Abstract
Background
Stereotactic body radiation therapy (SBRT) given in 1-5 fractions is an effective treatment for vertebral metastases. Real-time magnetic resonance-guided radiotherapy (MRgRT) improves soft tissue contrast, which translates into accurate delivery of spine SBRT. Here we report on clinical implementation of MRgRT for spine SBRT, the quality of MRgRT plans compared to TrueBeam based volumetric modulated arc therapy (VMAT) plans in the treatment of spine metastases and benefits of MRgRT MR scan.
Patients and methods
Ten metastatic lesions were included in this study for plan comparison. Lesions were spread across thoracic spine and lumbosacral spine. Three fraction spine SBRT plans: 27Gy to planning target volume (PTV) and 30Gy to gross tumor volume (GTV) were generated on the ViewRay MRIdian Linac system and compared to TrueBeamTM STx based VMAT plans. Plans were compared using metrics such as minimum dose, maximum dose, mean dose, ratio of the dose to 50% of the volume (R50), conformity index, homogeneity index and dose to the spinal cord.
Results
MRIdian plans achieved equivalent target coverage and spinal cord dose compared to VMAT plans. The maximum and minimum PTV doses and homogeneity index were equivalent for both planning systems. R50 was lower for MRIdian plans compared to VMAT plans, indicating a lower spread of intermediate doses with MRIdian system (5.16 vs. 6.11, p = 0.03).
Conclusions
MRgRT can deliver high-quality spine SBRT plans comparable to TrueBeam volumetric modulated arc therapy (VMAT) plans.
Key words: MR guided radiotherapy, spine radiotherapy, SBRT, treatment planning, VMAT
Introduction
Stereotactic body radiation therapy (SBRT) given in 1–5 fractions is an effective treatment for spinal metastases.1 Spine SBRT involves tight planning margins and steep dose gradients to the surrounding organs at risk (OAR). Spinal cord, which is a serial organ, is the most important dose-limiting structure in spine SBRT planning. The risk of radiation myelopathy can be kept to ≤ 1% with meticulous radiotherapy planning and delivery.2 Multiple studies have demonstrated the safety and feasibility of using stereotactic radiotherapy for spinal metastases.3, 4 In many published de novo and adjuvant studies, spine SBRT has led to one year local control rates of 80–90%.5, 6 Given its safety and efficacy, use of spine SBRT in the United States has increased from 2% to 20% over the last decade.7
Spine SBRT is often delivered with dynamic arc, static intensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) on linear accelerator (Linac), non-isocentric robotic delivery (CyberKnife) and Tomotherapy using computed tomography (CT)-based platforms.8, 9 At the time of planning, spinal cord volume is usually defined on diagnostic magnetic resonance (MR) images fused with planning kilovoltage computed tomography (kVCT). This approach can result in fusion errors on the order of 2 mm.10 Despite near-rigid full body immobilization and on-board CT-based imaging, inter-fraction and intra-fraction motion of the spinal cord necessitates a planning risk volume (PRV) margin of 1–2 mm to ensure safe treatment delivery.11
In a study that evaluated the effect of setup errors on dose distribution for spine SBRT12, investigators used cone beam computed tomography (CBCT) scans to assess the actual dose to the spinal cord PRV generated by expanding spinal cord by 2 mm. The difference in minimum dose to the upper 10% of the PRV (i.e., D10) was 0.03 ± 0.3 Gy (maximum, 0.9 Gy). Thus, although setup errors using CT-based image-guidance are often small and could result in non-significant change to the planned OAR dose, they could very easily become clinically significant given the steep dose gradient associated with these highly specialized treatments, especially if the error was found to be in the direction of the spinal cord. Compared to CT-guided radiotherapy, magnetic resonance (MRI)-guided platform improves soft tissue contrast, which can translate into accurate contouring of target and/or OARs.12
Magnetic resonance-guided radiotherapy (MRgRT) delivery systems have now entered clinical practice at several major treatment centers. One such system is the ViewRay MRIdian LinacTM (ViewRay, Inc., Oakwood Village, Ohio, USA), which combines a 0.345T field strength split-bore magnet MRI with a 28 cm gap that contains 6 MV flattening filter free linear accelerator (Linac).13 The imaging field of view is 50 cm wide with 70 cm diameter of bore body coil, with a capability to acquire scans as fast as 17 sec or 25 sec using the true FISP (TRUFI) imaging sequence. The TRUFI imaging sequence on the ViewRay MRIdian LinacTM platform enables real-time visualization of the spinal cord and surrounding cerebrospinal fluid, thereby making MRIdian an optimal modality for image guided radiotherapy.14 This novel Linac system is equipped with a slightly de-focused double-stack multi-leaf collimator (MLC). This system is designed so that the beams have sharp penumbra with minimal leakage through the leaves. MLCs are designed to project field sizes from 0.2 x 0.4 cm2 up to 27.4 x 24.1 cm2. The MRIdian system uses step-and-shoot intensity modulated radiation therapy (IMRT) technique to deliver dose that is calculated with a Monte Carlo algorithm.
Previous studies have compared dosimetric data for normal tissues and target for different treatment planning stations and delivery techniques.9,15 In this study, we report on the quality of ViewRay MRIdian Linac treatment plans compared to TrueBeamTM STx (Varian Medical Systems, Palo Alto, CA) volumetric modulated arc therapy (VMAT) plans and clinical implementation of MRgRT for spine SBRT along with benefits of using MRgRT for spine SBRT.
Patients and methods
Patients previously treated with vertebral body metastases between 2015 and 2018 were included in this retrospective study. This study was approved by the local institutional review board. For simulation, patients were immobilized in a BodyFIX bluebag (Elekta, Stockholm, Sweden) with vacuum wrap.16 They were scanned in supine position with arms elevated above the head. All scans were acquired on a Siemens SOMATOM Definition Edge scanner, with a slice thickness of 1 to 2 mm.
Planning CT and diagnostic MR scans were exported to MIM Maestro (MIM Software, Cleveland, OH, USA) for segmentation of target and OARs. Rigid registration was performed between CT simulation scan and the diagnostic MRI scan (MRI Dx) using the MIM optimization algorithm. Segmentation was done by radiation oncologists with expertise in spine SBRT. Radiographically visible tumor was contoured as gross tumor volume (GTV). Clinical target volume (CTV) was contoured using the international consensus guidelines.17 A geometric margin of 3 mm excluding the spinal cord was used to generate planning target volume (PTV), and a 2 mm margin was used for spinal cord planning risk volume (PRV). Similar principles were used to contour cauda equina.
Common practice for spine SBRT at our institution involves a prescription dose of 27 Gy to PTV in 3 fractions with a simultaneous integrated boost (SIB) of 30 Gy to GTV, which is based on published prospective studies.3,5 For dosimetric comparison, the same set of contours was used to generate both MRIdian and VMAT plans. Treatment planning objectives were: at least 95% of the target volume receives the prescribed dose; hotspots were limited to 110% within 1 cm of the target volume and 105% outside. For spinal cord and spinal cord PVR maximum dose was constrained to 18 Gy and 20 Gy respectively. Mean dose to kidneys was restricted to less than or equal to 10 Gy. For lungs, volume of lungs receiving 5 Gy, 12.5 Gy, 20 Gy and 12 Gy was restricted to 50%, 15%, 10% and 1000 cc respectively.
CT scans and contours were exported to Pinnacle treatment planning system (TPS) to generate VMAT plans for TrueBeam TM STx. VMAT plans were generated using three co-planar 6 MV arcs with gantry angles varying from 178° to 182° (CCW), 183° to 178° (CW), 178° to 182° (CCW) and collimator set at 330°, 25° and 320°. TrueBeamTM STx is equipped with a six degree of freedom (DOF) couch, which allows for more variable beam arrangement. Final dose distribution was calculated with the anisotropic analytic algorithm with dose grid of 2 mm.
For the MRIdian plans, CT images and contours that were used for VMAT plans were imported into MRIdian TPS. On average, 10 to 15 beams spaced 20° to 28° apart (110° to 221°) were used to generate a step-and-shoot IMRT plan. Beams entering through the corners of the couch were removed to avoid dosimetric uncertainty. The isocenter was placed in the PTV. Final dose calculation was done using grid size of 2 mm with Monte Carlo. Final dose distribution from MRIdian and Pinnacle were exported to MIM to tabulate and compare clinically relevant DVH parameters.
For dosimetric analysis, VMAT and MRIdian Linac plans were compared using plan metrics such as near minimum dose (D98%-Dose to 98% of PTV), near maximum dose (D2%), median dose (D50%), conformity index (CI) and dose homogeneity index (HI) for PTV. CI and HI were calculated as shown below18:
To evaluate the impact of intermediate dose on the normal tissue R50, the ratio of volume inside 50% isodose line to the PTV volume was calculated.
Dosimetric data for cord was compared between the two plans. Wilcoxon matched pairs signed-rank test, a non-parametric equivalent of paired t-test, was used to compare dosimetric parameters between VMAT and MRIdian Linac plans. For all statistical analysis SPPS version 25 was used.
Results
Ten metastatic lesions from nine patients were included in this study for plan comparison. Lesions were spread across thoracic spine (T6-T12) and also lumbosacral spine (L2-S1). Dose-volume histogram (DVH) parameters for both plans are shown in Table 1. Detailed dosimetric results for all cases are summarized in Table 2. All plans were able to meet the planning parameters. R50 was lower for MRIdian Linac plans when compared to VMAT plans, indicating a lower spread of intermediate doses with MRIdian (5.16 vs. 6.11, p = 0.056) for PTV. Average D98% (Near Minimum), D2% (Near Maximum) and D50% (Median dose) were similar between the two plans. HI and CI were also similar between VMAT and MRIdian Linac plans. The percentage difference for D98%, D2%, and D50% were 3%, 4.5% and 3.3%, respectively, between both the plans for PTV and D50% for GTV was within 0.3% and spinal cord maximum dose was within 5.5% for both the plans. Dose to other OARs were within acceptable limits for plans and there no significant difference between the plans.
Table 1.
Dose volume parameters for PTV, GTV, Spinal Cord and Cauda Equina for MRIdian Linac IMRT plans Vs TrueBeamTM STx VMAT plans
| Structures | TrueBeamTM STx VMAT | MRIdian Linac IMRT | p-value |
|---|---|---|---|
| PTV | Median(range) | Median(range) | |
| D98% (Gy) | 25.7 (15.6–29.5) | 26.5 (17.7–29.7) | 0.20 |
| D50% (Gy) | 29.0 (27.9–39.1) | 30.0 (26.4–33.2) | 0.77 |
| Conformity Index | 0.97 (0.93–1.0) | 0.97 (0.90–1.0) | 0.13 |
| Dose Homogeneity Index | 0.22 (0.05–0.6) | 0.19 (0.1–0.57) | 0.49 |
| R50 | 6.1 (2.9–16.7) | 5.2 (2.8–11.9) | 0.05 |
| GTV | |||
| D50% (Gy) | 31.6 (30.4–34.2) | 31.7 (31.0–34.6) | 0.36 |
| D98% (Gy) | 30.12 (25–33.63) | 30.35 (29.17–32.89) | 0.23 |
| Conformity Index | 0.99 (0.95–1.0) | 0.99 (0.96–1.0) | 0.58 |
| Dose Homogeneity Index | 0.09 (0.04–0.33) | 0.08 (0.01–0.18) | 0.30 |
| R50 | 32 (6.08–69.0) | 29 (5.8–66.0) | 0.01 |
| Spinal Cord | |||
| Max dose (Gy) | 12.6 (9.1–15.6) | 13.3 (10.35–17.3) | 0.13 |
| D0.03 (Gy) | 12 (9.0–15.3) | 12.8 (9.5–16.5) | 0.25 |
| Cauda Equina | |||
| Max dose (Gy) | 16 (0.22–28.0) | 18 (0.3–32) | 0.38 |
| D0.03 (Gy) | 13 (0.2–20.0) | 16 (0.25–31) | 0.07 |
Table 2.
Detailed Dose volume histogram parameters for PTV, GTV, Spinal Cord and Cauda Equina for MRIdian Linac IMRT plans Vs TrueBeamTM STx VMAT plans
| PTV | GTV | Spinal Cord | Cauda Equina | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case # | Volume | Maximum | Dose (Gy) | D95 ( | Gy) | Volume | Maximum | Dose (Gy) | D95 ( | Gy) | Maximum | Dose (Gy) | D0.03 cc | (Gy) | Maximum | Dose (Gy) | D0.03 | cc (Gy) |
| (cc) | MRIdian Linac IMRT | True Beam VMAT | MRIdian Linac IMRT | True Beam VMAT | (cc) | MRIdian Linac IMRT | True Beam VMAT | MRIdian Linac IMRT | True Beam VMAT | MRIdian Linac IMRT | True Beam VMAT | MRIdian Linac IMRT | True Beam VMAT | MRIdian Linac IMRT | True Beam VMAT | MRIdian Linac IMRT | True Beam VMAT | |
| 1 | 60.37 | 34.69 | 35.44 | 27.43 | 27.50 | 7.02 | 34.69 | 35.44 | 32.46 | 33.76 | 14.82 | 14.77 | 14.67 | 14.47 | 19.70 | 21.72 | 19.67 | 20.19 |
| 2 | 50.63 | 32.45 | 32.27 | 27.00 | 27.31 | 6.82 | 32.94 | 32.41 | 30.11 | 30.05 | 13.33 | 13.45 | 13.02 | 13.10 | 20.00 | 21.15 | 19.92 | 18.95 |
| 3 | 66.3 | 36.41 | 37.00 | 27.75 | 27.12 | 27.67 | 36.41 | 37.00 | 32.58 | 29.61 | 17.01 | 11.69 | 16.56 | 10.90 | 21.07 | 14.12 | 20.23 | 13.58 |
| 4 | 47.63 | 31.68 | 30.85 | 27.19 | 27.94 | 18.8 | 34.02 | 33.45 | 30.06 | 30.52 | 13.25 | 15.65 | 12.62 | 15.34 | 24.08 | 21.19 | 22.48 | 19.79 |
| 5 | 2.6 | 33.87 | 30.71 | 27.73 | 27.26 | 0.9 | 33.87 | 32.02 | 30.24 | 30.21 | 12.58 | 13.81 | 11.92 | 12.90 | 9.31 | 12.37 | 7.73 | 7.56 |
| 6 | 3.93 | 33.52 | 31.40 | 27.67 | 27.89 | 1.65 | 33.52 | 34.96 | 32.17 | 27.39 | 10.35 | 9.10 | 10.32 | 9.09 | 16.44 | 18.01 | 12.58 | 12.32 |
| 7 | 3.05 | 32.64 | 30.88 | 27.50 | 27.82 | 1.07 | 32.65 | 33.70 | 30.44 | 29.02 | 11.17 | 10.10 | 11.15 | 10.09 | 32.13 | 28.01 | 31.16 | 19.32 |
| 8 | 25.43 | 32.73 | 34.07 | 27.92 | 27.99 | 2.31 | 32.73 | 34.07 | 30.31 | 31.43 | 13.72 | 10.98 | 13.22 | 10.36 | 13.72 | 10.6 | 13.19 | 10.25 |
| 9 | 29.02 | 33.95 | 34.14 | 27.50 | 27.60 | 1.89 | 35.70 | 34.14 | 30.05 | 30.52 | 11.04 | 9.25 | 9.53 | 8.96 | 0.32 | 0.22 | 0.25 | 0.21 |
| 10 | 38.29 | 33.02 | 33.95 | 27.88 | 27.02 | 2.51 | 33.02 | 33.95 | 30.45 | 30.51 | 17.30 | 14.11 | 14.51 | 13.75 | 3.54 | 3.67 | 3.20 | 3.40 |
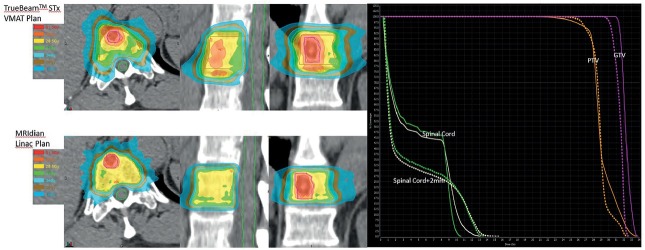
The average beam on time for the VMAT plans was 7 minutes compared to 12 minutes for the MRIdian Linac plans. Dose to the spinal cord and Cauda equina was also calculated and shown to be comparable between MRIdian Linac and VMAT plans (Table 1). Isodose distribution and DVH for MRIdian Linac vs. VMAT are shown in Figure 1. MRIdian Linac setup image quality was superior to isolate the target and spinal cord for each fraction compared to kVCT and megavoltage computed tomography (MVCT) (Figure 3, 4) thus minimizing setup errors.
Figure 1.
Isodose distribution (right) for TrueBeamTM STx VMAT and MRIdian Linac Plans: 30 Gy to gross tumor volume (GTV) and 27 Gy to planning target volume (PTV) in 3 fractions. On left, solid and dashed lines represents dose volume histogram for TrueBeamTM STx VMAT and MRIdian Linac Plans for GTV, PTV, Spinal Cord and Spinal Cord+2mm.
Figure 3.
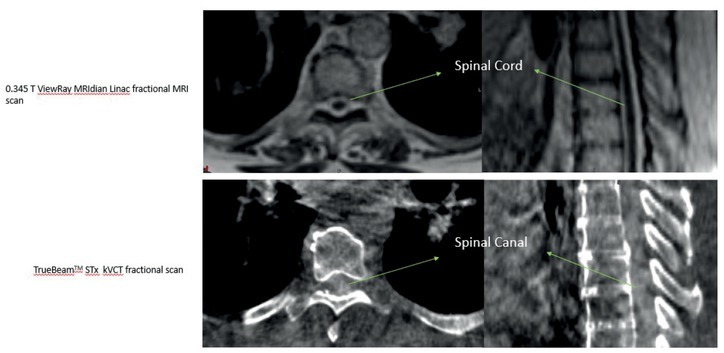
Fractional 0.345T MRIdian Linac MR scan (Top) and Kilo Voltage Cone Beam (Bottom) used for daily setup verification before radiotherapy treatment. Visibility of spinal cord on 0.345T is demonstrated increasing the accuracy on treatment delivery.
Figure 4.
Fractional 0.345T MRIdian Linac MR scan (Left) and TomoTherapy® Mega Voltage CT (Right) used for daily setup verification before radiotherapy treatment. Visibility of target (red) on 0.345T is demonstrated.
Discussion
Increased global incidence of cancer in combination with improved systemic therapies has led to an increase in the prevalence of oligometastatic disease involving bone.19 Synchronous or metachronous bony vertebral/spinal metastases are diagnosed in approximately 40–70% of patients with cancer, mainly secondary to breast, lung, or prostate adenocarcinoma.20, 21 Aggressive treatment of isolated metastases in select patients may lead to improved outcomes.22, 23
Multiple studies have compared treatment planning quality among dynamic conformal arcs, static IMRT, VMAT and tomotherapy for spine.24, 25, 26 Matuszak et al. study concludes that VMAT improved the isodose conformality and reduced the treatment time by 37% compared to IMRT.27 In this study, we have shown that MRIdian Linac plans are comparable to TrueBeamTM STx VMAT plans with respect to target metrics and spinal cord dosimetry. MRIdian Linac plans generated with manual beam angle selection helped to limit the beam angles entering through the critical structures. Beam-on times were higher for MRIdian Linac compared to TrueBeamTM STx given the lack of dynamic treatment delivery with ViewRay MRIdian Linac system. Dosimetric results of this study have helped us to clinically start treating spine SBRT on the MRIdian Linac system. Although MRIdian Linac plans resulted in comparable dose to spine yet clinical relevance of these dosimetric differences is unknown.
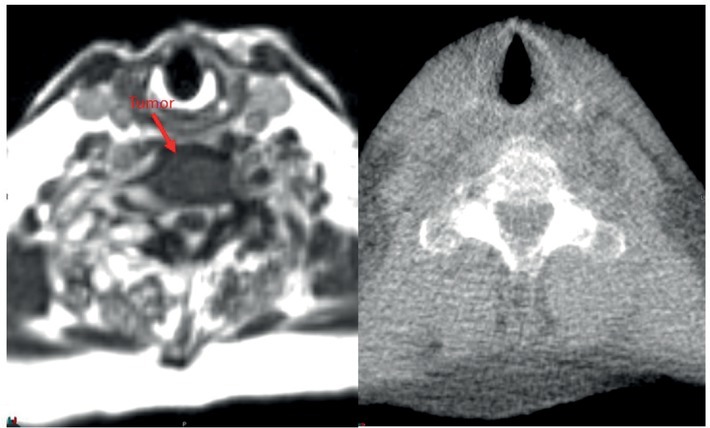
In our experience, the ability to accurately visualize the spinal cord is a significant advantage with the ViewRay MRgRT for several reasons. MRI-guided radiotherapy allows physician contouring to rely on the optimally visualized spinal cord on MR simulation images and not on CT-MRI fusion with accompanying errors associated with registration.10 Figure 2 shows a representative image of a T1 weighted image obtained on a 1.5T diagnostic MRI compared to TRUFI sequence on 0.345T ViewRay MRIdian system demonstrating excellent tumor demarcation on both images sets. At several clinics, including our institution spine SBRT treatments are delivered not only on TrueBeam but also Tomotherapy. Figures 3 and 4 show examples of 0.345T MRI images compared to kV CBCT from TrueBeamTM STx and to MVCT acquired on TomoTherapy®. As can be seen, MRI provides images with superior soft tissue contrast, allowing better visualization of the spinal cord and spinal canal on the volumetric image acquired for setup verification. This enables accurate patient setup for treatment delivery and could potentially minimize set up errors.
Figure 2.
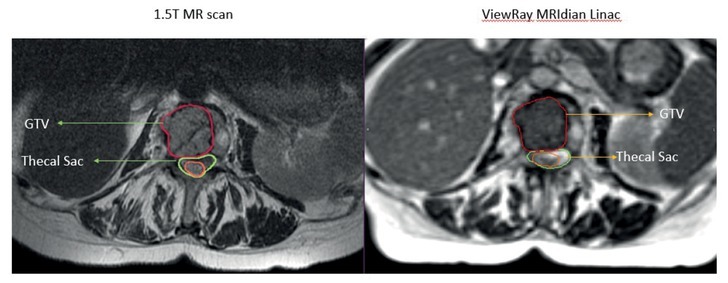
A metastatic tumor in the vertebral body of lumbar spine and adjacent spinal cord scan acquired on 1.5T diagnostic magnetic resonance (MR) and 0.345T ViewRay MRIdian Linac system. MRIdian scan allows for accurate delineation of the tumor and the spinal cord without having to rely on the fused diagnostic MR scan.
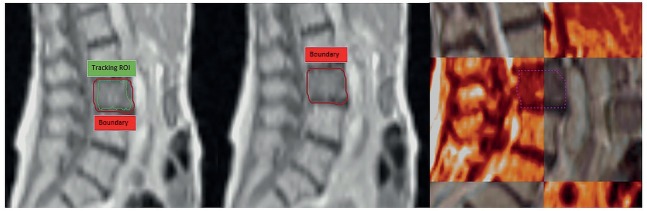
Spine SBRT plans usually have a steep dose gradient at the spinal cord/PTV interface. In a systematic review by Chang et al., crude risk of local failure at 1-year was 21.4%. Around 67% of these failures occurred within the epidural space.28 Sahgal et al. have reported that the majority of their local failures occurred at the spinal cord PRV-PTV interface. Their data suggest that as the cord PRV to PTV distance decreases, the risk of local failure increases.29 This is another area where MRIdian treatment delivery system could have advantages. Spinal cord PRV margin comprises patient set up uncertainty, organ motion, intrafraction patient motion and, contouring uncertainty. MRIdian can help minimize the margin required for contouring uncertainty, organ motion and intrafraction motion, allowing us to treat without having to add a separate PRV or a minimal PRV margin to the spinal cord. This thereby increases the distance between PTV and dose-limiting OAR and limits underdosing of epidural component of the PTV, where significant local failures tend to occur. By using “tracking region of interest (ROI)”, accurate online tracking of the target can be performed (Figure 5). This in turn has significant impact on dose deposition, given the close proximity of this tracking ROI to spinal cord, where a steep dose gradient exists. Additionally, the online adaptive workflow is a great advantage of the MRIdian system that can be utilized for challenging patients with minimal separation between the tumor and spinal cord.30
Figure 5.
0.345T MRIdian Linac cine acquired in sagittal plane. Any changes in reference setup results in shutting the radiation beam off as tracking structure (green) moves outside the boundary (red).
MRI-guided therapy with MRIdian does have a few limitations. The MRIdian couch does not allow for six degrees of freedom or non-coplanar beam angles. It also does not permit modification of collimator angle or allow dynamic treatment delivery. Beam entry from couch edges for treatment plans are restricted due to high couch attenuation. Also, the number of beams used for planning are restricted to keep the total treatment time reasonable. Another limitation of the system is the lowest monitor units (MU) that can be delivered with the MRIdian Linac system is 1. Additionally, MRgRT is contraindicated in the post-operative setting if patients have magnetic resonance imaging (MRI)-incompatible metal implants. In many cases, however, the benefits of substantially improving soft tissue contrast, ability of real-time tracking and online adaptation may outweigh any planning and delivery difficulties encountered with MRgRT.
Conclusions
Here, we have shown that 3-fraction spine SBRT plans are dosimetrically comparable between MRIdian and TrueBeamTM STx VMAT plans and MRgRT can be successfully used for SBRT spine with reasonable delivery time.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Husain ZA, Sahgal A, De Salles A, Funaro M, Glover J, Hayashi M. Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg-Spine. 2017;27:295–302. doi: 10.3171/2017.1.SPINE16684. et al. [DOI] [PubMed] [Google Scholar]
- 2.Chang JH, Shin JH, Yamada YJ, Mesfin A, Fehlings MG, Rhines LD. Stereotactic body radiotherapy for spinal metastases: What are the risks and how do we minimize them? Spine. 2016;41(Suppl 20):S238–45. doi: 10.1097/BRS.0000000000001823. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XS, Rhines LD, Shiu AS, Yang JN, Selek U, Gning I. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13:395–402. doi: 10.1016/S1470-2045(11)70384-9. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryu S, Pugh SL, Gerszten PC, Yin FF, Timmerman RD, Hitchcock YJ. RTOG 0631 Phase II/III study of image-guided stereotactic radiosurgery for localized (1-3) spine metastases: phase II results. Int J Radiat Oncol Biol Phys. 2011;81:S131–2. doi: 10.1016/j.prro.2013.05.001. et al. [DOI] [PubMed] [Google Scholar]
- 5.Katsoulakis E, Kumar K, Laufer I, Yamada Y. Stereotactic body radiotherapy in the treatment of spinal metastases. Semin Radiat Oncol. 2017;27:209–17. doi: 10.1016/j.semradonc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Tseng CL, Soliman H, Myrehaug S, Lee YK, Ruschin M, Atenafu EG. Imaging-based outcomes for 24 Gy in 2 daily fractions for patients with de novo spinal metastases treated with spine stereotactic body radiotherapy (SBRT) Int J Radiat Oncol Biol Phys. 2018;3:499–507. doi: 10.1016/j.ijrobp.2018.06.047. et al. [DOI] [PubMed] [Google Scholar]
- 7.McClelland S, Kim E, Passias PG, Murphy JD, Attia A, Jaboin JJ. Spinal stereotactic body radiotherapy in the United States: A decade-long nationwide analysis of patient demographics, practice patterns, and trends over time. J Clin Neurosci. 2017;46:109–12. doi: 10.1016/j.jocn.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Nalichowski A, Kaufman I, Gallo J, Bossenberger T, Solberg T, Ramirez E. Single fraction radiosurgery/stereotactic body radiation therapy (SBRT) for spine metastasis: A dosimetric comparison of multiple delivery platforms. J Appl Clin Med Phys. 2017;18:164–9. doi: 10.1002/acm2.12022. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, Djemil T, Zhuang T, Andrews M, Chao ST, Suh JH. Treatment plan quality and delivery accuracy assessments on 3 IMRT delivery methods of stereotactic body radiotherapy for spine tumors. Med Dosim. 2019;44:11–4. doi: 10.1016/j.meddos.2017.12.009. et al. [DOI] [PubMed] [Google Scholar]
- 10.Sharpe M, Brock KK. Quality assurance of serial 3D image registration, fusion, and segmentation. Int J Radiat Oncol. 2008;71:S33–7. doi: 10.1016/j.ijrobp.2007.06.087. [DOI] [PubMed] [Google Scholar]
- 11.Li WN, Sahgal A, Foote M, Millar BA, Jaffray DA, Letourneau D. Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int J Radiat Oncol. 2012;84:520–6. doi: 10.1016/j.ijrobp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Gutfeld O, Kretzler AE, Kashani R, Tatro D, Balter JM. Influence of rotations on dose distributions in spinal stereotactic body radiotherapy (SBRT) Int J Radiat Oncol. 2009;73:1596–601. doi: 10.1016/j.ijrobp.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menard C, van der Heide UA. Introduction: Magnetic resonance imaging comes of age in radiation oncology. Semin Radiat Oncol. 2014;24:149–50. doi: 10.1016/j.semradonc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Dirix P, Haustermans K, Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol 2014. 24:151–9. doi: 10.1016/j.semradonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Saenz DL, Crownover R, Stathakis S, Papanikolaou N. A dosimetric analysis of a spine SBRT specific treatment planning system. J Appl Clin Med Phys. 2019;20:154–9. doi: 10.1002/acm2.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubie C, Shaw M, Bydder S, Lane J, Waters G, McNabb M. A randomised comparison of three different immobilisation devices for thoracic and abdominal cancers. J Med Radiat Sci. 2017;64:90–6. doi: 10.1002/jmrs.202. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, Ryu S. International Spine Radiosurgery Consortium Consensus Guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol. 2012;83:E597–E605. doi: 10.1016/j.ijrobp.2012.03.009. et al. [DOI] [PubMed] [Google Scholar]
- 18.Gregoire V, Mackie TR. State of the art on dose prescription, reporting and recording in intensity-modulated radiation therapy (ICRU report No. 83) Cancer Radiother. 2011;15:555–9. doi: 10.1016/j.canrad.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R. NCCN task force report: bone health in cancer care. J Natl Compr Canc Ne. 2013;11:S1–S50. doi: 10.6004/jnccn.2013.0215. et al. [DOI] [PubMed] [Google Scholar]
- 20.Bollen L, van der Linden YM, Pondaag W, Fiocco M, Pattynama BPM, Marijnen CAM. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1 043 patients. Neuro-Oncology. 2014;16:991–8. doi: 10.1093/neuonc/not318. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constans JP, Dedivitiis E, Donzelli R, Spaziante R, Meder JF, Haye C. Spinal metastases with neurological manifestations - review of 600 cases. J Neurosurg. 1983;59:111–8. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- 22.Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–82. doi: 10.1016/S1470-2045(16)30532-0. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a Phase 2 randomized clinical trial. Jama Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Ma L, Wang XS, Xu WX, Cong XH, Xu SP. Dosimetric evaluation of 4 different treatment modalities for curative-intent stereotactic body radiation therapy for isolated thoracic spinal metastases. Med Dosim. 2016;41:105–12. doi: 10.1016/j.meddos.2015.10.003. et al. [DOI] [PubMed] [Google Scholar]
- 25.Wu QJ, Yoo S, Kirkpatrick JP, Thongphiew D, Yin FF. Volumetric arc intensity-modulated therapy for spine body radiotherapy: comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys. 2009;75:1596604. doi: 10.1016/j.ijrobp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Rao M, Yang W, Chen F, Sheng K, Ye J, Mehta V. Comparison of Elekta VMAT with helical tomotherapy and fixed field IMRT: plan quality, delivery efficiency and accuracy. Med Phys. 2010;37:1350–9. doi: 10.1118/1.3326965. et al. [DOI] [PubMed] [Google Scholar]
- 27.Matuszak MM, Yan D, Grills I, Martinez A. Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;77:608–16. doi: 10.1016/j.ijrobp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg-Spine. 2007;7:151–60. doi: 10.3171/SPI-07/08/151. et al. [DOI] [PubMed] [Google Scholar]
- 29.Sahgal A, Ames C, Chou D, Ma LJ, Huang K, Xu W. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol. 2009;74:723–31. doi: 10.1016/j.ijrobp.2008.09.020. et al. [DOI] [PubMed] [Google Scholar]
- 30.Henke L, Kashani R, Yang DS, Zhao TY, Green O, Olsen L. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: Characterization of potential advantages. Int J Radiat Oncol. 2016;96:1078–86. doi: 10.1016/j.ijrobp.2016.08.036. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]