Abstract
Background
Human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP) substantially reduces the risk of HIV acquisition, yet significant barriers exist to its prescription and use. Incorporating pharmacists in the PrEP care process may help increase access to PrEP services.
Methods
Our pharmacist-led PrEP program (P-PrEP) included pharmacists from a university-based HIV clinic, a community pharmacy, and 2 community-based clinics. Through a collaborative practice agreement, pharmacists conducted PrEP visits with potential candidates for PrEP, according to the recommended Centers for Disease Control and Prevention guidelines, and authorized emtricitabine-tenofovir disoproxil fumarate prescriptions. Demographics and retention in care over 12 months were summarized, and participant satisfaction and pharmacist acceptability with the P-PrEP program were assessed by Likert-scale questionnaires.
Results
Sixty patients enrolled in the P-PrEP program between January and June 2017 completing 139 visits. The mean age was 34 years (range, 20–61 years), and 88% identified as men who have sex with men, 91.7% were men, 83.3% were white, 80% were commercially insured, and 89.8% had completed some college education or higher. Participant retention at 3, 6, 9, and 12 months was 73%, 58%, 43%, and 28%, respectively. To date, no participant has seroconverted. One hundred percent of the participants who completed the patient satisfaction questionnaire would recommend the P-PrEP program. Pharmacists reported feeling comfortable performing point-of-care testing and rarely reported feeling uncomfortable during PrEP visits (3 occasions, 2.2%) or experiencing workflow disruption (1 occasion, 0.7%).
Conclusions
Implementation of a pharmacist-led PrEP program is feasible and associated with high rates of patient satisfaction and pharmacist acceptability.
Keywords: HIV pre-exposure prophylaxis, HIV prevention, pharmacist-led
Preventing human immunodeficiency virus (HIV) acquisition remains a challenge more than 3 decades after the discovery of the virus. Currently available biomedical HIV prevention approaches include the diagnosis and treatment of HIV, daily oral HIV pre-exposure prophylaxis (PrEP), and HIV postexposure prophylaxis. When taken once daily, emtricitabine/tenofovir disoproxil fumarate (F/TDF) is safe and highly effective in the prevention of HIV acquisition, but its utilization is limited for many reasons including lack of patient and provider knowledge and awareness, treatment access, and stigma [1–3].
There has been much debate about the optimal setting in which to provide PrEP. Human immunodeficiency virus practitioners believe primary care physicians (PCP) are best suited to prescribe PrEP because of their access to HIV-uninfected populations [4]. Although efforts are being made to educate PCP’s about PrEP, PCP’s are often uncomfortable with PrEP management [5–7]. Furthermore, in a survey of men who have sex with men (MSM), 80% stated that they did not want to talk to their PCP about PrEP [8].
Many areas of the Midwestern United States are designated as federal medically underserved areas, demonstrating reduced access to primary care services in general and particularly for specialty healthcare services in rural areas. Persons at risk for HIV acquisition may have difficulty accessing preventive services despite actively seeking PrEP. Furthermore, 1 in 8 PrEP-eligible patients would require greater than 30 minutes of travel to visit a PrEP provider in most rural areas [9]. Implementation of home-based and telehealth PrEP have demonstrated some acceptability and feasibility as an alternative to medical clinic-based PrEP [10–13]. With over 60 000 community pharmacies in the United States, representing 13 billion pharmacy visits annually, the community pharmacy potentially offers an alternative setting to reach individuals at risk for HIV acquisition [14].
Prior studies have demonstrated successful collaboration of pharmacists with other healthcare providers and health departments in HIV prevention efforts through programs ranging from HIV screening to pharmacy-based syringe distribution and postexposure prophylaxis services [15–18]. Individual states regulate pharmacists’ patient care services through scope-of-practice laws and related rules. Depending on state laws, pharmacists may provide an array of patient care services through collaborative practice agreements (CPA) with medical providers. Collaborative practice agreements create a formal relationship between a pharmacist and a prescriber and allow the prescriber to delegate specified patient care responsibilities to the pharmacist under negotiated conditions within the agreement. Nebraska state law explicitly authorizes CPA, allowing pharmacists to facilitate and manage a variety of patient care services including point-of-care testing (POCT) and treatment for bacterial and viral respiratory illnesses, such as streptococcal pharyngitis and influenza [19, 20]. Building on these models, a collaborative drug therapy management plan would allow pharmacists working within the context of a defined HIV PrEP protocol to assume professional responsibility for all aspects of PrEP administration, including performing patient assessments, ordering and interpreting laboratory tests, performing POCT, patient counseling, and dispensing and monitoring PrEP treatment [21].
Clinical pharmacists have been incorporated into PrEP delivery models in Miami, Florida, and Seattle, Washington [22, 23]. The Miami Veterans Affairs Health System model utilized pharmacists to optimize adherence and retention in care in between the scheduled quarterly visits but did not incorporate independent pharmacist visits at the quarterly clinical visits. Tung and colleagues have described a robust pharmacist-led PrEP delivery program, but they are unique in their ability to perform phlebotomy and other procedures outside the scope of traditional community pharmacies.
This pilot study investigated the acceptability and feasibility of a pharmacist-led HIV screening and PrEP program (P-PrEP) for individuals at risk for HIV acquisition in Omaha, Nebraska, including the number of patients initiated on PrEP, retention in PrEP care, and patient and pharmacist satisfaction with the program.
METHODS
Patient Participants
Participants were recruited in the P-PrEP program through self-referral or referral by friends or partners, their PCP, local HIV/acquired immune deficiency syndrome (AIDS) service organizations, county sexually transmitted infection (STI) clinics, or local HIV clinics. Eligibility for P-PrEP inclusion was as follows: (1) HIV-uninfected patients aged greater than 19 years of age (the age of majority in Nebraska); (2) at high-risk of acquiring HIV (naive or PrEP-experienced) based on 1 or more of the following risk factors—(a) MSM who engage in condomless anal intercourse, (b) individuals who are in a serodiscordant sexual relationship with a known HIV-positive partner, (c) transgender individuals who engage in condomless intercourse, (d) individuals engaging in transactional sex, (e) injection drug users, (e) individuals who use stimulant drugs associated with high-risk behaviors, such as methamphetamine, (f) individuals diagnosed (self-reported or by recent STI testing) with at least 1 anogenital STI in the last year, (g) individuals who have ever been prescribed nonoccupational postexposure prophylaxis (nPEP) demonstrating continued high-risk behavior or have used 2 or more courses of nPEP—(3) English-speaking; (4) serum creatinine less than 1.5 times the upper limit of normal; (5) nonreactive hepatitis B surface antigen; and (6) no signs or symptoms of acute HIV infection within the past 30 days. This study was approved by the University of Nebraska Medical Center Investigational Review Board, and all participants provided written informed consent.
Participating Pharmacists
Pharmacists at a university-based HIV clinic, a community pharmacy, a university-based primary care clinic, and a Federally Qualified Health Center primary care clinic were recruited as P-PrEP pharmacist providers based on proximity and access to high-risk populations, willingness to participate, and acceptance by their leadership management team.
Intervention
Pharmacist-led PrEP was designed as a pharmacist-led program allowing participating pharmacists to serve as PrEP providers through the utilization of a CPA. A CPA specifying pharmacist responsibilities within the P-PrEP program was completed between the university-based HIV medical providers and each participating P-PrEP pharmacist. Each participating P-PrEP pharmacist completed the National Association of Chain Drug Stores Point-of-Care certificate program [24]. The P-PrEP pharmacists were provided additional education on HIV risk assessment, testing, risk reduction counseling, and administration of PrEP from faculty of the Nebraska AIDS Education and Training Center located at the University of Nebraska Medical Center. Upon completion of training, P-PrEP pharmacists assumed responsibility for the PrEP care of individuals enrolled in P-PrEP through the CPA.
The screening and initial visit was conducted by the P-PrEP pharmacist at the university-based HIV clinic site to ensure complete collection of all baseline laboratory tests and consenting procedures. The university-based HIV clinic pharmacist collected basic demographic and socioeconomic information, completed a medical history, an HIV risk assessment based on the study eligibility criteria through conduction of sexual, STI (self-reported chlamydia, gonorrhea, and syphilis infections), and substance use histories, PrEP counseling, baseline laboratory testing, and performed HIV and STI screening. If clinical information such as laboratory testing, STI testing, etc performed at another healthcare facility was needed, the participant signed a Health Insurance Portability and Accountability Act of 1996 release form to gain access to such data. Eligible P-PrEP participants were provided a 90-day F/TDF prescription. Each participant was given the option to continue PrEP care at the university-based HIV clinic or at 1 of the other 3 participating sites (community pharmacy, university-based primary care clinic, or Federally Qualified Health Center primary care clinic). Primary care services were integrated with PrEP at the university-based and Federally Qualified Health Center primary care clinics through a patient-centered medical home model. All participants were encouraged to engage in care with a PCP if no current PCP relationship was in place.
Pharmacist-led PrEP participants presented for follow-up visits every 3 months after PrEP initiation, and laboratory monitoring was performed according to Centers for Disease Control and Prevention HIV PrEP guidelines [25]. Follow-up visits completed at a clinic-based site (university-based HIV clinic, university-based primary care clinic, and Federally Qualified Health Center primary care clinic) were conducted in clinic exam rooms, and all charting and laboratory test collections were performed by each clinic’s standard procedures. Follow-up visits conducted at the community pharmacy site occurred in a private room, and all POCT was performed at the community pharmacy and interpreted by the pharmacist.
At all follow-up sites, a sample of whole blood by finger stick was collected for HIV screening using a fourth-generation HIV 1/2 Antibody/Antigen test (Alere Determine; Abbott Laboratories, Chicago, IL), and urine, rectal, and pharyngeal specimens were obtained for Chlamydia trachomatis and Neisseria gonorrheae by deoxyribonucleic acid probe assay (Aptima Combo 2; Hologic, Marlborough, MA). Sexually transmitted infection screenings for chlamydia and gonorrhea at the community pharmacy site were self-collected by the participant. Participants provided urine specimens and were educated on self-collection of pharyngeal and rectal specimens for STI screening. Chlamydia and gonorrhea STI specimens were collected by the pharmacy for delivery by courier to a local hospital-based laboratory for processing with the results reported to university-based HIV clinic clinical staff for interpretation and coordination of STI treatment if applicable.
At the community pharmacy site, whole blood by finger stick was collected for rapid analysis of blood creatinine (i-STAT Handheld Analyzer; Abbott Laboratories, Chicago, IL) and Treponema palladium antibody screening (Syphilis Health Check; Diagnostics Direct, LLC, Stone Harbor, NJ). All safety blood tests conducted at the clinic-based sites (university-based HIV clinic, university-based primary care clinic, and Federally Qualified Health Center primary care clinic), including syphilis screening (BioPlex 2200 Syphilis Total and RPR Kit; Bio-Rad, Hercules, CA), were collected by venipuncture in compliance with standard practices for follow-up visits. If appropriate, the pharmacist initiated a new 3-month F/TDF prescription as designated through the CPA bylaws. Follow-up visits were scheduled for all clinic-based sites but not for the community pharmacy site. Instead, the participant was able to walk-in to the community pharmacy at their convenience for the next quarterly P-PrEP visit.
Study Assessments
The primary outcome measure was the total number of participants initiated on F/TDF for PrEP. Secondary outcome measures included the following: (1) adherence to F/TDF, (2) number of patients retained at 3, 6, 9, and 12 months, (3) patient satisfaction with P-PrEP services, and (4) pharmacist satisfaction with P-PrEP services.
Assessment of adherence to F/TDF while engaged in the P-PrEP program occurred at each follow-up visit for the preceding 3 months by calculation of a medication possession ratio (total number of F/TDF doses dispensed/total number of days between study follow-up visits) [26]. Retention in PrEP care within the P-PrEP program was determined as the total number of patients completing follow-up visits at 3, 6, 9, and 12 months.
Protocol-derived questionnaires, designed with Likert-scale and open-ended questions, to assess participant satisfaction and pharmacist experience of the P-PrEP program were completed by participants retained at the 6-month visit and at each visit for the pharmacists. The respondents completed questionnaires independently, and identifying information was not included on the questionnaires. Participants were asked to describe their P-PrEP experience regarding the quality of PrEP education provided, interactions with pharmacists, privacy, collection of laboratory specimens, timeliness of follow-up visits, ease of medication access, and maximal amount participants would pay for P-PrEP if offered (community pharmacy site only). Pharmacists were asked to describe their comfort level with conducting P-PrEP visits and performing POCT. Time requirements for performing POCT, PrEP consultation, and visit entirety were recorded.
Descriptive statistics were used to summarize all study data including the participant demographics, baseline characteristics, and participant/pharmacist P-PrEP satisfaction. Survival analysis via Kaplan-Meier estimators and log-rank tests were used to evaluate demographic characteristics associated with retention in PrEP care.
RESULTS
Participant Characteristics
From January 1, 2017 through June 30, 2017, 60 participants enrolled in the P-PrEP program and started F/TDF. The majority, 91.7% (55 of 60), were men, 83.3% (50 of 60) were white, 80% (48 of 60) were commercially insured, and 89.8% (54 of 60) had completed some college or higher. The mean age of participants was 34 years (range, 20–61 years), and 88.3% (53 of 60) identified as MSM. The mean creatinine clearance was 130 mL/minute (range, 89–172 mL/minute). Fourteen participants (23%) were diagnosed with chlamydia or gonorrhea at the baseline visit for a total number of 22 diagnosed infections. Rectal and pharyngeal infections were most common at baseline (4 rectal gonorrhea, 9 rectal chlamydia, and 6 pharyngeal gonorrhea). No incident syphilis infections were diagnosed at baseline; however, 10 participants (17%) had a previous history of syphilis infection and treatment (Table 1).
Table 1.
Baseline Characteristics of Participants
| Baseline Characteristic | All Participants, n = 60 |
|---|---|
| Age (years), mean (range) | 34 (20–61) |
| Gender, n (%) | |
| Male | 55 (91.7) |
| Female | 3 (5.0) |
| Transgender male | 2 (3.3) |
| Transgender female | 0 (0) |
| Race and Ethnicity, n (%)a | |
| White | 50 (83.3) |
| Latinx | 5 (8.3) |
| African American | 5 (8.3) |
| Asian or Pacific Islander | 2 (3.3) |
| Other | 1 (1.7) |
| Insurance Coverage, n (%) | |
| Private/Commercial | 48 (80.0) |
| Medicare | 1 (1.7) |
| Medicaid | 0 (0) |
| Uninsured | 11 (18.3) |
| Education Completed, n (%) | |
| Less than high school diploma or GED | 0 (0) |
| High school diploma or GED | 6 (10.2) |
| Some college, no degree | 19 (32.2) |
| Two-Year Associates Degree | 3 (5.1) |
| Bachelor Degree | 17 (28.8) |
| Some Postgraduate education | 2 (3.4) |
| Postgraduate or professional degree | 12 (20.3) |
| HIV Risk Factor, n (%)b | |
| MSM-UAI | 53 (88.3) |
| Sexually active with HIV+ partner | 17 (28.3) |
| Transgender person engaging in high-risk behavior | 2 (3.3) |
| Transactional sex | 1 (1.7) |
| Injection drug use | 0 (0) |
| Use of stimulant (eg, methamphetamine, MDMA) | 1 (1.7) |
| Anogenital STI within 1 year | 19 (31.7) |
| Previous nPEP prescription | 3 (5.0) |
| Any Baseline Sexually Transmitted Infection, n (%)c | 14 (23) |
| Chlamydia | |
| Anal | 9 (15.0) |
| Pharyngeal | 0 (0) |
| Urogenital | 1 (1.7) |
| Gonorrhea | |
| Anal | 4 (6.7) |
| Pharyngeal | 6 (10.0) |
| Urogenital | 2 (3.3) |
| Syphilis | |
| New diagnosis | 0 (0) |
| History of infection | 10 (17.0) |
Abbreviations: GED, General Education Diploma; HIV, human immunodeficiency virus; MDMA, 3,4-methylenedioxymethamphetamine, “Ecstasy”; MSM-UAI, men who have sex with men-unprotected anal intercourse; nPEP, nonhealthcare HIV postexposure prophylaxis; STI, sexually transmitted infections.
NOTE: Except where indicated, data are presented as n (%) of study group participants.
aSome participants reported multiple categories leading to unequal total proportion.
bSome participants reported multiple categories leading to unequal total proportion.
cSome participants reported multiple categories leading to unequal total proportion.
Retention and Adherence
Almost all of the participants (55 of 60; 91.7%) chose either the university-based HIV clinic or community pharmacy as their preferred follow-up site (university-based HIV clinic, 28, 46.7%; community pharmacy, 27, 45%). A total of 139 P-PrEP follow-up visits occurred over the course of the study period totaling 30.75 person years of follow-up. There were zero HIV seroconversions.
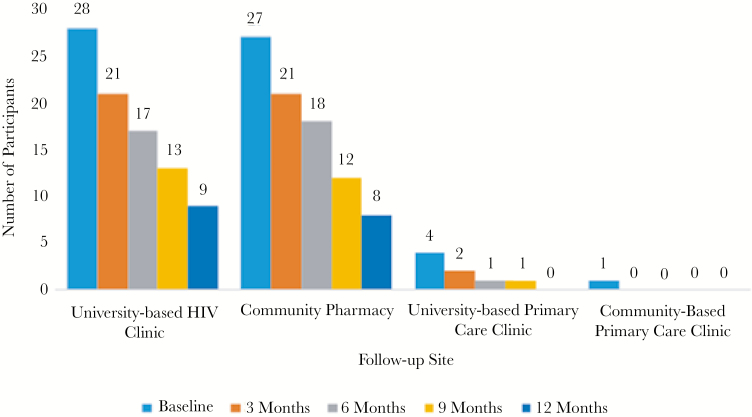
Retention within the P-PrEP program fell throughout the duration of the study with 73%, 58%, 43%, and 28% of the participants retained at 3, 6, 9, and 12 months, respectively. Participant retention per site is described in Figure 1. Participants without private insurance and those who were not MSM engaging in unprotected anal intercourse dropped out significantly sooner than other participants (log-rank test P = .033 and P = .001, respectively). Among participants retained throughout the study, adherence to F/TDF remained high with a mean medication possession ratio of 93%.
Figure 1.
Pharmacist-led pre-exposure prophylaxis program participant totals through study duration notated separately by follow-up site. All baseline visits were conducted at the university-based human immunodeficiency virus (HIV) clinic site. The participant totals for the baseline visit is representative of the participant’s choice of follow-up site after study entry.
Sexually Transmitted Infections
A total of 29 STIs (11 chlamydia, 17 gonorrhea, 1 syphilis) were diagnosed throughout the study demonstrating 0.94 incident STI infections per person years of follow-up. The majority were observed at the baseline visit upon P-PrEP enrollment. Overall STI prevalence decreased over the course of the study (data not shown).
Participant Satisfaction
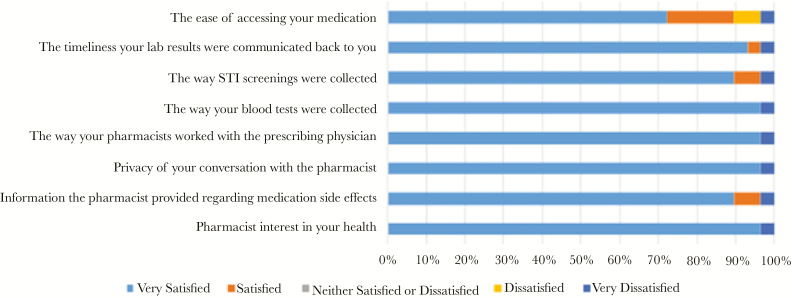
Of the 35 participants completing the 6-month visit, a total of 29 participants completed the P-PrEP satisfaction questionnaire: 13 (44.8%) from the community pharmacy site, 16 (55.2%) from the university-based HIV clinic site, and none from either the university-based primary care clinic or the Federally Qualified Health Center primary care clinic sites. All of the respondents stated they would definitely recommend the P-PrEP program. Respondents reported the P-PrEP program allowed for ease of PrEP care, quick service, extended hours for follow-up visits, and friendly and honest pharmacists. The ease of medication access, confusion with the collection of rectal and pharyngeal STI swabs, and delayed communication between pharmacist providers and medical providers were noted as areas needing improvement for the P-PrEP program (Figure 2). Of the participants who completed follow-up visits at the community pharmacy, half (6 of 12) stated they would be willing to pay at least $20 quarterly for continued PrEP visits with the remaining participants willing to pay up to $60 quarterly. One participant at the community pharmacy site did not respond to the cost consideration question.
Figure 2.
Participant satisfaction with the pharmacist-led pre-exposure prophylaxis program. STI, sexually transmitted infection.
Pharmacist Satisfaction
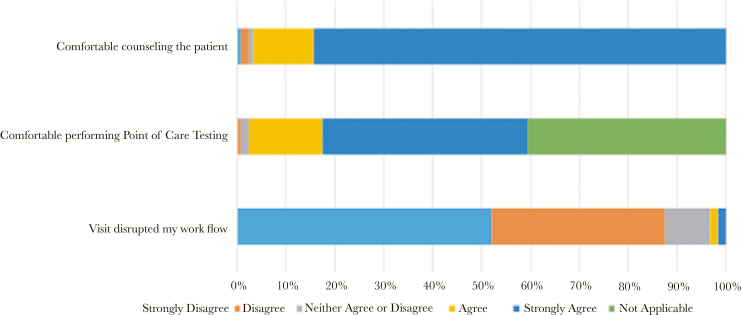
A total of 7 pharmacists (1 university-based HIV clinic, 3 community pharmacy, 1 university-based primary care clinic, and 2 Federally Qualified Health Center primary care clinic) participated in P-PrEP. The P-PrEP pharmacists felt comfortable performing POCT at all visits except on 1 occasion (0.7%). Furthermore, 1 pharmacist at the community pharmacy site reported 3 occasions (2.2%) in which they felt uncomfortable conducting sexual histories during P-PrEP follow-up visits. Workflow disruption at the community pharmacy site was reported only once (0.7%) throughout the study. The mean reported times for performing POCT, PrEP counseling, and total visit times were 8.7, 16, and 28 minutes, respectively (Figure 3).
Figure 3.
Pharmacist assessment of workflow disruption and comfortability with point-of-care testing and pre-exposure prophylaxis (PrEP) counseling, per pharmacist-led PrEP visit.
Discussion
In this pilot investigation of a P-PrEP, we successfully initiated PrEP in 60 participants at risk for HIV acquisition and found a high overall acceptance rate by both the participants and pharmacists. These data support P-PrEP as a desirable and feasible option for PrEP delivery and scale-up.
Retention in care decreased over the course of the study, with just over half retained at 6 months and approximately one quarter retained at 12 months. Although disappointing, these retention rates are similar to retention rates seen in other real-world PrEP implementation studies [27–29]. Furthermore, we identified similar drivers of poor retention that included both structural (insurance coverage) and individual (HIV risk factor) factors. More important, a high rate of medication adherence was seen in retained participants. The effectiveness of PrEP ultimately depends on adherence to the prescribed medication, so the high rates of adherence among those retained in care are encouraging.
The P-PrEP follow-up visits performed at the community pharmacy were quick and convenient for participants. The adoption of a P-PrEP may allow for increased PrEP access in rural settings by reducing travel times and potentially offering a lower cost option for PrEP follow-up, which is an important consideration in light of the Trump administration’s plan to end the HIV epidemic [30]. Furthermore, the pharmacy may be an acceptable setting for future PrEP formulations, such as intramuscular cabotegravir, considering pharmacists’ current integration into other long-acting medical treatments and vaccination administration [31–34].
The community pharmacy site used in this pilot study was a small, independent pharmacy with significant buy-in by its pharmacists and pharmacist owner. Pharmacy workflow disruption and leadership acceptance should be considered as potential barriers to implementation of P-PrEPs at other sites as evident from a recent survey of Midwest pharmacists in which concerns of workflow disruption and acceptance by leadership were cited as a concern, in spite of high pharmacist interest in provision of PrEP services [35]. In addition, the balance of cost to the patient and compensation to the pharmacy should be considered for program sustainability. Challenges remain in pharmacist compensation for these services because not all states allow pharmacists to bill for professional services [15]. The One-Step PrEP program, another P-PrEP in Seattle, Washington, was financially sustainable, but pharmacists are permitted to legally bill Medicaid for services provided in the state, allowing this model to be implemented more easily in Washington in comparison to states without this provision [13]. The P-PrEP participants at the community pharmacy in our study received PrEP care, POCT, and STI screening free of charge. All participants receiving care at a clinic-based P-PrEP site were not charged for the PrEP visit, but all laboratory and STI screenings were billed by standard procedures. Participants at the community pharmacy were willing to pay from $20 to $60 quarterly, amounting to approximately the cost of the POCT supplies and allowing for little compensation for the pharmacist’s time and effort for PrEP services. However, additional pharmacy revenue through F/TDF prescription reimbursements could potentially subsidize some of those costs.
Laboratory management and STI screening was a logistical challenge at the community pharmacy site. No CLIA-waived POC test is currently available to distinguish between previous and incident syphilis infections nor for hepatitis B screening, and, thus, both require venipuncture and subsequent processing at a clinical laboratory. The PrEP@Home study used the rapid plasma reagin card for syphilis screening (Arlington Scientific, Inc, Springville, UT), eliminating the need for venipuncture, although it still requires processing at a clinical laboratory [7]. The collection and couriered delivery of STI specimens and subsequent communication of results back to patients and providers are challenging to execute from a community pharmacy setting. Given these laboratory considerations, initial prescription of PrEP in the community pharmacy setting is likely not practical. However, P-PrEP care in the community pharmacy setting may be well suited for PrEP follow-up and potentially for HIV screening and linkage to care and postexposure prophylaxis.
There were some limitations to our study. The P-PrEP was a pilot study and thus included a small number of participants. Larger scale studies may help further determine the reproducibility of this model of PrEP delivery in various regions. Key patient populations were not well represented within our study population, including women, transgender persons, minority populations, and non-English-speaking patients. Research to help engage and retain these high-risk populations should continue to be explored. Finally, patient satisfaction may have been skewed towards the positive given that questionnaires were collected only from those who remained engaged in PrEP care at the 6-month visit at which time retention had dropped off.
Conclusions
Implementation of a P-PrEP is feasible with high rates of patient satisfaction and pharmacist acceptability. Its utilization may be of specific benefit to patients living in underserved or rural areas to increase PrEP access and allow for patient convenience. Despite concerns of patient cost, pharmacist compensation and workflow disruption, and the logistical challenges of laboratory and STI screening, the community pharmacy should remain a potential option for PrEP follow-up.
Acknowledgments
We thank the patients as well as the pharmacists (Jeff Kilborn, Stephanie Nitz, Crystal Pfieffer, Kristen Cook, Jessica Downes, and Jennifer Foster) who participated in this study.
Author contributions. S. H. B. designed the study. S. H. B. and J. P. H. contributed to data collection. All authors analyzed and interpreted the data. J. P. H. and S. H. B. drafted the manuscript. All authors reviewed, critically revised, and approved the final manuscript.
Financial support. This work was funded by Gilead Sciences, Inc.
Potential conflicts of interest. S. H. B. has received grants to her institution from Gilead. S. S. has received grants to her institution from ViiVHealthcare. D. G. K. has received personal fees from the NACDS Foundation and grants to his institution from the NACDS Foundation and Gilead. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 2. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffman S, Guidry JA, Collier KL, et al. A clinical home for preexposure prophylaxis: diverse health care providers’ perspectives on the “Purview Paradox”. J Int Assoc Provid AIDS Care 2016; 15:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krakower D, Ware N, Mitty JA, et al. HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav 2014; 18:1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer KH, Hosek S, Cohen S, et al. Antiretroviral pre-exposure prophylaxis implementation in the United States: a work in progress. J Int AIDS Soc 2015; 18:19980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav 2017; 21:1256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krakower DS, Oldenburg CE, Mimiaga MJ, et al. Patient-provider communication about sexual behaviors and pre-exposure prophylaxis: results from a national online survey of men who have sex with men in the United States [abstract TUPEC506]. IAS 2015. 8th Conference on HIV Pathogenesis, Treatment and Prevention (Vancouver). July 19–22, 2015. [Google Scholar]
- 9. Weiss K, Bratcher A, Sullivan PS, Siegler AJ. Geographic access to PrEP clinics among US MSM: documenting PrEP deserts [poster]. The Conference of Retrovirus and Opportunistic Infections (Boston, MA). March 4–7, 2018. [Google Scholar]
- 10. Siegler AJ, Mayer KH, Liu AY, et al. Developing and assessing the feasibility of a home-based preexposure prophylaxis monitoring and support program. Clin Infect Dis 2019; 68:501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Touger R, Wood BR. A review of telehealth innovations for HIV pre-exposure prophylaxis (PrEP). Curr HIV/AIDS Rep 2019; 16:113–9. [DOI] [PubMed] [Google Scholar]
- 12. Stekler JD, McMahan V, Ballinger L, et al. HIV pre-exposure prophylaxis prescribing through telehealth. J Acquir Immune Defic Syndr 2018; 77:e40–2. [DOI] [PubMed] [Google Scholar]
- 13. John SA, Rendina HJ, Grov C, Parsons JT. Home-based pre-exposure prophylaxis (PrEP) services for gay and bisexual men: an opportunity to address barriers to PrEP uptake and persistence. PLoS One 2017; 12:e0189794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The Adequacy of Pharmacist Supply: 2004 to 2030. Department of Health and Human Services. The adequacy of pharmacist supply: 2004 to 2030, 2008. http://bhpr.hrsa.gov/healthworkforce/reports/pharmsupply20042030.pdf. Accessed April 28, 2019. [Google Scholar]
- 15. Tesoriero JM, Battles HB, Klein SJ, Kaufman E, Birkhead GS. Expanding access to sterile syringes through pharmacies: assessment of New York’s Expanded Syringe Access Program. J Am Pharm Assoc (2003) 2009; 49:407–16. [DOI] [PubMed] [Google Scholar]
- 16. Rose VJ, Lutnick A, Kral AH. Feasibility of providing interventions for injection drug users in pharmacy settings: a case study among San Francisco pharmacists. J Psychoactive Drugs 2014; 46:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuller CM, Turner AK, Hernandez D, et al. Attitudes toward web application supporting pharmacist-clinician comanagement of postexposure prophylaxis patients. J Am Pharm Assoc (2003) 2013; 53:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darin KM, Klepser ME, Klepser DE, et al. Pharmacist-provided rapid HIV testing in two community pharmacies. J Am Pharm Assoc (2003) 2015; 55:81–8. [DOI] [PubMed] [Google Scholar]
- 19. Weber NC, Klepser ME, Akers JM, Klepser DG, Adams AJ. Use of CLIA-waived point-of-care tests for infectious diseases in community pharmacies in the United States. Expert Rev Mol Diagn 2016; 16:253–64. [DOI] [PubMed] [Google Scholar]
- 20. Klepser ME, Adams AJ, Klepser DG. Antimicrobial stewardship in outpatient settings: leveraging innovative physician-pharmacist collaborations to reduce antibiotic resistance. Health Secur 2015; 13:166–73. [DOI] [PubMed] [Google Scholar]
- 21. Hammond RW, Schwartz AH, Campbell MJ, et al. Collaborative drug therapy management by pharmacists–2003. Pharmacotherapy 2003; 23:1210–25. [DOI] [PubMed] [Google Scholar]
- 22. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health 2018; 15:556–61. [DOI] [PubMed] [Google Scholar]
- 23. Gauthier TP, Toro M, Carrasquillo MZ, et al. A PrEP Model incorporating clinical pharmacist encounters and antimicrobial stewardship program oversight may improve retention in care. Clin Infect Dis 2019; 68:347–9. [DOI] [PubMed] [Google Scholar]
- 24. Darin KM, Klepser ME, Klepser DE, et al. Pharmacist-provided rapid HIV testing in two community pharmacies. J Am Pharm Assoc (2003) 2014: e7–14. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline. Available at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed 10 April 2019. [Google Scholar]
- 26. Patel RR, Harrison LC, Liu AY, et al. Self-report and medication possession ratio are accurate measures of HIV pre-exposure prophylaxis use in a real-world clinical setting [abstract WEAC0105]. The International AIDS Society Conference on HIV Science (Paris, France). July 23–26, 2017. [Google Scholar]
- 27. Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc 2016; 19:20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hojilla JC, Vlahov D, Crouch PC, et al. HIV pre-exposure prophylaxis (PrEP) uptake and retention among men who have sex with men in a community-based sexual health clinic. AIDS Behav 2018; 22:1096–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rusie LK, Orengo C, Burrell D, et al. Preexposure prophylaxis initiation and retention in care over 5 years, 2012–2017: are quarterly visits too much? Clin Infect Dis 2018; 67:283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 31. Murray MI, Markowitz M, Frank I, et al. Satisfaction and acceptability of cabotegravir long-acting injectable suspension for prevention of HIV: patient perspectives from the ECLAIR trial. HIV Clin Trials 2018; 19:129–38. [DOI] [PubMed] [Google Scholar]
- 32. Mooney EV, Hamper JG, Willis RT, Farinha TL, Ricchetti CA. Evaluating patient satisfaction with pharmacist-administered long-acting injectable antipsychotics in the community pharmacy. J Am Pharm Assoc (2003) 2018; 58: S24–9.e2. [DOI] [PubMed] [Google Scholar]
- 33. Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018; 15:e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerges S, Peter E, Bowles SK, et al. Pharmacists as vaccinators: an analysis of their experiences and perceptions of their new role. Hum Vaccin Immunother 2018; 14:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Broekhuis JM, Scarsi KK, Sayles HR, et al. Midwest pharmacists’ familiarity, experience, and willingness to provide pre-exposure prophylaxis (PrEP) for HIV. PLoS One 2018; 13:e0207372. [DOI] [PMC free article] [PubMed] [Google Scholar]