Abstract
Significance: Pulmonary arterial hypertension (PAH) is a progressive disease of the lung vasculature characterized by the proliferation of all vascular wall cell types, including endothelial, smooth muscle, and fibroblasts. The disease rapidly advances into a form with extensive pulmonary vascular remodeling, leading to a rapid increase in pulmonary vascular resistance, which results in right heart failure.
Recent Advances: Most current research in the PAH field has been focused on the late stage of the disease, largely due to an urgent need for patient treatment options in clinics. Further, the pathobiology of PAH is multifaceted in the advanced disease, and there has been promising recent progress in identifying various pathological pathways related to the late clinical picture.
Critical Issues: Early stage PAH still requires additional attention from the scientific community, and although the survival of patients with early diagnosis is comparatively higher, the disease develops in patients asymptomatically, making it difficult to identify and treat early.
Future Directions: There are several reasons to focus on the early stage of PAH. First, the complexity of late stage disease, owing to multiple pathways being activated in a complex system with intra- and intercellular signaling, leads to an unclear picture of the key contributors to the pathobiology. Second, an understanding of early pathophysiological events can increase the ability to identify PAH patients earlier than what is currently possible. Third, the prompt diagnosis of PAH would allow for the therapy to start earlier, which has proved to be a more successful strategy, and it ensures better survival in PAH patients.
Keywords: pulmonary hypertension, metabolism, inflammation, vascular damage, hemolysis, oxidative stress
Introduction
Pulmonary arterial hypertension (PAH)/pulmonary hypertension (PH) is a fatal disease characterized by pathologic vascular remodeling, leading to right heart failure. Remodeling of the pulmonary arteries results in the thickening of the intima, media, and adventitia. Disease progression is associated with vessel lumen narrowing and the eventual occlusion, intimal fibrosis, and the development of the concentric and plexiform lesions. Despite the recent development of new therapeutics, survival remains poor. This is because at the advanced stage of the disease, the complexity of disturbed pathways is overwhelmingly complicated for delineation of causative or consequential mechanisms. Patients are usually diagnosed after the disease has had considerable progression and this is largely due to the consequence of the disease being asymptomatic early on. Thus, from a diagnostic standpoint, it would be of great benefit if more research efforts are focused on dissecting the pathways involved in the early initiation stages of the disease and the following chronological PAH progression.
Since this endeavor is clinically challenging, a critical approach would be to utilize preclinical animal models during the early and intermediate time-points in longitudinal studies (29) (Table 1; Fig. 1), rather than the endpoints on which the vast majority of literature is based. By focusing on the early disease development stages, it is highly probable that an understanding of the critical initiating disturbances in those signaling pathways can be attained. Moreover, it may be possible to study those disturbances in a more isolated environment that would alleviate some of the complexity and a vast number of cellular and molecular reprogramming that occurs in late-stage advanced disease. It is important to understand the early vital mechanisms that could help to treat familial cases of PAH and other predisposed PAH conditions such as connective tissue disease, hemolytic anemias, HIV, etc. There is also the importance of identifying early biomarkers that can move forward the diagnosis of PAH at an early, potentially reversible stage. This review is focused on the early mechanisms that were described in literature, and the potential early biomarkers of the disease. By discussing the current progress in understanding the mechanisms responsible for PAH initiation and early progression, we expect to highlight the importance to continue this line of research to advance the early PAH diagnostics and treatment.
Table 1.
Preclinical Animal Models Utilized to Study Pulmonary Hypertension at Early and Intermediate Time-Points in the Longitudinal Studies
| Early PH animal models | Treatment | Hemodynamic changes | References |
|---|---|---|---|
| MCT 3, 10, 15 days | MCT 60 mg/kg i.p. at day 0, then analysis on days 3, 10, and 15 | RVSP slightly elevated at days 10 and 15 in 25–30 mmHg range. Fulton index unchanged | (73, 139, 140) |
| Sugen/hypoxia 7 and 14 days | Sugen 25 mg–50 mg/kg s.c. and hypoxia 10% O2 at day 0, then analysis on days 7 and 14 | RVSP significantly increased at 7 and 14 days in 50–70 mmHg range. Fulton index significantly increased by day 15 | (29, 135, 144) |
| Sugen/hypoxia 21 and 35 days | Sugen 20 mg/kg s.c. and hypoxia 10% O2 (3 weeks) at day 0, then analysis on days 21 and 35 | RVWT significantly elevated at 21 and 35 days | (123) |
| AA 6 and 12 days | AA 0.35 mg/kg i.v. at days 0, 3, and 6, then analysis on days 6 and 12 | RVSP slightly elevated at days 6 and 12 in 40–45 mmHg range. Fulton index unchanged | (143) |
AA, antimycin A; i.p., intraperitoneal; , i.v., intravenous; MCT, monocrotaline; PH, pulmonary hypertension; RVSP, right ventricular systolic pressure; RVWT, right ventricle wall thickness; s.c., subcutaneous.
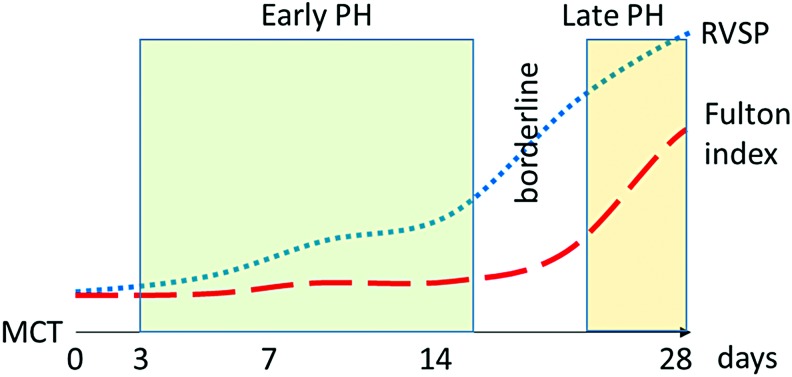
FIG. 1.
Illustration based on longitudinal MCT model that explains the selection of early time-points for the study. Early time-points for this model are indicated in green, where RVSP is slightly raised but without compensatory effect from the heart. Late pulmonary hypertension (red) is showed as a pronounced increase in both RVSP and Fulton index. MCT, monocrotaline; PH, pulmonary hypertension; RVSP, right ventricular systolic pressure. Color images are available online.
Metabolic Reprogramming
Recently, with the availability of untargeted metabolomic approaches, several reports were published that used metabolic profiling to evaluate possible biomarkers and shifts in metabolites in PAH patients (103, 116, 149, 207–209), animal models of PH (72, 140, 144, 210), and cells (36, 86, 182). The cancer-like proliferation of pulmonary vascular cells requires metabolic adaptation for the heightened demand of dividing cells. Different vascular cell types can adapt differently. Metabolomic analyses of human pulmonary microvascular endothelial cells (ECs) with PAH-causing mutations in the bone morphogenetic protein receptor type 2 (BMPR2) showed the upregulation of glycolysis and pentose phosphate pathways (36). Glucose, ribose, and their phosphorylated intermediates were also significantly increased. On the other hand, these cells also have exhibited a decrease in carnitine homeostasis, fatty acid oxidation pathways, and tricarboxylic acid (TCA) cycle metabolites. Thus, the upregulation of cytosolic glycolysis in ECs was accompanied by a decrease in mitochondrial-based metabolic pathways. It was also demonstrated that in the model of increased pulmonary blood flow due to heart defects, carnitine homeostasis is downregulated via the inhibition of the carnitine acetylation/deacetylation system, which is responsible for the transport of fatty acid into the mitochondrion (178). Therefore, the altered transport of fatty acids could be a significant contributor to the decrease in fatty acid oxidation and subsequent reduction of TCA cycle activity as well as oxidative phosphorylation. On the contrary, smooth muscle cells (SMCs) from PAH patients instead exhibited decreased glucose metabolism and an increase in fatty acid biosynthesis (86). Other groups found that SMCs isolated from monocrotaline (MCT) animals showed reduced oxidative phosphorylation and an increased rate of glycolysis (107, 139). This discrepancy could be from many factors, such as stability of metabolic phenotype in relation to methods of isolation, cell growth, the influence of cell culture media, etc. The question remains, however, whether or not endothelial and SMC lineages have a similar metabolic shift in PAH. Our published data indicate that inhibition of mitochondrial respiration by antimycin A (AA) shifts ECs toward glycolysis more effectively than in SMCs (144).
Currently, hypoxia-inducible factor (HIF) and RAC-alpha serine/threonine-protein kinase (Akt) are considered to play a regulatory role in the metabolic switch in PAH (15, 37, 107, 173). HIF upregulates expression of glycolysis genes such as hexokinase-2, Glut1,3, PFK, and growth factors that, in turn, can induce Akt (102, 129, 202). Akt activation can lead to cell proliferation, apoptosis resistance and further activation of glycolysis by the induction of HIF signaling, and the translocation of Glut4 and mammalian target of rapamycin (mTOR) phosphorylation. It has been shown that mTOR kinase is an important regulator of lung vasculature remodeling and right heart hypertrophy (51, 65, 124). Moreover, increased oxidative stress contributes to HIF and Akt activation (11, 53, 80, 138); this generates a feedforward loop. Besides, HIF-1α upregulates the expression of pyruvate dehydrogenase kinase (PDK), an inhibitor of the mitochondrial enzyme pyruvate dehydrogenase (PDH), and decreases intramitochondrial calcium, thus additionally impairing Ca2+-dependent activity of PDH (7, 28). Inactivation of PDH was previously found to be an underlying cause of the disease pathology as the inactivation of PDH limited pyruvate influx into the TCA cycle (179). By limiting the formation of oxidative phosphorylation substrates in the mitochondrial matrix, PDH insufficiency can contribute to a glycolytic switch. Indeed, ex vivo treatment of human PAH lungs with the PDK inhibitor dichloroacetate (DCA) induced the activation of PDH, increased mitochondrial respiration (112), and improved right ventricle (RV) function in animal models (127). DCA administrated to idiopathic PAH patients reduced mean pulmonary artery pressure and pulmonary vascular resistance and brought improvement in functional capacity, confirming the critical role of PDH activity in PAH pathogenesis, although showing a range of individual responses (112).
However, it is still not clear whether the role of mitochondrial dysfunction in the process of the glycolytic switch of vascular cells is a causative event or a consequence of the disease. We have recently demonstrated that the chronic inhibition of mitochondrial respiration by AA resulted in an increased pulmonary pressure and proliferation of the vascular wall (144). Interestingly, lung glucose and pulmonary pressure demonstrated a strong correlation in animals treated with AA. Metabolic assessment of the lung tissue from the AA model showed an upregulation of glycolytic intermediates with rising levels of glucose, ribose, and phosphorylated sugars, as previously seen in ECs with BMPR2 mutation. A recent study on the chronic hypoxia, Sugen SU5614 (SU)/hypoxia, and MCT rodent models also confirmed the upregulation of glycolysis and the downregulation of mitochondria-centered metabolism (63, 96, 98, 107). Studies showed that adventitia fibroblasts isolated from hypoxic animals exhibited an upregulation of glycolysis (173). Glycolytic fibroblasts, in turn, lead to the activation of inflammatory pathways via fibroblast–macrophage interactions, resulting in secretion of cytokines and chemokines such as interleukin (IL)-1β, IL-6, and vascular endothelial growth factor (VEGF)-A, which contribute to vascular remodeling. Therefore, we can conclude that the vasculature metabolic reprogramming could start both from ECs (inside) and from adventitia (outside).
Importantly, the metabolic shift in PAH discussed earlier occurs much earlier than the increase in pulmonary arterial pressure and right heart hypertrophy. By using rats just after 14 days of MCT injection, we found that before significantly increased pulmonary pressure and right ventricle hypertrophy, a complete metabolic reprogramming occurs in the lungs (140). Metabolic profiling indicated that at the early stage in disease development there were significant changes in glycolysis and fatty acids beta-oxidation as well as inflammatory, oxidative stress, and fibrosis biomarkers, which were previously described for developing PH (103, 116, 149, 208). Thus, metabolic reprogramming is an early event that takes place faster than the pathophysiologic changes in the pulmonary vasculature. Specifically, the accumulation of glycolytic intermediates and reductions in acyl-carnitine long-chain fatty acid metabolites were reported (140). Therefore, the increase in glycolysis with decreased fatty acid oxidation appears to occur very early in the disease. Kynurenine and kynurenate, metabolites of tryptophan degradation that are produced by activated indoleamine 2,3-dioxygenase during inflammation, could activate the production of inflammatory molecules such as IL-6 and could be involved in cell proliferation (175). Both pro- and anti-inflammatory prostaglandins, as well as omega-6 fatty acids, were increased during the development stage. Increased levels of glucosamine, its derivatives, and hydroxyproline indicate toward the predisposition to extracellular matrix (ECM) remodeling. Together with adventitial fibroblasts activation, this could involve profibrotic changes in the vasculature. Also, elevated levels of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase (NOS), and the upregulation of arginases (75, 174) could increase the consumption of arginine via the urea cycle with a 14-fold increase in urea, ornithine, and polyamines content. All these events indicate a disrupted arginine homeostasis and suggest the bypass of normal nitric oxide (NO) signaling. Profiling an early PH animal model may lead not only to finding the key pathways that modulate cells reprogramming but also to the discovery of metabolites that comprise the blueprint for early PAH diagnosis.
Early changes in metabolic footprints in the MCT model were mostly equal to the late stage changes found in patients with PAH. The plasma of patients with PAH show significantly increased TCA cycle intermediates, acyl-carnitines, urea, tryptophan, and polyamine metabolites, and they are similar to the early MCT model in the observed upregulation of these pathways (103, 140, 149). Interestingly, responders to vasorelaxation therapy, patients whose mean pulmonary artery pressure dropped >10 mmHg to <40 mmHg with preserved cardiac output in response to an acute pulmonary vasodilator challenge and remained stable on calcium channel blocker therapy alone, showed normalization of their metabolic profile back to healthy controls, providing the evidence of a strong association between the severity of PAH and metabolic alterations (149). Similar changes in arginine metabolism, ECM metabolites, polyamines, TCA intermediates, as well as increased heme degradation were found in lung tissues collected from PAH patients (207, 208). Common metabolites upregulated in PAH patients and the early model of MCT PH are indicated in Figure 2. This figure illustrates three main imbalances, which give rise to other metabolic disturbances. First, the shift from oxidative phosphorylation to glycolysis results in the activation of glycolysis-fed pathways involved in nucleotide biosynthesis and ECM remodeling. Second, the imbalance between the NO cycle and the urea cycle led to decreased NO production and increased polyamine synthesis, which contributed to vasoconstriction and proliferation. Third, decreased fatty acid oxidation and the demand for oxidative phosphorylation modify the TCA cycle by promoting anaplerotic reactions via glutaminolysis (12, 128), or pyruvate to oxaloacetate reactions, that are necessary for proliferating cells due to an increased need in building blocks for protein, fatty acids, and heme biosynthesis. Although those metabolic switches occur in the whole lung at the development stage of a preclinical model, they recapitulate metabolic disturbances in patients during the progressive phase of PAH. Metabolons of the whole lung are the most inclusive in terms of their variety of altered pathways; however, the plasma profile showed similar changes even with a reduced number of metabolites. Indeed, metabolites in plasma are well regulated by the liver, kidneys, and gut and do not reflect only lung status. However, availability of the plasma as well as difficulties to obtain lung biopsy should also be taken into account. Endothelial and SMCs isolated from animals or patients showed different results in metabolic profiling that could be explained by phenotype loss after isolation and cell culture. Thus, individual cell analyses are not so sensitive and could be cumbersome in clinical practice. Therefore, the profiling of the plasma samples could be important in the future diagnosis of PAH at the early stage.
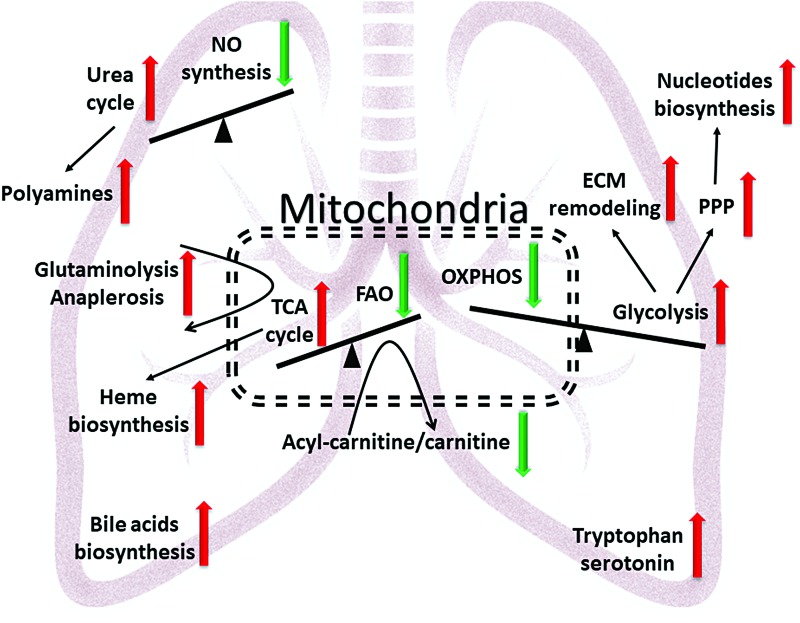
FIG. 2.
The generalized metabolic disturbances in PAH lungs indicated by the untargeted metabolic profiling. A central role of mitochondrial metabolism in reprogramming is attributed to a decrease in oxidative phosphorylation due to respiratory chain deficiency and reduced glucose oxidation due to pyruvate dehydrogenase deficiency. Reduction in fatty acid transport leads to a decrease in fatty acid oxidation. TCA cycle metabolites flux is upregulated by anaplerotic reactions that are required for the building blocks production for cellular proliferation instead of energy metabolism. This mitochondrial metabolic shift is accompanied with an increase in glycolysis for the energy demand and downstream of glycolysis metabolic pathways such as pentose phosphate pathway, nucleotide biosynthesis, and increased glycosylation, leading to proliferation and ECM remodeling. Another point of dysregulation is the nitric oxide and urea cycles, leading to vasoconstriction, increased reactive oxygen species/reactive nitrogen species, and polyamines production. Overall metabolic shift in lungs favors proliferation and dysfunction of cellular roles in all varieties of vascular cells. ECM, extracellular matrix; NO, nitric oxide; PAH, pulmonary arterial hypertension; TCA, tricarboxylic acid. Color images are available online.
Pulmonary Vascular Permeability
Inflammatory pathways in PAH are well accepted as important mechanisms of pathogenesis (21, 62, 134). PAH patients have increased infiltration of inflammatory cells (macrophages, lymphocytes, mast cells) in the perivascular region and an increased number of circulating cytokines in the plasma, such as monocyte chemoattractant protein 1 (MCP-1)/CCL2, regulated on activation normal T cell expressed and secreted/CCL5, tumor necrosis factor (TNF)-α, IL-1, and IL-6 (70, 91, 122, 130, 160). Importantly, the overexpression of IL-6 and TNF-α can induce a spontaneous development of PH in mice (43, 171). The mechanism behind cytokine-induced PH is based on studies that showed that a proliferative response could be induced in ECs, SMCs, and fibroblasts via yes associate protein/TAZ/SLUG, STAT3, mitogen-activated protein kinase (MAPK), protein kinase C (PKC), and AKT signaling cascades (24, 172, 192, 201). Thus, this promotes pulmonary vascular remodeling and subsequent increases in pulmonary arterial pressure. In the resolution phase of perivascular inflammation, ECM remodeling and the formation of fibrotic tissue can occur in the pulmonary vasculature (183). Interestingly, sex is an important component in the predisposition to a fibrotic phenotype of vascular remodeling. Our group found that the male sex is associated with increased inflammatory response and fibrosis of the pulmonary arteries (143). In a recent paper by Samokhin et al., it was found that important crosstalk exists between developmentally downregulated protein 9 (NEDD9) and mothers against decapentaplegic homolog 3 (SMAD3) within profibrotic transforming growth factor (TGF)β signaling (156). Oxidative stress-mediated post-translational modification in NEDD9 impairs its complex with SMAD3, leading to increased collagen production independent from ligand stimulation. Thus, this work established an important direct link between oxidative stress and vascular fibrosis. It is well accepted that female hormones mediate antioxidant responses (169, 206), and, thus, this mechanism may explain the predisposition of males to fibrotic changes in the vasculature. Reciprocally, connective tissue disease is known to be associated with a high risk of PH development (23, 58, 108). Fibrosis of the arteries increases vascular stiffness and results in an increased load to the heart through the shear stress of underlying fibrotic tissue vascular cells.
Although the complexity of inflammatory response in the pathogenesis of PAH is recognized (4, 17, 194), the initial trigger of inflammation that is involved in PAH development is not identified. It is still unclear whether initial damage to pulmonary vasculature activates an inflammatory response or whether pathogen/toxin-mediated inflammation damages the lung vasculature. However, both events must occur early in the development of PH. Initial damage to endothelial and SMCs induced via toxins or oxidative stress (1, 95, 196) can lead to damage-associated molecular patterns (DAMPs) activation, and a release that subsequently activates inflammatory cells via pattern recognition receptors (PRRs). Ruptured red blood cells (RBCs) can also be a source of DAMPs (77, 111). The pathogen can release pathogen-associated molecular patents (PAMPs) that also work by activating the inflammatory response via PRRs. The importance of one of the PRRs, receptor for advanced glycation endproducts, in PH was proven by elegant studies involving patient sample analysis and different animal models (31, 110). Therefore, a potentially feasible strategy is that PRRs and DAMPs/PAMPs inhibitors should be tested for clinical relevance in PAH management.
Another unanswered question in inflammation-mediated pathogenesis of PAH is why inflammatory cells have predominantly perivascular localization. This question may point to the increased infiltration of inflammatory cells through the vascular wall, which can be potentiated by the dysfunctional endothelial barrier. We and others have reported that chronic endothelial dysfunction due to different pathologies could be linked to PH (25, 106, 145, 196). In our study, we focused on the product of hemolysis, heme. Hemolysis leads to the accumulation of free hemoglobin in plasma, and this has correlated significantly with disease progression in PAH patients (145). Free hemoglobin can release free heme that, on the one hand, can damage the endothelium (77, 166), and, on the other hand, can induce disruptive endothelial barrier pathways leading to perivascular edema formation (145). Data indicated that the formation of perivascular edema and endothelial barrier dysfunction are early events in PH. In both SU/hypoxia and MCT models of PH, the increase in lung vascular leakage usually occurs during the first 2 weeks of disease induction and strongly correlates with the initial increase in pulmonary pressure (Fig. 3A, B). At an advanced stage of PAH, perivascular edema is diminished with highly increased thickness of the vasculature (145). Therefore, the perivascular inflammation due to infiltration of inflammatory cells into the perivascular area, crosstalk with adventitial fibroblasts, and the accumulation of cytokines in perivascular fluids could all be important contributors in the disease development phase. Moreover, fluid leakage in the perivascular area could impose local hypoxia to the flooded vasculature and induce vascular stiffness. Indeed, our data indicate the increased expression of carbonic anhydrase IX, the marker of hypoxic tissue, in the vasculature surrounded by perivascular fluid (Fig. 3C), but they showed no expression in the vessel without edema. This will induce HIF-mediated metabolic reprogramming in the lungs and increase the load on the right heart, leading to hypertrophy.
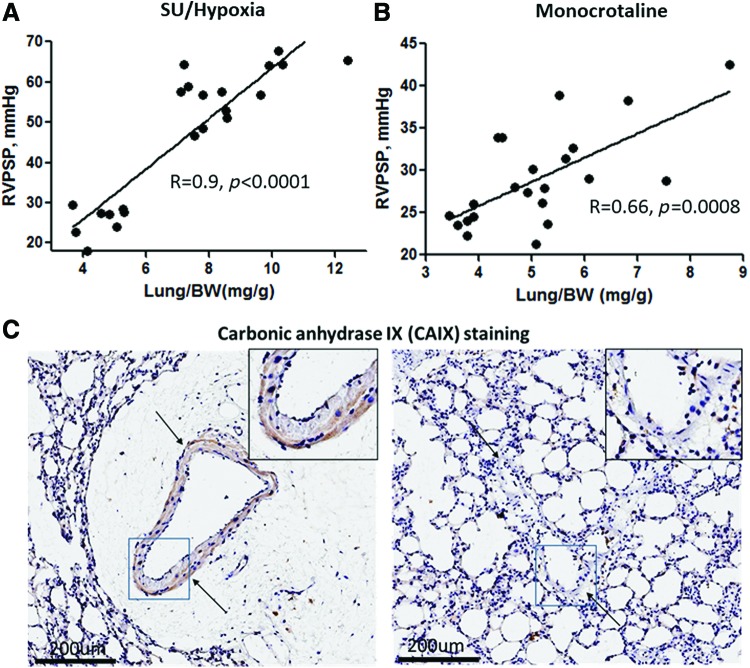
FIG. 3.
Correlation between RVPSP and lung weight as a measure of vascular leakage. Both parameters were measured at the early stages (0–14 days) of MCT (A) and SU/hypoxia models (B). In SU/hypoxia rats, PAH was induced by a single injection of Sugen 5416 (50 mg/kg, subcutaneous) followed by exposure to hypoxia (10% O2), as previously published (145). In the MCT group, the disease was initiated by MCT injection (60 mg/kg intraperitoneal.). Lungs from the MCT model were stained with carbonic anhydrase IX, the marker of hypoxia, that clearly indicates the presence of hypoxia in vessels surrounded by perivascular edema (arrows indicate vessel) (C). RVPSP, right ventricle peak systolic pressure; SU, Sugen SU5614. Color images are available online.
Vascular Damage and Inflammation
Injury of pulmonary vascular cells is one of the most recognized and established early mechanisms involved in PAH initiation and progression. In 2001, Voelkel and colleagues reported that inhibition of the primary endothelial prosurvival signaling molecule, VEGF, in combination with hypoxia induces apoptosis of pulmonary ECs and manifests as a severe angioproliferative PAH (184). This SU/hypoxia model now becomes a “gold standard” of experimental PAH that closely replicates the histological features seen in humans, including the formation of plexiform lesions composed of highly proliferative pulmonary vascular cells. It has been proposed that the initial apoptosis of pulmonary ECs induces a transition of and the selection for the remaining surviving cells. This causes a highly proliferative and apoptosis-resistant subpopulation, which, in turn, mediates PAH progression (154).
This conclusion may be viewed as paradoxical since it appears to require the initial inhibition of growth factors before it can promote the emergence of hyperproliferative cells. However, it seems likely that it is not growth inhibition itself, rather the induction of vascular cell damage, which is the main driving force in the stimulation of the proliferative potential of surviving cells. Indeed, the same apoptosis resistance could be achieved by exposure of naive cells directly to a conditioned medium from apoptotic cells (154). PAH could also be induced by the activation of apoptotic pathways (195). Apoptosis-inducing stimuli, including ultraviolet irradiation (49), drugs and toxins (46, 126), shear stress (155), mitochondrial dysfunction (203), oxidative/nitrative stress (186, 199), as well as genetic factors, such as loss of BMPR2 signaling (185), were shown to be directly involved in the pathogenesis of PAH.
In contrast, apoptosis inhibitors instead protect against PAH development. In two different studies, overexpression of VEGF in lungs blunts the onset of either hypoxia-induced PAH (35, 123) or PH associated with pulmonary fibrosis. Hameed et al. showed that the blockade or genetic deletion of a TNF-related apoptosis-inducing ligand that is responsible for endothelial apoptosis prevents the development of PAH in three independent rodent models (60). Another line of evidence comes from the research published by White et al. showing that attenuation of the programmed cell death 4/caspase-3/apoptotic pathway potently blocks SU/hypoxia PAH (195). Antagonizing the biosynthesis of macrophage-derived leukotriene B4 and directly inducing the apoptosis of pulmonary artery ECs reverses established PAH in animal models, as shown by Tian et al. (187). In contrast, overexpression of 5-lipoxygenase, which catalyzes leukotriene biosynthesis, was shown to have enhanced MCT-induced PAH (73). This last evidence supports the role of apoptotic cell death as not only in the initiation of PAH but also for its role in the continued progression during the developed stage. Indeed, it seems that PAH-associated damage is not a “hit and run” effect but rather an ongoing process continuously mediating disease progression. Thus, it was noticed that proapoptotic factors, such as caspase 3 and p53, remain elevated in the pulmonary arteries of patients with advanced irreversible disease (93). Another study reported that patients with a developed PAH continue to have circulating markers of vascular injury (167).
Although the causal role of initial vascular wall damage in PAH is widely accepted, the particular mechanisms responsible for the transformation of apoptosis into the proliferation of pulmonary vascular cells are still debated. In general, apoptosis serves as an essential regulator of tissue integrity and homeostasis that removes damaged or no longer functional cells. It represents a silent type of cellular damage with no detectable activation of the immune system. However, this silence is not complete; phagocytic clearance of apoptotic cells requires the production of chemoattractants that were described as “find me,” “eat me,” “listen to me,” or “stay away” signals that are critical for the recognition and the engulfment of the dying cell by the phagocytes (39, 55). This signaling along with the later discovered ability of apoptotic cells to regulate their local environment by releasing regulators of proapoptotic, antiapoptotic, and mitogenic pathways may explain the ultimate role of ongoing vascular apoptosis in the following proliferation of surrounding cells.
One of the examples of apoptotic cell communication is the releasing of long-range death factors. Thus, it was discovered that the induction of apoptosis in one part of the tissue stimulates an apoptotic death in remote cells through secretion of TNF-α (125). Other mechanisms inducing propagation of cell death require functional gap junctions. Two different research groups described that cell death signaling was propagated via gap junctions using two different systems. Krutovskikh et al. found that apoptotic cells use gap junction protein Connexin43 (Cx43) to couple with their nonapoptotic neighbors and propagate cell death (85). The formation of clusters of dying cells was inhibited in cells by expressing a dominant negative mutant of Cx43. Azzam et al. described that damage signals might be transmitted from irradiated to nonirradiated “bystander” cells that start expressing the genetic damage or changes in the expression of stress-induced genes through the same Cx43-mediated intercellular communication (8). Since gap junctions can typically pass molecules of up to 1000–1500 Da, the spreading of the damage signaling could occur through the exchange of metabolites: ions such as Ca2+, nucleotides, or peptides. Besides, these death messengers could be released from the cell itself. Lipid peroxide products, inosine nucleotides, and cytokines such as TNF-α, as well as reactive oxygen species (ROS), are the proposed candidates for transmission of damage signals from apoptotic to healthy cells (132). The same spread of the cell killing signaling cascade could coordinate apoptotic cell death in the pulmonary vasculature in response to various damaging stimuli discussed earlier. If confirmed, the propagation of death signaling could explain simultaneous apoptotic cell death in the remote parts of the pulmonary vascular tree.
Apoptotic cells are also known to mediate apoptosis-induced death resistance. The important survival signaling relevant to PAH is mediated through VEGF. Secreted from dying ECs (49), it induces apoptosis resistance in surviving ECs and vascular smooth muscle cells (VSMCs) (155). Another critical endothelial survival factor, angiopoietin-1 (121), is produced not only by apoptotic cells but also by monocytes interacting with apoptotic cells (161). Inflammatory cells are also a source of IL-6, a pleiotropic cytokine that serves to block apoptosis in different vascular cells exposed to the toxic environment (45). In contrast, apoptotic ECs promotes the survival of macrophages in a sphingosine-1-phosphate dependent manner, thus ensuring the rapid phagocytosis of dying cells (193). This double-sided protection represents a functional network between different types of cells that cooperatively stabilize the vascular wall against damage.
Such a combination of pro- and antiapoptotic stimuli does not fully explain the process of making the survival-versus-apoptosis decision for each affected cell. The current opinion tends to view each population of cells as a mixture of cells that are more sensitive or more resistant to apoptotic stimuli. That means that under the conditions of persistent self-perturbed apoptosis, the situation favors the selection of apoptosis-resistant cells due to the death of sensitive cells and the stimulation of apoptosis resistance in survivors. The secretion of prosurvival factors by apoptotic cells additionally shifts this balance toward survival, growth, and, eventually, vascular remodeling. Thus, TGF-1β released from the apoptotic ECs promotes intimal hyperplasia (105), SMC proliferation and migration (155), endothelial–mesenchymal transition with EC-derived SMC accumulation (94), and ECM deposition (64). The conditioned media were collected from the ECs exposed to hypoxia, a condition known to promote EC apoptosis (109), and stimulated proliferation of SMCs through the prostaglandin-mediated mechanism and the growth of fibroblasts via secretion on basic fibroblast growth factor (113). It was also reported that fibroblasts exposed to apoptotic media undergo myofibroblast differentiation in a connective tissue growth factor responsive manner (88). Endothelial injury impairs the secretion of NO, a main paracrine vasodilator with antimitogenic properties, and then stimulates the secretion of endothelin-1 (76), a potent vasoconstrictor and mitogen. Apoptosis is also associated with the increased production of ROS, which can initiate and control different aspects of apoptosis-induced proliferation (33).
Even in the context of immune system modulation, apoptotic cell death is now considered not “silent,” but rather anti-inflammatory (52). Inhibition of inflammation is achieved through a number of sequential steps. First, it requires a prompt and efficient engulfment of apoptotic cells by phagocytes to prevent an uncontrolled release of intracellular content. This is normally achieved by producing the “find me” and “eat me” factors. The examples of such attractants are lipid lysophosphatidylcholine, chemokine CX3CL1 (fractalkine [FKN]), the nucleotides adenosine triphosphate and uridine triphosphate, endothelial monocyte-activating polypeptide II, thrombospondin 1, TGF-β, annexin I, and oxidized phospholipids of apoptotic cells (55). Some of these attractants are also recognized as anti-inflammatory mediators. For example, apoptotic cell-derived TGF-β and FKN suppress macrophage proinflammatory responses (118).
Dying cells are typically known to be a source of DAMPs, which initiate a cascade of inflammatory reactions. For example, high mobility group box 1 (HMGB1) stimulates inflammatory cell activation via the activation of PRRs. However, as opposed to necrosis, apoptotic cells were shown to produce HMGB1 in considerably lower amounts due to its ability to stay attached to the apoptotic chromatin (13). This is especially true for human pulmonary artery endothelial cells (HPAECs, Fig. 4A, B). ROS produced by apoptotic cells oxidize intracellular proteins (78) as well as proteins in the extracellular environment (Fig. 4C); therefore, HMGB1 and other redox-sensitive DAMPS would be likely secreted in an already preoxidized inactive form (78) or will be quickly inactivated by oxidation after secretion. Importantly, an oxidized HMGB1 is known to be responsible for the resolution of the inflammation due to its ability to initiate immune tolerance (78). Apoptotic cells are also capable of mediating innate immunosuppression via a mechanism uncoupled from paracrine effects or phagocytosis. This process consists of direct transcriptional inhibition of genes encoding inflammatory cytokines (14) and could be activated in macrophages, fibroblasts, and potentially all contacting neighbors of apoptotic cells.
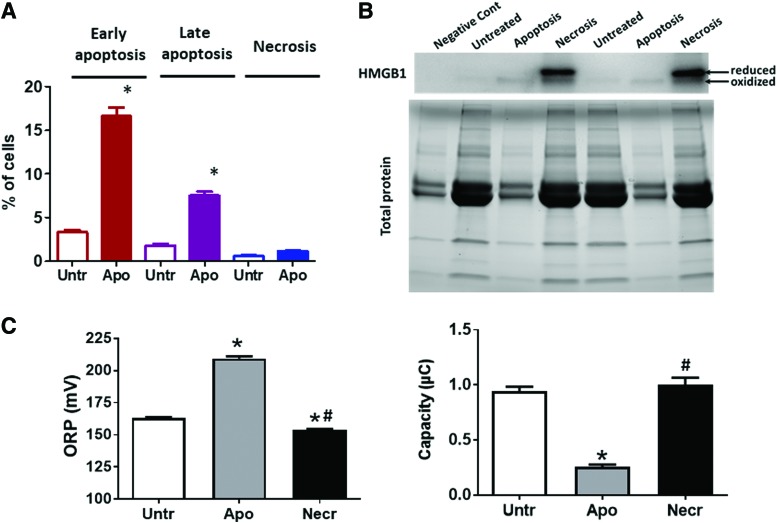
FIG. 4.
Apoptosis is the primary fate of endothelial cell death resulting in oxidized HMGB1 release and increases oxidative potential in the media. (A) HPAECs (ScienCell, Carlsbad, CA) were cultured in ECM growth media supplemented with 5% FBS and penicillin–streptomycin in a humidified incubator (21% O2, 5% CO2) at 37°C. To induce cell death, cells were treated with Sugen 5416 (20 μM) in 0.5% FBS for 24 h, as published (101). Apoptosis and necrosis were quantified by using Apoptosis and Necrosis Quantification Kit (Biotum, Fremont, CA) according to the manufacturer's protocol, as published (136). Sugen treatment stimulated apoptosis (Early and Late) but not necrosis in HPAEC. (B) Media from untreated HPAEC, HPAEC with apoptosis induced as described in (A) and necrosis induced by three to four cycles of cell freezing and thawing, as published (137) were used for Western blot analysis as previously described (137) to measure reduced and oxidized forms of HMGB1. Necrotic but not apoptotic cells produced a marked extracellular HMGB1 signal and showed an accumulation of the reduced form of HMGB1 in cell culture media. The difference in the total protein in the media related to the difference in FBS amount used in the experiment—negative control (media that were not used for cell culturing) and apoptotic media contain 0.5% FBS, untreated and necrotic media—5% FBS. The difference in total media proteins does not correlate with the HMGB1 signal that is absent in the negative control, and the media collected from untreated cells show a light signal from oxidized HMGB1 in the apoptotic media and a strong but mostly reduced signal in the necrotic media. (C) Same media samples collected for (B) were used to measure plasma redox homeostasis by analyzing ORP and total antioxidant capacity (Cap) electrochemically by using RedoxSys® Diagnostic System, as reported (137). Apoptotic media showed a significant increase in oxidative potential and a decrease in antioxidant capacity, indicating the development of oxidative stress in apoptotic cells and the release of oxidized cellular content. Necrotic media show a significant reduction in ORP signal, suggesting that necrosis produces release cellular components in a reduced state. N = 4; *p < 0.05 versus untreated; #p < 0.05 versus apoptosis. FBS, fetal bovine serum; HMGB1, high mobility group box 1; HPAEC, human pulmonary artery endothelial cell; ORP, oxidation–reduction potential. Color images are available online.
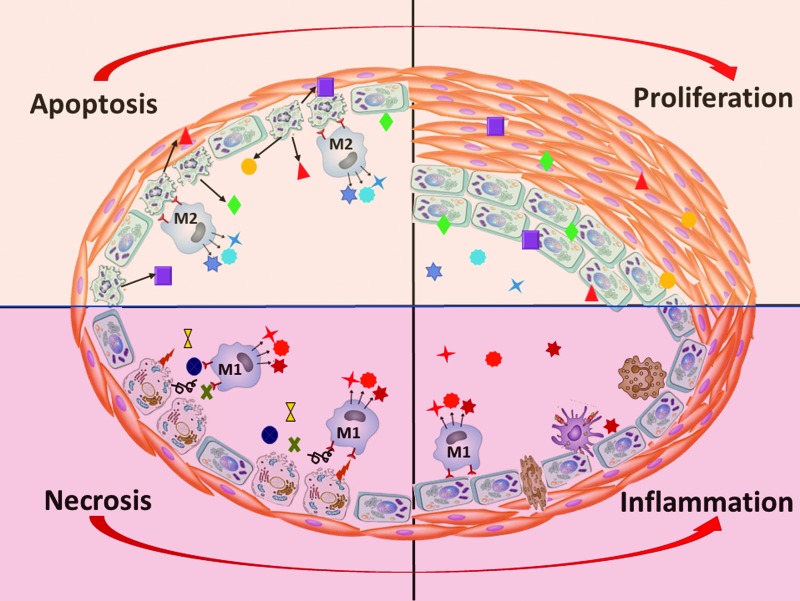
It is also well established that once macrophages engulf the apoptotic cell, they resemble an alternative mode of activation and transform into the anti-inflammatory M2 phenotype macrophages, in contrast to the classical, proinflammatory M1 phenotype (193). Such M2 macrophages produce a spectrum of anti-inflammatory mediators including TGF-β, IL-10, and prostaglandins. Besides, the uptake of apoptotic cells by already activated M1 macrophages will suppress the production of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, IL-8, and IL-12 (34, 55, 198). Thus, apoptosis could not only prevent the development of the inflammatory response but also cease any ongoing inflammation. Indeed, it was noticed that the addition of macrophages that ingest apoptotic cells into lipopolysaccharide-induced inflammatory cells reduces inflammatory pathways (52). Aside from anti-inflammatory activity, M2 macrophages also play an important role in tissue repair by secreting the ECM components, as well as angiogenic and chemotactic factors (151), and, thus, could directly contribute to vascular remodeling.
Despite the anti-inflammatory nature of apoptosis, PAH is associated with severe inflammatory changes in the pulmonary vascular wall (131, 135). Oddly, this activation of the innate and adaptive immune system in PAH is known to be directly connected to pulmonary vascular damage, which produces an apparent paradox. However, it is important to consider that apoptosis is not the only outcome for the damaged cell. The severe cell damage in PAH could also trigger necroptosis or necrosis. Surprisingly, the contribution of these types of cell death in the pathogenesis of PAH is almost unstudied. Both necroptotic and necrotic cells are the source of many DAMPs, known activators of the inflammatory response. Indeed, it was reported that the HMGB1 released into the extracellular space during the early stage of MCT-induced PAH contributes to PAH development (153, 200). Our group has also confirmed an accumulation of extranuclear HMGB1 in the pulmonary artery wall of male rats with PAH induced by SU/hypoxia (137). Apoptotic cells do not release HMGB1 even after they undergo secondary necrosis because of the hypoacetylation of one or more of the chromatin components that occurs during apoptosis and retains HMGB1 firmly bound to chromatin (158). Therefore, since the release of HMGB1 distinguishes the necrotic cells from apoptotic ones, it could be expected that MCT or SU/hypoxia induced PAH is associated with necrotic cell death. This necrosis is capable of initiating inflammatory signaling in pulmonary arteries through activation of TLR4/IL1β/E-Selectin axis (137) that activates ECs and increases inflammatory cell recruitment.
Activated monocytes and macrophages, in turn, are other cell types that could produce HMGB1, although not due to the passive release, but due to active secretion. This process requires HMGB1 hyperacetylation and, thus, is opposite to the hypoacetylation and nuclear retention that happens in apoptotic cells. However, the proinflammatory activation of phagocytes that should precede any cytokine production could not happen if the surrounding vascular cells died by apoptosis. In contrast, HMGB1 released from necrotic cells is an active facilitator of macrophage reprogramming toward the M1-like phenotype, whereas its neutralization reduces M1 macrophage infiltration (177). Therefore, necrosis, rather than apoptosis, appears to be responsible for the initiation of proinflammatory mechanisms (Fig. 5). By skewing macrophage differentiation toward M1-like phenotypes, HMGB1 also diminishes the amount of M2 macrophages and disturbs the normal clearance of apoptotic cells (159). Therefore, once released into the extracellular milieu, HMGB1 shifts the normally maintained balance between pro- and anti-inflammatory events toward inflammation, which, in turn, becomes self-perpetuating (204).
FIG. 5.
Apoptosis of vascular cells stimulates anti-inflammatory M2 macrophages, which release proliferative factors for damage resolution and activate vascular remodeling. In contrast, necrotic damage of vascular cells stimulates M1 macrophages with proinflammatory properties, increasing perivascular inflammation, vascular permeability, and fibrosis. Color images are available online.
There are many other DAMPs produced by dying cells that have nucleic, cytosolic, or mitochondrial origin and could either participate in tissue healing through stimulation of prosurvival/proliferative pathways or induce inflammation (19, 30, 47, 100, 111, 120, 133, 147, 205). The particular contributions of all these factors are yet to be established. However, some of them have been already reported as promising biomarkers associated with PAH (74, 111, 137, 145). It, therefore, follows that further validation of such biomarkers that could be routinely screened, at least in the PAH patient's family members or in high-risk patients, could help to identify this deadly disease at the early, curable stage.
Oxidative/Nitrative Stress
ROS are a family of oxygen-based highly chemically active species that include the parent oxygen free-radical superoxide (O2−•), its direct dismutation product hydrogen peroxide (H2O2), hydroxyl radical (OH−•), hypochlorite (OCl−), and hydroperoxyl radical (HO2•) (3a). Cellular sources of ROS include the NADPH oxidase (Nox) family of enzymes, mitochondrial electron transport chain complexes (mainly I and III), xanthine oxidoreductase (XO), cytochrome P450, lipid oxygenases including cyclooxygenases and lipoxygenases, peroxidases, and uncoupled NOSs (3a, 10, 20, 189, 197). Nox proteins are considered the only “professional” ROS generators in that ROS are their main product rather than a byproduct of their chemical reactivity (3a). Nox1, 2, 4, and 5 are expressed in the lung vasculature (3a, 10, 48). To counterbalance the sources of ROS in the cell, a number of endogenous antioxidant systems exist. These include superoxide dismutases (SODs), which convert O2−• to H2O2, catalase, which converts H2O2 to water, glutathione peroxidase, heme oxygenase, peroxiredoxins, glutaredoxin, and thioredoxin (41). Nonenzymatic antioxidant systems also contribute to the balance and include vitamins C and E, retinol, glutathione, and β-carotene (41). Similar to ROS, reactive nitrogen species (RNS) are chemical moieties derived from the free radical NO and include nitrogen dioxide (NO2), dinitrogen trioxide (N2O3), nitroxyl anion (NO−), and nitrosonium cation (NO+) (44, 181). The major enzymatic source of NO is the NOS enzymes, of which there are three isoforms: neuronal (nNOS or NOSI), inducible (iNOS or NOSII), and endothelial (eNOS or NOSIII) (44, 181). NO can also be produced through the reduction of nitrite by several nitrite reductases (81). NO can react with protein cysteine residues, heme iron in iron-containing proteins, and with lipids. Importantly, NO can also react with O2−• to produce the highly reactive peroxynitrite (ONOO−), which can lead to protein nitration (tyrosine residues modification to nitrotyrosine) (181).
ROS, RNS, and their sources (e.g., Nox enzymes for ROS) have been implicated in PH and associated RV responses in both in vivo and in vitro studies, and there is mounting evidence that ROS levels are increased in PH patients (16, 38, 40, 42, 44, 57, 181). For example, systemic and pulmonary vascular EC, VSMCs, and adventitial fibroblasts express Nox1, 2, and 4 and these enzymes have all been linked to PH in multiple studies (9, 22, 26, 32, 38, 41, 42, 48, 56, 57, 61, 71). ROS scavenging molecules glutathione and vitamin E were observed to be low in PH patient plasma, whereas lipid peroxidation products such as malondialdehyde and lipid hydroperoxide were elevated (148). Levels of nitrotyrosine, 5-oxo-eicosatetraenoic acid, and hydroxyeicosatetraenoic acid were also elevated (16, 144). A role of NO and XO in PH has been shown in vivo and in pulmonary VSMCs (211). A global effect of ROS/RNS is the decrease in NO bioavailability through chemical scavenging, similar to what occurs when ONOO− is formed. This contributes to increased vasoconstriction and impaired vasorelaxation, and thereby to the development of PH (142, 181). NO is a major vasodilator and regulator of the basal vasomotor tone. Interruptions in NO signaling or its levels can lead to impaired vasorelaxation and are associated with PH (44, 81, 82, 181). Moreover, sources of ROS such as Nox enzymes and XO can interrupt NO production and signaling, further contributing to the decreased NO bioavailability (181). Indeed, it was demonstrated that the use of nitrite as an NO source has been associated with an attenuation of experimental PH (211). ROS/RNS have also been associated with increasing levels and signaling of the potent pulmonary vasoconstrictor endothelin 1, which is the main factor in PH development, further contributing to an overall imbalance in motor tone and PH (181).
ROS and RNS also have well-documented effects on cellular and molecular changes that promote vascular cell proliferation, migration, hypertrophy, and apoptosis, all processes linked to PH development (3a, 9, 10, 44, 57, 141, 144, 181), and have been implicated in promoting endothelial, smooth muscle, and fibroblast proliferation (9, 41, 48, 141). Interruption of Nox1 was associated with attenuation of pulmonary arterial EC proliferation, and targeting of Nox4 was linked to a reduction in pulmonary artery smooth muscle and fibroblast proliferation (9) as well as with increased fibroblast apoptosis. Both isoforms were upregulated in PH patient lung tissue (48, 97, 115). The Nox4 effect appears to be associated with activation of mTOR complex 2, mTORC2, and AMP-activated protein kinase (50). In models of persistent PH of newborn targeting ROS by the pharmacologic inhibitors, apocynin and N-acetyl-cysteine, was associated with improvement in angiogenesis in ECs (186). Generally, in addition to direct oxidation and free-radical damage of DNA, proteins, and lipids, ROS such as H2O2 affect key signaling pathways and transcription factors that drive the cellular pathophysiologic processes associated with PH and pulmonary vascular remodeling (141). These include the MAPKs pathways p38 MAPK and Erk1/2, the c-Jun N-terminal kinase pathway, the Akt/PKB pathway, the NF-κB transcription factor, p53, and AP1 (3a, 10, 44, 145, 181). Also, the cAMP-response element binding transcription factor, which is selectively activated in the lungs of hypoxic PAH mice and the hypoxic human lung microvascular EC (92), was implicated as an ROS-inducible transcription factor (157, 164, 165). Other examples include a role for Nox1 in driving pulmonary arterial EC proliferation via upregulation of sonic hedgehog-mediated expression of the bone morphogenetic protein (BMP)-signaling antagonist Gremlin1 (18, 48, 66, 212). BMP receptor and signaling impairments are pervasive in hereditary forms of PAH and are also implicated in a subset of idiopathic PAH (68, 152). In addition, Gremlin1 has been linked to HIF2α and the vascular smooth muscle proliferation, migration, and angiogenesis (18, 80a, 104, 170), further supporting a role for ROS in these processes and PH development. The link between ROS sources and cellular growth pathways is strengthened by studies that show a reduction in the hypoxia-induced pulmonary vascular endothelial and smooth muscle proliferation by Nox inhibitory drugs and a link of this reduction to reduction in TGFβ and rescue of peroxisome proliferator-activated receptor γ (54). Moreover, Nox1-mediated proliferation was linked to modulations of SOD2, Nrf2, cyclin D1, and cofilin in pulmonary arterial SMCs (191). Upstream, caveolin-1 (Cav-1) was associated with the inhibition of Nox enzymes through the modulation of NF-κB and the reduced expression of Cav-1 observed in PH models; this was linked to increased Nox activity and ROS elevation (22). Also upstream, evidence implicates TGFβ, which is a potent mediator of cellular phenotypes in PH, in upregulation of Nox, specifically Nox4, via activation of insulin-like growth factor binding protein 3 and Akt/PKB in pulmonary arterial SMCs (69).
ROS have been shown to be involved in both acute and chronic regulation of hypoxic pulmonary vasoconstriction, including associated processes such as vascular cell proliferation and medial hypertrophy, in part through modulation of calcium and potassium channels; however, the precise mechanisms remain elusive (41, 119, 162, 180, 190). For example, in pulmonary arterial SMCs from Nox1 null animals, there was a decrease in the Kv1.5 voltage-dependent potassium channel, an increase in intracellular potassium levels, and an associated reduction in apoptotic cells (71). On the other hand, interruption of ROS and Nox4 was shown to lift hypoxia-induced decreases in the potassium current via direct association with Kv1.5 (114). These discrepancies allude to the complexity of these processes but do support a clear link between ROS and potassium currents in PH. Genetic deletion of Nox2 or administration of SOD also reduced vasoconstriction in hypoxic mice, supporting the role of ROS and further supporting a potential interplay with the reduction in NO bioavailability as discussed earlier (99). Further, the mechanism by which ROS affects intracellular calcium and calcium channels, namely the Cav1.2 channel, may involve downregulation of the glycolytic pyruvate kinase M2 (59) and involve contributions from PKC signaling (146). One mechanism by which ROS carry out their second-messenger effects involves shifting the balance toward activation between the kinases and phosphatases of these major pathways by oxidizing and turning off phosphatases. This mostly occurs through oxidation of key cysteine residues of effector proteins. Also, the RNS ONOO− can induce direct protein tyrosine nitration, which inhibits and stimulates degradation of important enzymes involved in vasorelaxation, such as eNOS and protein kinase G (2, 181). Tyrosine nitration can also mimic phosphorylation and induce activation of Akt in ECs (138). Nitration of Akt increases as early as 1 week from SU/hypoxia model induction (Varghese VM, Niihori M, Eccles CA, Kurdyukov S, James J, Rafikova O, Rafikov R.). Nitrite treatment has been shown to effectively increase NO bioavailability and reduce manifestations of PH both in vivo and in vitro through a mechanism that involves inhibition of smooth muscle proliferation and upregulation of the cyclin-dependent kinase inhibitor p21/cip (211).
It can, therefore, be seen that ROS/RNS plays important roles in the development and progression of PH and PH-driving processes. They pose as viable therapeutic targets for future development. Indeed, some evidence supports this. For example, administration of recombinant SOD and SOD mimetics, eNOS inhibitor l-NG-nitroarginine methyl ester, Nox inhibitors, and ROS scavengers have all been shown to reduce PH in animal models (9, 27, 44, 54, 181). Future modalities aimed at tissue-specific inhibition of ROS or ROS sources could prove valuable additional add-on therapies to existing drugs that could protect from the vascular remodeling consequences of the disease.
Early Diagnosis/Biomarkers of PAH
Initial diagnosis of PAH in patients is still problematic as patients experienced a very broad range of symptoms, and even at the earliest stage, PAH onset is asymptomatic (89, 90). The earliest symptom of PH, the dyspnea on exercise, is also a common symptom of asthma, chronic obstructive pulmonary disease, myocardial infarction, and pneumonia, among others. However, it is important to improve the early detection of PAH because functional class (FC) 1 and 2 patients with PAH have better survival rates than patients diagnosed in more severe conditions (67, 188). It can be argued that there is tremendous benefit in patients with risk factors such as familial PAH, sickle cell disease, thalassemia, glucose-6-phosphate dehydrogenase deficiency(87), portal hypertension, congenital heart disease, HIV infection, acute pulmonary embolism, and connective tissue disease(79), to undergo preventive assessment by echocardiography even in the absence of symptoms of PAH.
However, the accuracy of noninvasive echocardiography is not perfect, especially in mild conditions, and the gold standard of PAH diagnosis remains to be right heart catheterization (RHC). Nonetheless, RHC is an invasive procedure and monitoring even predisposed patients every year may be difficult and even unrealistic from a clinical perspective. Therefore, the importance of concerted efforts for the discovery of early biomarker “fingerprints” of PAH development at an early, asymptomatic phase becomes glaringly evident. The closest resemblance of a biomarker for PAH that exists today is the plasma levels of the probrain natriuretic peptide (pro-BNP). This is potentially a very useful, minimally invasive approach to assessing PAH that only requires plasma collection from patients (5). pro-BNP is a peptide that is released from heart tissue that is stressed by the extra load, similar to what is experienced by the right heart during PAH as a result of the rise in pulmonary vascular resistance and pressure. However, this approach presents important limitations. First, it can be a nonspecific marker of ventricular stress, be that from the right or left ventricles of the heart. Second, and more importantly, because an increase in pulmonary pressure is needed to induce RV stress, this biomarker cannot be used to assess early disease development that precedes pressure elevation.
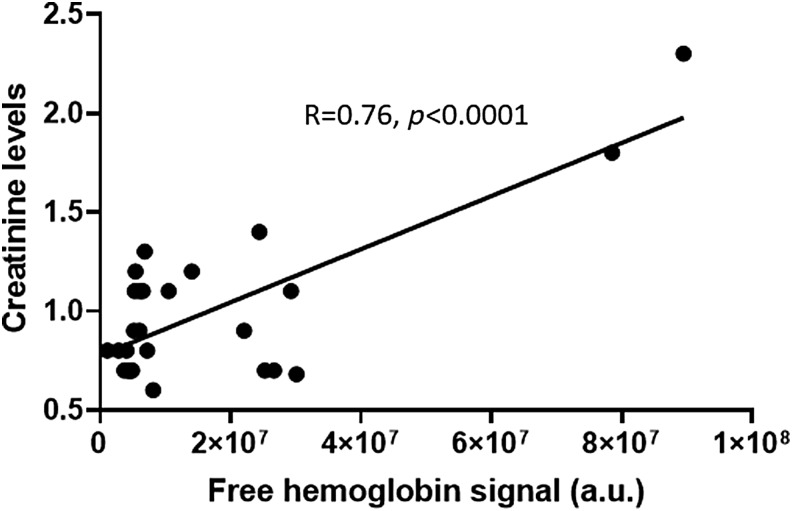
As discussed earlier, several conditions that initiate disease development could be used to assess early PAH. For example, several reports showed that metabolic changes in the lungs undergo significant disturbances. The importance of metabolic biomarkers in PAH is widely discussed. We found that even before hemodynamic changes occur in the pulmonary vasculature or right heart in the MCT model of PAH, more than 500 metabolites were significantly altered (140). Those metabolic changes could be used to generate the needed “fingerprint” of PAH for metabolic analysis. With the focus on the changes characteristic to PAH, these “fingerprints” could differentiate between other lung or heart diseases. PAH is accompanied with the glycolytic switch in metabolism, and indeed, our data indicate a more than 10-fold upregulation of glucose associated with the disease progression. Although a promising approach, the fact that these changes were assessed directly in lung tissue makes it infeasible due to the invasive nature of the needed procedures to collect lung biopsies. Building on these findings by examination of blood plasma for similar and specific metabolite changes, however, would be a favorable and feasible approach. Also, inflammatory markers, such as cytokines and DAMPs, could be used for early detection of PAH. The underlying hemolysis in PAH patients, which contributes to the inflammatory response can be assessed in plasma (111, 145). Therefore, erythroid-derived DAMPs may be promising targets for early detection. Indeed, signals from the free hemoglobin in plasma followed PAH progression, as demonstrated by a significant correlation with mean pulmonary arterial pressure (mPAP), pulmonary vascular resistance, and cardiac index. Free hemoglobin may not be an ideal reporter of hemolysis in PAH as it has a specific haptoglobin-mediated sequestering system; therefore, at the early phases of disease, it could be controlled by the detoxification system. Therefore, other markers of hemolysis may be utilized. Interestingly, an increase in plasma creatinine levels in PAH patients is common and correlates with disease progression; this is partially attributable to the worsening of renal function. Creatinine could also be found at a high level in RBCs and reticulocytes; thus, the release of creatinine from damaged RBCs could indicate ongoing hemolysis. Indeed, our data indicate that in PAH patients (N = 30) creatinine levels are strongly (p < 0.0001) correlated with free hemoglobin levels (Fig. 6). Two samples with increased free hemoglobin are probably producing the effect on correlation; thus, this required future studies with an analysis of the large cohort of patients.
FIG. 6.
Plasma creatinine levels in PAH patients (N = 30) correlate significantly (p < 0.0001) with the level of hemolysis. Free hemoglobin levels were measured as recently published (144).
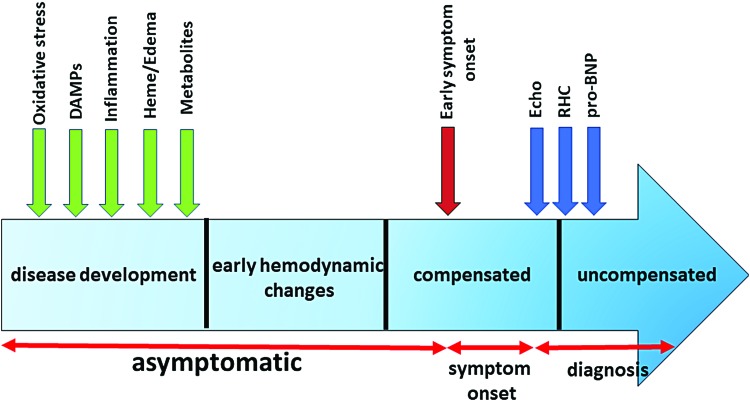
It was also found that RBCs size distribution width (RDW) is an important biomarker of PAH progression and outcome (3, 168). RDW predicted survival of PAH patients independently of the pro-BNP and the 6-minute walk distance. Interestingly, increased RDW is common for iron deficit anemias and blood loss conditions, again pointing to the role of hemolysis in PAH. Other plasma biomarkers that we tested in the study that are related to the inflammatory response are IL-6 and the growth differentiation factor 15 (150). Both biomarkers demonstrated a significant correlation with hemodynamics and survival estimates. Therefore, early diagnosis of PAH could be based on circulating biomarkers that reflect the characteristic “blueprint” of PAH based on oxidative stress(117), inflammatory/DAMPs, hemolysis, and metabolic reprogramming markers (Fig. 7). Although the identification and initial validation of the promising biomarkers have to be done in a preclinical animal model, they could be further validated in the patients with exercise-induced PAH or patients with a mild borderline PH (mPAP 20–25 mmHg) that represent patient groups with early disease(84, 163). Although still uncommon compared with patients with more advanced PAH, such early cases are occasionally diagnosed, and their frequency has been increasing in the recent years.
FIG. 7.
The markers of early pathological events related to oxidative stress, vascular damage, inflammation, hemolysis, and metabolic reprogramming could lead to the earlier detection of PAH. The particular sequence of the events shown is approximate, as they could vary in different patients and simultaneously co-occur, thus, cross-amplifying each other. However, multiple reports suggested preceding the symptom onset. Therefore, identification of specific biomarkers or their combination could not only decrease the time between symptom onset and diagnosis based on echocardiography (Echo), RHC, and circulating pro-BNP but also identify patients at risk even during the asymptomatic phase of PAH. DAMPs, damage-associated molecular patterns; pro-BNP, probrain natriuretic peptide; RHC, right heart catheterization. Color images are available online.
Conclusion
The recent advances make PH more treatable than it was earlier. However, the early diagnosis of PH still remains the biggest challenge. The nonspecific symptoms of PH significantly delay its diagnosis. Currently, the time to diagnosis (TTD) was reported as being close to 4 years (176). On average, it takes a patient about 12 months after the initial symptom onset to initiate the first medical contact, five general practitioner visits, and three specialist visits before being referred to a PH specialist and before undergoing RV catheterization. During this period, the disease progresses from FC II (95%)/FC III (5%) at the time of symptom onset to FC II (5%)/FC III (90%)/FC IV (5%) at diagnosis (176). At the same time, a few national registry studies confirmed that FC I or II patients have significantly better long-term survival than patients with FC III/IV (67). Therefore, an approach that would allow decreasing TTD would help to change the distribution between early and advanced PAH and toward less progressive and more responsive PAH therapy. A further shift toward diagnosing PH at an asymptomatic stage could be expected to significantly improve the survival and prognosis for this deadly disease. The accumulated body of literature strongly supports the idea that such early diagnosis is highly possible. PH patients undergo significant changes in metabolism, redox status, chronic unrecognized hemolysis, cellular damage, and activation of inflammatory pathways that precede PH manifestation. All these early pathological events can be used to establish a combination of biomarkers that could serve as a blueprint of the early stage of PH and could be used for the prescreening of PH patients. Therefore, we believe that the effort of the research community has to be shifted from an attempt to treat the advanced stages of PH, which is hardly achievable due to nonreversible changes of a failing heart, toward getting a better understanding about mechanisms involved in PH initiation and early progression. Such an approach would not only advance our current knowledge regarding the early pathogenic events but also significantly advance PH diagnostics, increase therapy effectiveness, and improve patient survival.
Acknowledgments
This work was supported by NIH grants R01HL133085 (O.R.), R01HL132918 (R.R.), AHA grant 15SDG24910003, ALA grant RG-515656, and Gilead grant 0059493 (I.A.G.).
Abbreviations Used
- AA
antimycin A
- Akt
RAC-alpha serine/threonine-protein kinase
- BMP
bone morphogenetic protein
- BMPR2
bone morphogenetic protein receptor type 2
- Cav-1
caveolin-1
- Cx43
connexin43
- DAMP
damage-associated molecular pattern
- DCA
dichloroacetate
- EC
endothelial cell
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- FC
functional class
- FKN
fractalkine
- H2O2
hydrogen peroxide
- HIF
hypoxia-inducible factor
- HMGB1
high mobility group box 1
- HPAEC
human pulmonary artery endothelial cell
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MCT
monocrotaline
- mPAP
mean pulmonary arterial pressure
- mTOR
mammalian target of rapamycin
- NEDD9
developmentally downregulated protein 9
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- O2−•
superoxide
- ONOO−
peroxynitrite
- PAH
pulmonary arterial hypertension
- PAMP
pathogen-associated molecular pattern
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- PH
pulmonary hypertension
- PKC
protein kinase C
- pro-BNP
probrain natriuretic peptide
- PRR
pattern recognition receptor
- RBC
red blood cell
- RDW
RBCs size distribution width
- RHC
right heart catheterization
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RV
right ventricle
- SMAD3
mothers against decapentaplegic homolog 3
- SMCs
smooth muscle cells
- SOD
superoxide dismutase
- SU
Sugen SU5614
- TCA
tricarboxylic acid
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TTD
time to diagnosis
- VEGF
vascular endothelial growth factor
- VSMCs
vascular smooth muscle cells
- XO
xanthine oxidoreductase
References
- 1. Abu El-Asrar AM, Alam K, Garcia-Ramirez M, Ahmad A, Siddiquei MM, Mohammad G, Mousa A, De Hertogh G, Opdenakker G, and Simo R. Association of HMGB1 with oxidative stress markers and regulators in PDR. Mol Vis 23: 853–871, 2017 [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal S, Gross CM, Rafikov R, Kumar S, Fineman JR, Ludewig B, Jonigk D, and Black SM. Nitration of tyrosine 247 inhibits protein kinase G-1alpha activity by attenuating cyclic guanosine monophosphate binding. J Biol Chem 289: 7948–7961, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akkermans MD, Uijterschout L, Vloemans J, Teunisse PP, Hudig F, Bubbers S, Verbruggen S, Veldhorst M, de Leeuw TG, van Goudoever JB, and Brus F. Red blood cell distribution width and the platelet count in iron-deficient children aged 0.5–3 years. Pediatr Hematol Oncol 32: 624–632, 2015 [DOI] [PubMed] [Google Scholar]
- 3a. Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, and Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Husseini A, Wijesinghe DS, Farkas L, Kraskauskas D, Drake JI, Van Tassel B, Abbate A, Chalfant CE, and Voelkel NF. Increased eicosanoid levels in the Sugen/chronic hypoxia model of severe pulmonary hypertension. PLoS One 10: e0120157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al-Naamani N, Palevsky HI, Lederer DJ, Horn EM, Mathai SC, Roberts KE, Tracy RP, Hassoun PM, Girgis RE, Shimbo D, Post WS, Kawut SM, and ASA-STAT Group. Prognostic significance of biomarkers in pulmonary arterial hypertension. Ann Am Thorac Soc 13: 25–30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. This reference has been deleted.
- 7. Archer SL. Pyruvate kinase and warburg metabolism in pulmonary arterial hypertension: uncoupled glycolysis and the cancer-like phenotype of pulmonary arterial hypertension. Circulation 136: 2486–2490, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azzam EI, de Toledo SM, and Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A 98: 473–478, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, and Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 34: 1704–1715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 11. BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, and Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem 385: 249–257, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J, 3rd, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, and Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bianchi ME. and Manfredi A. Chromatin and cell death. Biochim Biophys Acta 1677: 181–186, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Birge RB. and Ucker DS. Innate apoptotic immunity: the calming touch of death. Cell Death Differ 15: 1096–1102, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Blum JI, Bijli KM, Murphy TC, Kleinhenz JM, and Hart CM. Time-dependent PPARgamma modulation of HIF-1alpha signaling in hypoxic pulmonary artery smooth muscle cells. Am J Med Sci 352: 71–79, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, and Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 169: 764–769, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Breitling S, Hui Z, Zabini D, Hu Y, Hoffmann J, Goldenberg NM, Tabuchi A, Buelow R, Dos Santos C, and Kuebler WM. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L710–L721, 2017 [DOI] [PubMed] [Google Scholar]
- 18. Cahill E, Costello CM, Rowan SC, Harkin S, Howell K, Leonard MO, Southwood M, Cummins EP, Fitzpatrick SF, Taylor CT, Morrell NW, Martin F, and McLoughlin P. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 125: 920–930, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Camp SM, Ceco E, Evenoski CL, Danilov SM, Zhou T, Chiang ET, Moreno-Vinasco L, Mapes B, Zhao J, Gursoy G, Brown ME, Adyshev DM, Siddiqui SS, Quijada H, Sammani S, Letsiou E, Saadat L, Yousef M, Wang T, Liang J, and Garcia JG. Unique toll-like receptor 4 activation by NAMPT/PBEF induces NFkappaB signaling and inflammatory lung injury. Sci Rep 5: 13135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chalupsky K. and Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 102: 9056–9061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan SY. and Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44: 14–30, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen F, Barman S, Yu Y, Haigh S, Wang Y, Black SM, Rafikov R, Dou H, Bagi Z, Han W, Su Y, and Fulton DJ. Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 73: 201–213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cojocaru M, Cojocaru IM, Silosi I, and Vrabie CD. Associated pulmonary arterial hypertension in connective tissue diseases. Maedica (Buchar) 6: 141–145, 2011 [PMC free article] [PubMed] [Google Scholar]
- 24. Coll-Bonfill N, Peinado VI, Pisano MV, Parrizas M, Blanco I, Evers M, Engelmann JC, Garcia-Lucio J, Tura-Ceide O, Meister G, Barbera JA, and Musri MM. Slug is increased in vascular remodeling and induces a smooth muscle cell proliferative phenotype. PLoS One 11: e0159460, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coudert J, Antezana G, and Bedu M. [Acute altitude-induced edema of the lung]. Rev Pneumol Clin 41: 264–272, 1985. (Article in French) [PubMed] [Google Scholar]
- 26. Csanyi G, Taylor WR, and Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47: 1254–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, and Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 54: 668–675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Culley MK. and Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest 128: 3704–3715, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Raaf MA, Schalij I, Gomez-Arroyo J, Rol N, Happe C, de Man FS, Vonk-Noordegraaf A, Westerhof N, Voelkel NF, and Bogaard HJ. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J 44: 160–168, 2014 [DOI] [PubMed] [Google Scholar]
- 30. Dela Cruz CS and Kang MJ.. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 41: 37–44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, Rabinovitch M, and Maclean MR. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res 12: 159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, and Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diwanji N. and Bergmann A. An unexpected friend—ROS in apoptosis-induced compensatory proliferation: implications for regeneration and cancer. Semin Cell Dev Biol 80: 74–82, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, and Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, and Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 119: 1298–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, and West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ 2: 201–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, Zhen L, Mao T, Richter A, Erzurum SC, and Tuder RM. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol 176: 1130–1138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, and Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fogarty CE. and Bergmann A. The sound of silence: signaling by apoptotic cells. Curr Top Dev Biol 114: 241–265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frazziano G, Al Ghouleh I, Baust J, Shiva S, Champion HC, and Pagano PJ. Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4. Am J Physiol Heart Circ Physiol 306: H197–H205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frazziano G, Champion HC, and Pagano PJ. NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. Am J Physiol Heart Circ Physiol 302: H2166–H2177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fresquet F, Pourageaud F, Leblais V, Brandes RP, Savineau JP, Marthan R, and Muller B. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol 148: 714–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, and Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 280: L39–L49, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Fulton DJR, Li X, Bordan Z, Haigh S, Bentley A, Chen F, and Barman SA. Reactive oxygen and nitrogen species in the development of pulmonary hypertension. Antioxidants (Basel) 6: pii:, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Furuya Y, Satoh T, and Kuwana M. Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int J Rheumatol 2010: 720305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garg L, Akbar G, Agrawal S, Agarwal M, Khaddour L, Handa R, Garg A, Shah M, Patel B, and Dalal BD. Drug-induced pulmonary arterial hypertension: a review. Heart Fail Rev 22: 289–297, 2017 [DOI] [PubMed] [Google Scholar]
- 47. Garten A, Petzold S, Schuster S, Korner A, Kratzsch J, and Kiess W. Nampt and its potential role in inflammation and type 2 diabetes. Handb Exp Pharmacol 147–164, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Ghouleh IA, Sahoo S, Meijles DN, Amaral JH, de Jesus DS, Sembrat J, Rojas M, Goncharov DA, Goncharova EA, and Pagano PJ. Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation. Clin Sci (Lond) 131: 2019–2035, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, and Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 18: 1716–1718, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, and Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129: 864–874, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goncharova EA. mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects. FASEB J 27: 1796–1807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gordon S. and Pluddemann A. Macrophage clearance of apoptotic cells: a critical assessment. Front Immunol 9: 127, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, and Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ Res 89: 47–54, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gregory CD. and Pound JD. Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol 223: 177–194, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart 90: 491–493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, and Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grunig E, Maier F, Ehlken N, Fischer C, Lichtblau M, Blank N, Fiehn C, Stockl F, Prange F, Staehler G, Reichenberger F, Tiede H, Halank M, Seyfarth HJ, Wagner S, and Nagel C. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Res Ther 14: R148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo D, Gu J, Jiang H, Ahmed A, Zhang Z, and Gu Y. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to the development of pulmonary arterial hypertension. J Mol Cell Cardiol 91: 179–187, 2016 [DOI] [PubMed] [Google Scholar]
- 60. Hameed AG, Arnold ND, Chamberlain J, Pickworth JA, Paiva C, Dawson S, Cross S, Long L, Zhao L, Morrell NW, Crossman DC, Newman CM, Kiely DG, Francis SE, and Lawrie A. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med 209: 1919–1935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harijith A, Natarajan V, and Fu P. The role of nicotinamide adenine dinucleotide phosphate oxidases in lung architecture remodeling. Antioxidants (Basel) 6: pii:, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, and Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 63. He X, Song S, Ayon RJ, Balisterieri A, Black SM, Makino A, Wier WG, Zang WJ, and Yuan JX. Hypoxia selectively upregulates cation channels and increases cytosolic [Ca(2+)] in pulmonary, but not coronary, arterial smooth muscle cells. Am J Physiol Cell Physiol 314: C504–C517, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hinz B. The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol 47: 54–65, 2015 [DOI] [PubMed] [Google Scholar]
- 65. Houssaini A. and Adnot S. mTOR: a key to both pulmonary vessel remodeling and right ventricular function in pulmonary arterial hypertension? Am J Respir Cell Mol Biol 57: 509–511, 2017 [DOI] [PubMed] [Google Scholar]
- 66. Hsu DR, Economides AN, Wang X, Eimon PM, and Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1: 673–683, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Humbert M, Gerry Coghlan J, and Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev 21: 306–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. International PPHC, Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, and Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 26: 81–84, 2000 [DOI] [PubMed] [Google Scholar]
- 69. Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, and Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Itoh T, Nagaya N, Ishibashi-Ueda H, Kyotani S, Oya H, Sakamaki F, Kimura H, and Nakanishi N. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 11: 158–163, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Iwata K, Ikami K, Matsuno K, Yamashita T, Shiba D, Ibi M, Matsumoto M, Katsuyama M, Cui W, Zhang J, Zhu K, Takei N, Kokai Y, Ohneda O, Yokoyama T, and Yabe-Nishimura C. Deficiency of NOX1/nicotinamide adenine dinucleotide phosphate, reduced form oxidase leads to pulmonary vascular remodeling. Arterioscler Thromb Vasc Biol 34: 110–119, 2014 [DOI] [PubMed] [Google Scholar]
- 72. Izquierdo-Garcia JL, Arias T, Rojas Y, Garcia-Ruiz V, Santos A, Martin-Puig S, and Ruiz-Cabello J. Metabolic reprogramming in the heart and lung in a murine model of pulmonary arterial hypertension. Front Cardiovasc Med 5: 110, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jones JE, Walker JL, Song Y, Weiss N, Cardoso WV, Tuder RM, Loscalzo J, and Zhang YY. Effect of 5-lipoxygenase on the development of pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 286: H1775–H1784, 2004 [DOI] [PubMed] [Google Scholar]
- 74. Joshi K, Anjum F, Gowda S, Damania D, Graham-Hill S, Gillette P, Zein J, Jamaleddine G, Demetis S, and Wadgaonkar R. Uric Acid as a potential biomarker of pulmonary arterial hypertension in patients with sickle cell disease. Indian J Hematol Blood Transfus 27: 96–100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jung C, Grun K, Betge S, Pernow J, Kelm M, Muessig J, Masyuk M, Kuethe F, Ndongson-Dongmo B, Bauer R, Lauten A, Schulze PC, Berndt A, and Franz M. Arginase inhibition reverses monocrotaline-induced pulmonary hypertension. Int J Mol Sci 18: 1609, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kanmogne GD, Primeaux C, and Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun 333: 1107–1115, 2005 [DOI] [PubMed] [Google Scholar]
- 77. Kato GJ, Steinberg MH, and Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127: 750–760, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, and Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29: 21–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Khanna D. and McLaughlin V. Screening and early detection of pulmonary arterial hypertension in connective tissue diseases. it is time to institute it! Am J Respir Crit Care Med 192: 1032–1033, 2015 [DOI] [PubMed] [Google Scholar]
- 80. Kietzmann T. and Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol 16: 474–486, 2005 [DOI] [PubMed] [Google Scholar]
- 80a. Kim M, Yoon S, Lee S, Ha SA, Kim HK, Kim JW, and Chung J. Gremlin-1 induces BMP-independent tumor cell proliferation, migration, and invasion. PLoS One 7: e35100, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim-Shapiro DB. and Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide 38: 58–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim-Shapiro DB. and Gladwin MT. Pitfalls in measuring NO bioavailability using NOx. Nitric Oxide 44: 1–2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. This reference has been deleted.
- 84. Kovacs G, Maier R, Aberer E, Brodmann M, Graninger W, Kqiku X, Scheidl S, Troster N, Hesse C, Rubin L, and Olschewski H. Pulmonary arterial hypertension therapy may be safe and effective in patients with systemic sclerosis and borderline pulmonary artery pressure. Arthritis Rheum 64: 1257–1262, 2012 [DOI] [PubMed] [Google Scholar]
- 85. Krutovskikh VA, Piccoli C, and Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene 21: 1989–1999, 2002 [DOI] [PubMed] [Google Scholar]
- 86. Kudryashova TV, Goncharov DA, Pena A, Ihida-Stansbury K, DeLisser H, Kawut SM, and Goncharova EA. Profiling the role of mammalian target of rapamycin in the vascular smooth muscle metabolome in pulmonary arterial hypertension. Pulm Circ 5: 667–680, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kurdyukov S, Eccles CA, Desai AA, Gonzalez-Garay M, Yuan JX, Garcia JGN, Rafikova O, and Rafikov R. New cases of glucose-6-phosphate dehydrogenase deficiency in pulmonary arterial hypertension. PLoS One 13: e0203493, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Laplante P, Sirois I, Raymond MA, Kokta V, Beliveau A, Prat A, Pshezhetsky AV, and Hebert MJ. Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ 17: 291–303, 2010 [DOI] [PubMed] [Google Scholar]
- 89. Lau EM, Humbert M, and Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol 12: 143–155, 2015 [DOI] [PubMed] [Google Scholar]
- 90. Lau EM, Manes A, Celermajer DS, and Galie N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. Eur Heart J 32: 2489–2498, 2011 [DOI] [PubMed] [Google Scholar]
- 91. Lei Y, Zhen J, Ming XL,and Jian HK. Induction of higher expression of IL-beta and TNF-alpha, lower expression of IL-10 and cyclic guanosine monophosphate by pulmonary arterial hypertension following cardiopulmonary bypass. Asian J Surg 25: 203–208, 2002 [DOI] [PubMed] [Google Scholar]
- 92. Leonard MO, Howell K, Madden SF, Costello CM, Higgins DG, Taylor CT, and McLoughlin P. Hypoxia selectively activates the CREB family of transcription factors in the in vivo lung. Am J Respir Crit Care Med 178: 977–983, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Levy M, Maurey C, Celermajer DS, Vouhe PR, Danel C, Bonnet D, and Israel-Biet D. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol 49: 803–810, 2007 [DOI] [PubMed] [Google Scholar]
- 94. Li J, Xiong J, Yang B, Zhou Q, Wu Y, Luo H, Zhou H, Liu N, Li Y, Song Z, and Zheng Q. Endothelial cell apoptosis induces TGF-beta signaling-dependent host endothelial-mesenchymal transition to promote transplant arteriosclerosis. Am J Transplant 15: 3095–3111, 2015 [DOI] [PubMed] [Google Scholar]
- 95. Li L, Cai L, Zheng L, Hu Y, Yuan W, Guo Z, and Li W. Gefitinib inhibits bleomycin-induced pulmonary fibrosis via alleviating the oxidative damage in Mice. Oxid Med Cell Longev 2018: 8249693, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li M, Riddle S, Zhang H, D'Alessandro A, Flockton A, Serkova NJ, Hansen KC, Moldvan R, McKeon BA, Frid M, Kumar S, Li H, Liu H, Caanovas A, Medrano JF, Thomas MG, Iloska D, Plecita-Hlavata L, Jezek P, Pullamsetti S, Fini MA, El Kasmi KC, Zhang Q, and Stenmark KR. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C-terminal binding protein-1. Circulation 134: 1105–1121, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, and Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008 [DOI] [PubMed] [Google Scholar]
- 98. Lin T, Gu J, Huang C, Zheng S, Lin X, Xie L, and Lin D. (1)H NMR-based analysis of serum metabolites in monocrotaline-induced pulmonary arterial hypertensive rats. Dis Markers 2016: 5803031, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu JQ, Zelko IN, Erbynn EM, Sham JS, and Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 100. Liu P, Li H, Cepeda J, Zhang LQ, Cui X, Garcia JG, and Ye SQ. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Biol Int 33: 19–30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lu Q. Transforming growth factor-beta1 protects against pulmonary artery endothelial cell apoptosis via ALK5. Am J Physiol Lung Cell Mol Physiol 295: L123–L133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, and Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev 21: 1037–1049, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo N, Craig D, Ilkayeva O, Muehlbauer M, Kraus WE, Newgard CB, Shah SH, and Rajagopal S. Plasma acylcarnitines are associated with pulmonary hypertension. Pulm Circ 7: 211–218, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maciel TT, Melo RS, Schor N, and Campos AH. Gremlin promotes vascular smooth muscle cell proliferation and migration. J Mol Cell Cardiol 44: 370–379, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Majesky MW, Lindner V, Twardzik DR, Schwartz SM, and Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest 88: 904–910, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Markov AK, Warren ET, Cohly HH, Sauls DJ, and Skelton TN. Influence of fructose-1,6-diphosphate on endotoxin-induced lung injuries in sheep. J Surg Res 138: 45–50, 2007 [DOI] [PubMed] [Google Scholar]
- 107. Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, and Archer SL. Lung (1)(8)F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med 185: 670–679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mathai SC. and Hassoun PM. Pulmonary arterial hypertension in connective tissue diseases. Heart Fail Clin 8: 413–425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Matsushita H, Morishita R, Nata T, Aoki M, Nakagami H, Taniyama Y, Yamamoto K, Higaki J, Yasufumi K, and Ogihara T. Hypoxia-induced endothelial apoptosis through nuclear factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: in vivo evidence of the importance of NF-kappaB in endothelial cell regulation. Circ Res 86: 974–981, 2000 [DOI] [PubMed] [Google Scholar]
- 110. Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, Lauzon-Joset JF, Breuils-Bonnet S, Tremblay E, Biardel S, Racine C, Courture C, Bonnet P, Majka SM, Deshaies Y, Picard F, Provencher S, and Bonnet S. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc 2: e005157, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mendonca R, Silveira AA, and Conran N. Red cell DAMPs and inflammation. Inflamm Res 65: 665–678, 2016 [DOI] [PubMed] [Google Scholar]
- 112. Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O'Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JSR, and Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 9: pii:, 2017 [DOI] [PubMed] [Google Scholar]
- 113. Michiels C, De Leener F, Arnould T, Dieu M, and Remacle J. Hypoxia stimulates human endothelial cells to release smooth muscle cell mitogens: role of prostaglandins and bFGF. Exp Cell Res 213: 43–54, 1994 [DOI] [PubMed] [Google Scholar]
- 114. Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, and Weissmann N. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 52: 1033–1042, 2012 [DOI] [PubMed] [Google Scholar]
- 115. Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 116. Nagy BM, Nagaraj C, Meinitzer A, Sharma N, Papp R, Foris V, Ghanim B, Kwapiszewska G, Kovacs G, Klepetko W, Pieber TR, Mangge H, Olschewski H, and Olschewski A. Importance of kynurenine in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 313: L741–L751, 2017 [DOI] [PubMed] [Google Scholar]
- 117. Nguyen QL, Corey C, White P, Watson A, Gladwin MT, Simon MA, and Shiva S. Platelets from pulmonary hypertension patients show increased mitochondrial reserve capacity. JCI Insight 2: e91415, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ollivier V, Faure S, Tarantino N, Chollet-Martin S, Deterre P, Combadiere C, and de Prost D. Fractalkine/CX3CL1 production by human aortic smooth muscle cells impairs monocyte procoagulant and inflammatory responses. Cytokine 21: 303–311, 2003 [DOI] [PubMed] [Google Scholar]
- 119. Olschewski A. and Weir EK. Redox regulation of ion channels in the pulmonary circulation. Antioxid Redox Signal 22: 465–485, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pandey S, Singh S, Anang V, Bhatt AN, Natarajan K, and Dwarakanath BS. Pattern recognition receptors in cancer progression and metastasis. Cancer Growth Metastasis 8: 25–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, and Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest 79: 213–223, 1999 [PubMed] [Google Scholar]
- 122. Parpaleix A, Amsellem V, Houssaini A, Abid S, Breau M, Marcos E, Sawaki D, Delcroix M, Quarck R, Maillard A, Couillin I, Ryffel B, and Adnot S. Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur Respir J 48: 470–483, 2016 [DOI] [PubMed] [Google Scholar]
- 123. Partovian C, Adnot S, Raffestin B, Louzier V, Levame M, Mavier IM, Lemarchand P, and Eddahibi S. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 23: 762–771, 2000 [DOI] [PubMed] [Google Scholar]
- 124. Pena A, Kobir A, Goncharov D, Goda A, Kudryashova TV, Ray A, Vanderpool R, Baust J, Chang B, Mora AL, Gorcsan J, 3rd, and Goncharova EA. Pharmacological inhibition of mTOR kinase reverses right ventricle remodeling and improves right ventricle structure and function in rats. Am J Respir Cell Mol Biol 57: 615–625, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Perez-Garijo A, Fuchs Y, and Steller H. Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. Elife 2: e01004, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Perez VAdJ. Drug-induced pulmonary hypertension: the first 50 years. Adv Pulm Hypertens 15: 133–137, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, and Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 88: 47–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT, and Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 91: 1185–1197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Porporato PE, Dhup S, Dadhich RK, Copetti T, and Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol 2: 49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Price LC, Caramori G, Perros F, Meng C, Gambaryan N, Dorfmuller P, Montani D, Casolari P, Zhu J, Dimopoulos K, Shao D, Girerd B, Mumby S, Proudfoot A, Griffiths M, Papi A, Humbert M, Adcock IM, and Wort SJ. Nuclear factor kappa-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS One 8: e75415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, Cohen-Kaminsky S, and Humbert M. Inflammation in pulmonary arterial hypertension. Chest 141: 210–221, 2012 [DOI] [PubMed] [Google Scholar]
- 132. Prise KM. and O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 9: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pulla VK, Sriram DS, Soni V, Viswanadha S, Sriram D, and Yogeeswari P. Targeting NAMPT for therapeutic intervention in cancer and inflammation: structure-based drug design and biological screening. Chem Biol Drug Des 86: 881–894, 2015 [DOI] [PubMed] [Google Scholar]
- 134. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118: 2372–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rabinovitch M, Guignabert C, Humbert M, and Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rafikov R, McBride ML, Zemskova M, Kurdyukov S, McClain N, Niihori M, Langlais PR, and Rafikova O. Inositol monophosphatase 1 as a novel interacting partner of RAGE in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 316: L428–L444, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rafikov R, Nair V, Sinari S, Babu H, Sullivan JC, Yuan JX, Desai AA, and Rafikova O. Gender difference in damage-mediated signaling contributes to pulmonary arterial hypertension. Antioxid Redox Signal [Epub ahead of print]; DOI: 10.1089/ars.2018.7664, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rafikov R, Rafikova O, Aggarwal S, Gross C, Sun X, Desai J, Fulton D, and Black SM. Asymmetric dimethylarginine induces endothelial nitric-oxide synthase mitochondrial redistribution through the nitration-mediated activation of Akt1. J Biol Chem 288: 6212–6226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rafikov R, Sun X, Rafikova O, Meadows ML, Desai AA, Khalpey Z, Yuan JX, Fineman JR, and Black SM. Complex I dysfunction underlies the glycolytic switch in pulmonary hypertensive smooth muscle cells. Redox Biol 6: 278–286, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rafikova O, Meadows ML, Kinchen JM, Mohney RP, Maltepe E, Desai AA, Yuan JX, Garcia JG, Fineman JR, Rafikov R, and Black SM. Metabolic changes precede the development of pulmonary hypertension in the monocrotaline exposed rat lung. PLoS One 11: e0150480, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Rafikova O, Rafikov R, Kangath A, Qu N, Aggarwal S, Sharma S, Desai J, Fields T, Ludewig B, Yuan JX, Jonigk D, and Black SM. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free Radic Biol Med 95: 96–111, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rafikova O, Rafikov R, Kumar S, Sharma S, Aggarwal S, Schneider F, Jonigk D, Black SM, and Tofovic SP. Bosentan inhibits oxidative and nitrosative stress and rescues occlusive pulmonary hypertension. Free Radic Biol Med 56: 28–43, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rafikova O, Rafikov R, Meadows ML, Kangath A, Jonigk D, and Black SM. The sexual dimorphism associated with pulmonary hypertension corresponds to a fibrotic phenotype. Pulm Circ 5: 184–197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rafikova O, Srivastava A, Desai AA, Rafikov R, and Tofovic SP. Recurrent inhibition of mitochondrial complex III induces chronic pulmonary vasoconstriction and glycolytic switch in the rat lung. Respir Res 19: 69, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rafikova O, Williams ER, McBride ML, Zemskova M, Srivastava A, Nair V, Desai AA, Langlais PR, Zemskov E, Simon M, Mandarino LJ, and Rafikov R. Hemolysis-induced lung vascular leakage contributes to the development of pulmonary hypertension. Am J Respir Cell Mol Biol 59: 334–345, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, and Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Raucci A, Di Maggio S, Scavello F, D'Ambrosio A, Bianchi ME, and Capogrossi MC. The janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci 76: 211–229, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Reis GS, Augusto VS, Silveira AP, Jordao AA, Jr., Baddini-Martinez J, Poli Neto O, Rodrigues AJ, and Evora PR. Oxidative-stress biomarkers in patients with pulmonary hypertension. Pulm Circ 3: 856–861, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris PA, Howard LS, Kiely DG, Peacock AJ, Pepke-Zaba J, Toshner MR, Wort SJ, Gibbs JS, Lawrie A, Graf S, Morrell NW, and Wilkins MR. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 135: 460–475, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rhodes CJ, Wharton J, Howard LS, Gibbs JS, and Wilkins MR. Red cell distribution width outperforms other potential circulating biomarkers in predicting survival in idiopathic pulmonary arterial hypertension. Heart 97: 1054–1060, 2011 [DOI] [PubMed] [Google Scholar]
- 151. Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015: 816460, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, and Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 11: 1517–1525, 2002 [DOI] [PubMed] [Google Scholar]
- 153. Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H, and Maruyama I. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One 9: e102482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, and Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005 [DOI] [PubMed] [Google Scholar]
- 155. Sakao S, Taraseviciene-Stewart L, Wood K, Cool CD, and Voelkel NF. Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol 291: L362–L368, 2006 [DOI] [PubMed] [Google Scholar]
- 156. Samokhin AO, Stephens T, Wertheim BM, Wang RS, Vargas SO, Yung LM, Cao M, Brown M, Arons E, Dieffenbach PB, Fewell JG, Matar M, Bowman FP, Haley KJ, Alba GA, Marino SM, Kumar R, Rosas IO, Waxman AB, Oldham WM, Khanna D, Graham BB, Seo S, Gladyshev VN, Yu PB, Fredenburgh LE, Loscalzo J, Leopold JA, and Maron BA. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci Transl Med 10: pii:, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, and Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 89: 661–669, 2001 [DOI] [PubMed] [Google Scholar]
- 158. Scaffidi P, Misteli T, and Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 159. Schaper F, de Leeuw K, Horst G, Bootsma H, Limburg PC, Heeringa P, Bijl M, and Westra J. High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells. Rheumatology (Oxford) 55: 2260–2270, 2016 [DOI] [PubMed] [Google Scholar]
- 160. Schlosser K, Taha M, Deng Y, Jiang B, McIntyre LA, Mei SH, and Stewart DJ. Lack of elevation in plasma levels of pro-inflammatory cytokines in common rodent models of pulmonary arterial hypertension: questions of construct validity for human patients. Pulm Circ 7: 476–485, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Schubert SY, Benarroch A, Monter-Solans J, and Edelman ER. Primary monocytes regulate endothelial cell survival through secretion of angiopoietin-1 and activation of endothelial Tie2. Arterioscler Thromb Vasc Biol 31: 870–875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Schumacker PT. Lung cell hypoxia: role of mitochondrial reactive oxygen species signaling in triggering responses. Proc Am Thorac Soc 8: 477–484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Schwaiblmair M, Faul C, von Scheidt W, and Berghaus TM. Detection of exercise-induced pulmonary arterial hypertension by cardiopulmonary exercise testing. Clin Cardiol 35: 548–553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, and Chandrasekar B. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol 50: 928–938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shimizu H, Saito S, Higashiyama Y, Nishijima F, and Niwa T. CREB NF-kappaB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am J Physiol Cell Physiol, 2013 [DOI] [PubMed] [Google Scholar]
- 166. Singla S, Sysol JR, Dille B, Jones N, Chen J, and Machado RF. Hemin causes lung microvascular endothelial barrier dysfunction by necroptotic cell death. Am J Respir Cell Mol Biol 57: 307–314, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Smadja DM, Mauge L, Sanchez O, Silvestre JS, Guerin C, Godier A, Henno P, Gaussem P, and Israel-Biet D. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J 36: 1284–1293, 2010 [DOI] [PubMed] [Google Scholar]
- 168. Smukowska-Gorynia A, Tomaszewska I, Malaczynska-Rajpold K, Marcinkowska J, Komosa A, Janus M, Olasinska-Wisniewska A, Slawek S, Araszkiewicz A, Jankiewicz S, and Mularek-Kubzdela T. Red blood cells distribution width as a potential prognostic biomarker in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Heart Lung Circ 27: 842–848, 2018 [DOI] [PubMed] [Google Scholar]
- 169. Song JY, Kim MJ, Jo HH, Hwang SJ, Chae B, Chung JE, Kwon DJ, Lew YO, Lim YT, Kim JH, Kim JH, and Kim MR. Antioxidant effect of estrogen on bovine aortic endothelial cells. J Steroid Biochem Mol Biol 117: 74–80, 2009 [DOI] [PubMed] [Google Scholar]
- 170. Stabile H, Mitola S, Moroni E, Belleri M, Nicoli S, Coltrini D, Peri F, Pessi A, Orsatti L, Talamo F, Castronovo V, Waltregny D, Cotelli F, Ribatti D, and Presta M. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood 109: 1834–1840, 2007 [DOI] [PubMed] [Google Scholar]
- 171. Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, and Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 28p following 244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Stenmark KR, Bouchey D, Nemenoff R, Dempsey EC, and Das M. Hypoxia-induced pulmonary vascular remodeling: contribution of the adventitial fibroblasts. Physiol Res 49: 503–517, 2000 [PubMed] [Google Scholar]
- 173. Stenmark KR, Tuder RM, and El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 119: 1164–1172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Steppan J, Tran HT, Bead VR, Oh YJ, Sikka G, Bivalacqua TJ, Burnett AL, Berkowitz DE, and Santhanam L. Arginase inhibition reverses endothelial dysfunction, pulmonary hypertension, and vascular stiffness in transgenic sickle cell mice. Anesth Analg 123: 652–658, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Stone TW, Stoy N, and Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 34: 136–143, 2013 [DOI] [PubMed] [Google Scholar]
- 176. Strange G, Gabbay E, Kermeen F, Williams T, Carrington M, Stewart S, and Keogh A. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ 3: 89–94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Su Z, Zhang P, Yu Y, Lu H, Liu Y, Ni P, Su X, Wang D, Liu Y, Wang J, Shen H, Xu W, and Xu H. HMGB1 facilitated macrophage reprogramming towards a proinflammatory M1-like phenotype in experimental autoimmune myocarditis development. Sci Rep 6: 21884, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178. Sun X, Sharma S, Fratz S, Kumar S, Rafikov R, Aggarwal S, Rafikova O, Lu Q, Burns T, Dasarathy S, Wright J, Schreiber C, Radman M, Fineman JR, and Black SM. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid Redox Signal 18: 1739–1752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Sutendra G, Dromparis P, Bonnet S, Haromy A, McMurtry MS, Bleackley RC, and Michelakis ED. Pyruvate dehydrogenase inhibition by the inflammatory cytokine TNFalpha contributes to the pathogenesis of pulmonary arterial hypertension. J Mol Med (Berl) 89: 771–783, 2011 [DOI] [PubMed] [Google Scholar]
- 180. Sylvester JT, Shimoda LA, Aaronson PI, and Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Tabima DM, Frizzell S, and Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med 52: 1970–1986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Talati MH, Brittain EL, Fessel JP, Penner N, Atkinson J, Funke M, Grueter C, Jerome WG, Freeman M, Newman JH, West J, and Hemnes AR. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med 194: 719–728, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tan W, Madhavan K, Hunter KS, Park D, and Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 4: 560–580, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, and Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001 [DOI] [PubMed] [Google Scholar]
- 185. Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, and Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 98: 209–217, 2006 [DOI] [PubMed] [Google Scholar]
- 186. Teng RJ, Eis A, Bakhutashvili I, Arul N, and Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L184–L195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, and Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 5: 200ra117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Trow TK, Napoletano J, and White SL. Early diagnosis of pulmonary arterial hypertension: a plea for help. Conn Med 80: 433–434, 2016 [PubMed] [Google Scholar]
- 189. Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, and Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289: H2649–H2656, 2005 [DOI] [PubMed] [Google Scholar]
- 190. Veit F, Pak O, Brandes RP, and Weissmann N. Hypoxia-dependent reactive oxygen species signaling in the pulmonary circulation: focus on ion channels. Antioxid Redox Signal 22: 537–552, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Veit F, Pak O, Egemnazarov B, Roth M, Kosanovic D, Seimetz M, Sommer N, Ghofrani HA, Seeger W, Grimminger F, Brandes RP, Schermuly RT, and Weissmann N. Function of NADPH oxidase 1 in pulmonary arterial smooth muscle cells after monocrotaline-induced pulmonary vascular remodeling. Antioxid Redox Signal 19: 2213–2231, 2013 [DOI] [PubMed] [Google Scholar]
- 192. Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, Velegala S, Seeger W, McKinsey TA, Sucharov CC, and Stenmark KR. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res 114: 67–78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Weigert A, Johann AM, von Knethen A, Schmidt H, Geisslinger G, and Brune B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 108: 1635–1642, 2006 [DOI] [PubMed] [Google Scholar]
- 194. West J. and Hemnes A. Experimental and transgenic models of pulmonary hypertension. Compr Physiol 1: 769–782, 2011 [DOI] [PubMed] [Google Scholar]
- 195. White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Van Rooij E, Morrell NW, MacLean MR, and Baker AH. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 64: 185–194, 2014 [DOI] [PubMed] [Google Scholar]
- 196. Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, and Schutte H. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med 34: 1947–1954, 2006 [DOI] [PubMed] [Google Scholar]
- 197. Wolin MS, Ahmad M, and Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–L173, 2005 [DOI] [PubMed] [Google Scholar]
- 198. Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, and van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood 107: 4930–4937, 2006 [DOI] [PubMed] [Google Scholar]
- 199. Xue C, Sowden M, and Berk BC. Extracellular cyclophilin A, especially acetylated, causes pulmonary hypertension by stimulating endothelial apoptosis, redox stress, and inflammation. Arterioscler Thromb Vasc Biol 37: 1138–1146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Yang PS, Kim DH, Lee YJ, Lee SE, Kang WJ, Chang HJ, and Shin JS. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir Res 15: 148, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Yao JS, Zhai W, Young WL, and Yang GY. Interleukin-6 triggers human cerebral endothelial cells proliferation and migration: the role for KDR and MMP-9. Biochem Biophys Res Commun 342: 1396–1404, 2006 [DOI] [PubMed] [Google Scholar]
- 202. Yeung SJ, Pan J, and Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci 65: 3981–3999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yu Q. and Chan SY. Mitochondrial and metabolic drivers of pulmonary vascular endothelial dysfunction in pulmonary hypertension. Adv Exp Med Biol 967: 373–383, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, and Yang P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol 454: 103–111, 2017 [DOI] [PubMed] [Google Scholar]
- 205. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, and Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhao PW, Lee DY, Ma ZZ, Huang LS, Sun LP, Li YD, Chen JX, and Niu JZ. The antioxidant effect of carnosol in bovine aortic endothelial cells is mainly mediated via estrogen receptor alpha pathway. Biol Pharm Bull 35: 1947–1955, 2012 [DOI] [PubMed] [Google Scholar]
- 207. Zhao Y, Peng J, Lu C, Hsin M, Mura M, Wu L, Chu L, Zamel R, Machuca T, Waddell T, Liu M, Keshavjee S, Granton J, and de Perrot M. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One 9: e88727, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Zhao YD, Chu L, Lin K, Granton E, Yin L, Peng J, Hsin M, Wu L, Yu A, Waddell T, Keshavjee S, Granton J, and de Perrot M. A Biochemical approach to understand the pathogenesis of advanced pulmonary arterial hypertension: metabolomic profiles of arginine, sphingosine-1-phosphate, and heme of human lung. PLoS One 10: e0134958, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhao YD, Yun HZH, Peng J, Yin L, Chu L, Wu L, Michalek R, Liu M, Keshavjee S, Waddell T, Granton J, and de Perrot M. De novo synthesize of bile acids in pulmonary arterial hypertension lung. Metabolomics 10: 1169–1175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zheng HK, Zhao JH, Yan Y, Lian TY, Ye J, Wang XJ, Wang Z, Jing ZC, He YY, and Yang P. Metabolic reprogramming of the urea cycle pathway in experimental pulmonary arterial hypertension rats induced by monocrotaline. Respir Res 19: 94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, and Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation 121: 98–109, 2010 [DOI] [PubMed] [Google Scholar]
- 212. Zuniga A, Laurent F, Lopez-Rios J, Klasen C, Matt N, and Zeller R. Conserved cis-regulatory regions in a large genomic landscape control SHH and BMP-regulated Gremlin1 expression in mouse limb buds. BMC Dev Biol 12: 23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]