Significance
Helicobacter pylori is the strongest risk factor for gastric adenocarcinoma and has been deemed a class I carcinogen by the World Health Organization. One of the most important H. pylori virulence factors is the cag pathogenicity island (PAI); however, the precise mechanisms through which H. pylori induces gastric adenocarcinoma are incompletely defined. In human samples, Lrig1 expression is enhanced in lesions with premalignant potential. In mouse models, chronic H. pylori infection stimulates Lrig1+ progenitor cells in a cag-dependent manner, and these stem cells give rise to differentiated gastric cells. Thus, the cag PAI is a key mediator of the epithelial progenitor cell responsiveness that develops following chronic H. pylori infection.
Keywords: Helicobacter pylori, Lrig1, gastric, progenitor
Abstract
Helicobacter pylori-induced gastritis is the strongest risk factor for gastric adenocarcinoma, a malignancy preceded by a series of well-defined histological stages, including metaplasia. One microbial constituent that augments cancer risk is the cag type 4 secretion system (T4SS), which translocates the oncoprotein CagA into host cells. Aberrant stem cell activation is linked to carcinogenesis, and Lrig1 (leucine-rich repeats and Ig-like domains 1) marks a distinct population of progenitor cells. We investigated whether microbial effectors with carcinogenic potential influence Lrig1 progenitor cells ex vivo and via lineage expansion within H. pylori-infected gastric mucosa. Lineage tracing was induced in Lrig1-CreERT2/+;R26R-YFP/+ (Lrig1/YFP) mice that were uninfected or subsequently infected with cag+ H. pylori or an isogenic cagE− mutant (nonfunctional T4SS). In contrast to infection with wild-type (WT) H. pylori for 2 wk, infection for 8 wk resulted in significantly increased inflammation and proliferation in the corpus and antrum compared with uninfected or mice infected with the cagE− mutant. WT H. pylori-infected mice harbored significantly higher numbers of Lrig1/YFP epithelial cells that coexpressed UEA1 (surface cell marker). The number of cells coexpressing intrinsic factor (chief cell marker), YFP (lineage marker), and GSII lectin (spasmolytic polypeptide-expressing metaplasia marker) were increased only by WT H. pylori. In human samples, Lrig1 expression was significantly increased in lesions with premalignant potential compared with normal mucosa or nonatrophic gastritis. In conclusion, chronic H. pylori infection stimulates Lrig1-expressing progenitor cells in a cag-dependent manner, and these reprogrammed cells give rise to a full spectrum of differentiated cells.
Helicobacter pylori colonizes the gastric mucosa of more than one-half of the world’s population. Although most colonized persons remain asymptomatic, infection with this pathogen confers the strongest known risk for developing gastric adenocarcinoma, the third most lethal cancer worldwide. Intestinal-type gastric cancer, the most frequent histological subtype, is preceded by a series of well-defined and orchestrated stages progressing temporally through chronic gastritis, atrophy without metaplasia, pseudopyloric metaplasia/spasmolytic polypeptide-expressing metaplasia (SPEM), intestinal metaplasia, and dysplasia (1).
Strain-specific bacterial constituents clearly influence the outcomes of H. pylori infection, and strains that possess a functional cag pathogenicity island (PAI) incur a significantly higher risk for gastric cancer than non–cag-bearing strains. The cag PAI is a 40-kB DNA insertion element that contains 27 to 31 genes encoding proteins that form a type IV bacterial secretion system (T4SS). The cag T4SS exports CagA from adherent H. pylori across bacterial and epithelial membranes and into host cells (2–5). Translocated CagA is rapidly phosphorylated by Src and Abl kinases, and phosphorylated CagA activates a host phosphatase (SHP-2), leading to changes in cell motility and proliferation (6).
One downstream eukaryotic target of CagA with carcinogenic potential is β-catenin. Under homeostatic conditions, β-catenin is either complexed at the membrane in the adherens junction or sequestered in the cytosol by a multiprotein complex composed of adenomatous polyposis coli (APC), Axin1, casein, and glycogen synthase kinase-3β (GSK-3β) that constitutively targets β-catenin for proteosomal degradation. Following H. pylori infection, β-catenin can be activated via inactivation of GSK-3β (7–9). However, CagA also interacts with membrane-associated β-catenin to drive signaling and promote mitogenic responses (10, 11). Furthermore, increased expression of β-catenin, mutations within APC, and/or inhibition of GSK-3β, which function to stabilize β-catenin in the cytoplasm, are frequently observed in gastric cancer specimens (12).
Within gastric glandular units, stem cells are critical for regulating self-renewal and maintaining tissue homeostasis and are under tight regulation by β-catenin. Lgr5 is a well-studied marker of highly proliferative stem cells in both the intestine and the stomach, and within the gastric niche, H. pylori functionally activates Lgr5 (13, 14). However, provocative data have recently demonstrated that H. pylori can also activate Lgr5− stem cells within gastric glandular units (14). Lrig1 (leucine-rich repeats and Ig-like domains 1) is a transmembrane protein that acts as a pan-ErbB− regulator (15). Lineage tracing has identified Lrig1 as a marker of a subset of intestinal stem/progenitor cells that are less proliferative than Lgr5 stem cells and are long-lived under homeostatic conditions, but become proliferative on injury to repopulate damaged crypts (16). Disruption of 1 allele of the tumor-suppressor gene Apc in Lrig1+ stem cells results in highly dysplastic adenomas in the intestine and colon, suggesting that initiating events in Lrig1+ cells may drive tumorigenesis (16, 17). In the stomach, Lrig1 marks a distinct population of progenitor cells in the antrum and corpus. In the corpus, Lrig1 is expressed in the isthmus of the gastric glands and parietal cells (18), and haploinsufficiency of Apc in Lrig1+ cells in this niche leads to the development of high-grade dysplasia in the distal antrum/pylorus and increased proliferation in the corpus (17).
In this study, we hypothesized that H. pylori infection drives the progression of gastric injury through mobilization of Lrig1+ progenitor cell populations in the gastric epithelium. The aim of this study was to define whether strain-specific microbial effectors with carcinogenic potential influence Lrig1+ progenitor cells and the subsequent expansion of daughter cells within H. pylori-infected gastric mucosa and within ex vivo model systems. Furthermore, we sought to validate these findings within the endogenous human gastric niche colonized by this pathogen.
Results
Gastric Stem Cell Activity, Inflammation, and Proliferation Are Selectively Increased by Infection with H. pylori cag+ Strains.
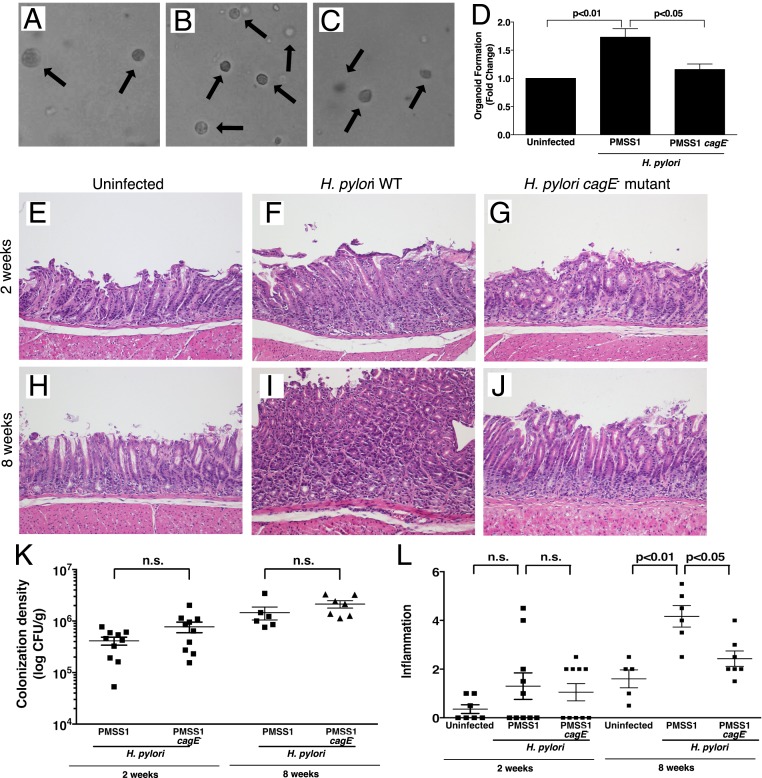
The number of organoids generated ex vivo directly reflects in vivo progenitor cell activity (13). To investigate stem cell activation by H. pylori, Lrig1+/YFP mice were challenged for 6 wk with Brucella broth as an uninfected control, the wild-type (WT) cag+ H. pylori strain PMSS1, or a PMSS1 cagE− isogenic mutant harboring a nonfunctional cag island. Infection of Lrig1+/YFP mice with WT H. pylori primed gastric stem cell populations, leading to generation of a significantly higher number of gastroids compared with either uninfected mice or mice infected with the cagE− isogenic mutant (Fig. 1 A–D).
Fig. 1.
Gastric stem cell activity and inflammation is increased by H. pylori infection in a cag-dependent manner. (A–C) Bright-field images (10× magnification) of organoids isolated from mice challenged for 6 wk with Brucella broth as an uninfected negative control (A), H. pylori WT strain PMSS1 (B), or H. pylori PMSS1 cagE− mutant (C). (D) Quantification of organoids formed from isolated gastric glands. (E–J) Lrig1/YFP mice were challenged for 2 wk (E–G) or 8 wk (H–J) with Brucella broth (E and H), H. pylori WT strain PMSS1 (F and I), or a PMSS1 cagE− isogenic mutant (G and J). Representative H&E images are shown (20× magnification). (K) Gastric tissue from H. pylori-challenged mice was homogenized and plated on selective trypticase soy agar plates with 5% sheep’s blood for isolation of H. pylori. Plates were incubated for 5 to 7 d, and colonization density was determined and expressed as log colony-forming units (CFU) per gram of tissue. (L) A single pathologist, blinded to treatment groups, assessed and scored inflammation at 2 wk and 8 wk. Acute and chronic inflammation in both the antrum and corpus was scored on a scale of 0 to 3, leading to a possible maximum score of 12. Each data point represents an individual animal, and mean values are shown. ANOVA and the Bonferroni test were used to determine statistical significance between groups, n.s., nonsignificant.
APC is frequently mutated in gastric cancer, and loss of a single Apc allele in Lrig1+ cells drives the formation of gastric preneoplastic lesions (17). Based on our findings demonstrating activation of stem cells by WT H. pylori (Fig. 1 A–D), we next determined whether infection targets populations of Lrig1+ cells in gastric epithelium in a cag-dependent manner. Lrig1/YFP mice were challenged with Brucella broth alone, WT H. pylori strain PMSS1, or a PMSS1 cagE− mutant for 2 wk (Fig. 1 E–G, K, and L) or 8 wk (Fig. 1 H–L). Colonization efficiency, defined as the percentage of successfully colonized mice, was 100% for both strains at all time points, and colonization density was similar in the infected groups (Fig. 1K). As expected, an 8-wk, but not a 2-wk, infection with H. pylori WT strain PMSS1 significantly increased gastric inflammation compared with uninfected controls, while levels of inflammation following infection with the cagE− mutant strain were no different from those in controls (Fig. 1L).
H. pylori strains that translocate CagA augment gastric cancer risk. Therefore, to investigate the relationship between Lrig1 expression and progenitor cell number, we performed immunohistochemistry for Ki67 (SI Appendix, Fig. S1). Ki67 staining localized to the base and isthmus in uninfected antrum and corpus gastric epithelium, respectively (SI Appendix, Fig. S1). At 2 wk and 8 wk after H. pylori challenge, antral proliferation was significantly increased following infection with WT H. pylori strain PMSS1, while proliferation in mice infected with the cagE− mutant was no different from that in controls (SI Appendix, Fig. S1). At 2 wk, no significant changes were identified in corpus proliferation among the 3 groups, which mirrored the results for inflammation (Fig. 1). In contrast, H. pylori WT strain PMSS1 significantly increased corpus proliferation at 8 wk, but this was not seen following infection with the H. pylori cagE− isogenic mutant (SI Appendix, Fig. S1). Changes in proliferation were likely not due to increased apoptosis, as activated caspase-3 expression was not significantly different between uninfected controls, mice infected with WT H. pylori strain PMSS1, and mice infected with the cagE− mutant at either time point analyzed (SI Appendix, Fig. S2).
The Effects of H. pylori on Lrig1 Progenitor Cells Are cag T4SS- Dependent.
We next determined whether H. pylori could mobilize Lrig1+ progenitor cells by performing lineage tracing using an Lrig1-inducible mouse model (Lrig1/YFP). At 2 wk after H. pylori challenge, prior to the onset of inflammation, approximately 40% of antral glands and 10% of corpus glands were Lrig1 lineage-labeled; however, there was no significant difference in Lrig1 lineage labeling in either the antrum or the corpus following infection with H. pylori WT strain PMSS1 or the cagE− isogenic mutant compared with controls (Fig. 2). At 8 wk postchallenge, there were scattered lineage-labeled glands in uninfected gastric antrum and corpus; however, H. pylori WT strain PMSS1 significantly increased Lrig1 progeny in both the antrum and corpus at this time point, and this was not seen in response to the cagE− isogenic mutant (Fig. 2). The pattern of this stem cell response to H. pylori is distinctly different temporally and topographically from the effects of H. pylori on Lgr5 cells, which are increased at both 2 wk and 8 wk of infection, but only in the antrum (13).
Fig. 2.
H. pylori increases Lrig1 progenitor activity in a cag-dependent manner. Lrig1/YFP mice were challenged for 2 wk (A–H) or 8 wk (I–P) with Brucella broth (A, E, I, and M), with H. pylori WT strain PMSS1 (B, F, J, and N), or with a PMSS1 cagE− isogenic mutant (C, G, K, and O). Lrig1 lineage tracing was assessed by YFP (green) immunostaining; nuclei are stained blue. Lineage-labeled glands were quantified by enumerating positive glands, defined as those with 2 or more adjacent YFP+ cells, and at least 2 fields per animal in both the antrum and corpus were quantified (D, H, L, and P). Images were acquired at 20× magnification. The Bonferroni test was used to determine statistical significance between groups, n.s., nonsignificant.
To determine whether the effects of H. pylori on Lrig1+ progenitor cells may be due to direct effects of CagA translocation into progenitor cells, we coimmunostained gastric tissue with antibodies targeting H. pylori as well as YFP. As previously reported with H. pylori strain PMSS1, the majority of H. pylori was found in the antrum (19). In uninfected mouse antrum, there was minimal YFP lineage labeling, and no H. pylori were detected (SI Appendix, Fig. S3). Following an 8-wk infection with H. pylori WT strain PMSS1, Lrig1+ progeny were increased and H. pylori was identified at the base of gastric glands closely juxtaposed to YFP+ cells (SI Appendix, Fig. S3). These findings suggest that H. pylori may exert direct effects on Lrig1+ progeny through translocation of CagA.
Lrig1-YFP–Marked Cells Differentiate into Surface Cells, Chief Cells, and SPEM, Which Is Accelerated in Response to H. pylori cag+ Strains.
We next defined specific cell lineages that derived from H. pylori-stimulated Lrig1+ stem cells at 8 wk postchallenge. Lineage tracing demonstrated that Lrig1+-marked cells engendered surface mucus cells, which was greatly enhanced in the presence of cag+ H. pylori (SI Appendix, Fig. S4). In uninfected mouse antrum and corpus, UEA1, which labels surface cells, was localized to the gastric pit, as expected, and there was minimal Lrig1 lineage tracing (SI Appendix, Fig. S4 A and D). WT H. pylori increased Lrig1+ progenitor activity, and a significantly higher number of YFP+ cells coexpressed UEA1. This was not seen in response to the cagE− mutant, which was no different compared with uninfected mice (SI Appendix, Fig. S4). These findings indicate that Lrig1+ cells undergo accelerated differentiation into surface mucus cells, specifically in response to cag+ H. pylori strains.
To define specific gastric lineages derived from activated Lrig1+ cells within the context of chronic H. pylori infection, we next coimmunostained gastric tissue with antibodies targeting gastric lineage markers as well as YFP. In uninfected mouse corpus, intrinsic factor localized to the glandular base and GS-II lectin localized to the midregion where mucus neck cells reside, in conjunction with minimal YFP lineage labeling (Fig. 3A). Following an 8-wk infection with H. pylori WT strain PMSS1, Lrig1+ progeny were increased and a significantly higher number of YFP+ cells coexpressed intrinsic factor and GS-II lectin at the base of gastric glands. Coexpression of these markers at this locale signifies the presence of metaplastic SPEM lineages (Fig. 3 B and G). Importantly, these changes were not present following infection with the cagE− isogenic mutant, which resembled uninfected mucosa (Fig. 3C). These results demonstrate that Lrig1+ cells differentiate into chief cells, and that labeled chief cells transdifferentiate into SPEM in response to H. pylori strains harboring a functional cag locus.
Fig. 3.
Lrig1 lineage-traced cells colocalize with chief cells and SPEM cells in response to cag+ H. pylori. Lrig1/YFP mice were challenged for 8 wk with Brucella broth, with H. pylori WT strain PMSS1, or with a PMSS1 cagE− isogenic mutant. Induced Lrig1 is labeled in green, nuclei in blue, GSII lectin in magenta, and intrinsic factor in red. In uninfected corpus, intrinsic factor localized to the gland base, GSII lectin localized to the midregion, and there is minimal lineage tracing (A). WT H. pylori increased Lrig1-marked cells, and a subset of these cells coexpressed intrinsic factor and GSII lectin (B); magnified images are shown (individual staining in D–F; merged image in G); this was not seen in response to the PMSS1 cagE− isogenic mutant (C). Cells triple-positive for YFP, GSII lectin, and intrinsic factor were quantified from at least 2 fields per animal (H), and ANOVA and the Bonferroni test were used to determine statistical significance between groups. Images were acquired at 20× magnification.
Lrig1 Expression Is Increased in Human Gastric Premalignant Lesions.
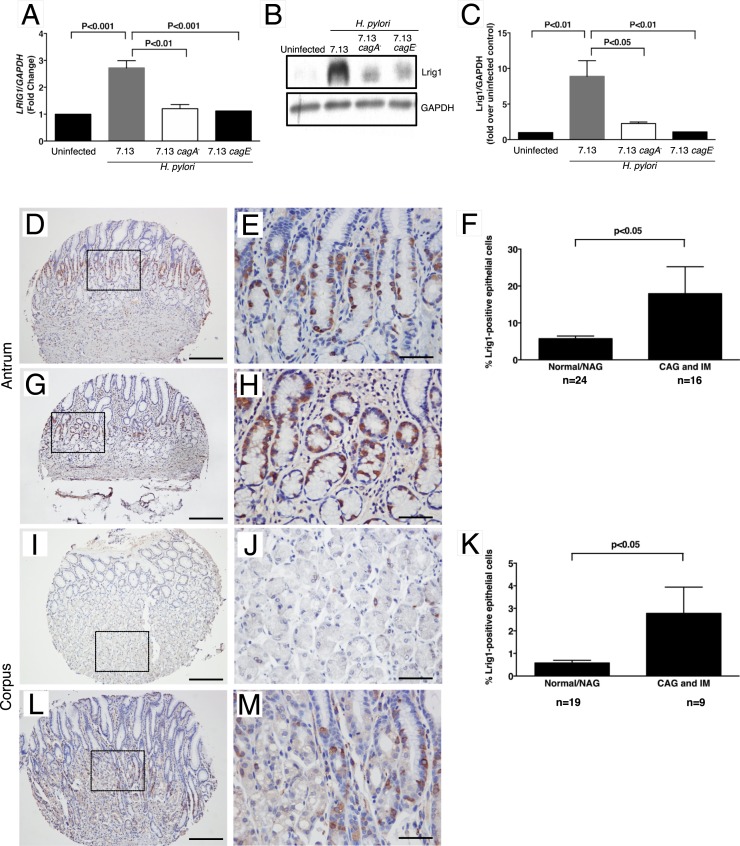
Having determined that the effects of H. pylori on Lrig1 progenitor cells in mice are cag T4SS-dependent, we next sought to validate these findings in a human model of H. pylori infection, as well as to determine whether CagA per se is required for Lrig1 responses to H. pylori. In human gastric monolayers cocultured with WT H. pylori, Lrig1 mRNA expression was significantly increased compared with uninfected monolayers (Fig. 4A). In contrast, there were no significant differences in Lrig1 mRNA expression following infection with either a 7.13 cagA− or cagE− isogenic mutant compared with uninfected controls (Fig. 4A). These results were recapitulated at the protein level using Western blot analysis, which demonstrated a similar increase in Lrig protein expression in a CagA- and CagE-dependent manner (Fig. 4 B and C). These results indicate that CagA, as well as a functional cag T4SS, are required for H. pylori-induced alterations in Lrig1.
Fig. 4.
Lrig1 expression is increased in human gastric monolayers and gastric premalignant lesions in humans. Human gastric monolayers were cocultured with the H. pylori cag+ strain 7.13 or isogenic 7.13 cagA− or cagE− mutant. Levels of LRIG1 mRNA (A) and Lrig1 protein (B and C) were quantified. (A) Real-time RT-PCR was performed on isolated RNA and quantified. (B) Representative Western blot for Lrig1 and glyceraldehyde 3-phosphate dehydrogenase in human gastric monolayers in the presence or absence of H. pylori strain 7.13, 7.13 cagA−, or 7.13 cagE−. (C) Densitometric analysis demonstrating increased expression of Lrig1 in WT H. pylori strain 7.13-infected cells. (D–M) Lrig1 expression was evaluated by immunohistochemistry in human gastric tissue samples. Representative images are show from normal antral gastric mucosa (D and E), chronic atrophic gastritis in antral gastric mucosa (G and H), normal corpus gastric mucosa (I and J), and chronic atrophic gastritis in corpus gastric mucosa (L and M). Low-magnification (10×) images are shown in D, G, I, and L, and high magnification images (40×) are shown in E, H, J, and M. Magnified areas are denoted by the rectangle. Quantification of Lrig1 in the antrum is shown in F and corpus (K). The unpaired Student’s t test was used to determine statistical significance between groups. NAG, nonatrophic gastritis. Premalignant lesions include chronic atrophic gastritis without intestinal metaplasia (CAG) and intestinal metaplasia (IM). (Scale bars: D, G, I, L, 200 μm; and E, H, J, M, 50 μm.)
Having established that H. pylori cag+ strains accelerate differentiation of Lrig1-YFP–marked cells into chief cells and SPEM, we next determined whether Lrig1 expression is differentially expressed in human gastric tissue when stratified by premalignant potential (Fig. 4 and SI Appendix, Table S1). In uninfected individuals with normal gastric mucosa, Lrig1+ cells were identified within the proliferative zone in the antrum and scattered throughout glands in the corpus (Fig. 4 D, E, I, and J). In the corpus, the number of Lrig1+ cells were significantly increased in premalignant lesions (e.g., chronic atrophic gastritis, intestinal metaplasia) compared with normal mucosa or gastritis-only samples (Fig. 4 I–M). Similar to the pattern observed in the corpus, the number of Lrig1+ cells within the antrum was also increased in patients with premalignant lesions (Fig. 4 D–H). Furthermore, the topography of Lrig1 expression was altered within premalignant lesions, such as intestinal metaplasia, where staining localized to the base of gastric glands. These results suggest that alterations in Lrig1+ progeny may contribute to the ability of H. pylori to induce injury and promote carcinogenesis within the human gastric niche.
Discussion
Gastric cancer carries a poor prognosis and is the third-leading cause of cancer-related death worldwide. Approximately 950,000 new cases of gastric cancer per year are attributable to H. pylori, making this pathogen the most common infectious agent linked to malignancy (20). However, only a minority of colonized persons develop gastric cancer, and enhanced risk is related to a combination of H. pylori strain differences, host responses governed by genetic diversity, and/or specific interactions among host, microbial, and environmental determinants (21).
Within gastric glands, stem cells are critical for regulating self-renewal and maintaining tissue integrity. Aberrant β-catenin signaling within a susceptible stem cell population such as Lrig1+ lineages may lower the threshold for carcinogenesis (22, 23), and our findings demonstrate that chronic H. pylori infection induces progenitor cell activity in Lrig1+ cells in a cag-dependent manner. Lrig1 is differentially expressed in many human cancers (24). Studies focused on colorectal cancer have shown patterns of both overexpression and underexpression of LRIG1, and variable expression is seen in metaplasia (25). Conversely, little is known about the function of Lrig1 in the stomach and specifically its role in gastric cancer. Recently, LRIG1 expression was linked to survival in patients with gastric cancer, with higher LRIG1 expression levels in gastric tumors associated with a decreased risk of disease relapse (26). In a metaplastic mouse model, the number of Lrig1+ cells were increased in metaplastic regions of the fundus, and furthermore, these cells were found to be proliferative (26). In the normal mouse stomach, Lrig1 is present in the isthmus of both antral and corpus glands where stem cells reside, suggesting a role for Lrig1 in proliferation (18).
Recent studies have confirmed that Lrig1 marks gastric epithelial progenitor cells, and under homeostatic conditions, these cells have the ability to self-renew and differentiate into all gastric lineages (18, 27). Schweiger et al. (27) recently demonstrated that cells isolated from gastric mucosa expressing high levels of Lrig1 have greater organoid-forming potential than cells with lower levels of Lrig1, further emphasizing the role of Lrig1 as a progenitor cell marker. In the context of acute injury induced by a parietal cell-specific protonophore, DMP-777, Lrig1-YFP–marked cells are able to regenerate gastric mucosa (18). In this study, the total number of glands with YFP+ lineage-labeled cells were significantly increased following DMP-777 treatment, and this was further increased during recovery, suggesting that Lrig1-YFP–marked cells expand during the recovery phase response to acute injury. Furthermore, Lrig1-YFP–marked cells that were mobilized in response to injury coexpressed H+K+ATPase (a parietal cell marker), GS-II lectin (a mucus neck cell marker), or intrinsic factor (a chief cell marker), suggesting that Lrig1+ cells have the capacity to respond to acute injury in the corpus and can give rise to gastric lineages during injury repair (16). Within the context of carcinogenesis, activation of RAS proteins can occur in up to 40% of human gastric cancers, and in a mouse model where Kras was specifically activated in Lrig1+ cells, foveolar hyperplasia developed with loss of parietal cells and no change in chief cells (28).
Our present findings provide insights into these observations and demonstrate that during chronic injury induced by H. pylori, cells arising from Lrig1+ cells differentiate into both surface mucus cells and chief cells, and that some chief cells ultimately produce SPEM, depending on the presence of a virulence factor, the cag PAI.
Within the corpus, cells derived from Lrig1+ cells migrated bidirectionally, which was augmented in response to cag+ H. pylori infection. These findings are concordant with findings reported by Choi et al. (18), who demonstrated that under normal conditions, Lrig1+ cells can give rise to all the major gastric cell lineages, including surface mucus cells. In addition to the corpus, progenitor cells can also influence disease in the antrum. In mouse models in which the tumor-suppressor gene APC was deleted from Lgr5+ stem cells, highly proliferative adenomas developed in the antrum (29). Disruption of the tumor-suppressor gene Klf4 in villin-positive gastric progenitor cells also resulted in the development of spontaneous gastric tumors in the antrum (30). Moreover, chronic inactivation of Klf4 in villin-positive gastric progenitor cells permitted increased susceptibility to chemically induced gastric carcinogenesis and increased rates of gastric tumor initiation (30). Our findings have now implicated aberrant activation of stem/progenitor cells in microbial carcinogenesis by using an endogenous and tractable in vivo model of H. pylori-induced injury.
Not all strains of H. pylori initiate the cascade to gastric cancer, but the bacterial oncoprotein CagA has been consistently shown to exert a critical role in carcinogenesis. For example, CagA has been shown to exert reprogramming potential, inducing epithelial-to-mesenchymal transition (EMT). In the process of CagA-induced EMT, cells lose key features of epithelial differentiation and undergo phenotypic and molecular changes associated with the emergence of stem cell-like cells as well as metastasis (6, 31–34). CagA can also activate β-catenin and thereby induce activation of WNT target genes, such as the transcription factor CDX1. CDX1 can subsequently induce the expression of several stemness-associated reprogramming factors, such as SALL4 and KLF5, potentially contributing to the plasticity of cells and endowing cells with pluripotent potential (35). Consistent with these findings, we have previously demonstrated that H. pylori promotes the expression of KLF5 in mouse gastric glands (36).
H. pylori activates Lgr5+ stem cells through direct colonization of the gastric glands (13); however, the mechanism involved in H. pylori T4SS-dependent effects on Lrig1 progeny likely involves both direct colonization and translocation of CagA by cag+ H. pylori, as well as indirect effects through cag-mediated inflammation. In the epidermis, IL-17A–mediated activation of the IL-17R–EGFR axis in Lrig1+ cells has been linked to the expansion and migration of Lrig1+ cells and their progeny, which has important implications for wound healing and tumorigenesis (37). Within the context of H. pylori pathogenesis, the proinflammatory cytokine IL-17 is significantly increased by H. pylori infection and is associated with disease severity, and future studies will focus on elucidating the importance of this pathway in our model.
In conclusion, we have demonstrated that chronic H. pylori infection stimulates Lrig1+ progenitor cell populations in a cag-dependent manner, and that these cells give rise to a full spectrum of differentiated cells. Moreover, the cag pathogenicity island is a key mediator of the epithelial progenitor cell responsiveness that develops following chronic H. pylori infection. In human samples, Lrig1 expression is enhanced in lesions with premalignant potential. Collectively, these data provide further insight into detrimental events that develop in response to H. pylori infection.
Materials and Methods
Animals and Gastroid Culture.
All procedures were approved by the Institutional Animal Care Committee of Vanderbilt University Medical Center. The generation of Lrig1-CreERT2/+ mice has been described previously (16). For lineage-tracing experiments of Lrig1-expressing cells, Lrig1-CreERT2/+ mice were crossed with R26R-YFP/+ mice, hereinafter referred to as Lrig1+/YFP mice. Further details are provided in SI Appendix, Materials and Methods.
Primary Human Gastric Organoid 2D Monolayers.
Human fundus was collected during sleeve gastrectomies according to a University of Cincinnati Institutional Review Board-approved protocol (IRB protocol no. 2015-4869), and informed consent was obtained. Gastric tissue was processed as described in SI Appendix, Materials and Methods.
H. pylori Strains and Culture Conditions.
The H. pylori cag+ strain PMSS1, a PMSS1 cagE− isogenic mutant, the WT carcinogenic cag+ H. pylori strain 7.13, and 7.13 isogenic cagA− and cagE− mutants were grown in Brucella broth with 10% FBS for 16 h and then harvested by centrifugation (6).
H. pylori quantitative culture.
To assess H. pylori colonization, one-quarter of the stomach was harvested and homogenized in sterile PBS. Samples were processed for quantification as described in SI Appendix, Materials and Methods.
Analysis of inflammation in murine gastric tissue.
The severity of acute and chronic inflammation was graded on a scale of 0 to 3 in both the gastric antrum and corpus, leading to a maximum combined score of 12, as described previously (38). Details of tissue processing are provided in SI Appendix, Materials and Methods.
Immunofluorescence.
Immunofluorescence analysis was performed to assess YFP expression in murine gastric tissue, the relationship between YFP expression and terminally differentiated cells, and proliferation, as described in SI Appendix, Materials and Methods.
Immunohistochemistry.
Lrig1 expression in human gastric tissue was assessed by immunohistochemistry analysis of deparaffinized gastric tissue sections as described in SI Appendix, Materials and Methods.
Western Blot Analysis.
For analysis of cellular protein, human gastric monolayers were cocultured with or without H. pylori for 24 h, and cells were processed for Western blot analysis as described in SI Appendix, Materials and Methods.
Quantitative Real-Time Reverse-Transcriptase PCR.
Human gastric monolayers were cocultured with or without H. pylori for 6 h. RNA was isolated, and reverse-transcriptase PCR and quantitative real-time PCR were performed as described in SI Appendix, Materials and Methods.
Statistical Analysis.
Results are expressed as mean ± SEM. Comparisons were made using ANOVA and post hoc examination of significant means with the Bonferroni test; results were considered significant at P ≤ 0.05. For human tissue data, comparisons were made using an unpaired t test.
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grants DK 58587, CA 77955, CA 116087, and DK 058404 (to R.M.P.), VA Shared Investment Grant IBX003097, and NIH Grant R01 DK101332 (to J.R.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903798116/-/DCSupplemental.
References
- 1.Wroblewski L. E., et al. , Helicobacter pylori induces aberrant Lrig1 stem cell activity within the stomach in a cag type IV secretion system-dependent manner. Gastroenterology 156, S-171–S-172 (2019). [Google Scholar]
- 2.Odenbreit S., et al. , Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287, 1497–1500 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Fischer W., et al. , Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: Essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42, 1337–1348 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Kwok T., et al. , Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449, 862–866 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Shaffer C. L., et al. , Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathog. 7, e1002237 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wroblewski L. E., et al. , Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut 64, 720–730 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M., et al. , Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J. Biol. Chem. 284, 1612–1619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki K., et al. , SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch. 440, 318–324 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Franco A. T., et al. , Activation of β-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A. 102, 10646–10651 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurashima Y., et al. , Deregulation of beta-catenin signal by Helicobacter pylori CagA requires the CagA-multimerization sequence. Int. J. Cancer 122, 823–831 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Murata-Kamiya N., et al. , Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26, 4617–4626 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Tsukashita S., et al. , Beta-catenin expression in intramucosal neoplastic lesions of the stomach: Comparative analysis of adenoma/dysplasia, adenocarcinoma and signet-ring cell carcinoma. Oncology 64, 251–258 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Sigal M., et al. , Helicobacter pylori activates and expands Lgr5 stem cells through direct colonization of the gastric glands. Gastroenterology 148, 1392–404.e21 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Sigal M., et al. , Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 548, 451–455 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Laederich M. B., et al. , The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. J. Biol. Chem. 279, 47050–47056 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Powell A. E., et al. , The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell A. E., et al. , Inducible loss of one Apc allele in Lrig1-expressing progenitor cells results in multiple distal colonic tumors with features of familial adenomatous polyposis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G16–G23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi E., et al. , Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut 67, 1595–1605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung C., et al. , High-resolution mapping reveals that microniches in the gastric glands control Helicobacter pylori colonization of the stomach. PLoS Biol. 17, e3000231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torre L. A., et al. , Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Polk D. B., Peek R. M. Jr, Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 10, 403–414 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagnaire A., Nadel B., Raoult D., Neefjes J., Gorvel J. P., Collateral damage: Insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Microbiol. 15, 109–128 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Naumann M., Sokolova O., Tegtmeyer N., Backert S., Helicobacter pylori: A paradigm pathogen for subverting host cell signal transmission. Trends Microbiol. 25, 316–328 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Poulin E. J., Coffey R. J., LRIG1 is a triple threat: ERBB negative regulator, intestinal stem cell marker and tumour suppressor. Br. J. Cancer 108, 1765–1770 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindquist D., et al. , Expression of LRIG1 is associated with good prognosis and human papillomavirus status in oropharyngeal cancer. Br. J. Cancer 110, 1793–1800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu S., et al. , Expression of LRIG1, a negative regulator of EGFR, is dynamically altered during different stages of gastric carcinogenesis. Am. J. Pathol. 188, 2912–2923 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweiger P. J., et al. , Lrig1 marks a population of gastric epithelial cells capable of long-term tissue maintenance and growth in vitro. Sci. Rep. 8, 15255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi E., Means A. L., Coffey R. J., Goldenring J. R., Active Kras expression in gastric isthmal progenitor cells induces foveolar hyperplasia but not metaplasia. Cell. Mol. Gastroenterol. Hepatol. 7, 251–253.e1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker N., et al. , Lgr5(+) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Li Q., et al. , Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology 142, 531–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagnoli F., Buti L., Tompkins L., Covacci A., Amieva M. R., Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc. Natl. Acad. Sci. U.S.A. 102, 16339–16344 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baud J., et al. , Helicobacter pylori initiates a mesenchymal transition through ZEB1 in gastric epithelial cells. PLoS One 8, e60315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bessède E., et al. , Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene 33, 4123–4131 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Yin Y., et al. , Helicobacter pylori potentiates epithelial:mesenchymal transition in gastric cancer: Links to soluble HB-EGF, gastrin and matrix metalloproteinase-7. Gut 59, 1037–1045 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii Y., et al. , CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc. Natl. Acad. Sci. U.S.A. 109, 20584–20589 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noto J. M., et al. , Helicobacter pylori promotes the expression of Krüppel-like factor 5, a mediator of carcinogenesis, in vitro and in vivo. PLoS One 8, e54344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., et al. , IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1+ stem cells. J. Exp. Med. 216, 195–214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco A. T., et al. , Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 68, 379–387 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.