ABSTRACT
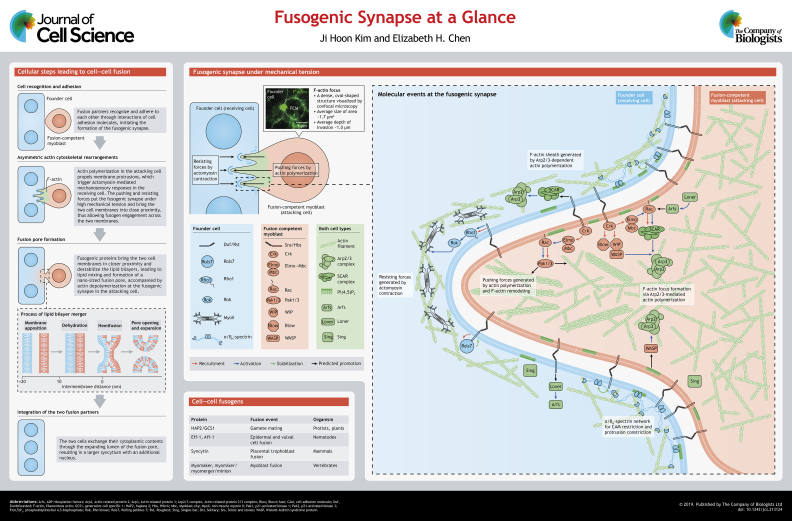
Cell–cell fusion is a fundamental process underlying fertilization, development, regeneration and physiology of metazoans. It is a multi-step process involving cell recognition and adhesion, actin cytoskeletal rearrangements, fusogen engagement, lipid mixing and fusion pore formation, ultimately resulting in the integration of two fusion partners. Here, we focus on the asymmetric actin cytoskeletal rearrangements at the site of fusion, known as the fusogenic synapse, which was first discovered during myoblast fusion in Drosophila embryos and later also found in mammalian muscle and non-muscle cells. At the asymmetric fusogenic synapse, actin-propelled invasive membrane protrusions from an attacking fusion partner trigger actomyosin-based mechanosensory responses in the receiving cell. The interplay between the invasive and resisting forces generated by the two fusion partners puts the fusogenic synapse under high mechanical tension and brings the two cell membranes into close proximity, promoting the engagement of fusogens to initiate fusion pore formation. In this Cell Science at a Glance article and the accompanying poster, we highlight the molecular, cellular and biophysical events at the asymmetric fusogenic synapse using Drosophila myoblast fusion as a model.
KEY WORDS: Actin cytoskeleton, Cell–cell fusion, Fusogenic synapse, Invasive protrusion, Mechanical force, Mechanosensory response, Myoblast fusion, Podosome
Summary: Cell–cell fusion is promoted by invasive protrusions and mechanosensory responses at the asymmetric fusogenic synapse.
Introduction
Cell–cell fusion is a fundamental cellular event in many developmental and physiological processes, including fertilization, muscle development and regeneration, bone remodeling, immune response, placental formation, and cancer metastasis (Aguilar et al., 2013; Chen and Olson, 2005; Gast et al., 2018; Hernández and Podbilewicz, 2017). As with any membrane fusion event, the rate-limiting step in cell–cell fusion is to bring the two cell membranes into close proximity to initiate fusion pore formation. Studies of cell–cell fusion in various model systems have revealed several sequential cellular events that bring the two cell membranes together. These events include cell recognition and adhesion, actin cytoskeletal rearrangements, fusogen engagement, lipid mixing, and fusion pore formation, leading to the integration of the two fusion partners (see poster). Studies of myoblast fusion in Drosophila embryos have provided major insights into the mechanisms underlying cell recognition, adhesion and actin cytoskeletal rearrangements (Abmayr and Pavlath, 2012; Deng et al., 2017; Kim et al., 2015a; Lee and Chen, 2019; Önel et al., 2014; Schejter, 2016). Invasive membrane protrusions and mechanosensory responses at the site of myoblast fusion, known as the fusogenic synapse, were first discovered in Drosophila embryos (Sens et al., 2010; Kim et al., 2015b). Similar protrusions were later found in mammalian muscle and non-muscle cells that undergo fusion (Randrianarison-Huetz et al., 2018; Shin et al., 2014), suggesting that these protrusions may play conserved roles in cell fusion across species from insects to mammals. Meanwhile, studies of protist and plant mating, Caenorhabditis elegans embryonic development, vertebrate myogenesis, and placenta formation have identified fusogens, which are transmembrane proteins specifically required for initiating fusion pore formation. The functions of these fusogens have been discussed in excellent recent reviews (Brukman et al., 2019; Hernández and Podbilewicz, 2017) and will not be a major focus of this article (see Box 1). In this Cell Science at a Glance, we summarize the molecular, cellular and biophysical events leading to the formation and dynamics of the actin-based asymmetric fusogenic synapse using Drosophila myoblast fusion as a model.
Box 1. Cell-cell fusogens.
Fusogens are specialized proteins that mediate fusion between membranes (Brukman et al., 2019; Hernández and Podbilewicz, 2017). They drive membrane fusion by bringing two membranes at a distance of <10 nm into direct contact, leading to the formation of a fusion intermediate (hemifusion stalk) and eventually the opening of a fusion pore (see poster) (Chernomordik and Kozlov, 2005; Sapir et al., 2008). Although the fusogen(s) that mediate Drosophila myoblast fusion remain unknown, diverse cell–cell fusogens that act in the fusion of placental trophoblasts, C. elegans somatic cells, protist and plant gametes, and vertebrate myoblasts have been identified. While syncytins are captured virus fusogens in trophoblasts (Blond et al., 2000; Huppertz and Borges, 2008; Mi et al., 2000), Eff-1 and its paralog Aff-1 in C. elegans epithelial and vulval cells, respectively (Mohler et al., 2002; Sapir et al., 2007), and HAP2 (also known as GCS1) in protist and plant gametes (Liu et al., 2008; Pinello et al., 2017; Valansi et al., 2017) resemble type II viral fusogens (Pérez-Vargas et al., 2014; Fédry et al., 2017). Interestingly, vertebrate myoblast fusion utilizes a bipartite fusogen comprising a seven-pass transmembrane protein myomaker (Millay et al., 2013), and a micropeptide myomixer (also known as myomerger or minion) (Bi et al., 2017; Quinn et al., 2017; Zhang et al., 2017). These two proteins work independently to control distinct steps of membrane remodeling during myoblast fusion, with myomaker involved in membrane hemifusion and myomixer in generating the membrane stress necessary for fusion pore formation (Leikina et al., 2018). Interestingly, while similar actin polymerization machineries and actin-propelled invasive membrane protrusions are used to promote cell–cell fusion from insects to mammals, fusogens are mostly species- and/or tissue-specific. For example, syncytins are only required in placental mammals, Eff-1 and Aff-1 are mainly used in nematodes, HAP2 acts in a range of protist and plant gametes, and myomaker and myomixer function in vertebrate skeletal muscle.
Two types of muscle cells in Drosophila embryos
During Drosophila embryogenesis, muscle progenitor cells in the somatic mesoderm are specified into two populations, muscle founder cells and fusion-competent myoblasts (FCMs) (Chen and Olson, 2005; Rochlin et al., 2010). Although different subsets of muscle founder cells are specified by distinct sets of transcription factors (Baylies et al., 1998), the fate of all FCMs is determined by a single transcription factor, Lame duck (Lmd) (Duan et al., 2001). An abdominal hemi-segment contains 30 muscle founder cells, each of which acts as a seed to attract the surrounding FCMs to fuse with them. Through multiple rounds of fusion, a multinucleated muscle fiber with specific position, orientation, size and nerve innervation pattern is generated. To initiate fusion, founder cells and FCMs express cell type-specific immunoglobulin (Ig) domain-containing cell adhesion molecules (CAMs) to mediate the recognition and adhesion between the two types of cells. The founder cell-specific CAMs, Dumbfounded (Duf, also known as Kirre) and its paralog Roughest (Rst), have redundant functions in myoblast fusion (Ruiz-Gómez et al., 2000; Strünkelnberg et al., 2001), whereas the FCM-specific CAMs, Sticks and stones (Sns) and its paralog Hibris (Hbs), are only partially redundant, with Sns playing a major role (Artero et al., 2001; Bour et al., 2000; Galletta et al., 2004; Shelton et al., 2009). Trans-interactions between founder cell- and FCM-specific CAMs establish a muscle cell contact site, which will become the future site of fusion. These CAMs, however, cannot induce cell–cell fusion by themselves, as demonstrated by S2 cell aggregation assays (Chen et al., 2003; Shilagardi et al., 2013). This is because the ectodomains of these CAMs form a rigid structure that props the two cell membranes apart by ∼45 nm, a distance too large for membrane fusion (Özkan et al., 2014). Therefore, the CAMs must engage additional cellular machineries to bring the two cell membranes into closer proximity (see poster). Forward genetic analyses in Drosophila identified a number of actin cytoskeletal regulators in myoblast fusion, leading to the discovery that actin cytoskeletal rearrangements triggered by CAM engagement play a key role beyond cell adhesion to promoting cell membrane juxtaposition and fusion.
The attacking cell: fusion-competent myoblast
Despite the general function of the actin cytoskeleton in cell motility, shape change and protrusion formation, the actin cytoskeleton has a specific and direct function in mediating cell–cell fusion. The initial evidence came from the discovery of a dense, oval-shaped F-actin focus at the site of myoblast fusion (Kesper et al., 2007; Kim et al., 2007; Richardson et al., 2007) (see poster). Genetic and cell biological studies have localized the actin focus exclusively in the FCM (Sens et al., 2010). The actin focus appears to exert an invasive force toward the apposing founder cell and create an inward curvature on the founder cell membrane (Sens et al., 2010). Viewed along the axis of FCM invasion, the actin focus is encircled by CAMs Duf and Sns (Kesper et al., 2007; Sens et al., 2010). Such FCM-specific bipartite structure, consisting of a ring of CAMs encircling a protrusive actin core, resembles a podosome and is thus named a podosome-like structure (PLS) (Chen, 2011; Sens et al., 2010). The actin focus is highly dynamic and rapidly changes its shape by continuous actin polymerization during its life span (Jin et al., 2011; Sens et al., 2010). At the ultrastructural level, revealed by electron microscopy (EM), each actin focus is composed of multiple finger-like protrusions emanating from a wide actin-enriched base and drilling into the apposing founder cell (Duan et al., 2012, 2018; Jin et al., 2011; Kim et al., 2015b; Sens et al., 2010) (see poster). The dynamic extension and/or retraction of the protrusions make fixation for EM challenging, often resulting in fuzzy membranes along the protrusions, especially at the tips (Sens et al., 2010). The generation of long and narrow invasive protrusions by the FCM is essential for fusion pore formation, because stubby and/or wide protrusions fail to promote fusion pore formation in myoblast fusion mutants (Duan et al., 2012, 2018; Jin et al., 2011; Kim et al., 2015b; Sens et al., 2010).
The formation of the F-actin focus depends on the seven-subunit Arp2/3 complex, which mediates the nucleation of branched actin filaments (Berger et al., 2008; Richardson et al., 2007). The activity of the Arp2/3 complex is regulated by two actin nucleation-promoting factors (NPFs), WASP (the single Drosophila ortholog of mammalian WASP and N-WASP) and SCAR (the single Drosophila ortholog of mammalian WAVEs) (Ben-Yaacov et al., 2001; Stradal and Scita, 2006; Zallen et al., 2002). WASP forms a tight protein complex with the WASP-interacting protein (WIP), also known as Solitary (Sltr) or as Verprolin 1 (Vrp1), which is specifically expressed in FCMs and colocalizes with the F-actin focus at the fusogenic synapse (Kim et al., 2007; Massarwa et al., 2007). The WASP–WIP complex is likely to be recruited to the fusogenic synapse by the SH2–SH3 domain-containing adaptor proteins. Of the three SH2–SH3 adaptor proteins in Drosophila (Crk, Dock and Drk), both Crk and Dock have been shown to interact with CAMs and actin cytoskeletal regulators, suggesting that they may link cell adhesion with the actin cytoskeleton (Kim et al., 2007; Kaipa et al., 2013). The stability of the WASP–WIP complex is regulated by a PH domain-containing protein, Blown fuse (Blow) (Doberstein et al., 1997; Jin et al., 2011). Blow colocalizes with the F-actin focus at the fusogenic synapse and competes with WASP for WIP binding (Jin et al., 2011). Destabilization of the WASP–WIP complex by Blow results in the rapid dissociation of WASP from the barbed ends of actin filaments, which leads to the initiation of short branched actin filaments capable of exerting mechanical force (Jin et al., 2011). Consistent with their roles in regulating the dynamics of branched actin polymerization, the WASP–WIP complex and Blow are all required for generating finger-like invasive protrusions in FCMs (Sens et al., 2010; Jin et al., 2011) (see poster).
The second Arp2/3 NPF, SCAR, is required in both founder cells and FCMs (Sens et al., 2010). Scar is a component of the pentameric SCAR complex that also includes Kette (also known as Nap1), Sra1, Abi and HSPC300 (Campellone and Welch, 2010; Stradal and Scita, 2006; Takenawa and Suetsugu, 2007). In founder cells, Scar mediates the formation of a thin sheath of actin underlying the cell membrane at the fusogenic synapse (Sens et al., 2010) (see poster). However, the upstream regulation of SCAR in founder cells remains unclear. In FCMs, SCAR functions together with WASP to generate the F-actin foci (Sens et al., 2010). Compared to the WASP–WIP complex, SCAR does not seem to affect the formation of invasive protrusions, as evidenced by the morphologically normal FCM protrusions in the absence of the SCAR complex (Jin et al., 2011; Sens et al., 2010). In FCMs, the SCAR complex is recruited to the fusogenic synapse and activated by the Rac GTPases (Rac hereafter) (Gildor et al., 2009). The binding of Rac to Sra1 releases the verprolin-homology, central and acidic (VCA) domain of SCAR which in turn activates the Arp2/3 complex (Chen et al., 2010). Consistent with being a SCAR regulator, Rac is required for myoblast fusion (Hakeda-Suzuki et al., 2002; Luo et al., 1994) and it is activated by the bipartite guanine nucleotide exchange factor (GEF) Elmo (also known as Ced-12)–Myoblast city (Mbc) in FCMs (Erickson et al., 1997; Geisbrecht et al., 2008; Haralalka et al., 2011). Besides the SCAR complex, Rac also recruits and activates the Drosophila group I p21-activated kinase (Pak) proteins Pak3 and Pak1, which function specifically in FCMs (Duan et al., 2012). The two Pak proteins have partially redundant functions in myoblast fusion, with Pak3 playing a major role (Duan et al., 2012). Both Pak proteins colocalize with the F-actin foci at the fusogenic synapse and are required for organizing branched actin filaments into a dense structure that is mechanically strong to generate invasive protrusions (Duan et al., 2012). The localization of both WASP and Scar is also proposed to be regulated by the Drosophila formin Diaphanous (Dia), which nucleates linear actin filaments (Deng et al., 2015). Dia colocalizes with the F-actin foci at the fusogenic synapse (Deng et al., 2015). However, the mechanism by which Dia regulates WASP and Scar localization remains unclear. In addition, phosphatidylinositol 4,5-bisphosphate (PIP2) is enriched at the fusogenic synapse and has been proposed to recruit Rac, Scar and WASP through interacting with pleckstrin homology (PH) domain-containing proteins (Bothe et al., 2014).
Conserved functions for invasive protrusions in cell–cell fusion
Actin-propelled invasive membrane protrusions are not only observed during myoblast fusion in Drosophila embryos. During Drosophila adult myogenesis, myoblast fusion occurs between individual myoblasts and/or with larval template myofibers. Here, WASP and Scar are also required for the fusion process and co-localize with the F-actin foci specifically generated in myoblasts (Mukherjee et al., 2011). Correspondingly, these myoblasts have been observed to project invasive protrusions toward the myofibers visualized by EM (Dhanyasi et al., 2015), similar to those observed at the fusogenic synapse during embryonic myoblast fusion (Sens et al., 2010). In Drosophila S2R+ cells (of hemolymph origin) induced to fuse by co-expressing the FCM-specific CAM Sns and a C. elegans fusogen Eff-1 (Mohler et al., 2002; Podbilewicz et al., 2006), similar actin-propelled invasive protrusions are also found to facilitate cell fusion (Shilagardi et al., 2013). These protrusions promote fusogen engagement across the two membranes, presumably by pushing the apposing plasma membranes into close proximity (Shilagardi et al., 2013). When Arp2/3-mediated actin polymerization is inhibited by Scar knockdown, S2R+ cell fusion fails to occur no matter how much Eff-1 is expressed (Shilagardi et al., 2013). Moreover, S2R+ cell fusion does not occur in the absence of a fusogen. Thus, actin cytoskeletal rearrangements and fusogens are two indispensable components in cell–cell fusion.
In addition to playing an essential role in Drosophila cell–cell fusion, actin cytoskeletal rearrangements are also required for the fusion of mammalian cells. Pharmacological perturbation of the actin cytoskeleton inhibits mammalian myoblast fusion, despite overexpression of a fusogenic protein involved in fusion pore formation (Millay et al., 2013). Furthermore, most, if not all, actin cytoskeletal regulators discovered in Drosophila myoblast fusion have mammalian homologs and have been shown to promote mammalian myoblast fusion (Gruenbaum-Cohen et al., 2012; Hamoud et al., 2014; Kim et al., 2015a; Laurin et al., 2008; Nowak et al., 2009; Pajcini et al., 2008; Rochlin et al., 2010; Vasyutina et al., 2009). In particular, actin-propelled invasive protrusions are observed in cultured mouse myoblasts and osteoblasts (E.H.C., D. Luvsanjav, Johns Hopkins University School of Medicine, USA, unpublished observation; Randrianarison-Huetz et al., 2018; Shin et al., 2014). Besides insect and mammalian cells, WASP and Arp2/3 have also been implicated in the fusion between seam cells and the hyp7 cell in the C. elegans larval epithelium (Yang et al., 2017). Although epidermal cell fusion proceeds in C. elegans embryos mutant for Scar and Arp2/3 (Patel et al., 2008), it is unclear whether maternal rescue could have masked any potential fusion defect. Interestingly, whereas Eff-1 in embryos does not appear to co-localize with actin (Smurova and Podbilewicz, 2016), Eff-1 in the larval seam cells is enriched at the cell cortex together with actin-propelled membrane protrusions projected toward the hyp7 cell (Yang et al., 2017). Future ultrastructural analyses are required to assess the invasiveness of these protrusions. Notably, fusion between walled cells, such as yeast, also requires the actin cytoskeleton. It has been shown that an actin-enriched focus is organized by the formin Fus1 at the site of yeast mating and serves to focalize the delivery of cell wall-degrading enzymes (Dudin et al., 2015). Whether the Fus-1-mediated actin focus exerts a protrusive force on the cell membrane following cell wall degradation requires future investigations. Taken together, invasive membrane protrusions are likely used as an evolutionarily conserved mechanism to promote cell–cell fusion, at least across animal families from insects to mammals. These protrusions not only increase the contact areas between the two cell membranes, but also dynamically push the membranes into close proximity, allowing fusogen engagement across the membranes.
The receiving cell: muscle founder cell
Although FCMs are the more aggressive partners in Drosophila myoblast fusion, the founder cells also exhibit dynamic cellular activities to facilitate the fusion process. In each fusion event, the founder cell actively transports CAMs to the fusogenic synapse and mounts mechanosensory responses to increase mechanical tension and promote cell membrane juxtaposition and fusion.
The transport of the founder cell CAM Duf to the fusogenic synapse is regulated by the founder cell-specific adaptor protein Rolling pebbles 7 [Rols7, also known as Antisocial (Ants) and Rols]. Rols7 is a protein containing ankyrin repeats, tetratricopeptide repeats (TPRs), a RING finger domain, and a coiled-coil domain (Chen and Olson, 2001; Menon and Chia, 2001; Rau et al., 2001), which is co-translocated with Duf in vesicles to the fusogenic synapse via Duf–Rols7 interaction (Menon et al., 2005). Through the Duf–Rols7 positive feedback loop, Duf gradually accumulates at the fusogenic synapse, which in turn stabilizes Sns in the apposing FCM by trans-interactions (Galletta et al., 2004; Menon et al., 2005) (see poster). It is unclear, however, which vesicle proteins are involved in transporting Duf and Rols7. A candidate vesicle protein is Singles bar (Sing), a MARVEL domain protein like synaptophysin, the latter of which is a major membrane protein of neuronal synaptic vesicles (Estrada et al., 2007). However, it has been shown that Duf and Sns still accumulate at the fusogenic synapse in sing mutant embryos, raising the question of whether Sing is indeed involved in their transport (Estrada et al., 2007).
The mechanosensory responses in the founder cell have been shown to be mediated by two proteins to date, non-muscle Myosin II (MyoII) and Spectrin (Duan et al., 2018; Kim et al., 2015b). The actin motor MyoII specifically functions in the founder cell and accumulates at the fusogenic synapse, despite the presence of only a thin sheath of actin underlying the founder cell membrane (Kim et al., 2015b) (see poster). Biophysical analyses demonstrate that MyoII rapidly accumulates to the cell cortex in response to local mechanical stimuli prior to the accumulation of its upstream regulators, the GTPase Rho1 and Rho kinase (Rok), suggesting that MyoII functions as a mechanosensor (Kim et al., 2015b). The mechanosensory response of MyoII is further stabilized and amplified by the CAM-initiated chemical signaling through Rho1 and Rok (Kim et al., 2015b). The accumulated MyoII, in turn, increases the cortical tension and/or stiffness in the founder cell to resist the FCM invasion (Kim et al., 2015b). The pushing and resisting forces from the founder cell and FCM, respectively, put the fusogenic synapse under high mechanical tension to help overcome the energy barrier between the two membranes and promote their fusion.
The second mechanoresponsive component in founder cells is the α/βH-spectrin heterotetramer (see poster). Spectrin is best known as a structural protein that forms a static polygonal lattice structure or an ordered periodic longitudinal array underneath the plasma membrane to protect cells from mechanical damage (Bennett and Lorenzo, 2013; Machnicka et al., 2014). α/βH-spectrin is specifically required in founder cells during myoblast fusion. However, instead of forming a stable network, α/βH-spectrin dynamically accumulates at the fusogenic synapse in response to FCM invasion and dissolves once fusion is completed, just as in the case of MyoII. Noticeably, α/βH-spectrin exhibits mechanosensitive accumulation to areas of shear deformation, corresponding to the base areas of invasive protrusions (Duan et al., 2018). In contrast, MyoII exhibits mechanosensitive accumulation to areas of dilation deformation, corresponding to the tip areas of invasive protrusions (Kim et al., 2015b; Duan et al., 2018) (see poster). Thus, protrusions arising from an FCM trigger α/βH-spectrin accumulation at the base areas in the apposing founder cell. Accumulated spectrin becomes a physical barrier for future protrusions, such that new protrusions may only penetrate through spectrin-free areas and trigger additional spectrin accumulation within these areas. Thus, over time, spectrin forms an uneven network at the fusogenic synapse with increasingly smaller spectrin-free domains and gradually constricts the diameter of invasive protrusions from the FCM (Duan et al., 2018). The narrow protrusions from the FCM, in turn, increase local mechanical tension to promote cell fusion. In addition to its role as a cellular sieve to constrict invasive protrusions, the α/βH-spectrin network functions as a cellular fence to restrict the founder cell CAM Duf to the fusogenic synapse through biochemical interactions and steric hindrance (Duan et al., 2018). As a consequence, the FCM-specific CAM Sns is also restricted to the fusogenic synapse through its trans-interactions with Duf, and continues to organize invasive protrusions that exert mechanical forces on the founder cell (Duan et al., 2018). Taken together, these studies demonstrate that the mechanosensitive accumulation of MyoII and α/βH-spectrin in the founder cell increases the mechanical tension at the fusogenic synapse to drive cell membrane juxtaposition and fusion. Interestingly, both MyoII and spectrin have been implicated in mouse myoblast fusion (Duan and Gallagher, 2009; Duan et al., 2018), with their potential roles in mechanosensory response in mammalian cell fusion yet to be tested.
Fusion pore formation: fusogens and lipids
Despite the requirement for actin cytoskeletal dynamics in cell–cell fusion, actin-propelled membrane protrusions are not sufficient to induce cell fusion without fusogens (Shilagardi et al., 2013). Once the two cell membranes are brought into close proximity by the actin-mediated mechanical interactions, fusogens are required to further decrease the distance between the two membranes (from ∼10 nm to 0 nm). This leads to local lipid mixing of the outer leaflets and the formation of a hemifusion stalk, followed by lipid mixing of the inner leaflets and the formation of a nano-sized fusion pore (see poster and Box 1) (Brukman et al., 2019; Chernomordik and Kozlov, 2005; Hernández and Podbilewicz, 2017). However, fusogens require a functional actin cytoskeleton to induce cell fusion, as revealed by studies of the heterologous S2R+ cells co-expressing Sns and Eff-1 (Shilagardi et al., 2013). This has been confirmed by experiments in cultured mammalian muscle cells showing that myomaker overexpression does not overcome the deleterious effects of actin inhibition on mouse myoblast fusion (Millay et al., 2013). The interdependent relationship between the fusogens and the actin cytoskeleton suggests that these two components may be intimately linked during the fusion process. Indeed, Eff-1 expressed in S2R+ cells is enriched along the actin-propelled invasive protrusions at the fusogenic synapse (Shilagardi et al., 2013), although the localization of endogenous cell–cell fusogens relative to the fusogenic synapse awaits future investigations. In addition, the Eff-1-interacting protein Spectraplakin (also known as Vab-10A) has been shown to promote C. elegans epithelial cell fusion by linking Eff-1 to the actin cytoskeleton, whereas Eff-1 enhances Spectraplakin's F-actin bundling activity in vitro and regulates actin dynamics at the cortex of fusing cells (Yang et al., 2017). Moreover, myomaker and myomixer co-overexpression induces massive actin polymerization at the cell cortex in fibroblasts (Zhang et al., 2017), suggesting that fusogens may also affect the rearrangements of the actin cytoskeleton.
Although it is well known that fusogens induce lipid mixing and fusion pore formation, the specific types of lipids that are involved in cell–cell fusion have only begun to be revealed. Besides PIP2 (Bothe et al., 2014), phosphatidylserine (PS) and very long chain fatty acids (VLCFAs), the latter of which are present in sphingolipids and glycerophospholipids, have been implicated in enhancing myoblast fusion. Although PS is normally enriched in the inner leaflet of the plasma membrane, it has been suggested that PS is flipped to the outer leaflet at the cell contact sites during mammalian myoblast fusion (Jeong and Conboy, 2011; Leikina et al., 2013) and axonal fusion in C. elegans (Neumann et al., 2015). In addition, genetic analyses revealed the function of several PS receptors, Bai1, Bai3 (also known as Adgrb1, Adgrb3) and stabilin-2, in myoblast fusion during vertebrate muscle development and regeneration (Hamoud et al., 2014; Hochreiter-Hufford et al., 2013; Park et al., 2016). Regarding VLCFAs, the endoplasmic reticulum-resident enzyme 3-hydroxyacyl-CoA dehydratase 1 (HACD1), which is involved in the synthesis of VLCFAs, has been shown to promote myoblast fusion by increasing plasma membrane fluidity (Blondelle et al., 2015). How these lipids coordinate with the fusogens and the actin cytoskeleton to promote plasma membrane fusion requires future investigation.
Conclusions and perspectives
Work in the past decade has led to the discovery of the asymmetric fusogenic synapse where mechanical interactions between two fusion partners promote membrane juxtaposition and fusogen engagement, eventually leading to the fusion of the two cells. The fusogenic synapse is analogous to two other types of synapses, the neural synapse and the immunological synapse (Billadeau et al., 2007; Dillon and Goda, 2005; Dustin, 2005; Salinas and Price, 2005; Stinchcombe and Griffiths, 2007); all three are relatively stable adherent structures with asymmetric cellular activities, despite using different CAMs and performing different physiological functions. Among these three synapses, only the fusogenic synapse leads to the fusion between two adherent cells, presumably owing to the expression of fusogens. Interestingly, both fusogenic synapse and immunological synapse utilize invasive protrusions to increase cell membrane contact areas, either between two fusion partners or between an immune cell and its target cell (Kim et al., 2015a; Sage et al., 2012; Sens et al., 2010; Ueda et al., 2011). Future studies may uncover additional commonalities among these different types of synapses. Regarding the fusogenic synapse, several major questions remain to be explored. First, what are the cell–cell fusogens for mammalian fertilization, invertebrate myoblasts, osteoclasts in bone remodeling, giant cells in immune response, and how do they compare with known fusogens? Second, how do the actin cytoskeleton and fusogens coordinate in various cell–cell fusion events? Third, are there additional types of lipids that modulate cell–cell fusion? Finally, once a nano-sized fusion pore has formed, what controls its expansion? We fully anticipate that the next few years will bring about exciting new insights into these questions and reveal more of the novel mechanisms underlying the fusogenic synapse and cell–cell fusion.
Acknowledgements
We apologize to colleagues whose work could not be cited due to space limitations.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health grants R01 AR053173 and R01 GM098816, an American Heart Association Established Investigator Award and a Howard Hughes Medical Institute Faculty Scholar Award to E.H.C. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.213124.supplemental.
References
- Abmayr S. M. and Pavlath G. K. (2012). Myoblast fusion: lessons from flies and mice. Development 139, 641-656. 10.1242/dev.068353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar P. S., Baylies M. K., Fleissner A., Helming L., Inoue N., Podbilewicz ., Wang H. and Wong M. (2013). Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29, 427-437. 10.1016/j.tig.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero R. D., Castanon I. and Baylies M. K. (2001). The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development 128, 4251-4264. [DOI] [PubMed] [Google Scholar]
- Baylies M. K., Bate M. and Ruiz Gomez M. (1998). Myogenesis: a view from Drosophila. Cell 93, 921-927. 10.1016/S0092-8674(00)81198-8 [DOI] [PubMed] [Google Scholar]
- Bennett V. and Lorenzo D. N. (2013). Spectrin- and Ankyrin-based membrane domains and the evolution of vertebrates. Curr. Top. Membr. 72, 1-37. 10.1016/B978-0-12-417027-8.00001-5 [DOI] [PubMed] [Google Scholar]
- Ben-Yaacov S., Le Borgne R., Abramson I., Schweisguth F. and Schejter E. D. (2001). Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J. Cell Biol. 152, 1-13. 10.1083/jcb.152.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S., Schäfer G., Kesper D. A., Holz A., Eriksson T., Palmer R. H., Beck L., Klämbt C., Renkawitz-Pohl R. and Onel S.-F. (2008). WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J. Cell Sci. 121, 1303-1313. 10.1242/jcs.022269 [DOI] [PubMed] [Google Scholar]
- Bi P., Ramirez-Martinez A., Li H., Cannavino J., McAnally J. R., Shelton J. M., Sánchez-Ortiz E., Bassel-Duby R. and Olson E. N. (2017). Control of muscle formation by the fusogenic micropeptide myomixer. Science 356, 323-327. 10.1126/science.aam9361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billadeau D. D., Nolz J. C. and Gomez T. S. (2007). Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 7, 131-143. 10.1038/nri2021 [DOI] [PubMed] [Google Scholar]
- Blond J.-L., Lavillette D., Cheynet V., Bouton O., Oriol G., Chapel-Fernandes S., Mandrand B., Mallet F. and Cosset F.-L. (2000). An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74, 3321-3329. 10.1128/JVI.74.7.3321-3329.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondelle J., Ohno Y., Gache V., Guyot S., Storck S., Blanchard-Gutton N., Barthélémy I., Walmsley G., Rahier A., Gadin S. et al. (2015). HACD1, a regulator of membrane composition and fluidity, promotes myoblast fusion and skeletal muscle growth. J Mol. Cell Biol. 7, 429-440. 10.1093/jmcb/mjv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe I., Deng S. and Baylies M. (2014). PI(4,5)P2 regulates myoblast fusion through Arp2/3 regulator localization at the fusion site. Development 141, 2289-2301. 10.1242/dev.100743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour B. A., Chakravarti M., West J. M. and Abmayr S. M. (2000). Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 14, 1498-1511. [PMC free article] [PubMed] [Google Scholar]
- Brukman N. G., Uygur B., Podbilewicz B. and Chernomordik L. V. (2019). How cells fuse. J. Cell Biol. 218, 1436-1451. 10.1083/jcb.201901017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone K. G. and Welch M. D. (2010). A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11, 237-251. 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. H. (2011). Invasive podosomes and myoblast fusion. Curr. Top. Membr. 68, 235-258. 10.1016/B978-0-12-385891-7.00010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. H. and Olson E. N. (2001). Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev. Cell 1, 705-715. 10.1016/S1534-5807(01)00084-3 [DOI] [PubMed] [Google Scholar]
- Chen E. H. and Olson E. N. (2005). Unveiling the mechanisms of cell-cell fusion. Science 308, 369-373. 10.1126/science.1104799 [DOI] [PubMed] [Google Scholar]
- Chen E. H., Pryce B. A., Tzeng J. A., Gonzalez G. A. and Olson E. N. (2003). Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell 114, 751-762. 10.1016/S0092-8674(03)00720-7 [DOI] [PubMed] [Google Scholar]
- Chen Z., Borek D., Padrick S. B., Gomez T. S., Metlagel Z., Ismail A. M., Umetani J., Billadeau D. D., Otwinowski Z. and Rosen M. K. (2010). Structure and control of the actin regulatory WAVE complex. Nature 468, 533-538. 10.1038/nature09623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V. and Kozlov M. M. (2005). Membrane hemifusion: crossing a chasm in two leaps. Cell 123, 375-382. 10.1016/j.cell.2005.10.015 [DOI] [PubMed] [Google Scholar]
- Deng S., Bothe I. and Baylies M. K. (2015). The formin diaphanous regulates myoblast fusion through actin polymerization and Arp2/3 regulation. PLoS. Genet. 11, e1005381 10.1371/journal.pgen.1005381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Azevedo M. and Baylies M. (2017). Acting on identity: myoblast fusion and the formation of the syncytial muscle fiber. Semin. Cell Dev. Biol. 72, 45-55. 10.1016/j.semcdb.2017.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanyasi N., Segal D., Shimoni E., Shinder V., Shilo B.-Z., VijayRaghavan K. and Schejter E. D. (2015). Surface apposition and multiple cell contacts promote myoblast fusion in Drosophila flight muscles. J. Cell Biol. 211, 191-203. 10.1083/jcb.201503005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon C. and Goda Y. (2005). The actin cytoskeleton: integrating form and function at the synapse. Annu. Rev. Neurosci. 28, 25-55. 10.1146/annurev.neuro.28.061604.135757 [DOI] [PubMed] [Google Scholar]
- Doberstein S. K., Fetter R. D., Mehta A. Y. and Goodman C. S. (1997). Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 136, 1249-1261. 10.1083/jcb.136.6.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R. and Gallagher P. J. (2009). Dependence of myoblast fusion on a cortical actin wall and nonmuscle myosin IIA. Dev. Biol. 325, 374-385. 10.1016/j.ydbio.2008.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Skeath J. B. and Nguyen H. T. (2001). Drosophila Lame duck, a novel member of the Gli superfamily, acts as a key regulator of myogenesis by controlling fusion-competent myoblast development. Development 128, 4489-4500. [DOI] [PubMed] [Google Scholar]
- Duan R., Jin P., Luo F., Zhang G., Anderson N. and Chen E. H. (2012). Group I PAKs function downstream of Rac to promote podosome invasion during myoblast fusion in vivo. J. Cell Biol. 199, 169-185. 10.1083/jcb.201204065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R., Kim J. H., Shilagardi K., Schiffhauer E. E., Lee D. M., Son S., Li S., Thomas C., Luo T., Fletcher D. A. et al. (2018). Spectrin is a mechanoresponsive protein shaping fusogenic synapse architecture during myoblast fusion. Nat. Cell Biol. 20, 688-698. 10.1038/s41556-018-0106-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudin O., Bendezú F. O., Groux R., Laroche T., Seitz A. and Martin S. G. (2015). A formin-nucleated actin aster concentrates cell wall hydrolases for cell fusion in fission yeast. J. Cell Biol. 208, 897-911. 10.1083/jcb.201411124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L. (2005). A dynamic view of the immunological synapse. Semin. Immunol. 17, 400-410. 10.1016/j.smim.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Erickson M. R. S., Galletta B. J. and Abmayr S. M. (1997). Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 138, 589-603. 10.1083/jcb.138.3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B., Maeland A. D., Gisselbrecht S. S., Bloor J. W., Brown N. H. and Michelson A. M. (2007). The MARVEL domain protein, Singles Bar, is required for progression past the pre-fusion complex stage of myoblast fusion. Dev. Biol. 307, 328-339. 10.1016/j.ydbio.2007.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fédry J., Liu Y., Péhau-Arnaudet G., Pei J., Li W., Tortorici M. A., Traincard F., Meola A., Bricogne G., Grishin N. V. et al. (2017). The ancient gamete fusogen HAP2 is a eukaryotic class II fusion protein. Cell 168, 904-915.e10. 10.1016/j.cell.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletta B. J., Chakravarti M., Banerjee R. and Abmayr S. M. (2004). SNS: adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech. Dev. 121, 1455-1468. 10.1016/j.mod.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Gast C. E., Silk A. D., Zarour L., Riegler L., Burkhart J. G., Gustafson K. T., Parappilly M. S., Roh-Johnson M., Goodman J. R., Olson B. et al. (2018). Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 4, eaat7828 10.1126/sciadv.aat7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbrecht E. R., Haralalka S., Swanson S. K., Florens L., Washburn M. P. and Abmayr S. M. (2008). Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev. Biol. 314, 137-149. 10.1016/j.ydbio.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildor B., Massarwa R., Shilo B.-Z. and Schejter E. D. (2009). The SCAR and WASp nucleation-promoting factors act sequentially to mediate Drosophila myoblast fusion. EMBO Rep. 10, 1043-1050. 10.1038/embor.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum-Cohen Y., Harel I., Umansky K.-B., Tzahor E., Snapper S. B., Shilo B.-Z. and Schejter E. D. (2012). The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc. Natl. Acad. Sci. USA 109, 11211-11216. 10.1073/pnas.1116065109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L. and Dickson B. J. (2002). Rac function and regulation during Drosophila development. Nature 416, 438-442. 10.1038/416438a [DOI] [PubMed] [Google Scholar]
- Hamoud N., Tran V., Croteau L.-P., Kania A. and Côté J.-F. (2014). G-protein coupled receptor BAI3 promotes myoblast fusion in vertebrates. Proc. Natl. Acad. Sci. USA 111, 3745-3750. 10.1073/pnas.1313886111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralalka S., Shelton C., Cartwright H. N., Katzfey E., Janzen E. and Abmayr S. M. (2011). Asymmetric Mbc, active Rac1 and F-actin foci in the fusion-competent myoblasts during myoblast fusion in Drosophila. Development 138, 1551-1562. 10.1242/dev.057653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández J. M. and Podbilewicz B. (2017). The hallmarks of cell-cell fusion. Development 144, 4481-4495. 10.1242/dev.155523 [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A. E., Lee C. S., Kinchen J. M., Sokolowski J. D., Arandjelovic S., Call J. A., Klibanov A. L., Yan Z., Mandell J. W. and Ravichandran K. S. (2013). Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 497, 263-267. 10.1038/nature12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B. and Borges M. (2008). Placenta trophoblast fusion. Methods Mol. Biol. 475, 135-147. 10.1007/978-1-59745-250-2_8 [DOI] [PubMed] [Google Scholar]
- Jeong J. and Conboy I. M. (2011). Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem. Biophys. Res. Commun. 414, 9-13. 10.1016/j.bbrc.2011.08.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Duan R., Luo F., Zhang G., Hong S. N. and Chen E. H. (2011). Competition between Blown fuse and WASP for WIP binding regulates the dynamics of WASP-dependent actin polymerization in vivo. Dev. Cell 20, 623-638. 10.1016/j.devcel.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipa B. R., Shao H., Schäfer G., Trinkewitz T., Groth V., Liu J., Beck L., Bogdan S., Abmayr S. M. and Önel S.-F. (2013). Dock mediates Scar- and WASp-dependent actin polymerization through interaction with cell adhesion molecules in founder cells and fusion-competent myoblasts. J. Cell Sci. 126, 360-372. 10.1242/jcs.113860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesper D. A., Stute C., Buttgereit D., Kreisköther N., Vishnu S., Fischbach K.-F. and Renkawitz-Pohl R. (2007). Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS). Dev. Dyn. 236, 404-415. 10.1002/dvdy.21035 [DOI] [PubMed] [Google Scholar]
- Kim S., Shilagardi K., Zhang S., Hong S. N., Sens K. L., Bo J., Gonzalez G. A. and Chen E. H. (2007). A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev. Cell 12, 571-586. 10.1016/j.devcel.2007.02.019 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Jin P., Duan R. and Chen E. H. (2015a). Mechanisms of myoblast fusion during muscle development. Curr. Opin. Genet. Dev. 32, 162-170. 10.1016/j.gde.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Ren Y., Ng W. P., Li S., Son S., Kee Y.-S., Zhang S., Zhang G., Fletcher D. A., Robinson D. N. et al. (2015b). Mechanical tension drives cell membrane fusion. Dev. Cell 32, 561-573. 10.1016/j.devcel.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin M., Fradet N., Blangy A., Hall A., Vuori K. and Côté J.-F. (2008). The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc. Natl. Acad. Sci USA 105, 15446-15451. 10.1073/pnas.0805546105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. M. and Chen E. H. (2019). Drosophila myoblast fusion: invasion and resistance for the ultimate union. Annu. Rev. Genet. 53 [Epub ahead of print] 10.1146/annurev-genet-120116-024603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E., Melikov K., Sanyal S., Verma S. K., Eun B., Gebert C., Pfeifer K., Lizunov V. A., Kozlov M. M. and Chernomordik L. V. (2013). Extracellular annexins and dynamin are important for sequential steps in myoblast fusion. J. Cell Biol. 200, 109-123. 10.1083/jcb.201207012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E., Gamage D. G., Prasad V., Goykhberg J., Crowe M., Diao J., Kozlov M. M., Chernomordik L. V. and Millay D. P. (2018). Myomaker and myomerger work independently to control distinct steps of membrane remodeling during myoblast fusion. Dev. Cell 46, 767-780.e7. 10.1016/j.devcel.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tewari R., Ning J., Blagborough A. M., Garbom S., Pei J., Grishin N. V., Steele R. E., Sinden R. E., Snell W. J. et al. (2008). The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 22, 1051-1068. 10.1101/gad.1656508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y. and Jan Y. N. (1994). Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8, 1787-1802. 10.1101/gad.8.15.1787 [DOI] [PubMed] [Google Scholar]
- Machnicka B., Czogalla A., Hryniewicz-Jankowska A., Bogusławska D. M., Grochowalska R., Heger E. and Sikorski A. F. (2014). Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim. Biophys. Acta. 1838, 620-634. 10.1016/j.bbamem.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Massarwa R., Carmon S., Shilo B.-Z. and Schejter E. D. (2007). WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev. Cell 12, 557-569. 10.1016/j.devcel.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Menon S. D. and Chia W. (2001). Drosophila rolling pebbles: a multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast attractant Dumbfounded. Dev. Cell 1, 691-703. 10.1016/S1534-5807(01)00075-2 [DOI] [PubMed] [Google Scholar]
- Menon S. D., Osman Z., Chenchill K. and Chia W. (2005). A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J. Cell Biol. 169, 909-920. 10.1083/jcb.200501126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Lee X., Li X.-P., Veldman G. M., Finnerty H., Racie L., LaVallie E., Tang X.-Y., Edouard P., Howes S. et al. (2000). Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785-789. 10.1038/35001608 [DOI] [PubMed] [Google Scholar]
- Millay D. P., O'Rourke J. R., Sutherland L. B., Bezprozvannaya S., Shelton J. M., Bassel-Duby R. and Olson E. N. (2013). Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 499, 301-305. 10.1038/nature12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler W. A., Shemer G., del Campo J. J., Valansi C., Opoku-Serebuoh E., Scranton V., Assaf N., White J. G. and Podbilewicz B. (2002). The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev. Cell 2, 355-362. 10.1016/S1534-5807(02)00129-6 [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Gildor B., Shilo B.-Z., VijayRaghavan K. and Schejter E. D. (2011). The actin nucleator WASp is required for myoblast fusion during adult Drosophila myogenesis. Development 138, 2347-2357. 10.1242/dev.055012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Coakley S., Giordano-Santini R., Linton C., Lee E. S., Nakagawa A., Xue D. and Hilliard M. A. (2015). EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature 517, 219-222. 10.1038/nature14102 [DOI] [PubMed] [Google Scholar]
- Nowak S. J., Nahirney P. C., Hadjantonakis A.-K. and Baylies M. K. (2009). Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J. Cell Sci. 122, 3282-3293. 10.1242/jcs.047597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önel S. F., Rust M. B., Jacob R. and Renkawitz-Pohl R. (2014). Tethering membrane fusion: common and different players in myoblasts and at the synapse. J. Neurogenet. 28, 302-315. 10.3109/01677063.2014.936014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkan E., Chia P. H., Wang R. R., Goriatcheva N., Borek D., Otwinowski Z., Walz T., Shen K. and Garcia K. C. (2014). Extracellular architecture of the SYG-1/SYG-2 adhesion complex instructs synaptogenesis. Cell 156, 482-494. 10.1016/j.cell.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajcini K. V., Pomerantz J. H., Alkan O., Doyonnas R. and Blau H. M. (2008). Myoblasts and macrophages share molecular components that contribute to cell–cell fusion. J. Cell Biol. 180, 1005-1019. 10.1083/jcb.200707191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-Y., Yun Y., Lim J.-S., Kim M.-J., Kim S.-Y., Kim J.-E. and Kim I.-S. (2016). Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nat. Commun 7, 10871 10.1038/ncomms10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel F. B., Bernadskaya Y. Y., Chen E., Jobanputra A., Pooladi Z., Freeman K. L., Gally C., Mohler W. A. and Soto M. C. (2008). The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev. Biol. 324, 297-309. 10.1016/j.ydbio.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Vargas J., Krey T., Valansi C., Avinoam O., Haouz A., Jamin M., Raveh-Barak H., Podbilewicz B. and Rey F. A. (2014). Structural basis of eukaryotic cell–cell fusion. Cell 157, 407-419. 10.1016/j.cell.2014.02.020 [DOI] [PubMed] [Google Scholar]
- Pinello J. F., Lai A. L., Millet J. K., Cassidy-Hanley D., Freed J. H. and Clark T. G. (2017). Structure-function studies link class II viral fusogens with the ancestral gamete fusion protein HAP2. Curr. Biol. 27, 651-660. 10.1016/j.cub.2017.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbilewicz B., Leikina E., Sapir A., Valansi C., Suissa M., Shemer G. and Chernomordik L. V. (2006). The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev. Cell 11, 471-481. 10.1016/j.devcel.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Quinn M. E., Goh Q., Kurosaka M., Gamage D. G., Petrany M. J., Prasad V. and Millay D. P. (2017). Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 8, 15665 10.1038/ncomms15665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrianarison-Huetz V., Papaefthymiou A., Herledan G., Noviello C., Faradova U., Collard L., Pincini A., Schol E., Decaux J. F., Maire P. et al. (2018). Srf controls satellite cell fusion through the maintenance of actin architecture. J. Cell Biol. 217, 685-700. 10.1083/jcb.201705130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau A., Buttgereit D., Holz A., Fetter R., Doberstein S. K., Paululat A., Staudt N., Skeath J., Michelson A. M. and Renkawitz-Pohl R. (2001). rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development 128, 5061-5073. [DOI] [PubMed] [Google Scholar]
- Richardson B. E., Beckett K., Nowak S. J. and Baylies M. K. (2007). SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development 134, 4357-4367. 10.1242/dev.010678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin K., Yu S., Roy S. and Baylies M. K. (2010). Myoblast fusion: when it takes more to make one. Dev. Biol. 341, 66-83. 10.1016/j.ydbio.2009.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Coutts N., Price A., Taylor M. V. and Bate M. (2000). Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell 102, 189-198. 10.1016/S0092-8674(00)00024-6 [DOI] [PubMed] [Google Scholar]
- Sage P. T., Varghese L. M., Martinelli R., Sciuto T. E., Kamei M., Dvorak A. M., Springer T. A., Sharpe A. H. and Carman C. V. (2012). Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells. J. Immunol. 188, 3686-3699. 10.4049/jimmunol.1102594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas P. C. and Price S. R. (2005). Cadherins and catenins in synapse development. Curr. Opin. Neurobiol. 15, 73-80. 10.1016/j.conb.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Sapir A., Choi J., Leikina E., Avinoam O., Valansi C., Chernomordik L. V., Newman A. P. and Podbilewicz B. (2007). AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans. Dev. Cell 12, 683-698. 10.1016/j.devcel.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A., Avinoam O., Podbilewicz B. and Chernomordik L. V. (2008). Viral and developmental cell fusion mechanisms: conservation and divergence. Dev. Cell 14, 22-21. 10.1016/j.devcel.2007.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejter E. D. (2016). Myoblast fusion: Experimental systems and cellular mechanisms. Semin. Cell Dev. Biol. 60, 112-120. 10.1016/j.semcdb.2016.07.016 [DOI] [PubMed] [Google Scholar]
- Sens K. L., Zhang S., Jin P., Duan R., Zhang G., Luo F., Parachini L. and Chen E. H. (2010). An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J. Cell Biol. 191, 1013-1027. 10.1083/jcb.201006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton C., Kocherlakota K. S., Zhuang S. and Abmayr S. M. (2009). The immunoglobulin superfamily member Hbs functions redundantly with Sns in interactions between founder and fusion-competent myoblasts. Development 136, 1159-1168. 10.1242/dev.026302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilagardi K., Li S., Luo F., Marikar F., Duan R., Jin P., Kim J. H., Murnen K. and Chen E. H. (2013). Actin-propelled invasive membrane protrusions promote fusogenic protein engagement during cell–cell fusion. Science 340, 359-363. 10.1126/science.1234781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin N.-Y., Choi H., Neff L., Wu Y., Saito H., Ferguson S. M., De Camilli P. and Baron R. (2014). Dynamin and endocytosis are required for the fusion of osteoclasts and myoblasts. J. Cell Biol. 207, 73-89. 10.1083/jcb.201401137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurova K. and Podbilewicz B. (2016). RAB-5- and DYNAMIN-1-mediated endocytosis of EFF-1 fusogen controls cell–cell fusion. Cell Rep. 14, 1517-1527. 10.1016/j.celrep.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe J. C. and Griffiths G. M. (2007). Secretory mechanisms in cell-mediated cytotoxicity. Annu. Rev. Cell Dev. Biol. 23, 495-517. 10.1146/annurev.cellbio.23.090506.123521 [DOI] [PubMed] [Google Scholar]
- Stradal T. E. B. and Scita G. (2006). Protein complexes regulating Arp2/3-mediated actin assembly. Curr. Opin. Cell Biol. 18, 4-10. 10.1016/j.ceb.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Strünkelnberg M., Bonengel B., Moda L. M., Hertenstein A., de Couet H. G., Ramos R. G. and Fischbach K. F. (2001). rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development 128, 4229-4239. [DOI] [PubMed] [Google Scholar]
- Takenawa T. and Suetsugu S. (2007). The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37-48. 10.1038/nrm2069 [DOI] [PubMed] [Google Scholar]
- Ueda H., Morphew M. K., McIntosh J. R. and Davis M. M. (2011). CD4+ T-cell synapses involve multiple distinct stages. Proc. Natl. Acad. Sci. USA 108, 17099-17104. 10.1073/pnas.1113703108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valansi C., Moi D., Leikina E., Matveev E., Graña M., Chernomordik L. V., Romero H., Aguilar P. S. and Podbilewicz B. (2017). Arabidopsis HAP2/GCS1 is a gamete fusion protein homologous to somatic and viral fusogens. J. Cell Biol. 216, 571-581. 10.1083/jcb.201610093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyutina E., Martarelli B., Brakebusch C., Wende H. and Birchmeier C. (2009). The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc. Natl. Acad. Sci. USA 106, 8935-8940. 10.1073/pnas.0902501106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang Y., Li W.-J., Jiang Y., Zhu Z., Hu H., Li W., Wu J.-W., Wang Z.-X., Dong M.-Q. et al. (2017). Spectraplakin induces positive feedback between fusogens and the actin cytoskeleton to promote cell–cell fusion. Dev. Cell 41, 107-120.e4. 10.1016/j.devcel.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Zallen J. A., Cohen Y., Hudson A. M., Cooley L., Wieschaus E. and Schejter E. D. (2002). SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 156, 689-701. 10.1083/jcb.200109057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Vashisht A. A., O'Rourke J., Corbel S. Y., Moran R., Romero A., Miraglia L., Zhang J., Durrant E., Schmedt C. et al. (2017). The microprotein Minion controls cell fusion and muscle formation. Nat. Commun. 8, 15664 10.1038/ncomms15664 [DOI] [PMC free article] [PubMed] [Google Scholar]