Abstract
Background
Tachyplesin III, an antimicrobial peptide (AMP), provides protection against multidrug-resistant (MDR) bacterial infections and shows cytotoxicity to mammalian cells. Mixed bacterial infections, of which P. aeruginosa plus A. baumannii is the most common and dangerous combination, are critical contributors to the morbidity and mortality of long-term in-hospital respiratory medicine patients. Therefore, the development of effective therapeutic approaches to mixed bacterial infections is urgently needed.
Methods and results
In this study, we demonstrated that compared with individual infections, mixed infections with MDR bacteria P. aeruginosa and A. baumannii cause more serious diseases, with increased pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and chemokines (MCP-1/MIP-2) and reduced mouse survival. In vitro treatment with Tachyplesin III enhanced phagocytosis in a mouse alveolar macrophage cell line (MH-S). Strikingly, in vivo, Tachyplesin III demonstrated a potential role against mixed-MDR bacterial coinfection. The bacterial burden in bronchoalveolar lavage fluid (BALF) was significantly reduced in the Tachyplesin III-treated group. In addition, a systemic reduction in pro-inflammatory cytokines and decreased lung injury occurred with Tachyplesin III therapy.
Conclusion
Therefore, our study demonstrated that Tachyplesin III represents a potential therapeutic treatment against mixed-MDR bacterial infection in vivo, which sheds light on the development of therapeutic strategies against mixed-MDR bacterial infections.
Keywords: Tachyplesin III, antimicrobial peptides, multidrug-resistant bacterial, coinfection, phagocytosis
Introduction
The use of antibiotics, first discovered approximately 80 years ago,1 is a double-edged sword.2 Antibiotics play an extremely important role in the human against various bacterial and viral infections by killing pathogens directly, reducing tissue damage and inhibiting the production of pro-inflammatory cytokines. However, long-term antibiotic misuse induces the emergence of multidrug-resistant (MDR) bacteria.3 Decreased antibiotic uptake, like reducing the entry of antibiotics into the bacteria and activating efflux system, and inactivation antibiotics targets are the most common resistance mechanism for bacterial. Frequently, ventilator-associated pneumonia (VAP) and intensive care unit (ICU) patients suffer lung bacterial pneumonia and are infected with multiple bacterial pathogens.4 Among these, Gram-negative P. aeruginosa and A. baumannii are the worst MDR bacteria that generally affect individuals with bacterial pneumonia-induced morbidity and mortality, particularly opportunistic and cross-infected bacteria within long-term in-hospital respiratory medicine patients.5,6 Staphylococcus aureus alpha toxin (AT) has recently been identified as a risk factor for host mucosal defense and macrophage function. Coinfection with S. aureus expressing AT improves the dissemination and propagation of gram-negative bacteria, such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii.7 No drugs can effectively eliminate and clear MDR bacteria. Therefore, therapeutic strategies for MDR bacterial coinfection remain to be elucidated.
Antimicrobial peptides (AMPs), which are widely present in a range of organisms, exhibit a variety of biological activities, including antibacterial,4,8,9 antifungal,10 antiparasitic,11 antiviral,12 and anticancer13 activities. Generally, AMPs are distinguished into four structural classes: α-helical peptides, β-sheet peptides, extended peptides, and loop peptides,14–17 of which α-helical peptides represent the largest proportion of the AMPs that have been found. β-Sheet peptides are also widely studied, because of their intramolecular disulfide bond-mediated rigidity in aqueous solution. Tachyplesin III, a β-sheet AMP,18 not only shows potential activity against MDR bacterial infection but also exhibits low cytotoxicity to mammalian cells.19 The cationic β-hairpin structure of Tachyplesin III provides strong stability under serum conditions and provides the foundation for bacteria killing by directly interacting with lipid membranes20 or binding to bacterial DNA.21 Recently, our group reported a novel mechanism by which Tachyplesin III may target FabG in bacteria killing.9
Except the capability of AMPs killing bacterial directly, AMPs also show potential role in innate immune cell regulation.16 Cathelicidin-BF, an α-helix structure AMP, shown a broad and strong ability in anti-MDR bacterial infection in vitro and in vivo. Pretreatment with Cathelicidin-BF significantly recruit neutrophils and macrophage to the lung and induce neutrophil extracellular traps formation which reduced bacterial burden and improve mice survival.8 LL-37 regulates the differentiation and phagocytosis of DC cells which is dependent on overexpression of phagocytosis receptor and costimulatory molecule CD86\CD80\CD40.22 Whether Tachyplesin III mediate innate immune cells especially monocytes remains unclear, we doubt that Tachyplesin III could remarkably increase mice against MDR bacterial infection by the directly killing mechanism collaboration with regulate innate immune cells infiltration to the infection site. Thus, it is essential to evaluate the ability of Tachyplesin III to achieve mixed bacterial clearance in vivo. Herein, we identified a potential role for the AMP Tachyplesin III in host defense against P. aeruginosa and A. baumannii coinfection.
Materials and methods
Ethics statement
The animal experiments were approved by the Ethics Committee of Animal Care and Welfare of the Institute of Medical Biology, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC) (Permit Number: SYXK (dian) 2010-0007) in accordance with the animal ethics guidelines of the Chinese National Health and Medical Research Council (NHMRC) and the Office of Laboratory Animal Management of Yunnan Province, China. All efforts were made to minimize animal.
Cell culture and treatment
MH-S (mouse alveolar macrophage), which was purchased from Chinese Academy of Sciences cell bank, was cultured in RPMI 1640 medium containing 10% FBS, 1% penicillin–streptomycin, 1% nonessential amino acids, and 1% L-glutamine (Solarbio® Life Science). Cells were seeded onto 12-well plates at a million cells per well and incubated overnight. One hour before Tachyplesin III (Southeast Asian horseshoe crab, KWCFRVCYRGICYRKCR-NH2, Sangon® Biotech, China) treatment, the cells were washed three times with PBS, incubated with fresh RPMI 1640 medium without antibiotics, and then treated with Tachyplesin III at concentrations of 0 µg/mL, 10 µg/mL, and 100 µg/mL for 3 hrs. Tachyplesin III was resuspended in fresh RPMI 1640 medium.
Neutral red phagocytosis
Briefly, after pretreatment with Tachyplesin III, MH-S cells were washed three times with PBS before neutral red staining. Next, 500 µL fresh RPMI 1640 medium containing 0.05% neutral red medium that was filtered with a 0.22 µm filter was added to the 12-well plates and incubated for 4 mins. After removing the medium and washing the cells three times with PBS, multiple red bubbles could be observed inside the treated cells under a microscope (Nikon, Japan). Acid-ethanol solution consisting of 50% ethanol, 1% acetic acid, and 49% ddH2O was subsequently used for neutral red extraction. Finally, the results were determined by absorbance measurements with a NanoDrop 2000 at 540 nm, which is the absorption peak for neutral red solution. The peptide Cramp (GLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPEQ) was employed as control peptide.
Animals
Six- to eight-week-old female BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. and maintained in the Central Animal Care Services of our institute under specific pathogen-free (SPF) conditions.
Bacterial strains and infection of mice
Two MDR clinical isolates that were inventory from our own laboratory, P. aeruginosa 1409 and A. baumannii 1408, were identified with a Vitek 32 system (bioMerieux, France) and further verified by 16S rDNA sequencing with the universal primers 27f and 1492R as previously described.4 Both P. aeruginosa 1409 and A. baumannii 1408 were grown overnight with shaking in Luria-Bertani (LB) medium at 37°C and then subcultured with fresh LB medium for 4 hrs. When the bacterial concentration corresponding to an OD600 value reached 0.5, the culture was centrifuged at 5000× g for 10 mins, and the bacteria were washed twice with PBS (8 g/L NaCl, 0.2 g/L KCl, 1.44 g/L Na2HPO4, 0.24 g/L KH2PO4, pH 7.4) and then resuspended in PBS.
Bacterial infection of mice and survival
To establish the murine bacteremia model, mice were intranasally injected with a 50 µL total volume bacterial solution. For P. aeruginosa mono-infection group, 2.5×106 CFU bacterial were used for mice challenge. For A. baumannii mono-infection group, 1.5×108 CFU bacterial were used for mice challenge. For coinfection group 2.5×106 CFU P. aeruginosa plus 1.5×108 CFU A. baumannii were used for mice challenge. To generate P. aeruginosa plus S. aureus co-infection model, mice were also intranasally injected with a 50 µL total volume bacterial solution. A lethal dose of P. aeruginosa which contains 5×106 CFU bacterial were used for mice challenge. For sub-lethal P. aeruginosa mono-infection group, 2.5×106 CFU bacterial were used for mice challenge. A total of 1.5×109 CFU and 7.5×108 CFU S. aureus were used for mono-infection group, respectively. For coinfection group, 2.5×106 CFU P. aeruginosa plus 7.5×108 CFU S. aureus were used for mice challenge. After bacterial challenge, mice survival rate was determined by continuous monitoring for 7 days, which was considered the experiment endpoint, and then all living animals were euthanized.
BALF bacterial burden
To evaluate the BALF bacterial burden, mice were sacrificed 24 hrs after treatment. One milliliter fresh PBS was injected to the lung via minimally invasive tracheostomy, and then repeated lavage of lung tissues for three times. BALF bacterial burden was measured with a 10-fold dilution on LB plates, which were incubated at 37°C overnight. The next day, the CFU was counted, and 30~300 spots/plate were selected for statistical analysis and expressed as CFU/mL BALF.
Cytokine and chemokine measurements
The BALF extraction supernatants were analyzed for Il-6, TNF-α, MCP-1 (eBioscience), IL-1β, and MIP-2 (Abcam) concentration by an enzyme-linked immune sorbent assay (ELISA) kit following the manufacturer’s instructions.16
Lung histological analysis
The lung tissue histology analysis was performed as previously described.35 Briefly, the left lung tissues were harvested from mice the day after bacterial infection and fixed in 4% paraformaldehyde at room temperature for 24 hrs. Subsequently, the tissues were embedded in paraffin and stained with a hematoxylin and eosin (H & E) kit purchased from Solarbio® Life Science.
Statistical analysis
All data were analyzed using GraphPad Prism 7.0 and presented as the mean±standard deviation (SD). The nonparametric log-rank test was used for survival rate comparisons, and the other comparisons were performed using Student’s t-test or one-way ANOVA between two or three groups, respectively. *indicates P-value<0.05, **indicates P-value<0.01, ***indicates P-value<0.001.
Results
Mixed infection with P. aeruginosa and other bacteria reduces mouse survival
To identify the damage of P. aeruginosa and A. baumannii mixed infection in vivo, we mimicked the pathogenic pattern of these two MDR bacteria. Therefore, three infected BALB/c mouse groups, representing P. aeruginosa and A. baumannii single and mixed infections, containing eight mice per group, were employed. The mixed-infection mouse group were intranasally infected with a 50 µL total volume of mixed bacteria consisting of 2.5×106 CFU of live P. aeruginosa and 1.5×108 CFU of live A. baumannii; the same doses were used in the single infection groups. Although the mice were coinfected with sub-lethal doses of bacteria, fewer than 40% of the mice survived to the 7-day study endpoint in the coinfection group, whereas only one P. aeruginosa-infected mouse died (P<0.05), and none of the A. baumannii-infected mice died (P<0.01) (Figure 1). To investigate whether P. aeruginosa could potentiate Gram-positive bacterial infections, mice were intranasally infected with P. aeruginosa in combination with S. aureus. As expected, most animals died in the coinfection group (Figure S1), which is consistent with recent studies.7,23 Our data indicate that compared with single infection, mixed bacterial infection with P. aeruginosa and Gram-negative A. baumannii or gram-positive S. aureus significantly reduced the survival rate.
Figure 1.
Coinfection of P. aeruginosa and A. baumannii reduces mouse survival. Survival curve of BALB/c mice (n=8) infected with P. aeruginosa (2.5×106 CFU) (Pa) alone, A. baumannii (1.5×108 CFU) (Aba) alone, or P. aeruginosa+ A. baumannii. Survival rates were monitored daily for a week, and survival rate significance was determined by log-rank test. *P<0.05 compared to mono-P. aeruginosa infection, **P<0.01 compared to A. baumannii mono-infection, and data represent at least three trials. The animals were euthanized the day after observation endpoint.
Coinfection of P. aeruginosa and A. baumannii causes serious disease
Gram-negative bacterial pathogen infection can trigger a robust systemic response of pro-inflammatory cytokines and chemokines.24 To investigate the pathogenic inflammatory reaction caused by bacterial infection, we first detected the levels of pro-inflammatory cytokines and chemokines in BALF collected at 24 hrs after bacterial infection. During P. aeruginosa and/or A. baumannii infection, the expression of IL-1β, IL-6, TNF-α, MIP-2, and MCP-1 was significantly induced in the infection groups compared with the control (Ctrl) groups. The production of cytokine IL-1β and chemokine MCP-1 in the mixed-infection group was significantly higher than that in the mono-infection groups. Interestingly, there were no differences in IL-6, TNF-α, and MIP-2 production between the P. aeruginosa plus A. baumannii mixed-infection group and the P. aeruginosa mono-infection group, but the expression of IL-6 and TNF-α in the mixed-infection group was higher than that in the A. baumannii mono-infection group (Figure 2A). Histological analysis show that, in contrast to WT mice, bacterial infection induced histological lung injury and the coinfection group presented the most serious injury. Increased vascular leakage and bleeding, inflammatory cell infiltration and alveolar disruption occurred in the single-bacterial infection group, and this condition was worse in the mixed-infection group (Figure 2B). In line with these results, the H & E score also consist with pathological section results (Figure 2C). In summary, our results demonstrate that pro-inflammatory cytokine IL-1β and chemokine MCP-1 were significantly increased and lung injury was significantly increased in the coinfection group.
Figure 2.
Bacterial coinfection induced more cytokines production in BALF and worse lung injury in vivo than mono-infection. (A) Mice were intranasally infected with co- and mono-bacterial solutions, and BALF was collected in 1 mL cold phosphate buffer saline (PBS) at 24 hrs after injection. BALF was collected after inoculation, and the levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and chemokines MIP-2 and MCP-1 were determined by ELISA. Coinfection enhanced cytokine and chemokine production. Bars represent the mean±SEM for six mice. (B) Hematoxylin-eosin (H & E) staining of the lung tissues from mice at 24 hrs after infection. Representative sections of control mice and mono- and co-infected mice are shown. Obvious bleeding and inflammatory cell infiltration were observed, indicating that bacterial coinfection enhances worse lung injury in vivo. (C) H & E pathological score results are shown as histograms. The data are shown as the mean±SEM. Comparisons among two groups were calculated using paired t-test and one-way ANOVA for three or more groups (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Abbreviation: ns, no significance.
Pretreatment with Tachyplesin III protects mice against mixed bacterial infection
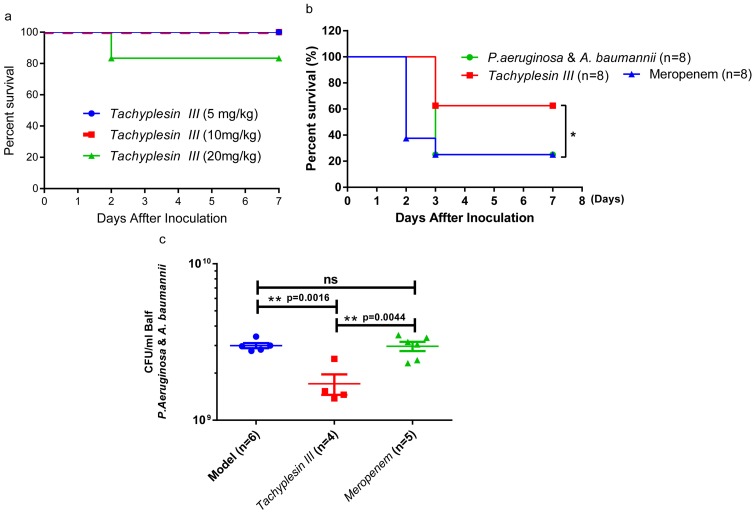
Tachyplesin III is cytotoxic to mammalian cells and shows MDR bacterial-killing activity.19 To test the toxicity of Tachyplesin III in vivo, the mice were intravenously (i.v.) injected with a 5, 10, and 20 mg/kg gradient peptide dose. Only one mouse in the 20 mg/kg group died, and none of the mice in the 5 and 10 mg/kg dose groups died (Figure 3A). The 10 mg/kg dose was chosen for the infection study. First, we try a therapeutic plan to i.v. inject with 10 mg/kg Tachyplesin III and control peptide Cathelicidin-BF 2 hrs after lethal dose of P. aeruginosa infection, Cathelicidin-BF significantly increased the survival rate as we described earlier, but Tachyplesin III did not show any potential activity in vivo (Figure S2). Then, BALB/c mice were treated (i.v.) with a single dose of 10 mg/kg Tachyplesin III, and Meropenem was used as a control. Both P. aeruginosa and A. baumannii were intranasally injected 24 hrs after peptide inoculation. As expected, no deaths occurred within 24 hrs after peptide and bacterial inoculation. Strikingly, in the preventive treatment condition, the mortality rate was significantly reduced within a week in the Tachyplesin III peptide-treated group but not in the Meropenem-treated group (Figure 3B). To further investigate the pivotal role of Tachyplesin III in the coinfection model, the bacterial burden in BALF was measured at 24 hrs after infection. As expected, the bacterial loads in the Tachyplesin III peptide-treated group were significantly lower than those in the model and control groups (Figure 3C). Taken together, our findings strongly suggest a potential role for Tachyplesin III in triggering an antimicrobial response against mixed bacterial infection, reducing the bacterial burden in lung tissues and improving survival.
Figure 3.
Protective efficacy of Tachyplesin III therapy in a mixed bacterial infection murine model of pneumonia. (A) Survival curve of BALB/c mice (n=6) intravenously infected with Tachyplesin III at different doses of 5, 10, and 20 mg/kg. Only one mouse died in the 20 mg/kg group, and no mice died in the 5, 10 mg/kg group, indicating that Tachyplesin III was safe through intravenous injection at 10 mg/kg. (B) The therapeutic potential of Tachyplesin III in bacterial coinfection pneumonia. Pretreatment with 10 mg/kg Tachyplesin III significantly increased the mouse survival rate compared with that of the model group, while meropenem-treated mice had no effect on MDR bacterial infection. (C) BALF bacterial burden in the bacterial coinfection group. Viable bacteria in the BALF that were collected at 24 hrs after bacterial infection were decreased in the pretreatment with Tachyplesin III group compared with the non-treatment (Model) and Meropenem-treated (Ctrl) groups. Bars represent the mean±SEM, survival rates were monitored daily for a week, and survival rate significance was determined by log-rank test, *P<0.05; BALF comparisons among two groups were calculated using paired t-test and one-way ANOVA for three or more groups (*P<0.05, **P<0.01). The animals were euthanized the day after observation endpoint.
Tachyplesin III reduces pro-inflammatory cytokines and chemokines production and ameliorates tissue damage in vivo
To determine whether Tachyplesin III reduces the systemic response of pro-inflammatory cytokines induced by the mixed bacterial infection with P. aeruginosa plus A. baumannii, the level of serum cytokines and chemokines was measured. As expected, Tachyplesin III therapy reduced the production of pro-inflammatory cytokines as IL-1β, IL-6, and TNF-α, but there was no difference in the production of chemokine MCP-1 and MIP-2 (Figure 4A). Histological examination showed no difference between the Tachyplesin III group and the wild-type group, which indicated that injection (i.v.) with 10 mg/kg Tachyplesin III did not obviously cause lung injury (Figure 4B). Treatment with Tachyplesin III resulted in decreased inflammatory cell infiltration, vascular leakage, and alveolar disruption compared with those in the coinfected groups or in the Meropenem-treated group (Figure 4B). In line with these results, the H & E score also consist with pathological section results (Figure 4C). Collectively, these data indicated that Tachyplesin III plays a crucial role in the prophylaxis of MDR bacterial mixed infection-induced lung injury and inflammatory cytokine production.
Figure 4.
Pulmonary pathological changes of Tachyplesin III-treated mice. (A) Cytokine and chemokine production was reduced in Tachyplesin III-pretreated mice. Serum was collected 24 hrs after bacterial infection, and IL-1β, IL-6, TNF-α, MIP-2, and MCP-1 were identified by ELISA. (B) Lung tissue was stained with H & E, no significant tissue damage occurred in Tachyplesin III (10 mg/kg), and less bleeding and inflammatory immune cell infiltration occurred in Tachyplesin III-treated mice than in the model and control groups. (C) H & E pathology was scored blindly in mouse lungs after infection with P. aeruginosa and A. baumannii. The data are shown as the mean±SEM. Serum cytokine and lung pathology score comparisons among the two groups were calculated using paired t-test and one-way ANOVA for three or more groups (*P<0.05, **P<0.01, ***P<0.001).
Tachyplesin III enhances the phagocytic function of mouse alveolar macrophages
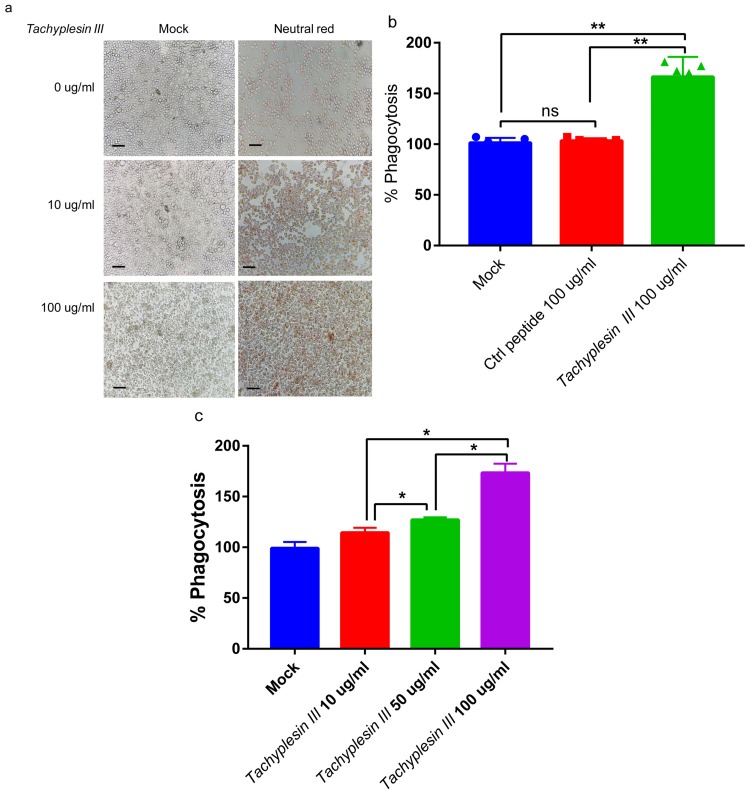
Macrophage phagocytosis, which is a secondary line of defense line in host innate immunity against bacterial infection, plays an important role in bacterial clearance.25 To evaluate the effect of Tachyplesin III on macrophage phagocytosis, we treated the mouse alveolar macrophage cell line MH-S with different Tachyplesin III concentrations in vitro. After MH-S cells were exposed to Tachyplesin III for 3 hrs, a significant dose-dependent phagocytosis of neutral red was observed (Figure 5A). In addition, after extracting the neutral red from MH-S cells, the concentration of neutral red was significantly increased in Tachyplesin III-treated cells than that in control peptide- (Cramp) treated cells (Figure 5B). Macrophage phagocytosis was increased in a dose-dependent manner (Figure 5C). Altogether, these results indicated that Tachyplesin III induced obvious alveolar macrophage phagocytosis.
Figure 5.
Tachyplesin III enhanced the phagocytosis of mouse alveolar macrophages in vitro. (A) Bright-field image showing neutral red phagocytosis of MH-S cells in a dose-dependent manner with Tachyplesin III treatment. (B) Quantification of phagocytic capacity via the OD540 nm measurement value of neutral red extract. (C) MH-S cells were incubated with Tachyplesin III and control peptides. The levels of neutral red phagocytosis were measured by OD540 nm detection. The data are shown as the mean±SD, and comparisons among two groups were calculated using paired t-tests and one-way ANOVA for three or more groups (*P<0.05, **P<0.01).
Discussion
For nearly 40 years, no novel antibiotics have been discovered or developed, while multiple natural substances, such as AMPs, have recently been described.1 However, the entire process from identifying to refining antimicrobial products, which are urgently needed for contemporary clinical therapy, is slow and time-consuming, although several representative AMPs, such P-113, MSI-78, MBI-226, IB-367, and rBPI21, have been applied in clinical trials.26,27,28 According to a report by the World Health Organization (WHO), the use of antibiotics extends the human life span at least 20 years. Nevertheless, this protective effect is becoming increasingly weaker with bacterial evolution, particularly the emergence and spread of MDR bacteria, which may contribute to 30 million deaths by 2050.29
Clinically, the Gram-positive bacterium S. aureus is often observed in coinfections with gram-negative bacteria, including P. aeruginosa, K. pneumoniae, and A. baumannii, which depend on the influence of toxins on mucosal host defense.7 Carbapenem-resistant (CSE) A. baumannii and P. aeruginosa, two Gram-negative bacteria, are the most critical global priority opportunistic pathogens. In the clinic, P. aeruginosa plus A. baumannii is the most common and dangerous combination30 and a critical contributor to the morbidity and mortality of long-term in-hospital respiratory medicine patients, particularly VAP patients.5,6 Our study revealed that compared with monoinfection, coinfection with P. aeruginosa plus A. baumannii significantly reduced mouse survival, and only one mouse dead with P. aeruginosa infection alone (Figure 1A). Interestingly, none of the mice intranasally infected with 1.5×108 CFU live A. baumannii died, whereas the addition of less than 3×106 CFU P. aeruginosa significantly reduced the survival rate, suggesting that the synergistic interactions of these bacteria increased disease severity, which coincides with the clinical phenomenon.31 Therefore, this lethal pneumonia model was further analyzed. Gram-negative bacterial pathogen infections can trigger a robust systemic response of pro-inflammatory cytokines and chemokines.32 In the current study, we indicated that, in the case of mixed bacterial infection, the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, known as a “cytokine storm”, were significantly increased compared with those in the single-bacterial infection group, which may be the main cause of multiple organ failure or death. Furthermore, MDR bacterial coinfection induced worse lung injury as well as multiple inflammatory cell infiltration, alveolar disruption, and vascular leakage (Figure 2).
Our previous work demonstrated that Tachyplesin III provides an antibacterial effect against MDR bacteria in vitro33 which may present strong anti-MDR bacteria activity by interacting with FabG to trigger bacterial death9 and this treatment is hypothesized to provide bacterial clearance in vivo. Therefore, to further examine the influence of Tachyplesin III on mixed bacterial infection, we proposed a murine pneumonia model that enabled us to evaluate Tachyplesin III activity in vivo. Herein, we found that although Tachyplesin III showed cytotoxicity to mammalian cells in vivo, no animal death or lung injury occurred in mice injected (i.v.) with a 10 mg/kg dose (Figures 3A and 4A–E). Strangely, we found that Tachyplesin III i.v. injection had non-therapeutic effect in the lethal dose of P. aeruginosa challenge (Figure S2) which reminds us that future research should focus on the development of detoxified form of Tachyplesin III. In a murine pneumonia model, a low lethal dose of P. aeruginosa significantly reduced the survival rate of A. baumannii-infected and S. aureus-infected mice (Figure S1), which was prevented by pretreatment with Tachyplesin III (Figure 3B). Consistent with the reduced mortality, pretreatment with Tachyplesin III significantly reduced the BALF bacterial burden, decreased the cytokine storm, and reduced lung injury, which may be the main causes of animal death (Figures 3B and 4A–C). Compared with mono-infections, mixed infections with P. aeruginosa and A. baumannii trigger even more serious disease in vivo.
Alveolar macrophages and neutrophils are the first immune cells in the host innate response to bacterial pathogen infection.34 Our recent work indicated that Cathelicidin-BF, another AMP subtype, enhanced neutrophil extracellular traps, which are the main facilitators of the capture and clearance of bacteria through the activation of innate immunity.35 Other studies have indicated that macrophages also contribute to the clearance of bacterial pathogen infection.36 In this study, our results demonstrated that Tachyplesin III improved alveolar macrophage phagocytosis in a dose-dependent manner in vitro (Figure 5), which may directly reduce the BALF burden (Figure 3C). Thus, our study reports a novel therapeutic strategy for gram-negative MDR bacterial coinfection using the AMP Tachyplesin III.
Taken together, the present work illustrated that coinfection with P. aeruginosa plus A. baumannii causes more serious disease than individual bacterial infections, especially by the release of pro-inflammatory cytokine IL-1β and a decrease in immune cell infiltration, thereby triggering lung injury. Dramatically, the prophylactic capability of Tachyplesin III against clinical MDR bacterial isotype infection was identified. This phenomenon could be partially explained by significantly enhanced survival, reduced BALF bacterial burden, decreased pro-inflammatory cytokine and chemokine levels, and reduced lung injury. Simultaneously, the phagocytic function of macrophages was significantly increased under Tachyplesin III in vitro.
Conclusion
In conclusion, our findings indicate that the AMP Tachyplesin III plays a potential role against MDR bacterial coinfection in vivo. Pretreatment with Tachyplesin III ameliorates bacterial coinfection in mice by enhancing macrophage phagocytosis and reducing the production of pro-inflammatory cytokines. Our results highlight the importance of external AMPs against MDR bacterial mixed infection and present a possible therapeutic strategy for controlling multiple bacterial coinfections that should be confirmed in clinical studies.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant numbers 81503117 and 81460322), the Innovation Fund of IMBCAMS grant number 2018018001, the Fundamental Research Funds for the Central Universities of China (grant numbers 2018018001), the CAMS Initiative for Innovative Medicine (grant number 2017-I2M-3-022 and 2016-I2M-019), and the Opening Foundation of the Key Laboratory of Bioactive Peptides of Yunnan Province (grant number AMHD-2017-1). The co-first authors of this paper are Jialong Qi, Ruiyu Gao, and Cunbao Liu.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Kang S-J, Park SJ, Mishig-Ochir T, Lee B-J. Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther. 2014;12(12):1477–1486. doi: 10.1586/14787210.2014.976613 [DOI] [PubMed] [Google Scholar]
- 2.Francis AM. The wages of sin: how the discovery of penicillin reshaped modern sexuality. Arch Sex Behav. 2013;42(1):5–13. doi: 10.1007/s10508-012-0018-4 [DOI] [PubMed] [Google Scholar]
- 3.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Shan B, Qi J, Ma Y. Systemic responses of multidrug-resistant pseudomonas aeruginosa and acinetobacter baumannii following exposure to the antimicrobial peptide cathelicidin-BF imply multiple intracellular targets. Front Cell Infect Microbiol. 2017;7. doi: 10.3389/fcimb.2017.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacy MK, Stryjewski ME, Wang W, et al. Telavancin hospital-acquired pneumonia trials: impact of Gram-negative infections and inadequate Gram-negative coverage on clinical efficacy and all-cause mortality. Clin Infect Dis. 2015;61(Suppl 2):S87–S93. doi: 10.1093/cid/civ536 [DOI] [PubMed] [Google Scholar]
- 6.Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Friedland IR, Sahm DF. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47(5):1681–1688. doi: 10.1128/aac.47.5.1681-1688.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen TS, Hilliard JJ, Jones-Nelson O, et al. Staphylococcus aureus α toxin potentiates opportunistic bacterial lung infections. Sci Transl Med. 2016;8(329):329ra31–329ra31. doi: 10.1126/scitranslmed.aad9922 [DOI] [PubMed] [Google Scholar]
- 8.Cai S, Qiao X, Feng L, et al. Python cathelicidin CATHPb1 protects against multidrug-resistant staphylococcal infections by antimicrobial-immunomodulatory duality. J Med Chem. 2018;61(5):2075–2086. doi: 10.1021/acs.jmedchem.8b00036 [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Qi J, Shan B, Ma Y. Tachyplesin causes membrane instability that kills multidrug-resistant bacteria by inhibiting the 3-Ketoacyl carrier protein reductase FabG. Front Microbiol. 2018;9:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Ong ZY, Liu S, et al. Synthetic beta-sheet forming peptide amphiphiles for treatment of fungal keratitis. Biomaterials. 2015;43:44–49. doi: 10.1016/j.biomaterials.2014.11.052 [DOI] [PubMed] [Google Scholar]
- 11.Lacerda AF, Pelegrini PB, de Oliveira DM, Vasconcelos EA, Grossi-de-Sa MF. Anti-parasitic peptides from arthropods and their application in drug therapy. Front Microbiol. 2016;7:91. doi: 10.3389/fmicb.2016.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X, Guo Y, Bao J, et al. Characterization and antivirus activities of a novel bovine IFN-omega24. Mol Immunol. 2015;66(2):357–363. doi: 10.1016/j.molimm.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 13.Pan CY, Lin CN, Chiou MT, Yu CY, Chen JY, Chien CH. The antimicrobial peptide pardaxin exerts potent anti-tumor activity against canine perianal gland adenoma. Oncotarget. 2015;6(4):2290–2301. doi: 10.18632/oncotarget.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karal MA, Alam JM, Takahashi T, Levadny V, Yamazaki M. Stretch-activated pore of the antimicrobial peptide, magainin 2. Langmuir. 2015;31(11):3391–3401. doi: 10.1021/la503318z [DOI] [PubMed] [Google Scholar]
- 15.Cirioni O, Simonetti O, Pierpaoli E, et al. Enhanced efficacy of combinations of pexiganan with colistin versus acinetobacter baumannii in experimental sepsis. Shock. 2016;46(2):219–225. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Lu W. Defensins in innate immunity. Curr Opin Hematol. 2014;21(1):37–42. doi: 10.1097/MOH.0000000000000005 [DOI] [PubMed] [Google Scholar]
- 17.Kuzmin DV, Emelianova AA, Kalashnikova MB, et al. Comparative in vitro study on cytotoxicity of recombinant beta-hairpin peptides. Chem Biol Drug Des. 2018;91(1):294–303. doi: 10.1111/cbdd.13081 [DOI] [PubMed] [Google Scholar]
- 18.Miyata T, Tokunaga F, Yoneya T, et al. Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J Biochem. 1989;106(4):663–668. doi: 10.1093/oxfordjournals.jbchem.a122913 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Furunaka H, Miyata T, et al. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem. 1988;263(32):16709–16713. [PubMed] [Google Scholar]
- 20.Katsu T, Nakao S, Iwanaga S. Mode of action of an antimicrobial peptide, tachyplesin I, on biomembranes. Biol Pharm Bull. 1993;16(2):178–181. doi: 10.1248/bpb.16.178 [DOI] [PubMed] [Google Scholar]
- 21.Yonezawa A, Kuwahara J, Fujii N, Sugiura Y. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry. 1992;31(11):2998–3004. doi: 10.1021/bi00126a022 [DOI] [PubMed] [Google Scholar]
- 22.Davidson DJ, Currie AJ, Reid GS, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172(2):1146–1156. doi: 10.4049/jimmunol.172.2.1146 [DOI] [PubMed] [Google Scholar]
- 23.Cohen TS, Hilliard JJ, Jones-Nelson O, et al. Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci Transl Med. 2016;8(329):329ra31. doi: 10.1126/scitranslmed.aaf0746 [DOI] [PubMed] [Google Scholar]
- 24.Shang D, Meng X, Zhang D, Kou Z. Antibacterial activity of chensinin-1b, a peptide with a random coil conformation, against multiple-drug-resistant Pseudomonas aeruginosa. Biochem Pharmacol. 2017;143:65–78. doi: 10.1016/j.bcp.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 25.Camberlein E, Cohen JM, Jose R, et al. Importance of bacterial replication and alveolar macrophage-independent clearance mechanisms during early lung infection with Streptococcus pneumoniae. Infect Immun. 2015;83(3):1181–1189. doi: 10.1128/IAI.02788-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50(1):43–48. doi: 10.1128/AAC.50.1.43-48.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giles FJ, Rodriguez R, Weisdorf D, et al. A phase III, randomized, double-blind, placebo-controlled, study of iseganan for the reduction of stomatitis in patients receiving stomatotoxic chemotherapy. Leuk Res. 2004;28(6):559–565. doi: 10.1016/j.leukres.2003.10.021 [DOI] [PubMed] [Google Scholar]
- 28.Bommarius B, Jenssen H, Elliott M, et al. Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides. 2010;31(11):1957–1965. doi: 10.1016/j.peptides.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker AT, Leonard SP, DuBois CD, et al. Discovery of next-generation antimicrobials through bacterial self-screening of surface-displayed peptide libraries. Cell. 2018;172(3):618–628.e13. doi: 10.1016/j.cell.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Q, Luo R, Bodin L, et al. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology. 2012;117(6):1335–1347. doi: 10.1097/ALN.0b013e31827515de [DOI] [PubMed] [Google Scholar]
- 31.Korgaonkar A, Trivedi U, Rumbaugh KP, Whiteley M. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc Natl Acad Sci U S A. 2013;110(3):1059–1064. doi: 10.1073/pnas.1214550110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S-A, Xiang Y, Wang Y-J, Liu J, Lee W-H, Zhang Y. Naturally occurring antimicrobial peptide OH-CATH30 selectively regulates the innate immune response to protect against sepsis. J Med Chem. 2013;56(22):9136–9145. doi: 10.1021/jm401134n [DOI] [PubMed] [Google Scholar]
- 33.Liu CB, Shan B, Bai HM, Tang J, Yan LZ, Ma YB. Hydrophilic/hydrophobic characters of antimicrobial peptides derived from animals and their effects on multidrug resistant clinical isolates. Zool Res. 2015;36(1):41–47. doi: 10.13918/j.issn.2095-8137.2015.1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones MCYG, Romela IR, Hebroni F, Seema BP, Caiyun X, Lloyd SM. Neutrophil-derived IL-1b is sufficient for abscess formation in immunity against Staphylococcu aureus in mice. Plos Pathogen. 2012;8(11):e1003047. doi: 10.1371/journal.ppat.1003047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu CB, Qi JL, Shan B, et al. Pretreatment with cathelicidin-BF ameliorates Pseudomonas aeruginosa pneumonia in mice by enhancing NETosis and the autophagy of recruited neutrophils and macrophages. Int Immunopharmacol 2018;65:382–391. doi: 10.1016/j.intimp.2018.10.030 [DOI] [PubMed] [Google Scholar]
- 36.Wan M, van der Does AM, Tang X, Lindbom L, Agerberth B, Haeggström JZ. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J Leukoc Biol. 2014;95(6):971–981. doi: 10.1189/jlb.0513304 [DOI] [PubMed] [Google Scholar]