Abstract
BACKGROUND:
Dengue fever is the most serious consequence of mosquito-borne infection worldwide. The pathophysiology of DHF in human is complex, which involve endothelial cell activation and impaired endothelial barrier leading to plasma leakage triggering the activation of the haemostatic system. The increased vascular permeability may lead to hypovolemia, hypotension and shock, which is life-threatening.
AIM:
The objective of the study was to determine the effects of dengue haemorrhagic fever on the vascular endothelium.
METHODS:
Fifty patients (males 34, females 16), were recruited, Grade 1 (n = 41), Grade 2 (n = 6), Grade 3 (n = 2) and Grade 4 (n = 1) DHF. Blood sampling was performed at the febrile, defervescence and convalescent phases for the determination of haemoglobin, haematocrit, platelets, prothrombin fragment F1 + 2, Von Willebrand Factor (VWF), vascular endothelial growth factor (VEGF) and D-dimer levels. Fifteen normal subjects were recruited to serve as normal controls.
RESULTS:
The patients aged between 4 and 54 years old. Grades 1 & 2 DHF showed no significant differences in the parameters studied. However, thrombocytopenia, elevated F1 + 2, VWF, VEGF and D-dimer levels were evident in febrile, defervescence and convalescent phases suggesting endothelial activation and plasma leakage. Pleural effusion was observed only in severe DHF. The three patients with Grades 3 and 4 DHF had similar study results. No mortality was recorded in the study.
CONCLUSION:
In dengue haemorrhagic fever, the vascular endothelium is activated, causing plasma leakage triggering the activation of the haemostatic system creating a hypercoagulable and enhanced fibrinolytic state evident by marked fibrinolysis.
Keywords: DHF, Endothelium status
Introduction
Dengue fever is the most serious consequence of mosquito-borne infection worldwide. There are more than 2.5 billion persons at risk of infection and occur mainly in the sub-tropical regions of Asia, Africa, and America [1] and the attacks have shifted mainly to adults [2]. The actual numbers of dengue cases are underreported or misclassified [3]. One study estimated that 3.9 billion people in 128 countries are at risk of infection with dengue viruses [4]. In Indonesia, the overall incidence increased significantly from 0.05 / 100,000 in 1968 to 35-40 / 100,000 in 2013 [5]. Clinical manifestations of DF include mild or marked febrile syndromes of abrupt onset with headache, pain behind the eyes muscle and bone pain, nausea, vomiting and rash. There is no specific treatment for dengue fever, but maintaining patients’ body fluid volume is critical. Dengue as defined by WHO [6] as dengue with and without warning signs of plasma leakage and defined into four grades (Grades 1 to 4).
The pathophysiology of DHF in human is complex and the clinical symptoms due mainly to immune response, which also involve endothelial cell activation leading to plasma leakage and triggering the activation of the haemostatic system. The endothelium plays an important regulatory role in the circulation as a physical barrier and involved in the control of thrombosis and thrombolysis, vascular tone and growth of blood vessels [7]. It plays a critical role in a variety of human disorders. Endothelial injury is associated with elevated Von Willebrand Factor (VWF) and vascular endothelial growth factor (VEGF) a known potent regulator of vascular permeability and angiogenesis is released by platelets [8], [9]. The platelets are the main transporter of VEGF [10]. Endothelial activation may be responsible for plasma leakage and shock [11]. D-dimer, the lysis product of cross-linked fibrin indicates hyperfibrinolysis in response to clotting activation and fibrin formation [12] It is also a marker for hypercoagulability and has been used to determine thrombosis in myeloproliferative disease [13], [14]. Thrombocytopenia is commonly observed in both mild and severe dengue syndrome and associated with clinical outcome [6], [15], [16], [17]. This may be due to bone marrow suppression, destruction and lengthening of the platelet life cycle [18], [19]. The level of platelet count correlates with severity of DHF, and high haematocrit with marked thrombocytopenia support the diagnosis of dengue shock syndrome (DSS) [2]. It has been considered as an important factor responsible for bleeding events in DHF [20]. Platelet activation is significantly increased in dengue-patients, especially with thrombocytopenia, which exhibited signs of apoptosis pathway activation [21]. Increased activation of coagulation (prothrombin fragment 1 + 2) was reported in a critical phase of severe dengue infection associated with plasma leakage and thrombocytopenia [2]. In the Brazilian study, it was reported that elevated D-dimer and thrombocytopenia with reduced thrombin generation and excessive fibrinolysis are associated with bleeding complications [23].
The objective of the study was to determine the effects of dengue haemorrhagic fever on the vascular endothelium.
Material and Method
The study received ethical approval from the Health Research Ethical Committee No 418 / TGL / KEPK FK USU-HAM / 2018, Faculty of Medicine, University of North Sumatera, Indonesia. The study was conducted at the Murni Teguh Memorial Hospital, Medan Indonesia.
Subjects
The patients admitted to the hospital were mainly from grade 1 DHF with some grade 2 and a few severe DHF. Fifty patients (males 34, females 16) admitted to the hospital with fever were recruited and diagnosed according to WHO protocol (6) to have Grade 1 (n = 41), Grade 2 (n = 6), Grade 3 (n = 2) and Grade 4 (n = 1) DHF. The Inclusion criteria: patients who met WHO criteria for dengue fever and willing to take part in the study and had one or more dengue serology positive for either IgM/IgG antibodies or NS1antigen, Exclusion criteria: patients with other infections and systemic diseases and not willing to take part in the study.
Normal Controls
Fifteen normal subjects (males n = 14, female n = 1) who are normotensive, had not taken any medication recently and no history of health issues was recruited to serve as normal controls for the DHF study. Their mean age was 22.9 ± 1.1 years and ranged between 18 years and 33 years old.
Blood Sampling and Laboratory Investigation
From a clean venepuncture 3 mL EDTA blood was used for routine determination of haemoglobin (Hb), haematocrit (Hct) and platelets performed in the Siemens high volume haematological analyser (ADVIA 2120 / 1), and plasma for serological tests for IgG / IgM antibodies and NS1 antigen (SD Bioscience, Ingbert, Germany). 10 mL of citrated blood was spun in the refrigerated centrifuge at 2500g for10 minutes and the plasma aliquoted and stored at -80°C. Citrated-plasma was used for Elisa analysis of prothrombin fragment F1 + 2 (F1+2), Von Willebrand Factor (VWF), vascular endothelial growth factor (VEGF) (USCN Life Sciences, Wuhan, China) and D-dimer (Vidas D-dimer Exclusion II, Biomerieux SA France).
Statistical Analysis
The Statistical Package for Social Sciences (SPSS 22 IBM Corp) was used to perform statistical analysis. The independent t-test for differences between groups at different DHF phases was performed together with one-way Analysis of Variance (ANOVA). A P value of < 0.05 was considered statistically significant.
Results
Characteristics of patients with dengue haemorrhagic fever
Petechiae or rash, headaches/bone and pain behind eyes are seen in all patients; Epistaxis is seen in grades 2 and 3 patients while the grade 4 patient was unconscious at admission, had bled into the brain at defervescence phase as evident from CT-scan. Pleural effusion was only observed in grades 3 and 4 DHF. The liver enlargement was seen in grades 2, 3 and 4 and 14.6% (6 / 41) in grade 1. The patients were discharged in an afebrile state. The clinical characteristics of DHF patients are shown in Table 1.
Table 1.
Characteristics of patients with dengue haemorrhagic fever
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| N | 41 | 6 | 2 | 1 |
| Age mean (SD) years | 20.6 (11.8) | 30.8 (8.7) | 38, 43 | 54 |
| Sex males/females | 28/13 | 4/2 | 2/0 | 1/0 |
| Petechiae/rash | 41 | 6 | 2 | 1 |
| Pain: headache/bones/behind eyes | 41 | 6 | 2 | 1 |
| Nausea | 19 | 6 | 2 | 1 |
| Cough | 15 | 4 | 1 | 0 |
| Bleeding: epitaxis | 0 | 6 | 2 | 1* |
| Pleural effusion | 0 | 0 | 2 | 1 |
| Liver enlargement | 6 | 6 | 2 | 1 |
Bleeding to the brain (CT scan).
Comparison of parameters studied in DHF (Grade 1) between cohorts at age seventeen years and below and above seventeen years.
There were twenty cohorts (males n = 13, females n = 7) at seventeen years and below and twenty-one cohorts (males n = 14, females n = 7) above 17 years old. Except for the significance in age (P ≤ 0.001) and lower mean trend for platelets in the above 17 years cohorts which did not reach statistical significance (P = 0.05), there were no statistical differences in the other parameters studied (not shown). They were therefore combined (Grade 1) for further statistical analysis.
Dengue haemorrhagic fever: Comparison of parameters studied between Grades 1 and 2 at febrile, defervescence and convalescence phases and comparison to febrile phase.
The combined Grade 1 DHF cohorts are significantly younger than the Grade 2 cohorts (P = 0.03). There were no significant differences in the other parameters studied between the two groups of cohorts at different phases of DHF.
Platelets had higher mean numbers at a convalescent phase in both grades 1 and 2 DHF, but they did not reach statistical differences even when compared to the febrile phase. Thrombocytopenia with elevated F1 + 2, VWF, VEGF and D-dimer was observed. Moreover, there was also no significant differences in the parameters studied when defervescence and convalescence phases were compared to febrile phase, except for D-dimer (Grade 2 DHF) which showed a significant decrease (P = 0.01) at convalescence compared with febrile phase even though it remained elevated (Table 2).
Table 2.
Dengue haemorrhage fever: Comparison of parameters studied (mean ± SD) between Grades 1 & 2 at febrile, defervescence and convalescence phases and compared to febrile phase
| Grade 1 | Gr1 – P vs Febrile phase | Grade 2 | Gr2- P vs Febrile phase | P Gr1 vs Gr2) | |
|---|---|---|---|---|---|
| Febrile | |||||
| N (male/female) | 41 (28/13) | 6 (4/2) | |||
| Age years | 20.6 (11.8) | 30.8 (8.7) | 0.03 | ||
| Haemoglobin g//L | 13.6 (1.8) | 14.3 (2.1) | 0.46 | ||
| Haematocrit % | 40.6 (5.6) | 42.8 (6.1 | 0.45 | ||
| Platelets x 109/L | 94.8 (70.9 | 70.5 (60.4) | 0.40 | ||
| F1 + 2 pg/mL | 293.1 (171.5) | 296.0 (157.0) | 0.97 | ||
| VWF ng/mL | 109.7 (29.6) | 120.0 (29.1) | 0.45 | ||
| VEGF pg/mL | 270.4 (248.6 | 253.5 (78.1) | 0.74 | ||
| D-dimer ng/mL | 1770.4 (789.3) | 1988.6 (472.1) | 0.42 | ||
| Defervescence | |||||
| Haemoglobin g//L | 13.6 (1.7) | 0.93 | 14.2 (2.0) | 0.91 | 0.49 |
| Haematocrit % | 41.2 (6.6) | 0.64 | 41.4 (8.3) | 0.75 | 0.96 |
| Platelets x 109/L | 78.4 (51.3) | 0.23 | 66.5 (413) | 0.90 | 0.54 |
| F1 + 2 pg/mL | 350.5 (197.4) | 0.93 | 378.9 (129.7) | 0.34 | 0.66 |
| VWF ng/mL | 114.0 (24.9) | 0.48 | 112.3 (26.1) | 0.37 | 0.89 |
| VEGF pg/mL | 384.7(430.2) | 0.27 | 312.2 (108.5) | 0.31 | 0.65 |
| D-dimer ng/mL | 1829.0 (1499.4) | 0.85 | 1525.2 (617.7) | 0.22 | 0.45 |
| Convalescence | |||||
| Haemoglobin g//L | 13.2 (1.7) | 0.35 | 13.6 (1.8) | 0.65 | 0.63 |
| Haematocrit % | 40.1 (5.3) | 0.68 | 40.4 (5.6) | 0.50 | 0.91 |
| Platelets x 109/L | 101.8 (58.8) | 0.63 | 97.0 (55.3) | 0.45 | 0.85 |
| F1 + 2 pg/mL | 313.8 (264,8) | 0.67 | 336.8 (176.2) | 0.68 | 0.79 |
| VWF ng/mL | 113.7 (24.8) | 0.51 | 114.3 (13. 0) | 0.68 | 0.93 |
| VEGF pg/mL | 349.0 (433.8 | 0.32 | 467.5 (534.0) | 0.37 | 0.62 |
| D-dimer ng/mL | 1528.1 (1422.8) | 0.47 | 1085.2 (480.7) | 0.01 | 0.16 |
Analysis of Variance (ANOVA), One-way ANOVA analysis for Hb, Hct, platelets, F1 + 2, VWF. VEGF in either Grades 1 or 2 between different DHF phases showed no statistical differences except for D-dimer (Grade 2 DHF) showed a significant decrease ((P = 0.04) at convalescence (not shown). When combined {Grades 1 & 2}, ANOVA analysis showed no significant differences in the parameters studied.
Comparison between normal controls against combined DHF (Grades 1 & 2) at different phases for F1 + 2, VWF, VEGF and D-dimer.
Grades 1 & 2 DHF were combined to analyse against normal controls. There were significant differences (P ≤ 0.001) at all phases of DHF for elevated F1 + 2, VWF, VEGF and D-dimer levels compared with normal controls (Table 3).
Table 3.
Comparison between normal controls and combined DHF (Grades 1 & 2) at different phases for F1+2, VWF, VEGF and D-dimer (mean ± SD)
| Normal-Control | DHF-Febrile | DHF-Defervescence | DHF-Convalescence | |
|---|---|---|---|---|
| N | 15 | 47 | 47 | 47 |
| Prothrombin | ||||
| Fragment F1+2 pg/mL | ND 293.4 (166.1) | 354.1 (189.2) | 316.8 (263.4) | |
| P | < 0.001 | < 0.001 | < 0.001 | |
| VWF ng/mL | 1.9 (31.4)* | 111.0 (29.6) | 113.8 (24.8) | 113.8 (23.6) |
| P | < 0.001 | < 0.001 | < 0.001 | |
| VEGF pg/mL | 71.7 (27.9) | 268.3 (233.4) | 340.5 (323.3) | 364.2 (442.9 |
| P | < 0.001 | < 0.001 | < 0.001 | |
| D-dimer ng/mL | < 500 | 1800.7 (751.9) | 1785.6 (1405.1) | 1463.8 (1334.8) |
| P | < 0.001 | < 0.001 | < 0.001 |
ND = not detectable (F1 + 2 sensitivity < 28.1 pg/mL);
ND (n = 13), VWF sensitivity < 0.94 ng/mL.
This suggests that there is endothelial activation, plasma leakage triggering the activation of coagulation, creating a hypercoagulable and fibrinolytic state in DHF.
The combined Grades 1 & 2 DHF for VWF, VEGF, F1 + 2 and D-dimer at febrile, defervescence and convalescence phases with ANOVA analysis and normal controls are shown in Figure 1. The results from the three severe DHF patients recruited had elevated VWF, F1 + 2, VEGF and D-dimer with thrombocytopenia similar with grades 1&2 DHF but had lower haemoglobin levels. However, the patient with severe DHF (grade 4) was unconscious when admitted and found to have cerebral bleeding (CT scan) at defervescence phase with pleural effusion, hypervolemic shock. Thrombocytopenia with platelets at 43 x 109/L and elevated D-dimer of 1620 ng/mL at admission were given electrolyte and crystalloid infusions. The platelet rose to 88 x 109/L, and D-dimer level fell to 809 ng/mL at convalescence. His condition improved and discharged after two weeks in the hospital. The other two patients with grade 3 DHF also had pleural effusion and enlarged livers.
Figure 1.
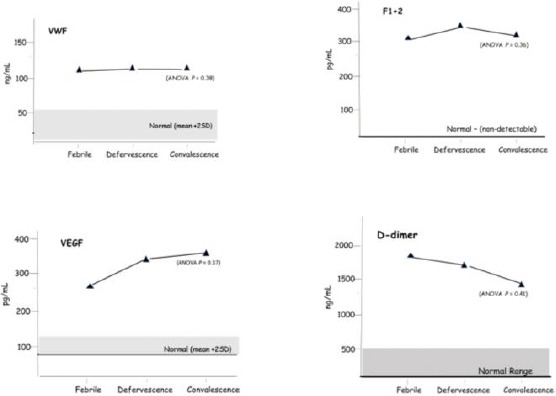
Mean levels of VWF, VEGF, F1 + 2 and D-dimer levels and ANOVA analysis in combined DHF Grades 1 & 2 at febrile, defervescence and convalescence phases
Thrombocytopenia was seen in one patient (platelets 87x 109/L) at febrile phase and fell to 11 x 109/L at defervescence phase but rose to 141 x 109/L at convalescence. The D-dimer levels of 5296.2 ng/mL at the febrile phase fell to 620.4 ng/mL at the convalescence phase. The other patient had normal platelets at admission (173 x 109/L), but severe thrombocytopenia was seen in defervescence and convalescence phases (4 x 109/L and 37 x 109/l) respectively. The D-dimer was 2307.8 ng/mL at admission and 2060 ng/ml at convalescence. They were given electrolyte infusion and other medications and discharged one week later in an afebrile state.
Discussion
Dengue fever is the most serious consequence of mosquito-borne infection worldwide. The pathophysiology of DHF in human is complex as its clinical symptoms are mainly due to an immune response involving the production of cytokine/chemokines as well as endothelial activation, T-lymphocytes, monocytes and platelets. Endothelial damage may also be caused by the virus itself. Thrombocytopenia is responsible for bleeding events in DHF [20], [23] but many factors can contribute to the onset of thrombocytopenia from a reactive immune response against platelets and decreased platelet production [11], [24], platelet activation and apoptosis [21], Dengue virus could bind directly to prothrombin inhibiting the conversion to thrombin [24] causing decreased coagulation activation, reduced thrombin generation and may be associated with bleeding complications in Brazilians with DHF [23] The relationship between dengue and activation of coagulation is controversial [27]. However, activation of coagulation in critical DHF phase was reported in Indonesian patients (22), which was contrary to the Brazilian study [23]. Bleeding manifestations and plasma leakage are complications seen in dengue and bleeding manifestation in adults may occur in the absence of plasma leakage [28].
In our study, petechiae or rash was observed in DHF besides the symptoms of pain in the bones, behind the eyes and headaches. Bleeding episodes like epitaxial were seen in grades 2 and 3 DHF while bleeding to the brain occurred in our grade 4 patient. Pleural effusion was seen only in severe DHF with liver enlargement present in Grades 2, 3 and 4 and about 14.6% (6/41) in Grade 1 DHF. Thrombocytopenia was observed in all phases of DHF even though in the convalescence phase, the mean platelet numbers were higher than in febrile and defervescence phases they did not reach statistical significance between grades 1 & 2 DHF. Normal haemoglobin and no haemo-concentration were observed, but elevated activation of coagulation (F1 + 2), VWF, VEGF and D-dimer suggest endothelial activation, plasma leakage and activation of coagulation in DHF. Activation of coagulation was reported earlier in critical DHF [22] in Indonesian patients, but contrary to this, Orsi and co-workers [23] reported reduced thrombin generation and enhanced fibrinolysis contributing to the bleeding episodes in Brazilian patients. Reduced thrombin generation could result from the dengue virus binding directly to prothrombin inhibiting the conversion to thrombin [26]. Activation of coagulation and elevated D-dimer levels also indicates hypercoagulability and enhanced fibrinolysis. Endothelial activation evident by elevated VWF and VEGF suggests plasma leakage triggering the activation of the coagulation system, creating hypercoagulation and enhanced fibrin-lysis state. Elevated D-dimer was seen in DHF even at convalescence. Normal haemoglobin and no haemoconcentration was observed in DHF grades 1 and 2. No mortality was recorded. Demographic differences and genetic make-up may contribute to these differences. Identifying the mechanisms affecting DHF would improve diagnosis and management therapy limiting morbidity and mortality.
In conclusion, in dengue haemorrhagic fever, the vascular endothelium is activated, causing plasma leakage triggering the activation of the haemostatic system creating a hypercoagulable and enhanced fibrinolytic state evident by marked fibrin-lysis.
Acknowledgements
The authors wish to express their sincere gratitude to the staff of the Research Laboratories at the Medical Faculty, Universitas Sumatera Utara and Murni Teguh Memorial Hospital for their expert technical assistance.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.World Health Organisation (WHO) Dengue and dengue haemorrhagic fever Factsheet No. 117. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 2.World Health Organisation (WHO) Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded version WHO; 2011. [Google Scholar]
- 3.World Health Organisation Media Centre. Dengue and severe dengue. WHO Factsheet updated. 2017 [Google Scholar]
- 4.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS neglected tropical diseases. 2012;6(8):e1760. doi: 10.1371/journal.pntd.0001760. https://doi.org/10.1371/journal.pntd.0001760 PMid:22880140 PMCid:PMC3413714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karayanti MK, Ulterwaal CSPM, Kusriantuti R, et al. The changing incidence of dengue haemorrhagic fever in Indonesia:a 45-year registry-based analysis. BMC Infectious Diseases. 2014;14:412. doi: 10.1186/1471-2334-14-412. https://doi.org/10.1186/1471-2334-14-412 PMid:25064368 PMCid:PMC4122763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation (WHO) Dengue:guidelines for diagnosis, treatment, prevention and control New ed. Geneva Switzerland: World Health Organisation; 2009. [Google Scholar]
- 7.Verhamme P, Hoylaerts MF. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clinica Belgica. 2006;61(5):213–9. doi: 10.1179/acb.2006.036. https://doi.org/10.1179/acb.2006.036 PMid:17240734. [DOI] [PubMed] [Google Scholar]
- 8.Connolly DT. Vascular permeability factor:a unique regulator of blood vessel function. J Cell Biochem. 1991;47:219–23. doi: 10.1002/jcb.240470306. https://doi.org/10.1002/jcb.240470306 PMid:1791186. [DOI] [PubMed] [Google Scholar]
- 9.Mohle R, Green D, Moore MAS, Nachman RL, Rafil S. Constitutive production of thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelet. Proc Natl Acad Sci. 1997;94:663–8. doi: 10.1073/pnas.94.2.663. https://doi.org/10.1073/pnas.94.2.663 PMid:9012841 PMCid:PMC19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhuel HM, Hoekman K, Lulx-de Bakker S, et al. Platelet transporter of vascular growth factor. Clin Cs Res. 1997;3(12 Pt1):9815–90. [PubMed] [Google Scholar]
- 11.De Castro RA, De Castro JA, Barez MY, Frias MV, Dixit J, Genereux M. Thrombocytopenia associated with dengue hemorrhagic fever responds to intravenous administration of anti-D(Rh(O)-1) immune globulin. Am J Trop Med Hyg. 2007;76:737–42. https://doi.org/10.4269/ajtmh.2007.76.737 PMid:17426181. [PubMed] [Google Scholar]
- 12.Falanga AM, Marchett M, Vignoli A. Coagulation and cancer:Biological and clinical aspects. J Thromb Haemostas. 2013;11:223–33. doi: 10.1111/jth.12075. https://doi.org/10.1111/jth.12075 PMid:23279708. [DOI] [PubMed] [Google Scholar]
- 13.Kleinegris MC, Ten Cate H, Ten Cate HAJ. D-dimer as a marker for cardiovascular and arterial thrombotic events in patients with peripheral arterial disease. A systemic review. Thromb Haemostas. 2013;110(2):23. doi: 10.1160/TH13-01-0032. https://doi.org/10.1160/TH13-01-0032 PMid:23784703. [DOI] [PubMed] [Google Scholar]
- 14.Gomez K, Tudderham EGD, McVoy JH. Normal Haemostasis. 6th ed. Post Graduate Haematology Wiley Blackwell; 2011. pp. 747–771. https://doi.org/10.1002/9781444323160.ch39. [Google Scholar]
- 15.Mourao MP, Lacerda MV, Macedo VO, Santos JB. Thrombocytopenia in patients with dengue virus infection in the Brazilian Amazon. Platelets. 2007;18:605–1. doi: 10.1080/09537100701426604. https://doi.org/10.1080/09537100701426604 PMid:18041652. [DOI] [PubMed] [Google Scholar]
- 16.Schexneider KL, Reedy EA. Thrombocytopenia in dengue fever. Curr Hematol Rep. 2005:145–8. [PubMed] [Google Scholar]
- 17.Honda S, Saito M, Dimano EM, et al. Increased of phagocytosis of platelets from patients with secondary dengue virus infection by human macrophages. Am J Trop Med Hyg. 2009;80:841–5. https://doi.org/10.4269/ajtmh.2009.80.841. [PubMed] [Google Scholar]
- 18.Nimmanitya S. Dengue hemorrhagic fever:disorders of hemostasis. Proceeding International Congress of Hematology. Asia-Pacific Division. 1999 [Google Scholar]
- 19.Srichaikul T, Nammannitya S. Hematology in dengue and dengue hemorrhagic fever. Baillieres Best Pract Res Clin Hematol. 2000;13:261–76. doi: 10.1053/beha.2000.0073. https://doi.org/10.1053/beha.2000.0073 PMid:10942625. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Quijano FA, Villa-Centeno LA, Marinez-Vega RA. Predictors of spontaneous bleeding in patients with acute febrile syndrome from a dengue endemic area. J Clin Virol. 2010;49:11–5. doi: 10.1016/j.jcv.2010.06.011. https://doi.org/10.1016/j.jcv.2010.06.011 PMid:20663710. [DOI] [PubMed] [Google Scholar]
- 21.Hottz ED, Oliviera MF, Nunes CG, et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J Thromb Haemostas. 2013;11:951–62. doi: 10.1111/jth.12178. https://doi.org/10.1111/jth.12178 PMid:23433144 PMCid:PMC3971842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pudjianto S, Setiabudy RD, Nainggolan L, Setiabudy R. Prothrombin fragment 1.2 (F1.2) in relation with plasma leakage and thrombocytopenia in dengue infection. Health Sci J Indonesia. 2016;7:37–43. https://doi.org/10.22435/hsji.v7i1.4913.37-43. [Google Scholar]
- 23.Orsi FA, Angerami RN, Mazetto BM, et al. Reduced thrombin formation and excessive fibrinolysis are associated with bleeding complications in patients with dengue fever:a case-control study comparing dengue fever patients with and without bleeding. BMC Infect Dis. 2013;13:250–6. doi: 10.1186/1471-2334-13-350. https://doi.org/10.1186/1471-2334-13-350 PMid:23890510 PMCid:PMC3733705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63(2):143–9. https://doi.org/10.1002/1096-9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L. [PubMed] [Google Scholar]
- 25.Saito M, Oishi K, Inoue S, et al. Association of increased platelet-associated immunoglobulins with thrombocytopenia and the severity of disease in secondary dengue virus infections. Clin Exp Immunol. 2004;138(2):299–303. doi: 10.1111/j.1365-2249.2004.02626.x. https://doi.org/10.1111/j.1365-2249.2004.02626.x PMid:15498040 PMCid:PMC1809201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SW, Chuang YC, Lin YS, Lei HY, Liu HS, Yeh TM. Dengue virus non-structured protein NS1 binds to prothrombin/thrombin and inhibits prothrombin activation. J Infect. 2011;64(3):325–334. doi: 10.1016/j.jinf.2011.11.023. https://doi.org/10.1016/j.jinf.2011.11.023 PMid:22138554. [DOI] [PubMed] [Google Scholar]
- 27.Mairuhu AT, Mac Gillavry MR, Setiati TE, et al. Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis?A critical review of the evidence. Lancet Infect Dis. 2003;3(1):33–41. doi: 10.1016/s1473-3099(03)00487-0. https://doi.org/10.1016/S1473-3099(03)00487-0. [DOI] [PubMed] [Google Scholar]
- 28.Wichmann O, Hongsinwon S, Bowonwatanuwong C, Chotivanich K, Sukthana K, Pukrittayakmee S. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health. 2004;9(9):1022–9. doi: 10.1111/j.1365-3156.2004.01295.x. https://doi.org/10.1111/j.1365-3156.2004.01295.x PMid:15361117. [DOI] [PubMed] [Google Scholar]


