Abstract
Frankliniella occidentalis (Pergande) is an invasive pest that endangers a wide variety of horticultural and agronomic crops. HSP70 is the most important member of the heat shock protein (HSP) family and plays an important role in insect thermal tolerance. In this study, a new gene encoding HSP70 from F. occidentalis, Fohsp706, was selected from the F. occidentalis transcriptome exposed to thermal stress (40 °C) and cloned by RT-PCR and RACE. Further characterization indicated that Fohsp706 localizes to the cytoplasm and does not contain introns. Quantitative real-time reverse transcriptase PCR indicated that Fohsp706 expression was significantly up-regulated by thermal stress; furthermore, there were significant differences in Fohsp706 expression in adults and second instar nymphs after heat stress. Our results indicated that Fohsp706 contributes to thermotolerance in F. occidentalis and provides another example of how this pest adapts to unfavorable environmental conditions.
Keywords: HSP70, Frankliniella occidentalis, Temperature, Recovery time, Expression pattern
Introduction
According to the Intergovernmental Panel on Climate Change (IPCC), the mean global surface temperature will be 0.3–0.7 °C higher in years 2016–2035 than 1986–2005 (IPCC, 2013). Over the past 55 years in China, the mean number of high-temperature days has increased by 28.4% (Wang et al., 2016). Environmental temperature determines geographic distribution and abundance of insect (Bowler & Terblanche, 2008). As poikilothermic animals, the growth, development, and reproduction of insects can be directly impacted by temperature, thus resulting in behavioral changes. Temperature can alter ecosystem stability and may result in recurrent insect outbreaks (Nelson, Bjørnstad & Yamanaka, 2013). Extreme temperatures can cause the destruction of insect cuticles, water loss, ion imbalances, and inactivation of proteins (Zhang, 2011).
Heat shock proteins (HSPs) are widely distributed in eukaryotic organisms. They are generally synthesized rapidly after temperature stress and help organisms adapt to adverse environments (Lindquist, 1986; Sǿrensen, Kristensen & Loeschcke, 2010; Sun et al., 2014). Heat shock proteins often function as molecular chaperones and facilitate the refolding of denatured proteins (Hartl, 1996); they are classified into HSP100, HSP90, HSP70, HSP60, HSP40, and small HSPs families according to homology and molecular weight (Lindquist & Craig, 1988; Morimoto, Tissieres & Georgopoulos, 1990; Kim, Kim & Kim, 1998). Among them, HSP90 and HSP40 are related to HSP70 in function. HSP90 chaperone activity requires collaboration with a subset of the many HSP90 cochaperones, including the HSP70 chaperone. In higher eukaryotes, the indirect collaboration between HSP90 and HSP70 involves Hop, a cochaperone interacting with both HSP90 and HSP70 (Kravats et al., 2018). HSP40 proteins contain the J domain which they bind to HSP70s. HSP40 proteins stimulate the ATPase activity of HSP70 and actually determine the activity of HSP70 by stabilizing their interaction with substrate proteins (Qiu et al., 2006).
The HSP70 family members are further subdivided into inducible (HSP70) and cognate forms (HSC70s). Inducible HSP70 genes have no introns or have relatively short introns and are preferentially translated; thus, HSP70s rapidly accumulate in response to adverse environmental stimuli (Gkouvitsas, Kontogiannatos & Kourti, 2009; Sǿrensen, 2010; Zhang & Denlinger, 2010). Cognate forms of HSP70 genes contain more introns, and intron numbers are conserved in vertebrates and are variable in invertebrates (Chuang, Ho & Song, 2007). HSP70s also have different localization signals; e.g. EEVD, HDEL, and PEAEYEEAKK for localization to the cytoplasm, endoplasmic reticulum, and mitochondria, respectively (Guy & Li, 1998).
Frankliniella occidentalis occurs worldwide and threatens both horticultural and agronomic crops. The pest has invaded many parts of China and exhibits a pattern of expansion from northern to southern regions (Zhang et al., 2003; Lv et al., 2011). The strong temperature tolerance and rapid domestication of F. occidentalis contribute to its fast, unrestricted dissemination in China. Studies have shown that antioxidant enzymes in F. occidentalis can effectively reduce the oxidative damage caused by high temperatures (Zheng, 2015). In addition, temperature tolerance in F. occidentalis is also conferred by inducible hsp genes including Fohsp40, Fohsp60, Fohsp70, and Fohsp90 (Li et al., 2014; Lu et al., 2016; Qin et al., 2017).
The expression of hsp genes in F. occidentalis is related to the intensity and duration of stress. Differences in hsp expression were previously in F. occidentalis in response to high-temperature stress; for example, the expression of Fohsp90 and Fohsc70 reached a maximum at two hours, while Fohsp60 reached maximal levels at six hours (Li, 2013). Genes encoding six forms of HSP70 were previously identified in F. occidentalis and differ in selected characteristics and responses to thermal stress (Lu et al., 2016; Qin et al., 2017; Qin et al., 2018). In this study, we isolate and analyze characteristic of Fohsp70, a new gene encoding an HSP70 form in F. occidentalis. Furthermore, we evaluated and compared Fohsp70 expression during both high- and low-temperature stress and after different recovery times. The results provide a foundation for future studies on the mechanism of thermotolerance in F. occidentalis.
Materials and Methods
Insects
Frankliniella occidentalis adults were originally collected from Zhejiang Academy of Agricultural Sciences in September 2008 and the adults reared in the laboratory according to Li et al. (2011). The experimental colony was fed on Phaseolus vulgaris maintained in a QHX-300BS-III climate chamber at 25 ± 1 °C, 70–80% RH, and a 16:8 h light: dark photoperiod.
High and low temperature treatments
Second instar larvae (n = 120) were collected, placed in glass tubes and exposed to various temperatures for 1 h. Larvae were exposed to cold (−6, −8, −10, −12, −14 °C) and hot (33, 35, 37, 39, 41 °C) conditions using a temperature controller (DC-3010, Ningbo, Zhejiang, China). The control group consisted of thrips maintained at 26 °C, and all treatments were replicated four times.
Recovery times
Adults (n = 200) were collected and placed together in glass tubes; two replicates of each sample were prepared. The adults were exposed to 40 °C for 1 h in a constant temperature water bath and allowed to recover at 26 °C for 0, 1, 1.5, 2 and 2.5 h. Treated and control samples were frozen in liquid nitrogen for 5 min and then stored at −80 °C. Each recovery period was replicated four times. The same protocol was used for second instar nymphs and pupae.
RNA extraction and cDNA synthesis
Total RNA was extracted from F. occidentalis adults using the SV Total RNA Isolation System (Promega, San Luis Obispo, CA, USA). The concentration and quality of RNA were analyzed by spectrophotometry (Eppendorf Bio Photometer Plus, Hamburg, Germany) and agarose gel electrophoresis. Total RNA (1 μg) was used as a template and oligo(dT)18 primers were used to generate the first strand cDNA as recommended in the First Strand cDNA Synthesis Kit (Clontech, Mountain View, CA, USA).
Cloning full-length Fohsp706
Primers (Table 1) were designed to amplify DNA fragments of F. occidentalis based on sequences obtained from the transcriptome. PCR reactions were as follows: 94 °C for 3 min, 19 cycles of 94 °C for 30 s, 64–44 °C (decreasing by 1 °C/cycle) for 30 s, 72 °C for 1 min, and then 30 cycles of 94 °C for 30 s, 45 °C for 30 s, and 72 °C for 1 min, with extension at 72 °C for 10 min. Purified products were cloned into the pGEM-T Easy vector (Promega, Madison, WI, USA) and transformed into competent Escherichia coli DH5α cells for sequencing.
Table 1. Primers in this study.
| Primer name | Primer sequences (5′-3′) | Purpose |
|---|---|---|
|
hsp706 DP-F hsp706 DP-R |
GCTTGATTGGCAGACGATTTGAG CAAGTGAAAGAGGGGTAACATCC |
Amplification of internal fragment |
|
hsp706 RACE-5′-1 hsp706 RACE-5′-2 |
GTGAACTAAGTCTCAATCTC TGGTTGTTGTTGGTATAAGAAGACGA |
5′RACE |
|
hsp706 RACE-3′-1 hsp706 RACE-3′-2 |
ATACACCAGAATCTCACTTG TGTGCTCCGAGGACTTTATTTCAGGG |
3′RACE |
|
hsp706 cDNA-F hsp706 cDNA-R |
AGCAGGCTGGCAGGCACAAC GGGACTGGTAACAGGAGCCG |
Verification of full-length cDNA |
|
hsp706 DNA-F hsp706 DNA-R |
AGCAGGCTGGCAGGCACAAC GGGACTGGTAACAGGAGCCG |
Verification of genome |
|
hsp706 RT-F hsp706 RT-R |
CTTTAGCGGCGACAGTTGGA GGAGCACAAACCGTGACCAA |
Real-time quantitative PCR |
Gene-specific primers (Table 1) were designed to obtain 5′ and 3′ regions using the SMART RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA) based on the sequence of partial fragments. PCR parameters were as follows: 94 °C for 3 min, 35 cycles of 94 °C for 30 s, 68 °C for 30 s, and 72 °C for 3 min, followed by extension at 72 °C for 10 min. Bands of the expected size were cloned and sequenced as described above.
Sequence analysis of Fohsp706
Nucleotide and amino acid sequence similarities were evaluated using the BLAST program available at NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) was used to identify complete open reading frame. Amino acid sequences (Gasteiger et al., 2003) were deduced using ExPASy sequence analysis tools, and motifs were identified using ScanProsite software (http://www.expasy.org/tools/scanprosite). Homology searches were carried out using Blast programs of NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). A phylogenetic tree of insect HSP70s was constructed by neighboring joining, minimum evolution, maximum likelihood, and maximum reduction methods using MEGA X (Kumar et al., 2018).
Amplification of genomic DNA
Genomic DNA of F. occidentalis adults was extracted according to AxyPrep instructions, and samples were stored at −20 °C. Based on the full-length cDNA sequence of Fohsp706 in F. occidentalis, multiple primers (Table 1) were designed to amplify the genomic sequence. PCR products were cloned and sequenced as described above. Homologous sequences were obtained using BlastN. After confirmation, full-length hsp70 genomic sequences and the DNA sequence of the new hsp70 gene (Fohsp706) were compared, and intron distribution was analyzed.
Quantitative real-time reverse transcriptase PCR (qRT-PCR)
Real-time quantitative cDNA was synthesized using instructions provided with the PrimeScript RT Reagent Kit (Bio-Rad, Berkeley, CA, USA), and primers were designed using Primer 5.0 software (Table 1). Reactions were conducted using a CFX-96 Real-Time PCR System (Bio-Rad, Berkeley, CA, USA), and each PCR reaction included three replicates. PCR reactions included iTaq Universal SYBR Green Supermix (2×) (Bio-Rad, Berkeley, CA, USA), one μL of each forward and reverse primer (10 μmol L–1) (Table 1), two μL of cDNA template (2.5 × 10–4 μg μL–1), and six μL of ddH2O. Quantitative real-time reverse transcriptase PCR conditions were as follows: 95 °C for 30 s, 40 cycles of 95 °C for 30 s, and 56.3 °C for 15 s; melting curve analysis was then performed to determine the specificity of PCR products.
Statistical analysis
Expression levels in each treatment were analyzed using the 2–ΔΔCt method (Livak & Schmittgen, 2001) and differences were detected using Tukey’s multiple comparison method. All data were analyzed and processed using SPSS16.0 software, and the significance level was P < 0.05.
Results
Characterization of a new hsp70 in F. occidentalis
A 257-bp fragment was amplified by PCR with internal primers (hsp706DP-F/R, Table 1) using F. occidentalis cDNA as a template and then cloned and sequenced. BlastN alignment revealed 75–91% identity with hsp70 in other insects, suggesting that the fragment encoded part of an hsp70 gene in F. occidentalis. The 5′-terminal (563 bp) and 3′-terminal (1519 bp) fragments of the putative F. occidentalis hsp70 were subsequently amplified by RACE. The full-length hsp70 was then obtained by splicing the 257-, 563-, and 1519-bp fragments together with DNAMAN (version 6.0, Lynnon Biosoft, America). The full-length sequence was verified by the cDNA sequence and was deposited in GenBank as Fohsp706 (accession no. MK603518).
The ORF Finder revealed that Fohsp706 contained a 5′ untranslated region (159 bp 5′ UTR), a 1917-bp ORF and 3′ untranslated region (1713 bp 3′UTR); the 3′UTR contained a typical polyA tail. ProtParam analysis tool (ExPASy) showed that Fohsp706 encoded 638 amino acids with a theoretical molecular weight of 70.1 kDa, a formula of C3051H4912N876O981S16 and an isoelectric point of 5.31. Alanine (Ala) was the most prevalent amino acid (9.6% of total), followed by aspartic acid (Asp), which accounted for 7.7%. The predicted protein contained 94 negatively charged residues (Asp + Glu) and 81 positively charged residues (Arg + Lys). The overall stability index of the protein is 40.51 (≤40 is considered stable), the aliphatic index is 81.66, and the hydrophilicity index is −0.461 (non-hydrophilic). ScanProsite showed that the deduced FoHSP706 amino acid sequence contained three HSP70 signature sequences: IDLGTTYS (6–13 aa), IFDLGGGTFDVSVL (194–207 aa) and VVLVGGSTRIPKVQS (332–346 aa). The C-terminal end is a typical EEVD (Glu-Glu-Val-Asp), indicating that the protein exists in the cytoplasm of F. occidentalis (Fig. S1).
Genome structure of Fohsp706
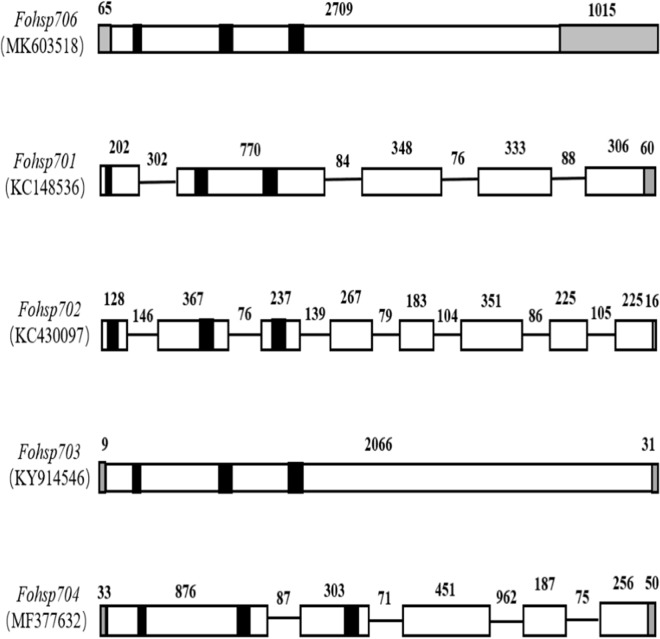
A pair of primers, hsp706DNA-F/R (Table 1.) was designed to amplify the genomic copy of Fohsp706, and a 2022-bp sequence was obtained. In Table 2, the genomic structures of Fohsps from F. occidentalis were compared, except Fohsc705, the genomic structure of which was not available. The genomic form of Fohsp706 had no introns, which suggested that the gene might be inducible. In contrast, Fohsp701 (accession no. KC148536), Fohsp702 (accession no. KC430097), and Fohsp704 (accession no. MF377632) have 4, 7, and 4 introns, respectively; whereas, Fohsp703 (accession no. KY914546) has no introns. Therefore, this indicates that not only the number of introns, but also the distribution positions of introns in HSP70 gene of the same species may be different (Fig. 1).
Table 2. Characteristics of six hsp70s in F. occidentalis.
| Genes | MW (kDa) | Number of introns | Response to heat stress | Response to cold stress |
|---|---|---|---|---|
| Fohsc701 | 69.81 | 4 | N | N |
| Fohsc702 | 72.93 | 7 | N | N |
| Fohsp703 | 73.6 | 0 | M | M |
| Fohsc704 | 75.0 | 4 | N | N |
| Fohsc705 | 54.5 | 6 | M | M |
| Fohsp706 | 70.1 | 0 | H | M |
Notes:
H, highly induced; M, moderately induced; N, not induced; and ND, not determined.
Figure 1. Genomic forms of Fohsp70s in F. occidentalis.
Grey boxes represent non-coding regions, black boxes represent signatural sequences of HSP70 family, blank boxes represent exons, straight lines represent introns, numbers indicate the lengths of exons, introns and non-coding regions.
Phylogenetic analysis of FoHSP706
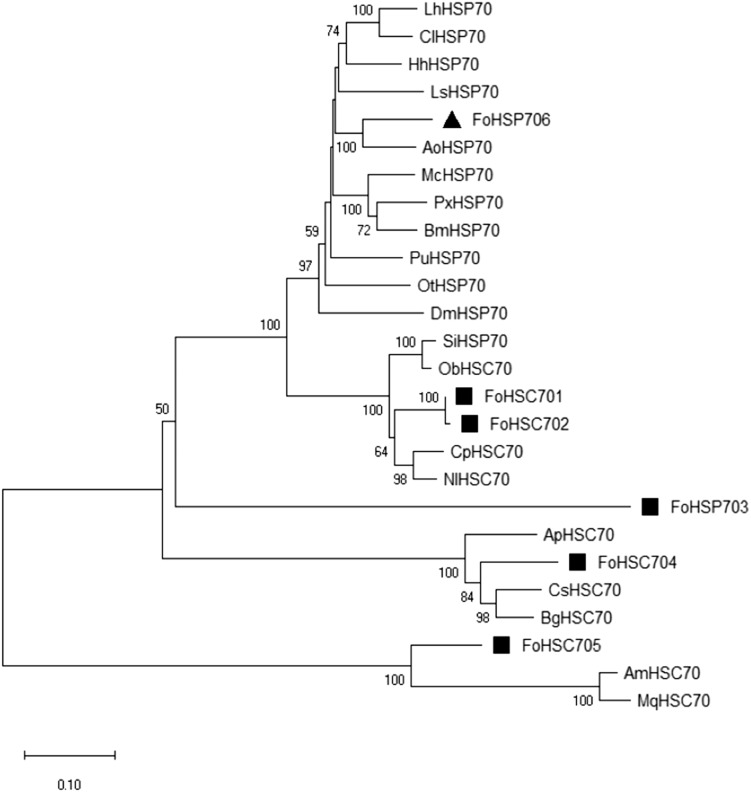
ClustalW (Thompson, Gibson & Higgins, 2002) indicated that the deduced amino acid sequence of FoHSP706 was over 70% similar to other HSP70s (Fig. S2). Several HSP70 amino acid sequences, including the FoHSP703 mentioned above, were compared with FoHSP706. Phylogenetic trees of HSP70s were constructed using MEGA X and the neighbor joining, minimum evolution, maximum reduction, and maximum likelihood methods (Zuckerkandl & Pauling, 1965; Kumar et al., 2018). The tree was divided into five branches, and these six FoHSP70s were distributed in different branches (Fig. 2). Phylogenetic analysis showed that FoHSP706 has a close evolutionary relationship with HSP70 of Anaphothrips obscurus (Muller), which also belongs to Thripinae (Gao & Feng, 2018).
Figure 2. Phylogenetic tree of HSP70s from multiple insects based on the maximum likelihood method.
FoHsp70 (this study) is marked with a solid square. HSP70 proteins and GenBank accession numbers are as follows: FoHSP706 (MK603518), FoHSC701 (JX002706.1), FoHSC702 (KC148536.1), FoHSP703 (KY986660.1), FoHSC704 (KY914547.1), FoHSC705 (MF377632.1), Bombyx mori BmHSP70 (AB035326.1), Plutella xylostella PxHSP70 (AB325801.1), Drosophila melanogaster DmHSP70 (AH007395.2), Anaphothrips obscurus (AXB26576.1), Halyomorpha halys (XP_024214745.1), Paratlanticus ussuriensis (AFP54305.1), Laodelphax striatella (AQP31338.1), Papilio polytes (XP_013148831.1), Onthophagus taurus (XP_022912059.1), Melitaea cinxia (AGR84224.1), Plutella xylostella (ADK94699.1), Lygus hesperus (AFX84560.1), Cyrtorhinus lividipennis (AXU24955.1), Solenopsis invicta (XP_025995000.1), Chrysopa phyllochroma (AHY95944.1), Nilaparvata lugens (ADE34170.1), Ooceraea biroi (XP_011348101.1), Cryptotermes secundus (XP_023710212.1), Blattella germanica, (PSN32841.1), Agrilus planipennis (XP_025835266.1), Apis mellifera (XP_623199.2), and Melipona quadrifasciata (KOX73871.1). Numbers on branches represent bootstrap values obtained from 1,000 replicates.
Expression of Fohsp706 in response to cold and heat shock
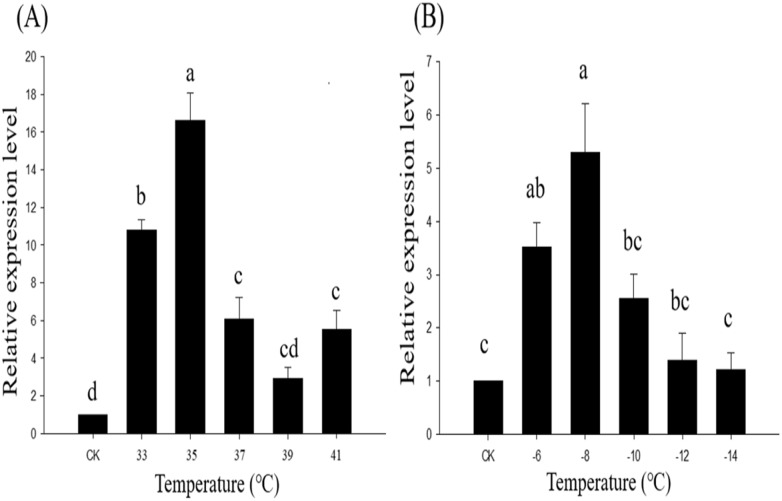
RT-PCR was used to study expression profiles of Fohsp706 in second instar larvae of F. occidentalis. qRT-PCR assays resulted in the production of single amplicons with efficiency values between 93.5 and 107.3% and an R2 value of 0.980, which meets the basic requirements of real-time quantitative research. The relative mRNA levels of Fohsp706 were compared at −14, −12, −10, −8, −6, 26, 33, 35, 37, 39 and 41 °C. Fohsp706 was significantly induced in response to hot and cold temperatures as compared with the control (26°C). With respect to high-temperature stress, expression of Fohsp706 was highest at 35 °C (Fig. 3A); in response to cold stress, expression of Fohsp706 was highest at −8 °C (Fig. 3B).
Figure 3. Expression levels of Fohsp706 during a 2-hexposure to Frankliniella occidentalis larvae.
(A) Fohsp706 expression in response to heat stress at 33, 35, 37, 39, 41 °C (B). Expression in response to cold stress at −6, −8, −10, −12, and −14 °C. Expression levels were normalized with respect to RPL32 for heat stress and 18S rRNA for cold stress, and histograms indicate relative expression levels. All statistics indicate means ± SE. Columns labeled with different letters indicate significant differences using a one-way ANOVA followed by Tukey’s multiple comparison analysis. All statistics are presented as means ± SE.
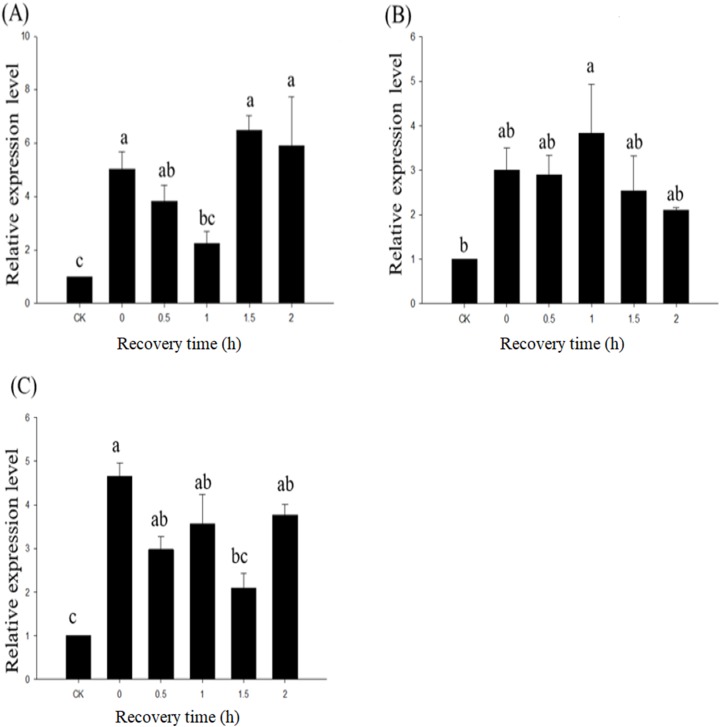
In response to high-temperature stress (40 °C), the expression of Fohsp706 in F. occidentalis adults after recovery times of 0, 0.5, 1.5, and 2 h remained significantly induced in comparison to the control group. However, the recovery time of 1.0 h was not significantly different from the control group (Fig. 4A). Statistical analysis showed that different recovery times after high-temperature exposure had no effect on Fohsp706 expression in F. occidentalis adults (F5, 17 = 9.529, P < 0.001). Contrary to adults, Fohsp706 expression in pupae was significantly induced after the recovery time of 1 h, but not at other time intervals (Fig. 4B). Fohsp706 expression in larvae showed significant induction after recovery times of 0, 0.5, 1, and 2 h (but not 1.5 h) as compared to the control (Fig. 4C). Thus, different recovery times after high-temperature treatment impact Fohsp706 expression in F. occidentalis pupae and larvae.
Figure 4. Relative expression of Fohsp706 in F. occidentalis at 0, 0.5, 1, 1.5 and 2 h recovery times at 26 °C after exposure to heatshock at 40 °C.
Relative expression of Fohsp706 in F. occidentalis adults (A), pupae (B), and (C) larvae. CK: expression level at 25 °C. All statistics are presented as means ± SE. Columns labeled with different letters indicate significant differences using one-way ANOVA followed by Tukey’s multiple comparison analysis.
Discussion
HSP70 family members often contain motifs for subcellular localization; for example, the seven hsp70 genes encode different signature sequences that target the protein products to different subcellular locations. In this study, the C-terminal motif in FoHSP706 contains EEVD, indicating that the protein localizes to the cytoplasm. Genes encoding five forms of HSP70 were previously identified in F. occidentalis which are separately named Fohsc701, Fohsc702, Fohsp703, Fohsc704, Fohsc705, thus, the new hsp70 was named Fohsp706. Some HSP70s contain KDEL at the C-terminus, which targets the protein to the endoplasmic reticulum. Most members of the HSP70 family in F. occidentalis end with EEVD and KDEL (Lu et al., 2016). Recently, Qin et al. (2017) described FoHSP703, a heat-induced HSP70 protein that contains a unique APAA motif at its C-terminus, which is distinct from previously described insect forms of HSP70.
In this study, multiple sequence alignment and phylogenetic tree construction identified Fohsp706 as a member of the HSP70 family. The phylogenetic tree showed the decentralized distribution of FoHSP70s, indicating that HSP70s in insecta are diverse and mutated. The FoHSP706 in this study was not located in the same branch with other FoHSP70s, but they were close to Hemiptera. The result suggested that the evolutionary history of FoHSP706 be parallel to insects in Hemiptera. Fohsp706 did not contain introns and was induced by high and low temperature stress; thus, it resembles with Fohsp703 (Table 2). In F. occidentalis larvae, the expression of Fohsp706 in this study peaked at 35 °C. According to Qin (2018), all F. occidentalis larvae survived after 1-h exposure at 35 °C. This might be related to the expression of Fohsp706 which also peaked at 35 °C. After 1-h exposure at cold stress in the range of −8 °C to −14 °C, the expression level of Fohsp706 decreased with the decrease of temperature showing a negative correlation to the mortality of F. occidentalis larvae after cold stress.
Interestingly, we discovered that intron numbers and positions were quite different in Fohsps. Fohsc701, Fohsc702, Fohsc704, and Fohsc705 contain introns (Table 2); however, only Fohsc705 was induced by temperature (Lu et al., 2016, Qin et al., 2018). It supported that highly expressed genes either lack introns or have relatively short introns relative to weakly expressed genes (Castillo-Davis et al., 2002). Thus, distinct differences exist among hsp70 genes within F. occidentalis and intra-specific variation can lead to differences in genomic structure and function. Besides, Wang et al. (2014) also did some research about responses of HSP70 genes from F. occidentalis to thermal stress and FoHSP70 was strongly induced by both heat and cold stress in their study, but no information of genome structure was involved. We presume that F. occidentalis HSP70 in their research may lack introns with such high expression level in response to heat stress, whereas this is only our assumption.
Numerous reports have shown that HSP70 plays an important role in insect tolerance to thermal stress (Huang & Kang, 2007; Udaka, Ueda & Goto, 2010; Lu et al., 2016). In this study, we investigated whether Fohsp706 expression in adult, larval, and pupal forms of F. occidentalis was impacted by different recovery times after high-temperature stress (40 °C). Our results indicated that Fohsp706 was differentially expressed in different developmental stages. In F. occidentalis adults, Fohsp706 expression peaked at the 1.5-h recovery time point. However, Fohsp706 expression reached a maximal level at the 0-h time point in larvae. In pupae, Fohsp706 expression was significantly induced at the 1-h time point but no significant differences were observed among different recovery times. To some extent, these results reflect the short-term nature of HSP70, which has been observed for some plant forms of the protein. Plant HSPs accumulated within 3–5 min after heat shock and reached maximal levels at 1–2 h; protein levels were significantly reduced at 6 h and undetectable at 12 h (Kimpel et al., 1990). In Drosophila melanogaster exposed to 0 °C, hsp70Aa expression peaked at the 2-h recovery time point (25 °C), and then gradually decreased (Colinet, Lee & Hoffmann, 2010).
Frankliniella occidentalis is an important pest on vegetables and horticultural crops worldwide. It can spread quickly and cause severe damage due to its small size, diverse modes of reproduction, rapid multiplication, and resistance to pesticides. In this study, we investigate the impact of thermal stress on FoHSP706, a new form of HSP70 in F. occidentalis. Additional studies on the HSP70 family in F. occidentalis are warranted and will hopefully provide a theoretical basis for prevention and control of this invasive pest.
Conclusion
Frankliniella occidentalis is an important pest on vegetables and horticultural crops worldwide. It can spread quickly and cause severe damage due to its small size, diverse modes of reproduction, rapid multiplication, and resistance to pesticides. In this study, we investigate the impact of thermal stress on FoHSP706, a new form of HSP70 in F. occidentalis. Additional studies on the HSP70 family in F. occidentalis are warranted and will hopefully provide a theoretical basis for prevention and control of this invasive pest.
Supplemental Information
Multiple sequence alignment of deduced amino acids from FoHSP706 with analogous proteins from other species.
A represents Adults, L represents larvae, P represents pupae.
Acknowledgments
We sincerely thank Dr. Carol L. Bender for editing English and helpful comments on the manuscript.
Funding Statement
This research was supported by the Special Fund for Agro-Scientific Research in the Public Interest of China (201103026, 200803025) and the Science and Technology Innovation Project of Student in Yangzhou University, China (X20160637). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Xiao-xiang Zhang conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Jing Qin conceived and designed the experiments, performed the experiments, analyzed the data.
Jia-Wen Yuan performed the experiments.
Ming-Xing Lu conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Yu-Zhou Du conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The Fohsp706 sequence is accessible at GenBank: MK603518.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in a Supplemental File.
References
- Bowler & Terblanche (2008).Bowler K, Terblanche JS. Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biological Reviews. 2008;83(3):339–355. doi: 10.1111/j.1469-185X.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- Castillo-Davis et al. (2002).Castillo-Davis CI, Mekhedov SL, Hartl DL, Koonin EV, Kondrashov FA. Selection for short introns in highly expressed genes. Nature Genetics. 2002;31(4):415–418. doi: 10.1038/ng940. [DOI] [PubMed] [Google Scholar]
- Chuang, Ho & Song (2007).Chuang K-H, Ho S-H, Song Y-L. Cloning and expression analysis of heat shock cognate 70 gene promoter in tiger shrimp (Penaeus monodon) Gene (Amsterdam) 2007;405(1–2):10–18. doi: 10.1016/j.gene.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Colinet, Lee & Hoffmann (2010).Colinet H, Lee SF, Hoffmann A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. Febs Journal. 2010;277(1):174–185. doi: 10.1111/j.1742-4658.2009.07470.x. [DOI] [PubMed] [Google Scholar]
- Gao & Feng (2018).Gao X-J, Feng J-N. Comparisons of expression levels of heat shock proteins (hsp70 and hsp90) from Anaphothrips obscurus (Thysanoptera: Thripidae) in polymorphic adults exposed to different heat shock treatments. Journal of Insect Science. 2018;18(3):1–10. doi: 10.1093/jisesa/iey059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger et al. (2003).Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 2003;31(13):3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkouvitsas, Kontogiannatos & Kourti (2009).Gkouvitsas T, Kontogiannatos D, Kourti A. Cognate Hsp70 gene is induced during deep larval diapause in the moth Sesamia nonagrioides. Insect Molecular Biology. 2009;18(2):253–264. doi: 10.1111/j.1365-2583.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- Guy & Li (1998).Guy CL, Li Q-B. The organization and evolution of the spinach stress 70 molecular chaperone gene family. The Plant Cell. 1998;10(4):539–556. doi: 10.1105/tpc.10.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl (1996).Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Huang & Kang (2007).Huang L-H, Kang L. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Molecular Biology. 2007;16(4):491–500. doi: 10.1111/j.1365-2583.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- IPCC (2013).IPCC . Climate change 2013: the physical science basis. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Kim, Kim & Kim (1998).Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394(6693):595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kimpel et al. (1990).Kimpel JA, Nagao RT, Goekjian V, Key JL. Regulation of the heat shock response in soybean seedlings. Plant Physiology. 1990;94(3):988–995. doi: 10.1104/pp.94.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravats et al. (2018).Kravats AN, Hoskins JR, Reidy M, Johnson JL, Doyle SM, Genest O, Wickner S. Functional and physical interaction between yeast Hsp90 and Hsp70. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(10):E2210–E2219. doi: 10.1073/pnas.1719969115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2018).Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li (2013).Li HB. Response to thermal press in western flower thrips, Frankliniella occidentalis. 2013. D. PWhil. thesis, Yangzhou University [in Chinese]
- Li et al. (2011).Li HB, Shi L, Lu MX, Wang JJ, Du YZ. Thermal tolerance of Frankliniella occidentalis: effects of temperature, exposure time, and gender. Journal of Thermal Biology. 2011;36(7):437–442. doi: 10.1016/j.jtherbio.2011.07.010. [DOI] [Google Scholar]
- Li et al. (2014).Li H-B, Zheng Y-T, Sun D-D, Wang J-J, Du Y-Z. Combined effects of temperature and avermectins on life history and stress response of the western flower thrips, Frankliniella occidentalis. Pesticide Biochemistry and Physiology. 2014;108(1):42–48. doi: 10.1016/j.pestbp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Lindquist (1986).Lindquist S. The heat-shock response. Annual Review of Biochemistry. 1986;55(1):1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist & Craig (1988).Lindquist S, Craig EA. The heat-shock proteins. Annual Review of Genetics. 1988;22(1):631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2016).Lu MX, Li HB, Zheng YT, Shi L, Du Y-Z. Identification, genomic organization and expression profiles of four heat shock protein genes in the western flower thrips, Frankliniella occidentalis. Journal of Thermal Biology. 2016;57:110–118. doi: 10.1016/j.jtherbio.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Lv et al. (2011).Lv YB, Zhang ZJ, Wu QJ, Du YZ, Zhang HR, Yu Y, Wang ED, Wang MH, Wang MC, Tong XL, Lu LH, Tan XQ, Fu WD. Research progress of the monitoring, forecast and sustainable management of invasive alien pest Frankliniella occidentalis in China. Journal of Applied Insects. 2011;48(3):488–496. [in Chinese] [Google Scholar]
- Morimoto, Tissieres & Georgopoulos (1990).Morimoto RI, Tissieres A, Georgopoulos C. The stress response, function of the proteins, and perspectives. Cold Spring Harbor Monograph Archive. 1990;19:1–36. [Google Scholar]
- Nelson, Bjørnstad & Yamanaka (2013).Nelson WA, Bjørnstad ON, Yamanaka T. Recurrent insect outbreaks caused by temperature-driven changes in system stability. Science. 2013;341(6147):796–799. doi: 10.1126/science.1238477. [DOI] [PubMed] [Google Scholar]
- Qin (2018).Qin J. Studies on thermotolerance of Frankliniella occidentalis, cloning and expression of heat shock protein 70 genes. 2018. D. Phil. thesis, Yangzhou University [in Chinese]
- Qin et al. (2018).Qin J, Gao P, Zhang XX, Lu MX, Du YZ. Characterization of two novel heat shock protein 70s and their transcriptional expression patterns in response to thermal stress in adult of Frankliniella occidentalis (Thysanoptera: Thripidae) Journal of Integrative Agriculture. 2018;17(5):1023–1031. doi: 10.1016/S2095-3119(17)61725-8. [DOI] [Google Scholar]
- Qin et al. (2017).Qin J, Zhang XX, Gao P, Zhang XX, Lu MX, Du YZ. Cloning and expression profile of novel, thermal, inducible HSP70 gene in insects. Chinese Journal of Applied Entomology. 2017;54(3):380–391. [in Chinese] [Google Scholar]
- Qiu et al. (2006).Qiu X-B, Shao Y-M, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cellular and Molecular Life Sciences. 2006;63(22):2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sǿrensen (2010).Sǿrensen JG. Application of heat shock protein expression for detecting natural adaptation and exposure to stress in natural populations. Current Zoologyy. 2010;56(6):703–713. [Google Scholar]
- Sǿrensen, Kristensen & Loeschcke (2010).Sǿrensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecology Letters. 2010;6(11):1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Sun et al. (2014).Sun M, Lu MX, Tang XT, Du YZ. Molecular cloning and sequence analysis of the HSP83 gene in Sesamia inferens (Walker) (Lepidoptera: Noctuidae) Chinese Journal of Applied Entomology. 2014;51(5):1246–1254. doi: 10.3390/ijms151223196. [DOI] [Google Scholar]
- Thompson, Gibson & Higgins (2002).Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Current Protocols in Bioinformatics. 2002 doi: 10.1002/0471250953.bi0203s00. Chapter 2: Unit 2.3. [DOI] [PubMed] [Google Scholar]
- Udaka, Ueda & Goto (2010).Udaka H, Ueda C, Goto SG. Survival rate and expression of Heat-shock protein 70 and Frost genes after temperature stress in Drosophila melanogaster lines that are selected for recovery time from temperature coma. Journal of Insect Physiology. 2010;56(12):1889–1894. doi: 10.1016/j.jinsphys.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang HH, Reitz SR, Wang LX, Wang SY, Li X, Lei ZR. The mRNA expression profiles of five heat shock protein genes from Frankliniella occidentalis at different stages and their responses to temperatures and insecticides. Journal of Integrative Agriculture. 2014;13(10):2196–2210. doi: 10.1016/S2095-3119(13)60680-2. [DOI] [Google Scholar]
- Wang et al. (2016).Wang YJ, Zhou BT, Ren YY, Sun CH. Impacts of global climate change on China climate security. Journal of Applied Meteorology Science. 2016;27(6):750–758. [in Chinese] [Google Scholar]
- Zhang (2011).Zhang XY. Insect ecology and pest prediction. Beijing: China Agriculture Press; 2011. [in Chinese] [Google Scholar]
- Zhang & Denlinger (2010).Zhang Q, Denlinger DL. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. Journal of Insect Physiology. 2010;56(2):138–150. doi: 10.1016/j.jinsphys.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2003).Zhang YJ, Wu QJ, Xu BY, Zhu GR. The occurrence and damage of Frankliniella occidentalis (Thysanoptera: Thripidae): a dangerous alien invasive pest in Beijing. Plant Protection. 2003;24:58–59. [in Chinese] [Google Scholar]
- Zheng (2015).Zheng YT. Effect of the high temperature stress on antioxidant enzyme activity and cloning and expression of CAT gene of Frankliniella Occidentalis. 2015. D. Phil. thesis, Yangzhou University [in Chinese]
- Zuckerkandl & Pauling (1965).Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel HJ, editors. Evolving Genes and Proteins. New York: Academic Press; 1965. pp. 97–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignment of deduced amino acids from FoHSP706 with analogous proteins from other species.
A represents Adults, L represents larvae, P represents pupae.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in a Supplemental File.