Abstract
Background
The spinocerebellar ataxias (SCAs) are a group of autosomal dominant degenerative diseases characterized by cerebellar ataxia. Classified according to gene discovery, specific features of the SCAs – clinical, laboratorial, and neuroradiological (NR) – can facilitate establishing the diagnosis. The purpose of this study was to review the particular NR abnormalities in the main SCAs.
Methods
We conducted a literature search on this topic.
Results
The main NR characteristics of brain imaging (magnetic resonance imaging or computerized tomography) in SCAs were: (1) pure cerebellar atrophy; (2) cerebellar atrophy with other findings (e.g., pontine, olivopontocerebellar, spinal, cortical, or subcortical atrophy; “hot cross bun sign”, and demyelinating lesions); (3) selective cerebellar atrophy; (4) no cerebellar atrophy.
Discussion
The main NR abnormalities in the commonest SCAs, are not pathognomonic of any specific genotype, but can be helpful in limiting the diagnostic options. We are progressing to a better understanding of the SCAs, not only genetically, but also pathologically; NR is helpful in the challenge of diagnosing the specific genotype of SCA.
Keywords: Spinocerebellar ataxia, brain imaging, magnetic resonance imaging, ataxia, gait ataxia, cerebellar diseases
Introduction
The spinocerebellar ataxias (SCA) are a group of autosomal dominant degenerative diseases characterized by cerebellar ataxia. The most accepted classification of the SCAs is to name them according to the order of gene discovery, SCA 1–SCA 48. Although genetic testing is increasingly available, it is essential to know the specific features of the SCAs – clinical, laboratorial, and neuroradiological (NR) – to improve diagnostic accuracy, especially when genetic testing is not available. Neuroimaging cannot predict the genotype of any specific person with an SCA due to the overlap between symptomatology and macroscopic pathology; however, it is possible to cluster subgroups of SCAs, thereby limiting the diagnostic possibilities. Magnetic resonance imaging (MRI) and computed tomography (CT) are widely available, and can facilitate investigation of symptomatic cases. In the presymptomatic scenario, the relevance of neuroimaging is not yet widely accepted, although quantitative and functional imaging is becoming more and more useful in understanding the disease progression and its preclinical stage.1,2
The main findings in NR examinations are cortical cerebellar atrophy (known as “pure” cerebellar degeneration), spinal atrophy, or olivopontocerebellar atrophy. These are not characteristic of any subtype of SCA, but together with other information (specifically ethnicity, and extracerebellar motor and nonmotor symptoms and signs, including systemic findings), these studies can guide the genetic diagnosis. It is also of note that, within the same genotype, there may be some individuals showing atrophy presymptomatically and others with normal neuroimaging, despite the presence of symptoms3,4,5 (Table 1).
Table 1.
Main Neuroradiological (NR) Characteristics of Brain Imaging (Magnetic Resonance Imaging or Computerized Tomography) in the Spinocerebellar Ataxias (SCAs)
| NR Finding | SCAs |
|---|---|
| Pure cerebellar atrophy | 4, 5S, 6S, 10, 11, 13S, 14S, 15/16V, 18Mi, 19/22Mi, 21Mi, 24Mi,, 25–28, 29S,V, 30–32, 35, 37V, 38, 41Mi,V, 42V, 43Mo,V, 44, 47Mi,V |
| Cerebellar atrophy with other findings | |
| Pontine atrophy | 3, 7, 8S, 13Mo, 34, 40 |
| Olivopontocerebellar atrophy | 1, 2S, 36 |
| Spinal cord atrophy | 3, 7 |
| Cortical atrophy | 2, 3, 12 |
| Pontocerebellar, cortical, and subcortical atrophy | 17 (frontotemporal lobes, basal ganglia), 23S (frontotemporal lobes) |
| Atrophy of the brainstem, superior cerebellar peduncle, and abnormal signals in the brainstem, cerebellum, and thalamus | DRPLA |
| Calcifications of the dentate nuclei | 20Mo |
| “Hot cross bun sign” | 1, 2, 3, 6, 7, 8, 34 |
| Demyelinating lesions on brain MRI | 9 |
| Selective cerebellar atrophy | |
| Cerebellar vermis atrophy and hemosiderin deposits in the mesencephalon. | 45 |
| Posterior area of the vermis and paravermis | 48 |
| No cerebellar atrophy | |
| Generally normal, when present, mild cerebellar atrophy | 46 |
Source: Adapted from references 2,3,62
Abbreviations: V, Main Involvement of Vermis; S, Severe Cerebellar Atrophy; Mi, Mild Cerebellar Atrophy; Mo, Moderate Cerebellar Atrophy;
DRPLA, Dentatorubral-Pallidoluysian Atrophy; SCA, Spinocerebellar Ataxias; NR, Neuroradiological; MRI, Magnetic Resonance Imaging.
The most prevalent SCAs worldwide are 1, 2, 3, 6, 7, 10, and DRPLA. Genetic testing for these subtypes is currently widely available, but is often not economically accessible. The SCAs can be grouped into two major groups on the basis of their common genetic mechanisms: microsatellite repeat expansions and point mutations (e.g., SPBTN2 for SCA 5). The first group can be divided into those caused by polyglutamine coding CAG repeat expansion in the gene (e.g., ATXN1 for SCA 1, ATXN2 for SCA 2, ATXN3 for SCA 3 [Machado Joseph disease], CACNA1A for SCA 6, ATXN7 for SCA 7, ATN1 for DRPLA), and expansion of intronic repeats or molecular changes in other noncoding regions (e.g., ATXN10 for SCA 10). Further details are reviewed elsewhere.4,5–9
Atrophy of the cerebellum and pons worsens with disease progression; using diffusion tensor imaging (DTI) and fixel-based analysis (FBA), a novel tractography method, it was possible to detect cross-sectional microstructural alterations in patients, especially with FBA. These methodologies allow the use of volumetric markers in large studies, with more reproducible and reliable measurement procedures than simple scales.10
Some specific abnormalities, although uncommon, could help in identifying specific genotypes. These include calcifications of the dentate nucleus (SCA 20), signal abnormalities in the basal ganglia (SCAs 2 and 17), atrophy in the basal ganglia (SCAs 3 and 17), and severe atrophy of the pons (SCAs 2 and 7). If present, these features can be very helpful, but their absence does not exclude the diagnosis2 (Table 1).
Several sophisticated assessments using functional and quantitative imaging can also contribute to the diagnosis, although these are in more limited use (Table 2). Cerebellar nuclei can be visualized by susceptibility imaging, due to the paramagnetic property of iron, which is substantially present in these structures. T2-weighted images, susceptibility-weighted images (SWI), and quantitative susceptibility mapping (QSM, a more precise postprocessing method) are promising noninvasive methods of quantifying iron load in the cerebellar nuclei in the degenerative ataxias.3
Table 2.
Main Neuroimaging Modalities Available for Spinocerebellar Ataxias Evaluation
| Modality | Information Afforded | Clinical Utility | Current Availability |
|---|---|---|---|
| CT | Identification of gross brain abnormalities. | Useful to characterize the main partners of atrophy, but not specifically. | Widely available. |
| MRI | Identification of abnormalities with accuracy to gray matter, white matter, and cerebrospinal fluid. | Useful to characterize the main partners of atrophy and signal changes, specifically; more sensitive to subtle alterations. | Available in developed geographic regions. Limited availability in underdeveloped areas. |
| Three-dimensional (3D) true volumetric methods and voxel-based morphometry allow automated segmentation and comparison between groups, in both cross-sectional and longitudinal studies. | Limited to specialized research centers. | ||
| MRS | Identification and interpretation of altered metabolites concentration in specific areas. | Assess physiological function of the observable metabolites and their concentration changes. Specific areas should be assessed. | Limited to specialized centers. |
| fMRI | Analyses of perfusion-induced changes in image contrast, during performance of a task. | Color maps superimposed on morphological images allows visualizing both positive correlation (greater activation during task) and negative correlation (reduced activation during task). | Limited to specialized research centers. |
| FDG-PET | Regional brain glucose metabolism | Downstream marker of neuronal injury and neurodegeneration. Important for presymptomatic evaluation. | Limited to specialized centers. |
A systematic review of quantitative central nervous system MRI findings demonstrated that the cerebellar hemispheres and vermis, whole brainstem, midbrain, pons, medulla oblongata, cervical spine, striatum, and thalamus were significantly atrophied in SCA 3 (in the majority of studies). This article also emphasized the necessity of cross-sectional and prospective volumetric analysis, MR spectroscopy, DTI studies, and preclinical (presymptomatic) disease biomarker studies.11
Currently, there are no preventive or curative treatments for the SCAs, although emerging therapeutic strategies, mainly in the genetic field, are being developed and are under investigation. Thus neuroimaging biomarkers are very important to identify patients in the presymptomatic disease stages, with the aim of performing successful clinical trials of disease-modifying drugs (DMDs). Use of biomarkers can improve clinical trials in two ways: first, the preclinical patient is the target, and thus clinical scales are not applicable; and second, using biomarkers as primary outcome measures can enable reduction of the sample size of the study. It should be noted, however, that reduction of a biomarker measure does not necessarily correlate with a reduction in disease progression.6,12
The purpose of this study is to review the particular NR abnormalities in the main SCAs. The authors do not intend to review clinical, genetic and pathological evolution related to each one. The text presented here seeks to summarize the main NR observations in the commonest SCAs. Table 1 summarizes the findings of all SCAs already known.
SCA 1
Structural MRI typically shows olivopontocerebellar atrophy (OPCA) and white matter (WM) atrophy, less severe than in SCA 2. In VBM studies, there is atrophy of frontal and cerebellar hemispheric white matter surrounding the dentate nuclei.2,13 In one study using spectroscopy, middle cerebellar peduncle (MCP) and medulla oblongata signals were significantly decreased compared to asymptomatic correlates.14 The “hot cross bun sign” or “cross sign,” a typical but not pathognomonic sign of multiple system atrophy (MSA – Figure 1), can also be found in SCA 1.15,16 Another finding reported is midline hyperintensity in the basis pontis.15
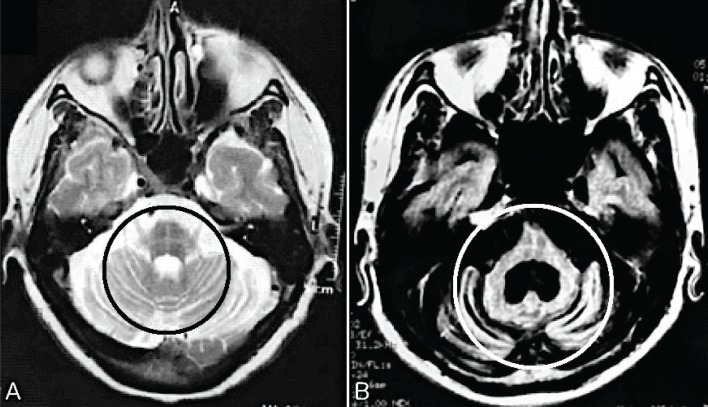
Figure 1.
The “Hot Cross Bun Sign” or “Cross Sign”, Typical but Not Pathognomonic Sign of Multiple System Atrophy (MSA), Can be Found in the Spinocerebellar Ataxias (SCAs): (A) a case of MSA and (B) SCA 2. Circles – T2 hyperintensity forms a cross on axial images through the pons, representing selective degeneration of pontocerebellar tracts. (A) Axial T2 image shows atrophy in the pons, bilateral middle cerebellar peduncle, and cerebellum. (B) Axial T2 FLAIR image shows severe atrophy in pons and cerebellum. A larger dilatation of the fourth ventricle is also noted.
Morphometric analyses of spinal cord (SC) area and eccentricity show significant reductions compared to controls; area reduction significantly correlates with scale for the assessment and rating of ataxia (SARA) scores, CAGn expansion, and disease duration in the multiple variable regression analysis.17
One study of supratentorial/spinal damage, in which the structural findings on MRI were correlated with the clinical features of the disease, demonstrated association of deep gray matter (amygdalae and red nuclei) atrophy with motor handicap (SARA) and poor cognition (memory) skills, and correlation of spinal cord atrophy with motor handicap. This study showed another interesting positive correlation; of red nuclei volumes and CAG repeat length. The authors identified that the spinal cord, lobule IX of the cerebellum, and brainstem WM tracts were the initial structures involved in SCA 1.18
SCA 2
As in SCA 1, but more severe, MRI in SCA 2 shows typical OPCA. In both VBM and subjective studies, there is WM atrophy of the pons, mesencephalon, the MCP, cerebellar hemispheric WM surrounding dentate nuclei, frontal, temporal, and precuneus lobes.2,13,19 The most prominent finding is the pontine atrophy, with flattening of the inferior part. The degree of OPCA correlates with clinical disability, but not with CAGn length.2,19 Again, the “hot cross bun sign” (Figure 1), the midline basis pontis, and basal ganglia hyperintensity can be found in SCA 2.2,15,20 In early disease stages, hypometabolism of glucose in the pontocerebellar area can be detected with positron emission tomography (PET), and later, frontotemporal atrophy and general ventricular enlargement can be visualized.21
In one study comparing preclinical and symptomatic SCA 2 mutation carriers, using semi-automated quantitative volumetry of the cerebellum and brainstem, atrophy was found in both the cerebellum and the pons, but was more pronounced in the latter. CAG repeats correlated with atrophy of the cerebellum, brainstem, pons, and age of onset. Pontine atrophy correlated with saccade velocities preclinically, with earlier age of onset, and cerebellar volume correlated with subjects’ ability to suppress responses to stimuli.22 Cognition is related to cerebellar atrophy in SCA 2: decrease in gray matter volume of posterior lobules correlated with visuospatial, verbal memory, and executive functions, while motor anterior and posterior lobule volume loss were correlated with motor and planning tasks.23
SCA 3
Also known as Machado-Joseph disease (MJD), SCA 3 is both the most common genotype of SCA worldwide and the most clinically heterogeneous. The clinical characteristics are much more important to the diagnostic approach than neuroimaging; the phenotype can vary from pure cerebellar ataxia to apparent hereditary neuropathy. Bulging eyes, facial myokymia, and dystonia are informative signs.24–27
VBM studies of presymptomatic patients demonstrate decreases in WM volume, mainly in the cerebellar peduncles and crus cerebri, and also volumetric reduction of the midbrain, spinal cord, and substantia nigra. These abnormalities began in the infratentorial structures, and evolved supratentorially with disease progression.28,29
Progression of the disease is accompanied by progressive intracranial atrophy, with the pathological process involving more structures. In early disease stages, atrophy of the cerebellar vermis and hemispheres, pons, brainstem, and the MCP are noted, although are less pronounced than in SCA 2. These alterations, mainly in structures close to the fourth ventricle, result in enlargement of the fourth ventricle as a common NR finding in SCA 3 (Figure 2). In addition to areas in common with SCA 2, other areas are also affected in SCA 3, specifically the caudate nucleus, putamen, and superior cerebellar peduncles. As the disease progresses, frontal and temporal lobes, and globus pallidus can be affected. As with SCA 1 and SCA 2, the “hot cross bun sign” in the pontine axial images can be observed (Figure 1).2,20,26,30–33
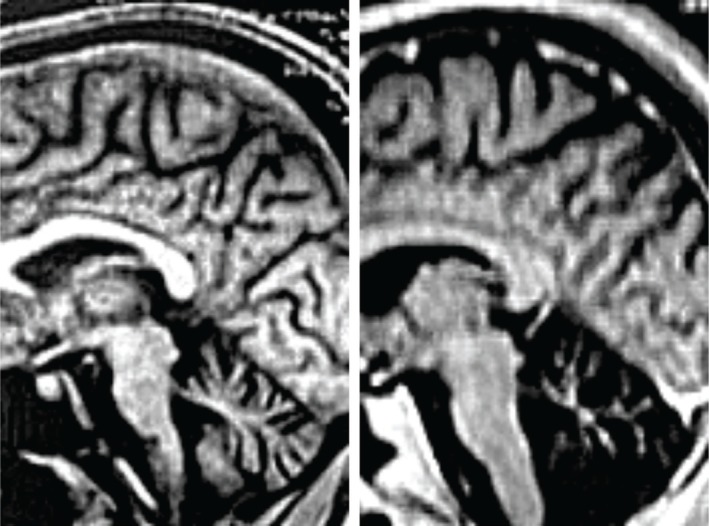
Figure 2.
Spinocerebellar Ataxia 3 (SCA 3): Initial And Advanced Stages of the Disease. Sagittal T1-weighted images of the brain in patient with SCA 3. Brain stem and cerebellum atrophy are clearly visible from the onset of clinical manifestations. With the evolution of the disease, there is an increase mainly in cortical and cerebellar atrophy.
Gait and limbic ataxia and posture instability, as measured by SARA, are inversely correlated to the brainstem and cerebellar hemispheric volumes, and spinal cord area.29,34 Dentate nuclei volumes are also reduced compared to controls, as measured by SWI. A correlation between CAGn expansion and SARA with the degree of atrophy of cerebellar cortex and nuclei has not yet been clearly documented.31,35,36
One VBM study of neocortical structures in MJD patients demonstrated decreased gray matter density in frontal, parietal, and temporal and occipital lobes. CAGn, disease duration, and age were significant factors in determining the degree of atrophy of these areas. Changes in these areas were important determinants of the final International Cooperative Ataxia Rating Scale (ICARS) score.37 This study did not demonstrate any differences in WM volumes; other authors, however, found significant losses of both gray and white matter in the cerebellar hemispheres, brainstem and in lateral thalamus, and the WM network, which strongly and positively correlated with SARA scores.38
SCA 6
SCA 6 is a late-onset slowly progressive pure cerebellar ataxia, mainly found in the people of Japanese ancestry. The main NR abnormalities are cortical cerebellar vermis and hemisphere atrophy, with less pronounced atrophy of pons, MCP, and red nucleus. No abnormalities of signal, SC and WM are found.32,39 The cerebellar volume, including the nuclei, is smaller when compared to SCA 3, while the brainstem is less atrophic than in SCA 3.31,40 The major previous studies regarding SCA 6 NR and its clinical features failed to demonstrate any significant correlation between clinical scores and volumetric abnormalities.32,39,40
A study of cognitive function in patients with SCA 6 demonstrated that the integrity of medial superior hemispheric regions is important in motor task performance as measured by several cognitive tests, while impairments in executive cognitive function are related to disease involvement of different inferior regions in the cerebellum.41
SCA 7
The hallmark of SCA 7 is visual loss due to macular degeneration (pigmentary retinal dystrophy), which leads to progressive and irreversible blindness. This is followed by slowly progressive cerebellar ataxia (typically at a younger age than in the other autosomal dominant cerebellar ataxias), in addition to other findings, such as cognitive impairment and ophthalmoplegia.42 The main NR findings in VBM studies are pontocerebellar atrophy, with greater involvement of the pons than in the other SCAs; cerebellar atrophy correlates with disease progression.43 SARA scores are inversely correlated to cerebellar volume, mainly due to atrophy of WM of the hemispheres, and also some cortical fields (parahippocampal gyrus, post- and precentral gyrus, cingulate gyrus, insula, inferior and medial frontal, parietal inferior, and parahippocampal and occipital cortices).44 A decrement in fractional anisotropy in other areas, including corona radiata, optical radiations, and occipital lobe WM, clearly indicates that neurodegeneration in SCA 7 extends beyond the cerebellum and the pons.45
SCA 10
SCA 10 was first described in Mexico, and in this region a typical feature of this type of SCA is epilepsy. In southern Brazil, however, this characteristic is absent, and the main phenotype is pure cerebellar ataxia.46 Additional symptoms such as ocular dyskinesia, pyramidal signs, cognitive impairment, and behavioral disturbances can be found.47–49 NR subjective imaging reveals predominant atrophy of the cerebellar hemispheres and the vermis (Figure 3).2,49
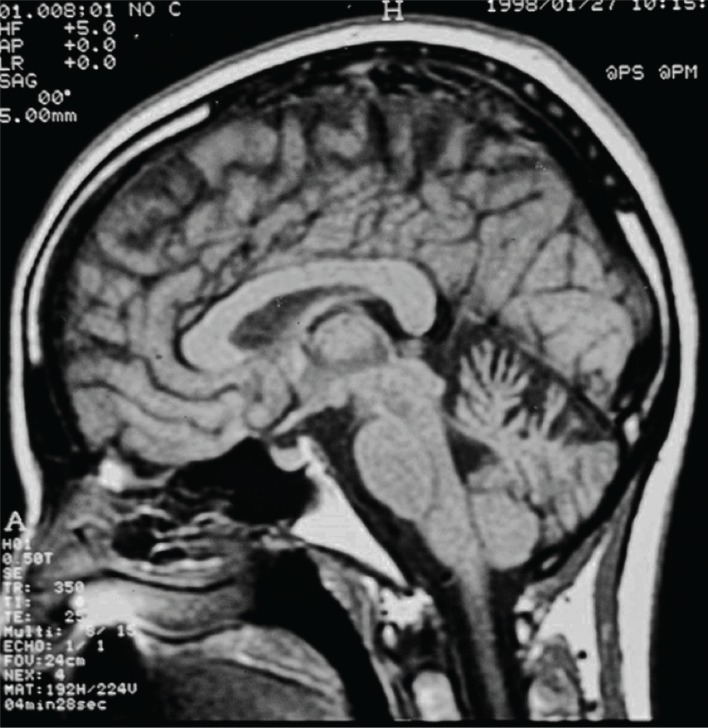
Figure 3.
Spinocerebellar Ataxia 10 (SCA 10) Image. Sagittal T1-weighted image of the brain in patient with SCA 10 showing cerebellar atrophy.
Dentatorubral-pallidoluysian atrophy
Dentatorubral-pallidoluysian atrophy (DRPLA) is also included in the group of autosomal dominant spinocerebellar ataxias, specifically when it presents with a choreoathetoid phenotype. This disease is divided into the ataxo-choreoathetoid phenotype (late-onset), the pseudo-Huntington phenotype, and the myoclonic epilepsy phenotype (infantile-onset). The choreoathetoid form is mainly characterized in the brain imaging by T2-weighted MRI showing subjective symmetrical high-signal lesions in the cerebral WM, globus pallidus, thalamus, midbrain, inferior olive, and pons. Atrophy of the cerebellum, superior cerebellar peduncle, and brainstem (Figure 4). The characteristics of the infantile and juvenile-onset are different from those cited, but are outside the scope of this article.50–52
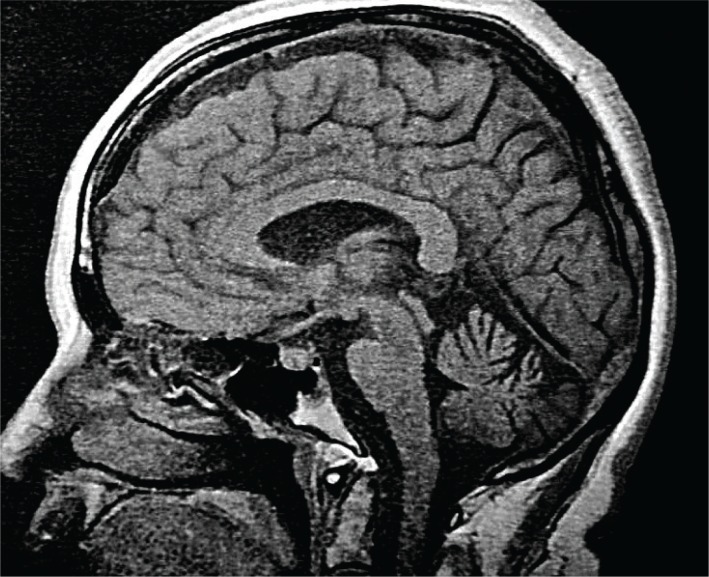
Figure 4.
Dentatorubral-pallidoluysian atrophy image. A T1-weighted midsagittal image of a patient with DRPLA shows atrophy of the brain stem and cerebellum.
Other SCAs
Although rare, some other specific SCAs have characteristics on subjective NR that should be noted. SCA 20 has three important hallmarks: dysphonia, palatal myoclonus (which correlates with olivary degeneration), and dentate calcification on CT scan. Cerebellar atrophy is another common finding.53 SCA 29 is an infantile-onset nonprogressive cerebellar ataxia, which causes hypotonia, gross motor delay, and mild-to-moderate cognitive impairment; on MRI, the main findings are nonspecific (cerebellar and pontine atrophy); however, one individual showed increased extra-axial fluid, possibly associated with cerebral atrophy.54 Patients with SCA 45 can have deposition of hemosiderin in the mesencephalon on brain MRI, in addition to atrophy of the cerebellar vermis.55
Advanced neuroimaging modalities
In a study of metabolite concentrations in the cerebellar vermis and pons of a cohort of patients with SCA 1, SCA 2, SCA 3, and SCA 7, patients were found to have lower concentrations of N-acetylaspartate and glutamate (neuronal loss/dysfunction), and elevated myoinositol (glial marker), compared to healthy controls. As in another polyglutamine disorder, Huntington’s disease, patients also showed elevated creatine. A strong correlation between SARA and neurometabolite levels was found in both cerebellar vermis and pons. The concentration of metabolites (N-acetylaspartate, glutamate, myoinositol, and total creatine) were able to distinguish SCA 2 and SCA 3, but not SCA 1 and SCA 7, from controls.56
Another study of spectroscopy correlated N-acetyl aspartate (NAA)/creatine (Cr) ratio with SARA score in patients with SCA 2, 3, and 6. The NAA/Cr ratio decreased with increasing age in patients, but this finding was not seen in controls. For SCA 2 and 3, NAA concentration in the vermis correlated with SARA, while for SCA 6 the score correlated with NAA of the right cerebellar hemisphere. In this study, the NAA concentration in the cerebellum was also correlated with CAGn and patient-reported age of onset. Because the NAA ratio was closely correlated with the SARA score, measurements of NAA could be used to predict the progression of SCA even when the CAG repeat number is controlled for. As NAA ratios correlate with the SARA score, NAA ratios could also be used as an indicator of the patient’s current disease severity.57
In a study using fluorine-18 fluorodeoxyglucose (18F-FDG) PET imaging of eight cerebellar regions, there was a significant decrease in the anterior/posterior ratio of 18F-FDG uptakes in patients with SCA (0.45) compared with controls (0.73). Glucose hypometabolism is preferentially decreased in the anterior rather than the posterior lobes, consistent with clinical observations.58 In another study, using dopamine transporter PET, the binding potential of [11C]d-threo-methylphenidate, which identifies dopamine terminal loss, was reduced in the striatum in SCA 2 and SCA 3, but without specific loss in the putamen, as is found in Parkinson’s disease. Cerebral glucose metabolism was decreased in the cerebellum (SCA 1, 2, 3, 6), brainstem (SCA 1, 2, 3), thalamus and putamen (SCA 3), parietal cortex (SCA 2), and was increased in the temporal cortex of all patients, mainly SCA 6.59
Functional MRI shows activation of the ventral dentate nucleus to be significantly higher than in controls in SCA 3 patients, with the implication that there is activation of pontocerebellar areas to compensate for the dysfunction of the spinocerebellum.31
SCA 36 is a late-onset, slowly progressive cerebellar ataxia syndrome, typically associated with sensorineural hearing loss. In one study, in which patients were assessed at asymptomatic, nonspecific symptomatic, and ataxic symptomatic stages (SARA ranged from 0 to 24.5), FDG-PET revealed hypometabolism in the asymptomatic stage in the cerebellum (vermis and right hemisphere). MRI was normal in asymptomatic and preataxic individuals. In the symptomatic stage, there was hypometabolism of both cerebellar hemispheres and brainstem.60 In SCA 38, FDG-PET studies in two patients showed selective hypometabolism of the cerebellar vermis and, to a lesser extent, of the cerebellar hemispheres.61
Table 2 summarizes the main neuroimaging modalities that are useful for evaluation of the SCAs, and their current utility and availability.
Conclusion
The main NR abnormalities (cerebellar, pontine, olivary, cortical, and spinal cord atrophy; demyelinating lesions; calcification of the dentate nuclei; “Hot cross bun sign”; hemosiderin deposits in the mesencephalon; signal abnormalities in white matter and basal ganglia) in the commonest SCAs are not pathognomonic of any specific genotype, but can be helpful in limiting the diagnostic options. Functional neuroimaging can be informative, and is increasingly used in clinical practice. The main findings with these studies are hypometabolism of the cerebellar structures; in addition, there can be loss of cerebral dopamine terminal (SCA 2 and 3). With further advances we will be able to develop NR biomarkers that will allow us to conduct clinical trials with DMD more effectively. We are progressing to a better understanding of the SCAs, not only genetically, but also pathologically; NR is helpful in the challenge of diagnosing the specific genotype of SCA.
Footnotes
Citation: Meira AT, Arruda WO, Ono SE, de Carvalho Neto A, Raskin S, Camargo CHF, et al. Neuroradiological Findings in the Spinocerebellar Ataxias. Tremor Other Hyperkinet Mov. 2019: 9. doi: 10.7916/tohm.v0.682
Editor: Ruth H. Walker, Mount Sinai School of Medicine, USA
Funding: None.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Arruda WO, Teive HA. Ataxias cerebelares hereditárias. Do martelo ao gen. Arq Neuropsiquiatr 1997;55(3 B):666–676. doi: 10.1590/S0004-282X1997000400027 [DOI] [PubMed] [Google Scholar]
- 2.Döhlinger S, Hauser T, Borkert J, Luft AR, Schulz JB. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum 2008;7(2):204–214. doi: 10.1007/s12311-008-0025-0 [DOI] [PubMed] [Google Scholar]
- 3.Deistung A, Stefanescu MR, Ernst TM, et al. . Structural and functional magnetic resonance imaging of the cerebellum: considerations for assessing cerebellar ataxias. Cerebellum 2016;15(1):21–25. doi: 10.1007/s12311-015-0738-9 [DOI] [PubMed] [Google Scholar]
- 4.Teive HAG, Ashizawa T. Primary and secondary ataxias. Curr Opin Neurol 2015;28:413–422. doi: 10.1097/WCO.0000000000000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9 [DOI] [PubMed] [Google Scholar]
- 6.Ashizawa T, Öz G, Paulson HL. Spinocerebellar ataxias: prospects and challenges for therapy development. Nat Rev Neurol 2018;14(10):590–605. doi: 10.1038/s41582-018-0051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klockgether T. The clinical diagnosis of autosomal dominant spinocerebellar ataxias. Cerebellum 2008;7(2):101–105. doi: 10.1007/s12311-008-0023-2 [DOI] [PubMed] [Google Scholar]
- 8.Giordano I, Harmuth F, Jacobi H, et al. . Clinical and genetic characteristics of sporadic adult-onset degenerative ataxia. Neurology 2017;89(10):1043–1049. doi: 10.1212/WNL.0000000000004311 [DOI] [PubMed] [Google Scholar]
- 9.Paulson HL, Shakkottai VG, Clark HB, Orr HT. Polyglutamine spinocerebellar ataxias-from genes to potential treatments. Nat Rev Neurosci 2017;18(10):613–626. doi: 10.1038/nrn.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adanyeguh IM, Perlbarg V, Henry PG, et al. . Autosomal dominant cerebellar ataxias: imaging biomarkers with high effect sizes. NeuroImage Clin 2018;19:858–867. doi: 10.1016/j.nicl.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaes A, Reckziegel E, Franca MC, et al. . MR imaging in spinocerebellar ataxias: a systematic review. Am J Neuroradiol 2016;37(8):1405–1412. doi: 10.3174/ajnr.A4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coarelli G, Brice A, Durr A. Recent advances in understanding dominant spinocerebellar ataxias from clinical and genetic points of view [version 1; peer review: 3 approved]. F1000Research 2018;7(F1000 Faculty Rev): 1781. doi: 10.12688/f1000research.15788.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel G, Pal PK, Ravishankar S, et al. . Gray matter volume deficits in spinocerebellar ataxia: an optimized voxel based morphometric study. Park Relat Disord 2011;17(7):521–527. doi: 10.1016/j.parkreldis.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 14.Mascalchi M, Tosetti M, Plasmati R, et al. . Proton magnetic resonance spectroscopy in an Italian family with spinocerebellar ataxia type 1. Ann Neurol 1998;43(2):244–252. doi: 10.1002/ana.410430215 [DOI] [PubMed] [Google Scholar]
- 15.Mandelli ML, de Simone T, Minati L, Bruzzone MG, Mariotti C, Fancellu R, et al. . Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. Am J Neuroradiol 2007;28(10):1996–2000. doi: 10.3174/ajnr.A0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namekawa M, Honda J, Shimazaki H. “Hot Cross Bun” sign associated with SCA1. Intern Med 2015;54(7):859–860. doi: 10.2169/internalmedicine.54.3460 [DOI] [PubMed] [Google Scholar]
- 17.Martins CR, Martinez ARM, de Rezende TJR, et al. . Spinal cord damage in spinocerebellar ataxia type 1. Cerebellum 2017;16(4):792–796. doi: 10.1007/s12311-017-0854-9 [DOI] [PubMed] [Google Scholar]
- 18.Martins Junior CR, Martinez ARM, Vasconcelos IF, et al. . Structural signature in SCA1: clinical correlates, determinants and natural history. J Neurol 2018;265(12):2949–2959. doi: 10.1007/s12311-017-0854-9 [DOI] [PubMed] [Google Scholar]
- 19.Giuffrida S, Saponara R, Restivo DA, et al. . Supratentorial atrophy in spinocerebellar ataxia type 2: MRI study of 20 patients. J Neurol 1999;246(5):383–388. doi: 10.1007/s004150050368 [DOI] [PubMed] [Google Scholar]
- 20.Bürk K, Skalej M, Dichgans J. Pontine MRI hyperintensities (“the cross sign”) are not pathognomonic for multiple system atrophy (MSA). Mov Disord 2001;16(3):535–535. [DOI] [PubMed] [Google Scholar]
- 21.Lastres-Becker I, Rüb U, Auburger G. Spinocerebellar ataxia 2 (SCA2). Cerebellum 2008;7(2):115–124. doi: 10.1007/s12311-008-0019-y [DOI] [PubMed] [Google Scholar]
- 22.Reetz K, Rodríguez-Labrada R, Dogan I, et al. . Brain atrophy measures in preclinical and manifest spinocerebellar ataxia type 2. Ann Clin Transl Neurol 2018;5(2):128–137. doi: 10.1002/acn3.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivito G, Lupo M, Iacobacci C, et al. . Structural cerebellar correlates of cognitive functions in spinocerebellar ataxia type 2. J Neurol 2018;265(3):597–606. doi: 10.1007/s00415-018-8738-6 [DOI] [PubMed] [Google Scholar]
- 24.Moro A, Munhoz RP, Arruda WO, Raskin S, Moscovich M, Teive HAG. Spinocerebellar ataxia type 3: subphenotypes in a cohort of brazilian patients. Arq Neuropsiquiatr 2014;72(9):659–662. doi: 10.1590/0004-282X20140129 [DOI] [PubMed] [Google Scholar]
- 25.Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology 1978;28(7):703–709. doi: 10.1212/WNL.28.7.703 [DOI] [PubMed] [Google Scholar]
- 26.Pedroso JL, Braga-Neto P, Radvany J, Barsottini OGP. Machado-Joseph disease in Brazil: from the first descriptions to the emergence as the most common spinocerebellar ataxia. Arq Neuropsiquiatr 2012;70(8):630–632. doi: 10.1590/S0004-282X2012000800013 [DOI] [PubMed] [Google Scholar]
- 27.Paulson H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb Clin Neurol 2012;103:437–449. doi: 10.1016/B978-0-444-51892-7.00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezende TJR, de Paiva JLR, Martinez ARM, et al. . Structural signature of SCA3: from presymptomatic to late disease stages. Ann Neurol 2018;84(3):401–408. doi: 10.1002/ana.25297 [DOI] [PubMed] [Google Scholar]
- 29.Fahl CN, Branco LMT, Bergo FPG, D’Abreu A, Lopes-Cendes I, França MC. Spinal cord damage in Machado-Joseph disease. The Cerebellum 2015;14(2):128–132. doi: 10.1007/s12311-014-0619-7 [DOI] [PubMed] [Google Scholar]
- 30.Klockgether T, Skalej M, Wedekind D, et al. . Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain 1998;121(9):1687–1693. doi: 10.1093/brain/121.9.1687 [DOI] [PubMed] [Google Scholar]
- 31.Stefanescu MR, Dohnalek M, Maderwald S, et al. . Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain 2015;138(5):1182–1197. doi: 10.1093/brain/awv064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukas C, Schöls L, Bellenberg B, et al. . Dissociation of grey and white matter reduction in spinocerebellar ataxia type 3 and 6: a voxel-based morphometry study. Neurosci Lett 2006;408(3):230–235. doi: 10.1016/j.neulet.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 33.Pedroso JL, Rivero RLM, Barsottini OGP " Hot cross bun" sign resembling multiple system atrophy in a patient with Machado-Joseph disease. Arq Neuropsiquiatr 2013;71(10):824. doi: 10.1590/0004-282X20130132 [DOI] [PubMed] [Google Scholar]
- 34.Jacobi H, Hauser TK, Giunti P, et al. . Spinocerebellar ataxia types 1, 2, 3 and 6: the clinical spectrum of ataxia and morphometric brainstem and cerebellar findings. Cerebellum 2012;11(1):155–166. doi: 10.1007/s12311-011-0292-z [DOI] [PubMed] [Google Scholar]
- 35.Huang SR, Wu Y Te, Jao CW, et al. . CAG repeat length does not associate with the rate of cerebellar degeneration in spinocerebellar ataxia type 3. NeuroImage Clin 2017;13:97–105. doi: 10.1016/j.nicl.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camargos ST, Marques W Jr, Santos AC. Brainstem and cerebellum volumetric analysis of Machado Joseph disease patients. Arq Neuropsiquiatr 2011;69(2B):292–296. doi: 10.1590/S0004-282X2011000300005 [DOI] [PubMed] [Google Scholar]
- 37.D’Abreu A, França MC, Yasuda CL, Campos BAG, Lopes-Cendes I, Cendes F. Neocortical atrophy in Machado-Joseph disease: a longitudinal neuroimaging study. J Neuroimaging 2012;22(3):285–291. doi: 10.1111/j.1552-6569.2011.00614.x [DOI] [PubMed] [Google Scholar]
- 38.Kang J-S, Klein JC, Baudrexel S, Deichmann R, Nolte D, Hilker R. White matter damage is related to ataxia severity in SCA3. J Neurol 2014;261(2):291–299. doi: 10.1007/s00415-013-7186-6 [DOI] [PubMed] [Google Scholar]
- 39.Lukas C, Hahn HK, Bellenberg B, et al. . Spinal cord atrophy in spinocerebellar ataxia type 3 and 6. J Neurol 2008;255(8):1244–1249. doi: 10.1007/s00415-008-0907-6 [DOI] [PubMed] [Google Scholar]
- 40.Eichler L, Bellenberg B, Hahn HK, Köster O, Schöls L, Lukas C. Quantitative assessment of brainstem and cerebellar atrophy in spinocerebellar ataxia types 3 and 6: impact on clinical status. Am J Neuroradiol 2011;32(5):890–897. doi: 10.3174/ajnr.A2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rentiya Z, Khan NS, Ergun E, Ying SH, Desmond JE. Distinct cerebellar regions related to motor and cognitive performance in SCA6 patients. Neuropsychologia 2017;107:25–30. doi: 10.1016/j.neuropsychologia.2017.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albuquerque MVC de, Pedroso JL, Braga Neto P, Barsottini OGP. Phenotype variability and early onset ataxia symptoms in spinocerebellar ataxia type 7: comparison and correlation with other spinocerebellar ataxias. Arq Neuropsiquiatr 2015;73(1):18–21. doi: 10.1590/0004-282X20140192 [DOI] [PubMed] [Google Scholar]
- 43.Bang OY, Lee PH, Kim SY, Kim HJ, Huh K. Pontine atrophy precedes cerebellar degeneration in spinocerebellar ataxia 7: MRI-based volumetric analysis. J Neurol Neurosurg Psychiatry 2004;75(10):1452–1456. doi: 10.1136/jnnp.2003.029819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez-Castillo CR, Galvez V, Diaz R, Fernandez-Ruiz J. Specific cerebellar and cortical degeneration correlates with ataxia severity in spinocerebellar ataxia type 7. Brain Imaging Behav 2016;10(1):252–257. doi: 10.1007/s11682-015-9389-1 [DOI] [PubMed] [Google Scholar]
- 45.Alcauter S, Barrios FA, Diaz R, Fernandez-Ruiz J. Gray and white matter alterations in spinocerebellar ataxia type 7: an in vivo DTI and VBM study. Neuroimage 2011;55(1):1–7. doi: 10.1016/j.neuroimage.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 46.Teive HAG, Arruda WO, Raskin S, Ashizawa T, Werneck LC. The history of spinocerebellar ataxia type 10 in Brazil: travels of a gene. Arq Neuropsiquiatr 2007;65(4A):965–968. doi: 10.1590/S0004-282X2007000600008 [DOI] [PubMed] [Google Scholar]
- 47.Moro A, Munhoz RP, Moscovich M, et al. . Nonmotor symptoms in patients with spinocerebellar ataxia type 10. Cerebellum 2017;16(5–6):938–944. doi: 10.1007/s12311-017-0869-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moro A, Afonso H, Teive G. Cognitive impairment in Spinocerebellar ataxia type 10. Dement Neuropsychol 2016;10(4):310–314. doi: 10.1590/s1980-5764-2016dn1004009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen A, Matsuura T, Ruano L, et al. . Clinical and genetic analysis of four Mexican families with spinocerebellar ataxia type 10. Ann Neurol 2001;50(2):234–239. doi: 10.1002/ana.1081 [DOI] [PubMed] [Google Scholar]
- 50.Uyama E, Kondo I, Uchino M, Fukushima T, Murayama N, Kuwano A, et al. . Dentatorubral-pallidoluysian atrophy (DRPLA): clinical, genetic, and neuroradiologic studies in a family. J Neurol Sci 1995;130(2):146–153. doi: 10.1016/0022-510X(95)00019-X [DOI] [PubMed] [Google Scholar]
- 51.Kin T, Hirano M, Taoka T, Yamada M, Mizutani T, Oyanagi K. Global and region-specific analyses of apparent diffusion coefficient in dentatorubral-pallidoluysian atrophy. Am J Neuroradiol 2011;27(7):1463–1436. [PMC free article] [PubMed] [Google Scholar]
- 52.Sugiyama A, Sato N, Nakata Y, et al. . Clinical and magnetic resonance imaging features of elderly onset dentatorubral–pallidoluysian atrophy. J Neurol 2018;265(2):322–329. doi: 10.1007/s00415-017-8705-7 [DOI] [PubMed] [Google Scholar]
- 53.Knight MA, Gardner RJMK, Bahlo M, et al. . Dominantly inherited ataxia and dysphonia with dentate calcification: spinocerebellar ataxia type 20. Brain 2014;127(5):1172–1181. doi: 10.1093/brain/awh139 [DOI] [PubMed] [Google Scholar]
- 54.Zambonin JL, Bellomo A, Ben-Pazi H, et al. . Spinocerebellar ataxia type 29 due to mutations in ITPR1: a case series and review of this emerging congenital ataxia. Orphanet J Rare Dis 2017;12(1):121. doi: 10.1186/s13023-017-0672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nibbeling EAR, Duarri A, Verschuuren-Bemelmans CC, et al. . Exome sequencing and network analysis identifies shared mechanisms underlying spinocerebellar ataxia. Brain 2017;140(11):2860–2878. doi: 10.1093/brain/awx251 [DOI] [PubMed] [Google Scholar]
- 56.Adanyeguh IM, Henry P-G, Nguyen TM, et al. . In vivo neurometabolic profiling in patients with spinocerebellar ataxia types 1, 2, 3, and 7. Mov Disord 2015;30(5):662–670. doi: 10.1002/mds.26181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang P-S, Chen H-C, Wu H-M, Lirng J-F, Wu Y-T, Soong B-W. Association between proton magnetic resonance spectroscopy measurements and CAG repeat number in patients with spinocerebellar ataxias 2, 3, or 6. Dermaut B, editor. PLoS One 2012;7(10):e47479. doi: 10.1371/journal.pone.0047479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh JS, Oh M, Chung SJ, Kim JS. Cerebellum-specific 18F-FDG PET analysis for the detection of subregional glucose metabolism changes in spinocerebellar ataxia. Neuroreport 2014;25(15):1198–1202. doi: 10.1097/WNR.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 59.Wüllner U, Reimold M, Abele M, et al. . Dopamine transporter positron emission tomography in spinocerebellar ataxias type 1, 2, 3, and 6. Arch Neurol 2005;62(8):1280–1285. doi: 10.1001/archneur.62.8.1280 [DOI] [PubMed] [Google Scholar]
- 60.Aguiar P, Pardo J, Arias M, et al. . PET and MRI detection of early and progressive neurodegeneration in spinocerebellar ataxia type 36. Mov Disord 2017;32(2):264–273. doi: 10.1002/mds.26854 [DOI] [PubMed] [Google Scholar]
- 61.Borroni B, Di Gregorio E, Orsi L, et al. . Clinical and neuroradiological features of spinocerebellar ataxia 38 (SCA38). Parkinsonism Relat Disord 2016;28:80–86. doi: 10.1016/j.parkreldis.2016.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minati L, Grisoli M, Bruzzone MG. MR spectroscopy, functional MRI, and diffusion-tensor imaging in the aging braIn: a conceptual review. J Geriatr Psychiatry Neurol 2007;20:3–21. doi: 10.1177/0891988706297089 [DOI] [PubMed] [Google Scholar]