Abstract
Allogeneic hematopoietic stem cell transplantation was until very recently, the only permanent curative option available for patients suffering from transfusion-dependent beta-thalassemia. Gene therapy, by autologous transplantation of genetically modified hematopoietic stem cells, currently represents a novel therapeutic promise, after many years of extensive preclinical research for the optimization of gene transfer protocols. Nowadays, clinical trials being held on a worldwide setting, have demonstrated that, by re-establishing effective hemoglobin production, patients may be rendered transfusion- and chelation-independent and evade the immunological complications that normally accompany allogeneic hematopoietic stem cell transplantation. The present review will offer a retrospective scope of the long way paved towards successful implementation of gene therapy for beta-thalassemia, and will pinpoint the latest strategies employed to increase globin expression that extend beyond the classic transgene addition perspective. A thorough search was performed using Pubmed in order to identify studies that provide a proof of principle on the aforementioned topic at a preclinical and clinical level. Inclusion criteria also regarded gene transfer technologies of the past two decades, as well as publications outlining the pitfalls that precluded earlier successful implementation of gene therapy for beta-thalassemia. Overall, after decades of research, that included both successes and pitfalls, the path towards a permanent, donor-irrespective cure for beta-thalassemia patients is steadily becoming a realistic approach.
Keywords: gene therapy, gene editing, thalassemia, mobilization, viral vectors, clinical trials, hematopoietic stem cells
Introduction
Beta-thalassemias are hereditary anemias that are caused by the absent or insufficient production of the beta-hemoglobin chain1 and constitute the most common monogenic disease with 270 million heterozygotes worldwide.2 The prevalence of beta-thalassemias was primarily favored in tropical and subtropical regions due to resistance against malaria incurred in the individuals carrying the pathological alleles. However, migration of populations, as well as implementation of effective prevention programs, have changed the epidemiological map for these syndromes.3
Without treatment, the severe form of the disease, known as beta-thalassemia major or Cooley’s anemia, is lethal within the first decade of life.4 The standard of care for these patients comprises lifelong transfusion therapy combined with pharmacological chelation to curb iron accumulation1,4 that substantially extends their life expectancy, if strictly followed.5 However, this lifelong treatment constitutes an unaffordable financial burden for many national economies and it severely compromises the quality of patients’ life, commonly resulting in treatment non-compliance and vital organ sequelae.6 Indeed, the major cause of death in these patients, following sub-optimal iron chelation, is cardiac failure due to secondary hemochromatosis.6 Older patients may be exposed to a higher risk of hepatocarcinoma.7
The traditional curative treatment is allogeneic bone marrow transplantation from a matched related donor. Nevertheless, significant drawbacks are associated with its implementation, including limited availability of major histocompatibility complex (MHC)-matched donors, the need for long-term immunosuppression, narrow application to the youngest patients and increased risk of immunological complications, as well as non-rejection mortality in older subjects with organ damage.8,9 This is because age-associated extramedullary hematopoiesis, chronic ineffective erythropoiesis and iron accumulation, may indeed adversely affect engraftment of the hematopoietic stem cell compartment. Moreover, transplants from alternative donors, such as matched unrelated or haploidentical donors, do not represent an approach devoid of complications, since they are associated with significantly lower disease-free survival and higher morbidity and mortality.10,11
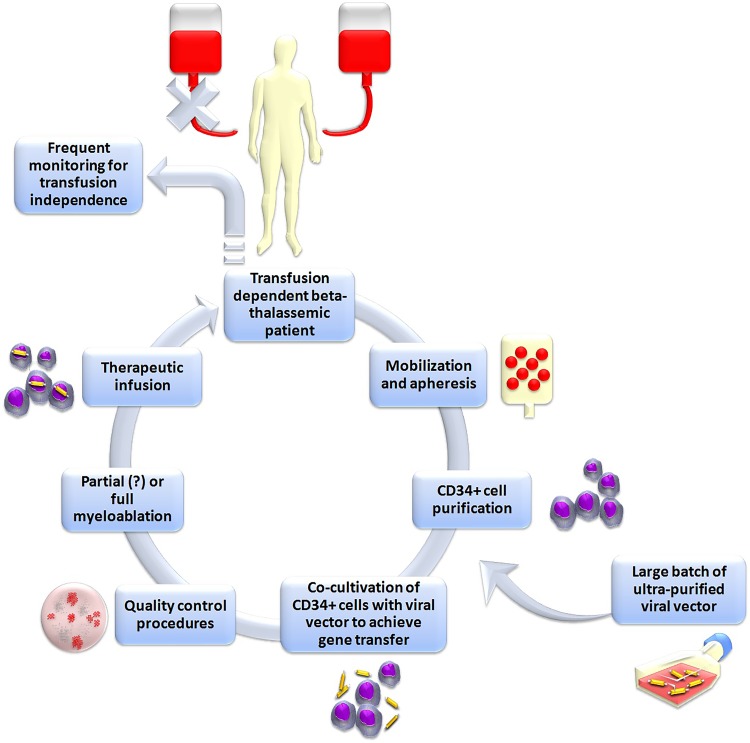
The goal of gene therapy for the treatment of beta-thalassemia is to achieve stable introduction of functional globin genes into the patient’s own hematopoietic stem cells (HSCs) in order to correct ineffective erythropoiesis and hemolytic anemia, thus obviating the need for transfusion12 (Figure 1). This was anticipated to offer a curative potential to those who could not undergo allogeneic transplantation or were lacking an MHC-compatible donor, thus avoiding the immunological risks of allogeneic hematopoietic stem cell transplantation (allo-HSCT) and without requiring immune suppression to avert them. Indeed, genetic treatment of beta-thalassemia has been very early singled out as one of the most promising areas for future gene therapies by the American Society of Gene and Cell Therapy.13
Figure 1.
Schematic representation of the classic gene addition protocol for the gene therapy of beta-thalassemia. In brief, Plerixafor+granulocyte-colony stimulating factor (G-CSF) is administered to the patient in order to mobilize hematopoietic stem cells into peripheral blood. Peripheral blood mononuclear cells are then collected with leukapheresis and enriched in CD34+ hematopoietic stem cells. These cells are co-cultured with a viral vector designed to express normal human beta-globin, followed by quality control. Ultimately, the patient is subjected to myelosuppression and then engrafted with the gene-corrected cells.
The two major hurdles posed for the implementation of safe and effective stem cell gene therapy in beta-thalassemia were firstly, to safely collect enough HSCs (the so-called CD34+ cells) and secondly, to transduce patients’ CD34+ HSCs at potentially therapeutic levels.14,15 Infusion of insufficient cell doses or poorly transduced cells would place the patient at risk of graft failure or futile infusion. These concerns were particularly highlighted in the case of gene therapy for beta-thalassemia, which requires complex tissue-specific vectors, that result in lower titers than smaller, cDNA-encoding vectors.16,17 Furthermore, adult subjects were the first to be recruited in these trials. This stood in contrast to the primarily pediatric subjects treated for metabolic disorders and severe immunodeficiencies, where the young age, the absence of potential medullary damage caused by iron accumulation and the proliferative advantage of genetically modified progenitors, were most favorable circumstances for HSC engraftment.18 Another issue that had to be addressed in the context of gene therapy for beta-thalassemia was the risk of insertional oncogenesis,19 as well as limited efficacy in certain cases, owing to non-myeloablative conditioning.20
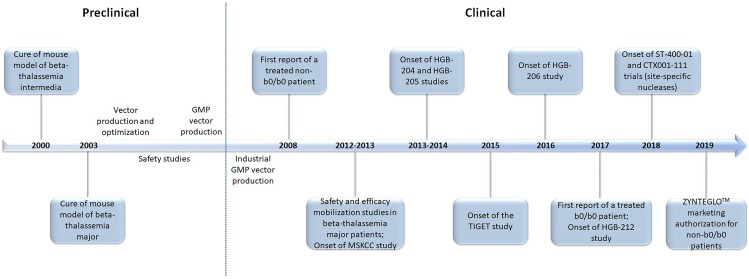
This study will retrospectively review the successes and pitfalls of the last two decades in the field of gene therapy for beta-thalassemia. Finally, it will discuss the most recent advances that extend beyond the classic gene addition protocols harnessing the semi-random integration of the transgene, such as in situ gene editing for the reactivation of the endogenous fetal hemoglobin, or gene addition in safe harbor genomic loci. A graphic timeline of the latest milestones for the gene therapy of beta-thalassemia is presented in Figure 2.
Figure 2.
The milestones of gene therapy for beta-thalassemia: a timeline of the last two decades.
Major Hurdle 1: Safe Collection Of Sufficient Numbers Of HSCs
The earliest assumption towards a molecular therapy for beta-thalassemia was the ex vivo gene addition strategy: target cells with a repopulating capacity, such as HSCs, are isolated from the patient and co-cultured with a viral vector carrying the therapeutic gene. These genetically modified cells are then reintroduced as a phenotypically functional graft into the patient from whom they were initially harvested and repopulate the bone marrow. To do so, a preparative conditioning regimen would have to be applied, in order to provide enough niches for engraftment of the gene-modified cells over the uncorrected endogenous ones21 (Figure 1).
It is well-established that the genetically modified cells lack a selective advantage at the level of stem/early progenitor cells in beta-thalassemia patients.14 Hence, large numbers of highly engraftable transduced CD34+ cells, need to be infused in order to achieve successful bone marrow reconstitution.22 The need for harvesting large numbers of HSCs from the patients is further intensified because a backup of unmodified CD34+ cells is additionally required, for rescue in case of engraftment failure.
Nowadays, peripheral blood-mobilized HSCs represent the preferable source for many autologous and allogeneic transplantation approaches.23 This is because this graft source provides, under a minimally invasive procedure, higher numbers of HSCs compared to conventional bone marrow harvest.24 Plerixafor, a most recent mobilizing agent, when used in combination with granulocyte-colony stimulating factor (G-CSF), results in rapid mobilization, within hours following administration,25 and exhibits a marked synergism, hence increasing CD34+ cell yields by several fold.26
Until recently, there was little information about the safety and efficacy of mobilization in adult patients with beta-thalassemia. The peculiarity of HSC mobilization in the context of beta-thalassemia mostly lied on the extensive extramedullary hematopoiesis that results in splenomegaly, as well as the hypercoagulability observed in these patients. Although G-CSF is known to be generally well-tolerated, the rare events of splenic rupture27,28 or thrombosis29 during mobilization in normal donors or patients with hematologic malignancies raised concerns for its safety in thalassemia.
Based on the results of two mobilization trials in patients with thalassemia,30–32 as well as preclinical studies in mice,33,34 the group of Yannaki suggested Plerixafor+G-CSF-mobilized CD34+ cells as the optimal graft source for thalassemia gene therapy. The two clinical trials (Figure 2) assessed the safety and efficacy of HSC mobilization, using a range of available agents (Hydroxyurea+G-CSF, G-CSF-alone, Plerixafor-alone, Plerixafor+G-CSF) in 40 beta-thalassemic adults. It was demonstrated that the combined treatment of Plerixafor+G-CSF leads to high yields of CD34+ cells in a single apheresis procedure, despite the mandatory dose reductions of G-CSF that were applied to splenectomized patients to avoid hyperleukocytosis. The Plerixafor+G-CSF-primed CD34+ cells were chosen as the optimal graft for thalassemia gene therapy because they provided, after lentiviral vector-driven genetic correction, increased beta-globin expression per vector copy and enhanced early human chimerism under non-myeloablative conditions in xenografts, over the differently mobilized cells.34 The superiority in engraftment of the Plerixafor+G-CSF CD34+ cell graft was probably associated with enrichment in cells bearing a more primitive phenotype.31 Ever since, Plerixafor+G-CSF mobilization has been adopted in all clinical gene therapy trials for beta-thalassemia.
Major Hurdle 2: Transduction Of Patients’ HSCs At Potentially Therapeutic Levels
The onset of the transfer of gene therapy for beta-thalassemia from the bench to the bedside was noted in two early clinical trials; in Paris, led by Philippe Leboulch, and New York, led by Michel Sadelain (Table 2). In the Paris trial, transfusion independence provided by infusion of gene-corrected autologous HSCs in one fully myeloablated patient, essentially reflected an HMGA2 dominant clone contribution to vector-derived erythropoiesis combined with the unusual activation of fetal hemoglobin post-transplant.19 Fortunately, the dominant clone did not progress to leukemic transformation and gradually decreased. Currently, it contributes for less than 10% of the circulating nucleated cells and the patient requires scarce transfusions.35 Similar in vivo gene transfer rates in the New York trial, reached with partial myeloablation, did not provide a therapeutic benefit to the patients.20 Although these trials demonstrated the feasibility and tolerability of gene therapy for beta-thalassemia, they also revealed safety and efficacy issues that needed to be addressed.
Table 2.
List Of The Clinical Trials For Beta-Thalassemia Conducted To Date
| Identifier And Phase | Sponsor | Country | Start Date | Vector | No Of Treated Patients And Age | Genotype | Graft Source | Conditioning | Administration Route | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| LG001 study Phase I/II | Bluebird Bio | France | September 2006 | HPV569 | 3 18–22 yrs |
Non-b0/b0 | BM, mPB(Plrxfr+G-CSF) | Busulfan 12.8 mg/kg | Intravenous | 1 transfusion independent patient with HMGA2 clonal dominance that regressed over years |
|
NCT01639690 Phase I |
MSKCC | USA | July 2012 | TNS9.3.55 | 4 18–23 yrs |
Non-b0/b0, b0/b0 |
mPB(G-CSF) | Busulfan 8 mg/kg | Intravenous | Decrease in transfusion requirements; this trial is currently suspended and has been amended for higher intensity conditioning |
| NCT01745120 HGB-204 Phase I/II | Bluebird Bio | Global | August 2013 | BB305 | 18 12–35 yrs |
Non-b0/b0 (n=10), b0/b0 (n=8) | mPB(Plrxfr+G-CSF) | Busulfan 12.8 mg/kg | Intravenous | 8 non-b0/b0 and 3 b0/b0 patients transfusion independent |
| NCT02151526 HGB-205 Phase I/II | Bluebird Bio | France | August 2013 | BB305 | 4 16–18 yrs |
Non-b0/b0 (n=3), b0/b0 [IVS1-110 homozygous] (n=1) | mPB(Plrxfr+G-CSF) | Busulfan 12.8 mg/kg | Intravenous | 4 transfusion independent patients |
| NCT02453477 TIGET-BTHAL Phase I/II | Telethon Foundation | Italy | May 2015 | GLOBE | 9 6–35 yrs |
Non-b0/b0 (n=7), b0/b0 (n=2) |
mPB(Plrxfr+G-CSF) | 42 g/m2 Treosulfan and 8 mg/kg thiotepa | Intra-BM | Reduced transfusion requirements in adults; 3 pediatric participants (1 b0/b0) transfusion independent |
| NCT02906202 HGB-207 Phase III | Bluebird Bio | Global | July 2016 | BB305 | 20 8–34 yrs |
Non-b0/b0 | mPB(Plrxfr+G-CSF) | Busulfan 12.8 mg/kg | Intravenous | 17 transfusion independent patients |
| NCT03207009 HGB-212 Phase III | Bluebird Bio | Global | June 2017 | BB305 | 11 ≤50 yrs | b0/b0 or IVS1-110 homozygous | mPB(Plrxfr+G-CSF) | Busulfan 12.8 mg/kg | Intravenous | 6 transfusion independent patients |
|
NCT03432364 ST-400-01 Phase I/II |
Sangamo Therapeutics/ Bioverativ Therapeutics Inc |
USA | March 2018 | ZFN (BCL11A enhancer) |
Est. 6 18–40 yrs |
TDT | mPB | Busulfan | N/A | 1 b0/b0 patient possibly transfusion independent 7 weeks post therapy |
|
NCT03655678 CTX001-111 Phase I/II |
Vertex Pharmaceuticals Inc/CRISPR Therapeutics |
Canada, Germany, UK | September 2018 | CRISPR/Cas9 (BCL11A enhancer) |
Est. 12 18–35 yrs |
Non-b0/b0 | N/A | Busulfan myeloablative | Intravenous | Currently recruiting |
Abbreviations: BM, bone marrow; MSKCC, Memorial Sloan-Kettering Cancer Center; mPB, mobilized peripheral blood; G-CSF, granulocyte-colony stimulating factor; Plrxfr, plerixafor; ZFN, zinc-finger nuclease; Est, estimate; TDT, transfusion-dependent beta-thalassemia; N/A, not applicable.
Efficacy Of Globin Vectors
Despite highly successful transduction of HSCs in gene therapy of immune deficiencies36 or lysosomal storage diseases,37 allowing for recent marketing authorization of specific gene therapy products,12 efficient globin gene transfer to HSCs was proved a challenge, mostly due to the traditionally low titers of the vectors utilized. Globin vectors need to possess extremely high transcriptional efficiency in order to be therapeutic, an issue that was initially addressed by the incorporation of elements of the beta-globin locus control region (LCR), at the expense, however, of a severe compromise of vector titers due to the substantial length of the micro-LCR cassettes.16,38
The group of Sadelain was the first to demonstrate successful gene therapy in murine models of beta-thalassemia intermedia and major, with a lentiviral vector which incorporated the human beta-globin promoter and regulatory elements to achieve erythroid-limited, developmental stage-specific globin expression (Figure 2).39–41 Extensive studies were followed, focusing on the design of a lentiviral vector encoding a unique combination of the globin gene/promoter/enhancer/LCR that was finally termed TNS9 (Table 1), which corrected anemia in beta-thalassemic mice.39–42 Several groups afterwards confirmed and enriched these findings in thalassemia models using various lentiviral vectors that encoded beta, gamma or mutated beta-globin genes.43–47 However, both gene transfer efficiency and titers remained suboptimal and consequently, full myeloblation was considered necessary to reach clinically relevant levels of engraftment. Additionally, the large vector production batches needed for the procedure, associated with high costs, hampered effective implementation of gene therapy for beta-thalassemia for many years.
Table 1.
Comparison Of The Vector Constructs Currently Utilized In Approved Clinical Trials For Beta-Thalassemia
| Vector | SIN | Insulator | Ψ | cPPT/RRE Elements | 3ʹ beta-globin Enhancer | Beta-globin Exon 3, Intron 2, Exon 2, Intron 1, Exon 1 | Beta-globin Promoter | LCR |
|---|---|---|---|---|---|---|---|---|
| TNS9.3.55 | Yes | No | Yes | Yes | Yes | Yes (372 bp segmental deletion of Intron 2) | Yes (615 bp) | Yes (HS2, HS3, HS4), 3.2 kb |
| TNS9.3.55.A1 | Yes | Yes (A1) | Yes | Yes | Yes | Yes (372 bp segmental deletion of Intron 2) | Yes (615 bp) | Yes (HS2, HS3, HS4), 3.2 kb |
| HPV569 | Yes | Yes (cHS4) | Yes | Yes | Yes | Yes (372 bp segmental deletion of Intron 2, betaT87Q in Exon 2) | Yes (265 bp) | Yes (HS2, HS3, HS4), 2.7 kb |
| BB305 | Yes | No | Yes | Yes | Yes | Yes (372 bp segmental deletion of Intron 2, betaT87Q in Exon 2) | Yes (265 bp) | Yes (HS2, HS3, HS4), 2.7 kb |
| GLOBE | Yes | No | Yes | Yes | Yes | Yes (372 bp segmental deletion of Intron 2) | Yes (265 bp) | Yes (HS2, HS3), 2.7 kb |
Abbreviations: LTR, long terminal repeat; Ψ, Psi sequence; RRE, Rev responsive element; cPPT, Central polypurine tract; HS, DNAseI hypersensitive site; bp, Base pair; kb, Kilobase; cHS4, chicken HS4 region insulator; bT87Q, b-globin T87Q mutation, which confers additional anti-sickling activity to the beta-chain.
The first vector ever reaching the clinic was the SIN-LentiGlobin HPV569,19 with two copies of the 250 bp core elements of the chicken beta-globin chromatin insulator (cHS4) flanking the LCR and an anti-sickling beta-globin transgene (Table 1). This transgene was utilized to discriminate the contribution of the vector to erythropoiesis, over any residual endogenous or transfusion-derived beta-globin expression. Three b0/bΕ thalassemic patients participated, after a full myeloablative conditioning. Aside from the sole patient who was rendered transfusion-independent, the two other individuals failed to benefit from the therapy. One patient had to receive his unmanipulated back-up cells due to engraftment failure of the gene-modified cells and long-lasting aplasia, while the other was presented with low gene marking in vivo and remained transfusion-dependent. Moreover, there was a strong belief at the time that even this one patient wouldn’t have been treated without the concurrent events of the dominant clone emergence and fetal hemoglobin activation.
On the other hand, the group of Sadelain, in the trial conducted in New York, made use of the TNS9.3.55 vector,17 which was an incremental version of the prototypical TNS9, and bore an uninsulated LCR with the wildtype beta-globin gene. Four b0/b+ thalassemic patients received their gene-corrected G-CSF-mobilized CD34+ cell grafts, after a reduced intensity conditioning with 8 mg/kg Busulfan. Although initially the patients were presented with increasing intervals between transfusions, they eventually remained transfusion-dependent, strongly indicating that partial myeloablation is not a realistic approach for engraftment of gene-modified cells in beta-thalassemia.
The above findings from the two trials also raised the concern that even higher in vivo gene transfer rates would be needed to ensure that the b0/b0 genotype would bear a chance to be cured, even after a full myeloablative conditioning. This is because non-b0/b0 genotypes are presented with residual endogenous globin production, which, if combined with transgenic beta-globin expression, it would be easier to reach transfusion independence.
Preparative Conditioning
The peculiarity of beta-thalassemia lies on the extremely expanded bone marrow that essentially hampers effective engraftment of gene-corrected HSCs, which, importantly, provide a selective advantage only at the level of erythroblasts and erythrocytes.22 Hence, the truly capable for long-term hematologic reconstitution genetically modified CD34+ cells, would have to be exposed in particularly competitive niche conditions upon transplantation.
Since beta-thalassemia is a non-malignant disease that requires complete elimination of endogenous HSCs to confer therapy, it was initially debated whether a partial or full myeloablation would provide the optimal balance of adequate vector-derived hematologic reconstitution, along with the minimum possible peri-transplant morbidity and mortality. Supporters of the former opinion stated that, to counterbalance the low engraftment expected from partially myeloablative conditions, very large numbers of gene-corrected HSCs would have to be administered.48 However, despite the encouraging engraftment rates achieved under competitive conditions of a non-myeloablative setting in mouse models with various vectors,17,34,49,50 it was soon discovered that this milder preparative regimen could not be transferred to the clinic.20 Nowadays, a reduced intensity conditioning with Busulfan at 8 mg/kg is sufficient to achieve therapeutic engraftment of modified HSCs only for disorders that possess a selective advantage at the HSC level and do not have an extramedullary hematopoiesis background.51,52
Ever since, several trials that implement a full myeloablation (described below), utilizing either 12.8 mg/kg Busulfan or 42 g/m2 Treosulfan and 8 mg/kg thiotepa have opened and are currently in data evaluation progress.35
BlueBird BIO has sponsored two Phase I/II trials utilizing 12.8 mg/kg Busulfan as the conditioning agent:53 the multicenter HGB-204 and the single-center HGB-205 (Table 2), using a modified version of Leboulch’s prototypical HPV569 beta-globin vector. The novel vector, called Lentiglobin BB305 (Table 1), has been improved by removing the insulator domains, that were found to be unstable, and by replacing the 5ʹ long-terminal repeat (LTR) with a CMV promoter.54 As a result, the titer of BB305 was 3–4 times higher than that of HPV569, and its transduction efficiency in CD34+ cells was 2–3 times greater.55 Both trials followed the exact same procedure as in the study conducted with HPV569, except that the BB305 vector was further purified by preparative chromatography and ultrafiltration.55 The HGB-205 trial enrolled 3 patients with the b0/bE genotype, who have discontinued transfusions and iron chelation.53 As of June 2019, in the completed HGB-204 study, 8 out of 10 patients with non-b0/b0 genotypes were able to cease transfusions at 0.3–5.8 months post gene therapy and had sustained transfusion independence for a duration of up to 45 months (data presented at the 24th European Hematology Association Congress) (https://library.ehaweb.org/eha/2019/24th/267342/mark.c.walters.clinical.outcomes.of.lentiglobin.gene.therapy.for.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dlentiglobin). All 10 patients of the HGB-204 trial are now enrolled in the long-term follow-up study, LTF-303. So far, no replication-competent lentivirus (RCL) has been detected, and no safety issue was attributed to the BB305 vector or the conditioning agent in either HGB-204 or HGB-205 study.
HGB-204 and HGB-205 were also the first studies to enroll patients bearing the severe b0/b0 phenotype or homozygosity for the IVS1-110 mutation, a b+ genotype with trace endogenous beta-globin expression that resembles the b0/b0 condition. Among the 9 patients with a b0/b0 genotype from both studies (HGB-204 n=8; HGB-205 n=1), 5 continued to be transfusion-dependent. However, the number of annual transfusions was reduced by 74%.53 The remaining 4 (HGB-204 n=3; HGB-205 n=1) patients had not received transfusions for 16 to 20 months.53 (https://library.ehaweb.org/eha/2019/24th/267342/mark.c.walters.clinical.outcomes.of.lentiglobin.gene.therapy.for.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dlentiglobin). The 8 patients in the HGB-204 trial are also enrolled in the LTF-303 study, as the 10 non-b0/b0 HBG-204 participants.
A more recent, Italy-based Phase I/II trial, sponsored by the Telethon Foundation, implemented a myeloablative conditioning with 42 g/m2 Treosulfan and 8 mg/kg thiotepa.35 Treosulfan was chosen because, in the context of allo-HCT for beta-thalassemia, demonstrated a more reduced toxicity than Busulfan.56 The vector utilized was GLOBE (Table 1), a beta-globin vector harboring a 2.7kb LCR cassete.57 Three b0/b+ adults (median age 33 years old) and 6 children (b+/b+ n=3; b0/b+ n=1; b0/b0 n=2; median age 9 years old) were treated, after intrabone administration of the gene-modified HSCs. Transfusion requirements were reduced in adults up to 28 months post gene therapy. Three out of 4 evaluable pediatric participants, including 1 with the b0/b0 genotype, were presented with transfusion independence during the last follow-up (up to 18 months post therapeutic infusion) (Table 2). The one child that did not reach transfusion independence also bore a b0/b0 background.57 Most importantly, this study verified that younger age and persistence of higher vector copy number (VCN) in the repopulating HSCs are associated with a better therapeutic outcome.
Safety Of Viral Vectors
The long way towards successful implementation of gene therapy was not devoid of pitfalls and complications. Leukemic transformation, also termed insertional oncogenesis, in children with X-SCID58 or Wiskott-Aldrich syndrome59 who were successfully treated by gamma-retroviral gene therapy, initially overshadowed the first successes of gene therapy and intensified the research towards the reduction of the genotoxicity risk of the procedure. Worries were also raised from the benign, yet potentially oncogenic dominant clone that emerged in the first beta-thalassemic patient ever treated with gene therapy.19 This was a result of the tandem cHS4 element contained in the HPV569 vector, which was proved prone to rearrange into a single cHS4 element upon vector insertion,19,60 and did not protect against gene activation when placed between enhancer and promoter regions.
Nowadays, lentiviral vectors encoding human beta-globin that are currently being used in clinical trials, bear safety features that should substantially reduce the risk of vector-mediated oncogenesis compared to the early generation gamma-retroviral vectors. The self-inactivating (SIN) vector design involving the deletion of the viral enhancers in the vector LTR abrogates these major determinants of genotoxicity.61 On the other hand, globin vectors contain a very powerful enhancer derived from elements of the LCR, and from the activating power of this enhancer the environment of the integrating vectors needs to be protected.38,62 In addition, genes expressed at ectopic sites, as in gene therapy applications, are subjected to the impact of the new chromosomal environment often resulting in differential expression and/or silencing.63
Chromatin insulators are naturally occurring DNA elements that help form functional boundaries between adjacent chromatin domains and have been proposed as a means to minimize vector-mediated genotoxicity (enhancer-blocking insulators) and limit transgene silencing (barrier insulators). For many years, the prototypic vertebrate insulator remained the 1.2 kb cHS4, derived from the DNAase hypersensitive site 4 of the chicken beta-globin locus control region.63 However, the utilization of cHS4 in viral vectors for clinical gene therapy up to date has been associated with several limitations including suboptimal titers, partial insulation and aberrant splicing.19,63,64
In recent years, novel, small-sized insulators have been identified in the human genome by powerful genomic technologies, allowing for the study of epigenetic marks on a genome-wide level and understanding the context in which gene regulation occurs. The majority of them display superior enhancer-blocking activity to that of the cHS4 insulator and substantially reduce the genotoxicity risk in a viral-vector-mediated carcinogenesis mouse model.65 Importantly, these insulators are small-sized (<300 bp) and can be easily accommodated in gene therapy vectors without having detrimental effects on vector titers. Indeed, the group of Sadelain extensively characterized one of these elements (termed A1) and incorporated it into the TNS9.3.55 vector, constructing its insulated version, TNS9.3.55.A1, which is aimed to be brought to the clinic.20
Most Recent Clinical Trials
Recently, BlueBird BIO launched the first two Phase III clinical trials; HGB-207 for patients with non-b0/b0 genotypes and HGB-212 for patients with a b0/b0 genotype or an IVS-I-110 mutation.35 The former plans to include 15 adult and adolescent patients together with 8 pediatric patients. The latter trial plans to enroll approximately fifteen adult, adolescent and pediatric patients.55 As of June 2019 (EHA Congress), 20 patients have been treated in the HGB-207 study and 11 in the HGB-212 study (Table 2). In the HGB-207 trial, 4 out of 5 non-b0/b0 patients are transfusion-free for a median follow-up duration of 13.6 months, and 13 of 14 non-b0/b0 patients with at least 3 months of follow-up are free from transfusions for at least 3 months. The number of pediatric patients enrolled in this study so far is 5. The first b0/b0 patient in the HGB-212 study was reported transfusion independent in late 2017 and, as of EHA 2019, 5 more patients have stopped transfusions for at least 3 months at the time of the last study visit (5–16 months post-treatment) (https://library.ehaweb.org/eha/2019/24th/267341/andreas.e.kulozik.results.from.the.phase.3.northstar-3.study.evaluating.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dhgb-212).
Most importantly, as of March 2019 (https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-zynteglo_en.pdf), BlueBird BIO received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) regarding conditional marketing authorization for ZYNTEGLO™ (autologous CD34+ cells encoding bA-T87Q-globin gene). The CHMP’s positive opinion was followed by recent marketing authorization for ZYNTEGLO™ in the European Union (EU) by the European Commission (EC) (https://ec.europa.eu/health/documents/community-register/2019/20190529144815/dec_144815_en.pdf). This product would be available to non-b0/b0 patients over 12 years old, for whom HSC transplantation is appropriate but a matched related HSC donor is not available. Hence, ZYNTEGLO™ became be the first gene therapy product for transfusion-dependent beta-thalassemia patients.
Beyond The Gene Addition Perspective
Gene editing, namely the in situ alteration of genes by specific nucleases, represents a novel strategy which, owing to the nuclease-associated creation of double stranded breaks in the DNA, replacement, insertion, or deletion of a sequence in a certain locus may become achievable. Such nucleases include zinc-finger nucleases (ZFNs), meganucleases (MNs), transcription-activator-like effector nucleases (TALENs), and the RNA-guided CRISPR/Cas9 system (Clustered, Regularly Interspaced Short Palindromic Repeats/CRISPR-Αssociated Ρrotein 9).66
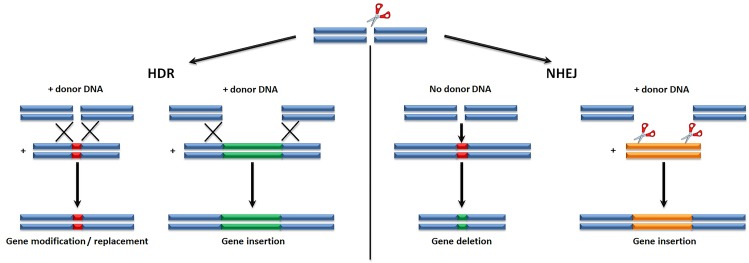
To carry out their function, these nucleases rely on specific DNA interaction modules and a nuclease domain. This procedure is rendered feasible either by homology-directed repair (HDR), or by non-homologous end-joining (NHEJ) (Figure 3). Although the latter is more efficient than the former, NHEJ is error-prone and the outcome of DNA modifications cannot be controlled. On the other hand, HDR allows for specific, predetermined changes to the target sequence67 and is thus the preferable emerging discipline for treating beta-thalassemia. Lately, many clinical gene editing trials for HIV, leukemia, hemophilia B and mucopolysaccharidosis I have been conducted.68 In the context of beta-thalassemia, two clinical trials, one utilizing a CRISPR/Cas9 system (https://clinicaltrials.gov/ct2/show/NCT03655678) and one implementing a ZFN (https://clinicaltrials.gov/ct2/show/NCT03432364) to treat transfusion-dependent beta-thalassemic patients are currently at the recruiting process (Table 2). In the latter trial (Table 2), early clinical data from one b0/b0 patient at 7 weeks post therapy were announced in April 2019 (https://www.prnewswire.com/news-releases/sangamo-provides-clinical-development-update-including-early-phase-12-beta-thalassemia-gene-edited-cell-therapy-data-300822611.html), stating cessation of transfusions. While these data are very early and will require confirmation in additional patients as well as longer follow-up to draw any clinical conclusion, they are promising, because of the patient’s genotype which has proved particularly difficult to treat. More data, including results from additional patients, are expected in the last quarter of 2019.
Figure 3.
Gene editing mechanisms are based on either homology-directed repair (HDR), or on non-homologous end-joining (NHEJ), both created by nuclease-associated creation of double stranded breaks in the DNA.
Therapeutic effects may also be driven by inserting the gene of interest into a specific locus (safe harbor),69 without affecting the transcriptional activity of adjacent genes. This method may also support minimization of transgene silencing. The AAVS1 site on chromosome 19, is well-known to serve as a common integration site of the human adeno-associated virus (AAV), and soon became a target for research.69,70 However, although the AAVS1 site has shown sustainable transgene expression upon incorporation in a different range of cell types, there is still a substantial amount of work to be done in order to be capable of providing a high level of safety confidence.70
A third option is reactivation of the gamma-globin gene, which is normally silenced after birth but it may prove sometimes capable of compensating the beta-globin deficiency, should expressed at a substantial level.71 Another approach, combining reactivation of the gamma-globin gene with correction of beta-globin mutations has also been reported.72 More recently, inactivation, by gene editing, of an erythroid intronic enhancer termed BCL11A, was shown to lead to fetal gamma-globin (HbF) reactivation.73–75 However, it has been observed that ubiquitous BCL11A knockdown impaired engraftment of both human and murine HSCs,76,77 suggesting that erythroid-specific disruption of BCL11A needs to be considered. Indeed, erythroid-specific BCL11A knockdown has previously been shown to support high-level and sustained reactivation of gamma-globin in Κ562 cells78 and human HSCs77 and also effectively bypass the engrafting inability of HSCs with ubiquitous BCL11A downregulation.77 Alternatively, disrupting the binding site of the GATA-1 transcription factor at the upstream enhancer of BCL11A may also lead to therapeutic expression levels of HbF.79 Generation of hereditary persistence of fetal hemoglobin (HPFH) mutations in HSCs by gene editing is also another promising therapeutic strategy applied in order to increase HbF levels in beta-thalassemic patients. Indeed, it has been demonstrated that introduction of the HPFH-175 T>C point mutation is associated with increased HbF expression in erythroid cell lines through de novo recruitment of a TAL1 activator.80
Remaining Questions
Despite the inarguable success of gene therapy in beta-thalassemic patients with non-b0/b0 genotypes, therapeutic outcomes for patients with the b0/b0 genotype still remain suboptimal, thus intensifying the need for further improvements in globin vector design, manufacturing and performance. It is widely acknowledged that gene-modified HSCs are prone to attenuation of their repopulating capacity because of ex vivo culturing conditions.22 Moreover, stable gene marking and effective engraftment of gene-modified HSCs may be challenged by the inevitable cryopreservation of the end-product prior to extensive quality assurance testing.12 All together, these interventions might lead to low engraftment levels post-transplant. Consequently, protocols should be further optimized using a range of various refinements such as: shortening the duration of ex-vivo culture of HSCs,81 enriching and transducing more primitive cells,82 amending culture and cytokine concentrations to accelerate platelet and granulocyte recovery,83 incorporating molecules to expand84,85 or to transduce HSCs,86–89 and administering the modified HSCs through alternative routes to favor engraftment and to reduce cell loss.57
Cationic additives, such as protamine sulfate, which neutralize membrane charges and enhance the cell-virus interaction, are well-known for increasing transduction efficiency and are already used in clinical studies.90 Other molecules, such as proteasome inhibitors,91 cyclin-dependent kinase p21,92 or mTOR,87 inhibit the post-entry trafficking from the plasma membrane to the nucleus, and may be clinically relevant in the future. Other compounds that support maintenance and expansion of HSCs include SR1 and UM729.93,94 On the other hand, using small molecules to increase VCN might risk transduction of only a particular cell subset95 and increase the risk of a dominant clone emergence.
An amended manufacturing process, using 2 small proprietary molecules as transduction enhancers,55 aims to generate higher VCN in vivo post gene therapy and is already applied for the BB305 vector in the HGB-207 trial for thalassemic patients with non-b0/b0 genotypes. This provides hope that such amendments may prove sufficient to confer transfusion independence in the most challenging cohort of b0/b0 patients participating in the HGB-212 trial.
Albeit no adverse events were so far observed in any of the clinical trials conducted with the BB305, the TNS9.3.55, or the GLOBE vector, the need for higher transduction efficiencies in order to treat b0/b0 genotypes requires patients’ close and long-term surveillance, since a higher VCN in vivo may entail increased risk of cell transformation. Alternatively, transducing cells at <1 VCN per cell and selecting only the gene-modified HSCs from the total cell population before infusion might alleviate the need for higher VCNs in vivo,95 as well as prevent possible gene silencing.96 However, this procedure is substantially longer in duration, which may ultimately lead to a loss of engraftment potential and a decrease in clonal diversity.97
Gene editing strategies by HDR, though they represent a theoretically safe and efficient way to repair the patient’s HSCs, do bear drawbacks. Such disadvantages include low rates of HDR in HSCs due to quiescence of this particular cell type,98 inefficient delivery of nucleases to the cells,99 potential off/oncotarget cleavage,100 low engraftment potential of HSCs bearing repaired genes,101 as well as on-target mutagenesis, such as large deletions and more complex rearrangements.102 In addition, since HDR is conducted with an exogenously supplied donor template, very large number of HDR products would have to be manufactured and authorized for human use in order to cover the >200 mutations for beta-thalassemia,55 unless only patients carrying the most common genotypes would be chosen to be treated. The aforementioned need to be extensively evaluated and addressed as this technology is becoming more widely adopted.
Conclusions
After years of research, a new era for the treatment of beta-thalassemia has begun and will soon offer curative potential with many alternate options. Since the long-term consequences of gene editing mechanisms in HSCs are not yet clarified, current gene editing cannot be considered safer than viral-mediated gene addition. Among novel therapies, the classic gene addition perspective remains the most promising strategy and soon after long-term data release from Phase III trials, gene therapy for beta-thalassemia might become available to a large cohort of patients.
Acknowledgments
This study was supported by a scholarship from the Greek State Scholarships Foundation, which was funded by the Operation “Support for post-doctoral researchers” from the resources of the Operational Program “Human Resources Development, Education and Lifelong Learning” with Priority Axes 6, 8 and 9 and is co-funded by the European Social Fund (ESF) and the Greek state. We would like to dedicate this work to our beloved, late mentor, George Stamatoyannopoulos and express our gratitude for his support over the years.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weatherall DJ, Clegg J. The Thalassemia Syndrome. Oxford: Blackwell Scientific Publishers; 1981. [Google Scholar]
- 2.Weatherall DJ. Thalassaemia: the long road from bedside to genome. Nature Rev Genet. 2004;5(8):625–631. doi: 10.1038/nrg1406 [DOI] [PubMed] [Google Scholar]
- 3.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–487. doi: 10.2471/blt.06.039818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatoyannopoulos G, Nienhuis AW, Majerus P, Varmus H. The Molecular Basis of Blood Diseases. Philadelphia: WB Saunders; 2001. [Google Scholar]
- 5.Levine L, Levine M. Health care transition in thalassemia: pediatric to adult-oriented care. Ann N Y Acad Sci. 2010;1202(1):244–247. doi: 10.1111/j.1749-6632.2010.05598.x [DOI] [PubMed] [Google Scholar]
- 6.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–1193. [PubMed] [Google Scholar]
- 7.Mancuso A, Sciarrino E, Renda MC, Maggio A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. 2006;30(1):119–124. doi: 10.1080/03630260500455565 [DOI] [PubMed] [Google Scholar]
- 8.Gaziev D, Polchi P, Galimberti M, et al. Graft-versus-host disease after bone marrow transplantation for thalassemia: an analysis of incidence and risk factors. Transplantation. 1997;63(6):854–860. doi: 10.1097/00007890-199703270-00011 [DOI] [PubMed] [Google Scholar]
- 9.Lucarelli G, Clift RA, Galimberti M, et al. Bone marrow transplantation in adult thalassemic patients. Blood. 1999;93(4):1164–1167. [PubMed] [Google Scholar]
- 10.Gaziev D, Galimberti M, Lucarelli G, et al. Bone marrow transplantation from alternative donors for thalassemia: HLA-phenotypically identical relative and HLA-nonidentical sibling or parent transplants. Bone Marrow Transplant. 2000;25(8):815–821. doi: 10.1038/sj.bmt.1702242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Nasa G, Caocci G, Argiolu F, et al. Unrelated donor stem cell transplantation in adult patients with thalassemia. Bone Marrow Transplant. 2005;36(11):971–975. doi: 10.1038/sj.bmt.1705100 [DOI] [PubMed] [Google Scholar]
- 12.Karponi G, Papayanni PG, Zervou F, Bouinta A, Anagnostopoulos A, Yannaki E. The functional effect of repeated cryopreservation on transduced CD34+ cells from patients with thalassemia. Hum Gene Ther Methods. 2018;29(5):220–227. doi: 10.1089/hgtb.2018.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weatherall DJ, Clegg JB. Genetic disorders of hemoglobin. Semin Hematol. 1999;36(4 Suppl 7):24–37. [PubMed] [Google Scholar]
- 14.Sadelain M, Rivière I, Wang X, et al. Strategy for a multicenter phase I clinical trial to evaluate globin gene transfer in beta-thalassemia. Ann N Y Acad Sci. 2010;1202(1):52–58. doi: 10.1111/j.1749-6632.2010.05597.x [DOI] [PubMed] [Google Scholar]
- 15.Yannaki E, Stamatoyannopoulos G. Hematopoietic stem cell mobilization strategies for gene therapy of beta thalassemia and sickle cell disease. Ann N Y Acad Sci. 2010;1202(1):59–63. doi: 10.1111/j.1749-6632.2010.05576.x [DOI] [PubMed] [Google Scholar]
- 16.Urbinati F, Arumugam P, Higashimoto T, et al. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3ʹLTR. Mol Ther. 2009;17(9):1527–1536. doi: 10.1038/mt.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulad F, Wang X, Qu J, et al. Safe mobilization of CD34+ cells in adults with beta-thalassemia and validation of effective globin gene transfer for clinical investigation. Blood. 2014;123(10):1483–1486. doi: 10.1182/blood-2013-06-507178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivière I, Dunbar CE, Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119(5):1107–1116. doi: 10.1182/blood-2011-10-388512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human b-thalassaemia. Nature. 2010;467(7313):318–322. doi: 10.1038/nature09328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansilla-Soto J, Riviere I, Boulad F, et al. Cell and gene therapy for the beta-thalassemias: advances and prospects. Hum Gene Ther. 2016;27(4):295–304. doi: 10.1089/hum.2016.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karponi G, Zogas N, Domvri K, et al. Prospects of gene therapy for pulmonary diseases: progress and limitations. Med Chem. 2017;13(4):308–318. doi: 10.2174/1573406413666170209122131 [DOI] [PubMed] [Google Scholar]
- 22.Psatha N, Karponi G, Yannaki E. Optimizing autologous cell grafts to improve stem cell gene therapy. Exp Hematol. 2016;44(7):528–539. doi: 10.1016/j.exphem.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dey BR, Shaffer J, Yee AJ, et al. Comparison of outcomes after transplantation of peripheral blood stem cells versus bone marrow following an identical nonmyeloablative conditioning regimen. Bone Marrow Transplant. 2007;40(1):19–27. doi: 10.1038/sj.bmt.1705688 [DOI] [PubMed] [Google Scholar]
- 24.To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89(7):2233–2258. [PubMed] [Google Scholar]
- 25.Broxmeyer HE, Orschell CM, Clapp DW. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20042565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nademanee AP, DiPersio JF, Maziarz RT, et al. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34+ hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34+ cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant. 2012;18(10):1564–1572. doi: 10.1016/j.bbmt.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 27.Falzetti F, Aversa F, Minelli O, Tabilio A. Spontaneous rupture of spleen during peripheral blood stem-cell mobilisation in a healthy donor. Lancet. 1999;13(9152):555. [DOI] [PubMed] [Google Scholar]
- 28.Balaguer H, Galmes A, Ventayol G, Bargay J, Besalduch J. Splenic rupture after granulocyte-colony-stimulating factor mobilization in a peripheral blood progenitor cell donor. Transfusion. 2004;44(8):1260–1261. doi: 10.1111/trf.2004.44.issue-8 [DOI] [PubMed] [Google Scholar]
- 29.Lindemann A, Rumberger B. Vascular complications in patients treated with granulocyte colony-stimulating factor (G-CSF). Eur J Cancer. 1993;29A(16):2338–2339. doi: 10.1016/0959-8049(93)90236-9 [DOI] [PubMed] [Google Scholar]
- 30.Yannaki E, Papayannopoulou T, Jonlin E, et al. Hematopoietic stem cell mobilization for gene therapy of adult patients with severe beta-thalassemia: results of clinical trials using G-CSF or Plerixafor in splenectomized and nonsplenectomized subjects. Mol Ther. 2012;20(1):230–238. doi: 10.1038/mt.2011.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yannaki E, Karponi G, Zervou F, et al. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with beta-thalassemia major. Hum Gene Ther. 2013;24(10):852–860. doi: 10.1089/hum.2013.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Constantinou VC, Bouinta A, Karponi G, et al. Poor stem cell harvest may not always be related to poor mobilization: lessons gained from a mobilization study in patients with β-thalassemia major. Transfusion. 2017;57(4):1031–1039. doi: 10.1111/trf.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yannaki E, Psatha N, Athanasiou E, et al. Mobilization of hematopoietic stem cells in a thalassemic mouse model: implications for human gene therapy of thalassemia. Hum Gene Ther. 2010;21(3):299–310. doi: 10.1089/hum.2009.077 [DOI] [PubMed] [Google Scholar]
- 34.Karponi G, Psatha N, Lederer CW, et al. Plerixafor+G-CSF-mobilized CD34+ cells represent an optimal graft source for thalassemia gene therapy. Blood. 2015;126(5):616–619. doi: 10.1182/blood-2015-03-629618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lidonnici MR, Ferrari G. Gene therapy and gene editing strategies for hemoglobinopathies. Blood Cells Mol Dis. 2018;70:87–101. doi: 10.1016/j.bcmd.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 36.Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151. doi: 10.1126/science.1233151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. doi: 10.1126/science.1233158 [DOI] [PubMed] [Google Scholar]
- 38.Hargrove PW, Kepes S, Hanawa H, et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in beta-thalassemic hematopoietic cells. Mol Ther. 2008;16(3):525–533. doi: 10.1038/sj.mt.6300394 [DOI] [PubMed] [Google Scholar]
- 39.May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in β-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406(6791):82–86. doi: 10.1038/35017565 [DOI] [PubMed] [Google Scholar]
- 40.May C, Rivella S, Chadburn A, Sadelain M. Successful treatment of murine beta-thalassemia intermedia by transfer of the human beta-globin gene. Blood. 2002;99(6):1902–1908. doi: 10.1182/blood.V99.6.1902 [DOI] [PubMed] [Google Scholar]
- 41.Rivella S, May C, Chadburn A, Rivière I, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human β-globin gene transfer. Blood. 2003;101(8):2932–2939. doi: 10.1182/blood-2002-10-3305 [DOI] [PubMed] [Google Scholar]
- 42.Lisowski L, Sadelain M. Locus control region elements HS1 and HS4 enhance the therapeutic efficacy of globin gene transfer in β-thalassemic mice. Blood. 2007;110(13):4175–4178. doi: 10.1182/blood-2007-08-108647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persons DA, Tisdale JF. Gene therapy for the hemoglobin disorders. Semin Hematol. 2004;41(4):279–286. doi: 10.1053/j.seminhematol.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 44.Sadelain M. Recent advances in globin gene transfer for the treatment of beta-thalassemia and sickle cell anemia. Curr Opin Hematol. 2006;13(3):142–148. doi: 10.1097/01.moh.0000219658.57915.d4 [DOI] [PubMed] [Google Scholar]
- 45.Sadelain M, Boulad F, Lisowki L, Moi P, Rivière I. Stem cell engineering for the treatment of severe hemoglobinopathies. Curr Mol Med. 2008;8(7):690–697. doi: 10.2174/156652408786241357 [DOI] [PubMed] [Google Scholar]
- 46.Perumbeti A, Malik P. Genetic correction of sickle cell anemia and beta-thalassemia: progress and new perspective. ScientificWorldJournal. 2010;10:644–654. doi: 10.1100/tsw.2010.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payen E, Colomb C, Negre O, Beuzard Y, Hehir K, Leboulch P. Lentivirus vectors in beta-thalassemia. Methods Enzymol. 2012;507:109–124. [DOI] [PubMed] [Google Scholar]
- 48.Yannaki E, Karponi G. Current status and developments in gene therapy for thalassemia and sickle cell disease. Thalass Rep. 2014;4(3):75–80. [Google Scholar]
- 49.Miccio A, Cesari R, Lotti F, et al. In vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of beta-thalassemia. Proc Natl Acad Sci U S A. 2008;105(30):10547–10552. doi: 10.1073/pnas.0711666105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miccio A, Poletti V, Tiboni F, et al. The GATA1-HS2 enhancer allows persistent and position-independent expression of a beta-globin transgene. PLoS One. 2011;6(12):e27955. doi: 10.1371/journal.pone.0027955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–409. doi: 10.1038/nm1393 [DOI] [PubMed] [Google Scholar]
- 52.Stein S, Ott MG, Schultze-Strasser S, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16(2):198–204. doi: 10.1038/nm1110-1167 [DOI] [PubMed] [Google Scholar]
- 53.Thompson AA, Walters MC, Kwiatkowski J, et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N Engl J Med. 2018;378(16):1479–1493. doi: 10.1056/NEJMc1711583 [DOI] [PubMed] [Google Scholar]
- 54.Negre O, Eggimann A-V, Beuzard Y, et al. Gene therapy of the beta-hemoglobinopathies by lentiviral transfer of the beta(A(T87Q))-globin gene. Hum Gene Ther. 2016;27(2):148–165. doi: 10.1089/hum.2016.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sii-Felice K, Giorgi M, Leboulch P, et al. Hemoglobin disorders: lentiviral gene therapy in the starting blocks to enter clinical practice. Exp Hematol. 2018;64:12–32. doi: 10.1016/j.exphem.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 56.Bernardo ME, Piras E, Vacca A, et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120(2):473–476. doi: 10.1182/blood-2012-04-423822 [DOI] [PubMed] [Google Scholar]
- 57.Marktel S, Scaramuzza S, Cicalese MP, et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent beta-thalassemia. Nat Med. 2019;25(2):234–241. doi: 10.1038/s41591-018-0301-6 [DOI] [PubMed] [Google Scholar]
- 58.Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143–3150. doi: 10.1172/JCI35798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braun CJ, Boztug K, Paruzynski A, et al. Gene therapy for Wiskott-Aldrich syndrome–long-term efficacy and genotoxicity. Sci Transl Med. 2014;6(227):227ra33. doi: 10.1126/scitranslmed.3007280 [DOI] [PubMed] [Google Scholar]
- 60.Ronen K, Negre O, Roth S, et al. Distribution of lentiviral vector integration sites in mice following therapeutic gene transfer to treat beta-thalassemia. Mol Ther. 2011;19(7):1273–1286. doi: 10.1038/mt.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montini E, Cesana D, Schmidt M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119(4):964–975. doi: 10.1172/JCI37630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arumugam PI, Urbinati F, Velu CS, Higashimoto T, Grimes HL, Malik P. The 3ʹ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PLoS One. 2009;4(9):e6995. doi: 10.1371/journal.pone.0006995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emery DW. The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum Gene Ther. 2011;22(6):761–774. doi: 10.1089/hum.2010.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Negre O, Bartholomae C, Beuzard Y, et al. Preclinical evaluation of efficacy and safety of an improved lentiviral vector for the treatment of beta-thalassemia and sickle cell disease. Curr Gene Ther. 2015;15(1):64–81. doi: 10.2174/1566523214666141127095336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu M, Maurano MT, Wang H, et al. Genomic discovery of potent chromatin insulators for human gene therapy. Nat Biotechnol. 2015;33(2):198–203. doi: 10.1038/nbt.3062 [DOI] [PubMed] [Google Scholar]
- 66.Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15(5):321–334. doi: 10.1038/nrg3686 [DOI] [PubMed] [Google Scholar]
- 67.Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016;26(1):52–64. doi: 10.1016/j.tcb.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cornu TI, Mussolino C, Cathomen T. Refining strategies to translate genome editing to the clinic. Nat Med. 2017;23(4):415–423. doi: 10.1038/nm.4265 [DOI] [PubMed] [Google Scholar]
- 69.Papapetrou EP, Lee G, Malani N, et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol. 2011;29(1):73–78. doi: 10.1038/nbt.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papapetrou EP, Schambach A. Gene insertion into genomic safe harbors for human gene therapy. Mol Ther. 2016;24(4):678–684. doi: 10.1038/mt.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng W, Rupon JW, Krivega I, et al. Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell. 2014;158(4):849–860. doi: 10.1016/j.cell.2014.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuccato C, Breda L, Salvatori F, et al. A combined approach for β-thalassemia based on gene therapy-mediated adult hemoglobin (HbA) production and fetal hemoglobin (HbF) induction. Ann Hematol. 2012;91(8):1201–1213. doi: 10.1007/s00277-012-1430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bauer DE, Kamran SC, Orkin SH. Reawakening fetal hemoglobin: prospects for new therapies for the β-globin disorders. Blood. 2012;120(15):2945–2953. doi: 10.1182/blood-2012-06-292078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patwardhan RP, Hiatt JB, Witten DM, et al. Massively parallel functional dissection of mammalian enhancers in vivo. Nat Biotechnol. 2012;30(3):265–270. doi: 10.1038/nbt.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melnikov A, Murugan A, Zhang X, et al. Systematic dissection and optimization of inducible enhancers in human cells using a massively parallel reporter assay. Nat Biotechnol. 2012;30(3):271–277. doi: 10.1038/nbt.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luc S, Huang J, McEldoon JL, et al. Bcl11a deficiency leads to hematopoietic stem cell defects with an aging-like phenotype. Cell Rep. 2016;16(12):3181–3194. doi: 10.1016/j.celrep.2016.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brendel C, Guda S, Renella R, et al. Lineage-specific BCL11A knockdown circumvents toxicities and reverses sickle phenotype. J Clin Invest. 2016;126(10):3868–3878. doi: 10.1172/JCI87885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khosravi MA, Abbasalipour M, Concordet JP, et al. Targeted deletion of BCL11A gene by CRISPR-Cas9 system for fetal hemoglobin reactivation: A promising approach for gene therapy of beta thalassemia disease. Eur J Pharmacol. 2019;854:398–405. doi: 10.1016/j.ejphar.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Zeng J, Roscoe BP, et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med. 2019;25(5):776–783. doi: 10.1038/s41591-019-0401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Traxler EA, Yao Y, Wang YD, et al. A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med. 2016;22(9):987–990. doi: 10.1038/nm.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larochelle A, Gillette JM, Desmond R, et al. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood. 2012;119(8):1848–1855. doi: 10.1182/blood-2011-10-388512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masiuk KE, Brown D, Laborada J, Hollis RP, Urbinati F, Kohn DB. Improving gene therapy efficiency through the enrichment of human hematopoietic stem cells. Mol Ther. 2017;25(9):2163–2175. doi: 10.1016/j.ymthe.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller PH, Knapp DJHF, Beer PA, et al. Early production of human neutrophils and platelets posttransplant is severely compromised by growth factor exposure. Exp Hematol. 2016;44(7):635–640. doi: 10.1016/j.exphem.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 84.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. doi: 10.1126/science.1191536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fares I, Chagraoui J, Gareau Y, et al. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509–1512. doi: 10.1126/science.1256337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fenard D, Ingrao D, Seye A, et al. Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells. Mol Ther Nucleic Acids. 2013;2:e90. doi: 10.1038/mtna.2013.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang CX, Sather BD, Wang X, et al. Rapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cells. Blood. 2014;124(6):913–923. doi: 10.1182/blood-2013-12-546218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heffner GC, Bonner M, Christiansen L, et al. Prostaglandin E 2 increases lentiviral vector transduction efficiency of adult human hematopoietic stem and progenitor cells. Mol Ther. 2018;26(1):320–328. doi: 10.1016/j.ymthe.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Masiuk KE, Zhang R, Osborne K, Hollis RP, Campo-Fernandez B, Kohn DB. PGE2 and poloxamer synperonic F108 enhance transduction of human HSPCs with a β-globin lentiviral vector. Mol Ther Methods Clin Dev. 2019;13:390–398. doi: 10.1016/j.omtm.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ribeil JA, Hacein-Bey-Abina S, Payen E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376(9):848–855. doi: 10.1056/NEJMoa1609677 [DOI] [PubMed] [Google Scholar]
- 91.Santoni De Sio FR, Cascio P, Zingale A, Gasparini M, Naldini L. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood. 2006;107(11):4257–4265. doi: 10.1182/blood-2005-10-4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Attar E, Cohen K, Crumpacker C, Scadden D. Silencing p21Waf1/Cip1/Sdi1 expression increases gene transduction efficiency in primitive human hematopoietic cells. Gene Ther. 2005;12(19):1444–1452. doi: 10.1038/sj.gt.3302544 [DOI] [PubMed] [Google Scholar]
- 93.Genovese P, Schiroli G, Escobar G, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510(7504):235–240. doi: 10.1038/nature13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adair JE, Waters T, Haworth KG, et al. Semi-automated closed system manufacturing of lentivirus gene-modified haematopoietic stem cells for gene therapy. Nat Commun. 2016;7:13173. doi: 10.1038/ncomms13173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhukhai K, de Dreuzy E, Giorgi M, et al. Ex vivo selection of transduced hematopoietic stem cells for gene therapy of beta-hemoglobinopathies. Mol Ther. 2018;26(2):480–495. doi: 10.1016/j.ymthe.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalberer CP, Pawliuk R, Imren S, et al. Preselection of retrovirally transduced bone marrow avoids subsequent stem cell gene silencing and age-dependent extinction of expression of human beta-globin in engrafted mice. Proc Natl Acad Sci. 2000;97(10):5411–5415. doi: 10.1073/pnas.100082597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kennedy DR, McLellan K, Moore PF, Henthorn PS, Felsburg PJ. Effect of ex vivo culture of CD34+ bone marrow cells on immune reconstitution of XSCID dogs following allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(6):662–670. doi: 10.1016/j.bbmt.2009.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33(5):538–542. doi: 10.1038/nbt.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuris JA, Thompson DB, Shu Y, et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80. doi: 10.1038/nbt.3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hendel A, Kildebeck EJ, Fine EJ, et al. Quantifying genome-editing outcomes at endogenous loci with SMRT sequencing. Cell Rep. 2014;7(1):293–305. doi: 10.1016/j.celrep.2014.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoban MD, Cost GJ, Mendel MC, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015;125(17):2597–2604. doi: 10.1182/blood-2014-07-591040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR–cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36(8):765–771. doi: 10.1038/nbt.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]