Abstract
Significance: Increased endothelial permeability and inflammation are two major hallmarks of the life-threatening conditions such as acute respiratory distress syndrome and sepsis. There is a growing consensus in the field that the Rho family of small guanosine triphosphates are critical regulators of endothelial function at both physiological and pathological states. A basal level of reactive oxygen species (ROS) is essential for maintaining metabolic homeostasis, vascular tone, and angiogenesis; however, excessive ROS generation impairs endothelial function and promotes lung inflammation. In this review, we will focus on the role of Rho in control of endothelial function and also briefly discuss a nexus between ROS generation and Rho activation during endothelial dysfunction.
Recent Advances: Extensive studies in the past decades have established that a wide range of barrier-disruptive and proinflammatory agonists activate the Rho pathway that, ultimately, leads to endothelial dysfunction via disruption of endothelial barrier and further escalation of inflammation. An increasing body of evidence suggests that a bidirectional interplay exists between the Rho pathway and ROS generation during endothelial dysfunction. Rac, a member of the Rho family, is directly involved in ROS production and ROS, in turn, activate RhoA, Rac, and Cdc42.
Critical Issues: A precise mechanism of interaction between ROS generation and Rho activation and its impact on endothelial function needs to be elucidated.
Future Directions: By employing advanced molecular techniques, the sequential cascades in the Rho-ROS crosstalk signaling axis need to be explored. The therapeutic potential of the Rho pathway inhibitors in endothelial-dysfunction associated cardiopulmonary disorders needs to be evaluated.
Keywords: endothelial barrier, permeability, inflammation, Rho, reactive oxygen species, pulmonary edema, Rho inhibitors
Introduction
Endothelial cell (EC) monolayer covering the luminal space of blood vessels creates a semi-permeable barrier that restricts the passage of fluids, macromolecules, and inflammatory cells between the blood and underlying tissue. The EC barrier is composed of a complex framework of proteins forming adherens junctions (AJ), tight junctions (TJ), and focal adhesions connected with the EC cytoskeleton (45, 107). The endothelium plays a key role in maintaining vascular tone, angiogenesis, and organ differentiation. During various pathological conditions, the disruption of the endothelial barrier results in an influx of plasma protein into extravascular space, leading to tissue edema, which is also accompanied by enhanced inflammatory responses, a common feature of numerous injury syndromes, including acute respiratory distress syndrome (ARDS) (86, 103, 117). Hence, the maintenance and recovery of the endothelial barrier integrity on inflammatory insults and pathologic interventions is regarded as an essential step toward prevention and treatment of a variety of inflammatory disorders, including acute lung injury (ALI).
Although effective pharmacotherapeutics to confront severe pulmonary inflammation and edema are yet to be developed, there has been a substantial advancement in the understanding of molecular and cellular mechanisms involved in the pathogenesis of these disorders.
Small guanosine triphosphates (GTPases), especially Ras-homologous (Rho) family proteins, have been identified as major regulators of endothelial barrier function by controlling endothelial permeability (138). Among these, RhoA is activated downstream of multiple barrier-disruptive agonists such as thrombin, histamine, vascular endothelial growth factor (VEGF), as well as pro-inflammatory molecules such as tumor necrosis factor-α (TNF-α) and gram-negative bacterial endotoxin lipopolysaccharide (LPS) (154, 160). An increase in phosphorylation of myosin light chain (MLC) resulting in the formation of actin stress fibers and activation of actin-myosin contractility has been postulated as a major mechanism of Rho-mediated endothelial hyperpermeability (84, 124).
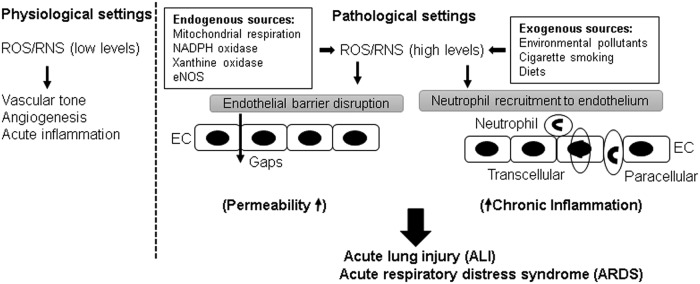
Reactive oxygen species (ROS) play a dual role in the regulation of endothelium (Fig. 1). At physiological levels, ROS-induced signaling axis is necessary for maintaining vascular tone by the endothelium and also facilitates angiogenesis and acute inflammatory responses to counter invading pathogens (34). In turn, excessive ROS generation in pathological settings, that is, hyperoxia, inflammation, environmental pollution has been implicated in endothelial barrier dysfunction (17), although delineation of precise pathologic mechanisms triggered by elevated ROS production awaits further investigation. Earlier studies have provided evidence that ROS increase endothelial permeability both in vitro and in vivo (128, 129). In response to injurious stimuli, EC and neutrophils may directly produce ROS that triggers permeability and inflammatory response. Herein, we will review the roles of Rho and ROS in regulation of endothelial function with the focus on the interconnection between these two signaling pathways in pathological settings of endothelial dysfunction.
FIG. 1.
Dual role of ROS in endothelial barrier function. A low level of ROS/RNS is essential for maintaining vital endothelial functions, but elevated levels of ROS/RNS from exogenous or endogenous sources disrupt endothelial barrier integrity and exacerbate endothelial inflammation. RNS, reactive nitrogen species; ROS, reactive oxygen species.
Rho GTPases: Master Regulators of Endothelial Barrier Function
EC comprise an intact endothelial barrier to control the passage of fluids and solutes between the circulation and the interstitial space. A highly selective permeability of endothelial barrier is essential to maintain tissue fluid homeostasis and to support a normal organ function. Dysregulation in endothelial barrier function often termed as “leaky” endothelium is a prominent feature of many cardiopulmonary disorders (80, 107). In the next sections, we will summarize the mechanisms of endothelial hyperpermeability and the role of Rho in these pathological cascades.
Endothelial permeability
The transport of fluids and macromolecules across the endothelium occurs via two routes: transcellular and paracellular pathways (86). The transcellular pathway is represented by caveolae-mediated vesicular transport of larger macromolecules such as albumin, immunoglobulins (86, 122). Studies have shown that Src kinase-mediated phosphorylation of caveolin-1, a major structural and regulatory component of caveolae, is involved in increased transcellular permeability (43, 110). The paracellular route is regulated by interendothelial junctions composed of AJ and TJ proteins that allow the majority of solutes, cytokines, and other macromolecules trafficking through the EC monolayer (45, 107). Vascular endothelial (VE)-cadherin is a key transmembrane AJ protein forming intercellular junctions in vascular endothelium by providing homophilic adhesion between neighboring EC and via its association with submembrane complex of α/β/γ- and p120-catenin family proteins linked to the actin cytoskeleton (33, 70). Numerous barrier-disruptive agonists increase endothelial permeability by causing phosphorylation-induced internalization and degradation of VE-cadherin, resulting in the weakened AJ assembly with the disruption of VE-cadherin-catenins association (36, 135). An increase in endothelial permeability not only causes an influx of protein-rich fluid into interstitial space but also allows for a rapid migration of neutrophils and uncontrolled flow of inflammatory cytokines, ultimately causing devastating respiratory illnesses that are best exemplified by ARDS.
Role of Rho in endothelial permeability
Vascular endothelium undergoes constant cytoskeletal remodeling in response to various circulating agonists such as thrombin and histamine, bacterial pathogens and endotoxins, and mechanical forces such as cyclic stretch and shear stress. Cytoskeletal reorganization caused by injurious stimuli promotes the formation of paracellular gaps, leading to increased endothelial permeability. Different members of the Rho family small GTPases have contrasting effects on cytoskeletal remodeling and EC permeability (154). Activation of RhoA triggers paracellular gap formation, cell contractility, and EC hyperpermeability response; whereas Rac1 and Cdc42 play a critical role in the maintenance of basal endothelial barrier function and recovery of EC barrier after injury (138). This review will focus on RhoA as a major trigger of EC barrier dysfunction caused by edemagenic agonists, inflammatory mediators, and pathologic mechanical forces. In addition, we will also discuss ROS-mediated regulation of the Rho pathway during endothelial dysfunction.
Small GTPases act as molecular switches for numerous signaling pathways of cell migration, adhesion, proliferation, and differentiation by cycling between GTP-bound active and GDP-bound inactive states (Fig. 2). The switch between Rho-GTPase-active and -inactive form is regulated by three different classes of regulators (9, 134). In resting cells, Rho is maintained in the inactive GDP-bound state by its interaction with GDP dissociation inhibitors (GDIs) present in the cytosol. On stimulation, Rho gets dissociated from GDI and translocates to the cell membrane where Rho-specific guanine nucleotide exchange factors (GEFs) such as GEF-H1, p115RhoGEF become activated and convert Rho to the GTP-bound active state. Once activated, Rho exerts its functions through its effectors, and GTPase activating proteins (GAPs) such as p190RhoGAP induce GTP hydrolysis and revert Rho to the cytosol in its GDP-bound inactive form.
FIG. 2.
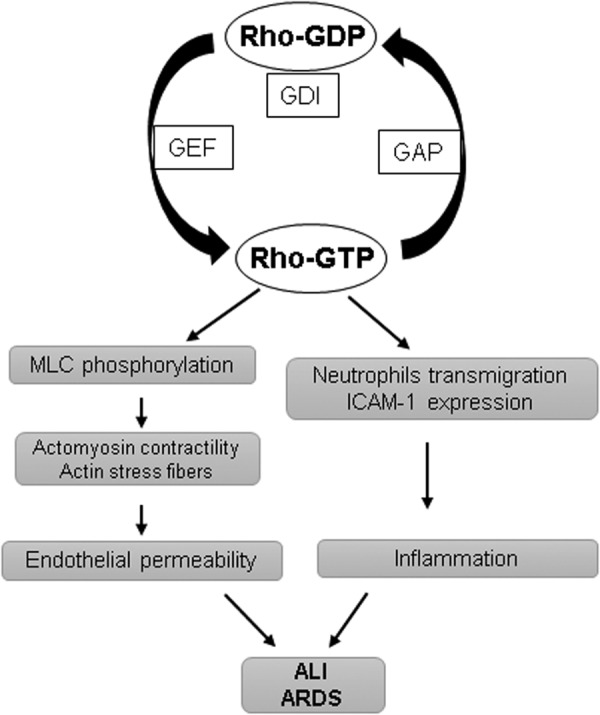
Activation of the Rho pathway mediates endothelial permeability and inflammation. Rho cycles between GTP-bound active and GDP-bound inactive states are regulated by GDI, GAP, and GEFs. Once activated, Rho induces MLC phosphorylation, resulting in an increase in actomyosin contractility and formation of actin stress fibers, leading to endothelial permeability. Simultaneously, activation of Rho also enhances ICAM-1 expression and facilitates neutrophil transmigration. GAP, GTPase activating protein; GDI, GDP dissociation inhibitor; GEF, guanine nucleotide exchange factor; ICAM-1, intercellular adhesion molecule-1; MLC, myosin light chain; Rho, Ras-homologous.
A negative role of Rho in endothelial barrier function has long been appreciated, with the observations that inhibition of RhoA and its effector Rho-associated kinase (ROCK) reduces baseline endothelial permeability both in vitro and in vivo (1, 8). However, this established dogma is challenged by a study reporting that basal Rho kinase activity is required for the maintenance of endothelial barrier (149). These opposing effects of RhoA have to be interpreted with caution since there is a wide variation in the signaling pathways activated by the Rho family GTPases depending on the cellular context and specific agonists. Moreover, a crosstalk between various Rho family members provides checks and balances for precise regulation of EC barrier function. Contrasting effects of Rho GTPases have been further illustrated by findings that Rac1, which is typically involved in the enhancement of endothelial barrier, may increase endothelial permeability in a p21-activated kinase-dependent manner in certain conditions (140, 141). Nevertheless, the role of increased RhoA activity in endothelial barrier dysfunction is more clearly understood and will be briefly overviewed next.
The increased actin-myosin contractility with the formation of actin stress fibers has been postulated as a major mechanism of RhoA-induced endothelial hyperpermeability (124). The actomyosin contractility is mediated by the phosphorylation of regulatory MLC, and Rho is directly involved in the regulation of MLC phosphorylation. RhoA, through its effector ROCK, can induce MLC phosphorylation at Ser19 and Thr18 or it can also increase MLC phosphorylation by inhibiting myosin phosphatase activity with the phosphorylation of its regulatory subunit MYPT1 at Thr686 and Thr850 residues (3, 84). Among various barrier-disruptive stimuli, the initial studies showed that thrombin induces endothelial permeability by the RhoA-MLC signaling axis (13, 52). In addition to RhoA activation, thrombin can also phosphorylate MLC in calcium/calmodulin-dependent activation of MLC kinase (37, 147). Further, Src-mediated Tyr phosphorylation in EC activates MLC kinase and thrombin utilizes this pathway to induce endothelial permeability (51, 131). The extensive studies have established thrombin-induced RhoA activation as a model of acute endothelial barrier disruption. The indispensable role of RhoA in endothelial dysfunction is further bolstered by the findings that during agonist-assisted recovery of endothelial barrier dysfunction, RhoA activity returns to basal levels, whereas barrier-protective Ras family small GTPase Rap1 becomes activated (11, 14). Accordingly, pharmacological inhibitors of the RhoA pathway have been shown to offer protection against endothelial barrier disruption in various models of endothelial dysfunction.
Besides thrombin, a plethora of studies has shown that activation of RhoA serves as a central pathway in mediating the barrier-disruptive effects of a wide range of other stimuli. For example, TNF-α-induced stress fiber formation and apoptosis in EC is mediated by RhoA activation (120, 121). However, later studies suggested a more prominent role of Rac1 inactivation due to a decrease in cyclic adenosine monophosphate (cAMP) levels rather than RhoA activation in the TNF-α-induced breakdown of endothelial junctions (127). Similar cAMP-dependent mechanisms as well as RhoA have been implicated in the endothelial barrier disruption caused by LPS (16, 46, 64, 77). VEGF, histamine, and lysophosphatidic acid also increase EC permeability via the RhoA signaling pathway (109, 142, 150). Transforming growth factor-β (TGF-β)–induced endothelial barrier disruption also involves the RhoA pathway (12, 31). Certain bacterial pathogens and their virulent cell wall components are also known to evoke endothelial permeability via RhoA activation (22, 159). Finally, activation of the RhoA pathway has been observed in EC exposed to pathologic mechanical forces, which led to endothelial barrier disruption (10, 132).
Rho in inflammation
Robust inflammatory pulmonary host response to various chemical, mechanical, and pathogenic challenges is a typical feature of ALI and ARDS (102). EC play a critical role in the initiation of inflammation. Inflammatory agents rapidly stimulate the expression of intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 by vascular EC, which facilitate the recruitment of neutrophils to the endothelium (90, 156). Rho GTPases mediate integrin-dependent signaling and, thus, regulate cell adhesion and migration (91). The RhoA pathway has been implicated in the trans-endothelial migration of neutrophils (30, 126). Consistently, fasudil, a Rho-kinase inhibitor, has been shown to inhibit leukocyte adhesion in large blood vessels (137). In line with the role of RhoA in exacerbating inflammation, various studies have demonstrated the protective effects of fasudil on different models of lung inflammation (42, 96, 163). Further, RhoA is activated in EC during inflammation (112), and RhoA activation is directly involved in the increased expression of ICAM-1 in EC exposed to thrombin and lysophosphatidic acid (4, 133). It is important to note that inflammation and endothelial permeability are inter-related pathological events. A number of inflammatory agonists such as TNF-α and LPS cause profound EC hyper-permeability (54, 162). It has been demonstrated that chemoattractant-mediated activation of neutrophils also induces a rapid increase in permeability (53). On the other hand, weakening of EC AJ by cell incubation with VE-cadherin blocking antibody simultaneously increased endothelial permeability and neutrophil transmigration (55). In turn, neutrophil extravasation further boosts endothelial inflammation and the generation of RhoA-activating pro-inflammatory cytokines (97, 119). Taken together, these results suggest that increased EC permeability, inflammation, and neutrophil transmigration form a vicious circle of vascular dysfunction, which is mediated by pathologic RhoA signaling.
Differential regulation of Rho GTPases in various EC types
EC originated from different vascular beds possess different characteristics. The best example is higher levels of basal transendothelial electrical resistance in microvascular EC compared with EC from large vessels (80). The studies have also shown the differential regulation of endothelial barrier function by Rho GTPases in various EC types. Baumer et al. demonstrated that cytotoxic necrotizing factor (CNF)-mediated simultaneous activation of RhoA, Rac, and Cdc42 increases permeability in macrovascular EC but stabilizes barrier function in microvascular EC (8). The inhibition of the Rho pathway with ROCK inhibitor Y-27632 prevents CNF-induced barrier disruption in macrovascular EC, suggesting that Rac-mediated neutralization of Rho effects is weak in these EC. Further, a cell-type-specific crosstalk between p38MAPK and Rho has been shown to exist during Staphylococcus aureus-derived lipoteichoic acid and peptidoglycan-induced endothelial dysfunction in micro-and macrovascular EC (157). The inhibition of p38MAPK with its pharmacological inhibitor SB203580 represses Rho activation in microvascular EC but has no effect on macrovascular EC. The molecular/cellular factors governing EC heterogeneity have now been actively investigated by various groups, including our own, and future studies will unravel the mechanisms defining cell type-specific regulation of Rho GTPases and their downstream effects in these processes.
Rho inhibitors as therapeutic targets for lung injury
Considering a major role for Rho as a trigger of endothelial permeability, inhibition of Rho signaling axis may serve as an attractive therapeutic target to treat lung syndromes with profound endothelial hyperpermeability. Indeed, the inhibitors of ROCK, the downstream effector or RhoA, have been shown to protect against lung injury and inflammation in various in vivo models. For example, ROCK inhibitor Y-27632 and fasudil prevented LPS-induced ALI, pulmonary inflammation, and coagulation in mice (41, 96). In other studies, fasudil inhibited LPS-induced vascular leak in guinea pigs (144), blunted systemic inflammation and endotoxin-induced ALI (42), attenuated sepsis-induced ALI (153), and alleviated various parameters of endothelial dysfunction in paraquat-induced ALI in rats (163). Importantly, these ROCK inhibitors have already been clinically approved for cerebral vasospasm and glaucoma in Japan and China. Ongoing clinical trials in Japan are evaluating therapeutic effects of fasudil, Y27632, and other Rho/ROCK inhibitors in various diseases such as idiopathic pulmonary fibrosis, renal failures, erectile dysfunction, and corneal endothelial disorders (47).
Notably, fasudil has shown promising therapeutic potential for another endothelial dysfunction-associated disease pulmonary arterial hypertension (PAH) with its effect to suppress hypertension in animals and humans as well as to decrease pulmonary artery pressure and improve vascular remodeling in rats [reviewed in Hensley et al. (61)]. The clinical trials have already provided evidence that fasudil therapy improves pulmonary artery pressure and pulmonary vascular resistance in PAH patients (49). Consistently, more recent clinical trials showed similar beneficial outcomes of fasudil in patients with congenital heart disease and severe PAH (158). Thus, its proven role in mitigating endothelial injury-related pathologies and encouraging findings from preclinical animal studies suggest the therapeutic potential of ROCK inhibitors against ARDS, a devastating respiratory illness with 40% mortality and currently without efficient pharmacological treatment.
ROS in Endothelial Regulation
ROS are generated during normal cellular metabolism from incomplete reduction of molecular oxygen. Superoxide anions (O2−), hydroxyl radicals (OH−), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) represent the most common ROS with high oxidizing potencies (146). At low physiological levels, ROS act as important signaling molecules in various cellular functions; initial ROS-mediated cellular responses protect cells against oxidative stress and play an important role in maintaining redox homeostasis (44). But an excessive ROS production during pathological conditions causes tissue injury and organ failure that has been ascribed to pathogenesis of ALI and ARDS (23, 81). Elevated ROS levels may directly induce EC dysfunction and, thus, lead to the development of various cardiopulmonary disorders (7, 101). Also, nitric oxide (NO) functions as an important signaling molecule in maintaining vascular tone and promoting angiogenesis. However, some of the NO metabolites known as reactive nitrogen species (RNS), such as peroxynitrite (ONOO−), formed after the reaction between NO and superoxide anion trigger endothelial dysfunction (105, 106). Deleterious cellular effects of both ROS and RNS have been attributed to their role in the oxidation of proteins, lipids, and oxidant-induced DNA damage.
Major exogenous sources of ROS/RNS for pulmonary endothelium include environmental pollutants, cigarette smoking, and diet (17). During infection and inflammation, the endothelium becomes exposed to a wide variety of ROS and inflammatory cytokines, a typical phenomenon associated with the development of ALI and its progression to ARDS. In these settings, neutrophils serve as a principal source of ROS for the endothelium, as activated neutrophils adhere to the EC surface. The induction of respiratory burst by neutrophils is facilitated by NADPH oxidase 2 (NOX2) present in these cells (87). Numerous studies have demonstrated an ample ROS generation by activated neutrophils in response to phorbol esters, TNF-α, and chemoattractants (100, 113).
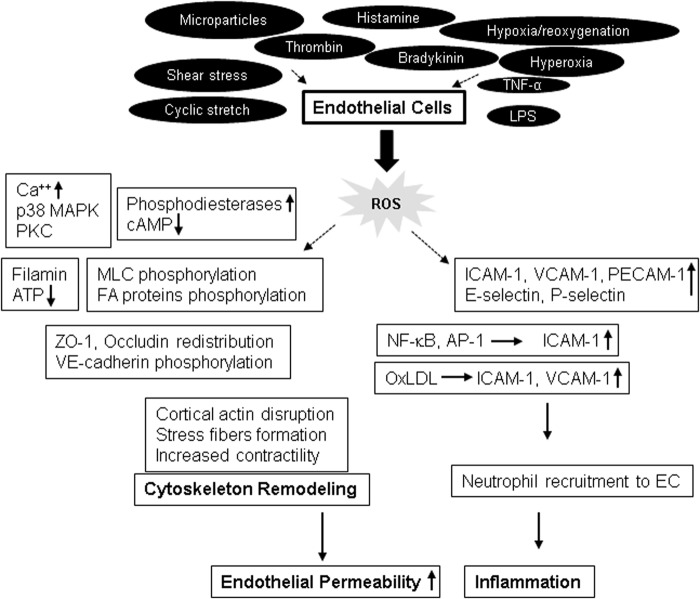
EC may also become a prominent source of ROS on challenge with injurious stimuli (Fig. 3). For example, an earlier study showed that EC produce superoxide ions after the treatment with interleukin (IL)-1 and interferon-γ (104). Consequently, other studies have demonstrated an increased ROS production by EC in response to various agonists, including shear stress (28), cyclic stretch (25), hypoxia/reoxygenation (99, 165), hyperoxia (19), microparticles (18, 108), TNF-α (27), LPS (118, 136), histamine (72), bradykinin (67), and thrombin (66). ROS production in EC is mediated by the mitochondrial electron transport chain, NOXs, especially NOX2 and NOX4 isoforms, uncoupled endothelial nitric oxide synthase, and xanthine oxidase (20, 74).
FIG. 3.
EC-generated ROS induce endothelial dysfunction via multiple mechanisms. In response to various agonist challenges, EC produce elevated levels of ROS that act on various signaling molecules, resulting in actin cytoskeleton remodeling and, ultimately, causing an abrupt increase in endothelial permeability. ROS-induced cytoskeletal remodeling is mediated by alterations in Ca++ and cAMP levels, phosphorylation and redistribution of junction proteins, and phosphorylation of MLC with enhanced actomyosin contractility. On the other hand, ROS also induce the increased expression of EC adhesion molecules that leads to augmented EC inflammatory responses. cAMP, cyclic adenosine monophosphate; EC, endothelial cell.
ROS-mediated endothelial permeability
Published studies provide substantial evidence that ROS have profound negative effects on endothelial barrier function (115, 148). Although the exact mechanism behind ROS-induced increase in endothelial permeability remains to be fully elucidated, elevation of intracellular Ca++ levels and activation of p38MAPK have been suggested as potential ROS targets. The role of ROS in disrupting endothelial barrier has been further supported by in vivo findings of pulmonary edema formation caused by ROS challenge (6, 7, 76, 128). Some of these studies showed a simultaneous increase in capillary hydrostatic pressure in conjunction with edema formation (76), whereas others showed that the development of edema without capillary pressure increases (6, 128). An involvement of ROS-induced endothelial dysfunction has also been observed in animal models of ischemia/reperfusion injury (5, 82). Briefly, Armstead et al. showed an increase in blood–brain barrier permeability after ischemia-induced injury in piglets (5), and Kennedy et al. found an increase in microvascular permeability and lung edema formation in their model of reperfusion injury in rabbit lungs (82).
Endothelial barrier integrity is maintained by a dynamic equilibrium between barrier-protective intercellular and cell-matrix adhesive forces and barrier-disruptive actomyosin contractile forces (45). Both sets of these forces are generated by the actin-cytoskeleton network, and the shift in the balance toward contractile forces increases endothelial permeability (15, 130). Analysis of potential mechanisms of ROS-induced endothelial barrier disruption has shown that ROS induce EC cytoskeletal remodeling and enhance the expression of surface adhesion molecules in affected endothelium (101). Exposure of EC to H2O2 or xanthine oxidase caused an abrupt cell shape change along with the formation of intercellular gaps (63, 129). Alterations in calcium homeostasis and activation of protein kinase C (PKC), p38MAPK, and phosphodiesterases have been attributed to ROS-induced increase in endothelial permeability in these and other studies (129, 143, 148). A role of ROS-mediated calcium signaling pathways in endothelial barrier regulation has been summarized in detail in an excellent recent review (38) and is not the scope of this review. Interestingly, ROS challenge caused a decrease in intracellular cAMP that was linked to increased EC permeability (60, 143). The studies from our group and others have established an important role of cAMP in upregulating EC barrier function by either PKA-dependent activation of Rac or PKA-independent Epac-Rap1 signaling cascade. Thus, ROS-mediated decrease in cAMP levels may induce endothelial permeability by regulating the activity of these Rho GTPases. Taken together with findings of decreased barrier-protective Rac1 activity and upregulation of RhoA activity as described earlier during endothelial dysfunction, these results highlight the functional crosstalk between ROS signaling and Rho GTPases in control of EC permeability.
ROS-induced cytoskeletal remodeling involves the formation of actin stress fibers and the disruption of cortical actin, a feature associated with increased cell contractility (99, 164). A role of oxidative stress in the reorganization of actin cytoskeleton was further addressed in the study by Crawford et al., where overexpression of superoxide dismutase inhibited reoxygenation-induced actin remodeling (35). ROS are known to cause changes in actin organization by targeting some actin-binding proteins such as filamin, which is involved in actin filament crosslinking and connecting actin microfilaments to membrane glycoproteins (59, 60). They reported that human umbilical vein endothelial cell (HUVEC) exposed to H2O2 show the rapid translocation of filamin from membrane cytoskeleton to cytosol, which was accompanied by a decrease in cAMP levels, resulting in the formation of interendothelial gaps. A drop in ATP levels after prolonged oxidative stress has also been reported to induce the disruption of actin microfilaments and depolymerization of microtubules (MT), leading to an increase in endothelial permeability (63, 68).
EC exposure to H2O2 increased MLC phosphorylation (164), suggesting ROS-induced activation of MLC phosphorylation-dependent signaling axis of endothelial dysfunction. EC exposure to ROS increased protein tyrosine phosphorylation at focal adhesions, which was also linked to ROS-induced increase in permeability and neutrophil adherence on endothelium (21, 56). The tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130cas in response to ROS exposure induced the formation of actin stress fibers and increased neutrophils adherence on HUVEC. ROS have also been implicated in disruption of TJ proteins. H2O2 caused redistribution of occludin on the cell surface, limiting its association with another TJ protein zonula occludens-1 (ZO-1) and leading to an increase in endothelial permeability (83). Likewise, H2O2-caused increase in brain EC permeability was mediated by altered expression and localization of actin, occludin, and ZO-1 (92). Rac overexpression-mediated ROS production has been shown to increase endothelial permeability due to ROS-mediated increase in tyrosine phosphorylation of VE-cadherin, leading to its disappearance from cell junctions and internalization (151). In agreement with the role of ROS-mediated VE-cadherin phosphorylation in inducing endothelial permeability, it has been shown that TNF-α causes endothelial dysfunction via NOX-mediated VE-cadherin phosphorylation (116). The increase in endothelial permeability due to loss of VE-cadherin from cell junctions via ROS-mediated phosphorylation represents a noncontractile mechanism of endothelial barrier disintegration where Rho GTPases are not involved. Further, redox-dependent activation of p38MAPK has also been shown to directly induce endothelial permeability (148). These evidences suggest that ROS can directly cause endothelial barrier disruption independent of Rho activation (Fig. 4).
FIG. 4.
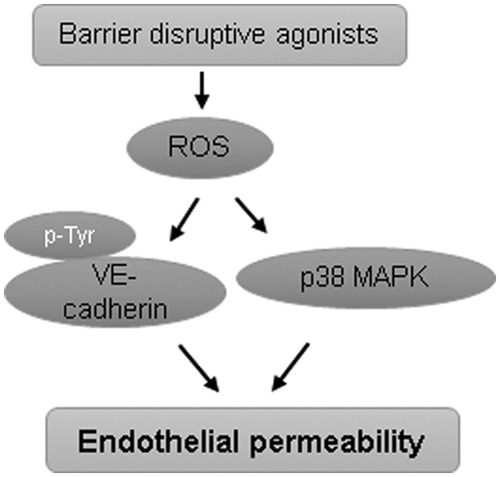
ROS induce endothelial permeability without Rho activation. Some barrier-disruptive agonists such as TNF-α induce the tyrosine phosphorylation of VE-cadherin, which undergoes internalization and loss from the membrane, leading to an increase in endothelial permeability. ROS-induced activation of p38MAPK also directly increases endothelial permeability independent of Rho activation. TNF-α, tumor necrosis factor-α; VE, vascular endothelial.
ROS in endothelial inflammation
EC play a major role in the immune and inflammatory responses in both the vasculature and surrounding tissues. Elevated levels of ROS increase the expression of EC adhesion molecules ICAM-1, VCAM-1 and platelet-endothelial cell adhesion molecule-1 (PECAM-1) that facilitate an enhanced adhesion and extravasation of neutrophils in the endothelium. Several studies have demonstrated that increased oxidative stress drives neutrophil recruitment and trafficking in the endothelium. Both in vitro and in vivo ROS exposure to EC resulted in an upregulated expression of ICAM-1, PECAM-1, and P-selectin and a subsequent increase of neutrophil adhesion to EC, which was abolished by EC incubation with antibodies against these EC adhesion proteins (50, 98). Conversely, antioxidants such as vitamin E and α-tocopherol have been shown to inhibit leukocyte adhesion to EC (75, 161). Along with the EC surface adhesion molecules, platelet-activating factor (PAF) is also suggested to play a role in neutrophil adhesion. This notion is supported by the experimental evidence that inhibition of PAF or its receptors blocked the ROS-induced leukocyte adhesion to EC (50, 71).
Another pathological consequence of ROS-induced neutrophil-EC interaction is the production of proinflammatory cytokines that also regulate the expression of EC adhesion molecules. The study by Griffin et al. demonstrated that TNF-α in conjunction with IL-17 activated EC by increasing the E-selectin and P-selectin expression, leading to enhanced leukocyte recruitment to EC (57). Likewise, LPS stimulation upregulated the expression of ICAM-1 in epithelial cells and VCAM-1 in renal mesangial cells in an ROS-dependent manner (29, 93). Another pro-inflammatory molecule, oxidized low-density lipoprotein (OxLDL), also induced ICAM-1 and VCAM-1 expression in vascular EC that was mediated by oxidative stress (32).
Some redox-dependent transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) control gene transcription of EC adhesion molecules and regulate ROS-dependent neutrophil adhesion in the endothelium. In this line, H2O2 rapidly increased ICAM-1 mRNA transcripts and protein expression at the EC surface, causing enhanced neutrophil adhesion (98). Further, a definitive role of ROS in transcriptional regulation of EC adhesion molecules was demonstrated in studies with anti-oxidants N-acetyl cysteine and NF-κB inhibitor pyrrolidine dithiocarbamate, which suppressed ICAM-1 expression (48, 73). In addition, changes in glutathione levels in EC showed both transcription-dependent and transcription-independent expression of adhesion molecules dictating neutrophil-endothelial adhesion (85). A study has also reported that OxLDL induces monocyte adhesion to EC via the ROS-p38MAPK-NF-κB signaling pathway (26).
Crosstalk Between Rho and ROS in Endothelial Regulation
The fact that both RhoA and ROS trigger endothelial dysfunction by inducing MLC phosphorylation suggests that a crosstalk may exist between these two signaling pathways (Fig. 5). An increasing body of evidence suggests that ROS regulate RhoA activity, and RhoA, in turn, controls cellular redox balance by modulating the enzymes involved in the generation of ROS (65, 111). It has been demonstrated that Rho GTPases RhoA, Rac1, and Cdc42 contain a conserved redox-sensitive motif, and ROS target this motif to induce GTP nucleotide exchange, leading to Rho GTPases activation (62). The reversible oxidation of cysteine on this motif causes the nucleotide displacement favoring the GTP exchange and activation of Rho GTPases. These findings were further validated by another study that showed that two cysteine residues located at the redox-sensitive motif of RhoA are critical for ROS-induced activation of RhoA and subsequent cytoskeletal reorganization (2). The presence of one extra redox-sensitive cysteine residue in RhoA that is absent in Rac1 and Cdc42 is suggested to regulate the functional differences between these GTPases (62). Oxidative stress also activates RhoA by PKC-dependent phosphorylation and activation of p115RhoGEF (24). This signaling axis activates endothelial arginase, which competes with NOS for l-arginine, resulting in reduced NO levels and increased superoxide production. Since NO is essential for normal vascular function, its low levels due to enhanced arginase activity have been linked to various disease states such as diabetes, aging, and hypertension.
FIG. 5.
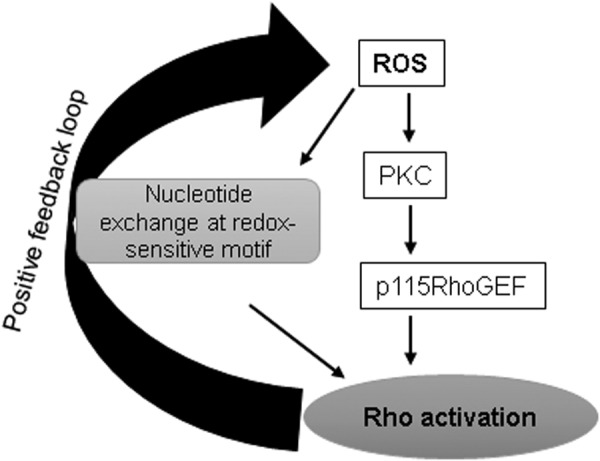
A crosstalk exists between ROS and Rho. ROS regulates Rho activation via modulating its activators, and Rho controls ROS generation by a positive feedback loop. This active crosstalk between ROS and Rho plays a crucial role in endothelial function.
Studies have suggested that nitrosative stress also regulates RhoA activity, thereby mediating endothelial dysfunction. For instance, LPS-induced endothelial barrier disruption was mediated by nitration of Tyr34 in RhoA (123). On the other hand, Rac-mediated NOX-dependent ROS generation activates p190RhoGAP, which inhibits RhoA activity (114). The opposing redox-dependent role of RhoA and Rac during hypoxia/reoxygenation-induced endothelial permeability has been described (155). Hypoxia inhibits Rac, leading to the activation of Rho, which causes stress fibers formation, breakdown of AJ, and increased endothelial permeability. In contrast, reoxygenation robustly activates Rac, inducing the enhancement of cortical actin cytoskeleton and strengthening AJ by inhibiting Rho activity. These findings suggest toward the existence of a redox-dependent, self-regulatory crosstalk between barrier-protective and barrier-disruptive members Rho GTPase family. It is likely that Rac-mediated ROS production inhibits Rho activity through NOX. This notion is supported by a report showing that activation of NOX1 inhibits Rho, whereas inhibition of NOX1 hyperactivates Rho (125).
The direct involvement of Rho GTPases in ROS generation is best exemplified by Rac-mediated, NOX-induced ROS production, where Rac plays the role of an essential component of an activated NOX complex at the cell membrane that, ultimately, produces ROS (69). Rac also directly interacts with NOS to regulate NO production (39) and interacts with SOD in a redox-dependent manner during the conversion of superoxide ion to hydrogen peroxide (58). The interaction of Rac1 with SOD has pathological implications as observed in amyotrophic lateral sclerosis. Some SOD mutants linked to the amyotrophic lateral sclerosis firmly are associated with Rac1, causing the sustained activation of Rac with excessive generation of ROS (58). Interestingly, Cdc42, an Rho GTPase closely related to Rac, is not involved in ROS generation by the NOX mechanism, but it directly binds to flavocytochrome b558 (40). This competitive binding of Cdc42 and Rac to flavocytochrome antagonizes the ROS-producing capabilities of Rac. Nonetheless, ROS-induced activation of Cdc42 by p47phox-mediated inhibition of Cdc42 negative regulator, Cdc42GAP, has been reported in smooth muscle cells (94).
Recent studies have suggested that Rho GTPases mediate a positive redox feedback loop. In this regard, actin binding protein profilin-1-mediated endothelial dysfunction is shown to be dependent on its positive feedback loop activation by ROS-activated RhoA/Rho-kinase (95). Briefly, advanced glycation end products-induced endothelial permeability is mediated by co-ordination of increased expression of profilin-1 and elevated production of ROS that leads to RhoA activation. Herein, a positive feedback loop consisting of prolifin-1-mediated ROS production and ROS-Rho-mediated expression of prolifin-1 regulates oxidative stress and endothelial dysfunction. An earlier study has demonstrated that neutrophil migration is controlled by a Rac-regulated and redox-mediated feedback loop (89). The authors proposed that Rac-mediated NOX activation inhibits phosphatase and tensin homologue (PTEN). Such inhibition of PTEN results in accumulation of PtdIns (3,4,5) P3. This signaling cascade creates a positive feedback loop to activate Rac and promote cell migration or activation of cortical cytoskeleton motility that is essential for recovery of intercellular gaps and reannealing of cell–cell junctions.
An active interplay between ROS production and RhoA activation in mediating EC dysfunction was established by a study from our group. The results showed that LPS-induced endothelial permeability and lung inflammation is mediated by oxidative stress-caused destabilization of MT and ensuing RhoA activation (88). LPS challenge increased ROS levels in EC that caused the disassembly of the MT network, leading to the release of the MT-bound RhoA activator, GEF-H1. ROS-mediated and MT-dependent activation of RhoA was responsible for enhanced endothelial permeability and inflammation both in vitro and in vivo. In agreement with these findings, recent data suggest that ROS-mediated MT destabilization plays a pivotal role in S. aureus-induced endothelial permeability and inflammation (78). The exposure of EC to bacteria increased ROS levels, which activated histone deacetylase 6 (HDAC6), causing MT disintegration. The destabilization of MT increases endothelial permeability and inflammation either via the breakdown of EC junction assembly or via release of GEF-H1 and activation of the Rho pathway. This pathway also mediated lung endothelial permeability and inflammation caused by air pollution factor, particulate matter (79). These findings strongly suggest that the ROS-HDAC6-MT-Rho signaling pathway of endothelial dysfunction appears to be a universal mechanism triggered by many barrier-disruptive and proinflammatory agonists (Fig. 6).
FIG. 6.
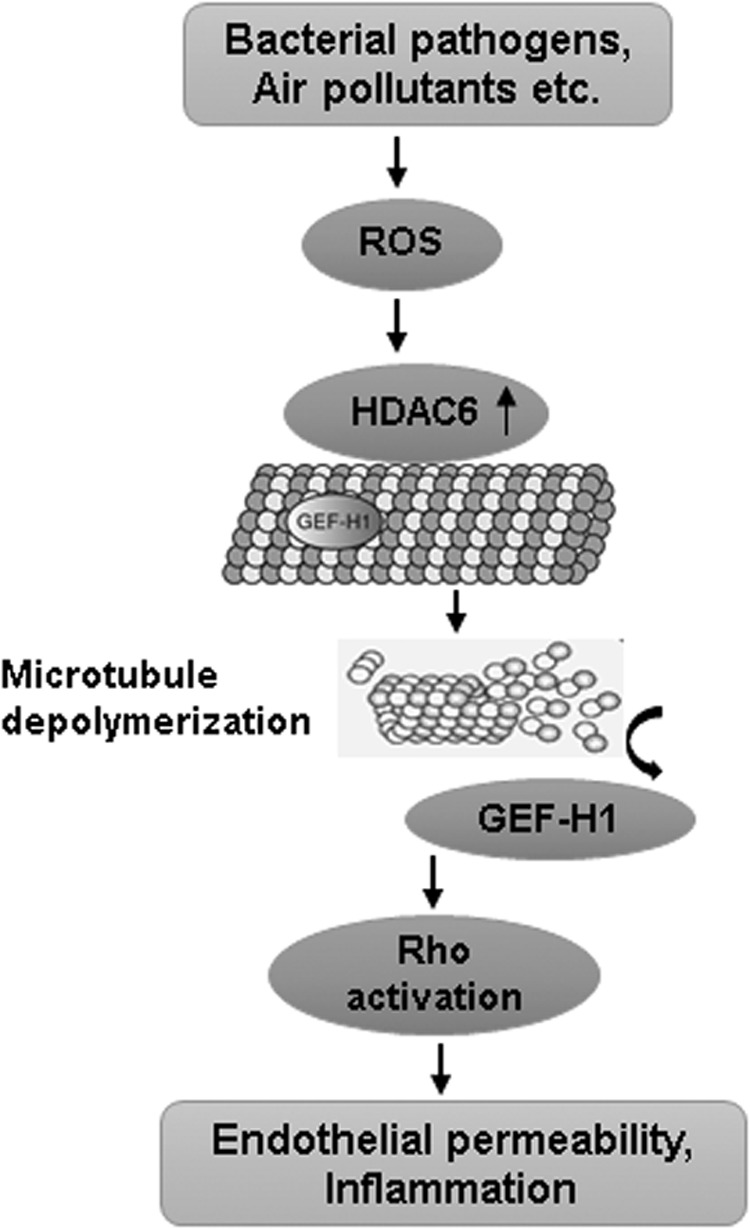
MT-dependent Rho activation plays a critical role in ROS-induced endothelial dysfunction. An increase in ROS levels activates HDAC6, which causes MT destabilization and release of MT-bound GEF-H1. The subsequent activation of Rho then mediates an increase in endothelial permeability as well as EC inflammatory responses. HDAC6, histone deacetylase 6; MT, microtubules.
Actin cytoskeletal reorganization represents another major impact of ROS-Rho GTPase crosstalk in endothelial function. Various Rho GTPases regulate endothelial barrier integrity by constant cytoskeletal remodeling based on the cell needs. The actin reorganization is essential for cell adhesion and migration, and ROS are known to influence these processes. Actin itself is prone to redox modification and oxidation of cysteine-374 is shown to alter its structural and functional organization on oxidation, resulting in decreased actin polymerization (145). ROS-mediated glutathionylation of actin is another mechanism that inhibits actin polymerization (152). Moreover, published studies demonstrate that Rac1 and other NOX components directly bind to actin and actin activates NOX (139). Taken together, these findings clearly suggest the existence of tri-directional interplay between Rho GTPases, ROS, and actin remodeling. It can be postulated that Rho GTPases, especially Rac1, are involved in the ROS production, and these GTPases regulate actin cytoskeleton organization either directly or via ROS-mediated redox signaling. In turn, ROS can directly activate Rho GTPases.
In summary, the bidirectional regulatory interaction between RhoA and ROS signaling appears to play a crucial role in inducing endothelial dysfunction and pathogenesis of several cardiopulmonary disorders, including ALI and ARDS. Basal physiological levels of ROS are essential for the maintenance of vascular homeostasis, whereas uncontrolled generation of excessive ROS impairs normal endothelial function by increasing permeability and inducing inflammation. RhoA activation is widely regarded as a central mechanism of endothelial permeability and inflammation in response to a broad range of barrier-disruptive and inflammatory agonists. A growing body of evidence suggests that ROS also activate RhoA to induce endothelial dysfunction, and Rho mediates a positive feedback loop of ROS generation. The existence of such a vicious cycle of ROS-RhoA signaling will have detrimental effects on endothelial barrier integrity, which may augment tissue injury and organ failure. A better understanding of ROS-induced regulation of Rho and interplay between the two in triggering endothelial dysfunction will be key to the development of new therapeutics against a broad range of disorders associated with dysregulated redox balance and hyperactivated RhoA signaling.
Acknowledgments
The studies in the authors laboratory are supported by the grants HL076259, HL087823 (K.G.B.) from the National Heart, Lung, and Blood Institute and GM122940 (K.G.B.) from the National Institute of General Medical Sciences.
Abbreviations Used
- AJ
adherens junctions
- ALI
acute lung injury
- AP-1
activator protein-1
- ARDS
acute respiratory distress syndrome
- cAMP
cyclic adenosine monophosphate
- CNF
cytotoxic necrotizing factor
- EC
endothelial cell
- GAP
GTPase activating protein
- GDI
GDP dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- GTPases
guanosine triphosphates
- H2O2
hydrogen peroxide
- HDAC6
histone deacetylase 6
- HUVEC
human umbilical vein endothelial cell
- ICAM-1
intercellular adhesion molecule-1
- LPS
lipopolysaccharide
- MLC
myosin light chain
- MT
microtubules
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- NOX
NADPH oxidase
- OxLDL
oxidized low-density lipoprotein
- PAF
platelet-activating factor
- PAH
pulmonary arterial hypertension
- PECAM
platelet-endothelial cell adhesion molecule
- PKC
protein kinase C
- PTEN
phosphatase and tensin homologue
- Rho
Ras-homologous
- ROCK
rho-associated kinase
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- TGF-β
transforming growth factor-β
- TJ
tight junctions
- TNF-α
tumor necrosis factor-α
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- ZO-1
zonula occludens-1
References
- 1. Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, and Drenckhahn D. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 539: 295–308, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aghajanian A, Wittchen ES, Campbell SL, and Burridge K. Direct activation of RhoA by reactive oxygen species requires a redox-sensitive motif. PLoS One 4: e8045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, and Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Anwar KN, Fazal F, Malik AB, and Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol 173: 6965–6972, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Armstead WM, Mirro R, Thelin OP, Shibata M, Zuckerman SL, Shanklin DR, Busija DW, and Leffler CW. Polyethylene glycol superoxide dismutase and catalase attenuate increased blood-brain barrier permeability after ischemia in piglets. Stroke 23: 755–762, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Barnard JW, Patterson CE, Hull MT, Wagner WW, Jr., and Rhoades RA. Role of microvascular pressure in reactive oxygen-induced lung edema. J Appl Physiol (1985) 66: 1486–1493, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Barnard ML. and Matalon S. Mechanisms of extracellular reactive oxygen species injury to the pulmonary microvasculature. J Appl Physiol (1985) 72: 1724–1729, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Baumer Y, Burger S, Curry FE, Golenhofen N, Drenckhahn D, and Waschke J. Differential role of Rho GTPases in endothelial barrier regulation dependent on endothelial cell origin. Histochem Cell Biol 129: 179–191, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Beckers CM, van Hinsbergh VW, and van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 103: 40–55, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, and Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Birukov KG. and Karki P. Injured lung endothelium: mechanisms of self-repair and agonist-assisted recovery (2017 Grover Conference Series). Pulm Circ 8: 2045893217752660, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, and Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 204: 934–947, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, and Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Birukova AA, Tian X, Tian Y, Higginbotham K, and Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 24: 2678–2688, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogatcheva NV. and Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res 76: 202–207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C, and Verin AD. Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvascular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol 221: 750–759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boueiz A. and Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res 77: 26–34, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Brodsky SV, Zhang F, Nasjletti A, and Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol 286: H1910–H1915, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, and Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 34: 453–463, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Cai H. and Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Carbajal JM. and Schaeffer RC., Jr. H2O2 and genistein differentially modulate protein tyrosine phosphorylation, endothelial morphology, and monolayer barrier function. Biochem Biophys Res Commun 249: 461–466, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Carles M, Lafargue M, Goolaerts A, Roux J, Song Y, Howard M, Weston D, Swindle JT, Hedgpeth J, Burel-Vandenbos F, and Pittet JF. Critical role of the small GTPase RhoA in the development of pulmonary edema induced by Pseudomonas aeruginosa in mice. Anesthesiology 113: 1134–1143, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Chabot F, Mitchell JA, Gutteridge JM, and Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757, 1998 [PubMed] [Google Scholar]
- 24. Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, and Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol 165: 506–519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, and Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L834–L841, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu JD, Mi MT, Zhang QY, Ling WH, and Yu B. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-kappaB pathway. Cell Biochem Biophys 61: 337–348, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Chen X, Andresen BT, Hill M, Zhang J, Booth F, and Zhang C. Role of reactive oxygen species in tumor necrosis factor-alpha induced endothelial dysfunction. Curr Hypertens Rev 4: 245–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chin LK, Yu JQ, Fu Y, Yu T, Liu AQ, and Luo KQ. Production of reactive oxygen species in endothelial cells under different pulsatile shear stresses and glucose concentrations. Lab Chip 11: 1856–1863, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Cho RL, Yang CC, Lee IT, Lin CC, Chi PL, Hsiao LD, and Yang CM. Lipopolysaccharide induces ICAM-1 expression via a c-Src/NADPH oxidase/ROS-dependent NF-kappaB pathway in human pulmonary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 310: L639–L657, 2016 [DOI] [PubMed] [Google Scholar]
- 30. Chu JY, McCormick B, and Vermeren S. Small GTPase-dependent regulation of leukocyte-endothelial interactions in inflammation. Biochem Soc Trans 46: 649–658, 2018 [DOI] [PubMed] [Google Scholar]
- 31. Clements RT, Minnear FL, Singer HA, Keller RS, and Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, Pastorino AM, and Lo Cascio V. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic Biol Med 22: 117–127, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, and Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A 96: 9815–9820, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Craige SM, Kant S, and Keaney JF., Jr. Reactive oxygen species in endothelial function—from disease to adaptation. Circ J 79: 1145–1155, 2015 [DOI] [PubMed] [Google Scholar]
- 35. Crawford LE, Milliken EE, Irani K, Zweier JL, Becker LC, Johnson TM, Eissa NT, Crystal RG, Finkel T, and Goldschmidt-Clermont PJ. Superoxide-mediated actin response in post-hypoxic endothelial cells. J Biol Chem 271: 26863–26867, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Dejana E, Orsenigo F, and Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Dery O, Corvera CU, Steinhoff M, and Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 274: C1429–C1452, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Di A, Mehta D, and Malik AB. ROS-activated calcium signaling mechanisms regulating endothelial barrier function. Cell Calcium 60: 163–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Di-Poi N, Faure J, Grizot S, Molnar G, Pick E, and Dagher MC. Mechanism of NADPH oxidase activation by the Rac/Rho-GDI complex. Biochemistry 40: 10014–10022, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Diebold BA, Fowler B, Lu J, Dinauer MC, and Bokoch GM. Antagonistic cross-talk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J Biol Chem 279: 28136–28142, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Ding R, Zhao D, Li X, Liu B, and Ma X. Rho-kinase inhibitor treatment prevents pulmonary inflammation and coagulation in lipopolysaccharide-induced lung injury. Thromb Res 150: 59–64, 2017 [DOI] [PubMed] [Google Scholar]
- 42. Ding RY, Zhao DM, Zhang ZD, Guo RX, and Ma XC. Pretreatment of Rho kinase inhibitor inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in mice. J Surg Res 171: e209–e214, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, and Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Dudek SM. and Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Essler M, Staddon JM, Weber PC, and Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol 164: 6543–6549, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Feng Y, LoGrasso PV, Defert O, and Li R. Rho kinase (ROCK) inhibitors and their therapeutic potential. J Med Chem 59: 2269–2300, 2016 [DOI] [PubMed] [Google Scholar]
- 48. Ferran C, Millan MT, Csizmadia V, Cooper JT, Brostjan C, Bach FH, and Winkler H. Inhibition of NF-kappa B by pyrrolidine dithiocarbamate blocks endothelial cell activation. Biochem Biophys Res Commun 214: 212–223, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, and Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels 25: 144–149, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Gaboury JP, Anderson DC, and Kubes P. Molecular mechanisms involved in superoxide-induced leukocyte-endothelial cell interactions in vivo. Am J Physiol 266: H637–H642, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Garcia JG, Davis HW, and Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Garcia JG, Verin AD, and Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost 22: 309–315, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Gautam N, Hedqvist P, and Lindbom L. Kinetics of leukocyte-induced changes in endothelial barrier function. Br J Pharmacol 125: 1109–1114, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goldblum SE, Hennig B, Jay M, Yoneda K, and McClain CJ. Tumor necrosis factor alpha-induced pulmonary vascular endothelial injury. Infect Immun 57: 1218–1226, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, and Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci 110(Pt 5): 583–588, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Gozin A, Franzini E, Andrieu V, Da Costa L, Rollet-Labelle E, and Pasquier C. Reactive oxygen species activate focal adhesion kinase, paxillin and p130cas tyrosine phosphorylation in endothelial cells. Free Radic Biol Med 25: 1021–1032, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, and Lichtman AH. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188: 6287–6299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, and Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest 118: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hastie LE, Patton WF, Hechtman HB, and Shepro D. Filamin redistribution in an endothelial cell reoxygenation injury model. Free Radic Biol Med 22: 955–966, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Hastie LE, Patton WF, Hechtman HB, and Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. J Cell Physiol 172: 373–381, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Hensley MK, Levine A, Gladwin MT, and Lai YC. Emerging therapeutics in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 314: L769–L781, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heo J. and Campbell SL. Mechanism of redox-mediated guanine nucleotide exchange on redox-active Rho GTPases. J Biol Chem 280: 31003–31010, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Hinshaw DB, Burger JM, Armstrong BC, and Hyslop PA. Mechanism of endothelial cell shape change in oxidant injury. J Surg Res 46: 339–349, 1989 [DOI] [PubMed] [Google Scholar]
- 64. Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber C, Goebeler M, Ludwig S, and Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 95: 3044–3051, 2000 [PubMed] [Google Scholar]
- 65. Hobbs GA, Zhou B, Cox AD, and Campbell SL. Rho GTPases, oxidation, and cell redox control. Small GTPases 5: e28579, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holland JA, Meyer JW, Chang MM, O'Donnell RW, Johnson DK, and Ziegler LM. Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium 6: 113–121, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Holland JA, Pritchard KA, Pappolla MA, Wolin MS, Rogers NJ, and Stemerman MB. Bradykinin induces superoxide anion release from human endothelial cells. J Cell Physiol 143: 21–25, 1990 [DOI] [PubMed] [Google Scholar]
- 68. Holman RG. and Maier RV. Oxidant-induced endothelial leak correlates with decreased cellular energy levels. Am Rev Respir Dis 141: 134–140, 1990 [DOI] [PubMed] [Google Scholar]
- 69. Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98: 453–462, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, and Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci 112(Pt 12): 1915–1923, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Hotter G, Closa D, Prats N, Pi F, Gelpi E, and Rosello-Catafau J. Free radical enhancement promotes leucocyte recruitment through a PAF and LTB4 dependent mechanism. Free Radic Biol Med 22: 947–954, 1997 [DOI] [PubMed] [Google Scholar]
- 72. Hu Q, Yu ZX, Ferrans VJ, Takeda K, Irani K, and Ziegelstein RC. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J Biol Chem 277: 32546–32551, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Ikeda M, Schroeder KK, Mosher LB, Woods CW, and Akeson AL. Suppressive effect of antioxidants on intercellular adhesion molecule-1 (ICAM-1) expression in human epidermal keratinocytes. J Invest Dermatol 103: 791–796, 1994 [DOI] [PubMed] [Google Scholar]
- 74. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, and Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 100: 1–19, 2018 [DOI] [PubMed] [Google Scholar]
- 75. Islam KN, Devaraj S, and Jialal I. alpha-Tocopherol enrichment of monocytes decreases agonist-induced adhesion to human endothelial cells. Circulation 98: 2255–2261, 1998 [DOI] [PubMed] [Google Scholar]
- 76. Johnson A, Phillips P, Hocking D, Tsan MF, and Ferro T. Protein kinase inhibitor prevents pulmonary edema in response to H2O2. Am J Physiol 256: H1012–H1022, 1989 [DOI] [PubMed] [Google Scholar]
- 77. Joshi AD, Dimitropoulou C, Thangjam G, Snead C, Feldman S, Barabutis N, Fulton D, Hou Y, Kumar S, Patel V, Gorshkov B, Verin AD, Black SM, and Catravas JD. Heat shock protein 90 inhibitors prevent LPS-induced endothelial barrier dysfunction by disrupting RhoA signaling. Am J Respir Cell Mol Biol 50: 170–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karki P, Ke Y, Tian Y, Ohmura T, Sitikov A, Sarich N, Montgomery CP, and Birukova AA. Staphylococcus aureus-induced endothelial permeability and inflammation are mediated by microtubule destabilization. J Biol Chem 294: 3369–3384, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Karki P, Meliton A, Sitikov A, Tian Y, Ohmura T, and Birukova AA. Microtubule destabilization caused by particulate matter contributes to lung endothelial barrier dysfunction and inflammation. Cell Signal 53: 246–255, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kasa A, Csortos C, and Verin AD. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers 3: e974448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, and Black SM. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol 967: 105–137, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kennedy TP, Rao NV, Hopkins C, Pennington L, Tolley E, and Hoidal JR. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest 83: 1326–1335, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kevil CG, Oshima T, Alexander B, Coe LL, and Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 279: C21–C30, 2000 [DOI] [PubMed] [Google Scholar]
- 84. Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, and Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996 [DOI] [PubMed] [Google Scholar]
- 85. Kokura S, Wolf RE, Yoshikawa T, Granger DN, and Aw TY. Molecular mechanisms of neutrophil-endothelial cell adhesion induced by redox imbalance. Circ Res 84: 516–524, 1999 [DOI] [PubMed] [Google Scholar]
- 86. Komarova Y. and Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72: 463–493, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Konior A, Schramm A, Czesnikiewicz-Guzik M, and Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal 20: 2794–2814, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, and Birukova AA. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol 47: 688–697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kuiper JW, Sun C, Magalhaes MA, and Glogauer M. Rac regulates PtdInsP(3) signaling and the chemotactic compass through a redox-mediated feedback loop. Blood 118: 6164–6171, 2011 [DOI] [PubMed] [Google Scholar]
- 90. Lawson C. and Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep 61: 22–32, 2009 [DOI] [PubMed] [Google Scholar]
- 91. Lawson CD. and Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases 5: e27958, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, and Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res 68: 231–238, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Lee IT, Shih RH, Lin CC, Chen JT, and Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun Signal 10: 33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li QF. and Tang DD. Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am J Physiol Cell Physiol 297: C1424–C1433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li X, Liu J, Chen B, and Fan L. A positive feedback loop of profilin-1 and RhoA/ROCK1 promotes endothelial dysfunction and oxidative stress. Oxid Med Cell Longev 2018: 4169575, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li Y, Wu Y, Wang Z, Zhang XH, and Wu WK. Fasudil attenuates lipopolysaccharide-induced acute lung injury in mice through the Rho/Rho kinase pathway. Med Sci Monit 16: BR112–BR118, 2010 [PubMed] [Google Scholar]
- 97. Liu H, Yu X, Yu S, and Kou J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int Immunopharmacol 29: 937–946, 2015 [DOI] [PubMed] [Google Scholar]
- 98. Lo SK, Janakidevi K, Lai L, and Malik AB. Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am J Physiol 264: L406–L412, 1993 [DOI] [PubMed] [Google Scholar]
- 99. Lum H, Barr DA, Shaffer JR, Gordon RJ, Ezrin AM, and Malik AB. Reoxygenation of endothelial cells increases permeability by oxidant-dependent mechanisms. Circ Res 70: 991–998, 1992 [DOI] [PubMed] [Google Scholar]
- 100. Lum H, Gibbs L, Lai L, and Malik AB. CD18 integrin-dependent endothelial injury: effects of opsonized zymosan and phorbol ester activation. J Leukoc Biol 55: 58–63, 1994 [DOI] [PubMed] [Google Scholar]
- 101. Lum H. and Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 280: C719–C741, 2001 [DOI] [PubMed] [Google Scholar]
- 102. Maniatis NA, Kotanidou A, Catravas JD, and Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 49: 119–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Maniatis NA. and Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care 14: 22–30, 2008 [DOI] [PubMed] [Google Scholar]
- 104. Matsubara T. and Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol 137: 3295–3298, 1986 [PubMed] [Google Scholar]
- 105. McQuaid KE. and Keenan AK. Endothelial barrier dysfunction and oxidative stress: roles for nitric oxide? Exp Physiol 82: 369–376, 1997 [DOI] [PubMed] [Google Scholar]
- 106. McQuaid KE, Smyth EM, and Keenan AK. Evidence for modulation of hydrogen peroxide-induced endothelial barrier dysfunction by nitric oxide in vitro. Eur J Pharmacol 307: 233–241, 1996 [DOI] [PubMed] [Google Scholar]
- 107. Mehta D. and Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, Goligorsky MS, and Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 289: H1106–H1114, 2005 [DOI] [PubMed] [Google Scholar]
- 109. Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, Offermanns S, Mochizuki N, Zheng Y, and Gutkind JS. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat Commun 6: 6725, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Minshall RD, Sessa WC, Stan RV, Anderson RG, and Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 285: L1179–L1183, 2003 [DOI] [PubMed] [Google Scholar]
- 111. Mitchell L, Hobbs GA, Aghajanian A, and Campbell SL. Redox regulation of Ras and Rho GTPases: mechanism and function. Antioxid Redox Signal 18: 250–258, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mong PY. and Wang Q. Activation of Rho kinase isoforms in lung endothelial cells during inflammation. J Immunol 182: 2385–2394, 2009 [DOI] [PubMed] [Google Scholar]
- 113. Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest 80: 1550–1560, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nimnual AS, Taylor LJ, and Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol 5: 236–241, 2003 [DOI] [PubMed] [Google Scholar]
- 115. Niwa K, Inanami O, Ohta T, Ito S, Karino T, and Kuwabara M. p38 MAPK and Ca2+ contribute to hydrogen peroxide-induced increase of permeability in vascular endothelial cells but ERK does not. Free Radic Res 35: 519–527, 2001 [DOI] [PubMed] [Google Scholar]
- 116. Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, and Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood 104: 3214–3220, 2004 [DOI] [PubMed] [Google Scholar]
- 117. Orfanos SE, Mavrommati I, Korovesi I, and Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med 30: 1702–1714, 2004 [DOI] [PubMed] [Google Scholar]
- 118. Park HS, Chun JN, Jung HY, Choi C, and Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006 [DOI] [PubMed] [Google Scholar]
- 119. Peters K, Unger RE, Brunner J, and Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res 60: 49–57, 2003 [DOI] [PubMed] [Google Scholar]
- 120. Petrache I, Crow MT, Neuss M, and Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun 306: 244–249, 2003 [DOI] [PubMed] [Google Scholar]
- 121. Petrache I, Verin AD, Crow MT, Birukova A, Liu F, and Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 280: L1168–L1178, 2001 [DOI] [PubMed] [Google Scholar]
- 122. Predescu SA, Predescu DN, and Palade GE. Endothelial transcytotic machinery involves supramolecular protein-lipid complexes. Mol Biol Cell 12: 1019–1033, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rafikov R, Dimitropoulou C, Aggarwal S, Kangath A, Gross C, Pardo D, Sharma S, Jezierska-Drutel A, Patel V, Snead C, Lucas R, Verin A, Fulton D, Catravas JD, and Black SM. Lipopolysaccharide-induced lung injury involves the nitration-mediated activation of RhoA. J Biol Chem 289: 4710–4722, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ridley AJ. and Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70: 389–399, 1992 [DOI] [PubMed] [Google Scholar]
- 125. Sadok A, Pierres A, Dahan L, Prevot C, Lehmann M, and Kovacic H. NADPH oxidase 1 controls the persistence of directed cell migration by a Rho-dependent switch of alpha2/alpha3 integrins. Mol Cell Biol 29: 3915–3928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Saito H, Minamiya Y, Saito S, and Ogawa J. Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol 72: 829–836, 2002 [PubMed] [Google Scholar]
- 127. Schlegel N. and Waschke J. Impaired cAMP and Rac 1 signaling contribute to TNF-alpha-induced endothelial barrier breakdown in microvascular endothelium. Microcirculation 16: 521–533, 2009 [DOI] [PubMed] [Google Scholar]
- 128. Seeger W, Hansen T, Rossig R, Schmehl T, Schutte H, Kramer HJ, Walmrath D, Weissmann N, Grimminger F, and Suttorp N. Hydrogen peroxide-induced increase in lung endothelial and epithelial permeability—effect of adenylate cyclase stimulation and phosphodiesterase inhibition. Microvasc Res 50: 1–17, 1995 [DOI] [PubMed] [Google Scholar]
- 129. Shasby DM, Lind SE, Shasby SS, Goldsmith JC, and Hunninghake GW. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood 65: 605–614, 1985 [PubMed] [Google Scholar]
- 130. Shasby DM, Shasby SS, Sullivan JM, and Peach MJ. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res 51: 657–661, 1982 [DOI] [PubMed] [Google Scholar]
- 131. Shi S, Verin AD, Schaphorst KL, Gilbert-McClain LI, Patterson CE, Irwin RP, Natarajan V, and Garcia JG. Role of tyrosine phosphorylation in thrombin-induced endothelial cell contraction and barrier function. Endothelium 6: 153–171, 1998 [DOI] [PubMed] [Google Scholar]
- 132. Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, and Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304: 40–49, 2005 [DOI] [PubMed] [Google Scholar]
- 133. Shimada H. and Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem 285: 12536–12542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Siderovski DP. and Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci 1: 51–66, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sidibe A. and Imhof BA. VE-cadherin phosphorylation decides: vascular permeability or diapedesis. Nat Immunol 15: 215–217, 2014 [DOI] [PubMed] [Google Scholar]
- 136. Simon F. and Fernandez R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J Hypertens 27: 1202–1216, 2009 [DOI] [PubMed] [Google Scholar]
- 137. Slotta JE, Braun OO, Menger MD, and Thorlacius H. Fasudil, a Rho-kinase inhibitor, inhibits leukocyte adhesion in inflamed large blood vessels in vivo. Inflamm Res 55: 364–367, 2006 [DOI] [PubMed] [Google Scholar]
- 138. Spindler V, Schlegel N, and Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 87: 243–253, 2010 [DOI] [PubMed] [Google Scholar]
- 139. Stanley A, Thompson K, Hynes A, Brakebusch C, and Quondamatteo F. NADPH oxidase complex-derived reactive oxygen species, the actin cytoskeleton, and Rho GTPases in cell migration. Antioxid Redox Signal 20: 2026–2042, 2014 [DOI] [PubMed] [Google Scholar]
- 140. Stockton R, Reutershan J, Scott D, Sanders J, Ley K, and Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell 18: 2346–2355, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Stockton RA, Schaefer E, and Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem 279: 46621–46630, 2004 [DOI] [PubMed] [Google Scholar]
- 142. Sun H, Breslin JW, Zhu J, Yuan SY, and Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 13: 237–247, 2006 [DOI] [PubMed] [Google Scholar]
- 143. Suttorp N, Weber U, Welsch T, and Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest 91: 1421–1428, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Suzuki K, Nemoto K, Ninomiya N, Kuno M, Kubota M, and Yokota H. Fasudil, a Rho-kinase inhibitor, attenuates lipopolysaccharide-induced vascular hyperpermeability and colonic muscle relaxation in guinea pigs. J Surg Res 178: 352–357, 2012 [DOI] [PubMed] [Google Scholar]
- 145. Terman JR. and Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol 25: 30–38, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thannickal VJ. and Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 147. Tinsley JH, De Lanerolle P, Wilson E, Ma W, and Yuan SY. Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am J Physiol Cell Physiol 279: C1285–C1289, 2000 [DOI] [PubMed] [Google Scholar]
- 148. Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, and Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal 5: 723–730, 2003 [DOI] [PubMed] [Google Scholar]
- 149. van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, and van Hinsbergh VW. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol 27: 2332–2339, 2007 [DOI] [PubMed] [Google Scholar]
- 150. van Nieuw Amerongen GP, Vermeer MA, and van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol 20: E127–E133, 2000 [DOI] [PubMed] [Google Scholar]
- 151. van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, and Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci 115: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 152. Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, and Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem 276: 47763–47766, 2001 [DOI] [PubMed] [Google Scholar]
- 153. Wang Y, Wang X, Liu W, and Zhang L. Role of the Rho/ROCK signaling pathway in the protective effects of fasudil against acute lung injury in septic rats. Mol Med Rep 18: 4486–4498, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wojciak-Stothard B. and Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 39: 187–199, 2002 [DOI] [PubMed] [Google Scholar]
- 155. Wojciak-Stothard B, Tsang LY, and Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005 [DOI] [PubMed] [Google Scholar]
- 156. Wojciak-Stothard B, Williams L, and Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol 145: 1293–1307, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wu T, Xing J, and Birukova AA. Cell-type-specific crosstalk between p38 MAPK and Rho signaling in lung micro- and macrovascular barrier dysfunction induced by Staphylococcus aureus-derived pathogens. Transl Res 162: 45–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xiao JW, Zhu XY, Wang QG, Zhang DZ, Cui CS, Zhang P, Chen HY, and Meng LL. Acute effects of Rho-kinase inhibitor fasudil on pulmonary arterial hypertension in patients with congenital heart defects. Circ J 79: 1342–1348, 2015 [DOI] [PubMed] [Google Scholar]
- 159. Xing J, Moldobaeva N, and Birukova AA. Atrial natriuretic peptide protects against Staphylococcus aureus-induced lung injury and endothelial barrier dysfunction. J Appl Physiol (1985) 110: 213–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yao L, Romero MJ, Toque HA, Yang G, Caldwell RB, and Caldwell RW. The role of RhoA/Rho kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res 1: 165–170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yoshida N, Yoshikawa T, Manabe H, Terasawa Y, Kondo M, Noguchi N, and Niki E. Vitamin E protects against polymorphonuclear leukocyte-dependent adhesion to endothelial cells. J Leukoc Biol 65: 757–763, 1999 [DOI] [PubMed] [Google Scholar]
- 162. Zhang H. and Sun GY. LPS induces permeability injury in lung microvascular endothelium via AT(1) receptor. Arch Biochem Biophys 441: 75–83, 2005 [DOI] [PubMed] [Google Scholar]
- 163. Zhang L, Li Q, Liu Z, Liu W, and Zhao M. Protective effects of a Rho kinase inhibitor on paraquat-induced acute lung injuries in rats. Inflammation 41: 2171–2183, 2018 [DOI] [PubMed] [Google Scholar]
- 164. Zhao Y. and Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol 174: 370–379, 1998 [DOI] [PubMed] [Google Scholar]
- 165. Zulueta JJ, Sawhney R, Yu FS, Cote CC, and Hassoun PM. Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. Am J Physiol 272: L897–902, 1997 [DOI] [PubMed] [Google Scholar]