Significance
The Em values of the 2 QB redox couples, Em(QB/QB•−) and Em(QB•−/QBH2), in Photosystem II are fundamental for understanding the enzyme function, but they remain vague. Using EPR signals arising from the semiquinone, QB•−, we have now measured both redox couples. The semiquinone is thermodynamically stable and has a relatively high potential. This minimizes back-reactions and electrons leaking onto O2, explaining the remarkable stability of QB•−. The release of QBH2 is thermodynamically favorable, and the binding of the substrate PQ is greatly favored over the product PQH2: This optimizes PSII function in the presence of a largely reduced plastoquinone pool. This work corrects and explains a recent report of anomalous values for the QB redox couples.
Keywords: photosynthesis, redox potential, proton-coupled electron transfer, photoinhibition
Abstract
Photosystem II (PSII), the light-driven water/plastoquinone photooxidoreductase, is of central importance in the planetary energy cycle. The product of the reaction, plastohydroquinone (PQH2), is released into the membrane from the QB site, where it is formed. A plastoquinone (PQ) from the membrane pool then binds into the QB site. Despite their functional importance, the thermodynamic properties of the PQ in the QB site, QB, in its different redox forms have received relatively little attention. Here we report the midpoint potentials (Em) of QB in PSII from Thermosynechococcus elongatus using electron paramagnetic resonance (EPR) spectroscopy: Em QB/QB•− ≈ 90 mV, and Em QB•−/QBH2 ≈ 40 mV. These data allow the following conclusions: 1) The semiquinone, QB•−, is stabilized thermodynamically; 2) the resulting Em QB/QBH2 (∼65 mV) is lower than the Em PQ/PQH2 (∼117 mV), and the difference (ΔE ≈ 50 meV) represents the driving force for QBH2 release into the pool; 3) PQ is ∼50× more tightly bound than PQH2; and 4) the difference between the Em QB/QB•− measured here and the Em QA/QA•− from the literature is ∼234 meV, in principle corresponding to the driving force for electron transfer from QA•− to QB. The pH dependence of the thermoluminescence associated with QB•− provided a functional estimate for this energy gap and gave a similar value (≥180 meV). These estimates are larger than the generally accepted value (∼70 meV), and this is discussed. The energetics of QB in PSII are comparable to those in the homologous purple bacterial reaction center.
In photosynthesis, light is absorbed by chlorophyll, resulting in charge separation within a photosynthetic reaction center. In Photosystem II (PSII), the water/plastoquinone photooxidoreductase, the electron hole is transferred from the chlorophyll cation radical, PD1•+, via a redox-active tyrosine (TyrZ) to the Mn4O5Ca cluster. After 4 sequential photochemical turnovers and the resulting oxidations of the Mn4O5Ca cluster (S0–4), 2 water molecules are oxidized (1). On the electron acceptor side, the electron is transferred from the pheophytin anion (PheoD1•−) via a nonexchangeable plastoquinone (QA), which acts as a one-electron relay, to an exchangeable plastoquinone (QB), the terminal electron acceptor (2).
QB•− decays in tens of seconds by charge recombination with S2 or S3 when present, but is stable for several hours if S0 or S1 are present (3). During the subsequent photochemical turnover, QB•− accepts a second electron from the newly formed QA•−. This is accompanied by the 2 protonation steps, thought to occur sequentially, one before and one after the arrival of the second electron (4), as occurs in the homologous site in purple bacteria (5). The QBH2 formed is released from the site and enters the PQ/PQH2 pool, from where it can deliver electrons to the cytochrome b6f complex (6–8).
Due to the 2-electron chemistry of QB, the QB•− state is the only state on the electron acceptor side that cannot be stabilized by forward electron transfer; i.e., there is no “kinetic control” on this step (9). “Kinetic control” of electron transfer occurs in photosynthetic reaction centers when the forward rate outruns both the backward rate and the charge pair recombination rate (9). As a stable intermediate, QB•− is therefore available to back-react via QA•− with the S2 and S3 states on the donor side (3). Two main competing back-reaction pathways occur within PSII: 1) the direct route via electron tunneling from QA•− to P•+ and 2) the indirect route via thermal repopulation of the P•+PheoD1•− state (10). Recombination from the P•+PheoD1•− state mainly forms the chlorophyll triplet state 3P680 (11), which reacts with oxygen to form highly reactive and damaging singlet oxygen 1O2 (12).
The driving force (ΔG) for electron transfer between QA and QB determines the QA•−QB ↔ QAQB•− equilibrium and therefore the extent to which back-reactions from QB•− (or QBH2) to QA can occur. This equilibrium is determined by the difference between the midpoint potentials (Em) of the donor and the acceptor, Em(QA/QA•−) and Em(QB/QB•−) or Em(QB•−/QBH2), according to the following equation:
| [1] |
The redox state of QA can be monitored relatively easily using fluorescence measurements. QA•− has an electrostatic effect on the potential of PheoD1/PheoD1•−, decreasing the already small energy gap between P* (the primary donor) and the first radical pair, which results in a decrease in the yield of charge separation and an increase in fluorescence. A wide range of values have been reported (13). This scatter of reported values is, at least in part, due to the potential being modulated to regulate forward and particularly back electron transfer reactions (9). The QA redox potential is affected by the binding of the Mn4O5Ca cluster to its site (10, 13–15) and by the bicarbonate binding to the nonheme iron (16) (Fig. 1). The redox potential of the QA/QA•− couple in the fully functional, bicarbonate-bound system is −144 mV (15, 16).
Fig. 1.
PSII acceptor side. Blue lines denote possible hydrogen bonds. Data from the 1.9-Å crystal structure (Protein Data Bank ID Code 3WU2) (53).
The measurement of the redox state of QB is more complicated than that of QA. Firstly, there is no easy experimental probe for the redox state of QB. Secondly, in contrast to QA, which undergoes a one-electron redox reaction forming QA•− without the involvement of protons, the QB reduction involves 2 electrons and 2 protons (Eq. 2).
| [2] |
Thirdly, QA is a tightly bound cofactor in both of its redox states, while QB has 2 of the 3 relevant redox states (QB and QBH2) that are relatively weakly bound and exchangeable with PQ or PQH2. As a consequence, kinetic data have been used to estimate the redox potential of QB by deriving the apparent equilibrium constant, K, for the electron transfer between QA•− and QB (17–19). Taking K as 15 (K = 10 to 20 in refs. 17–19), the energy gap is 70 meV, so, with the Em of QA/QA•− of −144 mV (15, 16), the Em of QB/QB•− Em would thus be −74 mV (17–19). Due to the complex nature of the experiments from which the kinetic parameters were extracted, uncertainties remain (17–20). A theoretical model based on thermoluminescence (TL) was used to obtain a similar value for the energy gap between the QA/QA•− and QB/QB•− couples (21, 22). Doubts remain, however, as experimental observables in TL do not always fit with thermodynamic parameters derived from the TL model (21, 23).
In summary, the mechanism and energetics of the PSII acceptor side and especially QB are still relatively poorly understood. The redox potentials for the 2 couples, Em(QB/QB•−) and Em(QB•−/QBH2), have received relatively little attention, despite being central to understanding the quinone reduction function of this enzyme. These redox potentials should provide thermodynamic information related to 1) the stability of the electron transfer intermediates and their tendency to back-react, or to react with O2; 2) the driving force for forward reactions; and 3) the (de)binding properties of the substrate (PQ) and product (PQH2).
An experimental estimate of the Em values of the 2 QB redox couples, QB/QB•− and QB•−/QBH2, based on equilibrium redox titrations was published recently (24). Fourier transform infrared (FTIR) was used to monitor QB•− formation upon illumination by a single flash as a function of the applied potential. The data seemed to show that QB•− was not thermodynamically stable, a surprising result in light of the mechanistic implications and given the conflicting results in the homologous purple bacterial reaction centers (20, 25–27).
Here we have used electron paramagnetic resonance (EPR) spectroscopy to estimate the redox potential of the 2 couples, QB/QB•− and QB•−/QBH2. Two different EPR signals were measured: a QB•−Fe2+ semiquinone signal (28) and a QA•−Fe2+QB•− biradical signal (29, 30). We also used TL to estimate empirically the energy gap between the QA/QA•− and QB/QB•− couples, without relying on the theoretical model previously employed. Our results differ from previous measurements and estimates and show that the semiquinone, QB•−, is stabilized thermodynamically and that the plastoquinone is preferentially bound compared to the plastoquinol.
Results
EPR spectra of PSII were measured at a series of electrode potentials. The D2-Y160F mutant lacking tyrosine D (TyrD) was used to eliminate the TyrD• signal, which would otherwise dominate the PSII EPR spectrum in the radical region (31). At each potential, dark spectra and spectra after illumination at 77 K were recorded. Fig. 2A shows a scan of the radical region around g = 2. The appearance and disappearance of the EPR signal as a function of potential can be observed. This signal has been assigned to the low-field edge of the ground-state doublet of the semiquinone, QB•−Fe2+ (28).
Fig. 2.
EPR spectra of PSII poised at different potentials from an equilibrium redox titration done in the dark. (A) Radical region spectra showing the QB•−Fe2+ at g = 2.0024 (microwave power, 205.1 mW; modulation amplitude, 10.53 G). (B) Wider scan of the same samples after 77-K illumination showing the QA•−Fe2+QB•− signal at g = 1.66 (microwave power, 20 mW; modulation amplitude, 25.35 G).
Fig. 2B shows a full spectrum scan of the same samples as used in Fig. 2A after illumination at 77 K. A peak at 4,000 G (g = 1.66) shows a potential dependence similar to that of the QB•−Fe2+ signal. The g = 1.66 signal has been assigned to the QA•−Fe2+QB•− biradical state (29, 30). The low-temperature illumination generates QA•− in nearly all of the centers. No electron transfer occurs from QA•− to QB or to QB•− at 77 K (30); therefore the biradical signal should only be observed if QB•− were present before the 77-K illumination. Thus, the biradical signal can be used to monitor the presence of QB•− independently of the QB•−Fe2+ signal.
To assess the proportion of QB•− formed during the titration, the signal size of the QB•−Fe2+ signal if present in 100% of the centers was estimated. In a dark-adapted sample, QB•− is present in ∼40% of the centers, while QB is present in the rest (3, 30). That proportionality can be inverted by illuminating at 77 K and subsequently thawing in darkness (3). Therefore, the sum of the amplitudes of the signals present before and after this treatment should yield the size of the signal when QB•− is present in all of the centers. This experiment was done, and the estimated value for 100% QB•− was used to calibrate the amplitudes of the EPR signals in the titrations (SI Appendix, Fig. S2).
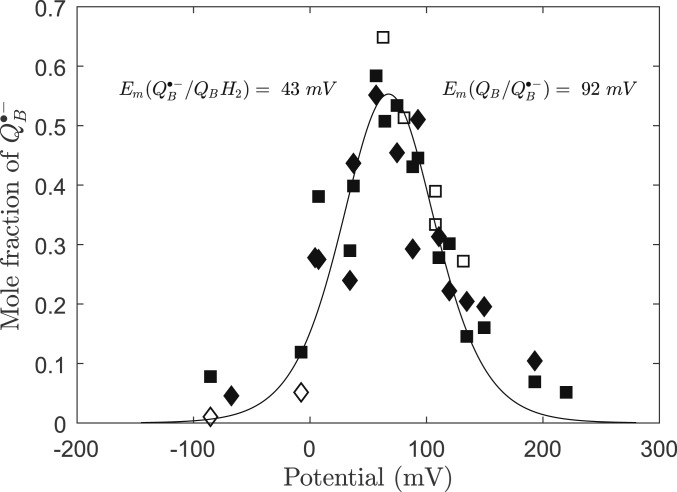
Fig. 3 shows a plot of the normalized QB•−Fe2+ and QA•−Fe2+QB•− signals versus the measured potential, combining data from 3 individual titrations (including those in Fig. 2). Titrations were carried out in oxidizing and reducing directions. Due to poor mediation between ∼0 mV and 50 mV, fewer reliable data points could be collected in that region, and the data are more scattered. The maximum amplitude, which occurs at 67 mV, represented about 55% of the centers in the stable QB•− state. Data were fitted using the model first established by Michaelis (32). The resulting potentials for the 2 couples were Em(QB/QB•−) = 92 ± 23 mV and Em(QB•−/QBH2) = 43 ± 23 mV.
Fig. 3.
Equilibrium redox titration of the QB•− semiquinone in the dark done using 2 different EPR signals. Open squares, oxidizing titration of the QB•−Fe2+ signal; closed squares, reducing titration of the QB•−Fe2+ signal; closed diamonds, reducing titration of the QA•−Fe2+QB•− signal; open diamonds, oxidizing titration of the QA•−Fe2+QB•− signal.
The retention of the Mn4O5Ca cluster during the course of the titration was verified in 2 ways: firstly, by the presence of free “hexaquo” Mn2+ signals in the spectra, representing a loss of the Mn4O5Ca cluster, and, secondly, by the ability to form the S2 multiline signal (33). The Mn4O5Ca cluster was retained in the majority of centers even after exposure to low potentials (SI Appendix, Fig. S5 and associated text).
In addition to the redox titrations of the EPR signals associated with QB•−, pH-dependent TL measurements were used to estimate the energy gap between the QA/QA•− and QB/QB•− couples in PSII cores containing TyrD. TL measures the emission of luminescence associated with the heating-induced back-reaction of a stable charge-separated state. The peak temperature of TL is indicative of the energy stored in the charge-separated state and is determined by redox potentials of both the recombination partners, in this case, S2/S1 and QB/QB•− (3, 21). Below pH of ∼7, the S2/S1 couple involves very little protonation and is almost independent of pH (34). The QB/QB•− couple involves proton release when QB•− is reoxidized and is expected to follow Nernst behavior (19, 35, 36), with the redox potential changing by −59 mV per pH unit. Thus, the pH dependence of the S2QB•− recombination peak position should reflect this process and can be used as an empirical calibration of the change in emission temperature in terms of the change in the redox potential of QB.
Fig. 4 shows the TL curves of long dark-adapted PSII samples after one saturating flash at different pH values. A clear shift of the peak positions to lower temperatures with increasing pH can be seen. Fig. 4, Inset shows a plot of peak temperature versus the pH, from which a linear dependence of −11.6 °C per pH unit can be observed. Using the ΔE relationship given by the Nernst equation, this translates to −5.1 meV °C−1. A similar slope was observed by Vass and Inoue (37) in their study of the pH dependence of TL in plant PSII (see also SI Appendix, Figs. S8–S13).
Fig. 4.
Thermoluminescence of long-dark-adapted PSII cores from T. elongatus. Blue, after one saturating flash at different values of pH; red, after the addition of DCMU. Inset shows a plot of the S2QB•− peak position versus the pH with a slope of −11.6 °C/pH unit and a plot of the S2QB•− peak over the restricted zone of pH in which it is pH independent. At pH 6.5, a TL band of ∼70 °C becomes the dominant emission but is not attributed to the S2QB•−; it is more likely to be from the so-called C band (54).
This calibration was then used to estimate the gap between the QA and QB couples. Fig. 4 (red circles) shows the S2QA•− recombination band in the presence of the QB-site inhibitor, DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) occurring at ∼14 °C. The difference in peak positions of 36 °C between the S2QA•− and S2QB•− peaks corresponds to an energy gap of 183 meV. This may be extended by taking into account the upshift of S2QA•− caused by the binding of DCMU of ∼52 mV in plant PSII (38). However, this effect of DCMU has yet to be demonstrated in Thermosynechococcus elongatus PSII.
Discussion
In the present work, the midpoint potential of the terminal electron acceptor of PSII, QB, was measured with EPR. The results show that the semiquinone, QB•−, is stabilized thermodynamically at pH 7. The redox potentials derived from the data for the 2 redox couples are Em(QB/QB•−) ≈ 90 mV and Em(QB•−/QBH2) ≈ 40 mV. In addition, we have estimated the difference in redox potentials between the QA/QA•− and QB/QB•− couples using the pH dependence of S2QA•− and S2QB•− recombination measured by TL. The energy difference obtained from this approach is ∼180 meV (230 meV if there is a DCMU-induced shift on the QA potential, as occurs in plant PSII). This value is similar to the ΔE of 234 meV between the Em = −144 mV for QA/QA•− (15, 16) and +90 mV for QB/QB•− presented here.
Stabilization of the Semiquinone State within the QB Site.
A fit of the Nernst model to our data shows the difference between the QB/QB•− and QB•−/QBH2 couples, ΔEm, to be ∼+50 mV, a value that agrees with the measured maximum of ∼55% of stable QB•− in the EPR signal generated in the titrations. This ΔEm is indicative of the degree of stabilization of the semiquinone radical, QB•−, in the site, with larger values equating to a more stable semiquinone. When titrating a free quinone in the membrane, the semiquinone is not stabilized, the redox transition occurs as a steeper n = 2 curve (i.e., a 2-electron transition), no semiquinone can be observed, and the ΔEm is negative. Although the membrane is aprotic, the quinone head group can access the aqueous solvent, and thus the semiquinone is destabilized [ΔEm ≈ −500 meV for ubiquinone (39)]. Our data indicate that QB•− is strongly bound and stabilized by the QB site in comparison to a quinone in the membrane. The structure of the QB site (Fig. 1) shows several features that likely contribute to the electrochemical properties of QB•−, including 1) the proximity to the nonheme ferrous iron, 2) hydrogen bonds from D1His215 and D1Ser264 to both carbonyls of the quinone, and 3) the likely protonation of the distal H-bonding D1Ser264/His252 pair (4).
This stabilization can be rationalized in part as a damage prevention mechanism. In PSII, back-reactions from P•+QA•− result in damage to the complex (9, 16). A large gap between the QA/QA•− and QB/QB•− couples would favor QB reduction and lower the equilibrium concentration of QA•−, thereby diminishing the likelihood of a damaging back-reaction from QA•−. This stabilization of the semiquinone would come at a cost, because a more positive Em(QB/QB•−) must yield a more negative Em(QB•−/QBH2) in order to maintain the chemical requirement that the average Em for the 2 couples remains unchanged (see below). Back-reactions from the fully reduced quinol, QBH2, to QA would therefore become more likely. These back-reactions, however, would only occur when the plastoquinone pool is reduced, because quinol/quinone exchange occurs orders of magnitude faster (∼10 ms; ref. 40) than the S2QBH2 back-reaction, which we predict to decay with kinetics between the recombination rates of QA•− and QB•− with S2 [i.e., between ∼1 s and ∼30 s (41)].
The high potential Em (∼90 mV) for the QB/QB•− also means that QB•− is a poor reductant for O2 forming superoxide (Em(O2/O2•−) ≈ −160 mV). It has been suggested that QB•− may be a source of reactive oxygen species (42); however, its very long lifetime, with half-times of hours in the presence of S1 and S0 (3, 30), argues against this. The present work showing the thermodynamic stabilization of QB•− provides a good explanation for its lack of reactivity with oxygen and for its very long lifetime in the dark.
The presence of a stabilized QB•− directly contradicts a recent report (24) (discussed in detail below) but is supported by other reports. A reductive titration of the g = 1.66 biradical signal with PSII particles from Phormidium laminosum was reported previously as part of the work done to identify the origin of the biradical signal (29). Although no reversibility was demonstrated and the signal size was not quantified, a thermodynamically stable QB•−, similar to that reported in this work, was clearly present in the reductive titration.
A thermodynamically stable QB•− was also present in the homologous purple bacterial reaction centers from Rhodobacter sphaeroides (25), Chromatium vinosum (26), and Blastochloris viridis (27). These titrations show ∼30 to 50% of the total quinone form as a stable semiquinone (see SI Appendix, Fig. S7 for a corrected fitting of these titrations). The exact ΔEm and Emavrg all differ from each other to some extent, perhaps reflecting different mechanistic requirements in the different species, but, in all reaction centers, the semiquinone, QB•−, is clearly thermodynamically stabilized. Together, these studies in the literature (25–27, 29) provide strong support for the current results.
Preferential Binding of the Quinone vs. the Quinol.
Although the redox potential of the plastoquinone in the pool from T. elongatus has not been measured, it is expected to be very similar to that in other organisms, i.e., a 2-electron transition at 117 mV (43, 44). The average for the 2 QB redox couples measured here (Emavrg ≈ 65 mV) is therefore about 50 mV lower than that of the plastoquinone pool. This represents a significant driving force for the release of the PQH2 to the pool. This should be considered as an additional energy loss in the ongoing effort to understand energy use in PSII. A smaller but qualitatively similar energy loss was reported earlier for UQH2 release from the QB site in the purple bacterial reaction center to the pool, based on a computational chemistry approach (39).
From the difference in redox potential, the ratio of the binding constants for the quinone and the quinol forms can be calculated (16) (see SI Appendix, Fig. S6 and associated text for details). It is found that the quinone is bound to the QB site ∼50 times more tightly than the quinol. Again, this fits qualitatively with the situation reported in purple bacterial reaction centers (39, 45). For PSII to function optimally, it makes sense that the binding of the substrate (the quinone) is favored over that of the product (the quinol). Preferential quinone binding will allow PSII to function when the pool is significantly reduced.
In the literature, it has often been assumed that the binding constants of the quinone and quinol in the QB site are equal both in PSII and in purple bacterial reaction centers (e.g., refs. 18 and 20). Nevertheless, the QB•− redox titrations in purple bacterial reaction centers indicated the preferential binding of the quinone over the quinol (25), and other reports favor this binding regime (6, 46), partly because of the experimental indications (25–27, 39) and partly because it seemed mechanistically more likely [see SI Appendix for further discussion and a possible exception in C. vinosum (26)]. The present work is good experimental evidence for preferential binding of the quinone over the quinol in PSII.
The Difference in Redox Potentials Between QA and QB.
The difference in equilibrium redox potentials between the QA/QA•− and QB/QB•− couples reported here, ∼234 meV, is larger than previously estimated (∼70 meV) from the kinetics of the forward and backward electron transfer reactions between QA and QB (21, 41). The following factors could contribute to the mismatch: 1) The previous dynamic measurements are based on assumptions that may be complicated by the so-called “gating effect,” which determines the rate-limiting step of electron transfer between QA and QB rather than the driving force (47). This dynamic effect is not accounted for in the previous estimates and may at least contribute to the discrepancy. 2) While the QB redox couples themselves do not suffer from the problem of being titrated adjacent to an already reduced cofactor, the Em of QA/QA•− may be systematically underestimated (a lower potential) in redox titrations due to the binding of QBH2 which would result in a smaller energy gap. 3) The QA/QA•− couple may be more specifically sensitive to modulation from the electron donor side, which would also affect the energy gap estimate (see SI Appendix for a more detailed discussion).
Given the uncertainty about the energy gap between the Em QA/QA•− from the literature and the EmQB/QB•− measured here, we made an independent estimate of this energy gap by estimating empirically the energy gap between S2QA•− and S2QB•− as a function of pH. It was assumed that the energy gap determining the peak position of the TL follows Nernst behavior and shifts by 59 mV per pH unit. This assumption seems justified given the experimentally determined proton stoichiometries in the literature (34, 36). The resulting value, ∼180 meV or ∼230 meV if we assume a DCMU binding effect (40), is similar to the energy gap obtained from the equilibrium redox titrations (∼234 meV). A similar value (∼212 meV) is obtained by using the data from Vass and Inoue (37). This estimate is done in the presence of S2 (as were the previous kinetic estimates) rather than S1 (as were the equilibrium redox titrations), thus indicating the absence of a significant influence of the S state on the energy gap. We remain somewhat skeptical about the similarity of the 2 values because in the TL method: 1) The determination of the S2QA•− and S2QB•− peak temperatures are not very accurate, and 2) the added DCMU shift of 50 mV has not yet been determined in PSII cores from T. elongatus. Despite these shortcomings, the TL measurements seem to provide independent support for the energy gap being significantly bigger than 70 meV estimated previously.
A large energy gap would make sense in functional terms. As QB•− is the terminal electron acceptor, it cannot be prevented from back-reacting by “kinetic control,” i.e., by ensuring the forward electron transfer is faster than the back-reactions (9). Thus wasteful and damaging back-reactions can be minimized by increasing the energy gap between the QA/QA•− and QB/QB•− couples.
Rationalizing the Conflicting Report in the Literature.
The findings in the present work differ significantly from a recently published study on the redox potentials of QB (24). In that work, redox titrations were performed in which the ability to form QB•− by a single saturating flash was monitored by FTIR measurements in a spectroelectrochemical thin cell (24). This is not a direct measurement of QB•−; rather, it is a measurement of the ability to form QB•− upon flash illumination (or to form QBH2 if QB•− were already present). This method should, in principle, provide a valid QB titration. The results in ref. 24, however, showed no evidence for stable QB•− formation. Instead a redox curve was reported that was essentially indistinguishable from an n = 2 curve, i.e., a 2-electron transition, with an Em = 155 mV at pH 6.5; this is equivalent to 125 mV at pH 7. The Em and the n = 2 curve are both characteristic of a titration of free plastoquinone (43, 44). While the PSII cores have no membrane and thus no membrane-localized quinone pool, they do contain 1 or 2 free quinones in addition to QB, and these quinones act as a limited plastoquinone pool (30, 48, 49).
Here we show that the Em values of the quinone couples in the QB site are more negative than that of free quinone; therefore, in a reductive titration, the free quinone will be reduced before the QB quinone. Since quinone exchange happens on the millisecond timescale, this free quinol will exchange with a quinone in the QB site, allowing the free quinone to be reduced. If the mediation with the QB site is insufficient, electrons will not be removed from QBH2, and therefore the redox state of the quinone in the QB site will reflect the redox state of the pool, irrespective of the true quinone potential in the QB site. Under these conditions, an n = 2 Nernst dependency at the Em of the pool would be observed even when probing exclusively the QB site. Given that 1) only 3 mediators were used in the titration, only one of which is in the appropriate range, and 2) the flash-induced state monitored had to be stable for several minutes in order to allow the data collection, it seems likely that the redox mediation was insufficient and therefore the Em of PQ was measured (24). See SI Appendix for a detailed analysis of ref. 24.
Conclusion
Fig. 5 summarizes the results of the present work and provides a consistent energetic description of PSII, now including the 2 redox potentials of the QB couples. This provides insights into the redox tuning of QB with respect to the redox potentials of its neighboring redox partners, QA and free plastoquinone. The energy gap between the QA/QA•− and the 2 QB redox couples reported here is significantly larger than previously assumed. The redox potentials need to be high enough compared to that of QA to provide for sufficient driving force and to minimize back-reactions. The redox potential of the plastoquinone pool limits the average value of the 2 QB couples. The measured value shows that, in PSII, ∼50 meV of driving force is expended to ensure rapid debinding of the quinol and the preferential binding of the quinone. These data indicate that the protein tunes the thermodynamics of the QB redox chemistry to optimize function over a wide range of plastoquinone pool reduction states, while minimizing back-reactions and side reactions with O2.
Fig. 5.
Redox scheme of PSII. Redox potentials values were taken from the present work for the QB couples and from ref. 16 for QA, from ref. 55 for PheoD1, from ref. 2 for P680, and from refs. 43 and 44 for the quinone pool. The yellow arrow represents excitation of the P680 chlorophyll. The straight black arrows are electron transfer events. The curved arrows show excitation transfer.
Materials and Methods
Isolation of PSII from T. elongatus.
PSII cores were isolated from D2-Y160F; CP43-His strain (31) using a method based on that of Sugiura and Inoue (50) with specific modifications described in SI Appendix.
EPR-Detected Potentiometric Titrations.
Multiple PSII preparations were pooled to yield 7 mL to 10 mL of purified PSII at 0.7 mg (Chl)⋅mL−1 in titration buffer. Redox titrations were carried out essentially as described by Dutton (51) at 15 °C under a bicarbonate-enriched argon atmosphere in absolute darkness and in the presence of the following redox mediators all at 50 μM: N,N,N′,N′-tetramethyl-p-phenylenediamine (300 mV), 2,6-Dichlorophenolindophenol (217 mV), Phenazine methosulfate (80 mV), Thionine (64 mV), Phenazine ethosulfate (55 mV), Methylene blue (11 mV), Pyocyanin (−34 mV), Indigotetrasulfonate (−46 mV), and Resorufin (−51 mV). Reductive titrations were carried out using sodium dithionite; oxidative titrations were carried out using potassium ferricyanide. EPR spectra were recorded on a Bruker ElexSys X-band spectrometer fitted with an Oxford Instruments liquid helium cryostat and temperature control system. Illumination at 77 K (20 min) was carried out in an unsilvered dewar with a halogen lamp (LQ 2600; Fiberoptic-Heim AG). See SI Appendix, Fig. S1 for further information on data treatment and fitting.
TL was measured using a laboratory-built apparatus (52). PSII cores (20 μg [Chl]⋅mL−1) in buffer 1 (MOPS was used instead of MES at pH > 7 and HEPES at pH > 8, 20% glycerol instead of 10%) were dark-adapted for >1 h at 4 °C, and 200-μL samples were loaded in darkness. If required, DCMU (in ethanol) was added to the sample on the sample plate (50 μM final concentration). The pH was measured in darkness directly prior to the experiment. Samples were cooled (<30 s) to −20 °C with liquid N2 directly after a ∼5-ns laser flash at 523 nm, the second harmonic of a Nd-YAG laser (Minilite II; Continuum). The frozen samples were then heated at a constant rate of 20 °C⋅min−1, and TL was detected with a photomultiplier (H7422-50; Hamamatsu). The signal was amplified using a transimpedance amplifier (C7319; Hamamatsu) and digitized using a microcontroller board based on the Atmel SAM3X8E ARM Cortex-M3 CPU (Arduino Due).
Supplementary Material
Acknowledgments
We are grateful for access to the EPR facilities available at the Aix-Marseille University EPR center and at the national TGE-RPE network (FR3443). This work was supported by the Royal Society Wolfson Research Merit Award (to A.W.R.) and by Biotechnology and Biological Sciences Research Council (BBSRC) Grants BB/K002627/1 and BB/R00921X (to A.W.R.). These BBSRC grants also supported A.F. and S.D.C., and S.D.C. was also supported by an Imperial College London Scholarship during his PhD studies. J.S.D. was supported by Engineering and Physical Science Research Council Standard Research Studentship EP/P504953/1 from the Energy Futures Laboratory of Imperial College.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910675116/-/DCSupplemental.
References
- 1.Dau H., Zaharieva I., Principles, efficiency, and blueprint character of solar-energy conversion in photosynthetic water oxidation. Acc. Chem. Res. 42, 1861–1870 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Rappaport F., Diner B. A., Primary photochemistry and energetics leading to the oxidation of the (Mn)4Ca cluster and to the evolution of molecular oxygen in photosystem II. Coord. Chem. Rev. 252, 259–272 (2008). [Google Scholar]
- 3.Rutherford A. W., Crofts A. R., Inoue Y., Thermoluminescence as a probe of photosystem II photochemistry. The origin of the flash-induced glow peaks. Biochim. Biophys. Acta Bioenerg. 682, 457–465 (1982). [Google Scholar]
- 4.Saito K., Rutherford A. W., Ishikita H., Mechanism of proton-coupled quinone reduction in Photosystem II. Proc. Natl. Acad. Sci. U.S.A. 110, 954–959 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wraight C. A., Proton and electron transfer in the acceptor quinone complex of photosynthetic reaction centers from Rhodobacter sphaeroides. Front. Biosci. 9, 309–337 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Wraight C. A., Oxidation‐reduction physical chemistry of the acceptor quinone complex in bacterial photosynthetic reaction centers: Evidence for a new model of herbicide activity. Isr. J. Chem. 21, 348–354 (1981). [Google Scholar]
- 7.Müh F., Glöckner C., Hellmich J., Zouni A., Light-induced quinone reduction in photosystem II. Biochim. Biophys. Acta 1817, 44–65 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Cardona T., Sedoud A., Cox N., Rutherford A. W., Charge separation in photosystem II: A comparative and evolutionary overview. Biochim. Biophys. Acta 1817, 26–43 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Rutherford A. W., Osyczka A., Rappaport F., Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O2. FEBS Lett. 586, 603–616 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Johnson G. N., Rutherford A. W., Krieger A., A change in the midpoint potential of the quinone QA in Photosystem II associated with photoactivation of oxygen evolution. Biochim. Biophys. Acta Bioenerg. 1229, 202–207 (1995). [Google Scholar]
- 11.Rutherford A. W., Paterson D. R., Mullet J. E., A light-induced spin-polarized triplet detected by EPR in Photosystem II reaction centers. Biochim. Biophys. Acta Bioenerg. 635, 205–214 (1981). [DOI] [PubMed] [Google Scholar]
- 12.Durrant J. R., Giorgi L. B., Barber J., Klug D. R., Porter G., Characterisation of triplet states in isolated Photosystem II reaction centres: Oxygen quenching as a mechanism for photodamage. Biochim. Biophys. Acta Bioenerg. 1017, 167–175 (1990). [Google Scholar]
- 13.Krieger A., Rutherford A. W., Johnson G. N., On the determination of redox midpoint potential of the primary quinone electron acceptor, QA, in Photosystem II. Biochim. Biophys. Acta Bioenerg. 1229, 193–201 (1995). [Google Scholar]
- 14.Krieger A., Weis E., Demeter S., Low-pH-induced Ca2+ion release in the water-splitting system is accompanied by a shift in the midpoint redox potential of the primary quinone acceptor QA. Biochim. Biophys. Acta Bioenerg. 1144, 411–418 (1993). [Google Scholar]
- 15.Shibamoto T., Kato Y., Sugiura M., Watanabe T., Redox potential of the primary plastoquinone electron acceptor Q(A) in photosystem II from Thermosynechococcus elongatus determined by spectroelectrochemistry. Biochemistry 48, 10682–10684 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Brinkert K., De Causmaecker S., Krieger-Liszkay A., Fantuzzi A., Rutherford A. W., Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. Proc. Natl. Acad. Sci. U.S.A. 113, 12144–12149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diner B. A., Dependence of the deactivation reactions of Photosystem II on the redox state of plastoquinone pool a varied under anaerobic conditions. Equilibria on the acceptor side of Photosystem II. Biochim. Biophys. Acta Bioenerg. 460, 247–258 (1977). [DOI] [PubMed] [Google Scholar]
- 18.Bouges-Bocquet B., “Electron acceptors of photosystem II” in Proceedings of the 3rd International Congress on Photosynthesis, Avron M., Ed. (Elsevier, 1975), pp. 579–588. [Google Scholar]
- 19.Robinson H. H., Crofts A. R., “Kinetics of proton uptake and the oxidation-reduction reactions of the quinone acceptor complex of PS II from pea chloroplasts” in Proceedings of the International Congress on Photosynthesis. Brussels, Belgium, Aug 1-6, 1983, C. Sybesma, Ed. (Martinus Nijhoff, The Hague, 1984), vol. I, pp. 477–480.
- 20.Crofts A. R., Wraight C. A., The electrochemical domain of photosynthesis. Biochim. Biophys. Acta Bioenerg. 726, 149–185 (1983). [Google Scholar]
- 21.Rappaport F., Lavergne J., Thermoluminescence: Theory. Photosynth. Res. 101, 205–216 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Rose S., et al. , D1-arginine257 mutants (R257E, K, and Q) of Chlamydomonas reinhardtii have a lowered QB redox potential: Analysis of thermoluminescence and fluorescence measurements. Photosynth. Res. 98, 449–468 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato Y., et al. , Influence of the PsbA1/PsbA3, Ca2+/Sr2+ and Cl−/Br− exchanges on the redox potential of the primary quinone QA in Photosystem II from Thermosynechococcus elongatus as revealed by spectroelectrochemistry. Biochim. Biophys. Acta 1817, 1998–2004 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Kato Y., Nagao R., Noguchi T., Redox potential of the terminal quinone electron acceptor QB in photosystem II reveals the mechanism of electron transfer regulation. Proc. Natl. Acad. Sci. U.S.A. 113, 620–625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutherford A. W., Evans M. C. W., Direct measurement of the redox potential of the primary and secondary quinone electron acceptors in Rhodopseudomonas sphaeroides (wild-type) by EPR spectrometry. FEBS Lett. 110, 257–261 (1980). [DOI] [PubMed] [Google Scholar]
- 26.Heathcote P., Rutherford A. W., “An EPR signal arising from QB-Fe in chromatium vinosum” in Progress in Photosynthesis Research (Dordrecht, 1986), vol. 1, pp. 201–204. [Google Scholar]
- 27.Rutherford A. W., Heathcote P., Evans M. C. W., Electron-paramagnetic-resonance measurements of the electron-transfer components of the reaction centre of Rhodopseudomonas viridis. Oxidation–reduction potentials and interactions of the electron acceptors. Biochem. J. 182, 515–523 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedoud A., et al. , Semiquinone-iron complex of photosystem II: EPR signals assigned to the low-field edge of the ground state doublet of QA•-Fe2+ and QB•-Fe2+. Biochemistry 50, 6012–6021 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Corrie A. R., Nugent J. H. A., Evans M. C. W., Identification of EPR signals from the states QaQb and Qb in photosystem II from Phormidium laminosum. Biochim. Biophys. Acta Bioenerg. 1057, 384–390 (1991). [Google Scholar]
- 30.Fufezan C., Zhang C., Krieger-Liszkay A., Rutherford A. W., Secondary quinone in photosystem II of Thermosynechococcus elongatus: Semiquinone-iron EPR signals and temperature dependence of electron transfer. Biochemistry 44, 12780–12789 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Sugiura M., et al. , Site-directed mutagenesis of Thermosynechococcus elongatus photosystem II: The O2-evolving enzyme lacking the redox-active tyrosine D. Biochemistry 43, 13549–13563 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Michaelis L., Theory of the reversible two-step oxidation. J. Biol. Chem. 96, 703–715 (1932). [Google Scholar]
- 33.Dismukes G. C., Siderer Y., EPR spectroscopic observations of a manganese center associated with water oxidation in spinach chloroplasts. FEBS Lett. 121, 78–80 (1980). [Google Scholar]
- 34.Rappaport F., Lavergne J., Proton release during successive oxidation steps of the photosynthetic water oxidation process: Stoichiometries and pH dependence. Biochemistry 30, 10004–10012 (1991). [DOI] [PubMed] [Google Scholar]
- 35.Rutherford A. W., Renger G., Koike H., Inoue Y., Thermoluminescence as a probe of photosystem II. The redox and protonation states of the secondary acceptor quinone and the O2-evolving enzyme. Biochim. Biophys. Acta Bioenerg. 767, 548–556 (1984). [Google Scholar]
- 36.Haumann M., Junge W., The rates of proton uptake and electron transfer at the reducing side of photosystem II in thylakoids. FEBS Lett. 347, 45–50 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Vass I., Inoue Y., pH dependent stabilization of S2QA− and S2QB− charge pairs studied by thermoluminescence. Photosynth. Res. 10, 431–436 (1986). [DOI] [PubMed] [Google Scholar]
- 38.Krieger-Liszkay A., Rutherford A. W., Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: Relevance to photodamage and phytotoxicity. Biochemistry 37, 17339–17344 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Zhu Z., Gunner M. R., Energetics of quinone-dependent electron and proton transfers in Rhodobacter sphaeroides photosynthetic reaction centers. Biochemistry 44, 82–96 (2005). [DOI] [PubMed] [Google Scholar]
- 40.de Wijn R., van Gorkom H. J., Kinetics of electron transfer from QA to QB in photosystem II. Biochemistry 40, 11912–11922 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Petrouleas V., Crofts A. R., “The iron-quinone acceptor complex” in Photosystem II, Wydrzynski T., Satoh K., Freeman J., Eds. (Springer-Verlag, Berlin, 2005), pp. 177–206. [Google Scholar]
- 42.Pospíšil P., Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta Bioenerg. 1817, 218–231 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Golbeck J. H., Kok B., Redox titration of electron acceptor Q and the plastoquinone pool in Photosystem II. Biochim. Biophys. Acta Bioenerg. 547, 347–360 (1979). [DOI] [PubMed] [Google Scholar]
- 44.Rich P. R., Bendall D. S., The kinetics and thermodynamics of the reduction of cytochrome c by substituted p-benzoquinols in solution. Biochim. Biophys. Acta Bioenerg. 592, 506–518 (1980). [DOI] [PubMed] [Google Scholar]
- 45.Gunner M. R., Madeo J., Zhu Z., Modification of quinone electrochemistry by the proteins in the biological electron transfer chains: Examples from photosynthetic reaction centers. J. Bioenerg. Biomembr. 40, 509–519 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diner B. A., Schenck C. C., De Vitry C., Effect of inhibitors, redox state and isoprenoid chain length on the affinity of ubiquinone for the secondary acceptor binding site in the reaction centers of photosynthetic bacteria. Biochim. Biophys. Acta Bioenerg. 766, 9–20 (1984). [Google Scholar]
- 47.Graige M. S., Feher G., Okamura M. Y., Conformational gating of the electron transfer reaction QA−.QB → QAQB−. in bacterial reaction centers of Rhodobacter sphaeroides determined by a driving force assay. Proc. Natl. Acad. Sci. U.S.A. 95, 11679–11684 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kern J., et al. , Purification, characterisation and crystallisation of photosystem II from Thermosynechococcus elongatus cultivated in a new type of photobioreactor. Biochim. Biophys. Acta Bioenerg. 1706, 147–157 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Krivanek R., Kern J., Zouni A., Dau H., Haumann M., Spare quinones in the QB cavity of crystallized photosystem II from Thermosynechococcus elongatus. Biochim. Biophys. Acta Bioenerg. 1767, 520–527 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Sugiura M., Inoue Y., Highly purified thermo-stable oxygen-evolving photosystem II core complex from the thermophilic cyanobacterium Synechococcus elongatus having His-tagged CP43. Plant Cell Physiol. 40, 1219–1231 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Dutton P. L. , Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77° K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim. Biophys. Acta 226, 63–80 (1971). [DOI] [PubMed] [Google Scholar]
- 52.De Causmaecker S., “Bioenergetic studies on the quinone electron acceptors of photosystem II,” PhD thesis, Imperial College London, London, UK (2018).
- 53.Umena Y., Kawakami K., Shen J.-R., Kamiya N., Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Johnson G. N., Boussac A., Rutherford A. W., The origin of 40-50°C thermoluminescence bands in Photosystem II. Biochim. Biophys. Acta Bioenerg. 1184, 85–92 (1994). [Google Scholar]
- 55.Kato Y., Sugiura M., Oda A., Watanabe T., Spectroelectrochemical determination of the redox potential of pheophytin a, the primary electron acceptor in photosystem II. Proc. Natl. Acad. Sci. U.S.A. 106, 17365–17370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.