Abstract
Significance: High-mobility group protein box 1 (HMGB1), a ubiquitous nuclear protein, regulates chromatin structure and modulates the expression of many genes involved in the pathogenesis of lung cancer and many other lung diseases, including those that regulate cell cycle control, cell death, and DNA replication and repair. Extracellular HMGB1, whether passively released or actively secreted, is a danger signal that elicits proinflammatory responses, impairs macrophage phagocytosis and efferocytosis, and alters vascular remodeling. This can result in excessive pulmonary inflammation and compromised host defense against lung infections, causing a deleterious feedback cycle.
Recent Advances: HMGB1 has been identified as a biomarker and mediator of the pathogenesis of numerous lung disorders. In addition, post-translational modifications of HMGB1, including acetylation, phosphorylation, and oxidation, have been postulated to affect its localization and physiological and pathophysiological effects, such as the initiation and progression of lung diseases.
Critical Issues: The molecular mechanisms underlying how HMGB1 drives the pathogenesis of different lung diseases and novel therapeutic approaches targeting HMGB1 remain to be elucidated.
Future Directions: Additional research is needed to identify the roles and functions of modified HMGB1 produced by different post-translational modifications and their significance in the pathogenesis of lung diseases. Such studies will provide information for novel approaches targeting HMGB1 as a treatment for lung diseases.
Keywords: HMGB1, inflammatory lung injury, pulmonary infection, pulmonary vascular remodeling, fibrosis, cancer
Introduction
High-mobility group protein box 1 (HMGB1) was first identified in 1973 as a nonhistone chromosomal nuclear protein (107). It was isolated from calf thymus chromatin and was categorized as a “high-mobility group” protein by its defining characteristic of rapid migration in polyacrylamide gel electrophoresis systems, without any signs of aggregation (107). In 1999, it was reported that extracellular HMGB1 was significantly increased in animal models of sepsis and in septic patients (336). Circulating HMGB1 can produce significant proinflammatory effects and cause lethal sepsis shock (336, 337). Over the last two decades, HMGB1 has been described as a damage-associated molecular pattern (DAMP) molecule or an alarmin that plays a role in the pathogenesis of an increasing number of diseases, including, but not limited to, sepsis, autoimmune diseases, cancer, liver diseases, and lung diseases (69, 145, 218, 262).
A number of reviews have been published in this journal that have discussed the (i) post-translational modifications of HMGB1 that occur during inflammatory responses; (ii) role of HMGB1 receptors in oxidative and inflammatory tissue injury; (iii) the involvement of HMGB1 in cardiovascular diseases, including pulmonary hypertension; and (iv) targeting HMGB1 for the treatment of various metabolic disorders resulting from sterile inflammation (44, 96, 289). In this review, we focus on recent studies regarding the potential cellular and molecular mechanisms underlying the roles of HMGB1, especially in the pathogenesis of lung diseases where it could represent a biomarker for disease prognosis and a target for therapeutic strategies in treating lung diseases.
HMGB1 Structure and Its Function in the Nucleus
HMGB1 is a 215 amino acid nuclear protein, weighing 25 kDa (123a). The gene that encodes for HMGB1 is located on chromosome 13q12.3 and was sequenced in 1996 (91). The primary structure of HMGB1 is well conserved across species and shares high sequence homology among species such as yeast, Drosophila, Chironomidae, bacteria, plants, and fish (101, 175, 282, 331, 347, 351, 354). Furthermore, mouse and rat HMGB1 shares 100% homology, while human and rodent HMGB1 has 99% homology (45, 91, 98, 271, 344).
The binding of nuclear HMGB1 in the minor groove of DNA can facilitate DNA bending, producing higher transcription factor activity, DNA repair, and remodeling of the chromatin (34, 208, 250, 264). As illustrated in Figure 1, HMGB1 binds to DNA via two unique binding domains, the A-box (amino acid residues 9–79) and the B-box (amino acid residues 95–163), which share high sequence similarity with each other (11, 32). The A-Box and B-Box are separated by a short interlinking peptide sequence (32, 264, 265). The C-terminal of HMGB1 (amino acid residues 186–215) is composed of a highly acidic tail containing aspartic and glutamic acid residues (22, 34). The acidic C-terminal tail of HMGB1, which is not required for binding, regulates its effects on transcriptional activity, as it is required for DNA bending (119, 300, 332). The C-terminal plays an essential role in the binding of protein p53 to DNA to regulate cell cycle and death pathways (6, 22).
FIG. 1.
The structure and function determining sequence of HMGB1. Human HMGB1 is a protein with 215 amino acids, encoded by the gene located at chromosome 13q12.3. HMGB1 contains two DNA-binding domains: the A Box (amino acids 9–79) and B-Box (amino acids 95–163), and a C-terminal tail (amino acids 186–215), which is involved in promoting the interaction of A and B box with DNA. HMGB1 contains two NLS, which are located at amino acids 28–44 (NLS1) and 179–185 (NLS2), responsible for the nuclear localization of HMGB1 and for regulating HMGB1's translocation between the nucleus and the cytoplasm on post-translational modifications, such as phosphorylation and acetylation. There are three critical cysteines (C23, C45, and C106) subject to redox modifications, which determine whether HMGB1 functions as a cytokine, a chemokine, or an inactive protein. HMGB1 also has a heparin binding site (amino acids 6–12), a TLR4 binding site (amino acids 89–108), and an RAGE binding site (amino acids 150–183). HMGB1, high-mobility group protein box 1; NLS, nuclear localization signals; RAGE, receptor for advanced glycation end products; TLR, toll-like receptor.
HMGB1 Localization and Lung Diseases
Wang et al. reported in 1999 that treatment of cultured macrophages with endotoxin lipopolysaccharide (LPS) caused a significant release of nuclear HMGB1 into cell culture media. They further demonstrated that extracellular HMGB1 in the serum of subjects with sepsis can act as a late mediator of inflammation for septic shock mice (336). Since then, excessive accumulation of extracellular HMGB1, especially airway and sputum HMGB1, has been reported in many studies of a variety of lung diseases, such as cystic fibrosis (CF), asthma, chronic obstructive pulmonary disease (COPD), acute lung injury (ALI), acute respiratory distress syndrome (ARDS), idiopathic pulmonary fibrosis, pneumonia, tuberculosis (TB), pulmonary arterial hypertension (PAH), and lung cancer (Table 1). Thus, blocking the accumulation of extracellular HMGB1 has been postulated in the treatment of these disorders.
Table 1.
Levels and Modifications of High-Mobility Group Protein Box 1 in Biological Samples in Lung Diseases
| Lung disease | Sample (origin) | Fold increase compared to control | HMGB1 modification | References |
|---|---|---|---|---|
| Asthma | Sputum | 5 | Reduced and disulfide | (71) |
| COPD | Sputum | 37 | — | (128) |
| Plasma | 3.83 | — | (128) | |
| Serum | 4.0 | — | (366) | |
| ALI/ARDS | BAL | 10–17 | Oxidized, reduced, and disulfide | (377) |
| PF | Serum | 1.97–5.66 | — | (296) |
| CF | Serum | 5.11 | — | (57) |
| Sputum | 4.64 | |||
| BAL | 20.11 | — | (86) | |
| Pneumonia | BAL | 4 | — | (378) |
| TB | Plasma | 1.68 | — | (368) |
| Sputum | 1.82 | — | (368) | |
| Lung cancer | Serum | 1.8–8.2 | — | (343) |
| PAH | Serum | 2.47 | — | (366) |
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; HMGB1, high-mobility group protein box 1; PAH, pulmonary arterial hypertension; PF, pulmonary fibrosis; TB, tuberculosis.
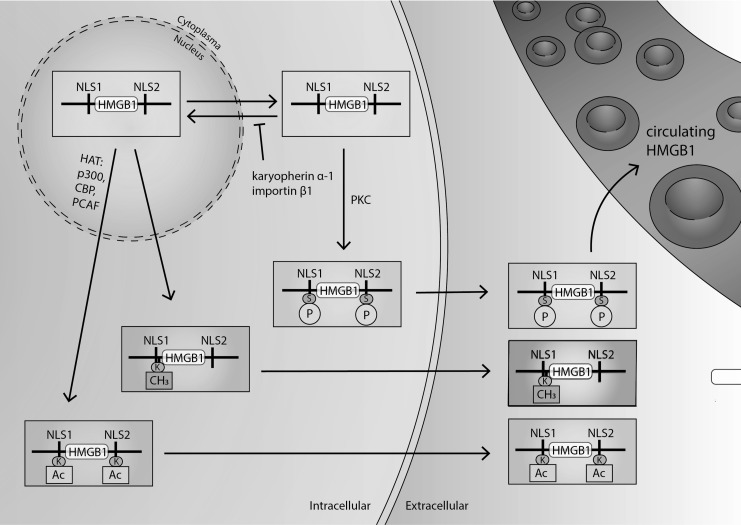
Under normal physiological conditions, HMGB1 is localized in the nucleus, due to its two nuclear localization signals (NLS), located at amino acids 28–44 (NLS1) and 179–185 (NLS2) (Fig. 2) (138, 253, 278). However, HMGB1 can be readily shuttled between the nucleus and the cytoplasm by modifications of NLS1 and NLS2 via acetylation and deacetylation (Fig. 2) (138, 280, 363). Acetylation and deacetylation of HMGB1 are mediated by histone acetyltransferase (HAT) family proteins and histone deacetylase, thus regulating its translocation between the nucleus and the cytoplasm (37, 201, 363).
FIG. 2.
Regulation of HMGB1 localization. HMGB1 is a nuclear nonhistone binding protein that can shuttle between the nucleus and the cytosol through nuclear pores. HMGB1 contains two nuclear localization sequences (NSL1 and NLS2). These NLS are post-translationally modified by hyperacetylating lysine residues within NLS1 and NLS2. Hyperacetylation of NLS by HAT (p300, PCAF, CBP) is required to induce nucleocytoplasmic translocation. Also, the phosphorylation of cytoplasmic HMGB1 by PKC can cause HMGB1 to bind with karyopherin-α-1 and importin-β-1, which can block its nuclear import, keeping it in the cytoplasm. In addition, the methylation of lysine-42 at NLS1 alters HMGB1 conformation, which can result in its decreased ability to bind with DNA and causing HMGB1's passive diffusion to the cytoplasm. Under conditions of oxidative stress, HMGB1 translocates from the nucleus to the cytoplasm through nuclear export factor 1 (CRM1). Cytoplasmic HMGB1 can then be secreted from the cell in secretory vesicle-mediated exocytosis. Alternatively, HMGB1 can be passively secreted from injured or necrotic cells. Whether passively or actively secreted, HMGB1 then can accumulate in extracellular spaces such as the airways and circulating blood. Extracellular HMGB1 also is subjected to redox modifications of three critical cysteine residues (C23, C45, and C106). When HMGB1 fully reduces it can function as a cytokine and when HMGB1 is oxidized it forms a disulfide bond between C23 and C45, which imparts chemokine and cytokine activity. HMGB1 can be terminally oxidized (sulfonic acid) at all three cysteines, which inactivates all activity. CRM1, chromosome region maintenance 1; HAT, histone acetyltransferase; PCAF, p300/CBP-associated factor; PKC, protein kinase C.
Under cellular stress, the translocation of HMGB1 from the nucleus to the cytoplasm can be favored by post-translational modifications of nuclear HMGB1, such as hyperacetylation, phosphorylation, methylation, and oxidation (37, 68, 99, 139, 166, 238, 314). The accumulation of HMGB1 in the cytoplasm can lead to active HMGB1 release from cells into the extracellular milieu by secretory lysosomes (37, 97, 238).
The translocation of HMGB1 from the nucleus to the cytoplasm was first shown in HMGB1 with hyperacetylated lysine residues in NLS1 and NLS2 (37, 201, 363). It was found that the acetylation of HMGB1 is primarily regulated by the HAT family protein p300/CBP and p300/CBP-associated factor (PCAF) (37, 362). In addition, under oxidative conditions, HMGB1 can form a complex with chromosome region maintenance 1 (CRM1), a nuclear export factor (NEF), and is readily translocated to the cytoplasm on acetylation (314). Hyperacetylated HMGB1 was found in cells or animals subjected to oxidative stress under conditions of exposure to hyperoxia or proinflammatory stimuli (66, 301).
Interestingly, it was recently discovered that oxidation of the critical cysteine residues of HMGB1 (Fig. 1) can directly affect its subcellular location (166). HMGB1 has three critical cysteine residues at amino acids C23, C45, and C106 (Fig. 1). Intracellular HMGB1 is present in the fully reduced state (85). In a recent study, Kwak et al. demonstrated that hydrogen peroxide generated in macrophages on exposure to LPS can facilitate the intramolecular disulfide formation between cysteines C23 and C45. Such oxidation of HMGB1 cysteines can stimulate its nucleocytoplasmic translocation and release from macrophages (166).
In addition to NLS acetylation-dependent subcellular localization, the phosphorylation of serine residues of NLS1 and NLS2 is another modification that regulates the shuttling of HMGB1 from the nucleus to the cytoplasm (99, 363). The phosphorylation of specific serine residues in HMGB1 is mediated by protein kinase C (PKC). Compounds that are specific PKC inhibitors (i.e., okadaic acid and baicalein) can cause a concentration-dependent reduction in HMGB1 phosphorylation (363), whereas activators of PKC (e.g., 12-O-tetradecanoylphorbol-13-acetate [PMA] and bryostatin-1) increase HMGB1 phosphorylation and its secretion (238). On phosphorylation, HMGB1 can bind to karyopherin-α1, a member of the importin-α family, which induces the sequestration of HMGB1 in the cytoplasm by blocking its translocation to the nucleus (Fig. 2) (363).
Nuclear HMGB1 can also be methylated at Lys42, which can alter HMGB1 protein conformation, decreasing HMGB1-DNA binding activity (139, 349). Decreased binding of HMGB1 to DNA increases its passive diffusion out of the nucleus and into the cytoplasm (139, 149, 280). This has been reported in neutrophils under chronic inflammatory conditions (139). Moreover, the secretion of HMGB1 from macrophages can be facilitated when HMGB1 is poly(ADP-ribosyl)ated, a post-translational modification produced by poly(ADP)-ribose polymerase (PARP)-1 (68, 360).
In summary, many post-translational modifications regulate HMGB1's translocation from the nucleus to the cytoplasm and the subsequent active release from cells (Fig. 2) (37, 68, 99, 139, 238, 314). Because of the critical role of extracellular HMGB1 in the pathogenesis of numerous lung diseases, blocking the release of HMGB1 has been postulated for therapeutic approaches. In fact, inhibiting PARP-1, either genetically or pharmacologically, has been shown to effectively attenuate LPS-stimulated HMGB1 release, suggesting its therapeutic application in treating lung disorders caused by bacterial infections (68). Other studies have shown that ascorbic acid and GTS-21, a partial agonist of α7 nicotinic acetylcholine receptors, can effectively attenuate ALI and enhance host defense against pulmonary infections by inhibiting HMGB1 hyperacetylation (247, 301). These studies are discussed further in this review.
HMGB1 can also be passively released by leaking necrotic cells, a process that can induce inflammation (37, 280). Interestingly, HMGB1 is not easily released by cells undergoing apoptosis due to its high affinity to chromatin and the low levels of NLS acetylation (280). The significant difference in HMGB1-induced clinical outcomes, by HMGB1 released from cells undergoing either necrosis or apoptosis, was elegantly demonstrated in a recent study by Rafikov et al. (258). In this study, the authors show that HMGB1 released from necrotic cells was in a reduced redox status, exhibiting a more potent and sustained inflammatory ability than HMGB1 released from apoptotic cells. The redox regulation of HMGB1 activities is further discussed in this review.
HMGB1 Signaling in Pathophysiology of Lung Diseases
Extracellular HMGB1, as a DAMP through similar pathways, can play a critical role in activating signaling pathways leading to inflammatory responses, vascular remodeling, and fibrotic progression in several lung diseases. Figure 3 illustrates these HMGB1-elicited signaling events mediated by HMGB1 receptors.
FIG. 3.
The role of HMGB1 in the pathogenesis of lung diseases. In the studies reviewed in this article, elevated extracellular HMGB1, whether it is in the airways, serum, or other extracellular milieu, can signal as a DAMP through similar pathways that lead to the pathogenesis of different lung diseases. The binding of extracellular HMGB1 to its receptors, such as TLR2, TLR4, TLR9, and RAGE, can initiate innate immune responses by increasing NF-κB and MAPK activities. The activation of NF-κB is responsible for the increased secretion of numerous proinflammatory mediators, including the active release of HMGB1 into the airways. Proinflammatory mediators can induce the infiltration of leukocytes into the lungs and airways, leading to lung inflammation. This further causes an elevation in the levels of proteases and ROS, which feeds back and contributes to increased NF-κB activity. A significant elevation in ROS can damage and cause dysfunction, injury, and death to lung cells, including macrophages and endothelial and epithelial cells. Both ROS and extracellular HMGB1 can directly disrupt epithelial and endothelial intercellular tight junctions, reduce their barrier integrity, and result in increased alveolar permeability. Macrophage injury reduces efferocytosis, which contributes to the higher leukocyte counts, as well as impairs the phagocytosis of invading bacteria, resulting in increased susceptibility to infections. Pulmonary infections and damage to the lung cells further increase the accumulation of extracellular HMGB1, establishing a negative feedback cycle. In addition, the binding of HMGB1 to RAGE receptors increases MAPK activity, altering cell proliferation and inducing vascular remodeling. Overall, HMGB1-induced inflammatory lung cell damage, in combination with vascular remodeling, can significantly compromise lung functions and cause lung diseases. DAMP, damage-associated molecular pattern; MAPK, mitogen-activated protein kinase; NF-κβ, nuclear factor kappa-light-chain-enhancer of activated B cells; ROS, reactive oxygen species.
Extracellular HMGB1 can function as a DAMP molecule that can activate immune cells via the pattern recognition receptors, including toll-like receptors (TLRs), TLR2, TLR4, TLR9, and receptor for advanced glycation end products (RAGE). The binding of HMGB1 to these cell surface receptors can elicit inflammatory responses by activating nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinase (JNK)/p38 pathways (Fig. 3) (56, 117, 193, 239, 334, 366). It has been shown that extracellular HMGB1 has high affinity for the TLR2, TLR4, and RAGE, on pneumocytes and leukocytes (137, 245).
Binding of extracellular HMGB1 to TLR2/4/9 receptors can activate the canonical pathway of NF-κB. Following the activation of TLR2 and TLR4 receptors, the phosphorylation of the inhibitory IκB-α protein by IκB kinase (IKK) causes the NF-κB subunit complex (p65 [NF-kappa-B p65 subunit/transcription factor p65] and p50) to translocate to the nucleus after p65 phosphorylation (17, 18, 58, 169, 170). On entering the nucleus, NF-κB upregulates the expression of more than 150 proteins (41, 195, 292). On HMGB1 binding to RAGE, it can activate the MAPK pathway to phosphorylate extracellular signal-regulated kinase (ERK) and p38, which also can activate NF-κB (188).
HMGB1-induced activation of canonical NF-κB pathways can induce the synthesis secretion and secretion of proinflammatory mediators, including interleukin (IL)-1β, IL-8, tumor necrosis factor-α (TNF-α), interferon (IFN)-γ, and monocyte chemoattractant protein-1 (MCP-1), which are involved in the recruitment of leukocytes and lymphocytes to the lung, and contribute to furthering lung inflammation (56, 59, 67, 106, 114, 334). The chemokine activity of HMGB1 was revealed following the pharmacological inhibition of α-chemokine receptors, which resulted in the attenuation of the HMGB1-induced increase in airway levels of infiltrated neutrophils, subsequent lung injury, or even death of the animals (192).
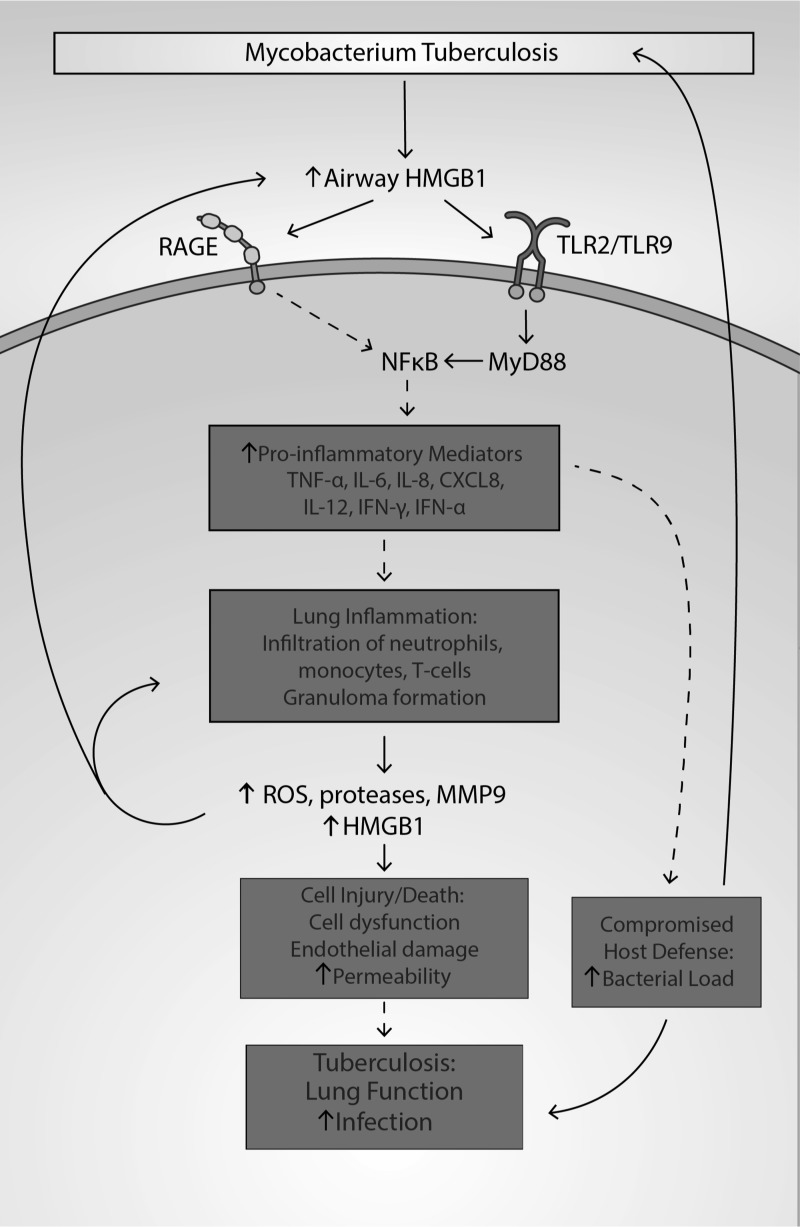
During pulmonary bacterial infection, pathogen-associated molecular pattern (PAMP) molecules, including bacterial components such as LPS, peptidoglycan, and lipoteichoic acid, can activate airway cells, including alveolar macrophages and epithelial cells, through surface receptor PRRs, TLR2, TLR4, TLR9, and RAGE (324, 376). The activation of these receptors can increase HMGB1 expression and induce the translocation and release of HMGB1 into the extracellular milieu (338, 376). Together, PAMPs and HMGB1 can cause synergetic effects on activating immune cells, producing exacerbated inflammatory lung injury (8, 130, 256). The binding of HMGB1 to RAGE, TLR2, and TLR9 receptors has been shown to increase the production of chemokines and cytokines that are critical in the host defense against TB, demonstrated in TB-infected TLR2 and TLR9 knockout mice and TLR2/9 double knockout mice (95, 116).
Although inflammation is essential for host defense against pulmonary infection, activated leukocytes in the lung can release myeloperoxidase (MPO), matrix metalloproteinase 9 (MMP9), proteases, and reactive oxygen species (ROS) (52, 270, 272). In addition, neutrophils undergoing cell death release various DAMPs (e.g., HMGB1, chemokine ligand 8 [CXCL8], IL-8, TNF-α, and IL-1β). When airway epithelial cells are exposed to these DAMPs, epithelial cells can release the potent chemotactic protein, CXCL8 (120, 205). CXCL8 can increase the infiltration of more neutrophils, thereby augmenting neutrophil-induced damage (120, 163, 205, 281). It has been reported that in epithelial cells exposed to necrotic neutrophil DAMPs, the pharmacological inhibition of TLR2, TLR4, and RAGE can significantly decrease CXCL8 levels (120).
The activation of HMGB1-mediated RAGE can compromise endothelial cell/cell junctions and increase endothelial barrier permeability (287, 348). The binding of HMGB1 to RAGE primarily activates the MAPK/p38 pathway (87, 160, 348). One of the downstream targets of MAPK/p38 activation catalyzes the phosphorylation of heat shock protein 27 (Hsp27), an actin binding protein (145, 313, 348). The phosphorylation of Hsp27 produces actin stress fiber formation, actin cytoskeletal disorganization, and cell/cell junction dissociation (108, 148, 348). These changes increase the permeability of endothelial cells, which contributes to decreased lung function (348).
The increase in ROS levels and extracellular HMGB1 can directly disrupt epithelial and endothelial intercellular tight junctions, damage alveolar architecture, and stimulate further upregulation of proinflammatory mediators and the release of HMGB1 into the airways, establishing a vicious feedback loop (156, 204). Thus, neutrophil-derived ROS and protease secretion can promote cell injury and death, disrupting the distribution of junction proteins, resulting in the loss of epithelial/endothelial barrier integrity, which contributes to poorer lung function, airway obstruction, and the progression of many lung diseases, including COPD, ALI/ARDS, PAH, and pneumonia (1, 103, 131, 302, 366).
In addition, extracellular HMGB1 can directly induce macrophage injury, causing reduced efferocytosis, which contributes to an increased number of leukocytes, and impairing the phagocytosis of invading bacteria, resulting in increased susceptibility to infections (86, 194, 248). Pulmonary infections and resulting damage to lung cells can further increase the accumulation of extracellular HMGB1, establishing a positive feedback cycle. Moreover, HMGB1 has been reported to mediate the recruitment of inflammatory cells in subjects with asthma, thereby exacerbating oxidative stress-mediated asthma pathology (126, 206). Therefore, blocking HMGB1 activities using neutralizing anti-HMGB1 antibodies could effectively enhance host defense against bacterial infections and attenuate lung injuries, as demonstrated in mouse models of ventilator-associated pneumonia (VAP) and CF (86, 248).
In addition, HMGB1-induced activation of MAPK pathways via binding to RAGE can lead to vascular remodeling by enhancing endothelial and epithelial cell proliferation (Fig. 3). HMGB1 has also been shown to directly increase the proliferation of primary arterial endothelial cells and pulmonary arterial smooth muscle cells, as evidenced by activation of MAPK/ERK/p38 and activator protein 1 (AP-1; c-fos/c-jun) (366). Silencing the expression of c-jun significantly decreased the HMGB1-induced proliferation of endothelial and epithelial cells (366).
HMGB1-mediated activation of NF-κB can also induce the upregulation of α-smooth muscle actin (α-sma) and transforming growth factor-β (TGF-β) (340). As a mediator of vascular remodeling, TGF-β1 can induce phosphorylation of SMAD2 and SMAD3, which can phosphorylate the transcription factor signal transducer and activator of transcription 3 (STAT-3) (251, 316). Activated STAT-3 can induce cell proliferation and TGF-β-dependent myofibroblast differentiation (56, 251, 340). TGF-β-induced proliferation is STAT-3 dependent and inhibition of STAT-3 decreased α-sma, collagen deposition, total airway leukocytes, and the transition of fibroblasts to myofibroblasts (251, 316). Zhang et al. also reported that the exposure of alveolar epithelial cells to TGF-β1 promoted HMGB1 expression and epithelial to mesenchymal transition (EMT), as well as decreased RAGE expression (372).
EMT is stimulated by abnormal cell proliferation and differentiation and is characterized by a reversible alteration in the phenotype of epithelial cells to motile mesenchymal cells (117, 181, 305). During this process, epithelial cells are transformed into myofibroblasts, which secrete extracellular matrix (ECM) components and collagen (305, 315). During EMT, cell/cell junction dissolution occurs and fibroblasts and myofibroblasts accumulate, producing profibrotic factors, fibroblast growth factor 2 (FGF2) and platelet-derived growth factor (PDGF), in addition to α-sma, collagen, and ECM proteins (239, 340). The accumulation of ECM proteins can further promote airway remodeling, through which ECM components are cleaved causing ECM dysregulation, resulting in increased collagen deposition and tissue stiffening (117, 305). Lung stiffening and increased deposition of collagen and ECM promote fibrosis of lung tissue (305).
It has been reported that COPD patients have not only increased levels of HMGB1 in cigarette smoke-exposed neutrophils but also an increase in neutrophil autophagy, which can induce excessive levels of intracellular ROS, incite the release of neutrophil elastase, and increase airway obstruction (120, 205, 297). Excessive levels of neutrophil MPO and MMP9 can damage lung elastic fibers and the ECM, which impairs vascular remodeling (59, 302). Thus, HMGB1 may play a role in vascular remodeling and airway obstruction in COPD.
Overall, data suggest that decreasing the accumulation of extracellular HMGB1 and attenuating its downstream signaling with TLRs and RAGE may decrease inflammation, vascular remodeling, and fibrotic lung injury. Therefore, compounds targeting HMGB1 signaling pathways have been postulated to treat patients with lung diseases. Reagents, such as anti-HMGB1 antibodies, thrombomodulin, pulmonary rehabilitation mixture, and ethyl pyruvate, have been tested in animal models of lung diseases and are discussed further in this review.
Redox Regulation of HMGB1 Pathophysiology
On its secretion into the extracellular milieu, HMGB1 is subject to redox modifications at three critical cysteine residues: C23, C45, and C106 (149, 276, 328). Redox modification of these cysteine residues determines whether HMGB1 will function as a cytokine, chemokine, or an inactive protein (118, 159, 359). The oxidation of HMGB1 can form disulfide (C23 and C45) or fully oxidized isoforms, such as sulfonic acid (276, 359). Redox-modified HMGB1 has been shown to present in bronchoalveolar lavage fluids (BALF) of mouse models of ALI (85, 301). Redox regulation of HMGB1 has also been shown to play an essential role in the gender differences in PAH (258).
HMGB1, whether in the fully reduced or disulfide isoform, produces cytokine activity (203, 358, 359). However, on terminal oxidation (i.e., the sulfonic acid isoform), HMGB1's cytokine activity is abrogated, further demonstrating the significant role of redox modifications on the functions of HMGB1 (141, 359). When all three critical cysteines of HMGB1 are fully reduced, HMGB1 retains chemoattractant activity (213, 328).
Reduced HMGB1 with cytokine activity can induce inflammatory responses following binding to its receptors, such as the receptor for RAGE at HMGB1 150–183 residues (Fig. 1), by stimulating the expression and secretion of TNF-α by macrophages (13, 125, 178, 280, 310). Reduced HMGB1 can also bind to TLR4 and TLR2 at the 89–108 residues to stimulate the expression and secretion of proinflammatory mediators such as IL-8, IL-6, and TNF-α (358). The cysteine present at residue 106 (Fig. 1) is critical for the binding of HMGB1 to TLR4 (178, 358).
Interestingly, the heparin-binding domain of HMGB1, located in the A-box at amino acid residues 6–12 (Fig. 1), also regulates its binding to cell surface receptors (140, 217, 333, 355). When heparin is bound to HMGB1, the binding affinity of HMGB1 to RAGE is significantly decreased, indicating a unique mechanism for modulating HMGB1 activity (140, 217, 303, 375). Studies in our laboratory using 2-O,3-O desulfated heparin (ODSH), a modified heparin, demonstrated that blocking HMGB1 binding to its receptors could effectively attenuate bacterial infection-induced lung injury (291).
The clinical relevance of redox regulation of HMGB1 in the pathophysiology was demonstrated in the gender differences between female and male patients with PAH (258). In this study, the authors showed that cells from males are more likely to die by necrosis, while cells from females more likely die by apoptosis. During necrotic cell death, many thiols were released into the extracellular milieu, creating a reduced environment for extracellular HMGB1. Reduced HMGB1 elicits further inflammation, prolonging the stimulation of downstream inflammatory pathways. On the contrary, extracellular HMGB1 is more oxidized in an inflammatory environment in females with the influx of thiols from necrotic cells, thus losing the ability to bind to PRRs and triggering further inflammatory responses (258). Thus, redox regulation of HMGB1 may provide another approach to modulate the impact of extracellular HMGB1 on the pathogenesis of lung diseases.
Asthma
Globally, 300 million people suffer from asthma (244, 249, 277, 322). More than 80% of asthma deaths occur in low-income to lower middle-income countries, and in 2015, it was estimated that 383,000 deaths occurred from asthma globally (244, 345). Although asthma has a relatively low fatality rate compared with other chronic diseases, many asthmatics have symptoms that can significantly reduce their quality of life (80, 237, 244, 249). Asthma is often underdiagnosed and undertreated, creating a substantial burden to individuals that can restrict various activities for the remainder of their lives (244, 249). Recurrent asthma symptoms frequently cause sleeplessness, daytime fatigue, reduced activity levels, and school and work absenteeism (237, 244, 249).
Asthma is typically characterized by reversible airway obstruction, airway remodeling, airway hyperresponsiveness, and inflammation (71, 123). Airway irritants, such as pollution, dust, pollen, cold air, tobacco smoke, and chemical irritants, may trigger asthmatic reactions (26, 244, 249, 322, 345). Airway smooth muscle in asthmatic patients is hyperresponsive to certain stimuli such as environmental allergens, resulting in airway constriction and obstruction (236, 254). In addition, there is an increase in inflammatory mediator release and the smooth muscle mass of the lungs (80, 123, 126, 171, 206, 323). Furthermore, asthma airflow obstruction and airway remodeling can be triggered by inflammatory mediators (80, 105, 123, 228, 277, 279).
Extracellular HMGB1 has been shown to be involved in mediating certain inflammatory responses in asthma (Fig. 4) (145, 180). Indeed, HMGB1 levels are significantly increased in the airways of asthmatic patients and in animal models of asthma (71, 126, 206, 295, 315). Di Candia et al. reported that sputum concentrations of HMGB1 and HMGB1 expression in smooth muscle were significantly increased in patients with severe asthma (71). An increase in the airway levels of HMGB1 has been shown to occur in tandem with increased inflammation in neutrophilic sputum, increased blood neutrophil counts, and increased levels of MMP, chemokines, and cytokines in severe asthmatic patients (123, 126).
FIG. 4.
The role of HMGB1 in the pathogenesis of asthma. Asthma is pathologically characterized by airway inflammation, vascular remodeling, airway hyperreactivity, and constriction. Extracellular HMGB1 levels have been reported to be increased in asthma patients. The increased levels of airway HMGB1 in asthma can produce inflammation and vascular remodeling. Airway HMGB1 can bind to RAGE, TLR2, and TLR4. The binding of HMGB1 to these receptors can activate NF-κB and MAPK pathways. NF-κB activation stimulates the expression and secretion of proinflammatory mediators such as IL-8, IL-4, IL-5, IFN-γ, IFN-α, IL-13, IL-6, and IL-17, initiating the infiltration of eosinophils, neutrophils, and helper T cells into the airway. The presence of activated neutrophils in the lungs can increase the levels of ROS, proinflammatory cytokines, proteases (e.g., MMP9), and HMGB1, which can produce cell injury, further increasing neutrophil infiltration. HMGB1-mediated NF-κB activation can also promote the secretion of VEGF and TGF-β, which are involved in vascular remodeling. The subsequent vascular remodeling is characterized by loss of epithelial integrity, increased smooth muscle mass, smooth muscle cell proliferation, and subepithelial fibrosis. TGF-β-induced remodeling can result in an increase in lung fibroblast migration, mucus production, and secretion of ECM components as well as levels of MMP9, collagen, and α-sma. The airway injury that occurs as a result of inflammation and vascular remodeling produces airway hyperreactivity and reversible airway obstruction. ECM, extracellular matrix; IFN, interferon; IL, interleukin; MMP9, matrix metalloproteinase 9; sma, smooth muscle actin; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
The use of anti-HMGB1 antibodies has provided information about the role of HMGB1 in the pathogenesis of asthma. In mouse models of asthma, generated by exposing animals to compounds such as toluene 2,6-diisocyanate (TDI) or ovalbumin, the treatment of these animals with anti-HMGB1 antibody significantly attenuated elevated airway levels of MMP9, cytokines, cell counts, collagen levels, and leukocyte infiltration (126, 206, 369). In addition, airway hyperresponsiveness, inflammatory responses, and airway remodeling were significantly decreased in mice administered anti-HMGB1 antibodies (126, 206).
Moreover, in a mouse model of ovalbumin-induced airway hypersensitivity that produces some of the pathogenic characteristics of asthma, an antibody to HMGB1 significantly (i) inhibited lung HMGB1 expression; (ii) suppressed HMGB1-induced infiltration of neutrophils in the airways; (iii) decreased IL-23 levels and airway hyperresponsiveness; and (iv) attenuated T helper cell type 17 (Th17) secretion of IL-17, IL-4, and IFN-γ (369). Furthermore, eosinophilic airway inflammation, nonspecific airway resistance, and airway responsiveness were also significantly decreased in these animals (295).
Data obtained from mouse models of asthma indicate that HMGB1 stimulates the secretion of proinflammatory mediators such as TNF-α, IL-17, IL-6, IL-8, IL-1β, IL-4, and IL-5 (126, 295, 369). Moreover, HMGB1 mediates the recruitment of inflammatory cells, thereby exacerbating oxidative stress-mediated asthma pathology (126, 206). Indeed, fully reduced HMGB1 significantly increased intracellular ROS levels in the smooth muscles of nonasthmatics (71). The induction of oxidative stress by activation of the HMGB1/TLR4 pathway can subsequently oxidize HMGB1, thereby attenuating inflammatory responses as oxidized HMGB1 is less effective than reduced HMGB1 in eliciting inflammation (258). Although it is not clear how an oxidative environment affects the pathology of asthma, it may facilitate asthmatic airway remodeling of smooth muscle cells (71).
In patients with severe asthma, airway remodeling involves the accumulation of ECM proteins such as fibulin-1 and collagen (193). HMGB1 has been implicated in the increased deposition of ECM proteins suggesting a potential role in the airway remodeling that occurs in asthma (126, 239). HMGB1's role in airway remodeling is, in part, due to the upregulation of several proteases (i.e., MMPs), other enzymes, and growth-related proteins such as vascular endothelial growth factor (VEGF) (76, 188). MMP9 and α-sma are involved in the remodeling of airway smooth muscle (89, 126, 171, 172). In mice with ovalbumin-induced airway hypersensitivity similar to asthma, HMGB1 can upregulate α-sma expression and increase MMP9 expression in the airways and interstitial lung tissue (126).
Excessive levels of airway HMGB1 in subjects with asthma are associated with increased lung levels of α-sma, increased number of fibroblasts (as a result of increased migratory activity), higher secretion of VEGF, TGF-β, higher collagen content, and airway hyperresponsiveness (126). In these mice, high levels of extracellular HMGB1 increased the hyperplasia of mucus secreting goblet cells, increasing mucus secretion (206). This is supported by findings showing that HMGB1 upregulates MUC5AC and MUC5B messenger RNA (mRNA) and protein in human bronchial epithelial cells via HMGB1-RAGE signaling (165).These changes were significantly attenuated by the systemic administration of an anti-HMGB1 antibody (206).
The HMGB1-induced increase in mucus could potentially contribute to the severity of rhinorrhea, airway hyperreactivity, and reversible airway obstruction in asthmatic patients. Overall, these studies suggest that HMGB1 may play a role in mediating inflammation and remodeling in asthmatic subjects. Thus, HMGB1 represents a potential therapeutic target for the treatment of asthma.
Studies using reagents that inhibit the release of HMGB1 or attenuate its downstream signaling pathways provide further support for the critical role of HMGB1 in the pathogenesis of asthma. In ovalbumin-induced asthmatic mice, the intravenous (via tail vein) administration of vitamin D significantly decreased airway resistance, inflammatory response, and inflammatory cytokine secretion, and increased the secretion of the anti-inflammatory cytokine, IL-10 (370). Importantly, attenuation of these effects was correlated with a significant decrease in HMGB1 levels and HMGB1 translocation from the nucleus to cytoplasm (370). In addition, 10–100 mg/kg of ethyl pyruvate interperitoneally administered to mice with TDI-induced asthma significantly decreased the number of immune, cells including macrophages, neutrophils, eosinophils, and lymphocytes, and significantly decreased the levels of extracellular HMGB1 (315).
Overall, there are data suggesting that in asthma, HMGB1 plays a deleterious role in disease progression and severity by inducing inflammation and airway remodeling. Therefore, HMGB1 may be a potential therapeutic target in the treatment of asthmatic patients.
Chronic Obstructive Pulmonary Disease
COPD (Fig. 5) is a major cause of morbidity and mortality worldwide. As of 2010, estimates suggest that ∼328 million people suffer from COPD (198). Approximately 3 million people die each year from COPD, making it the fourth largest cause of death since the year 2000 (198). About 70% of all cases of COPD are caused by cigarette smoking or chronic inhalation of environmental and occupational air pollutants (198, 335). COPD is characterized by emphysema and chronic bronchitis (112, 162, 207). Decreased airflow in the lungs of COPD patients is a symptom caused by fixed airway obstruction and increased pulmonary inflammation (112, 162, 207). Repeated injury to the respiratory system from cigarette smoke or air pollutants in patients with COPD can result in alveolar tissue destruction (i.e., emphysema), hypersecretion of mucus (i.e., chronic bronchitis), and fibrosis, which can hasten disease progression (112, 162, 207).
FIG. 5.
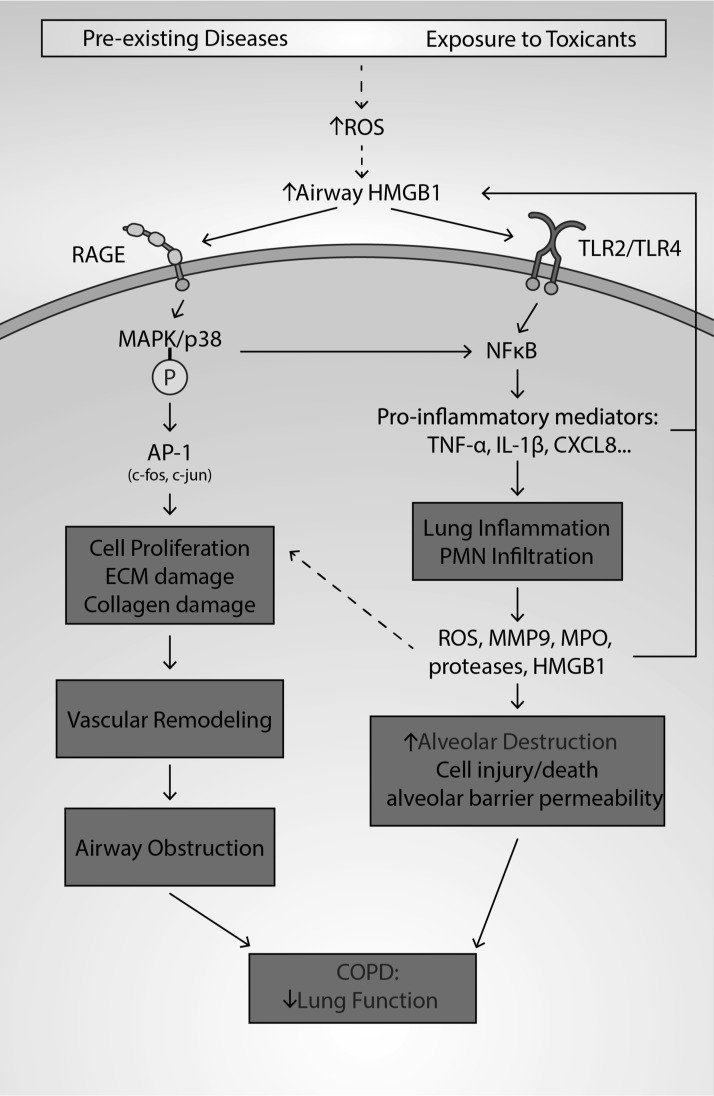
The role of HMGB1 in the pathogenesis of COPD. COPD, caused by chronic inhalation of environmental pollutants, cigarette smoking, or preexisting diseases, is pathologically characterized by chronic obstruction of airways, alveolar dysfunction, and airway inflammation. Increased levels of ROS in the lungs of subjects with COPD can induce the accumulation of airway HMGB1. Airway HMGB1, via a number of mechanisms, can exacerbate COPD by inducing excessive lung inflammation and vascular remodeling. Lung inflammation is induced by HMGB1 binding to TLR2, TLR4, and RAGE, which increases the likelihood of NF-κB translocating to the nucleus. Subsequently, NF-κB increases the expression and secretion of proinflammatory cytokines such as IL-1β, IL-8, TNF-β, IFN-γ, and MCP-1, which induce leukocyte infiltration and produce inflammation. Increased MPO activity, MMP9, and ROS levels from the neutrophils/monocytes can cause damage to the tight junctions of endothelial/epithelial cells, causing a loss of barrier integrity. Furthermore, the increased levels of elastase, proteases, MPO, and ROS can damage epithelial/endothelial cells, causing increased vascular and alveolar permeability, subsequently contributing to further HMGB1 accumulation in the airways and exacerbating lung inflammation. The injury and death of endothelial and epithelial cells can further result in alveolar destruction and a decline in lung function. HMGB1 also signals through RAGE to activate MAPK pathways. MAPK can activate the transcription factor AP-1, promoting proliferation of pulmonary endothelial and smooth muscle cells. Neutrophil elastase and MMP9 contribute to ECM and elastic fiber damage. Overall, these aforementioned changes promote vascular remodeling, which contributes to airway obstruction. AP-1, activator protein 1; COPD, chronic obstructive pulmonary disease; MCP-1, monocyte chemoattractant protein-1; MPO, myeloperoxidase; TNF, tumor necrosis factor.
Recently, studies in both patients and animal models have implicated the role of HMGB1 in the pathogenesis of COPD. First, HMGB1 levels in the lung tissue of COPD patients were either 17-fold (smokers) or 10-fold (nonsmokers) greater than those of healthy controls (158). In addition, the increase in the airway levels of HMGB1 is negatively correlated with a 60% (smokers with COPD) and 40% (nonsmokers with COPD) decrease in the forced expiratory volume 1% (FEV1%) (90, 144). Furthermore, BALF samples from 14 healthy nonsmokers, 13 smokers without COPD, and 30 smokers with COPD were analyzed, and the levels of peripheral (alveolus and bronchioles) airway HMGB1 were significantly increased in COPD patients. This increase was positively correlated with a significant decrease in the FEV1% score (90). The mean HMGB1 levels in the peripheral BALF of smokers with COPD, smokers without COPD, and healthy controls were 1496, 816, and 250 ng/mL, respectively. Thus, both smokers with COPD and nonsmokers with COPD had significantly higher (p < 0.01) levels of peripheral airway HMGB1 compared with healthy controls (144).
In addition, in the subset of patients with COPD who also have other lung diseases, such as idiopathic pulmonary arterial hypertension (COPD+IPAH), serum HMGB1 levels are further increased compared with healthy patients (366). The serum levels of HMGB1 from COPD+IPAH patients were 4.2 and 2.6 ng/mL for patients with only COPD, compared with 1.05 ng/mL in healthy volunteers (366). A study in 36 COPD patients reported a significant increase in serum HMGB1 compared with healthy controls. Increased HMGB1 levels were positively correlated with significant decreases in FEV1% and oxygen saturation (302). Interestingly, recent genome-wide association studies reported that HMGB1 is the top differentially expressed gene (p = 5.40 × 10−10) that is significantly correlated with decreased lung function (FEV1%) in COPD patients (224). Based on these results, it has been hypothesized that HMGB1 is involved in the pathogenesis of COPD (144, 224, 302, 366).
Numerous studies in animal models of COPD provide further compelling evidence for the role of HMGB1 in COPD pathology (31, 56, 366). While it is currently unknown whether HMGB1 accumulation in the lungs of COPD patients is due to disease progression or from direct injury to the lungs, it has been found that cigarette smoke induces the accumulation of HMGB1 in the airways of mice. The exposure of mice to five cigarettes four times a day for 3 days significantly increased HMGB1 accumulation in the airways and significantly upregulated TLR4 expression (56). Long-term exposure of mice to cigarette smoke can induce higher levels of HMGB1 in lung tissues (31). In addition, HMGB1 expression is increased in the lung tissue in animal models of COPD (59, 334). Furthermore, studies in in vitro models of COPD have revealed that hydrogen peroxide can induce the nuclear to cytoplasmic translocation of HMGB1, subsequently releasing significantly high levels of HMGB1 into the cell culture media (127).
Numerous studies indicate that excessive lung inflammation can also contribute to airway obstruction and exacerbation of COPD (59, 90, 158, 302, 366). It has been reported that in COPD patients, platelet-activating factor receptor (PAFR) levels are increased compared with nonsmoking healthy controls (297). The activation of PAFRs in COPD can elicit inflammatory cell recruitment, proinflammatory cytokine production, oncogenic cell growth, angiogenesis, and tumor growth and exacerbate inflammation (56, 205, 290, 297). HMGB1 levels are significantly increased in cigarette smoke-exposed neutrophils, which have an upregulated expression of PAFR (120, 297). In addition, the JNK, p38, and NF-κB pathways were activated by smoke exposure, and this effect was augmented by exogenous HMGB1 (56).
In TLR4-knockout mice exposed to cigarette smoke or in mice treated with an HMGB1 polyclonal antibody, the airway levels of IL-1β, TNF-α, IL-6, IFN-γ, IL-8, and MCP-1 were significantly decreased (56). In fact, in myeloid differentiation primary response 88 (MyD88) knockout mice exposed to cigarette smoke and exogenous HMGB1, there was no significant activation of JNK and p38, and phosphorylation of NF-κB inhibitor (IκB-α) (56). Ethyl pyruvate (20 mg/kg administered concomitantly with cigarette smoke), an inhibitor of NF-κB activation (56), significantly decreased cigarette smoke-induced HMGB1 airway accumulation. These studies in mouse models of COPD suggest that airway HMGB1 can activate NF-κB and JNK/p38 pathways by TLR4- and MyD88-mediated pathways (56). The inflammation in COPD caused by cigarette smoke has been shown to induce neutrophil infiltration into the lung (56, 120, 205). Interestingly, continuous exposure of airway neutrophils to cigarette smoke induced a shift from neutrophil apoptosis to necrosis (120, 162, 205). Neutrophil-derived ROS and protease secretion can promote the progression of COPD (1, 77, 93, 259).
Moreover, the binding of HMGB1 to RAGE activates MAPK/p38 pathways in mouse models of COPD to induce vascular remodeling, which occurs in the lungs of COPD patients (173, 334). Overall, these data suggest that (i) HMGB1 may play a role in vascular remodeling and airway obstruction, and (ii) oxidative stress, generated after exposure to smoke, can induce the accumulation of extracellular HMGB1 in animals, activating its downstream pathways, leading to the COPD pathologies (127). Thus, while it is unclear whether HMGB1 induces COPD or is secondary to COPD initiation, it is evident that HMGB1 plays an essential role in the pathogenesis of emphysema and chronic bronchitis in COPD.
Acute Lung Injury/Acute Respiratory Distress Syndrome
ALI and ARDS (Fig. 6) are major concerns for patients in intensive care units (ICUs) (29, 134, 227). A recent international study of 50 countries indicated that 10% of all admitted ICU patients were diagnosed with ALI/ARDS (29). Patients diagnosed with ALI/ARDS can develop respiratory failure and hypoxemia, and, currently, the only supportive measure is invasive mechanical ventilation (MV) with hyperoxia, which has been shown to exacerbate ALI and ARDS (29, 161, 227). The clinical outcomes for patients with ARDS are problematic, with mortality rates as high as 75% (367). ALI/ARDS is primarily caused by infection, sepsis, trauma, shock, and inhalation of toxicants, including particulate matter or other substances (161, 246, 252, 269). In addition, it is well known that animal models of ALI can be induced or exacerbated by prolonged exposure to hyperoxia (85, 115, 269, 319).
FIG. 6.
The role of HMGB1 in the pathogenesis of ALI and ARDS. ALI and ARDS are caused by various conditions and characterized by excessive lung inflammation, increased alveolar permeability, and impairment of respiratory function. Increased levels of airway HMGB1 in patients and animals with ALI and ARDS induce the accumulation of airway HMGB1. The deleterious effects of airway HMGB1 are mediated by excessive lung inflammation and alveolar destruction. Airway HMGB1 induces lung inflammation by binding to TLR2, TLR4, and RAGE, which increases the likelihood of NF-κB translocating to the nucleus, where it increases the transcription of genes that code for proinflammatory cytokines such as IL-1β, TNF-α, IL-4, and IL-6, causing leukocyte infiltration and inflammation. Leukocytes increase the levels of MPO activity, ROS production, and proteases, thereby damaging endothelial/epithelial cells and their tight junctions, causing the dysfunction of endothelial/epithelial and other lung cells, which compromises alveolar barrier and lung function. In addition, the activation of NF-κB is associated with HMGB1 hyperacetylation, which leads to its subsequent translocation to the cytoplasm and its active release into the airways. The increased production of ROS, such as NO, can inhibit SIRT-1 (a deacetylase enzyme) and can further stimulate HMGB1 release. Airway HMGB1 can also signal through RAGE-activated MAPK/p38 pathways, which can induce the phosphorylation of Hsp27, an actin-binding protein. Phosphorylated Hsp27 can produce endothelial cytoskeleton disorganization, paracellular gap formation, and the loss of peripheral organized actin fibers. These effects produced by phosphorylated Hsp27 can produce cell/cell junction dissociation and a loss of alveolar barrier integrity, which also contribute to a decrease in lung function. The accumulation of airway HMGB1 can be attenuated by cholinergic anti-inflammatory pathway modulators (e.g., endogenous acetylcholine or GTS-21) and NF-κB inhibitors (e.g., ethyl pyruvate or GTS-21). Antioxidants, such as ascorbic acid, hydrogen sulfide (in gaseous form), and resveratrol, can also decrease ROS levels and decrease airway HMGB1 levels. ALI, acute lung injury; ARDS, acute respiratory distress syndrome; Hsp27, heat shock protein 27; SIRT-1, Sirtuin-1.
ALI is characterized by excessive lung inflammation with neutrophil/monocyte infiltration and dysfunction of the alveolar endothelial/epithelial barrier, leading to increased alveolar capillary permeability, subsequent lung cell dysfunction, and pulmonary edema (43, 85, 113, 269). A major pathological feature of ALI/ARDS is increased levels of cytokines/chemokines in the airways and plasma, such as TNF-α, IL-1β, MCP-1, IL-6, and IL-8 (13, 106, 185). Proinflammatory cytokines, such as IL-1β and TNF-α, are early mediators of inflammation and are present primarily at the onset of ALI/ARDS (16).
After the first report of HMGB1's role as a late mediator of inflammation during septic shock, it was postulated that HMGB1 could be a major mediator of lung inflammation in endotoxin-induced lung injury (336). The intratracheal administration of HMGB1 can induce a concentration-dependent increase in the infiltration of interstitial/intra-alveolar neutrophils, alveolar red blood cells, levels of MPO and lung edema, similar to endotoxin-induced ALI. This suggests that HMGB1 may play a role in mediating inflammatory lung injury in ALI/ARDS (3, 326).
The level of HMGB1 in the airways of postsurgical patients who required MV for several days was 10-fold higher compared with patients who were ventilated for 5 h (377). However, the levels of airway HMGB1 in patients ventilated for 5 h were not significantly different from healthy controls. In addition, patients who developed postsurgery VAP had a 17-fold higher level of airway HMGB1 compared with healthy controls. These data suggest that prolonged exposure to MV is required to induce the accumulation of HMGB1 in the airways (377).
The role of HMGB1 in the pathogenesis of ALI has been further confirmed in mouse models. For example, the intratracheal administration of anti-HMGB1 antibodies (360 μg anti-HMGB1 antibody/mouse) ameliorated the development of lung injury and increased survival rates (3, 85, 248). In addition, airway HMGB1 can attenuate the phagocytosis of invading bacteria and apoptotic neutrophils by alveolar macrophages, exacerbating inflammatory lung injury (86, 94, 109, 248). Moreover, the HMGB1 box A protein, an antagonist of HMGB1 binding to the RAGE receptor, can significantly attenuate lung injury by decreasing airway inflammation, further suggesting that HMGB1 is involved in the initiation and progression of ALI/ARDS (103).
One major pathological feature of ALI/ARDS is the presence of excessive lung inflammation (191, 214, 342). The accumulation of airway HMGB1 has been reported to produce excessive lung inflammation (3, 13, 85). Extracellular HMGB1, on binding to pneumocytes, neutrophils, and macrophages, can produce potent proinflammatory effects that induce the release of other proinflammatory cytokines, such as TNF-α, IL-1β, IL-8, MCP-1, and IL-6 (13, 178). Consequently, lung cells are injured, cell/cell junctions are damaged, and endothelial/epithelial barrier permeability is increased, causing lung injury and loss of lung function (43, 85, 113, 269, 348).
The other major pathological feature of ALI/ARDS is the presence of lung edema, in part, mediated by an increase in the permeability of the endothelial/epithelial barrier (33, 78, 219, 346). The binding of extracellular HMGB1 to TLR2, TLR4, and RAGE can compromise endothelial cell/cell junction and increase endothelial barrier permeability (348). While the binding to TLR receptors can cause inflammatory damage to lung cells, HMGB1 can also affect the permeability of endothelial cells by activation of RAGE-mediated pathway (287), as illustrated in Figure 3. Several mechanisms have been implicated in the accumulation of HMGB1 in the airways during lung injury. Various biomolecules, such as endotoxins, TNF-α, IFN-β, and hydrogen peroxide, as well as hyperoxia, can induce hyperacetylation and release of HMGB1 from lung cells (85, 201, 248, 314, 336). These results suggest that HMGB1 is one of the mediators that induce the loss of endothelial barrier integrity, causing inflammatory lung injury in patients with ALI/ARDS.
Pulmonary Fibrosis
Pulmonary fibrosis (PF) is an interstitial lung disease that is often irreversible and lethal (153, 157, 284, 341). The median survival of PF is 3 years, and it affects 50,000 individuals annually in the United States (283, 341). Between 1988 and 2005, the estimated incidence of PF increased from 6.8 to 17.4 per 100,000 people in the United States (230). PF is characterized by lung scarring and the formation of fibroids, inflammation, vascular leakage, myofibroblast recruitment, and deposition of elevated amounts of ECM proteins (157, 180, 283). Lung tissue scarring impairs the diffusion of oxygen through alveolar walls into the bloodstream (5, 174, 284).
Patients with PF experience shortness of breath and dry cough (84, 157, 284). PF can increase susceptibility to other conditions such as pneumonia, pneumothorax, pulmonary hypertension, pulmonary embolism, and eventually, respiratory and heart failure (135, 284, 304). While individuals can be genetically predisposed to PF, exposure to hazardous materials (e.g., asbestos, pollutants, and cigarettes) and comorbidities such as autoimmune diseases, sarcoidosis, COPD, and viral infections can increase the risk of PF (157, 284, 294). Genetics can account for up to 30% of the risk of developing PF (7, 146, 284, 286, 360). Mutations in surfactant protein A2 and C, and mutations affecting telomere length are all associated with an increased risk of PF (7, 360).
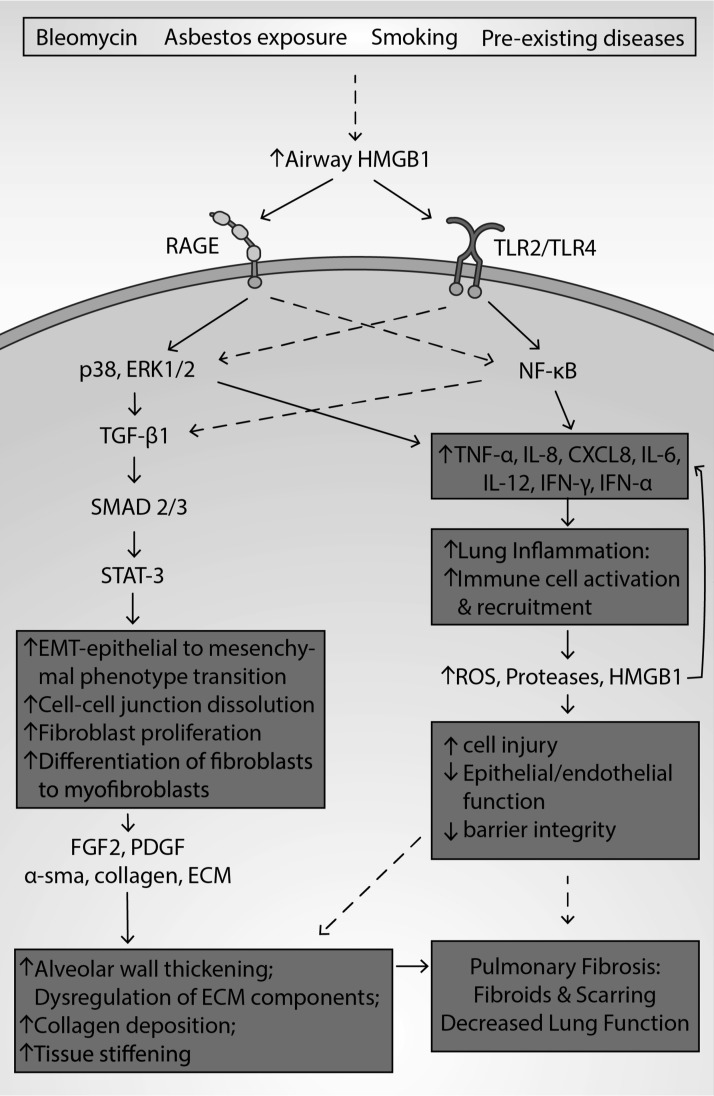
In patients and subjects with PF, decreased survival has been associated with increased levels of serum and airway HMGB1 (84, 114, 296, 372). In addition, in a mouse model of bleomycin-induced PF, the administration of anti-HMGB1 antibody significantly attenuated the thickening of alveolar walls, decreased collagen content in lung homogenate, and lowered the number of total immune cells present in the airways (114). These studies suggest that HMGB1 plays a role in PF pathogenesis and the degree of disease severity.
HMGB1 plays a role in mediating fibrosis and airway remodeling in PF. HMGB1 can stimulate ROS production, increase α-sma, collagen deposition, and TGF-β, induce the differentiation of fibroblasts to myofibroblasts, and increase the gene expression of MUC8, a mucin that denotes aberrant alveolar epithelia common in PF (154, 340). Zhang et al. also reported that in a model of PF, collagen fibers in the lung tissue of rats were significantly increased in bleomycin-treated rats compared with animals treated with vehicle (373).
The HMGB1-RAGE axis also plays a role in the inflammatory pathology of PF. In a bleomycin-induced PF model, 29% of wild-type mice survived after 14 days of bleomycin administration, while 100% of RAGE knockout mice survived (117). Furthermore, in this PF model, RAGE knockout mice had lower levels of fibrotic deposition and decreased airway levels of TGF-β1. However, total leukocyte, lymphocyte, macrophage, and neutrophil infiltration into the lung, and airway levels of HMGB1 were not reduced, suggesting that HMGB1 inflammatory signaling in PF may be more complicated than RAGE signaling alone (117). Treatment of animals with an anti-RAGE antibody and pharmacological inhibition of ERK1/2 significantly decreased epithelial barrier disruption and permeability associated with inflammation (117, 133). Furthermore, direct inhibition of NF-κB by pyrrolidinedithiocarbamate (PDTC) or upstream inhibition by targeting TLR2 in bleomycin-induced PF reduces edema, histological markers of inflammation in lung tissue, HMGB1-induced TGF-β1 release, collagen-1 levels, and α-sma expression (177, 340).
The role of HMGB1 and the levels of RAGE expression and its signaling pathways are not only limited to lung inflammation but are also associated with remodeling and deposition of fibrotic tissue in PF. Increased levels of RAGE are associated with ameliorated lung injury and decreased apoptosis in alveolar epithelial cells (372). However, in a rat model of PF, RAGE expression was significantly decreased compared with the controls (372). Furthermore, in a model of bleomycin-induced PF, RAGE null mice had less fibrotic injury, such as edematous alveolar thickening, cellular infiltration, fibrosis, and collagen deposition (84, 117). Thus, HMGB1/RAGE interaction and signaling in the profibrotic processes play a role in the development of PF.
HMGB1-mediated activation of NF-κB also plays a role in airway remodeling in PF. HMGB1-mediated activation of NF-κB can induce the upregulation of α-sma and TGF-β1, a mediator of vascular remodeling via SMAD-STAT-3 signaling (84, 180, 251, 316, 373). Activated STAT-3 can increase α-sma, collagen deposition, and total airway leukocytes and can induce cell proliferation and TGF-β-dependent myofibroblast differentiation (251, 316, 340). Zhang et al. also reported that the exposure of alveolar epithelial cells to TGF-β1 promoted HMGB1 expression and decreased RAGE expression (372). These studies suggest that HMGB1 may play a role in EMT via SMAD-STAT signaling.
Compounds targeting RAGE or HMGB1 have been used in PF models; however, the clinical efficacy of these compounds remains to be determined. Anti-HMGB1 antibodies and HMGB1 neutralizing reagents such as thrombomodulin decrease the levels of HMGB1 in animals and attenuate fibrotic lung injury (2, 140, 153, 187). Thrombomodulin binds directly to HMGB1 and cleaves it, degrading it to a less inflammatory form (2, 140, 153, 187). Thrombomodulin improves the survival rate of patients by inactivating coagulant factors and thrombin, as well as by competitively inhibiting the binding of HMGB1 to receptors such as TLR4 and RAGE (153, 180, 296).
Treatments targeting the RAGE-HMGB1 signaling pathway could significantly ameliorate the pathology and symptomatology of PF. Although current treatments, such as prednisone, azathioprine, and N-acetylcysteine, may improve the symptoms of PF, there is a need for PF-specific therapies targeting disease biomarkers such as HMGB1 (124, 211, 306). Overall, data suggest that decreasing the accumulation of HMGB1 and attenuating its downstream signaling with RAGE may decrease inflammation in PF. Thus, HMGB1 may be a novel therapeutic target that has the potential to decrease mortality and severity of symptoms in PF (Fig. 7) (153).
FIG. 7.
The role of HMGB1 in the pathogenesis of PF. PF, an interstitial lung disease, is characterized by fibroblast proliferation, EMT, lung inflammation and the formation of fibroids, producing lung scarring. Patients and animals with PF have been shown to have increased levels of airway HMGB1. HMGB1 may be involved in the pathogenesis of PF as it can produce fibroblast differentiation and proliferation, alveolar wall thickening or muscularization thickening, and collagen accumulation. HMGB1 binds to RAGE, TLR2, and TLR4, activating both NF-κB and MAPK/p38/ERK 1/2 pathways. NF-κB activation and the p38 pathway can increase the levels of proinflammatory mediators and TGF-β1. As a mediator of vascular remodeling, TGF-β1 can induce phosphorylation of SMAD2 and SMAD3, which phosphorylate the transcription factor STAT-3. STAT-3, when activated, induces cell proliferation and myofibroblast differentiation. STAT-3 activation promotes EMT, where epithelial cells develop mesenchymal characteristics such as loss of cell/cell adhesion. The increased number of fibroblasts and myofibroblasts can stimulate the formation of fibrotic tissue. TGF-β1 can elicit the release of α-sma, upregulate HMGB1 expression, and induce secretion of the profibrotic factors FGF2 and PDGF. α-sma directly stimulates the EMT process, causing an increased accumulation of ECM proteins, which augments airway remodeling. ECM-mediated remodeling involves the dysregulation of ECM composition, cleavage of ECM components, increased collagen deposition, and stiffening of tissue. In addition to inducing remodeling, the binding of HMGB1 to RAGE and TLR4 stimulates the expression and secretion of TNF-α, MCP-1, IL-8, and IL-1β. These proinflammatory mediators recruit and induce the accumulation of macrophages, neutrophils, and lymphocytes into the airways, increasing the levels of proteases, ROS, and HMGB1, establishing a deleterious feedback loop. The release of these molecules contributes to epithelial and endothelial injury and death, dissolution of cell/cell junctions, and loss of barrier integrity, thereby increasing alveolar permeability. Thus, HMGB1-mediated airway remodeling and inflammation contribute to lung fibrosis, scarring, and decreased lung function. EMT, epithelial to mesenchymal transition; ERK, extracellular signal-regulated kinase; FGF2, fibroblast growth factor 2; PF, pulmonary fibrosis; STAT-3, signal transducer and activator of transcription 3.
Cystic Fibrosis
CF (Fig. 8) is a genetic disease affecting 30,000 people, with ∼1000 new cases diagnosed each year in the United States (64, 81). CF is diagnosed by phenotypic features, including sinopulmonary disease, gastrointestinal and nutritional abnormalities, persistent cough with thick mucus, wheezing, recurrent lung infections, as well as poor weight gain and growth (82, 102, 285). Histopathological studies indicate that neutrophilic inflammation and pulmonary infections occur frequently in CF patients, producing acute pulmonary exacerbation and severe lung dysfunction (21, 35, 50, 57, 83, 102).
FIG. 8.
The role of HMGB1 in the pathogenesis of CF. CF is a disease caused by mutations in the CFTR gene and its pathological hallmarks include neutrophilic inflammation and bacterial infections. An increased expression and accumulation of HMGB1 are present in CF airways, resulting in an increase in HMGB1 levels in bronchoalveolar lavage fluids, sputum, and serum of CF patients. HMGB1 elicits chemotactic activity by interacting with the chemokine CXCR2 receptors on neutrophils, thereby recruiting them into the airways, compromising macrophage phagocytosis. In CF, HMGB1 may produce an impairment of macrophage efferocytosis, which decreases the removal of apoptotic neutrophils and the clearance of pathogenic bacteria. We postulate that the activation of the TLR4 receptor by HMGB1 attenuates macrophage function, producing a higher bacterial load and excessive inflammation in the airways and lung tissue, causing lung injury. The increase in PAMP and DAMP biomolecules from the increased bacterial load and excessive inflammation further stimulates the release of HMGB1 from airway cells, producing a deleterious feedback cycle between the release of HMGB1 and the development of lung injury. In addition, serum HMGB1 impairs the function of pancreatic β cells by activating RAGE and TLR4, producing insulin deficiency and insulin resistance, causing CFRD. Insulin and ODSH can reduce HMGB1 expression and translocation from the nucleus to cytoplasm in cells (epithelial cells and macrophages, respectively). ODSH can also reduce chronic neutrophil-mediated inflammation induced by neutrophil elastase in CF. CF, cystic fibrosis; CFRD, cystic fibrosis-related diabetes; CXCR, CXC-chemokine receptor; ODSH, 2-O,3-O desulfated heparin; PAMP, pathogen-associated molecular pattern.
The first clinical report on CF was published in 1938 and, until recently, the majority of individuals diagnosed with CF died in infancy or early childhood (10). As a result of improvements in symptomatic management, including the use of anti-inflammatory, antimicrobial drugs and nutritional supplements, more than two decades have been added to the life span of CF patients (82, 240). However, the median survival age is still only around 40 years, and thus, novel therapeutic approaches and biomarkers that are predictive of the development of CF are urgently needed (40, 64, 240).
Studies over the last two decades suggest that HMGB1 may represent a new therapeutic target for CF (86, 320). CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes the CFTR protein (151, 164). Almost 2000 mutations in the CFTR gene have been identified, which are classified into six major classes based on the effects of the mutations on the CFTR protein (28, 82, 164). The mutations alter the secondary and tertiary structure of CFTR protein, a chloride ion channel, resulting in impaired secretion of chloride and an increase in sodium absorption (164). This can cause abnormal levels of chloride, bicarbonate, sodium ions, and water, affecting the epithelial lining in the lungs and digestive system (38, 151).
Levels of HMGB1 are significantly increased in the BALF, sputum, and serum of CF patients (110). The two secondary pathogenic mechanisms of CF in the airway are the recruitment of neutrophils and susceptibility to Pseudomonas aeruginosa (PA) infections, which further increase HMGB1 release into the airways (110). A high load of neutrophil elastase, as a result of neutrophil recruitment, can further induce the in vitro and in vivo release of HMGB1 from macrophages (110). In Scnn1b-transgenic mice, which exhibit obstructed mucus production similar to the CF phenotype, and in CFTR−/− CF mouse models, HMGB1 levels are significantly increased in the lungs and airways, subsequently increasing the number of leukocytes in the airways through a CXC-chemokine receptor (CXCR)-dependent mechanism (86, 274). Moreover, an anti-HMGB1 antibody decreases the chemotactic efficacy of HMGB1 in CF patient sputum and in the bronchoalveolar lavage (BAL) of CF Scnn1b-transgenic mice (274). These findings are congruent with studies reporting that the administration of an anti-HMGB1 antibody to CF mice significantly decreased neutrophil infiltration into the airways and attenuated inflammatory lung injury (86, 274). Thus, these studies suggest that HMGB1 plays a role in mediating neutrophilic inflammation in CF.
Pulmonary infections, particularly those due to PA, occur frequently in CF patients, as a result of compromised immunity (42, 64, 86, 142, 167). For example, macrophages from CF patients have a significantly lower phagocytic activity compared with healthy individuals (142). The impaired macrophage phagocytosis compromised by CF patient BALF may be mediated by increased levels of airway HMGB1 as CF BALF-compromised macrophage phagocytic activity was attenuated following the administration of an anti-HMGB1 antibody (86). Furthermore, macrophages from TLR4−/− mice do not respond to the HMGB1-mediated impairment of macrophage phagocytosis, suggesting that HMGB1-mediated macrophage dysfunction occurs via HMGB1 activation of TLR4 (86).
HMGB1 can also inhibit neutrophil efferocytosis by macrophages in CF, resulting in the accumulation of apoptotic neutrophils in the airways, which can augment proinflammatory responses (194). Alveolar macrophages in CF are also characterized by the overproduction of sCD14 and inflammatory cytokines, as well as a decreased expression of CD11b and TLR5, leading to an overactive proinflammatory response and nonresolution of infection by CF macrophages (142). Pulmonary infections, such as PA, can further induce the release and subsequent accumulation of airway HMGB1, creating a detrimental feedback cycle between infections and HMGB1 accumulation in the lungs (200). However, PA in CF patients does not significantly alter the expression of HMGB1 mRNA, suggesting that PA induces HMGB1 extracellular accumulation by increasing HMGB1 nucleocytoplasmic translocation and release into the airways in CF (320).
At present, the mechanisms that induce HMGB1 accumulation in the airways of CF patients remain to be elucidated. Montanini et al. reported that HMGB1 expression in the CF epithelial cell line, CFBE41o-, is significantly higher than the normal control 16HBE14o- cells (222). The incubation of 16HBE14o- cells with a CFTR inhibitor (CFTR inh 172) increases HMGB1 expression, suggesting that loss of CFTR function can induce HMGB1 expression (222). In the same study, insulin significantly decreased HMGB1 gene expression in CF epithelial cells, suggesting a correlation between upregulated HMGB1 and the diabetic condition in CF subjects. It was postulated that HMGB1 can serve a potential therapeutic target for the treatment of patients with CF (222).
In conclusion, these studies suggest that HMGB1 can serve as a biomarker in CF. However further studies are needed to determine the sources and mechanisms of extracellular HMGB1 accumulation in CF patients, as well as to verify the role of HMGB1 as a therapeutic target in CF.
Pneumonia
Pneumonia (Fig. 9) is an inflammatory condition of the lung primarily affecting the alveoli. The main cause of pneumonia is infection of the lung parenchyma, and it is symptomatically characterized by productive or dry cough, chest pain, fever, and troubled breathing (325). Pneumonia is a leading cause of hospitalization among adults and children in the United States, and at least 1 million people seek medical care for the disease every year. In the United States, ∼50,000 people die from pneumonia annually (60, 61, 241). Globally, 1 million children younger than the age of 5 die from pneumonia each year, which is greater than the numbers of death by any other infectious disease in children, including HIV, malaria, and TB (47, 197, 209, 330). Pneumonia can be caused by bacteria, viruses, and less commonly, fungi. The risk of pneumonia is increased in patients with other health conditions such as asthma, COPD, diabetes, or compromised host defense due to treatments with drugs (60, 61, 232, 241). Based on the setting in which it develops, pneumonia can be categorized into (i) community-acquired pneumonia (CAP), (ii) hospital-acquired pneumonia, (iii) health care-acquired pneumonia, and (iv) aspiration pneumonia (23, 73, 241, 255, 268).
FIG. 9.
The role of HMGB1 in the pathogenesis of pneumonia. Pneumonia is a disease caused by bacteria, viruses, and fungi that can produce pulmonary inflammation. Risk factors include the presence of other diseases such as CF, COPD, and asthma. PAMP molecules released by viruses and bacteria interact with cell surface receptors such as TLRs and RAGE, which increases oxidative stress and the expression of HMGB1, leading to its increased translocation and subsequent release of HMGB1 from airway cells such as macrophages and epithelial cells. The release of HMGB1 induced by LPS is JNK dependent. The accumulation of HMGB1 in the airway activates NF-κB and ERK/p38 pathways by binding to TLRs and RAGE on airway cells, increasing the production of ROS, inflammatory cytokines, ICAM-1, and vascular cell adhesion molecule 1 (VCAM-1). Airway HMGB1 can also induce the expression of TLR2 on certain cells, further increasing inflammatory responses in the lung. These molecules mentioned above can elicit excessive inflammation and tissue damage in the lung. ICAM-1, intercellular adhesion molecule 1; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; VCAM-1, vascular cell adhesion molecule 1.
The levels of HMGB1 in the lung tissues and body fluids are altered in patients with pneumonia. Significantly higher levels of HMGB1 in BALF, sputum, and plasma have been reported in patients with different types of pneumonia compared with healthy individuals (8, 14, 122, 248, 338, 378). Interestingly, Wang et al. reported that plasma levels of HMGB1 are decreased in patients with pneumonia after antibiotic treatments (338). Furthermore, the plasma levels of HMGB1 were positively correlated with the pneumonia severity index (PSI) score, suggesting that HMGB1 levels may be indicative of pneumonia severity, although lack of such correlation was also observed in other studies (14, 122, 338). Such conflicting results may be due to small sample sizes, differences in the causes of pneumonia, and other confounding risk factors during the treatments and hospitalization (8). Nonetheless, these studies indicate that HMGB1 concentrations in plasma/serum were elevated in patients with pneumonia and remained elevated throughout the course of treatments in hospitals. In addition, the level of HMGB1 in the sputum is significantly higher in patients with pneumonia caused by Streptococcus pneumoniae (8). These studies suggest that significantly increased levels of HMGB1 can be a biomarker of pneumonia, but they do not indicate the prognosis of pneumonia. Thus, future research should be conducted under narrower scopes and focus on the role of HMGB1 in pneumonia with more specific settings.
Studies using anti-HMGB1 antibodies have demonstrated that HMGB1 mediates the pathology of pneumonia (129, 248, 317). In numerous mouse models of pneumonia, targeting HMGB1 with monoclonal antibodies not only attenuated inflammatory lung injury but also decreased bacterial burden in the lungs of mice with VAP, Staphylococcus aureus pneumonia, and pneumonia due to influenza A virus subtypes H5N1 and H1N1 (92, 129, 235, 248). By neutralizing HMGB1 with anti-HMGB1 antibodies in mouse and human adenovirus-infected epithelial cells, HMGB1-mediated inflammation can be significantly reduced, accompanied by a decrease in the expression of HMGB1 receptors, (e.g., TLR4, TLR9, RAGE), and decreased NF-κB activation (317).
PAMPs, including bacterial components such as LPS, peptidoglycan, and lipoteichoic acid, can activate airway cells via PRRs (alveolar macrophages and epithelial cells) to increase HMGB1 expression and induce the translocation and release of HMGB1 into the extracellular milieu (324, 376). Bacteria, including Mycoplasma pneumoniae, S. pneumoniae, S. aureus, and Escherichia coli, have been shown to induce the release of HMGB1 into the airways in mice (4, 74, 260). Viruses, such as human adenovirus and influenza A virus, can evoke HMGB1 release and subsequently induce an HMGB1-mediated inflammatory response via activation of NF-κB (317, 379).
In addition, these pathogens not only promote HMGB1 expression and HMGB1 release by interacting with PRRs such as RAGE but also upregulate the expression of these receptors, exacerbating the downstream cascades that can result in an inflammatory response. Morbini et al. reported that RAGE expression was significantly upregulated in patients with interstitial and postobstructive pneumonia (223). TLR receptors and RAGE are also upregulated during pneumonia caused by M. pneumoniae, S. pneumoniae, human adenovirus, and influenza A virus (74, 317, 379).
HMGB1 can be passively or actively released into the airways by injured cells and immune cells on exposure to respiratory infections, which contributes to the high levels of extracellular HMGB1 accumulation in the lung of patients with pneumonia (199, 248). However, the main source of the circulating HMGB1 in plasma/serum is from activated immune cells rather than injured cells in patients with CAP (8). Airway HMGB1 can function as a DAMP molecule that can activate immune cells via PRRs (e.g., TLR2, TLR4, and TLR9) and RAGE (267). During pulmonary bacterial infection, PRRs can be activated by both PAMPs and DAMPs such as HMGB1, which together can cause synergetic effects in activating immune cells, producing exacerbated inflammatory lung injury (8, 130, 256).
The critical roles that HMGB1 plays in the pathogenesis of pneumonia have also been confirmed by numerous studies using compounds that can either inhibit HMGB1 accumulation in the airways or block its downstream pathways. Compounds such as the α7 nicotinic acetylcholine receptor partial agonist, GTS-21, and the NF-κB inhibitors, BAY 11-7082 and ethacrynic acid, inhibit HMGB1 release by decreasing the post-translational hyperacetylation of HMGB1 (301, 339). Curcumin decreases inflammation-mediated ALI in rats by decreasing the release of both HMGB1 and the expression of its receptor RAGE (55). The heparin derivative ODSH blocks extracellular HMGB1 binding to TLR2 and TLR4 receptors, thereby decreasing TLR-dependent pathways (291). This subsequently attenuates lung injury and decreases bacterial burden in mice with P. aeruginosa pneumonia (291). Ethyl pyruvate can also inhibit HMGB1 release by attenuating NF-κB- and p38 MAPK-mediated signaling cascades without affecting the expression of HMGB1 (327). Ethyl pyruvate decreases the level of circulating HMGB1 in mice with sepsis, as well as in postsepsis pneumonia in mice infected with P. aeruginosa (51, 327). Together, these studies suggest that HMGB1 mediates the pathogenesis of pneumonia.
Tuberculosis
TB is a predominantly airborne disease caused by Mycobacterium tuberculosis (36, 75, 104, 210). Globally, TB (Fig. 10) is one of the leading causes of mortality (36, 104). In 2016, it was estimated that as least 10.4 million people were infected with TB, and 1.7 million died from the disease (36, 75, 104). Multidrug-resistant TB (MDR-TB) is a public health crisis in numerous countries. It was estimated that in 2013, 480,000 people were diagnosed with MDR-TB worldwide (150). A prominent pathological feature of TB is masses of mature immune cells, known as granulomas (210, 215, 242). On inhalation, M. tuberculosis infects alveoli in the lungs and can be phagocytosed (62, 242). This is followed by an early inflammatory response, induced by both bacterial and host factors, which results in the recruitment of leukocytes to the site of infection (62, 210). Individuals with compromised immunity, IFN-γ and IL-12 receptor dysfunction, and those undergoing treatment with TNF-α antibody are more vulnerable to TB infection (36, 70).
FIG. 10.
The role of HMGB1 in the pathogenesis of TB. TB is a respiratory infection caused by Mycobacterium tuberculosis and is associated with lung function impairment, pulmonary obstruction, and granulitic infiltrate. TB infection elicits an inflammatory response, the infiltration of neutrophils, monocytes, and T cells, and the formation of granulomas. HMGB1 contributes to the pathology of TB by inducing granuloma formation, increasing lung bacterial burden, and promoting excessive inflammation. In subjects with TB, there is an increase in the extracellular levels of HMGB1 in the airways and in lung tissues. Airway HMGB1 can bind to the receptors RAGE, TLR2, and TLR9, which can activate NF-κB and upregulate the expression of genes that code for proinflammatory mediators such as IFN-γ, TNF-α, IL-6, CXCL8, and IL-8. Increased levels of these proinflammatory mediators promote recruitment of monocytes and CD4/CD8 lymphocytes and neutrophils. The recruitment and accumulation of immune cells are associated with the formation of granulomas, which undergo necrosis and augment inflammation. Necrosis of cells can cause the release of cytokines and ROS, which perpetuates immune cell recruitment and activation. The accumulation of neutrophils exacerbates airway inflammation by inducing excessive levels of ROS, proteases, and HMGB1, causing cell dysfunction, endothelial damage, and increased permeability of the alveolar epithelium. These cellular changes contribute to the increase in the pulmonary bacterial burden that further augments airway HMGB1, producing a deleterious feedback cycle. Therefore, these factors contribute to infection and decreased pulmonary function in TB. CD, cluster of differentiation; TB, tuberculosis.
Studies indicate that extracellular HMGB1 levels are significantly increased in patients with active TB. In patients with TB, levels of HMGB1 in plasma and sputum were significantly increased compared with healthy subjects (368). Lui et al. reported that the levels of HMGB1 and inflammasome-associated factors were significantly increased in patients with TB (202). In addition, there was a positive correlation between plasma HMGB1 levels, fever duration, and respiratory failure (202).
It has been reported that in animal models of TB, there is a significant increase in the accumulation of HMGB1 in the airways (111, 121). In mice with early- and late-stage TB, anti-HMGB1 antibody treatment decreased lung bacterial burden, decreased the expression of IFN-γ, TNF-α, and IL-17, and increased the expression of the anti-inflammatory cytokine IL-10 (121). Furthermore, the intratracheal administration of recombinant HMGB1 to guinea pigs with TB further increases lung bacterial burden when compared with control TB animals (121). These findings suggest that lowering levels of airway HMGB1 attenuates proinflammatory signaling and reduces lung bacterial load.
The binding of HMGB1 to RAGE, TLR2, and TLR9 receptors can increase the production of chemokines and cytokines that are critical in the host defense against TB. In TB-infected TLR2 and TLR9 knockout mice and TLR2/9 double knockouts, there is a decrease in the secretion of TNF-α, IL-12, and IL-6 (95, 116). The activation of these receptors by HMGB1 can activate NF-κB, which can further increase the synthesis and release of proinflammatory mediators such as TNF-α, IL-8, CXCL8, IL-6, IL-12, IFN-γ, and IFN-α (19, 121, 202, 368). TLR2 primarily mediates the release of TNF-α and IL-6, while TLR9 primarily mediates the secretion of INF-γ and INF-α (19).
The generation of excessive proinflammatory mediators can result in pronounced lung inflammation, which can cause leukocyte-mediated cell injury and compromised lung function, thereby exacerbating TB (263). In TB patients, there is a significant increase in leukocyte, monocyte, and neutrophil counts in blood samples, which may be due to elevated airway levels of HMGB1, and an increase in the chemokine CXCL12 (152, 189, 281, 357, 368). HMGB1-RAGE signaling via CXCL12 and CXCL8 can stimulate chemotaxis and increase the infiltration of immune cells such as macrophages and neutrophils (152, 364). As more immune cells accumulate in the airways, granulomas form, which can promote disease severity (229). As neutrophils accumulate in the airway, they elicit granuloma formation and the generation and accumulation of ROS, proteases (e.g., MMP9), and HMGB1 in the airways (229, 231, 309). These biomolecules exacerbate lung inflammation and can drive a feedback cycle initiated by airway HMGB1. The accumulation of these deleterious biomolecules in combination with the HMGB1 feedback cycle can cause cell/cell junction dysfunction and endothelial damage, and increase the permeability of alveolar barriers (133).
The secretion of IL-8 into the airways can recruit cluster of differentiation (CD) 4 and CD8 lymphocytes, which can enhance the phagocytosis and killing of M. tuberculosis (163). The increased number of helper T cells can attenuate bacterial growth as CD4 helper cells can release IFN-γ (19). Furthermore, HIV patients with a CD4+ cell deficiency are more susceptible to contracting TB when compared with healthy controls (63). In IFN-γ knockout mice, there is a significant increase in necrotic granulomas and lung bacterial burden (63). Granuloma necrosis leads to increased intracellular ROS levels, which can cause the dysfunction of phagocytic cells, suggesting a connection to increased lung bacteria burden (229).
In TB, airway HMGB1 can mediate inflammation by interacting with the cell surface receptors TLR4 and RAGE. Through these interactions, proinflammatory mediators are released and immune cells are recruited, exacerbating lung inflammation. While RAGE is involved in proinflammatory signaling that may enhance disease progression, RAGE signaling is also vital in viral immunity. Lui et al. reported that in TB patients, RAGE expression was upregulated in monocytes and dendritic cells (DCs) (202). In TB-infected RAGE-knockout mice, there was increased pulmonary inflammation and mortality compared with wild-type mice (19, 368). Furthermore, in TB-infected RAGE-knockout mice, there was more lung edema, pleuritis, and increased neutrophil infiltration when compared with wild-type mice. After 3 weeks of TB infection, RAGE-knockout mice also had significantly elevated levels of airway TNF-α, IL-6, IL-1β, IL-4, MIP-2, and lung bacterial burden (380). Therefore, RAGE receptors may play a protective role in chronic TB infection due to the need of a fine-tuned level of inflammation in the lung (380).
Soluble RAGE (sRAGE), an unbound isoform of RAGE, antagonizes RAGE-mediated signaling and downstream inflammation as demonstrated in studies in patients with newly diagnosed active TB (202). These patients have significantly lower levels of sRAGE compared with individuals with latent asymptomatic TB patients, correlating with higher mycobacterial counts, lung inflammation, and significantly higher expression of RAGE and plasma levels of HMGB1 (202).
In a recent study, TB patients were found to have significantly higher levels of sRAGE in their serum when compared with healthy controls (299). In TB patients who were smokers, significantly lower levels of serum sRAGE were found, which correlated with higher mortality rates (299). Thus, sRAGE and RAGE levels in TB patients may be complicated as a result of comorbidities, smoking, age, and whether the infection is active or latent asymptomatic. Further studies are needed to study how the balance between sRAGE/RAGE/HMGB1 at certain time points in TB can be modulated to increase survival rates, decrease bacterial loads, and resolve inflammation (202, 299, 380).
Therefore, HMGB1-mediated inflammation plays a complex role in TB. HMGB1 can cause the secretion of proinflammatory mediators that augment and prolong inflammation, producing cell dysfunction and disease progression. Future studies are needed to (i) clarify HMGB1's role during different stages of TB and (ii) determine how to attenuate severe inflammation without reducing lymphocyte recruitment or impairing the host defense against TB.
Lung Cancer
Lung cancer (Fig. 11) is one of the leading causes of mortality in both men and women in the United States (48, 168, 321). Approximately 541,000 Americans are living with lung cancer and an estimated 154,050 were expected to die from lung cancer in 2018, accounting for ∼25% of all cancer deaths (234, 298). Among all the lung cancer cases, about 80–85% are nonsmall-cell lung cancer (NSCLC) (9).
FIG. 11.
The role of HMGB1 in the pathogenesis of lung cancer. The levels of HMGB1 expression in tumors and the subsequent release of HMGB1 into the blood serum are positively correlated to tumor prognosis. The role of HMGB1 in lung cancer results from its localization. HMGB1 in the nucleus facilitates the transcription of genes involved in tumor development. A high expression level of HMGB1 in the nucleus increases the transcription of genes involved in tumor cell growth (e.g., cdc25A, FGFR), proliferation (e.g., met), migration (e.g., MMP), and angiogenesis (e.g., FGFR). The passive or active release of HMGB1 into the airways can have opposing roles in terms of mediating tumor growth and development. HMGB1 can interact with cell surface receptors such as TLR4, RAGE, and CXCR4 on airway cells, which activates the inflammasome, NF-κB, JNK/p38, and Ras-Raf-Erk1/2 pathways and increases tumor cell proliferation, migration, and invasion. However, the acute release of HMGB1 can induce the maturation of antigen presenting cells through activation of TLR4, which further activates tumor antigen-specific T cells and produces antitumor effects. FGFR, fibroblast growth factor receptor.
Increased levels of extracellular HMGB1 in patients diagnosed with lung cancer have been correlated with an unfavorable prognosis. The serum levels of HMGB1 in patients with NSCLC are significantly increased compared with healthy individuals (233, 343, 353). Serum levels of HMGB1 are not only positively correlated with tumor size and the stages of lung cancer progression, but can also be significantly reduced 1 month after tumor removal (288). These studies suggest that elevated serum levels of HMGB1 can be a biomarker for the diagnosis and prognosis of NSCLC. In addition, the expression of HMGB1 in lung tissues from NSCLC patients is significantly higher than in healthy individuals (353). HMGB1 expression, assessed by the levels of mRNA and protein in tumor tissues, increases from stage I to II, whereas HMGB1 mRNA and protein levels are downregulated in stages III and IV (293). The mechanisms underlying the discrepancy in the levels of HMGB1 between serum and tumor tissues at different tumor stages are still unclear but likely due to inconsistency in sample size, therapy methods, and analysis strategies (350).
The increase in HMGB1 levels in the serum by lung cancer could result from an increase in HMGB1 production and release from cancer cells (46). Cell ischemia and necrosis are commonly found in solid tumors, including lung tumors (46, 88, 212). During the early phases of tumor development, rapid cell growth is greater than the limited blood supply and physiological space, resulting in an ischemic environment that induces local necrosis (46, 356). Necrosis is usually found in the core region of tumors and is primarily due to the depletion of oxygen and glucose, which further increases the production and release of HMGB1 (46, 190, 361).
HMGB1 can be actively released following the activation of immune cells, or passively by necrotic and ischemic cells (12, 139, 199, 273). As a nuclear protein, HMGB1 is involved in mediating transcription, replication, DNA repair, and nucleosome assembly (225, 318, 329, 365). The overexpression of HMGB1 within the cells is positively correlated with tumorigenesis and tumor metastasis (352, 353, 356). HMGB1 can alter the expression of genes that play important roles in neoplasm progression and metastasis, including the genes involved in cell cycle and growth regulators, cell adhesion, mobility, and invasion regulators of angiogenesis and cell/cell interaction (53, 65, 145, 182, 216, 266).
The presence of HMGB1 in the extracellular environment can further augment the growth and metastasis of tumors by interacting with cell surface receptors, including TLRs and RAGE. For example, activated TLRs interact with the protein MyD88 to induce the recruitment of interleukin-1 receptor-associated kinase 1 (IRAKs) and tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6) that activates mitogen-activated protein kinase kinase kinase 7 (MAP3K7, also known as TAK1) (183, 245). TAK1 phosphorylates IKK subsequently activating the NF-κB pathway, as well as the JNK and p38 pathways, which are prominently involved in tumor progression (183, 245). TLR4 plays a role in mediating tumorigenesis caused by chemically induced pulmonary inflammation (24, 132, 312, 361, 371). Extracellular HMGB1 released from keratinocytes following ultraviolet irradiation induced TLR4-mediated inflammation, which is essential during perivascular invasion and metastasis in melanoma (20).
Interestingly, the binding of RAGE to HMGB1 produces inflammation that appears to mediate mitochondrial ATP production and tumor cell growth in pancreatic cancer cells (145). Extracellular HMGB1's induction of inflammation produces precancerous lesion of NSCLC by activating the RAGE/JNK/NF-κB pathway (349, 350). In addition, HMGB1-RAGE signaling can activate NF-κB, MAPK, and type IV collagenase (MMP2/9) pathways that can elicit cancer cell growth, invasion, and metastasis (310, 311). The incubation of endothelial cells with recombinant HMGB1 increases the expression of TLR4 and RAGE, as well as the endogenous expression of HMGB1, establishing a positive feedback loop (310, 311). Extracellular HMGB1 also activates the NLRP3 inflammasome, producing caspase-1-dependent release of IL-1β and IL-18, creating an inflammatory environment and augmenting tumor cell invasion (27, 30, 356). Overall, these studies suggest that extracellular HMGB1 may play a role in tumor pathogenesis and progression.
HMGB1 has also been suggested to promote drug resistance by regulating autophagy in NSCLC (243). HMGB1 translocation from the nucleus to cytoplasm was observed in lung adenocarcinoma cells treated with docetaxel, which inhibited autophagy and promoted cell resistance to docetaxel. HMGB1 was shown to regulate the formation of autophagosome through activating the mitogen-activated protein kinase (MAPK)-ERK signaling pathway. By suppressing HMGB1 cytosolic translocation, the protection in drug-induced autophagy is diminished, and cells restore sensitivity to docetaxel (243).
These effects of HMGB1 may be due to the cellular environment and its concentration. It has been postulated that cancer is not simply a disease of a specific tissue or organ, but a disease that can result from a dysregulation of the host defense (15). Since HMGB1 regulates innate and adaptive immunity, research related to HMGB1 may offer novel treatments for regulating tumorigenesis, progression, and metastasis. For example, HMGB1 released by dying tumor cells can be utilized to activate host DCs to further process and present tumor antigens (15). Furthermore, the interaction of HMGB1 with TLR4 on DCs contributes to the priming of antitumor T cells (15). Clinically, TLR4 polymorphisms, which can decrease the interaction of HMGB1 with TLRs, are negatively correlated with an early relapse of breast cancer following chemotherapy (15). The increased production of HMGB1 in tissues induced by radiation therapy is positively correlated with a superior clinical outcome in esophageal squamous cell carcinoma due to the role of HMGB1 in promoting DC activation and migration (79, 308). However, the exact mechanism by which HMGB1-TLR4 interactions affect the tumor antigen presentation of DCs in immunogenic tumor cell death remains to be elucidated.
Pulmonary Arterial Hypertension
PAH (Fig. 12) is an inflammatory disease that has a high mortality rate due to right ventricular heart failure (307, 362). The median survival time of patients with PAH is 2.8 years (221). In 2010, the death rate of PAH was 6.5 per 100,000 people (100). A recent report indicates that men diagnosed with PAH typically have higher mortality rates than women, which is partly due to higher rates of vascular cell necrosis (258). Pathologically, PAH is characterized by increased peripheral vascular resistance caused by vasoconstriction and obstruction of the pulmonary arteries, abnormal proliferation of smooth muscle cells and endothelial cells, thrombosis, lung inflammation, and extensive vascular remodeling (25, 136, 221). PAH is likely due to an imbalance in various vasodilating (e.g., prostacyclins and nitric oxide) and vasoconstricting biomolecules (e.g., thromboxaneA2 [TAX2], endothelin-1 [ET-1], and serotonin [5-HT]) (131, 226). The risk of PAH is increased by mutations in the BMPR2 (TGF-β receptor bone morphogenetic protein receptor type 2) gene, elevated 5-HT levels, exposure to various toxins, and the presence of pre-existing diseases, such as HIV, schistosomiasis, sarcoidosis, and scleroderma (72, 100, 257).
FIG. 12.
The role of HMGB1 in the pathogenesis of PAH. PAH is a disease that is characterized by increased pulmonary arterial pressure, vasoconstriction, and thrombosis. Right ventricular heart failure results from an increase in peripheral vascular resistance, systolic and diastolic pressure, pulmonary arterial pressure, venous hypertrophy, and a thickening of arterial walls. Inflammation and vascular remodeling are major pathological changes in pulmonary hypertension. Patients with PAH have significantly elevated levels of airway HMGB1. These airway levels of HMGB1 can interact with RAGE to activate the p38/ERK/JNK pathway that signals via AP-1 to promote proliferation of smooth muscle cells and endothelial cells. This increased proliferation produces vascular remodeling, venous hypertrophy, and thickening of arterial walls. Vascular remodeling contributes to vasoconstriction, which can increase vascular pressure. HMGB1 also can bind to RAGE and TLR4 to induce excessive inflammation by activating NF-κB. The activation of NF-κB increases the transcription of TGF-β1 to elicit vascular remodeling. NF-κB also increases the synthesis of the proinflammatory mediators MCP-1, CXCL8, IL-6, and TNF-α. TNF-α can increase neutrophil infiltration and plays a role in vascular remodeling by eliciting ET-1 secretion. ET-1 stimulates vasoconstriction and further augments the pathology of pulmonary hypertension. Also, IL-6, MCP-1, and CXCL8 mediate neutrophil infiltration and recruitment of immune cells. This leads to increased levels of ROS, MMP9, MPO, proteases, HMGB1, and endothelial dysfunction, which can stimulate vascular remodeling, formation of vascular lesions, and a further increase in the levels of HMGB1. Vascular lesions and remodeling can cause cell injury and death, contributing to increased pulmonary arterial pressure and pulmonary hypertension. ET-1, endothelin-1; PAH, pulmonary arterial hypertension.
Studies in both patients with PAH and animal models of PAH indicate a role for HMGB1 in the pathogenesis of PAH. Patients with idiopathic PAH have significantly greater levels of HMGB1 in the serum and airways compared with healthy subjects (25). This elevation in HMGB1 levels was positively correlated with enhanced vasoconstriction, right ventricular hypertrophy, increased pulmonary arterial pressure, and proliferation of endothelial and smooth muscle cells (39, 54, 275, 362, 366). In addition, the administration of an anti-HMGB1 antibody (2 mg antibody/kg via intraperitoneal injection) to rats with PAH induced by monocrotaline and chronic hypoxia, significantly reduced systolic pressure, thickening of the pulmonary arterial wall, right ventricular hypertrophy, and pulmonary vascular remodeling, and significantly decreased the levels of ET-1 (275). Also, in animals administered an anti-HMGB1 antibody, there was an attenuation of lung inflammation and a greater survival rate (25, 275).
The adverse effects of HMGB1 in PAH may result from its interaction with the transmembrane receptors, TLR4 and RAGE (184). Both TLR4 and RAGE are involved in HMGB1-mediated vascular remodeling (25, 184). In mice with monocrotaline-induced PAH, a deficiency of TLR4 abolished HMGB1-induced PAH, as indicated by a lack of an increase in right ventricular systolic pressure, pulmonary vascular remodeling, and wall thickening (25). In addition, in a hypoxia-induced PAH model, HMGB1 and RAGE levels were significantly correlated with the extent of PAH pathology, indicating a critical role of HMGB1-RAGE signaling pathways in the pathogenesis of PAH (54, 184).
As illustrated in Figure 3, the activation of NF-κB and MAPK pathways can increase the expression of numerous genes, including those that code for various proinflammatory cytokines (e.g., IL-6, IL-1β, TNF-α, CXCL8) and TGF-β, contributing to inflammation and vascular remodeling in PAH (54, 275). In an animal model of monocrotaline-induced PAH, Iκβα-deficient mice or mice treated with an NF-κB inhibitor (pyrrolidine dithiocarbamate) had significantly reduced levels of cytokine production and an attenuated EMT process in vascular remodeling (179, 366). Overall, these data suggest a role for the HMGB1-NF-κB signaling pathways in the pathogenesis of PAH.
A significant increase in the airway levels of proinflammatory mediators, notably HMGB1, MCP-1, CXCL8, IL-6, and TNF-α, is observed in animal models of PAH (39, 54, 184, 275). Increased levels of cytokines and airway HMGB1 can contribute to the toxic environment by exacerbating inflammation, which can increase levels of ROS in PAH, as illustrated in Figure 3. Oxidative environments can further increase the release of HMGB1 into the airway and damage lung cells, such as endothelial cells (127, 258, 313). Interestingly, increased necrosis in the vasculature can induce reductive stress in the extracellular environment in PAH, producing highly reduced HMGB1, augmenting inflammation via TLR4-mediated signaling, establishing a feedback loop, and leading to excessive lung inflammation, cell death, and formation of vascular lesions (85, 127, 258, 313).
Vascular remodeling in PAH has also been correlated with elevated HMGB1 levels. HMGB1-induced TNF-α release can directly stimulate the secretion of ET-1 from endothelial cells, producing increased vasoconstriction of the airways (362). In addition, increased levels of TNF-α and IL-6 are significantly correlated with endothelial cell proliferation, increases in right ventricular systolic and diastolic pressure, and enhanced vascular remodeling in rats (39, 181, 275). HMGB1-TLR4 and RAGE signaling has been postulated to affect vascular remodeling, venous hypertrophy, and wall thickening in pulmonary arteries due to an increase in proliferation of endothelial and smooth muscle cells (143, 257, 340). Furthermore, cell proliferation is accompanied by increased TGF-β levels and the increased deposition of ECM proteins and α-sma (143, 340). These results suggest that HMGB1 may mediate peripheral vascular resistance, ventricular pressures, and vasculature remodeling in PAH.
Overall, these studies suggest that HMGB1 plays a critical role in mediating severe pulmonary hypertension by activating its downstream signaling pathways that can induce pathological changes associated with PAH. These downstream signaling pathways can be abrogated by regulating the environmental redox state (258). Thus, the reduced HMGB1 found in PAH patients may serve as a unique biomarker for disease severity and a pharmacological target in the treatment of PAH.
HMGB1 as a Therapeutic Target
Using anti-HMGB1 antibodies, we and others have demonstrated the critical role of HMGB1 in the pathogenesis of pulmonary diseases (i.e., CF, ALI/ARDS, COPD, pneumonia, TB, PF, PAH, and asthma). Although anti-HMGB1 antibodies are effective in attenuating the pathology associated with many of these lung diseases, other approaches targeting HMGB1 have been investigated (Fig. 13) to attenuate the release and airway accumulation of HMGB1, to neutralize extracellular HMGB1, or mitigate its downstream adverse effects. Certain approaches target the post-translational modifications of HMGB1 to attenuate its active release. Ethyl pyruvate inhibits HMGB1 release by inhibiting or decreasing its hyperacetylation (66, 85).
FIG. 13.
HMGB1 as a therapeutic target in pulmonary diseases. Preexisting trauma, exposure to toxicants, PAMPs, or DAMPs can induce the release of HMGB1, either active or passively, and subsequently the accumulation of HMGB1 in the airways of patients with pulmonary diseases. Post-translational modifications such as hyperacetylation and phosphorylation result in the translocation of HMGB1 into the cytoplasm, where HMGB1 can be actively released. The hyperacetylation of HMGB1 can be decreased by silent mating type information regulation 2 homolog 1 (SIRT)-1 activators (e.g., resveratrol), NF-κB inhibitors (e.g., ethyl pyruvate, GTS-21, naringin, hesperidin, FPS-ZMI), Nrf2 activators (e.g., baicalin, glycyrrhizin, hydrogen sulfide gas, resveratrol), and ODSH. Antioxidants (e.g., ascorbic acid, sulforaphane), as well as other compounds (e.g., vitamin D, insulin), can also inhibit the cytoplasmic translocation of HMGB1. Similar to antibodies, compounds such as ODSH, glycyrrhizin, and thrombomodulin can bind directly to, neutralize, or inactivate extracellular HMGB1, thereby decreasing the binding of HMGB1 to its receptors. Extracellular HMGB1 can bind to TLR2, TLR4, TLR9, and RAGE receptors to activate pathways involved in inflammation, tissue injury, and tissue remodeling. Following the binding of HMGB1 to TLR2 and TLR4, it can activate the NF-κB pathway, which ultimately increases the expression and secretion of proinflammatory mediators and further augments the secretion of HMGB1, creating a deleterious feedback cycle. Also, HMGB1 can bind with RAGE receptors and activate MAPK-dependent pathways that mediate tissue injury and remodeling. MAPK inhibitors (e.g., ulinastatin, naringin) can decrease the accumulation of airway HMGB1. The accumulation of airway HMGB1 and proinflammatory mediators increases the levels of ROS, MMP9, heat shock proteins, and certain proteases that produce oxidative stress, inflammation, tissue injury, and vascular remodeling. NF-κB inhibitors (e.g., ethyl pyruvate, GTS-21, naringin, hesperidin, FPS-ZMI) can attenuate cytokine and HMGB1 airway accumulation. Antioxidants, PPAR-γ, and Nrf2 activators (e.g., vitamin D, glycyrrhizin, baicalin, resveratrol, ethyl pyruvate) can decrease inflammation, lung injury, and remodeling by decreasing oxidative stress induced by the accumulation of HMGB1 in the airways. FPS-ZMI, N-benzyl-4-chloro-N-cyclohexylbenzamide; Nrf2, nuclear factor erythroid 2-related factor 2; PPAR, peroxisome proliferator-activated receptor.
GTS-21, a partial agonist of α7 nicotinic acetylcholine receptors, significantly decreases the active release of HMGB1 by decreasing its hyperacetylation in macrophages in the presence of a hyperoxic environment (301). The NF-κB inhibitors, BAY 11-7082 and ethacrynic acid, inhibit HMGB1 release by attenuating the post-translational hyperacetylation of HMGB1 (339). Ascorbic acid, in addition to modulating intracellular levels of ROS, inhibits the activation of the NF-κB pathway and subsequently attenuates the oxidative stress-induced HMGB1 translocation from the nucleus to the cytoplasm in hyperoxia-exposed macrophages (247). Similarly, Vitamin D also inhibits HMGB1 translocation by reducing the expression of NF-κB (261, 374). Other compounds, such as resveratrol, a Sirtuin-1 (SIRT-1) activator, can attenuate the active release of HMGB1 into the extracellular milieu (76). As a deacetylase, SIRT-1 activation can block the active release of HMGB1, by decreasing its acetylation, from lung endothelial cells, and attenuating lung epithelial barrier leakage induced by exposure to airway ventilation and LPS (78, 263, 362).
Antioxidants are effective in reducing HMGB1 release in lung cells. For example, the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) attenuates oxidative stress by upregulation of heme oxygenase-1 (HO-1) and downregulation of active HMGB1 release (49, 147, 156). Nrf2 activators, such as hydrogen sulfide gas and resveratrol, significantly decrease the levels of airway HMGB1, restoring epithelial barrier integrity, attenuating lung injury, and increase animal survival after bacterial infections. Other studies have investigated the effects of antioxidants in mediating lung injury by affecting HMGB1 release (76, 155, 156). Antioxidants, such as naringin, a flavanone glycoside, significantly decrease airway accumulation of HMGB1 and ALI by reducing the efficacy of the activation of the NF-κB and MAPK/ERK/p38/JNK pathways (103). The downregulation of these HMGB1-activated pathways decreases the airway accumulation of IL-1β, IL-4, IL-6, and TNF-α, leading to a decrease in airway neutrophil infiltration, edema, and hemorrhage (303). Hesperidin is an antioxidant flavonoid glycoside commonly found in citrus fruits that attenuates LPS-induced ALI by reducing HMGB1 release in mice (196). Hesperidin also attenuates LPS-induced MPO activity and significantly decreases the secretion of TNF-α, IL-6, and MCP-1, as well as lung damage (196). Baicalin and curcumin, compounds derived from plants with antioxidant properties, inhibit the translocation of HMGB1 (55, 56, 344). Therefore, targeting HMGB1 release into the airways by blocking its post-translational modifications of hyperacetylation and oxidation may prove to be an important therapeutic approach to treat lung diseases such as ALI and ARDS.
Other therapeutic strategies include the application of compounds that can neutralize extracellular HMGB1. For example, thrombomodulin, which binds and cleaves HMGB1, reduces HMGB1-mediated proinflammatory responses (2, 140, 153, 186). The heparin derivative ODSH inhibits the binding of extracellular HMGB1 to TLR2, TLR4, and RAGE, attenuating lung injury and bacterial burden in mice infected with P. aeruginosa (291). Glycyrrhizin, which binds to the A and B boxes of HMGB1, can decrease HMGB1-mediated adverse effects (220). Glycyrrhizin also decreases HMGB1 release by activating Nrf2, as previously reported for other Nrf2 activators, such as ethyl pyruvate and vitamin D (155, 176, 362).
Conclusions
HMGB1 has been implicated in various pathological processes in lung diseases, as either a nuclear or an extracellular protein. Thus, it has been postulated not only as a biomarker for pathology but also a therapeutic target for the treatment of lung diseases. As a DAMP molecule, HMGB1 can be released into the extracellular milieu either passively from injured cells, especially those undergoing necrosis, or secreted actively from activated immune cells. Extracellular HMGB1 can accumulate in the airways and/or in the serum and acts as a potent danger signal to neighboring cells. The extracellular levels of HMGB1 in the airway, sputum, and serum positively correlate with the stage or the severity of lung diseases, with often higher levels of accumulation in the local environments such as BAL and sputum than the systemic circulation (Tables 1 and 2).
Table 2.
Sources and Effects of Elevated HMGB1 in Lung Diseases
| Disease | Species | HMGB1 (source) | Affected cell/tissue types | References |
|---|---|---|---|---|
| Asthma | Mice | Elevated in BALF, sputum, lung homogenate | Total lung lysate, bronchial epithelial cells | (71, 126, 206) |
| COPD | Mice | Elevated in BALF and serum | Epithelial and endothelial cells, neutrophils, lung vasculature | (56, 120, 205, 366) |
| ALI/ARDS | Mice | Elevated in BALF | Neutrophils, monocytes, macrophages, lung tissue | (85, 301) |
| PF | Rats | Elevated in BALF and serum | Alveolar/bronchial epithelial cell, fibroblasts, leukocytes | (114, 153) |
| CF | Mice | Elevated in BALF | Neutrophils, monocytes, macrophages, epithelial cells | (38, 50, 86) |
| Pneumonia | Mice | Elevated in BALF and serum | Bronchoalveolar cells | (4, 235) |
| TB | Guinea pigs | Elevated in BALF | Macrophages, epithelial cells, endothelial cells, fibroblasts | (111) |
| Lung cancer | In vitro | Elevated in cell culture media | Keratinocytes, parenchyma cells | (20, 145) |
| PAH | Rat | Elevated in BALF | Pulmonary arterial smooth muscle cells, airway epithelial cells | (184, 275, 362) |
BALF, bronchoalveolar lavage fluids.
As a cytokine and an inflammatory mediator, extracellular HMGB1 can bind to its receptors on the neighboring cells and activates signaling pathways to further induce proinflammatory cytokine secretion, to cause dysfunctions of lung cells and cell junctions, to promote cell proliferation, and exacerbate HMGB1 accumulation in the extracellular milieu. Ultimately, these pathways can result in excessive infiltration of immune cells, endothelial/epithelial barrier integrity loss, vascular remodeling, fibrosis, increased susceptibility to infections, and tumorigenesis.
As a transcription factor, nuclear HMGB1 can regulate the transcription of various genes associated with inflammation, tumorigenesis, and metastasis. Herein, these pathological changes drive lung disease progression that can result in lung injury, decreased lung functions, and even mortality. Thus, elevated levels of HMGB1 in different lung diseases share a similar etiology with respect to the initiation of signaling pathways mediated by TLRs and RAGE, leading to activation of several signaling pathways, eliciting an inflammatory response. However, the pathology varies in different lung diseases, which may result from many other compounding factors.
The involvement of other compounding factors, such as genetic backgrounds including CFTR deficiency, exposure to xenobiotics including infectious agents, oxidative or reductive reagents/inducers including cigarette smoke and particulate matter, the use of pro- or anti-inflammatory reagents, could affect the expression levels and post-translational modifications of HMGB1. These factors can contribute to the different microenvironments, including different redox states, which can result in different levels of local HMGB1, different post-translational modifications of HMGB1, which can affect not only its sublocation but also the impact of HMGB1 on the physiology/pathophysiology through its interaction with different types of surrounding cells. Consequently, the outcome of HMGB1 downstream pathology in different lung diseases can be different. Thus, multiple factors, including biochemical modifications of HMGB1, local concentrations of HMGB1, cell types that HMGB1 interacts with, and signaling pathways activated by HMGB1, can drive different pathologies observed in different lung diseases. However, the detailed mechanisms driving these pathologies remain to be further delineated.
Therefore, there are still many questions about how HMGB1 mediates different lung diseases that remain unanswered. This urges future studies with standardized animal models of human lung diseases and better analyses on larger patient populations. Although many studies have been successful in animal models by targeting HMGB1 through modulating its redox modifications, extracellular neutralization, and its downstream effects, it is still not clear whether this can be translated to treating patients with these lung diseases. Future research with more accurate analysis of the time course of HMGB1 release, HMGB1 concentrations at local site and in systemic circulation, as well as post-translational modifications is needed to elucidate the function of HMGB1 on redox modifications, other post-translational modifications, and how these modifications can impact lung disease pathogenesis and outcomes. In addition, research is needed to illustrate signaling pathways, especially RAGE- and TLR-mediated pathways, as well as the cross talk between them. These future studies will no doubt help in the design of novel therapeutic strategies toward targeting HMGB1 as a treatment for lung diseases.
Acknowledgments
This work was supported by grants (L.L.M.) from the National Heart and Blood Institute (HL093708) and St. John's University. The authors thank Susana Solis for her help in editing this work.
Abbreviations Used
- α-sma
α-smooth muscle actin
- 5-HT
serotonin
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- CAP
community-acquired pneumonia
- CD
cluster of differentiation
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- COPD
chronic obstructive pulmonary disease
- CXCL8
chemokine ligand 8
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- ERK
extracellular signal-regulated kinase
- ET-1
endothelin-1
- FEV
forced expiratory volume
- HAT
histone acetyltransferase
- HMGB1
high-mobility group protein box 1
- Hsp27
heat shock protein 27
- ICUs
intensive care units
- IFN
interferon
- IKK
IκB kinase
- IL
interleukin
- IPAH
idiopathic pulmonary arterial hypertension
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharides
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MDR-TB
multidrug-resistant TB
- MMP9
matrix metalloproteinase 9
- MPO
myeloperoxidase
- mRNA
messenger RNA
- MV
mechanical ventilation
- MyD88
myeloid differentiation primary response 88
- NF-κβ
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLS
nuclear localization signals
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSCLC
nonsmall-cell lung cancer
- ODSH
2-O,3-O desulfated heparin
- p65
NF-kappa-B p65 subunit complex
- PA
Pseudomonas aeruginosa
- PAFR
platelet-activating factor receptor
- PAH
pulmonary arterial hypertension
- PAMP
pathogen-associated molecular pattern
- PARP
poly(ADP)-ribose polymerase
- PF
pulmonary fibrosis
- PKC
protein kinase C
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- SIRT-1
Sirtuin-1
- sRAGE
soluble RAGE
- STAT-3
signal transducer and activator of transcription 3
- TB
tuberculosis
- TDI
toluene 2,6-diisocyanate
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- VAP
ventilator-associated pneumonia
- VEGF
vascular endothelial growth factor
Author Disclosure Statement
The authors declare no competing financial interest.
References
- 1. Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, and Dales RE. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163: 349–355, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, Yamamoto Y, Yamamoto H, Iino S, Taniguchi N, and Maruyama I. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest 115: 1267–1274, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abraham E, Arcaroli J, Carmody A, Wang H, and Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 1950 165: 2950–2954, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Achouiti A, van der Meer AJ, Florquin S, Yang H, Tracey KJ, van't Veer C, de Vos AF, and van der Poll T. High-mobility group box 1 and the receptor for advanced glycation end products contribute to lung injury during Staphylococcus aureus pneumonia. Crit Care 17: R296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agustí AGN, Roca J, Gea J, Wagner PD, Xaubet A, and Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis 143: 219–225, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Aizawa S, Nishino H, Saito K, Kimura K, Shirakawa H, and Yoshida M. Stimulation of transcription in cultured cells by high mobility group protein 1: essential role of the acidic carboxyl-terminal region. Biochemistry 33: 14690–14695, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, and Armanios M. Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest 147: 1361–1368, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alpkvist H, Athlin S, Mölling P, Norrby-Teglund A, and Strålin K. High HMGB1 levels in sputum are related to pneumococcal bacteraemia but not to disease severity in community-acquired pneumonia. Sci Rep 8: 13428, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Lung Association. Lung cancer fact sheet. Chicago, IL: American Lung Association, 2018 [Google Scholar]
- 10. Andersen DH. Cystic Fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child 56: 344, 1938 [Google Scholar]
- 11. Andersson U, Erlandsson-Harris H, Yang H, and Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol 72: 1084–1091, 2002 [PubMed] [Google Scholar]
- 12. Andersson U. and Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29: 139–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, and Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192: 565–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L, and GenIMS Investigators. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 35: 1061–1067, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira J-P, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, André F, Delaloge S, Tursz T, Kroemer G, and Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13: 1050–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Armstrong L. and Millar AB. Relative production of tumour necrosis factor alpha and interleukin 10 in adult respiratory distress syndrome. Thorax 52: 442–446, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aupperle K, Bennett B, Han Z, Boyle D, Manning A, and Firestein G. NF-kappa B regulation by I kappa B kinase-2 in rheumatoid arthritis synoviocytes. J Immunol 1950 166: 2705–2711, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Aupperle KR, Bennett BL, Boyle DL, Tak PP, Manning AM, and Firestein GS. NF-kappa B regulation by I kappa B kinase in primary fibroblast-like synoviocytes. J Immunol 163: 427–433, 1999 [PubMed] [Google Scholar]
- 19. Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, and Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med 202: 1715–1724, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, and Tüting T. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507: 109–113, 2014 [DOI] [PubMed] [Google Scholar]
- 21. Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, and Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 20: 63–70, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Banerjee S. and Kundu TK. The acidic C-terminal domain and A-box of HMGB-1 regulates p53-mediated transcription. Nucleic Acids Res 31: 3236–3247, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barbier F, Andremont A, Wolff M, and Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 19: 216–228, 2013 [DOI] [PubMed] [Google Scholar]
- 24. Bauer AK, Dixon D, DeGraff LM, Cho H-Y, Walker CR, Malkinson AM, and Kleeberger SR. Toll-like receptor 4 in butylated hydroxytoluene-induced mouse pulmonary inflammation and tumorigenesis. J Natl Cancer Inst 97: 1778–1781, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, and Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of toll-like receptor 4. Mol Med 18: 1509–1518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beghé B, Fabbri LM, Contoli M, and Papi A. Update in asthma 2016. Am J Respir Crit Care Med 196: 548–557, 2017 [DOI] [PubMed] [Google Scholar]
- 27. van Beijnum JR, Nowak-Sliwinska P, van dem Boezem E, Hautvast P, Buurman WA, and Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene 32: 363–374, 2013 [DOI] [PubMed] [Google Scholar]
- 28. Bell SC, De Boeck K, and Amaral MD. New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther 145: 19–34, 2015 [DOI] [PubMed] [Google Scholar]
- 29. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, LUNG SAFE Investigators, and ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315: 788–800, 2016 [DOI] [PubMed] [Google Scholar]
- 30. Bertheloot D. and Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol 14: 43–64, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bezerra FS, Valença SS, Pires KMP, Lanzetti M, Pimenta WA, Schmidt AC, Porto LC, and Zin WA. Long-term exposure to cigarette smoke impairs lung function and increases HMGB-1 expression in mice. Respir Physiol Neurobiol 177: 120–126, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Bianchi ME, Falciola L, Ferrari S, and Lilley DM. The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J 11: 1055–1063, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, and Garcia JGN. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 26: 453–464, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Blair RH, Horn AE, Pazhani Y, Grado L, Goodrich JA, and Kugel JF. The HMGB1 C-terminal tail regulates DNA bending. J Mol Biol 428: 4060–4072, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, Paterson N, Jackson M, Lougheed MD, Kumar V, and Aaron SD. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 66: 680–685, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Boisson-Dupuis S, Bustamante J, El-Baghdadi J, Camcioglu Y, Parvaneh N, Azbaoui SE, Agader A, Hassani A, Hafidi NE, Mrani NA, Jouhadi Z, Ailal F, Najib J, Reisli I, Zamani A, Yosunkaya S, Gulle-Girit S, Yildiran A, Cipe FE, Torun SH, Metin A, Atikan BY, Hatipoglu N, Aydogmus C, Kilic SS, Dogu F, Karaca N, Aksu G, Kutukculer N, Keser-Emiroglu M, Somer A, Tanir G, Aytekin C, Adimi P, Mahdaviani SA, Mamishi S, Bousfiha A, Sanal O, Mansouri D, Casanova J-L, and Abel L. Inherited and acquired immunodeficiencies underlying tuberculosis in childhood. Immunol Rev 264: 103–120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, and Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551–5560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonfield TL, Konstan MW, and Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 104: 72–78, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Breitling S, Hui Z, Zabini D, Hu Y, Hoffmann J, Goldenberg NM, Tabuchi A, Buelow R, Dos Santos C, and Kuebler WM. The mast cell–B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol-Lung Cell Mol Physiol 312: L710–L721, 2017 [DOI] [PubMed] [Google Scholar]
- 40. Brown SD, White R, and Tobin P. Keep them breathing: cystic fibrosis pathophysiology, diagnosis, and treatment. J Am Acad Physician Assist 30: 23–27, 2017 [DOI] [PubMed] [Google Scholar]
- 41. Bunting K, Rao S, Hardy K, Woltring D, Denyer GS, Wang J, Gerondakis S, and Shannon MF. Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. J Immunol 178: 7097–7109, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, and Ramsey BW. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 183: 444–452, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Butt Y, Kurdowska A, and Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med 140: 345–350, 2016 [DOI] [PubMed] [Google Scholar]
- 44. Cai J, Wen J, Bauer E, Zhong H, Yuan H, and Chen AF. The role of HMGB1 in cardiovascular biology: danger signals. Antioxid Redox Signal 23: 1351–1369, 2015 [DOI] [PubMed] [Google Scholar]
- 45. Calogero S, Grassi F, Aguzzi A, Voigtländer T, Ferrier P, Ferrari S, and Bianchi ME. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet 22: 276–280, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention. Prevent pneumonia. 2017. https://www.cdc.gov/features/pneumonia/ September 18, 2018
- 48. Centers for Disease Control and Prevention. Compressed mortality file 1968–2016. 2017. https://wonder.cdc.gov/wonder/help/cmf.html August 20, 2018
- 49. Chai D, Zhang L, Xi S, Cheng Y, Jiang H, and Hu R. Nrf2 activation induced by Sirt1 ameliorates acute lung injury after intestinal ischemia/reperfusion through NOX4-mediated gene regulation. Cell Physiol Biochem 46: 781–792, 2018 [DOI] [PubMed] [Google Scholar]
- 50. Chen J. Cystic fibrosis transmembrane conductance regulator modulates acute lung injury: evidence from a genetic association study. Crit Care Med 40: 3101, 2012 [DOI] [PubMed] [Google Scholar]
- 51. Chen W, Lian J, Ye J, Mo Q, Qin J, Hong G, Chen L, Zhi S, Zhao G, and Lu Z. Ethyl pyruvate reverses development of Pseudomonas aeruginosa pneumonia during sepsis-induced immunosuppression. Int Immunopharmacol 52: 61–69, 2017 [DOI] [PubMed] [Google Scholar]
- 52. Chen W-Y, Huang Y-C, Yang M-L, Lee C-Y, Chen C-J, Yeh C-H, Pan P-H, Horng C-T, Kuo W-H, and Kuan Y-H. Protective effect of rutin on LPS-induced acute lung injury via down-regulation of MIP-2 expression and MMP-9 activation through inhibition of Akt phosphorylation. Int Immunopharmacol 22: 409–413, 2014 [DOI] [PubMed] [Google Scholar]
- 53. Chen Y, Lin C, Liu Y, and Jiang Y. HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2. Tumor Biol 37: 4399–4408, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen Z. and Wang Q. Activation of PPARγ by baicalin attenuates pulmonary hypertension in an infant rat model by suppressing HMGB1/RAGE signaling. FEBS Open Bio 7: 477–484, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng K, Yang A, Hu X, Zhu D, and Liu K. Curcumin attenuates pulmonary inflammation in lipopolysaccharide induced acute lung injury in neonatal rat model by activating peroxisome proliferator-activated receptor γ (PPARγ) pathway. Med Sci Monit 24: 1178–1184, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cheng Y, Wang D, Wang B, Li H, Xiong J, Xu S, Chen Q, Tao K, Yang X, Zhu Y, and He S. HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway. Mol Biol Cell 28: 201–209, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C, Di Dio G, Lionetti E, David A, Arrigo T, Salpietro C, and La Rosa M. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: high-mobility group box 1 (HMGB1) between inflammation and infection. Clin Microbiol Infect 21: 368.e1–368.e9, 2015 [DOI] [PubMed] [Google Scholar]
- 58. Christian F, Smith EL, and Carmody RJ. The regulation of NF-κB subunits by phosphorylation. Cells 5: 12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Churg A, Marshall CV, Sin DD, Bolton S, Zhou S, Thain K, Cadogan EB, Maltby J, Soars MG, Mallinder PR, and Wright JL. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am J Respir Crit Care Med 185: 34–43, 2012 [DOI] [PubMed] [Google Scholar]
- 60. Cillóniz C, Ewig S, Polverino E, Marcos MA, Prina E, Sellares J, Ferrer M, Ortega M, Gabarrús A, Mensa J, and Torres A. Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur Respir J 40: 931–938, 2012 [DOI] [PubMed] [Google Scholar]
- 61. Cilloniz C, Martin-Loeches I, Garcia-Vidal C, San Jose A, and Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci 17: 2120, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clay H, Davis JM, Beery D, Huttenlocher A, Lyons S, and Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2: 29–39, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cooper AM. Cell mediated immune responses in tuberculosis. Annu Rev Immunol 27: 393–422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cystic Fibrosis Foundation. 2016 Patient registry reports. 2016. https://www.cff.org/research/researcher-resources/patient-registry/2016-patient-registry-annual-data-report.pdf February 27, 2018
- 65. Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe J-P, Rodier F, and Campisi J. p53-Dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201: 613–629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Davé SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, and Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol 86: 633–643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davidson D. and Patel H. Cytokine-induced neutrophil chemotaxis assay. In: Cytokine Bioassays, Vol. 1172, edited by Vancurova I. New York, NY: Springer New York, 2014, pp. 107–113 [DOI] [PubMed] [Google Scholar]
- 68. Davis K, Banerjee S, Friggeri A, Bell C, Abraham E, and Zerfaoui M. Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol Med 18: 359–369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Deng M, Scott MJ, Fan J, and Billiar TR. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J Leukoc Biol 106: 161–169, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, and Zhang Y. The immunology of tuberculosis: from bench to bedside. Respirology 15: 433–450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Di Candia L, Gomez E, Venereau E, Chachi L, Kaur D, Bianchi ME, Challiss RAJ, Brightling CE, and Saunders RM. HMGB1 is upregulated in the airways in asthma and potentiates airway smooth muscle contraction via TLR4. J Allergy Clin Immunol 140: 584.e8–587.e8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Diaz-Guzman E, Farver C, Parambil J, and Culver DA. Pulmonary hypertension caused by sarcoidosis. Clin Chest Med 29: 549–563, x, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. DiBardino DM. and Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care 30: 40–48, 2015 [DOI] [PubMed] [Google Scholar]
- 74. Ding Y, Chu C, Li Y, Li G, Lei X, Zhou W, and Chen Z. High expression of HMGB1 in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect Dis 18: 439, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, and Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 5: e898–e906, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dong W-W, Liu Y-J, Lv Z, Mao Y-F, Wang Y-W, Zhu X-Y, and Jiang L. Lung endothelial barrier protection by resveratrol involves inhibition of HMGB1 release and HMGB1-induced mitochondrial oxidative damage via an Nrf2-dependent mechanism. Free Radic Biol Med 88: 404–416, 2015 [DOI] [PubMed] [Google Scholar]
- 77. Du Y, Ding Y, Chen X, Mei Z, Ding H, Wu Y, and Jie Z. MicroRNA-181c inhibits cigarette smoke–induced chronic obstructive pulmonary disease by regulating CCN1 expression. Respir Res 18: 155, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dudek SM, Birukov KG, Zhan X, and Garcia JGN. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun 298: 511–519, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, and Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81: 84–91, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Dunican EM. and Fahy JV. The role of type 2 inflammation in the pathogenesis of asthma exacerbations. Ann Am Thorac Soc 12: S144–S149, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Durmowicz AG, Lim R, Rogers H, Rosebraugh CJ, and Chowdhury BA. The U.S. Food and Drug Administration's experience with ivacaftor in cystic fibrosis. Establishing efficacy using in vitro data in lieu of a clinical trial. Ann Am Thorac Soc 15: 1–2, 2017 [DOI] [PubMed] [Google Scholar]
- 82. Elborn JS. Cystic fibrosis. Lancet 388: 2519–2531, 2016 [DOI] [PubMed] [Google Scholar]
- 83. Emerson J, Rosenfeld M, McNamara S, Ramsey B, and Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34: 91–100, 2002 [DOI] [PubMed] [Google Scholar]
- 84. Englert JM, Kliment CR, Ramsgaard L, Milutinovic PS, Crum L, Tobolewski JM, and Oury TD. Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int J Clin Exp Pathol 4: 241–254, 2011 [PMC free article] [PubMed] [Google Scholar]
- 85. Entezari M, Javdan M, Antoine DJ, Morrow DM, Sitapara RA, Patel V, Wang M, Sharma L, Gorasiya S, Zur M, Wu W, Li J, Yang H, Ashby CR, Thomas D, Wang H, and Mantell LL. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol 2: 314–322, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, and Mantell LL. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med 18: 477–485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, and Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol 178: 6573–6580, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Fan S, Xu Y, Li X, Tie L, Pan Y, and Li X. Opposite angiogenic outcome of curcumin against ischemia and Lewis lung cancer models: in silico, in vitro and in vivo studies. Biochim Biophys Acta 1842: 1742–1754, 2014 [DOI] [PubMed] [Google Scholar]
- 89. Fehrenbach H, Wagner C, and Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res 367: 551–569, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, Dombret M-C, Sims GP, Kolbeck R, Coyle AJ, Aubier M, and Pretolani M. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 917–927, 2010 [DOI] [PubMed] [Google Scholar]
- 91. Ferrari S, Finelli P, Rocchi M, and Bianchi ME. The active gene that encodes human high mobility group 1 protein (HMG1) contains introns and maps to chromosome 13. Genomics 35: 367–371, 1996 [DOI] [PubMed] [Google Scholar]
- 92. Fink MP. HMGB1 as a drug target in staphylococcal pneumonia. Crit Care 18: 131, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fiorini G, Crespi S, Rinaldi M, Oberti E, Vigorelli R, and Palmieri G. Serum ECP and MPO are increased during exacerbations of chronic bronchitis with airway obstruction. Biomed Pharmacother 54: 274–278, 2000 [DOI] [PubMed] [Google Scholar]
- 94. Friggeri A, Yang Y, Banerjee S, Park Y-J, Liu G, and Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. Am J Physiol Cell Physiol 299: C1267–C1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gangwani MR. and Kumar A. Multiple protein kinases via activation of transcription factors NF-κB, AP-1 and C/EBP-δ regulate the IL-6/IL-8 production by HIV-1 Vpr in astrocytes. PLoS One 10: e0135633, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Garcia-Martinez I, Shaker ME, and Mehal WZ. Therapeutic opportunities in damage-associated molecular pattern-driven metabolic diseases. Antioxid Redox Signal 23: 1305–1315, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, and Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3: 995–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gariboldi M, De Gregorio L, Ferrari S, Manenti G, Pierotti MA, Bianchi ME, and Dragani TA. Mapping of the Hmg1 gene and of seven related sequences in the mouse. Mamm Genome 6: 581–585, 1995 [DOI] [PubMed] [Google Scholar]
- 99. Ge X, Antoine DJ, Lu Y, Arriazu E, Leung T-M, Klepper AL, Branch AD, Fiel MI, and Nieto N. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem 289: 22672–22691, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. George MG, Schieb LJ, Ayala C, Talwalkar A, and Levant S. Pulmonary hypertension surveillance. Chest 146: 476–495, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Giavara S, Kosmidou E, Hande MP, Bianchi ME, Morgan A, d'Adda di Fagagna F, and Jackson SP. Yeast Nhp6A/B and mammalian Hmgb1 facilitate the maintenance of genome stability. Curr Biol 15: 68–72, 2005 [DOI] [PubMed] [Google Scholar]
- 102. Gibson RL, Burns JL, and Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168: 918–951, 2003 [DOI] [PubMed] [Google Scholar]
- 103. Gil M, Kim YK, Hong SB, and Lee KJ. Naringin decreases TNF-α and HMGB1 release from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. PloS One 11: e0164186, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Glaziou P, Sismanidis C, Floyd K, and Raviglione M. Global epidemiology of tuberculosis. Cold Spring Harb Perspect Med 5: a017798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gong W, Wang X, Zhang Y, Hao J, Xing C, Chu Q, Wang G, Zhao J, Wang J, Dong Q, Liu T, Zhang Y, and Dong L. Interleukin-20 promotes airway remodeling in asthma. Inflammation 37: 2099–2105, 2014 [DOI] [PubMed] [Google Scholar]
- 106. Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, and Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 154: 602–611, 1996 [DOI] [PubMed] [Google Scholar]
- 107. Goodwin GH, Sanders C, and Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem 38: 14–19, 1973 [DOI] [PubMed] [Google Scholar]
- 108. de Graauw M, Tijdens I, Cramer R, Corless S, Timms JF, and van de Water B. Heat shock protein 27 is the major differentially phosphorylated protein involved in renal epithelial cellular stress response and controls focal adhesion organization and apoptosis. J Biol Chem 280: 29885–29898, 2005 [DOI] [PubMed] [Google Scholar]
- 109. Grégoire M, Uhel F, Lesouhaitier M, Gacouin A, Guirriec M, Mourcin F, Dumontet E, Chalin A, Samson M, Berthelot L-L, Tissot A, Kerjouan M, Jouneau S, Le Tulzo Y, Tarte K, Zmijewski JW, and Tadié J-M. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur Respir J 52: 1702590, 2018 [DOI] [PubMed] [Google Scholar]
- 110. Griffin KL, Fischer BM, Kummarapurugu AB, Zheng S, Kennedy TP, Rao NV, Foster WM, and Voynow JA. 2-O, 3-O-desulfated heparin inhibits neutrophil elastase-induced HMGB-1 secretion and airway inflammation. Am J Respir Cell Mol Biol 50: 684–689, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Grover A, Taylor J, Troudt J, Keyser A, Sommersted K, Schenkel A, and Izzo AA. Mycobacterial infection induces the secretion of high-mobility group box 1 protein. Cell Microbiol 10: 1390–1404, 2008 [DOI] [PubMed] [Google Scholar]
- 112. Guiedem E, Ikomey GM, Nkenfou C, Walter P-YE, Mesembe M, Chegou NN, Jacobs GB, and Okomo Assoumou MC. Chronic obstructive pulmonary disease (COPD): neutrophils, macrophages and lymphocytes in patients with anterior tuberculosis compared to tobacco related COPD. BMC Res Notes 11: 192, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Günther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, and Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 153: 176–184, 1996 [DOI] [PubMed] [Google Scholar]
- 114. Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, Yamada S, Kuwano K, and Nakanishi Y. The role of high mobility group box 1 in pulmonary fibrosis. Am J Respir Cell Mol Biol 39: 440–447, 2008 [DOI] [PubMed] [Google Scholar]
- 115. Hay JG, Haslam PL, Turner-Warwick M, and Laurent GJ. The effects of iron and desferrioxamine on the lung injury induced by intravenous bleomycin and hyperoxia. Free Radic Res Commun 4: 109–114, 1987 [DOI] [PubMed] [Google Scholar]
- 116. Hayden MS. and Ghosh S. NF-κB in immunobiology. Cell Res 21: 223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, and Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 293: L1427–L1436, 2007 [DOI] [PubMed] [Google Scholar]
- 118. He Q, You H, Li X-M, Liu T-H, Wang P, and Wang B-E. HMGB1 promotes the synthesis of Pro-IL-1β and Pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pac J Cancer Prev 13: 1365–1370, 2012 [DOI] [PubMed] [Google Scholar]
- 119. He Y, Ding Y, Wang D, Zhang W, Chen W, Liu X, Qin W, Qian X, Chen H, and Guo Z. HMGB1 bound to cisplatin-DNA adducts undergoes extensive acetylation and phosphorylation in vivo. Chem Sci 6: 2074–2078, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Heijink IH, Pouwels SD, Leijendekker C, de Bruin HG, Zijlstra GJ, van der Vaart H, ten Hacken NHT, van Oosterhout AJM, Nawijn MC, and van der Toorn M. Cigarette smoke-induced damage-associated molecular pattern release from necrotic neutrophils triggers proinflammatory mediator release. Am J Respir Cell Mol Biol 52: 554–562, 2015 [DOI] [PubMed] [Google Scholar]
- 121. Hernández-Pando R, Barrios-Payán J, Mata-Espinosa D, Marquina-Castillo B, Hernández-Ramírez D, Botasso OA, and Bini EI. The role of high mobility group box 1 protein (HMGB1) in the immunopathology of experimental pulmonary tuberculosis. PLoS One 10: e0137263, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Higa F, Furugen M, Koide M, Karimata Y, Nabeya D, Iha Y, Kinjo T, Miyagi K, Haranaga S, Hokama A, Tateyama M, and Fujita J. Clinical evaluation of high mobility group box 1 protein in Legionella pneumophila pneumonia. J Infect Chemother 20: 289–292, 2014 [DOI] [PubMed] [Google Scholar]
- 123. Hinks TSC, Brown T, Lau LCK, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, Djukanović R, Kurukulaaratchy RJ, Chauhan A, and Howarth PH. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol 138: 61–75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123a. HMGB1 (human). Phosphosite. www.phosphosite.org/proteinAction?id=6934&showAllSites=true May 8, 2019
- 124. Homma S, Azuma A, Taniguchi H, Ogura T, Mochiduki Y, Sugiyama Y, Nakata K, Yoshimura K, Takeuchi M, Kudoh S, and Japan NAC Clinical Study Group. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 17: 467–477, 2012 [DOI] [PubMed] [Google Scholar]
- 125. Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, and Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270: 25752–25761, 1995 [DOI] [PubMed] [Google Scholar]
- 126. Hou C, Kong J, Liang Y, Huang H, Wen H, Zheng X, Wu L, and Chen Y. HMGB1 contributes to allergen-induced airway remodeling in a murine model of chronic asthma by modulating airway inflammation and activating lung fibroblasts. Cell Mol Immunol 12: 409–423, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hou C, Zhao H, Li W, and Cai S. Hydrogen peroxide induces high mobility group box 1 release in human bronchial epithelial cells [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 32: 1131–1134, 2012 [PubMed] [Google Scholar]
- 128. Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, Shen X, Liang Z, Cai S, and Zou F. High mobility group protein B1 (HMGB1) in asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med 17: 807–815, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hou XQ, Qin JL, Zheng XX, Wang L, Yang ST, Gao YW, and Xia XZH. Potential role of high-mobility group box 1 protein in the pathogenesis of influenza H5N1 virus infection. Acta Virol 58: 69–75, 2014 [DOI] [PubMed] [Google Scholar]
- 130. Hreggvidsdottir HS, Östberg T, Wähämaa H, Schierbeck H, Aveberger A-C, Klevenvall L, Palmblad K, Ottosson L, Andersson U, and Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol 86: 655–662, 2009 [DOI] [PubMed] [Google Scholar]
- 131. Hsu C-H, Ho W-J, Huang W-C, Chiu Y-W, Hsu T-S, Kuo P-H, Hsu H-H, Chang J-K, Cheng C-C, Lai C-L, Liang K-W, Lin S-L, Sung H-H, Tsai W-C, Weng K-P, Hsieh K-S, Yin W-H, Lin S-J, and Wang K-Y. 2014 Guidelines of Taiwan Society of Cardiology (TSOC) for the management of pulmonary arterial hypertension. Acta Cardiol Sin 30: 401–444, 2014 [PMC free article] [PubMed] [Google Scholar]
- 132. Huang B, Zhao J, Unkeless JC, Feng ZH, and Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 27: 218–224, 2008 [DOI] [PubMed] [Google Scholar]
- 133. Huang W, Zhao H, Dong H, Wu Y, Yao L, Zou F, and Cai S. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway. Int J Mol Med 37: 1189–1198, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huang X, Xiu H, Zhang S, and Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm 2018: 1264913, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hubbard RB, Smith C, Le Jeune I, Gribbin J, and Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease. Am J Respir Crit Care Med 178: 1257–1261, 2008 [DOI] [PubMed] [Google Scholar]
- 136. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, and Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: S13–S24, 2004 [DOI] [PubMed] [Google Scholar]
- 137. Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, and Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res 62: 4805–4811, 2002 [PubMed] [Google Scholar]
- 138. Isackson PJ, Bidney DL, Reeck GR, Neihart NK, and Bustin M. High mobility group chromosomal proteins isolated from nuclei and cytosol of cultured hepatoma cells are similar. Biochemistry 19: 4466–4471, 1980 [DOI] [PubMed] [Google Scholar]
- 139. Ito N, DeMarco RA, Mailliard RB, Han J, Rabinowich H, Kalinski P, Stolz DB, Zeh HJ, and Lotze MT. Cytolytic cells induce HMGB1 release from melanoma cell lines. J Leukoc Biol 81: 75–83, 2007 [DOI] [PubMed] [Google Scholar]
- 140. Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T, and Maruyama I. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 28: 1825–1830, 2008 [DOI] [PubMed] [Google Scholar]
- 141. Janko C, Filipović M, Munoz LE, Schorn C, Schett G, Ivanović-Burmazović I, and Herrmann M. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal 20: 1075–1085, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jeune KS-L, Jeune AL, Jouneau S, Belleguic C, Roux P-F, Jaguin M, Dimanche-Boitre M-T, Lecureur V, Leclercq C, Desrues B, Brinchault G, Gangneux J-P, and Martin-Chouly C. Impaired functions of macrophage from cystic fibrosis patients: CD11b, TLR-5 decrease and sCD14, inflammatory cytokines increase. PLoS One 8: e75667, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jia D, He Y, Zhu Q, Liu H, Zuo C, Chen G, Yu Y, and Lu A. RAGE-mediated extracellular matrix proteins accumulation exacerbates HySu-induced pulmonary hypertension. Cardiovasc Res 113: 586–597, 2017 [DOI] [PubMed] [Google Scholar]
- 144. Kanazawa H, Tochino Y, Asai K, Ichimaru Y, Watanabe T, and Hirata K. Validity of HMGB1 measurement in epithelial lining fluid in patients with COPD. Eur J Clin Invest 42: 419–426, 2012 [DOI] [PubMed] [Google Scholar]
- 145. Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan X, and Yan Z. HMGB1 in health and disease. Mol Aspects Med 40: 1–116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kaur A, Mathai SK, and Schwartz DA. Genetics in idiopathic pulmonary fibrosis pathogenesis, prognosis, and treatment. Front Med 4: 154, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kawahara K-I, Hashiguchi T, Masuda K, Saniabadi AR, Kikuchi K, Tancharoen S, Ito T, Miura N, Morimoto Y, Biswas KK, Nawa Y, Meng X, Oyama Y, Takenouchi K, Shrestha B, Sameshima H, Shimizu T, Adachi T, Adachi M, and Maruyama I. Mechanism of HMGB1 release inhibition from RAW264.7 cells by oleanolic acid in Prunus mume Sieb. et Zucc. Int J Mol Med 23: 615–620, 2009 [DOI] [PubMed] [Google Scholar]
- 148. Kawano F, Fujita R, Nakai N, Terada M, Ohira T, and Ohira Y. HSP25 can modulate myofibrillar desmin cytoskeleton following the phosphorylation at Ser15 in rat soleus muscle. J Appl Physiol 1985 112: 176–186, 2012 [DOI] [PubMed] [Google Scholar]
- 149. Kazama H, Ricci J-E, Herndon JM, Hoppe G, Green DR, and Ferguson TA. Induction of immunological tolerance by apoptotic cells requires Caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29: 21–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kendall EA, Azman AS, Cobelens FG, and Dowdy DW. MDR-TB treatment as prevention: the projected population-level impact of expanded treatment for multidrug-resistant tuberculosis. PLoS One 12: e0172748, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, and Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 245: 1073–1080, 1989 [DOI] [PubMed] [Google Scholar]
- 152. Kew RR, Penzo M, Habiel DM, and Marcu KB. The IKKα-dependent NF-κB p52/RelB non-canonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. J Immunol 1950 188: 2380–2386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kida T, Seno T, Nagahara H, Inoue T, Nakabayashi A, Kukida Y, Fujioka K, Fujii W, Wada M, Kohno M, and Kawahito Y. Roles of high-mobility group box 1 and thrombin in murine pulmonary fibrosis and the therapeutic potential of thrombomodulin. Am J Physiol Lung Cell Mol Physiol 314: L473–L483, 2017 [DOI] [PubMed] [Google Scholar]
- 154. Kim DE, Min K, Kim JS, and Kwon TK. High-mobility group box-1 protein induces mucin 8 expression through the activation of the JNK and PI3K/Akt signal pathways in human airway epithelial cells. Biochem Biophys Res Commun 421: 436–441, 2012 [DOI] [PubMed] [Google Scholar]
- 155. Kim SR, Ha YM, Kim YM, Park EJ, Kim JW, Park SW, Kim HJ, Chung HT, and Chang KC. Ascorbic acid reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and improves survival rate in septic mice by activation of Nrf2/HO-1 signals. Biochem Pharmacol 95: 279–289, 2015 [DOI] [PubMed] [Google Scholar]
- 156. Kim YM, Park EJ, Kim HJ, and Chang KC. Sirt1 S-nitrosylation induces acetylation of HMGB1 in LPS-activated RAW264.7 cells and endotoxemic mice. Biochem Biophys Res Commun 501: 73–79, 2018 [DOI] [PubMed] [Google Scholar]
- 157. King TE, Pardo A, and Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011 [DOI] [PubMed] [Google Scholar]
- 158. Ko H-K, Hsu W-H, Hsieh C-C, Lien T-C, Lee T-S, and Kou YR. High expression of high-mobility group box 1 in the blood and lungs is associated with the development of chronic obstructive pulmonary disease in smokers. Respirology 19: 253–261, 2014 [DOI] [PubMed] [Google Scholar]
- 159. Kohlstaedt LA, King DS, and Cole RD. Native state of high mobility group chromosomal proteins 1 and 2 is rapidly lost by oxidation of sulfhydryl groups during storage. Biochemistry 25: 4562–4565, 1986 [DOI] [PubMed] [Google Scholar]
- 160. Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger C-J, Arnold B, Nawroth P, Andersson U, Harris RA, and Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 61: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 161. Kollisch-Singule M, Jain S, Andrews P, Smith BJ, Hamlington-Smith KL, Roy S, DiStefano D, Nuss E, Satalin J, Meng Q, Marx W, Bates JHT, Gatto LA, Nieman GF, and Habashi NM. Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA Surg 151: 64–72, 2016 [DOI] [PubMed] [Google Scholar]
- 162. Kosmider B, Messier EM, Chu HW, and Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One 6: e26059, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Krupa A, Fol M, Dziadek BR, Kepka E, Wojciechowska D, Brzostek A, Torzewska A, Dziadek J, Baughman RP, Griffith D, and Kurdowska AK. Binding of CXCL8/IL-8 to Mycobacterium tuberculosis modulates the innate immune response. Mediators Inflamm 2015, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kumar S, Tana A, and Shankar A. Cystic fibrosis—what are the prospects for a cure? Eur J Intern Med 25: 803–807, 2014 [DOI] [PubMed] [Google Scholar]
- 165. Kummarapurugu AB, Zheng S, Ledford J, Karandashova S, and Voynow JA. High-mobility group box 1 upregulates MUC5AC and MUC5B expression in primary airway epithelial cells. Am J Respir Cell Mol Biol 58: 126–128, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kwak MS, Kim HS, Lkhamsuren K, Kim YH, Han MG, Shin JM, Park IH, Rhee WJ, Lee SK, Rhee SG, and Shin J-S. Peroxiredoxin-mediated disulfide bond formation is required for nucleocytoplasmic translocation and secretion of HMGB1 in response to inflammatory stimuli. Redox Biol 24: 101203, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Langan KM, Kotsimbos T, and Peleg AY. Managing Pseudomonas aeruginosa respiratory infections in cystic fibrosis. Curr Opin Infect Dis 28: 547–556, 2015 [DOI] [PubMed] [Google Scholar]
- 168. Langevin SM, Kratzke RA, and Kelsey KT. Epigenetics of lung cancer. Transl Res 165: 74–90, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lawrence T, Gilroy DW, Colville-Nash PR, and Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med 7: 1291–1297, 2001 [DOI] [PubMed] [Google Scholar]
- 171. Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, Holtzman MJ, Whitsett JA, and Hershey GK. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol 300: L414–L421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lee C-C, Lai Y-T, Chang H-T, Liao J-W, Shyu W-C, Li C-Y, and Wang C-N. Inhibition of high-mobility group box 1 in lung reduced airway inflammation and remodeling in a mouse model of chronic asthma. Biochem Pharmacol 86: 940–949, 2013 [DOI] [PubMed] [Google Scholar]
- 173. Lee H, Lee J, Hong S-H, Rahman I, and Yang S-R. Inhibition of RAGE attenuates cigarette smoke-induced lung epithelial cell damage via RAGE-mediated Nrf2/DAMP signaling. Front Pharmacol 9: 684, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lee RNC, Kelly E, Nolan G, Eigenheer S, Boylan D, Murphy D, Dodd J, Keane MP, and McNicholas WT. Disordered breathing during sleep and exercise in idiopathic pulmonary fibrosis and the role of biomarkers. QJM 108: 315–323, 2015 [DOI] [PubMed] [Google Scholar]
- 175. Lehming N, Thanos D, Brickman JM, Ma J, Maniatis T, and Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature 371: 175–179, 1994 [DOI] [PubMed] [Google Scholar]
- 176. Li C, Peng S, Liu X, Han C, Wang X, Jin T, Liu S, Wang W, Xie X, He X, Zhang H, Shan L, Fan C, Shan Z, and Teng W. Glycyrrhizin, a direct HMGB1 antagonist, ameliorates inflammatory infiltration in a model of autoimmune thyroiditis via inhibition of TLR2-HMGB1 signaling. Thyroid 27: 722–731, 2017 [DOI] [PubMed] [Google Scholar]
- 177. Li C, Yu Y, Li W, Liu B, Jiao X, Song X, Lv C, and Qin S. Phycocyanin attenuates pulmonary fibrosis via the TLR2-MyD88-NF-κB signaling pathway. Sci Rep 7: 5843, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, and Ulloa L. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med 9: 37–45, 2003 [PMC free article] [PubMed] [Google Scholar]
- 179. Li L, Wei C, Kim I-K, Janssen-Heininger Y, and Gupta S. Inhibition of nuclear factor-B in the lungs prevents monocrotaline-induced pulmonary hypertension in mice. Hypertension 63: 1260–1269, 2014 [DOI] [PubMed] [Google Scholar]
- 180. Li L-C, Gao J, and Li J. Emerging role of HMGB1 in fibrotic diseases. J Cell Mol Med 18: 2331–2339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Li L-C, Li D-L, Xu L, Mo X-T, Cui W-H, Zhao P, Zhou W-C, Gao J, and Li J. High-mobility group box 1 mediates epithelial-to-mesenchymal transition in pulmonary fibrosis involving transforming growth factor-β1/Smad2/3 signaling. J Pharmacol Exp Ther 354: 302–309, 2015 [DOI] [PubMed] [Google Scholar]
- 182. Li Q, Li J, Wen T, Zeng W, Peng C, Yan S, Tan J, Yang K, Liu S, Guo A, Zhang C, Su J, Jiang M, Liu Z, Zhou H, and Chen X. Overexpression of HMGB1 in melanoma predicts patient survival and suppression of HMGB1 induces cell cycle arrest and senescence in association with p21 (Waf1/Cip1) up-regulation via a p53-independent, Sp1-dependent pathway. Oncotarget 5: 6387–6403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Li T-T, Ogino S, and Qian ZR. Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol 20: 17699–17708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Li WJ, Hu K, Yang JP, Xu XY, Li N, Wen ZP, and Wang H. HMGB1 affects the development of pulmonary arterial hypertension via RAGE. Eur Rev Med Pharmacol Sci 21: 9, 2017 [PubMed] [Google Scholar]
- 185. Li XY, Donaldson K, Brown D, and MacNee W. The role of tumor necrosis factor in increased airspace epithelial permeability in acute lung inflammation. Am J Respir Cell Mol Biol 13: 185–195, 1995 [DOI] [PubMed] [Google Scholar]
- 186. Li Y, Paonessa JD, and Zhang Y. Mechanism of chemical activation of Nrf2. PLoS One 7: e35122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Li Y-H, Kuo C-H, Shi G-Y, and Wu H-L. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci 19: 34, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Liang Y, Hou C, Kong J, Wen H, Zheng X, Wu L, Huang H, and Chen Y. HMGB1 binding to receptor for advanced glycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol Cell Biochem 405: 63–71, 2015 [DOI] [PubMed] [Google Scholar]
- 189. Lim K, Hyun Y-M, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, and Kim M. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science 349: aaa4352, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lim S-C, Choi JE, Kim CH, Duong H-Q, Jeong G-A, Kang HS, and Han SI. Ethyl pyruvate induces necrosis-to-apoptosis switch and inhibits high mobility group box protein 1 release in A549 lung adenocarcinoma cells. Int J Mol Med 20: 187–192, 2007 [PubMed] [Google Scholar]
- 191. Lin W-C, Lin C-F, Chen C-L, Chen C-W, and Lin Y-S. Prediction of outcome in patients with acute respiratory distress syndrome by bronchoalveolar lavage inflammatory mediators. Exp Biol Med (Maywood) 235: 57–65, 2010 [DOI] [PubMed] [Google Scholar]
- 192. Lin X, Yang H, Sakuragi T, Hu M, Mantell LL, Hayashi S, Al-Abed Y, Tracey KJ, Ulloa L, and Miller EJ. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol 289: L583–L590, 2005 [DOI] [PubMed] [Google Scholar]
- 193. Liu G, Cooley MA, Nair PM, Donovan C, Hsu AC, Jarnicki AG, Haw TJ, Hansbro NG, Ge Q, Brown AC, Tay H, Foster PS, Wark PA, Horvat JC, Bourke JE, Grainge CL, Argraves WS, Oliver BG, Knight DA, Burgess JK, and Hansbro PM. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c: Fbln1c regulates the pathogenesis of chronic asthma. J Pathol 243: 510–523, 2017 [DOI] [PubMed] [Google Scholar]
- 194. Liu G, Wang J, Park Y-J, Tsuruta Y, Lorne EF, Zhao X, and Abraham E. HMGB1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol 1950 181: 4240–4246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Liu R, McEachin RC, and States DJ. Computationally identifying novel NF-kappa B-regulated immune genes in the human genome. Genome Res 13: 654–661, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Liu X, Yu D, Chen M, Sun T, Li G, Huang W, Nie H, Wang C, Zhang Y, Gong Q, and Ren B. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int Immunopharmacol 25: 370–376, 2015 [DOI] [PubMed] [Google Scholar]
- 197. López Del Prado GR, Hernán García C, Moreno Cea L, Fernández Espinilla V, Muñoz Moreno MF, Delgado Márquez A, Polo Polo MJ, and Andrés García I. Malaria in developing countries. J Infect Dev Ctries 8: 1–4, 2014 [DOI] [PubMed] [Google Scholar]
- 198. López-Campos JL, Tan W, and Soriano JB. Global burden of COPD. Respirology 21: 14–23, 2016 [DOI] [PubMed] [Google Scholar]
- 199. Lotze MT. and Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331–342, 2005 [DOI] [PubMed] [Google Scholar]
- 200. Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MAD, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, and Tracey KJ. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A 111: 3068–3073, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lu B, Wang C, Wang M, Li W, Chen F, Tracey KJ, and Wang H. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev Clin Immunol 10: 713–727, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lui G, Wong CK, Ip M, Chu YJ, Yung IMH, Cheung CSK, Zheng L, Lam JSY, Wong KT, Sin WWY, Choi KW, and Lee N. HMGB1/RAGE signaling and pro-inflammatory cytokine responses in non-HIV adults with active pulmonary tuberculosis. PLoS One 11: e0159132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Lundbäck P, Lea JD, Sowinska A, Ottosson L, Fürst CM, Steen J, Aulin C, Clarke JI, Kipar A, Klevenvall L, Yang H, Palmblad K, Park BK, Tracey KJ, Blom AM, Andersson U, Antoine DJ, and Erlandsson Harris H. A novel high mobility group box 1 neutralizing chimeric antibody attenuates drug-induced liver injury and postinjury inflammation in mice. Hepatology 64: 1699–1710, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Luo Y, Che W, and Zhao M. Ulinastatin post-treatment attenuates lipopolysaccharide-induced acute lung injury in rats and human alveolar epithelial cells. Int J Mol Med 39: 297–306, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lv X-X, Liu S-S, Li K, Cui B, Liu C, and Hu Z-W. Cigarette smoke promotes COPD by activating platelet-activating factor receptor and inducing neutrophil autophagic death in mice. Oncotarget 8: 74720–74735, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ma L, Zeng J, Mo B, Wang C, Huang J, Sun Y, Yu Y, and Liu S. High mobility group box 1: a novel mediator of Th2-type response- induced airway inflammation of acute allergic asthma. J Thorac Dis 7: 10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 258–266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Maher JF. and Nathans D. Multivalent DNA-binding properties of the HMG-1 proteins. Proc Natl Acad Sci U S A 93: 6716–6720, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Marais BJ. Tuberculosis in children. J Paediatr Child Health 50: 759–767, 2014 [DOI] [PubMed] [Google Scholar]
- 210. Marakalala MJ, Martinez FO, Plüddemann A, and Gordon S. Macrophage heterogeneity in the immunopathogenesis of tuberculosis. Front Microbiol 9: 1028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Martinez FJ, de Andrade JA, Anstrom KJ, King TE, and Raghu G. Randomized trial of N-acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 370: 2093–2101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Martínez-Sapiña Llanas MJ, Ríos Reboredo A, Romay Cousido G, and Romero González JA. Severe intestinal ischemia as a presenting feature of metastatic ileal carcinoid tumor: role of MDCT with coronal reformation in the early diagnosis. Abdom Imaging 37: 558–560, 2012 [DOI] [PubMed] [Google Scholar]
- 213. Martinotti S, Ranzato E, and Patrone M. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther 101: 101–109, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Matthay MA. and Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. McClean CM. and Tobin DM. Macrophage form, function, and phenotype in mycobacterial infection: lessons from tuberculosis and other diseases. Pathog Dis 74: ftw068, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu X, and Zheng M. HMGB1 promotes cellular proliferation and invasion, suppresses cellular apoptosis in osteosarcoma. Tumor Biol 35: 12265–12274, 2014 [DOI] [PubMed] [Google Scholar]
- 217. Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, and Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem 266: 16722–16729, 1991 [PubMed] [Google Scholar]
- 218. Mihm S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J Mol Sci 19: 3104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Mokra D. and Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol 209: 52–58, 2015 [DOI] [PubMed] [Google Scholar]
- 220. Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, and Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14: 431–441, 2007 [DOI] [PubMed] [Google Scholar]
- 221. Montani D, Günther S, Dorfmüller P, Perros F, Girerd B, Garcia G, Jaïs X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, and Sitbon O. Pulmonary arterial hypertension. Orphanet J Rare Dis 8: 97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Montanini L, Cirillo F, Smerieri A, Pisi G, Giardino I, d'Apolito M, Spaggiari C, Bernasconi S, Amarri S, and Street ME. HMGB1 Is increased by CFTR loss of function, is lowered by insulin, and increases in vivo at onset of CFRD. J Clin Endocrinol Metab 101: 1274–1281, 2016 [DOI] [PubMed] [Google Scholar]
- 223. Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, and Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol 19: 1437–1445, 2006 [DOI] [PubMed] [Google Scholar]
- 224. Morrow JD, Zhou X, Lao T, Jiang Z, DeMeo DL, Cho MH, Qiu W, Cloonan S, Pinto-Plata V, Celli B, Marchetti N, Criner GJ, Bueno R, Washko GR, Glass K, Quackenbush J, Choi AMK, Silverman EK, and Hersh CP. Functional interactors of three genome-wide association study genes are differentially expressed in severe chronic obstructive pulmonary disease lung tissue. Sci Rep 7: 44232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Müller S. The double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J 20: 4337–4340, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Naeije R. and Eddahibi S. Serotonin in pulmonary arterial hypertension. Am J Respir Crit Care Med 170: 209–210, 2004 [DOI] [PubMed] [Google Scholar]
- 227. Nanchal RS. and Truwit JD. Recent advances in understanding and treating acute respiratory distress syndrome. F1000Res 7: 1322, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Nayak AP, Deshpande DA, and Penn RB. New targets for resolution of airway remodeling in obstructive lung diseases. F1000Res 7: 680, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ndlovu H. and Marakalala MJ. Granulomas and inflammation: host-directed therapies for tuberculosis. Front Immunol 7: 434, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Nett RJ. Dental personnel treated for idiopathic pulmonary fibrosis at a tertiary care center—Virginia, 2000–2015. MMWR Morb Mortal Wkly Rep 67: 270–273, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Nguyen GT, Green ER, and Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol 7: 373, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. NHLBI. Pneumonia National Heart, Lung, and Blood Institute (NHLBI). 2011. https://www.nhlbi.nih.gov/health-topics/pneumonia#Risk-Factors September 18, 2018
- 233. Niki M, Yokoi T, Kurata T, and Nomura S. New prognostic biomarkers and therapeutic effect of bevacizumab for patients with non-small-cell lung cancer. Lung Cancer Targets Ther 8: 91–99, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, and Cronin K. (Eds). Cancer statistics review, 1975–2015—SEER statistics, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. Bethesda, MD: National Cancer Institute; https://seer.cancer.gov/csr/1975_2015/ August 20, 2018 [Google Scholar]
- 235. Nosaka N, Yashiro M, Yamada M, Fujii Y, Tsukahara H, Liu K, Nishibori M, Matsukawa A, and Morishima T. Anti-high mobility group box-1 monoclonal antibody treatment provides protection against influenza A virus (H1N1)-induced pneumonia in mice. Crit Care 19: 249, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. O'Byrne PM. and Inman MD. Airway hyperresponsiveness. Chest 123: 411S–6S, 2003 [DOI] [PubMed] [Google Scholar]
- 237. O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson L-G, and Busse WW. The poorly explored impact of uncontrolled asthma. Chest 143: 511–523, 2013 [DOI] [PubMed] [Google Scholar]
- 238. Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, and Shin J-S. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol 1950 182: 5800–5809, 2009 [DOI] [PubMed] [Google Scholar]
- 239. Ojo OO, Ryu MH, Jha A, Unruh H, and Halayko AJ. High-mobility group box 1 promotes extracellular matrix synthesis and wound repair in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 309: L1354–L1366, 2015 [DOI] [PubMed] [Google Scholar]
- 240. O'Sullivan BP. and Freedman SD. Cystic fibrosis. Lancet 373: 1891–1904, 2009 [DOI] [PubMed] [Google Scholar]
- 241. Ottosen J. and Evans H. Pneumonia: challenges in the definition, diagnosis, and management of disease. Surg Clin North Am 94: 1305–1317, 2014 [DOI] [PubMed] [Google Scholar]
- 242. Pagán AJ. and Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med 5, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Pan B, Chen D, Huang J, Wang R, Feng B, Song H, and Chen L. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma. Mol Cancer 13: 165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Papi A, Brightling C, Pedersen SE, and Reddel HK. Asthma. Lancet 391: 783–800, 2018 [DOI] [PubMed] [Google Scholar]
- 245. Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A, and Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377, 2004 [DOI] [PubMed] [Google Scholar]
- 246. Parker JC, Hernandez LA, and Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med 21: 131, 1993 [DOI] [PubMed] [Google Scholar]
- 247. Patel VS, Sampat V, Espey MG, Sitapara R, Wang H, Yang X, Ashby CR, Thomas DD, and Mantell LL. Ascorbic acid attenuates hyperoxia-compromised host defense against pulmonary bacterial infection. Am J Respir Cell Mol Biol 55: 511–520, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Patel VS, Sitapara RA, Gore A, Phan B, Sharma L, Sampat V, Li JH, Yang H, Chavan SS, Wang H, Tracey KJ, and Mantell LL. High mobility group box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol 48: 280–287, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J 7: 1–3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Payet D, Hillisch A, Lowe N, Diekmann S, and Travers A. The recognition of distorted DNA structures by HMG-D: a footprinting and molecular modelling study. J Mol Biol 294: 79–91, 1999 [DOI] [PubMed] [Google Scholar]
- 251. Pedroza M, Le TT, Lewis K, Karmouty-Quintana H, To S, George AT, Blackburn MR, Tweardy DJ, and Agarwal SK. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. FASEB J 30: 129–140, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Perlman CE, Lederer DJ, and Bhattacharya J. Micromechanics of alveolar edema. Am J Respir Cell Mol Biol 44: 34–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Phair RD, Scaffidi P, Elbi C, Vecerová J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, and Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol Cell Biol 24: 6393–6402, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Postma DS. and Kerstjens HAM. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: S187–S192, 1998 [DOI] [PubMed] [Google Scholar]
- 255. Prina E, Ranzani OT, and Torres A. Community-acquired pneumonia. Lancet 386: 1097–1108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Qin Y-H, Dai S-M, Tang G-S, Zhang J, Ren D, Wang Z-W, and Shen Q. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol 183: 6244–6250, 2009 [DOI] [PubMed] [Google Scholar]
- 257. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Rafikov R, Nair V, Sinari S, Babu H, Sullivan JC, Yuan JX-J, Desai AA, and Rafikova O. Gender difference in damage-mediated signaling contributes to pulmonary arterial hypertension. Antioxid Redox Signal 2019. [Epub ahead of print]; DOI: 10.1089/ars.2018.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Rahman I, Morrison D, Donaldson K, and MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 260. Ramsgaard L, Englert JM, Manni ML, Milutinovic PS, Gefter J, Tobolewski J, Crum L, Coudriet GM, Piganelli J, Zamora R, Vodovotz Y, Enghild JJ, and Oury TD. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice. PLoS One 6: e20132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Rao Z, Zhang N, Xu N, Pan Y, Xiao M, Wu J, Zhou H, Yang S, and Chen Y. 1,25-Dihydroxyvitamin D inhibits LPS-induced high-mobility group box 1 (HMGB1) secretion via targeting the NF-E2-related factor 2—hemeoxygenase-1—HMGB1 pathway in macrophages. Front Immunol 8: 1308, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Raucci A, Di Maggio S, Scavello F, D'Ambrosio A, Bianchi ME, and Capogrossi MC. The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci 76: 211–229, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Ravimohan S, Kornfeld H, Weissman D, and Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 27: 17077, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Read CM, Cary PD, Crane-Robinson C, Driscoll PC, and Norman DG. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res 21: 3427–3436, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Read CM, Cary PD, Preston NS, Lnenicek-Allen M, and Crane-Robinson C. The DNA sequence specificity of HMG boxes lies in the minor wing of the structure. EMBO J 13: 5639–5646, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Reeves R, Edberg DD, and Li Y. Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol 21: 575–594, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Ren D, Sun R, and Wang S. Role of inducible nitric oxide synthase expressed by alveolar macrophages in high mobility group box 1-induced acute lung injury. Inflamm Res 55: 207–215, 2006 [DOI] [PubMed] [Google Scholar]
- 268. Restrepo MI, Faverio P, and Anzueto A. Long-term prognosis in community-acquired pneumonia. Curr Opin Infect Dis 26: 151–158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, Larsson A, Hedenstierna G, Bugedo G, and Bruhn A. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care 18: 505, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Revak SD, Rice CL, Schraufstätter IU, Halsey WA, Bohl BP, Clancy RM, and Cochrane CG. Experimental pulmonary inflammatory injury in the monkey. J Clin Invest 76: 1182–1192, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ronfani L, Ferraguti M, Croci L, Ovitt CE, Scholer HR, Consalez GG, and Bianchi ME. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development 128: 1265–1273, 2001 [DOI] [PubMed] [Google Scholar]
- 272. Rotta AT. and Steinhorn DM. Partial liquid ventilation reduces pulmonary neutrophil accumulation in an experimental model of systemic endotoxemia and acute lung injury. Crit Care Med 26: 1707–1715, 1998 [DOI] [PubMed] [Google Scholar]
- 273. Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, Bianchi ME, and Manfredi AA. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5: 825–830, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, O'Neal W, Sorscher EJ, Abraham E, and Blalock JE. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med 178: 822–831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H, and Maruyama I. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS One 9: e102482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Sahu D, Debnath P, Takayama Y, and Iwahara J. Redox properties of the A-domain of the HMGB1 protein. FEBS Lett 582: 3973–3978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Saito A, Horie M, and Nagase T. TGF-β signaling in lung health and disease. Int J Mol Sci 19: 2460, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Sapojnikova N, Maman J, Myers FA, Thorne AW, Vorobyev VI, and Crane-Robinson C. Biochemical observation of the rapid mobility of nuclear HMGB1. Biochim Biophys Acta 1729: 57–63, 2005 [DOI] [PubMed] [Google Scholar]
- 279. Sastre B, Rodrigo-Muñoz JM, Garcia-Sanchez DA, Cañas JA, and Del Pozo V. Eosinophils: old players in a new game. J Investig Allergol Clin Immunol 28: 289–304, 2018 [DOI] [PubMed] [Google Scholar]
- 280. Scaffidi P, Misteli T, and Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 281. Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, and Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209: 551–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Schulman IG, Wang T, Wu M, Bowen J, Cook RG, Gorovsky MA, and Allis CD. Macronuclei and micronuclei in Tetrahymena thermophila contain high-mobility-group-like chromosomal proteins containing a highly conserved eleven-amino-acid putative DNA-binding sequence. Mol Cell Biol 11: 166–174, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Schwartz DA. Idopathich pulmonary fibrosis is a complex genetic disorder. Trans Am Clin Climatol Assoc 127: 34–45, 2016 [PMC free article] [PubMed] [Google Scholar]
- 284. Schwartz DA. Idiopathic pulmonary fibrosis—NORD (National Organization for Rare Disorders). 2017. https://rarediseases.org/rare-diseases/idiopathic-pulmonary-fibrosis/ August 21, 2018
- 285. Scott A. Cystic fibrosis. Radiol Technol 84: 493–518, 2013 [PubMed] [Google Scholar]
- 286. Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, and Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364: 1503–1512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Shang D, Peng T, Gou S, Li Y, Wu H, Wang C, and Yang Z. High mobility group box protein 1 boosts endothelial albumin transcytosis through the RAGE/Src/Caveolin-1 pathway. Sci Rep 6: 32180, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Shang G-H, Jia C-Q, Tian H, Xiao W, Li Y, Wang A-H, Dong L, and Lin D-J. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respir Med 103: 1949–1953, 2009 [DOI] [PubMed] [Google Scholar]
- 289. Shao Y, Nanayakkara G, Cheng J, Cueto R, Yang WY, Park J-Y, Wang H, and Yang X. Lysophospholipids and their receptors serve as conditional DAMPs and DAMP receptors in tissue oxidative and inflammatory injury. Antioxid Redox Signal 28: 973–986, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Sharma J, Young DM, Marentette JO, Rastogi P, Turk J, and McHowat J. Lung endothelial cell platelet-activating factor production and inflammatory cell adherence are increased in response to cigarette smoke component exposure. Am J Physiol Lung Cell Mol Physiol 302: L47–L55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Sharma L, Wu J, Patel V, Sitapara R, Rao NV, Kennedy TP, and Mantell LL. Partially-desulfated heparin improves survival in Pseudomonas pneumonia by enhancing bacterial clearance and ameliorating lung injury. J Immunotoxicol 11: 260–267, 2014 [DOI] [PubMed] [Google Scholar]
- 292. Shelest E, Kel AE, Göessling E, and Wingender E. Prediction of potential C/EBP/NF-kappaB composite elements using matrix-based search methods. In Silico Biol 3: 71–79, 2003 [PubMed] [Google Scholar]
- 293. Shen X, Hong L, Sun H, Shi M, and Song Y. The expression of high-mobility group protein box 1 correlates with the progression of non-small cell lung cancer. Oncol Rep 22: 535–539, 2009 [DOI] [PubMed] [Google Scholar]
- 294. Shigemitsu H. and Azuma A. Sarcoidosis and interstitial pulmonary fibrosis; two distinct disorders or two ends of the same spectrum: Curr Opin Pulm Med 17: 303–307, 2011 [DOI] [PubMed] [Google Scholar]
- 295. Shim E-J, Chun E, Lee H-S, Bang B-R, Kim T-W, Cho S-H, Min K-U, and Park H-W. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy 42: 958–965, 2012 [DOI] [PubMed] [Google Scholar]
- 296. Shimizu H, Sakamoto S, Isshiki T, Furuya K, Kurosaki A, and Homma S. Association of serum high-mobility group box protein 1 level with outcomes of acute exacerbation of idiopathic pulmonary fibrosis and fibrosing nonspecific interstitial pneumonia. PLoS One 13: e0196558, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Shukla SD, Muller HK, Latham R, Sohal SS, and Walters EH. Platelet-activating factor receptor (PAFr) is upregulated in small airways and alveoli of smokers and COPD patients. Respirology 21: 504–510, 2016 [DOI] [PubMed] [Google Scholar]
- 298. Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA Cancer J Clin 68: 7–30, 2018 [DOI] [PubMed] [Google Scholar]
- 299. da Silva LF, Skupien EC, Lazzari TK, Holler SR, de Almeida EGC, Zampieri LR, Coutinho SE, Andrades M, and Silva DR. Advanced glycation end products (AGE) and receptor for AGE (RAGE) in patients with active tuberculosis, and their relationship between food intake and nutritional status. PLoS One 14: e0213991, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Singh J. and Dixon GH. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry 29: 6295–6302, 1990 [DOI] [PubMed] [Google Scholar]
- 301. Sitapara RA, Antoine DJ, Sharma L, Patel VS, Ashby CR, Gorasiya S, Yang H, Zur M, and Mantell LL. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol Med 20: 238–247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 302. Sobh E, Elmadbouly AAAS, Ezzat H, and Abd-Allah M. Serum levels of high mobility group box 1 (HMGB1) and matrix metalloprotinase 9 (MMP9) are related to lung function indices in chronic obstructive pulmonary disease. Clin Med Diagn 7: 31–39, 2017 [Google Scholar]
- 303. Song JH, Kim JY, Piao C, Lee S, Kim B, Song SJ, Choi JS, and Lee M. Delivery of the high-mobility group box 1 box A peptide using heparin in the acute lung injury animal models. J Control Release 234: 33–40, 2016 [DOI] [PubMed] [Google Scholar]
- 304. Sprunger DB, Olson AL, Huie TJ, Fernandez-Perez ER, Fischer A, Solomon JJ, Brown KK, and Swigris JJ. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J 39: 125–132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, and Tomic-Canic M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365: 495–506, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sun T, Liu J, and Zhao DW. Efficacy of N-acetylcysteine in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Medicine (Baltimore) 95: e3629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Suzuki S, Nakazato K, Sugimoto K, Yoshihisa A, Yamaki T, Kunii H, Suzuki H, Saitoh S, and Takeishi Y. Plasma levels of receptor for advanced glycation end-products and high-mobility group box 1 in patients with pulmonary hypertension. Int Heart J 57: 234–240, 2016 [DOI] [PubMed] [Google Scholar]
- 308. Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, and Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 72: 3967–3976, 2012 [DOI] [PubMed] [Google Scholar]
- 309. Tadie J-M, Bae H-B, Jiang S, Park DW, Bell CP, Yang H, Pittet J-F, Tracey K, Thannickal VJ, Abraham E, and Zmijewski JW. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol 304: L342–L349, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, and Schmidt AM. Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature 405: 354–360, 2000 [DOI] [PubMed] [Google Scholar]
- 311. Takada M, Hirata K, Ajiki T, Suzuki Y, and Kuroda Y. Expression of receptor for advanced glycation end products (RAGE) and MMP-9 in human pancreatic cancer cells. Hepatogastroenterology 51: 928–930, 2004 [PubMed] [Google Scholar]
- 312. Takeda K. and Akira S. TLR signaling pathways. Semin Immunol 16: 3–9, 2004 [DOI] [PubMed] [Google Scholar]
- 313. Tang D, Kang R, Zeh HJ, and Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14: 1315–1335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, and Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol 81: 741–747, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Tang H, Zhao H, Song J, Dong H, Yao L, Liang Z, Lv Y, Zou F, and Cai S. Ethyl pyruvate decreases airway neutrophil infiltration partly through a high mobility group box 1-dependent mechanism in a chemical-induced murine asthma model. Int Immunopharmacol 21: 163–170, 2014 [DOI] [PubMed] [Google Scholar]
- 316. Tang L-Y, Heller M, Meng Z, Yu L-R, Tang Y, Zhou M, and Zhang YE. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J Biol Chem 292: 4302–4312, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Tang Z, Zang N, Fu Y, Ye Z, Chen S, Mo S, Ren L, and Liu E. HMGB1 mediates HAdV-7 infection-induced pulmonary inflammation in mice. Biochem Biophys Res Commun 501: 1–8, 2018 [DOI] [PubMed] [Google Scholar]
- 318. Thomas JO. and Travers AA. HMG1 and 2, and related “architectural” DNA-binding proteins. Trends Biochem Sci 26: 167–174, 2001 [DOI] [PubMed] [Google Scholar]
- 319. Tipping PG, Campbell DA, Boyce NW, and Holdsworth SR. Alveolar macrophage procoagulant activity is increased in acute hyperoxic lung injury. Am J Pathol 131: 206–212, 1988 [PMC free article] [PubMed] [Google Scholar]
- 320. Tiringer K, Treis A, Kanolzer S, Witt C, Ghanim B, Gruber S, Schmidthaler K, Renner S, Dehlink E, Nachbaur E, Frischer T, Klepetko W, Akdis CA, Szépfalusi Z, and Eiwegger T. Differential expression of IL-33 and HMGB1 in the lungs of stable cystic fibrosis patients. Eur Respir J 44: 802–805, 2014 [DOI] [PubMed] [Google Scholar]
- 321. Torre LA, Siegel RL, and Jemal A. Lung cancer statistics. Adv Exp Med Biol 893: 1–19, 2016 [DOI] [PubMed] [Google Scholar]
- 322. Toskala E. and Kennedy DW. Asthma risk factors: asthma risk factors. Int Forum Allergy Rhinol 5: S11–S16, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Trivedi A, Pavord ID, and Castro M. Bronchial thermoplasty and biological therapy as targeted treatments for severe uncontrolled asthma. Lancet Respir Med 4: 585–592, 2016 [DOI] [PubMed] [Google Scholar]
- 324. Tsai KS. and Grayson MH. Pulmonary defense mechanisms against pneumonia and sepsis. Curr Opin Pulm Med 14: 260–265, 2008 [DOI] [PubMed] [Google Scholar]
- 325. Turkington C. and Ashby B. The Encyclopedia of Infectious Diseases. New York: Infobase Publishing, 2007 [Google Scholar]
- 326. Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, and Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170: 1310–1316, 2004 [DOI] [PubMed] [Google Scholar]
- 327. Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, and Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A 99: 12351–12356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, and Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 209: 1519–1528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Venereau E, De Leo F, Mezzapelle R, Careccia G, Musco G, and Bianchi ME. HMGB1 as biomarker and drug target. Pharmacol Res 111: 534–544, 2016 [DOI] [PubMed] [Google Scholar]
- 330. Venturini E, Turkova A, Chiappini E, Galli L, de Martino M, and Thorne C. Tuberculosis and HIV co-infection in children. BMC Infect Dis 14(Suppl 1): S5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Wagner CR, Hamana K, and Elgin SC. A high-mobility-group protein and its cDNAs from Drosophila melanogaster. Mol Cell Biol 12: 1915–1923, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Wagner JP, Quill DM, and Pettijohn DE. Increased DNA-bending activity and higher affinity DNA binding of high mobility group protein HMG-1 prepared without acids. J Biol Chem 270: 7394–7398, 1995 [DOI] [PubMed] [Google Scholar]
- 333. Wake H, Mori S, Liu K, Takahashi HK, and Nishibori M. High mobility group box 1 complexed with heparin induced angiogenesis in a matrigel plug assay. Acta Med Okayama 63: 249–262, 2009 [DOI] [PubMed] [Google Scholar]
- 334. Wang C-M, Jiang M, and Wang H-J. Effect of NF-κB inhibitor on high-mobility group protein B1 expression in a COPD rat model. Mol Med Rep 7: 499–502, 2013 [DOI] [PubMed] [Google Scholar]
- 335. Wang F, Ni S-S, and Liu H. Pollutional haze and COPD: etiology, epidemiology, pathogenesis, pathology, biological markers and therapy. J Thorac Dis 8: E20–E30, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, and Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 337. Wang H, Yang H, Czura CJ, Sama AE, and Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med 164: 1768–1773, 2001 [DOI] [PubMed] [Google Scholar]
- 338. Wang H-L, Tsao S-M, Yeh C-B, Chou Y-E, and Yang S-F. Circulating level of high mobility group box-1 predicts the severity of community-acquired pneumonia: regulation of inflammatory responses via the c-Jun N-terminal signaling pathway in macrophages. Mol Med Rep 16: 2361–2366, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Wang M, Gorasiya S, Antoine DJ, Sitapara RA, Wu W, Sharma L, Yang H, Ashby CR, Vasudevan D, Zur M, Thomas DD, and Mantell LL. The compromise of macrophage functions by hyperoxia is attenuated by ethacrynic acid via inhibition of NF-κB-mediated release of high-mobility group box-1. Am J Respir Cell Mol Biol 52: 171–182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Wang Q, Wang J, Wang J, Hong S, Han F, Chen J, and Chen G. HMGB1 induces lung fibroblast to myofibroblast differentiation through NF-κB-mediated TGF-β1 release. Mol Med Rep 15: 3062–3068, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, and Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 84: 52–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA, and NHLBI ARDS Clinical Trials Network. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 137: 288–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Wei F, Yang F, Li J, Zheng Y, Yu W, Yang L, and Ren X. Soluble Toll-like receptor 4 is a potential serum biomarker in non-small cell lung cancer. Oncotarget 7: 40106–40114, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Wen L, Huang JK, Johnson BH, and Reeck GR. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res 17: 1197–1214, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. WHO. Asthma-key facts. 2017. http://www.who.int/news-room/fact-sheets/detail/asthma September 14, 2018
- 346. Wiener-Kronish JP, Albertine KH, and Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest 88: 864–875, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Wiśniewski JR, Schulze E, and Sapetto B. DNA binding and nuclear translocation of insect high-mobility-group-protein-1 (HMG1) proteins are inhibited by phosphorylation. Eur J Biochem 225: 687–693, 1994 [DOI] [PubMed] [Google Scholar]
- 348. Wolfson RK, Chiang ET, and Garcia JGN. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res 81: 189–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Wu F, Zhao Z-H, Ding S-T, Wu H-H, and Lu J-J. High mobility group box 1 protein is methylated and transported to cytoplasm in clear cell renal cell carcinoma. Asian Pac J Cancer Prev 14: 5789–5795, 2013 [DOI] [PubMed] [Google Scholar]
- 350. Wu L. and Yang L. The function and mechanism of HMGB1 in lung cancer and its potential therapeutic implications. Oncol Lett 15: 6799–6805, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Wu Q, Zhang W, Pwee K-H, and Kumar PP. Cloning and characterization of rice HMGB1 gene. Gene 312: 103–109, 2003 [DOI] [PubMed] [Google Scholar]
- 352. Wu X, Wang W, Chen Y, Liu X, Wang J, Qin X, Yuan D, Yu T, Chen G, Mi Y, Mou J, Cui J, Hu A E Y, and Pei D. High mobility group box protein 1 serves as a potential prognostic marker of lung cancer and promotes its invasion and metastasis by matrix metalloproteinase-2 in a nuclear factor-κB-dependent manner. Biomed Res Int 2018: 3453706, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Xia Q, Xu J, Chen H, Gao Y, Gong F, Hu L, and Yang L. Association between an elevated level of HMGB1 and non-small-cell lung cancer: a meta-analysis and literature review. Onco Targets Ther 9: 3917–3923, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Xie J, Hodgkinson JW, Li C, Kovacevic N, and Belosevic M. Identification and functional characterization of the goldfish (Carassius auratus L.) high mobility group box 1 (HMGB1) chromatin-binding protein. Dev Comp Immunol 44: 245–253, 2014 [DOI] [PubMed] [Google Scholar]
- 355. Xu D, Young J, Song D, and Esko JD. Heparin sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J Biol Chem 286: 41736–41744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SPS, Geller DA, Lotze MT, and Tsung A. High mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology 55: 1863–1875, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Yang D, Elner SG, Bian Z-M, Till GO, Petty HR, and Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res 85: 462–472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, and Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107: 11942–11947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Yang H, Lundbäck P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, and Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 18: 250–259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 360. Yang H, Wang H, Chavan SS, and Andersson U. High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med 21(Suppl 1): S6–S12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Yang J, Ma Z, Wang Y, Wang Z, Tian Y, Du Y, Bian W, Duan Y, and Liu J. Necrosis of osteosarcoma cells induces the production and release of high-mobility group box 1 protein. Exp Ther Med 15: 461–466, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Yang P-S, Kim D-H, Lee YJ, Lee S-E, Kang WJ, Chang H-J, and Shin J-S. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir Res 15: 148, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Youn JH. and Shin J-S. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol 1950 177: 7889–7897, 2006 [DOI] [PubMed] [Google Scholar]
- 364. Yun J, Jiang G, Wang Y, Xiao T, Zhao Y, Sun D, Kaplan HJ, and Shao H. The HMGB1–CXCL12 complex promotes inflammatory cell infiltration in uveitogenic T cell-induced chronic experimental autoimmune uveitis. Front Immunol 8: 142, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Yusein-Myashkova S, Stoykov I, Gospodinov A, Ugrinova I, and Pasheva E. The repair capacity of lung cancer cell lines A549 and H1299 depends on HMGB1 expression level and the p53 status. J Biochem 160: 37–47, 2016 [DOI] [PubMed] [Google Scholar]
- 366. Zabini D, Crnkovic S, Xu H, Tscherner M, Ghanim B, Klepetko W, Olschewski A, Kwapiszewska G, and Marsh LM. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J Cell Mol Med 19: 1151–1161, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Zambon M. and Vincent J-L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133: 1120–1127, 2008 [DOI] [PubMed] [Google Scholar]
- 368. Zeng J-C, Xiang W-Y, Lin D-Z, Zhang J-A, Liu G-B, Kong B, Gao Y-C, Lu Y-B, Wu X-J, Yi L-L, Zhong J-X, and Xu J-F. Elevated HMGB1-related interleukin-6 is associated with dynamic responses of monocytes in patients with active pulmonary tuberculosis. Int J Clin Exp Pathol 8: 1341–1353, 2015 [PMC free article] [PubMed] [Google Scholar]
- 369. Zhang F, Huang G, Hu B, Fang L-P, Cao E-H, Xin X-F, Song Y, and Shi Y. Anti-HMGB1 neutralizing antibody ameliorates neutrophilic airway inflammation by suppressing dendritic cell-mediated Th17 polarization. Mediators Inflamm 2014: 1–11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Zhang H, Yang N, Wang T, Dai B, and Shang Y. Vitamin D reduces inflammatory response in asthmatic mice through HMGB1/TLR4/NF-κB signaling pathway. Mol Med Rep 17: 2915–2920, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Zhang J, Clark K, Lawrence T, Peggie MW, and Cohen P. An unexpected twist to the activation of IKKβ: TAK1 primes IKKβ for activation by autophosphorylation. Biochem J 461: 531–537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Zhang L, Ji Y, Kang Z, Lv C, and Jiang W. Protocatechuic aldehyde ameliorates experimental pulmonary fibrosis by modulating HMGB1/RAGE pathway. Toxicol Appl Pharmacol 283: 50–56, 2015 [DOI] [PubMed] [Google Scholar]
- 373. Zhang L, Ji YX, Jiang WL, and Lv CJ. Protective roles of pulmonary rehabilitation mixture in experimental pulmonary fibrosis in vitro and in vivo. Braz J Med Biol Res 48: 545–552, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Zhang P, Xin X, Fang L, Jiang H, Xu X, Su X, and Shi Y. HMGB1 mediates Aspergillus fumigatus-induced inflammatory response in alveolar macrophages of COPD mice via activating MyD88/NF-κB and syk/PI3K signalings. Int Immunopharmacol 53: 125–132, 2017 [DOI] [PubMed] [Google Scholar]
- 375. Zheng S, Kummarapurugu AB, Afosah DK, Sankaranarayanan NV, Boothello RS, Desai UR, Kennedy T, and Voynow JA. 2-O, 3-O desulfated heparin blocks high mobility group box 1 release by inhibition of p300 acetyltransferase activity. Am J Respir Cell Mol Biol 56: 90–98, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. van Zoelen MA, Achouiti A, and van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit Care 15: 208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, and Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock 29: 441–445, 2008 [DOI] [PubMed] [Google Scholar]
- 378. van Zoelen MAD, Laterre P-F, van Veen SQ, van Till JWO, Wittebole X, Bresser P, Tanck MW, Dugernier T, Ishizaka A, Boermeester MA, and van der Poll T. Systemic and local high mobility group box 1 concentrations during severe infection. Crit Care Med 35: 2799–2804, 2007 [DOI] [PubMed] [Google Scholar]
- 379. van Zoelen MAD, van der Sluijs KF, Achouiti A, Florquin S, Braun-Pater JM, Yang H, Nawroth PP, Tracey KJ, Bierhaus A, and van der Poll T. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology 391: 265–273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. van Zoelen MAD, Wieland CW, van der Windt GJW, Florquin S, Nawroth PP, Bierhaus A, and van der Poll T. Receptor for advanced glycation end products is protective during murine tuberculosis. Mol Immunol 52: 183–189, 2012 [DOI] [PubMed] [Google Scholar]
- 381. This reference has been deleted.