Abstract
BACKGROUND:
Chronic Obstructive Pulmonary Disease (COPD) is a chronic inflammatory disease and disturbed bacterial clearance. Vitamin D deficiency is sometimes observed in COPD patients and as significant roles in increasing inflammation of airway obstruction and systemic obstruction, increasing pro-inflammatory cytokine including TNF-α, reduction of bacterial clearance and increase exacerbation risk due to infection. Also, vitamin D plays significant roles in the metabolism of calcium and mineralisation of bones and regulation system of immune. TNF-α also has essential roles in pathogenesis and inflammation of COPD. Several studies that investigate the relationship between vitamin D level and serum TNF-α concentration in COPD patients are relatively uncommon, including in Indonesia.
AIM:
This study aimed to assess the relationship between vitamin D level and TNF-α concentration in patients on the severity of the chronic obstructive pulmonary disease.
METHODS:
This study was a hospital-based descriptive cross-sectional study. Total samples were 50 COPD patients with the average age of older than 60 years during their enrollments at the Department of Pulmonology and Respiratory Medicine of the Dr Wahidin Sudirohusodo General Hospital Makassar in September 2018-January 2019. All procedures of the present study were reviewed and approved by the Research Ethics Committee of Medicine Faculty of Hasanuddin University. The severity of COPD was assessed according to the combination of COPD assessment stages that referred to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guideline 2015 that consisted of the combination of scoring COPD Assessment Test (CAT), the modified Medical Research Council (mMRC) questionnaire and results of the spirometry measurement. Assessment of airway obstruction levels referred to the GOLD spirometry criteria. Determination of thoracic photographs was conducted to verify the COPD diagnosis of the severity of COPD. Determination of serum TNF-α concentration and vitamin D3 [1,25(OH)2D3] level used the ELISA method.
RESULTS:
The majority of COPD patients were observed in the category of older than 60 years old accounted for 34 COPD patients (68%), and the majority of COPD patients were males accounted for 47 males with COPD (94%). The majority of COPD patients were observed in the group of D (38%). All the study subjects observed in this study were smokers, and 82% of them were in the category of heavy smokers. 21 study subjects had higher concentration of serum TNF-α (tertile 3 = 0.21-1.83 pg/dl), 20 study subjects and lower level of vitamin D (tertile 1 = 182.1-364.5 pg/dl). The majority of the study subjects (38%) were in the category of severe COPD (category D of the severity of COPD at the tertile 3) according to the GOLD Combine Assessment. Given the relationship between vitamin D level and serum TNF-α concentration on the airway obstruction, there were significant positive correlations between the increase of vitamin D levels and the increase of serum TNF-α concentrations on airway obstruction. Given the relationship between vitamin D level and serum TNF-α concentration on the severity of COPD, there were significant positive correlations between the increase of vitamin D levels (tertiles 1, 2 and 3) and the increase of serum TNF-α concentrations on the severity of COPD at p-value < 0.05. Overall, there were non-linear relationships between vitamin D level and serum TNF-α concentration on the severity of COPD.
CONCLUSIONS:
Serum TNF-α concentration was positively associated with airway obstruction level and severity of COPD. Low level of vitamin D was negatively associated with airway obstruction level and severity of COPD. Vitamin D3 level (1,25(OH)2D) was negatively associated with serum TNF-α concentration and airway obstruction level and severity of COPD.
Keywords: Vitamin D, TNF-α, Obstruction, COPD
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a chronic inflammatory disease characterized by persistent airflow limitation of the lungs [1] that includes a number of combinations of asthma bronchitis (spasms or involuntary contraction in the bronchial passages), emphysema (decreased elasticity of the alveoli) and chronic bronchitis (inflammation of the bronchi) [2], [3]. The mechanistic basis underlying COPD is complex and can involve recurrent inflammation, oxidative stress (i.e., oxidant / antioxidant imbalance), protease/antiprotease imbalance, environmental insult, and host genetics [4].
Cigarette smoking is the primary environmental risk factor for COPD. Also, other environmental (e.g., wood smoke) and occupational exposures, as well as genetics, contribute to COPD pathogenesis. Consequently, pathologic changes and clinical symptoms are linked to the interaction of host factors with the environment. These interactions generate the pathologic triad of COPD: persistent inflammation, protease-antiprotease imbalance, and oxidative stress. This triad results in mucous/goblet cell metaplasia and hyperplasia, hyper mucus secretion, fibrosis, smooth-muscle alterations, and lung-tissue destruction. Chronic smoking exposes the respiratory tree and lungs to reactive oxygen species (ROS), resulting in oxidative stress and injury. This triggers the production of other ROS and lipid peroxidation and subsequent pulmonary inflammation [5], mainly due to infections by bacteria and viruses [5], [6]. Based on a population-based survey in nine Asia-Pacific territories in 2012, the overall estimated prevalence of COPD was 6.2%, ranging from 4.5% in Indonesia to 9.5% in Taiwan [7]. COPD is a significant cause of morbidity and mortality worldwide, and it is the sixth leading cause of death world [8]. The determinants of COPD pathogenesis are not a separate entity by itself where smoking tobacco is the primary environmental risk factor for COPD and other determinants of COPD-associated inflammation related with smoking tobacco that includes reactive oxygen species (ROS), protease-antiprotease imbalance and genetic variations or polymorphisms [5], respiratory viruses and bacteria which infect the lower airway and increase airway inflammation [6].
Low level of vitamin D is sometimes found in patients with COPD. High prevalence vitamin D deficiency in COPD patients is caused by the reduction of synthesis of vitamin D in skin due to ageing, poor diet, low capacity to store at fat related to wasting [9]. Some publications show the inconsistency results about the role of vitamin D and COPD [10]; some studies reported a correlation between vitamin D deficiency and pulmonary function. In the placebo-controlled intervention study, Rafiq et al., [11] revealed that vitamin D had essential roles for the prevention of exacerbations (the increase of severity) in patients with COPD and vitamin D deficiency through vitamin D supplementation. Sank et al., [12] proved the positive relationship between vitamin D deficiency and the severity of Chronic Obstructive Pulmonary Disease.
Despite many mechanistic studies highlighting important anti-inflammatory and anti-infectious effects of vitamin D in laboratory experiments, the clinical evidence in cohorts of patients with COPD remains contradictory. Several studies have shown that vitamin D could regulate activities of immune cells [13], restore respiratory muscle strength and inflammatory responses [14]. In COPD, there is a down-regulation of local signalling of vitamin D, leading to insufficient control of pro-inflammatory processes in airways [15]. COPD is associated with chronic inflammation affecting predominantly lung parenchyma and peripheral airways and results in mostly irreversible and progressive airflow limitation. This inflammation is characterised by increased numbers of alveolar macrophages, neutrophils, and T lymphocytes, which are recruited from the circulation. Oxidative stress plays a crucial role in driving this inflammation. Pulmonary inflammation may enhance the development and growth of lung cancer. The peripheral inflammation extends into the circulation, resulting in systemic inflammation with the same inflammatory proteins. Systemic inflammation may worsen comorbidities. Treatment of pulmonary inflammation may, therefore, have beneficial effects [16].
Multiple cytokines play roles in inflammatory airway diseases, such as COPD, through the recruitment, activation, and survival of inflammatory cells [17]. This is proven from the increase of TNF-α concentration according to the examination of induced sputum and lung biopsy of COPD patients [18]. TNF-α is the most extensively studied cytokine in patients with COPD, and it is a potent activator of NF-κβ and amplifies the inflammatory response. TNF-α plays a significant role in many inflammatory diseases affecting the lung, such as chronic bronchitis (CB), COPD, asthma, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [19]. However, studies that investigate the relationship between vitamin D level and serum TNF-α concentration in COPD patients are relatively uncommon, including in Indonesia. For that reason, this study aimed to assess the relationship between vitamin D level and TNF-α concentration in patients on the severity of the chronic obstructive pulmonary disease.
Methods
This study was a hospital-based descriptive cross-sectional study. Total samples were 50 COPD patients with the average age of older than 60 years during their enrollments at the Department of Pulmonology and Respiratory Medicine of the Dr Wahidin Sudirohusodo General Hospital Makassar in September 2018-January 2019. All procedures of the present study were reviewed and approved by the Research Ethics Committee of Medicine Faculty of Hasanuddin University as stated in the Recommendation Letter of Research Ethics issued in the registration No.1052/H.4.8.4.5.31/PP36-KOMETIK/2018.
Inclusion criteria include COPD patients with dyspnea (difficulty in breathing) and chronic cough with sputum. There was a history of exposure of cigarette fog and other irritants, results of diagnosis using the spirometry test at FEV1/FVC < 70% and post-bronchodilator (after the use of certain drugs to reduces the tone of smooth muscle in the lungs’ bronchioles to increase their diameter) at FEV1 < 80%. The severity of COPD was assessed according to the combination of COPD assessment stages that referred to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guideline 2015 that consisted of the combination of scoring COPD Assessment Test (CAT), the modified Medical Research Council (mMRC) questionnaire and results of the spirometry measurement. Assessment of airway obstruction levels referred to the GOLD spirometry criteria. Determination of thoracic photographs was conducted to verify no other respiratory diseases except COPD. Determination of serum TNF-α concentration and vitamin D3 [1,25(OH)2D3] level used the ELISA method (Thermo Scientific Multiscan FC). Exclusion criteria include individuals with asthma, lung tuberculosis, bronchiectasis (dilatation of the bronchi) and patients with COPD in the category of acute exacerbation and the use of oral steroids.
Statistical analysis
The study data were processed and analysed using the Statistical Package for Social Science (SPSS) version 22. The analytical method used in this study was descriptive statistics using frequency distribution, whereas the statistical test used in this study was Kolmogorov-Smirnov’s test used to determine the normality of data. Chi-square test was used to assess the strength of the correlation or relationship between two categorical variables. Kruskal-Wallis test was used to compare mean values of numerical data based on the categorical variables of more than two correlated variables. Results of statistical tests were significant when p-value < 0.05. Results of data analyses were presented in the form tables and graphs along with their descriptions.
Results
A total number of study subjects were 50 COPD patients who fulfilled the inclusion criteria. The majority of COPD patients were observed in the category of older than 60 years old accounted for 34 COPD patients (68%), and the majority of COPD patients were males considered for 47 males with COPD (94%). The study subjects were statistically distributed. The majority of COPD patients were observed in group D (38%). Vitamin D levels were classified into 3 tertiles, and the most significant frequency of COPD patients was seen at the tertile 1 of vitamin D level accounted for 20 COPD patients (40%). Serum TNF-α concentrations were also classified into three tertiles, and the most considerable frequency of COPD patients was noticed at the tertile 3 of serum TNF-α level accounted for 21 COPD patients (42%). Baseline characteristics of the study subjects are shown in Table 1.
Table 1.
Baseline characteristics of the study subjects with COPD
| Variable | n | % | |
|---|---|---|---|
| Gender | Male | 47 | 94.0 |
| Female | 3 | 6.0 | |
| Age | 40-59 years old | 16 | 32.0 |
| ≥ 60 years old | 34 | 68.0 | |
| Smoking status | Mild | 1 | 2.0 |
| Moderate | 8 | 16.0 | |
| Severe | 41 | 82.0 | |
| Serum TNF-α concentrationa | Tertile 1 | 12 | 24.0 |
| Tertile 2 | 17 | 34.0 | |
| Tertile 3 | 21 | 42.0 | |
| Vitamin D Levelb | Tertile 1 | 20 | 40.0 |
| Tertile 2 | 15 | 30.0 | |
| Tertile 3 | 15 | 30.0 | |
| Severity of COPD | A | 5 | 10.0 |
| B | 18 | 36.0 | |
| C | 8 | 16.0 | |
| D | 19 | 38.0 | |
Tertile 1 (0. 00-0.06 pg/dl); Tertile 2 (0.07-0.20 pg/dl); Tertil 3 (0.21-1.83 pg/dl);
Tertile 1 (182. 1-364.5 pg/mL); Tertile 2 (364.7-630.7 pg/mL); Tertile 3 (632.6-3707.2 pg/mL).
TNF-α concentrations at the tertile 1 were dominant for COPD patients in the groups A and B (24%). TNF-α levels at the tertile 2 were prevailing for COPD patients in the groups B and C (34%) whereas TNF-α levels were dominant at the tertile 3 for COPD patients in the group D (42%).
Relationship between vitamin D level and serum TNF-α concentration on the airway obstruction
Table 3 shows the statistical correlations of vitamin D level and serum TNF-α concentration on airway obstruction. There were significant positive correlations between the increase of vitamin D levels and the increase of serum TNF-α concentrations on airway obstruction according to the rise of GOLD values (p < 0.05) at the tertile 1 of vitamin D levels with the highest concentration of serum TNF-α at the tertile 1 was 0.24 pg/dl. There were also significant positive correlations between the increase of vitamin D levels and the increase of serum TNF-α concentrations on airway obstruction according to the rise in GOLD values (p < 0.05) at the tertile 2 of vitamin D levels with the highest concentration of serum TNF-α at the tertile 2 was 0.93 pg/dl. In addition, there were positive significant correlations between the increase of vitamin D levels and the increase of serum TNF-α concentrations on airway obstruction according to the rise in GOLD values (p < 0.05) at the tertile 3 of vitamin D levels with the highest concentration of serum TNF-α at the tertile 3 was 0.48 pg/dl.
Table 3.
Statistical correlations of vitamin D level and TNF-α concentration on airway obstruction
| Vitamin D | Airway Obstruction | n | Mean TNF-α | SD | p |
|---|---|---|---|---|---|
| Tertile 1 | Mild | 3 | 0.04 | 0.02 | 0.024 |
| Moderate | 11 | 0.06 | 0.05 | ||
| Severe | 5 | 0.24 | 0.19 | ||
| Very severe | 1 | 0.23 | - | ||
| Tertile 2 | Mild | 1 | 0.04 | - | 0.012 |
| Moderate | 4 | 0.04 | 0.05 | ||
| Severe | 6 | 0.22 | 0.15 | ||
| Very severe | 4 | 0.93 | 0.63 | ||
| Tertile 3 | Moderate | 4 | 0.12 | 0.04 | 0.018 |
| Severe | 4 | 0.31 | 0.11 | ||
| Very severe | 7 | 0.48 | 0.24 |
Data in Table 3 also illustrate the relationship between serum TNF-α concentration and airway obstruction level. There was a positive significant linear correlation between vitamin D level and serum TNF-α concentration on the airway obstruction level with the correlation coefficient value = 0.502 at p-value = 0.000, and the most considerable airway obstruction was observed at the tertile 3.
Correlation pattern of vitamin D and TNF-α on the airway obstruction level airway was not linear, and the highest TNF-α concentration with vitamin D level was observed at the tertile 2 (Figure 1).
Figure 1.
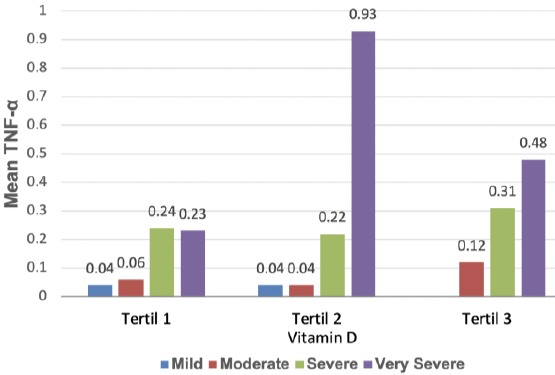
Linear relationship between vitamin D level and serum TNF-α concentration with the severity of airway obstruction
Relationship between vitamin D level and serum TNF-α concentration on the severity of COPD
On the whole, there were positive significant correlations between the increase of vitamin D levels (tertiles 1, 2 and 3) and the increase of serum TNF-α concentrations on the severity of COPD at p-value < 0.05 although the rise in serum TNF-α concentrations was not linear with the rise of vitamin D levels with the largest concentration of TNF-α was observed at the tertile 2 of vitamin D levels (Table 4).
Table 4.
Statistical correlation between vitamin D level and serum TNF-α concentration with the severity of COPD
| Vitamin D Level | Severity of COPD | n | Mean TNF-α | SD | p |
|---|---|---|---|---|---|
| Tertile 1 | A | 2 | 0.04 | 0.02 | 0.021 |
| B | 12 | 0.06 | 0.05 | ||
| C | 3 | 0.15 | 0.12 | ||
| D | 3 | 0.33 | 0.17 | ||
| Tertile 2 | A | 2 | 0.06 | 0.03 | 0.008 |
| B | 3 | 0.03 | 0.05 | ||
| C | 3 | 0.10 | 0.03 | ||
| D | 7 | 0.68 | 0.55 | ||
| Tertile 3 | A | 1 | 0.07 | - | 0.025 |
| B | 3 | 0.14 | 0.03 | ||
| C | 2 | 0.24 | 0.14 | ||
| D | 9 | 0.46 | 0.22 |
Overall, there were non-linear relationships between vitamin D level and serum TNF-α concentration on the severity of COPD (Figure 2).
Figure 2.
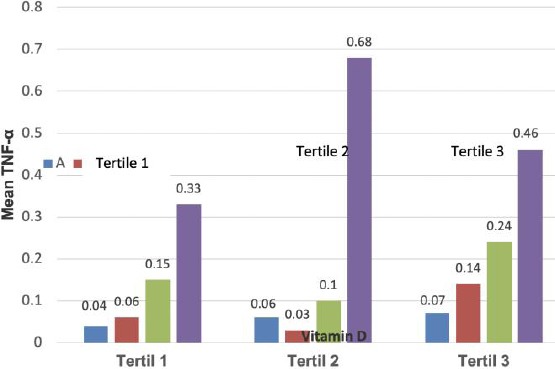
Non-linear relationships between vitamin D level and serum TNF-α concentration on the severity of COPD
Discussion
Characteristics of the study subjects
A total of the study subjects identified in this study were50 patients according to the history of the disease, clinical descriptions, and spirometry test. Of 50 study subjects, the majority of them were male accounted for 47 patients (94%) and 3 females (6%), and the majority of the study subjects are in the category of age older than 60 years old. Results of this study are consistent with those of the case-control study conducted by Sanket et al., for 81 COPD patients that consisted of 75 males (92.5%) [12]. The higher prevalence rate of COPD for the male group is associated with a higher prevalence rate of smokers for males compared to females [20]. All the study subjects observed in this study were smokers, and 82% of them were in the category of heavy smokers. Twenty-One study subjects had higher concentration of serum TNF-α (tertile 3 = 0.21-1.83 pg/dl), 20 study subjects and lower level of vitamin D (tertile 1 = 182.1-364.5 pg/dl). The majority of the study subjects (38%) were in the category of severe COPD (category D of the severity of COPD at the tertile 3) according to the GOLD Combine Assessment.
Distribution of severity of COPD according to serum TNF-α concentration
As shown in Table 2, 12 study subjects were classified into two categories (A and B) according to the severity of COPD. The lowest TNF-α level was observed at the tertile 1 (= 0.00-0.06 ng/dl) accounted for, 17 study subjects are seen at the tertile 2 (0.07-0.20 pg/dl) and the tertile 3 with 21 study subjects. With refers to the analysis of above data distribution, mild COPD had the lowest serum TNF-α concentration whereas patients with high severe COPD and highest TNF-α level. These results coincide with those of the study of Maharaj et al., [21] that serum TNF-α concentration was positively associated with airway obstruction level and severity of COPD.
Table 2.
Frequency distribution of severity of COPD according to TNF-α level
| Serum TNF-α Level | Severity of COPD | Total | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||
| Tertile 1 | n | 3 | 9 | 0 | 0 | 12 | |
| % | 25.0% | 75.0% | 0.0% | 0.0% | 100.0% | ||
| Tertile 2 | n | 2 | 9 | 6 | 0 | 17 | |
| % | 11.8% | 52.9% | 35.3% | 0.0% | 100.0% | ||
| Tertile 3 | n | 0 | 0 | 2 | 19 | 21 | |
| % | 0.0% | 0.0% | 9.5% | 90.5% | 100.0% | ||
| Total | n | 5 | 18 | 8 | 19 | 50 | |
| % | 10.0% | 36.0% | 16.0% | 38.0% | 100.0% | ||
All the study subjects in this study are smokers, and 82% are heavy smokers. This coincides with the research of Watanabe et al., [22] for 142 non-COPD subjects that serum TNF-α concentrations of smokers increased compared to non-smokers and hypothesized that light smoking was associated with an increase in WBC counts, while heavy smoking was responsible for TNF-α activation in Japanese male subjects with standard glucose tolerance and the study of Tanni et al., [23] pertaining to the association between inflammation, smoking status, and disease and showed that serum TNF-α was higher in COPD current smokers [4.8 (4.2-5.8) pg/mL] and in current smoker controls [4.8 (4.2-6.1) pg/mL] when compared to COPD ex-smokers [4.3 (3.9-4.9) pg/mL; p = 0.02] and to never-smoker controls [3.7 (3.4-4.0) pg/mL; p < 0.001] and concluded smoking may influence TNF-α mediated systemic inflammation, which, in turn, may account for some of the benefits observed in patients with COPD who stop smoking. In view of the association between TNF-α concentration and severity COPD, results of this study are coherent with those of the research performed by Healing V et al., [24] that spontaneous TNF-α production was 5.0 times higher in patients with severe COPD compared to mild-to-moderate COPD (p = 0.02), and serum TNF-α was significantly elevated in patients versus controls (2.1 ±0.3 vs 1.1 ±0.1) at p = 0.007 and concluded that increasing airflow obstruction and hypercapnia was associated with an enhanced TNF-α response in COPD.A meta-analysis study conducted by Wei et al., [25] revealed that patients with stable COPD had higher serum IL-6 concentrations than healthy controls. No evidence showing a positive or negative association between IL-6 concentrations and the severity of pulmonary function impairment was found. The correlation between IL-6 levels and pulmonary function was weak in different severities of stable COPD patients.
Low level of vitamin D is sometimes found in patients with COPD. High prevalence of vitamin D deficiency in COPD patients is caused by the reduction of synthesis of vitamin D in skin due to ageing, poor diet, low capacity to store at fat related to wasting [9]. Active metabolism of vitamin D3 (1,25(OH)2D3) increase the expression of an antimicrobial peptide, and reduce the expression of pro-inflammatory cytokines and can be used to explain the relationship between vitamin D and susceptibility of respiratory infection. Vitamin D deficiency also affects pulmonary function through various mechanisms, innate and adaptive immunity systems in the pathogenesis of COPD [27].
Overall, vitamin D level assessed in this study was not significantly associated with serum TNF-α concentration on airway obstruction level and severity of COPD. Various unobserved factors influencing vitamin D level and serum TNF-α concentration were not assessed in this study including physical activity, lifestyle, and nutritional status of the study subjects as investigated in the study of Bouillon et al., [28]. Moreover, levels of 1,25(OH)2D3 serum are also affected by serum Vitamin D Binding Protein (VDBP) level, phosphorus serum, parathyroid hormone [30]. In contrary, results of this study are consistent with those of the study Mekovet al., [31] (2015) and of the study Nishimura et al., [32] (2016) that there was not a significant positive correlation between vitamin D level and severity of COPD.
Results of this study were not consistent with the study of Sanket et al., [12] that COPD was associated with the risk increase of vitamin D deficiency, and there was a significant association between level vitamin D and severity of COPD. Zhu et al., (2015) [33] investigated the association between host serum 25-hydroxyvitamin D (25(OH)D) and the susceptibility and severity of COPD and found that low serum levels of 25(OH)D were not associated with COPD susceptibility, but the high deficiency rate of 25(OH)D was associated with COPD severity. Vitamin D supplementation may prevent COPD exacerbation. Vitamin D supplements could prevent COPD exacerbation as shown by Rafiq et al., (2015) [11] that a low level of vitamin D was positively associated with the severity of COPD.
This study has some limitations that did not evaluate other determinants influencing vitamin D3 status including lifestyles, eating behaviours, medications, serum vitamin D binding protein (VDBP), and serum vitamin D2 (25OHD). All information in this descriptive cross-sectional study was collected at the same time, and the study subjects were contacted only once. The temporal sequence of cause and effect could not be addressed in this study in assessing temporal relationships of vitamin D3 level, cytokine response, worsening of symptoms and severity of COPD, but it might be suggestive of an association that should be investigated more fully by further studies.
In conclusion, serum TNF-α concentration was positively associated with airway obstruction level and severity of COPD. Low level of vitamin D was negatively associated with airway obstruction level and severity of COPD. Vitamin D3 level {1,25(OH)2D} was negatively associated with serum TNF-α concentration and airway obstruction level and severity of COPD.
Further studies are necessary for determining more cytokines involved in pathogenesis and severity of COPD as well as search for determinants influencing vitamin D3 level to elucidate a more authentic relationship between the two pathological findings.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–135. doi: 10.1016/S0140-6736(11)60968-9. https://doi.org/10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. https://doi.org/10.1056/NEJMoa032158 PMid:15215480. [DOI] [PubMed] [Google Scholar]
- 3.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. https://doi.org/10.1056/NEJMoa1106955 PMid:22029978 PMCid:PMC3238466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD:oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–421. doi: 10.2147/COPD.S10770. https://doi.org/10.2147/COPD.S10770 PMid:21857781 PMCid:PMC3157944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer BM, Judith A, Voynow JA, Ghio AJ. COPD:balancing oxidants and antioxidants. International Journal of COPD. 2015;10:261–276. doi: 10.2147/COPD.S42414. https://doi.org/10.2147/COPD.S42414 PMid:25673984 PMCid:PMC4321570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Seemungal TA. COPD exacerbations:defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. https://doi.org/10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim S, Chi-Leung Lam D, Muttalif AR, Yunus F, Wongtim S, Lan LT. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region:the EPIC Asia population-based survey. Asia Pacific Family Medicine. 2015;14:4. doi: 10.1186/s12930-015-0020-9. https://doi.org/10.1186/s12930-015-0020-9 PMid:25937817 PMCid:PMC4416253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Initiative Obstructive Lung Disease (GOLD) Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease update. 2015;3 https://www.atsjournals.org/doi/full/10.1164/rccm.201204-0596PP. [Google Scholar]
- 9.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. https://doi.org/10.1056/NEJMra070553 PMid:17634462. [DOI] [PubMed] [Google Scholar]
- 10.Janssens W, Decramer M, Mathieu C, Korf H. Vitamin D, Chronic obstructive pulmonary disease:hype or reality. Lancet Respiratory Medicine. 2013;1:804–812. doi: 10.1016/S2213-2600(13)70102-4. https://doi.org/10.1016/S2213-2600(13)70102-4. [DOI] [PubMed] [Google Scholar]
- 11.Rafiq R, Alvera FE, Schrumpf JA. Prevention of exacerbation in patients with COPD and vitamin D deficiency through vitamin D supplementation (precovis):A study protocol. BMC Pulm Med. 2015;15:106. doi: 10.1186/s12890-015-0101-4. https://doi.org/10.1186/s12890-015-0101-4 PMid:26399451 PMCid:PMC4580355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanket S, Madireddi J, Stanley W, Sura P, Prabhu M. Relation between vitamin D Deficiency and Severity of Chronic Obstructive Pulmonary Disease-A Case-Control Study. Journal of Clinical and Diagnostic Research. 2016;10(1):16–19. doi: 10.7860/JCDR/2016/15404.7097. https://doi.org/10.7860/JCDR/2016/15404.7097 PMid:26894108 PMCid:PMC4740636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M. The role of Vitamin D in pulmonary disease:COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. https://doi.org/10.1186/1465-9921-12-31 PMid:21418564 PMCid:PMC3071319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkinson NS, Li KW, Kehoe A, Humphries SE, Roughton M, Moxham J. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–90. doi: 10.1093/ajcn/87.2.385. https://doi.org/10.1093/ajcn/87.2.385 PMid:18258629. [DOI] [PubMed] [Google Scholar]
- 15.Janssens W, Decramer M, Mathieu C, Korf H. Vitamin D, and chronic obstructive pulmonary disease:Hype or reality? Lancet Respir Med. 2013;1:804–12. doi: 10.1016/S2213-2600(13)70102-4. https://doi.org/10.1016/S2213-2600(13)70102-4. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ. Cellular and molecular mechanism of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. https://doi.org/10.1016/j.ccm.2013.10.004 PMid:24507838. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ. The cytokine network in COPD. Am J Respir Cell Mol Biol. 2009;41:631–638. doi: 10.1165/rcmb.2009-0220TR. https://doi.org/10.1165/rcmb.2009-0220TR PMid:19717810. [DOI] [PubMed] [Google Scholar]
- 18.Pitsiou G, Kyriaziz G, Hatzizisi O, Argyropoulou P, Mavrofridis E, Patakas D. Tumor necrosis factor alpha serum level, weight loss, and tissue oxygenation in chronic obstructive pulmonary disease. Respir Med. 2002;98(8):594–598. doi: 10.1053/rmed.2002.1322. https://doi.org/10.1053/rmed.2002.1322 PMid:12195840. [DOI] [PubMed] [Google Scholar]
- 19.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between Chronic Respiratory disease and systemic inflammation:A systemic review and meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. https://doi.org/10.1136/thx.2003.019588 PMid:15223864 PMCid:PMC1747070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hylkema MN, Sterk PJ, De Boer WI. Tobacco use in relation to COPD and Asthma. Eur RespirJ. 2007;29:438–445. doi: 10.1183/09031936.00124506. https://doi.org/10.1183/09031936.00124506 PMid:17329490. [DOI] [PubMed] [Google Scholar]
- 21.Mathanraj S, Kumar V, Yuvaranjan S, Reddy V. Correlation of serum TNF-αlevel with the severity of chronic obstructive pulmonary disease. Int J Res Med Sci. 2017;5(8):3309–3316. https://doi.org/10.18203/2320-6012.ijrms20173020. [Google Scholar]
- 22.Watanabe N, Fukushima M, Taniguchi A. Smoking, white blood cell counts, and TNF system activity in Japanese male subjects with normal glucose tolerance. Tobacco Induc Dis. 2011;9:1–6. doi: 10.1186/1617-9625-9-12. https://doi.org/10.1186/1617-9625-9-12 PMid:22117840 PMCid:PMC3254068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanni SE, Pellegrino N, Aparecida Y. Smoking status and tumor necrosis factor-alpha-mediated systemic inflammation in COPD patients. Journal of Inflammation. 2010:7–29. doi: 10.1186/1476-9255-7-29. https://doi.org/10.1186/1476-9255-7-29 PMid:20534161 PMCid:PMC2891738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haehling V, Hopkinson NS, Polkey MI, Niethammer M, Anker SD, Genth-Zotz S. Elevated TNFalpha production in whole blood in patients with severe COPD:the potential link to disease severity. (abstract). PubMed. https://www.ncbi.nlm.nih.gov/pubmed/19562291 . [DOI] [PubMed]
- 25.Wei J, Xiong X, Lin Y, Cheng D. Association between serum tumor necrosis concentrations and chronic αfactor-obstructive pulmonary disease. Current science. 2016;110(2):172–179. https://doi.org/10.18520/cs/v110/i2/172-179. [Google Scholar]
- 26.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J. Cutting edge:1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. https://doi.org/10.4049/jimmunol.173.5.2909 PMid:15322146. [DOI] [PubMed] [Google Scholar]
- 27.Cosio BG, Agusti A. Update in chronic obstructive pulmonary disease 2009. Am J Respir Crit Care Med. 2010;181(7):655–60. doi: 10.1164/rccm.201001-0111UP. https://doi.org/10.1164/rccm.201001-0111UP PMid:20335383. [DOI] [PubMed] [Google Scholar]
- 28.Bouillon B, Heyns W, De Moor P. Influence of the Vitamin D-binding Protein on the Serum Concentration of 1,25- Dihydroxyvitamin D3:Significance of the free 1,25-dihydroxy vitamin D3 concentration. J Clin Invest. 1981;67(3):589–596. doi: 10.1172/JCI110072. https://doi.org/10.1172/JCI110072 PMid:6894152 PMCid:PMC370606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D:Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. https://doi.org/10.1093/ajcn/79.3.362 PMid:14985208. [DOI] [PubMed] [Google Scholar]
- 30.Tsiaras WG, Weinstocks MA. Factors Influencing Vitamin D Status. Acta DermVenereol. 2011;91:115–124. doi: 10.2340/00015555-0980. https://doi.org/10.2340/00015555-0980 PMid:21384086. [DOI] [PubMed] [Google Scholar]
- 31.Mekov E, Slavova Y, Tsakova A. Vitamin D Deficiency and Insufficiency in Hospitalized COPD Patients. Plos One. 2015;10:1–14. doi: 10.1371/journal.pone.0129080. https://doi.org/10.1371/journal.pone.0129080 PMid:26047485 PMCid:PMC4457885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura K, Ogasarawa M, Makita M. Vitamin D and Diagnosis of COPD in a Working Population. J Pulm Respir Med. 2016;6:2–4. https://doi.org/10.4172/2161-105X.1000359. [Google Scholar]
- 33.Zhu B, Xiao C, Zheng Z. Vitamin D deficiency is associated with the severity of COPD:A systemic review and meta-analysis. International Journal of COPD. 2015;10:1907–1916. doi: 10.2147/COPD.S89763. https://doi.org/10.2147/COPD.S89763 PMid:26392765 PMCid:PMC4574800. [DOI] [PMC free article] [PubMed] [Google Scholar]


