Abstract
Background
Despite progress achieved in bladder cancer (BC) treatment, the prognosis of patients with advanced BC (ie, metastasized from the bladder to other organs) is poor. Although mortality in cases of low-grade BC is rare, the treatment, such as a radical cystectomy, often has a serious impact on the quality of life. Thus, research is needed to identify more effective treatment strategies and this work is aiming to examine the potential application of combination of radiofrequency ablation (RFA) and SB435142, a inhibitor of transforming growth factor β (TGFβ)/Smad pathway.
Methods
BC cells were transplanted into nude mice (thymusdeficiency Bal B/c) to form subcutaneous tumors. The mice with subcutaneous tumors were then treated with RFA and oral administration of SB431542, an inhibitor of TGFβ/Smad signaling pathway. The antitumor effect of RFA was measured by tumor proliferation curves and micro-positron emission computed tomography (micro-PET). The effect of SB431542 on epithelial–mesenchymal transition (EMT) related regulators in subcutaneous tumor tissues formed by BC cells were examined by quantitative real-time polymerase chain reaction (qPCR) experiments.
Results
The SB431542 treatment enhanced the antitumor effect of RFA on subcutaneous growth of BCs. SB431542 also decreased EMT-related regulators in subcutaneous tumor tissues formed by BC cells in nude mice.
Conclusion
SB431542 enhances the effect of RFA on BC.
Keywords: bladder cancer, radiofrequency ablation, SB431542, epithelial–mesenchymal transition
Introduction
Bladder cancer (BC) is one of the most common human cancers and is associated with high morbidity and mortality rates in the absence of optimal treatment.1 For primary BC or nonmuscle-invasive BC, the first treatment choice is complete resection of BC tissues (ie, radical treatment).2 Induction and maintenance immunotherapy with the Bacillus Calmette–Guérin vaccine or chemotherapies may prevent BC recurrence or lengthen the time to recurrence.2,3 Although radical treatments may prolong survival in cases of nonmuscle-invasive BC,4 they affect patients’ quality of life. In advanced BC, systemic cisplatin-based chemotherapies or immunotherapies may not control the progress of the disease. As a result, the prognosis of patients with advanced BC remains poor.5 Therefore, more effective treatment strategies for BC treatment are urgently needed.
Radiofrequency ablation (RFA), which is a kind of interventional therapeutic strategy, involves local ablative therapy to selectively destroy tumor tissues and minimize damage to tissues surrounding the tumor.6 RFA is a promising therapeutic strategy and has been widely used in human cancer treatment, such as advanced hepatocellular carcinomas.7,8 Although RFA selectively destroys tumor tissues or decelerates the progress of human cancers, the potential for rapid and aggressive recurrence of tumors after incomplete RFA treatment is a major obstacle.7,8 It is also impossible to infinitely increase the temperature during RFA, as this would result in serious organ damage. Therefore, research aimed at the development of adjuvant treatment strategies for BC that can improve the safety and effectiveness of RFA therapy is needed.
The ability of RFA to directly target tumor tissue for destruction makes it important in the treatment of BC.9 RFA can ablate BC tissues and avoid urinary system damage induced by extensive resection of bladder tissue in radical treatment of BC. There are only a few reports on the application of RFA in BC treatment.10 Previous studies confirmed that the epithelial–mesenchymal transition (EMT) played an important role in RFA resistance or tumor recurrence after RFA treatment.11–13 A combination of RFA with various molecular targeting agents (eg, sorafenib or apatinib) enhanced the antitumor effect of RFA by inhibiting the EMT process.7,13 Therefore, inhibition of the EMT is a promising approach to combine with RFA. The transforming growth factor beta (TGFβ)/Smad signaling pathway plays a central role in the EMT process in human cancer cells.14,15 In the present study, we used SB431542,16 an inhibitor of the TGFβ/Smad signaling pathway, to enhance the effect of RFA on BC and examined its antitumor effect in combination with RFA using in vivo and in vitro models.
Materials And Methods
Cell Lines And Agents
Clinical specimens of BC were obtained from five patients with bladder urothelial carcinomas during surgery as part of normal medical care. The specimens were preserved in our lab until used. The collection of the clinical specimens and protocols were in compliance with the Helsinki Declaration. The methods and research were approved by the ethics committee of the First People’s Hospital, Qujing City, Yunnan Province, People’s Republic of China. Informed written consent was obtained from all the patients. The represented pathological analysis image of clinical specimens of BC tissues was shown as Supplemental Figure 1. The antitumor agent SB431542 (Cat. No. S1067) was purchased from Selleck Corporation, Houston, Texas, USA.
Cell Culture And Survival Analysis
The BC cell lines, T24, 5637 or RT4, were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China), a Chinese government organization containing typical biological samples. The BC cells were cultured in DMEM (Thermo Fisher Corporation, Waltham, MA, USA) with 10% FBS (Thermo Fisher Corporation). SB431542 was initially dissolved in dimethyl sulfoxide and then diluted with DMEM without FBS. To determine the impact of SB431542 on BC cell survival/inhibition, the cells were treated with the following concentrations of SB431542 for 48 hrs: 10 μmol/L, 3 μmol/L, 1 μmol/L, 0.3 μmol/L, 0.1 μmol/L, 0.03 μmol/L, and 0.01 μmol/L. MTT assays were then performed, and the optical density of the cell samples was examined. The inhibition rates of SB431542 on BC cells’ survival were calculated using the methods described previously.17,18
Luciferase Experiments
The luciferase reporters (the vectors of luciferase reporters) of TGFβ/Smad signaling pathway, the SRB-Luc or the 3TP-Luc reporters, were gifts from Dr Fan Feng in Research Center for Clinical and Translational Medicine, the 302nd Hospital of Chinese PLA, Beijing, 100039, People’s Republic of China. The BC cells, which were transfected with luciferase reporters, were treated with indicated concentration of SB431542. Then, cells were harvested for luciferase activation-examination by using a kit purchased from Promega Corporation (Madison, Wisconsin, USA) following methods described by Yang et al (2013) or Lu et al (2013).19,20 The inhibition rated od SB431542 was calculated by using luciferase activation and the IC50 values of SB431542 of SB431542 was calculated based on the inhibition rates.
Quantitative Real-Time Polymerase Chain Reaction (qPCR) Experiments
mRNA was extracted from the BC cells and subcutaneous tumor samples and subjected to reverse transcription or the qPCR in accordance with methods described previously.21,22 The primers used in the qPCR experiments are listed in Table 1.
Table 1.
Primers Used in This Work
| Targets | Primers | Sequences (5ʹ-3ʹ) |
|---|---|---|
| β-Actin | Forward Sequence | CTCCATCCTGGCCTCGCTGT |
| Reverse Sequence | GCTGTCACCTTCACCGTTCC | |
| E-cadherin | Forward Sequence | AAGGCACGCCTGTCGAAGCA |
| Reverse Sequence | ACGTTGTCCCGGGTGTCATCCT | |
| N-cadherin | Forward Sequence | CCTCCAGAGTTTACTGCCATGAC |
| Reverse Sequence | GTAGGATCTCCGCCACTGATTC | |
| Vimentin | Forward Sequence | AGGCAAAGCAGGAGTCCACTGA |
| Reverse Sequence | ATCTGGCGTTCCAGGGACTCAT | |
| Snail | Forward Sequence | TGCCCTCAAGATGCACATCCGA |
| Reverse Sequence | GGGACAGGAGAAGGGCTTCTC | |
| Twist | Forward Sequence | GCCAGGTACATCGACTTCCTCT |
| Reverse Sequence | TCCATCCTCCAGACCGAGAAGG |
Subcutaneous Tumor And RFA Experiments
The BC cells were cultured and harvested to prepare a cell suspension. The cells were then injected into nude mice to form subcutaneous tumors. When the tumor volumes reached 1200–1500 mm3, the mice received RFA treatment.7,8 The RFA was performed in accordance with previous methods.7 RFA was performed at 65–70°C for 3–5 mins to attenuate the subcutaneous growth of these cells. Subsequently, the mice were treated with different concentrations of SB431542 or a solvent control (control) via oral administration. The antitumor effect of RFA was measured based on the tumor volumes and tumor weights. The volumes were calculated based on tumor length and width in accordance with the methods described previously.23,24
In Vivo Animal Micro-Positron Emission Computed Tomography (micro-PET) Experiments
The BC cells were cultured and then seeded into nude mice to form subcutaneous tumors. The tumors were then treated by RFA, and the nude mice were analyzed by micro-PET (positron emission computed tomography). The absorbance of 18F-FDG (fluorodeoxyglucose) by the subcutaneous tumors formed by the BC cells was examined using previously described methods.25,26
Western Blot
The subcutaneous tumor tissues formed by BC cells were harvested for Western blot experiments. The expression level of EMT-related proteins was examined by their antibodies (Abcam Corporation, Cambridge, CB2 0AX, UK). The images of Western blot were quantitative examined by Image J Software (National Institutes of Health, Bethesda, Maryland, USA). GAPDH was chosen as a loading control.
Transwell Experiments
The BC cells were separated from the tumor tissues. The methods have been described previously.8,23 Single cells were then separated and analyzed by transwell experiments as described in our previous study.27 SB431542- and RFA-induced inhibition of in vitro invasion or migration was calculated as follows: relative invasion/migration cell number in the control group – relative invasion/migration cell number in the SB431542/relative invasion/migration cell number*100% in the control group.
Ethics Statement
For the usage of cell lines or clinical specimens, the methods, research, protocol or collection of the clinical specimens were approved by the ethics committee of the First People’s Hospital, Qujing City, Yunnan Province, People’s Republic of China. All experiments were performed in compliance with the Helsinki Declaration. The collection of clinical specimens were the consent of patients by written consent from all the patients. For animal experiments, the methods, protocol or usage of animals were approved by the ethics committee of the First People’s Hospital, Qujing City, Yunnan Province, People’s Republic of China. All animal studies were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines.
Statistical Analysis
Statistical analysis was performed by Bonferroni correction, with or without a two-way analysis of variance using SPSS Software (IBM Corporation, Armonk, NY, USA). The IC50 or EC50 values (50% effective concentration on agents’ inhibition rate or 50% effective concentration on agents’ effective rate) were calculated using Origin software, version No 6.1 (OriginLab Corporation, Northampton, MA, USA). A P-value of <0.05 was considered statistically significant.
Results
SB431542 Inhibited The EMT Process In Cultured BC Cell Lines
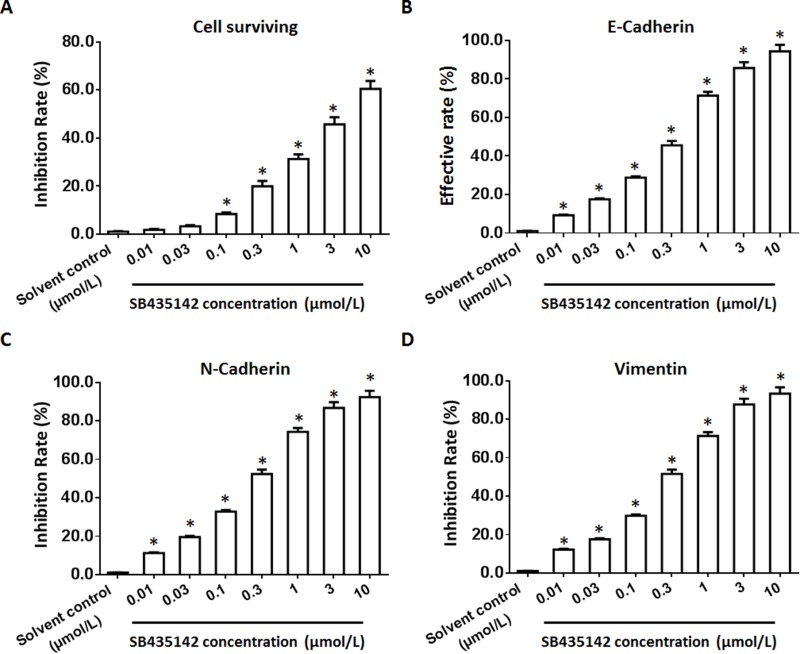
To examine the effect of SB431542 on BC cells, a patient-derived BC cell line was cultured and treated with the indicated concentrations of SB431542. As shown in Figure 1, SB431542 inhibited the survival and EMT of BC cells. The effect of the SB431542 treatment on the BC cell lines from the five patients is shown in Table 2. SB431542 inhibited the survival of the BC cells in a dose-dependent manner. In addition, the SB431542 treatment inhibited the EMT process in these cells (Table 3). The effective dose of SB431542 in terms of the EMT in BC cells was much lower than the effective dose of SB431542 on BC cell survival (Tables 2 and 3). To examine the specificity of SB435142, the activation of 3TP-Luc or SRB-Luc, two luciferase reporters of TGFβ/Smad was examined. As shown in Supplemental Table 1 and Supplemental Table 2, SB431542 repressed the activation of the two reporters in a dose-dependent manner in BC cells, both in current cell lines or PDCs. Therefore, SB431542 could be used in BC treatment.
Figure 1.
The effect of SB431542 on BC cells. A patient-derived BC cell line (patient no. 1) treated with the indicated concentrations of SB431542. The BC cells were harvested for MTT experiments (A) or qPCR experiments (B–D). The inhibition rates or effective rates of SB431542 on BC cell survival (A), E-cadherin (B), N-cadherin (C) and vimentin (D) are shown as the mean±SD. *P<0.05 versus a solvent control with SB431542.
Abbreviations: BC, bladder cancer; qPCR, quantitative real-time polymerase chain reaction.
Table 2.
The IC50 Values Of SB431542 On BC Cells’ Survival
| PDC | IC50 values (μmol/L) of SB431542 on cells’ survival |
|---|---|
| No. 1 | 4.37±0.35 |
| No. 2 | 2.52±0.22 |
| No. 3 | 5.55±0.44 |
| No. 4 | 3.33±0.10 |
| No. 5 | 2.67±0.66 |
| Averaged data | 3.69±1.27 |
Abbreviations: PDC, patients derived cells; BC, bladder cancer; IC50, half-effect concentration of inhibition rates.
Table 3.
The IC50 Values Of SB431542 On BC Cells’ EMT Process
| PDC | E-Cadherin | N-Cadherin | Vimentin |
|---|---|---|---|
| IC50 Or EC50 values of SB431542 on cells’ EMT | |||
| No. 1 | 0.33±0.01 | 0.21±0.02 | 0.25±0.05 |
| No. 2 | 0.26±0.01 | 0.28±0.04 | 0.30±0.03 |
| No. 3 | 0.51±0.05 | 0.32±0.02 | 0.46±0.04 |
| No. 4 | 0.25±0.04 | 0.25±0.04 | 0.25±0.04 |
| No. 5 | 0.38±0.03 | 0.38±0.03 | 0.38±0.03 |
| Averaged data | 0.35±0.10 | 0.29±0.06 | 0.33±0.09 |
Abbreviations: PDC, patients derived cells; BC, bladder cancer; IC50, half-effect concentration of inhibition rates; EC50, half-effect concentration of effective rates; EMT, epithelial–mesenchymal transition.
SB431542 Inhibited The EMT Of BC Cells In Subcutaneous Tumors
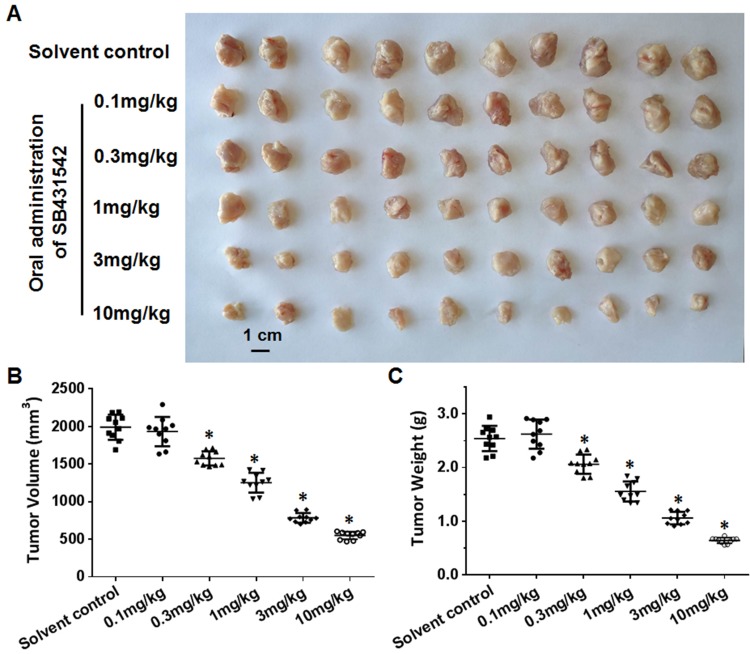
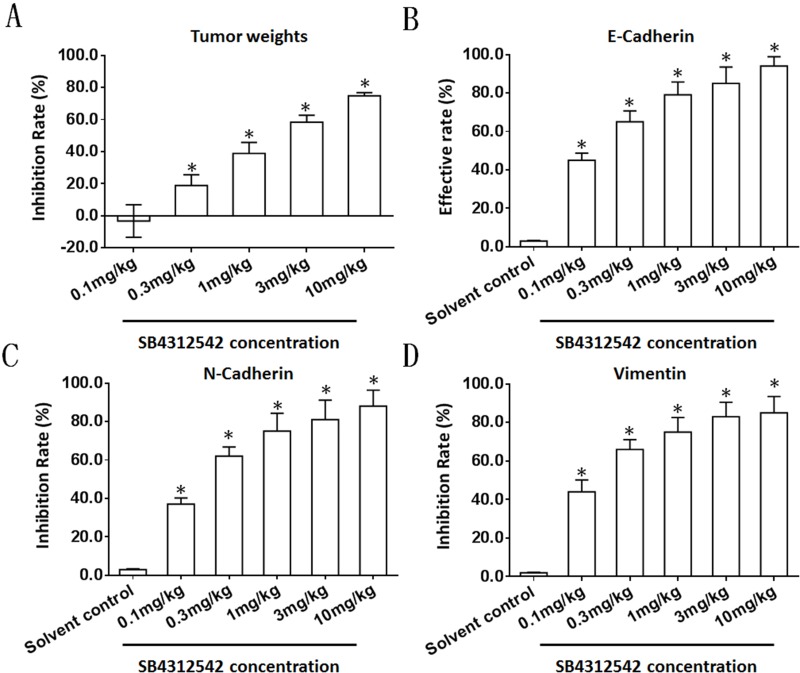
To further examine the effect of SB431542 on BC cells, the BC cells obtained from the five patients were injected into nude mice to form subcutaneous tumors. In this patient-derived tumor xenograft model, the mice were treated with various concentrations of SB431542. As shown in Figure 2 and Table 4, SB431542 inhibited subcutaneous growth of BC cells in a dose-dependent manner. Furthermore, SB431542 decreased the expression of N-cadherin or vimentin and enhanced the expression of E-cadherin in subcutaneous tumors formed by the BC cells (Figure 3). The SB431542 treatment inhibited the EMT in the BC cells in these subcutaneous tumors. Among the various concentrations of SB431542, concentrations of 0.3 mg/kg, 1 mg/kg, 3 mg/kg and 10 mg/kg inhibited the EMT process in the BC cells in subcutaneous tumors (Figures 2 and 3, Tables 4 and 5). A concentration of 1 mg/kg of SB431542 which could significantly inhibit the EMT process of BC cells was selected for use in subsequent experiments.
Figure 2.
The effect of SB431542 on subcutaneous growth of BC cells. A patient-derived BC cell line (patient no. 1) was injected into nude mice to form subcutaneous tumors. The mice were treated with the indicated concentrations of SB431542 via oral administration. Results of effect of SB431542 on BC cells’ subcutaneous growth were shown as photographs (A), tumor volumes (B) and tumor weights (C). *P<0.05.
Abbreviation: BC, bladder cancer.
Table 4.
The IC50 Values Of SB431542 On Subcutaneous Tumor Formed By BC Cells
| PDX | IC50 values (mg/kg) of SB431542 on cells’ survival |
|---|---|
| No. 1 | 1.24±0.45 |
| No. 2 | 0.93±0.22 |
| No. 3 | 1.10±0.30 |
| No. 4 | 1.46±0.67 |
| No. 5 | 1.02±0.35 |
| Averaged data | 1.15±0.20 |
Abbreviations: PDX, patient-derived tumor xenograft; BC, bladder cancer; IC50, half-effect concentration of inhibition rates.
Figure 3.
The effect of SB431542 on the EMT process in BC cells in subcutaneous tumors. A patient-derived BC cell line (patient no. 1) was injected into nude mice to form subcutaneous tumors. The mice were treated with the indicated concentrations of SB431542 via oral administration. Tumors were harvested for qPCR experiments. The inhibition rates or effective rates of SB431542 on tumor weights (A), E-cadherin (B), N-cadherin (C) and vimentin (D) are shown as the mean±SD. *P<0.05.
Abbreviations: BC, bladder cancer; EMT, epithelial–mesenchymal transition; qPCR, quantitative real-time polymerase chain reaction.
Table 5.
IC50 Values Of SB431542 On Subcutaneous Tumor Formed By BC Cells’ EMT
| PDX | E-Cadherin | N-Cadherin | Vimentin |
|---|---|---|---|
| IC50 or EC50 values (mg/kg) of SB431542 on cells’ EMT | |||
| No. 1 | 0.11±0.00 | 0.20±0.03 | 0.15±0.02 |
| No. 2 | 0.15±0.02 | 0.25±0.01 | 0.22±0.03 |
| No. 3 | 0.25±0.03 | 0.16±0.02 | 0.24±0.05 |
| No. 4 | 0.08±0.01 | 0.11±0.02 | 0.10±0.01 |
| No. 5 | 0.13±0.02 | 0.21±0.04 | 0.13±0.02 |
| Averaged data | 0.14±0.06 | 0.19±0.05 | 0.17±0.06 |
Abbreviations: PDX, patient-derived tumor xenograft; BC, bladder cancer; IC50, half-effect concentration of inhibition rates; EC50, half-effect concentration of effective rates; EMT, epithelial–mesenchymal transition.
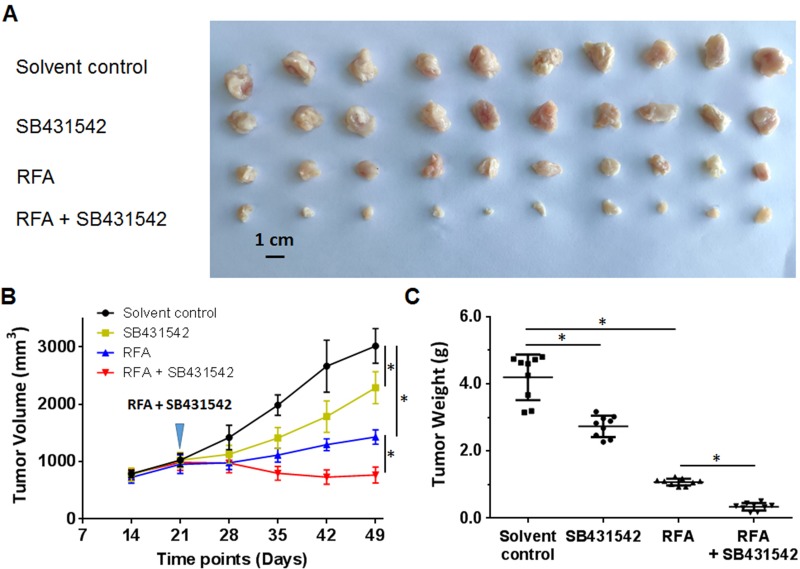
SB431542 Enhanced The Antitumor Effect Of RFA And Inhibited The EMT In BC Cells In Subcutaneous Tumors
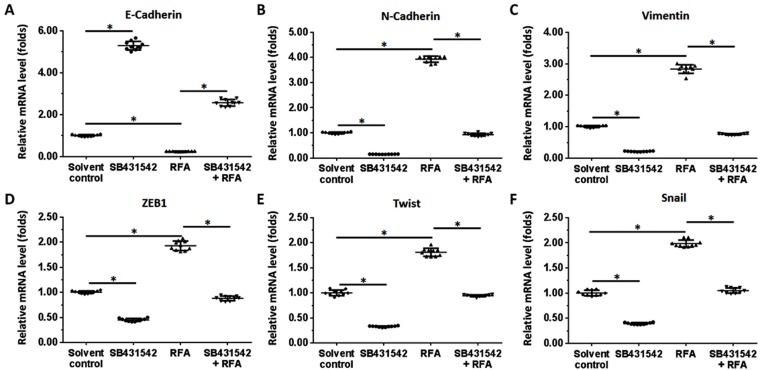
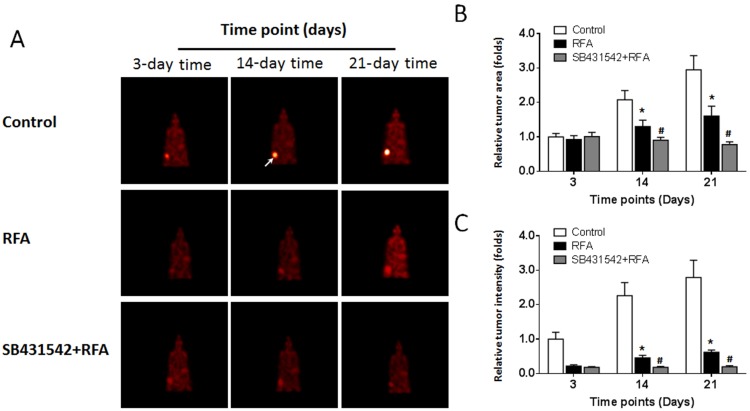
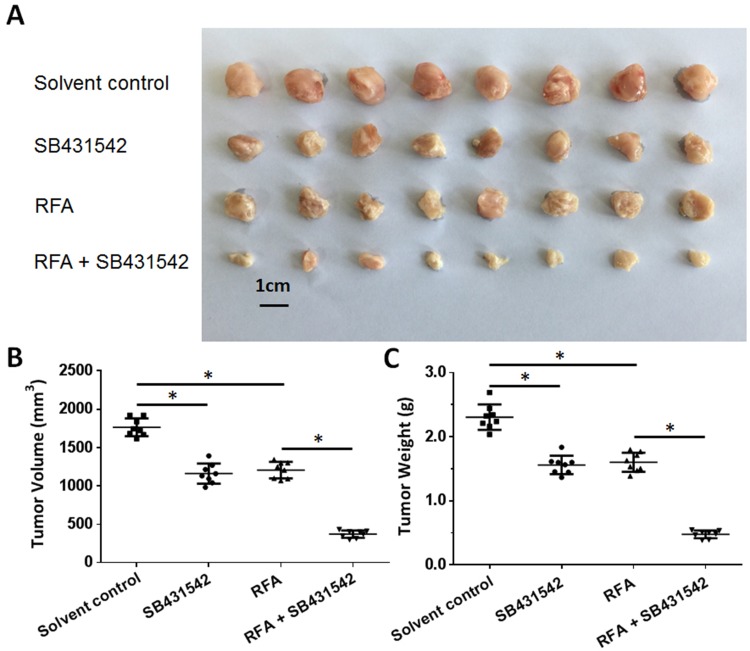
The BC cells were subcutaneously injected into nude mice. The results are shown in Figure 4. As depicted in Figure 4, the RFA treatment resulted in shrinkage of the subcutaneous tumors formed by the BC cells. Administration of a 1 mg/kg dose of SB431542 enhanced the antitumor effect of RFA. The expression of EMT-related factors in subcutaneous tumor tissues was then examined by qPCR experiments and similar results were obtained from Western blot (Supplemental Figure 2 and Supplemental Figure 3). The images of Western blot are shown as Supplemental Figure 2 and the results from quantitative analysis are shown as Supplemental Figure 3. Moreover, as shown in Figure 5, the RFA treatment induced the EMT process of cells in subcutaneous tumors, and the SB431542 treatment inhibited the EMT process induced by RFA. To further examine the antitumor effect of RFA on tumor tissues, micro-PET screening was performed to determine the vitality of the BC cells in the tumor tissues. As shown in Figure 6, the RFA treatment significantly inhibited the vitality of these cells, and a dose of 1 mg/kg of SB431542 enhanced the effect of RFA.
Figure 4.
The effects of a combination of SB431542 and RFA on subcutaneous growth of BC cells. A patient-derived BC cell line (patient no. 1) was injected into nude mice to form subcutaneous tumors. RFA of the tumor tissues was performed, and the mice were treated with SB431542 at a dose of 1 mg/kg via oral administration. The results are shown as photographs (A) of subcutaneous tumors, tumor-growth curves (B) or tumor weights (C) at the end of the experiment. *P<0.05.
Abbreviations: BC, bladder cancer; RFA, radiofrequency ablation.
Figure 5.
The effects of a combination of SB431542 and RFA on the EMT in subcutaneous tumor cells. A patient-derived BC cell line (patient no. 1) was injected into nude mice to form subcutaneous tumors. RFA of the tumor tissues was performed, and the mice were treated with SB431542 at a dose of 1 mg/kg via oral administration. Tumors were then harvested for qPCR experiments. The mRNA levels of E-cadherin (A), N-cadherin (B), vimentin (C), ZEB1 (D), twist (E) and Snail (F) in tumor tissues are shown as scatter diagrams. *P<0.05.
Abbreviations: BC, bladder cancer; RFA, radiofrequency ablation; EMT, epithelial–mesenchymal transition; ZEB1, zinc finger E-box binding homeobox 1.
Figure 6.
Results of the micro-PET analysis of the combined effect of SB431542 and RFA on subcutaneous growth of BC cells. A patient-derived BC cell line (patient no. 1) was injected into nude mice to form subcutaneous tumors. RFA of the tumor tissues was performed, and the mice were treated with SB431542 at a dose of 1 mg/kg via oral administration. The results of micro-PET at particular time points, showing (A) images of micro-PET, (B) tumor areas and (C) tumor intensity. In the micro-PET images, the arrows indicate the location of the tumor tissues (A). *P<0.05 versus control group with RFA treatment group; #P<0.05 versus RFA treatment group with RFA+SB431542 treatment group.
Abbreviations: BC, bladder cancer; qPCR, quantitative real-time polymerase chain reaction; RFA, radiofrequency ablation; PET, positron emission computed tomography.
To further examine the antitumor effect of SB431542, the cells were isolated from the subcutaneous tumors formed by the BC cells (Figure 4) for transwell assays. As depicted in Figure 7, both the SB431542 treatment and RFA treatment attenuated in vitro invasion or migration of single cells separated from the tumor tissues, and SB431542 enhanced the effect of RFA. Next, the in vivo growth of the single cells separated from the tumor tissues seeded into nude mice was examined. As presented in Figure 8, the RFA treatment decreased the subcutaneous growth of the cells, and the SB431542 treatment enhanced the effect of RFA on the BC cells. Thus, SB431542 enhanced the antitumor effect of the RFA treatment.
Figure 7.
In vitro invasion or migration of BC cells separated from subcutaneous tumors. The subcutaneous tumor tissues (Figure 4) were harvested, and single cells were separated. Transwell experiments were then performed to determine in vitro invasion or migration. Photographs of in vitro invasion (A) and migration (B). The results are the mean±SD. *P<0.05.
Abbreviation: BC, bladder cancer.
Figure 8.
Subcutaneous growth of single cells separated from subcutaneous tumors. Single cells were separated from subcutaneous tumors (Figure 4). The cells were seeded into nude mice to form subcutaneous tumors. Photographs showing the (A) tumor volumes (B) and tumor weights (C). *P<0.05.
Abbreviations: BC, bladder cancer; RFA, radiofrequency ablation.
Discussion
The important role of RFA in local therapy for advanced HCC treatment is well known.28 Recently, RFA has also been used to treat other cancers, such as non-small-cell lung cancer.29 However, previous studies demonstrated tumor recurrence after RFA treatment, as well as incomplete ablation, which can induce cellular stress and lead to pathological changes, such as the EMT.11–13 The same studies reported that EMT was related to RFA resistance or recurrence of tumors after RFA. The TGFβ signaling pathway is one of the foremost mediators of the EMT,15,16 and the TGFβ/Smad signaling pathway plays an important role in many important physiological processes, such as neuromuscular regulation and bone metabolism.30–32 The septicity of SB431542 was examined by luciferase experiments: treatment of SB431542 inhibited the activation of 3TP-Luc or SRB-Luc in a dose-dependent manner. The 3TP-Luc or SRB-Luc is the common and widely accepted tools to examine the activation of TGFβ/Smad pathway.33–35 The findings of the present study suggest that inhibition of activation of the TGFβ/Smad signaling pathway is a promising approach to enhance the antitumor effect of RFA.
In the present study, we established an RFA model based on subcutaneous growth of BC cells derived from BC patients. Subsequent treatment with SB431542 inhibited the EMT of BC cells and enhanced the antitumor effect of RFA in multi-models, eg, subcutaneous tumors. These findings point to the potential importance of SB431542 as an adjuvant in antitumor therapy. A urothelial carcinoma, also known as a transitional-epithelial tumor carcinoma, is the most common pathological subtype of BC.36–38 Given the histological features (epithelial source/origin) of urothelial carcinomas, the EMT may be closely related to the occurrence and progression of BC. The EMT may also play a role in BC cell tolerance to treatment.39,40
Radical treatment for BC, such as a cystectomy, can markedly prolong the survival of BC patients.1–3 However, both total and partial bladder resection affect the quality of life of the patient.1–3 In advanced BC, where metastasis or invasion has occurred, surgical treatment may be contraindicated.1–3 In this setting. RFA can play a role by precisely targeting and destroying local tumor tissue and reducing damage to tissues surrounding the tumor.7,8 There are few reports on the application of RFA in BC. The present study established a relevant research model that not only contributes to RFA-related research on BC but also to clinical treatment selection.
The methodological approach used in this study is transferable to the clinical setting. We also determined the weights of the subcutaneous tumors and generated tumor-growth curves, which quantitatively reflected the antitumor effects of RFA on tumor tissues. Furthermore, we used micro-PET, which is an effective in vivo imaging technique, to visually reflect the effect of RFA on the viability of tumor tissues. Further isolation/separation of cells from the tumor tissue and subsequent analysis further confirmed the antitumor effect of RFA and SB431542. It is reported that, some molecular targeting agents, including aorafenin or apatinib, could inhibit the EMT of cancer cells.41–45 However, these agents were targeting to the receptor tyrosine protein kinases represented by vascular endothelial growth factor receptor.46–52 Therefore, the inhibitor of TGF/Smad pathway may be a more useful or specific strategy to enhance the efficiency of RFA via repressing EMT process.
It is worth mentioning that the RFA conditions used in this study are similar to those of incomplete ablation. Thus, RFA can change the characteristics of tumor cells and induce the EMT process in tumor cells while inducing tumor cell injury and delaying the growth of tumor tissues. The tumor model established herein was a subcutaneous tumor model. Future research will focus on how to establish bladder in situ tumors or simulate the characteristics of bladder organs.
Conclusion
Only a few previous studies have reported antitumor effects SB431542 on BC.53–55 In the present study, SB431542 inhibited the effects of the RFA-induced EMT and upregulated the antitumor effect of RFA. Thus, SB431542 enhanced the effect of RFA on BC. The results indicate that SB435142 combined with RFA is a new and promising treatment strategy for BC.
Author contributions
All authors made substantial contributions to the design and conception, acquisition, analysis or interpretation of data. All authors took part in either drafting or revising the manuscript. All authors also gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388:2796–2810. doi: 10.1016/S0140-6736(16)30512-8 [DOI] [PubMed] [Google Scholar]
- 2.Voelker R. Immunotherapy for Bladder Cancer. JAMA. 2017;317:2363. doi: 10.1001/jama.2017.6976 [DOI] [PubMed] [Google Scholar]
- 3.Kouba EJ, Cheng L. Understanding the genetic landscape of small cell carcinoma of the urinary bladder and implications for diagnosis, prognosis, and treatment: a review. JAMA Oncol. 2017;3:1570–1578. doi: 10.1001/jamaoncol.2016.7013 [DOI] [PubMed] [Google Scholar]
- 4.Booth CM, Karim S, Peng Y, Siemens DR, Brennan K, Mackillop WJ. Radical treatment of the primary tumor in metastatic bladder cancer: potentially dangerous findings from observational data. J Clin Oncol. 2018;36:533–535. doi: 10.1200/JCO.2017.76.1759 [DOI] [PubMed] [Google Scholar]
- 5.Hu YW. Real-world role of adjuvant chemotherapy in bladder cancer. J Clin Oncol. 2016;34:3224. doi: 10.1200/JCO.2016.67.5884 [DOI] [PubMed] [Google Scholar]
- 6.Lee MW, Raman SS, Asvadi NH, et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology. 2017;65:1979–1990. doi: 10.1002/hep.29098 [DOI] [PubMed] [Google Scholar]
- 7.Xie H, Tian S, Yu H, et al. A new apatinib microcrystal formulation enhances the effect of radiofrequency ablation treatment on hepatocellular carcinoma. Onco Targets Ther. 2018;11:3257–3265. doi: 10.2147/OTT.S165000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie H, Yu H, Tian S, et al. MEIS-1 level in unresectable hepatocellular carcinoma can predict the post-treatment outcomes of radiofrequency ablation. Oncotarget. 2018;9:15252–15265. doi: 10.18632/oncotarget.24165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajdos C, Macdermott T, McCarter MD, Pearlman NW. Combined thermal-surgical ablation of locally advanced abdominopelvic malignancies. Ann Surg Oncol. 2011;18:1267–1273. doi: 10.1245/s10434-010-1467-4 [DOI] [PubMed] [Google Scholar]
- 10.Maugeri R, Graziano F, Basile L, et al. Reconstruction of vertebral body after radiofrequency ablation and augmentation in dorsolumbar metastatic vertebral fracture: analysis of clinical and radiological outcome in a clinical series of 18 patients. Acta Neurochir Suppl. 2017;124:81–86. doi: 10.1007/978-3-319-39546-3_13 [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Ma M, Lin XH, et al. Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment. BMC Cancer. 2018;18:901. doi: 10.1186/s12885-018-4820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwahashi S, Shimada M, Utsunomiya T, et al. Epithelial-mesenchymal transition-related genes are linked to aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablation. Cancer Lett. 2016;375:47–50. doi: 10.1016/j.canlet.2016.02.041 [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Kim SM, Kim BW, et al. Anti-cancer effects of HNHA and lenvatinib by the suppression of EMT-mediated drug resistance in cancer stem cells. Neoplasia. 2018;20:197–206. doi: 10.1016/j.neo.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moustakas A, Heldin CH. Mechanisms of TGFβ-induced epithelial-mesenchymal transition. J Clin Med. 2016;5:pii: E63. doi: 10.3390/jcm5070063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommerova L, Ondrouskova E, Vojtesek B, Hrstka R. Suppression of AGR2 in a TGF-β-induced Smad regulatory pathway mediates epithelial-mesenchymal transition. BMC Cancer. 2017;17:546. doi: 10.1186/s12885-017-3537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae JS, Jeon Y, Kim SM, et al. Depletion of MOB1A/B causes intestinal epithelial degeneration by suppressing Wnt activity and activating BMP/TGF-β signaling. Cell Death Dis. 2018;9:1083. doi: 10.1038/s41419-018-1138-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan F, Ding R, Zhang Q, et al. WX-132-18B, a novel microtubule inhibitor, exhibits promising anti-tumor effects. Oncotarget. 2017;8:71782–71796. doi: 10.18632/oncotarget.17710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Wei A, Bu L, et al. Procaspase-3-activating compound 1 stabilizes hypoxia-inducible factor 1α and induces DNA damage by sequestering ferrous iron. Cell Death Dis. 2018;9:1025. doi: 10.1038/s41419-018-1038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Q, Feng F, Zhang F, et al. LINE-1 ORF-1p functions as a novel HGF/ETS-1 signaling pathway co-activator and promotes the growth of MDA-MB-231 cell. Cell Signal. 2013;25:2652–2660. doi: 10.1016/j.cellsig.2013.08.029 [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Feng F, Yang Y, et al. LINE-1 ORF-1p functions as a novel androgen receptor co-activator and promotes the growth of human prostatic carcinoma cells. Cell Signal. 2013;25:479–489. doi: 10.1016/j.cellsig.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 21.Liang Y, Xu X, Wang T, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8:e2928. doi: 10.1038/cddis.2017.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji Q, Xu X, Li L, et al. miR-216a inhibits osteosarcoma cell proliferation, invasion and metastasis by targeting CDK14. Cell Death Dis. 2017;8:e3103. doi: 10.1038/cddis.2017.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Li D, Jiang Q, et al. Novel ADAM-17 inhibitor ZLDI-8 enhances the in vitro and in vivo chemotherapeutic effects of Sorafenib on hepatocellular carcinoma cells. Cell Death Dis. 2018;9:743. doi: 10.1038/s41419-018-0804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao Z, Li Y, Dai W, et al. ETS-1 induces Sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR. Pharmacol Res. 2018;135:188–200. doi: 10.1016/j.phrs.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 25.Li L, Liang Y, Kang L, et al. Transcriptional regulation of the warburg effect in cancer by SIX1. Cancer Cell. 2018;33:368–385.e7. doi: 10.1016/j.ccell.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Fan Z, Liang C, et al. A signature motif in LIM proteins mediates binding to checkpoint proteins and increases tumour radiosensitivity. Nat Commun. 2017;8:14059. doi: 10.1038/ncomms14059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Ma X, Song E, et al. Tubulin cofactor A functions as a novel positive regulator of ccRCC progression, invasion and metastasis. Int J Cancer. 2013;133:2801–2811. Epub 2013 Jul 9. doi: 10.1002/ijc.28306 [DOI] [PubMed] [Google Scholar]
- 28.Lee TY, Lin JT, Zeng YS, Chen YJ, Wu MS, Wu CY. Association between nucleos(t)ide analog and tumor recurrence in hepatitis B virus-related hepatocellular carcinoma after radiofrequency ablation. Hepatology. 2016;63:1517–1527. doi: 10.1002/hep.28266 [DOI] [PubMed] [Google Scholar]
- 29.Huang BY, Li XM, Song XY, et al. Long-term results of CT-guided percutaneous radiofrequency ablation of inoperable patients with stage Ia non-small cell lung cancer: a retrospective cohort study. Int J Surg. 2018;53:143–150. doi: 10.1016/j.ijsu.2018.03.034 [DOI] [PubMed] [Google Scholar]
- 30.Stylianou A, Gkretsi V, Stylianopoulos T. Transforming growth factor-β modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion. Biochim Biophys Acta Gen Subj. 2018;1862:1537–1546. doi: 10.1016/j.bbagen.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caraci F, Spampinato SF, Morgese MG, et al. Neurobiological links between depression and AD: the role of TGF-β1 signaling as a new pharmacological target. Pharmacol Res. 2018;130:374–384. doi: 10.1016/j.phrs.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 32.Xiang X, Cai HD, Su SL, et al. Salvia miltiorrhiza protects against diabetic nephropathy through metabolome regulation and wnt/β-catenin and TGF-β signaling inhibition. Pharmacol Res. 2019;139:26–40. doi: 10.1016/j.phrs.2018.10.030 [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y, Feng F, Yu J, et al. L1-ORF1p, a Smad4 interaction protein, promotes proliferation of HepG2 cells and tumorigenesis in mice. DNA Cell Biol. 2013;32:531–540. doi: 10.1089/dna.2013.2097 [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Cui J, Xue F, et al. Pokemon (FBI-1) interacts with Smad4 to repress TGF-β-induced transcriptional responses. Biochim Biophys Acta. 2015;1849:270–281. doi: 10.1016/j.bbagrm.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Ding L, Wang Z, Yan J, et al. Human four-and-a-half LIM family members suppress tumor cell growth through a TGF-beta-like signaling pathway. J Clin Invest. 2009;119:349–361. doi: 10.1172/JCI35930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, Phase Ib study. J Clin Oncol. 2017;35:2117–2124. doi: 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massari F, Di Nunno V. Atezolizumab for platinum-treated metastatic urothelial carcinoma. Lancet. 2018;391:716–718. doi: 10.1016/S0140-6736(17)33298-1 [DOI] [PubMed] [Google Scholar]
- 38.Hanna KS. A review of immune checkpoint inhibitors for the management of locally advanced or metastatic urothelial carcinoma. Pharmacotherapy. 2017;37:1391–1405. doi: 10.1002/phar.2033 [DOI] [PubMed] [Google Scholar]
- 39.Li A, Zhu X, Wang C, et al. Upregulation of NDRG1 predicts poor outcome and facilitates disease progression by influencing the EMT process in bladder cancer. Sci Rep. 2019;9:5166. doi: 10.1038/s41598-019-41660-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q, Miao S, Han X, et al. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018;9:960. doi: 10.1038/s41419-018-0986-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Tang Z. A novel long-sustaining system of apatinib for long-term inhibition of the proliferation of hepatocellular carcinoma cells. Onco Targets Ther. 2018;11:8529–8541. doi: 10.2147/OTT.S188209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Xia W, Chen L, et al. Synergistic antitumor effect of a γ-secretase inhibitor PF-03084014 and sorafenib in hepatocellular carcinoma. Oncotarget. 2018;9:34996–35007. doi: 10.18632/oncotarget.26209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu K, Huang J, Lai L, et al. Medium or large hepatocellular carcinoma: sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;288:300–307. doi: 10.1148/radiol.2018172028 [DOI] [PubMed] [Google Scholar]
- 44.Giorgio A, Merola MG, L M, et al. Sorafenib combined with radio-frequency ablation compared with sorafenib alone in treatment of hepatocellular carcinoma invading portal vein: a western randomized controlled trial. Anticancer Res. 2016;36:6179–6183. doi: 10.21873/anticanres.11211 [DOI] [PubMed] [Google Scholar]
- 45.Dong S, Kong J, Kong F, et al. Sorafenib suppresses the epithelial-mesenchymal transition of hepatocellular carcinoma cells after insufficient radiofrequency ablation. BMC Cancer. 2015;15:939. doi: 10.1186/s12885-015-1949-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Wu Y, Wang D, et al. Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacol Res. 2019;146:104313. doi: 10.1016/j.phrs.2019.104313 [DOI] [PubMed] [Google Scholar]
- 47.Roskoski R Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res. 2019;144:19–50. doi: 10.1016/j.phrs.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 48.Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97–107. doi: 10.1016/j.phrs.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 49.Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–132. doi: 10.1016/j.phrs.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 50.Feng F, Jiang Q, Cao S, et al. Pregnane X receptor mediates sorafenib resistance in advanced hepatocellular carcinoma. Biochim Biophys Acta Gen Subj. 2018;1862:1017–1030. doi: 10.1016/j.bbagen.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 51.Roskoski R Jr. The role of small molecule platelet-derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disorders. Pharmacol Res. 2018;129:65–83. doi: 10.1016/j.phrs.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 52.Roskoski R Jr, Sadeghi-Nejad A. Role of RET protein-tyrosine kinase inhibitors in the treatment RET-driven thyroid and lung cancers. Pharmacol Res. 2018;128:1–17. doi: 10.1016/j.phrs.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Zhang Y, Li M, et al. Combination of SB431542, CHIR99021 and PD0325901 has a synergic effect on abrogating valproic acid‑induced epithelial‑mesenchymal transition and stemness in HeLa, 5637 and SCC‑15 cells. Oncol Rep. 2019;41:3545–3554. doi: 10.3892/or.2019.7088 [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Zhong W, Zhu M, Hu S, Su X. Nodal regulates bladder cancer cell migration and invasion via the ALK/Smad signaling pathway. Onco Targets Ther. 2018;11:6589–6597. doi: 10.2147/OTT.S177514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong H, Yin H, Hossain MA, et al. Starvation-induced autophagy promotes the invasion and migration of human bladder cancer cells via TGF-β1/Smad3-mediated epithelial-mesenchymal transition activation. J Cell Biochem. 2019;120:5118–5127. doi: 10.1002/jcb.27788 [DOI] [PubMed] [Google Scholar]