 2016;42:1–11 [Google Scholar]
- 16. Martins ML, Watral V, Rodrigues-Soares JP, Kent ML. A method for collecting eggs of Pseudocapillaria tomentosa (Capillariidae) from zebrafish Danio rerio and efficacy of heat and chlorine for killing the nematode's eggs. J. Fish Dis 2017;40:166–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norris LJ, Watral V, Kent ML. Survival of bacterial and parasitic pathogens from zebrafish (Danio rerio) after cryopreservation and thawing. Zebrafish 2018;15:188–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghiglietti RP, Ramsan M, Colombi A. Temperature viability of Ascaris suum, Ascaris lumbricoides and Trichuris muris eggs to alkaline pH and different temperatures Parassitologia 1995:37:229–232 [PubMed] [Google Scholar]
- 19. Murray KN, Varga ZM, Kent ML. Biosecurity and health monitoring at the Zebrafish International Resource Center. Zebrafish 2016;13:S30–S38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brownell SA, Nelson KL. Inactivation of single-celled Ascaris suum eggs by low-pressure UV radiation Appl Environ Microbiol 2006;72:2178–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ware MW, Augustine SA, Erisman DO, See MJ, Wymer L, Hayes SL, et al. Determining UV inactivation of Toxoplasma gondii oocysts by using cell culture and a mouse bioassay. Appl Environ Microbiol 2010;76:5140–5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hobbs MR, Shankaran SS, James WL. Controlling endemic pathogens—challenges and opportunities. Zebrafish 2016;13:S66–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish Microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Dis Aquat Org 2007;76:205–214 [DOI] [PubMed] [Google Scholar]
- 24. Kent ML, Buchner C, Tanguay RL. Toxicity of chlorine to zebrafish embryos. Dis Aquat Org 2014;107:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villegas EN, Augustine SA, Villegas LF, Ware MW, See MJ, Lindquist HD, et al. Using quantitative reverse transcriptase PCR and cell culture plaque assays to determine resistance of Toxoplasma gondii oocysts to chemical sanitizers. J Microbiol Meth 2010;81:219–225 [DOI] [PubMed] [Google Scholar]
- 26. Perry RN. Desiccation survival of parasitic nematodes. Parasitology 1999;11:S19–S30 [PubMed] [Google Scholar]
- 27. Ernst I, Whittington ID, Corneille S, Talbot C. Effects of temperature, salinity, desiccation and chemical treatments on egg embryonation and hatching success of Benedenia seriolae (Monogenea: Capsalidae), a parasite of farmed Seriola spp. J Fish Dis 2005;28:157–164 [DOI] [PubMed] [Google Scholar]
- 28. Moravec F, Prokopic J, Shlikas AV. The biology of nematodes of the family Capillariidae Neveu-LeMaire, 1936. Folia Parasitol 1987;34:39–56 [PubMed] [Google Scholar]
- 29. Crim MJ, Lawrence C, Livingston RS, Rakitin A, Hurley SJ, Riley LK. Comparison of antemortem and environmental samples for zebrafish health monitoring and quarantine. J Am Ass Lab Anim Sci 2017;56:412–424 [PMC free article] [PubMed] [Google Scholar]