Summary
Background:
Drug–drug interactions between orally–administered antiretroviral therapy (ART) and hormones released from an intravaginal ring (IVR) are not known. We hypothesized that efavirenz–based ART and atazanavir/ritonavir–based ART would alter plasma concentrations of vaginally–administered etonogestrel/ethinyl estradiol, yet ART concentrations would be unchanged during IVR use.
Methods:
We conducted a parallel, three–group, pharmacokinetic evaluation at clinical research sites in Asia, South America, sub–Saharan Africa, and the United States between December 30, 2014 and September 12, 2016 (). We enrolled women with HIV who were either ART–naïve (Control group; n=25), receiving efavirenz–based ART (n=25), or receiving atazanavir/ritonavir–based ART (n=24). An IVR releasing etonogestrel/ethinyl estradiol was inserted at entry. Single plasma samples for hormone concentrations were collected 7, 14, and 21 days after IVR insertion. The primary outcome was the plasma concentration of etonogestrel and ethinyl estradiol on Day 21. Etonogestrel and ethinyl estradiol concentrations were compared between each ART group and the Control group by geometric mean ratio (GMR) with 90% confidence intervals (CI) and Wilcoxon Rank–Sum test. Secondarily, efavirenz or atazanavir/ritonavir concentrations were assessed by 8–hour intensive pharmacokinetic sampling at entry before IVR insertion and before IVR removal on Day 21. Antiretroviral areas under the concentration–time curve (AUC0–8h) were compared before and after IVR insertion by GMR (90% CI) and Wilcoxon Signed–Rank test.
Findings:
On Day 21 of IVR use, participants receiving efavirenz had 79% lower etonogestrel [GMR 0·21 (0·16–0·28); p<0·0001] and 59% lower ethinyl estradiol [GMR 0·41 (0·32–0·52); p<0·0001] concentrations compared to the Control group. In contrast, participants receiving atazanavir/ritonavir had 71% higher etonogestrel [GMR 1·71 (1·37–2·14); p<0·0001], yet 38% lower ethinyl estradiol [GMR 0·62 (0·49–0·79); p=0·0037] compared to controls. No statistically significant changes occurred in the AUC0–8h of efavirenz or atazanavir.
Interpretation:
We observed significantly lower hormone exposure when an IVR contraceptive was combined with efavirenz–based ART. Further studies designed to examine pharmacodynamic endpoints, such as ovulation, when IVR hormones are combined with efavirenz are warranted.
Keywords: antiretroviral therapy, atazanavir/ritonavir, efavirenz, contraception, drug interactions, pharmacokinetics, vaginal ring, ethinyl estradiol, etonogestrel
Research In Context
Evidence before this study:
Efavirenz–based antiretroviral therapy remains the most commonly used therapy for women living with HIV throughout the world. Efavirenz is known to significantly decrease exposure to contraceptive hormones, increasing the risk of unintended pregnancies in women using hormonal contraceptives in combination with efavirenz–based antiretroviral therapy. Atazanavir–ritonavir based antiretroviral therapy was a World Health Organization preferred option for second–line therapy until the 2018 updated guidelines. When given with oral contraceptives, atazanavir/ritonavir is known to increase progestin exposure, yet decrease ethinyl estradiol exposure. Because the etonogestrel/ethinyl estradiol intravaginal ring (IVR) for contraception must reach adequate systemic concentrations to suppress ovulation, the package insert advises that drugs which interfere with exogenous hormone metabolism, like efavirenz or protease inhibitor–containing antiretroviral therapy, should be avoided or used with caution in combination with the IVR. Despite this recommendation, no data are reported in the package insert to describe the impact of metabolism–related drug interactions on vaginally administered hormonal contraceptives. We searched PubMed on August 21, 2018 for studies reporting drug–drug or other pharmacokinetic interactions with hormones administered via an IVR. Pubmed search terms were “vaginal ring,” “interaction,” “pharmacokinetic,” or “drug interaction” in any field and were not restricted by language or publication date. Evidence regarding the influence of coadministered products on the pharmacokinetic exposure of hormones released from an IVR includes two co–administered oral antimicrobials (amoxicillin and doxycycline) and three co–administered topical products (spermicide, tampons, or vaginally administered miconazole). No influence on hormone exposure by these oral antimicrobials, spermicide or tampons was observed. When combined with miconazole suppositories, approximately 40% higher serum concentrations of vaginally administered hormones were observed.
Added value of this study:
We evaluated the systemic exposure of vaginally administered hormones when combined with antiretroviral therapy that either induce or inhibit the metabolism of hormones. We found that efavirenz–based antiretroviral therapy significantly reduced both the etonogestrel and ethinyl estradiol concentrations of the IVR administered contraceptive, which may jeopardize contraceptive effectiveness and tolerability. Atazanavir/ritonavir–based antiretroviral therapy significantly increased plasma etonogestrel concentrations and decreased plasma ethinyl estradiol concentrations compared to women in the Control group who were not receiving antiretroviral therapy.
Implications of all the available evidence:
Our findings are of particular interest for reproductive–aged women living with HIV, who require both effective contraception and antiretroviral therapy. We demonstrate that despite avoiding metabolism via the first–pass effect by administering hormones via an IVR, systemic hormone exposure was significantly influenced by concomitant oral medications that mediate hormone metabolism via the cytocholme P450 system. Further, the study highlights the importance of evaluating the potential interactive effects of other systemically administered medications, such as anticonvulsants or rifamycin antimicrobials, on vaginally administered medications. Evaluation of both local and systemic drug–drug interactions should be considered early in product development to provide evidence to support the appropriate combination therapy for women using IVRs. Finally, extending the understanding of hormone pharmacokinetic drug interactions with pharmacodynamic evaluations of contraceptive mechanisms of action will inform clinicians in how to effectively interpret and manage drug–drug interactions.
Introduction
An estimated 1·5 million pregnancies occur annually in women living with HIV in low–and middle–income countries.1,2 Over half of these pregnancies are unintended, and due to lack of access to, or failure of, effective contraception. Unintended pregnancies are associated with economic disparity, maternal and fetal morbidity and mortality, and risk of perinatal HIV transmission.3,4 Therefore, provision of effective contraception is an important component of comprehensive healthcare for people living with HIV who are of reproductive potential. Despite the benefits of hormonal contraceptives, drug–drug interactions (DDIs) represent a barrier to contraceptive options for women living with HIV, particularly with the most common global antiretroviral therapy (ART) regimen, efavirenz–based ART.5
Combination hormonal contraceptives containing a progestin and estrogen are widely available. In contraception, exogenous progestins prevent ovulation, thicken cervical mucus, and cause endometrial atrophy, while estrogens improve contraceptive tolerability by stabilizing the endometrial lining, reducing unpredictable bleeding.6 Intravaginal rings (IVRs) are devices currently used for contraception. While hormones administered orally undergo extensive first pass metabolism in the liver, hormones administered via an IVR avoid this first pass effect.7,8 For the etonogestrel/ethinyl estradiol IVR, the drugs are absorbed extensively from the vaginal tract, resulting in a systemic site of drug action to prevent ovulation as the mechanism of contraception.9 As such, the etonogestrel/ethinyl estradiol IVR product labeling recommends inserting the IVR during the first week of a menstrual cycle where it should remain in place for 21 days, followed by a seven day hormone–free period for menses.8 Estimated use of IVRs in the United States was 2·4% of contraceptive users in 2014;10 however, multipurpose IVRs for delivery of antiretrovirals for HIV prevention plus progestins for contraception are being developed.11,12
Efavirenz–based ART significantly reduces exposure to both estrogens and progestins given orally or via subdermal implant.13–17 In contrast, protease inhibitors like atazanavir/ritonavir increase progestin exposure, yet decrease estrogen exposure.18 These changes in hormone exposure are related to the influence of antiretrovirals on hormone metabolism via cytochrome P450 (CYP) 3A4 isoenzyme, the enzyme believed to be responsible for progestin metabolism, and multiple CYP and glucuronidation pathways associated with estrogen metabolism.18 Furthermore, some studies have identified lower antiretroviral exposure when combined with hormones,18 possibly due to the influence of progestin and estrogen on enzymes involved in antiretroviral metabolism.19,20
Few studies have been conducted to evaluate DDIs related to IVR administered hormones.8 Dogterom et al. found no significant effect of amoxicillin and doxycycline on etonogestrel/ethinyl estradiol serum concentrations.21 Vaginally administered topical miconazole increased IVR hormone PK,22 while tampons and spermicide did not affect hormone PK.8 Although the etonogestrel/ethinyl estradiol IVR product labeling cautions against coadministration of drugs that influence the metabolism of hormones contained in the IVR, no data were available to inform the extent of metabolism–related DDIs with IVR hormones. Studies conducted to assess the influence of ART on other non–oral hormones found both similar and contrasting findings to oral contraceptive DDI studies, making extrapolation across routes of hormone administration challenging.14,15,17,23,24
Our primary objective was to assess the effect of efavirenz–or atazanavir/ritonavir–based ART on the pharmacokinetics (PK) of etonogestrel/ethinyl estradiol administered via an IVR. Based on findings with ART and other contraceptives, we hypothesized that efavirenz–based ART and atazanavir/ritonavir–based ART would alter plasma concentrations of vaginally–administered etonogestrel/ethinyl estradiol, potentially in opposing directions. Secondary objectives assessed the impact of vaginally–administered hormones on the PK of efavirenz and atazanavir/ritonavir, ovarian function estimated by endogenous progesterone levels, and safety of the combination.
Methods
Study Design and Participants
We conducted a non–randomized, open–label, three–group parallel PK study among women living with HIV. All study procedures followed the Declaration of Helsinki and were approved by ethics boards at each research site and each participant provided written informed consent. Investigators enrolled participants at HIV specialized care clinics that were clinical research sites affiliated with the AIDS Clinical Trials Group (ACTG) or International Maternal Pediatric Adolescent AIDS Clinical Trials Network in Asia (two sites), South America (five sites), sub–Saharan Africa (three sites), and the United States (11 sites).
We enrolled participants who were 16 years of age or older and reported their last menstrual period within 6 months, or had a follicle–stimulating hormone level ≤40 mIU/mL at screening. All participants agreed to use condoms or a non–hormonal intrauterine device (IUD) in addition to the IVR. At screening, we required women receiving ART to be on the same regimen for at least 30 days, with a plasma HIV–1–RNA ≤400 copies per mL; women not receiving ART had CD4+ cell counts ≥350 cells per μL. We excluded participants who had a bilateral oophorectomy or conditions that were contraindicated in the IVR product labeling.8 Finally, participants did not receive other hormone therapies or medications that may interact with either hormones or ART prior to enrollment or during the study. The Appendix (pages 2–4) includes all protocol–defined inclusion and exclusion criteria.
Procedures
We enrolled all participants within 60 days of screening and allowed study entry (Day 0) to occur irrespective of time since participants’ last menses. We assigned participants to one of three study groups based on current ART. The Control group were women who had not yet begun ART. Women receiving efavirenz 600mg daily plus at least two nucleoside/nucleotide reverse transcriptase inhibitors (NRTI) were included in the efavirenz group and women receiving atazanavir/ritonavir 300/100mg daily, tenofovir disoproxil fumarate 300mg daily, plus at least one additional NRTI were included in the atazanavir/ritonavir group.
For all participants on Day 0, an IVR releasing etonogestrel/ethinyl estradiol 120/15 mcg/day was inserted by the participant or study personnel. Participants returned on Days 7, 14, 21, and 28. At each visit, investigators assessed adverse effects (AEs) and endogenous serum progesterone was measured. Severity of AEs were evaluated according to the Division of AIDS (DAIDS) Adverse Event Grading Tables. Site investigators determined the association between AEs and study procedures. Endogenous progesterone levels were measured centrally (Quest Diagnostics–Baltimore) using the FDA–cleared, Siemens Centaur method, a competitive immunoassay using direct chemiluminescent technology (limit of quantitation 0·5 ng/mL). We measured Plasma HIV–1–RNA at entry and at Day 21 by nucleic acid amplification tests (Abbott m2000sp/2000rt; Abbott Park, IL, USA). Investigators monitored participant adherence to ART and the IVR at each visit by standardized adherence self–report forms.25 Investigators asked participants about any missed doses of ART, the times of the last three doses of ART, and all time the IVR was outside the vagina since the last visit. The IVR was removed at the completion of the Day 21 visit and participants returned for a safety visit on Day 28.
To assess hormone PK, we collected a single plasma sample at entry, then weekly (±2 days) on Days 7, 14, and 21. To assess PK of efavirenz, atazanavir, or ritonavir, participants in the ART groups underwent 8–hour PK sampling on Day 0 prior to IVR placement and on Day 21 prior to IVR removal. Surrounding an observed dose of ART, plasma was collected pre–dose, then 1, 3, 4, 5, and 8 hours post–dose. PK sampling was rescheduled if any of the prior three ART doses were missed or the IVR was removed in the prior 12 hours.
All hormone and antiretroviral concentrations were analyzed via liquid chromatography tandem mass spectrometry (LC–MS/MS). The assay calibration range was 5–250 pg/mL for ethinyl estradiol, 25–25,000 pg/mL for etonogestrel, and 20–10,000 ng/mL for efavirenz, atazanavir, and ritonavir; all assays had a coefficient of variation <15%. All assays were validated in accordance with guidance from the Food and Drug Administration and were reviewed and approved by the DAIDS–funded Clinical Pharmacology Quality Assurance Program.26,27
Outcomes
Our primary endpoint was hormone concentrations in each ART group compared to the Control group on Day 21. We included participants in the hormone analysis if etonogestrel/ethinyl estradiol concentrations were available from both Days 0 and 21. Secondary endpoints were hormone concentrations on Days 7 and 14, and the area under the concentration time curves (AUC0–21days) over 21 days of IVR use. For ART, we compared the PK parameters of efavirenz, atazanavir, and ritonavir within each individual on Day 0 to Day 21. Antiretroviral PK parameters were the observed maximum concentration (Cmax), observed minimum concentration (Cmin), and the AUC for each antiretroviral. The AUCs were calculated over the 8–hour PK sampling period (AUC0–8h) and estimated over the dosing interval (AUC0–24h). We included participants in the ART PK analysis if efavirenz, atazanavir, or ritonavir concentrations were available on both Days 0 and 21.
We evaluated safety endpoints in all participants for whom treatment was initiated (IVR inserted), including the frequency of AEs and proportion of HIV–1–RNA below the limits of quantitation (<40 and <400 copies per mL). The proportion of participants with detectable endogenous serum progesterone was summarized at each visit as a measure of ovulation suppression. We included participants in the progesterone evaluation if the IVR was not outside the vagina for more than three hours during the first two weeks of use.8
Statistical analysis
Based on the primary endpoint and assuming a coefficient of variation of 25%, 22 participants per arm were estimated to provide at least 90% power to detect a 30% difference in hormone exposure between groups. We aimed to enroll 25 participants per group to allow for loss of evaluable data, and continued enrollment until the desired sample size was met.
All PK parameters were summarized per visit for each study group as the median and interquartile range (IQR) and as geometric means (GM) with 95% confidence intervals (CI). We calculated the etonogestrel and ethinyl estradiol AUC0–21days by the trapezoidal rule. We summarized the effect of ART on etonogestrel and ethinyl estradiol PK parameters by GM ratios (GMR) and associated 90% CI for each ART group relative to the Control group; these differences were also evaluated using the Wilcoxon Rank–Sum test. Efavirenz, atazanavir, and ritonavir PK parameters were compared between Day 0 and Day 21 by the intraindividual GMR (90% CI) and the Wilcoxon Signed–Rank test. To estimate the antiretrovirals AUC0–24h, we imputed the observed pre–dose concentration to estimate the concentration at the end of the dosing interval (24 hours). The ART AUCs were calculated by trapezoidal rule using Phoenix® WinNonLin® version 7.0 (Certara USA, Inc., Princeton, NJ). Detailed methods for calculating GM, GMR, and associated CIs are described on Appendix page 5.
Demographic characteristics, AEs, and progesterone concentrations were summarized by proportion or median (range) and compared by Wilcoxon Rank Sum or Fisher’s Exact Test, as appropriate. All statistical analyses were performed using SAS version 9.4, (SAS Institute Inc, Cary, NC, USA) without adjustment for multiple comparisons. The study was registered on clinicaltrials.gov ().
Role of the Funding Source
The funders of the study had an oversight role in the development and monitoring of the study, but had no role in the conduct, analyses, and conclusions of the study. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Results
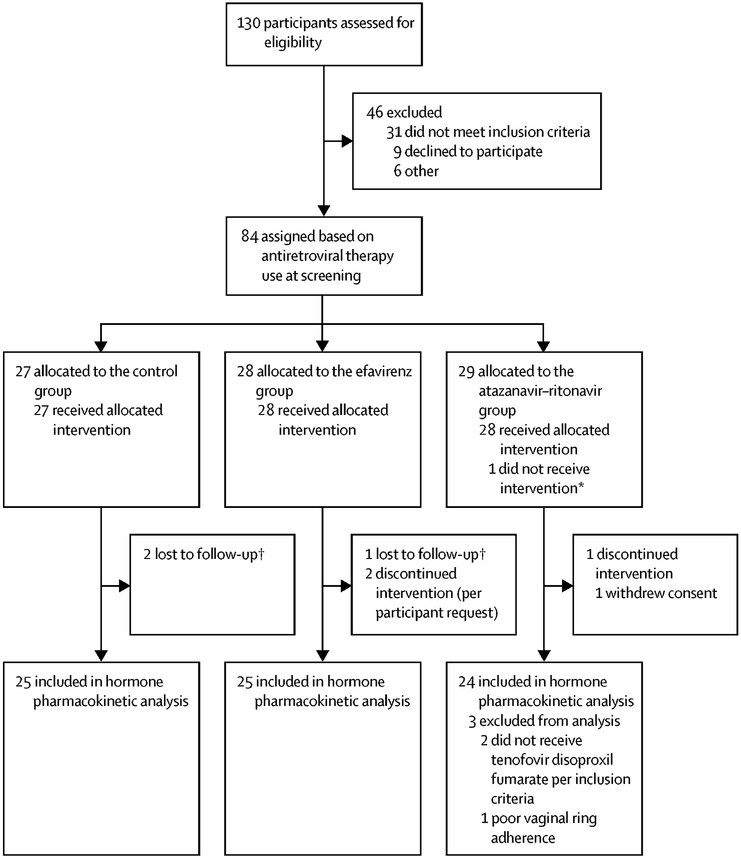
Between December 30, 2014 and September 12, 2016, we enrolled 84 participants in the study; ten participants were excluded from the primary hormone PK analysis for reasons described in Figure 1. Therefore, 74 participants met the primary endpoint, 25 in the Control group, 25 in the efavirenz group, and 24 in the atazanavir/ritonavir group. Of the 74 participants, the median age was 34·5 years, 37 (50%) were Black race, 26 (35%) were Hispanic ethnicity, and the median menstrual cycle day at enrollment was nine days in each group (Table 1). Participant characteristics were balanced between ART and Control groups, except atazanavir/ritonavir group participants were more likely to be older and enrolled in the United States compared to the Control group. In the ART groups, the mean (range) time on efavirenz was 4.02 (0.10–12.01) years, 3.80 (0.21–9.06) years on atazanavir, and 3.13 (0.12–9.06) years on ritonavir.
Figure 1:
Participant enrollment and follow–up for the primary endpoint of hormone concentrations on day 21
Table 1.
Baseline characteristics of participants included in the primary analysis by study group
| Total (n=74) |
Control Group (n=25) | Efavirenz Group (n=25) | Atazanavir/ritonavir Group (n=24) | |
|---|---|---|---|---|
| Age (years)a | 34·5 (22 – 55) | 31 (22 – 48) | 36 (24 – 55) | 36·5 (24 – 48) |
| United States | 33 (45) | 6 (24) | 12 (48) | 15 (63) |
| Hispanic | 26 (35) | 10 (40) | 8 (32) | 8 (33) |
| Weight (kg)c | 67·6 (36·9 – 170·6) | 66·8 (36·9 – 112·9) | 68·3 (46·5 – 170·6) | 65·9 (47·3 – 152·4) |
| BMI (kg/m2)c | 26·0 (16·3 – 64·5) | 25·8 (16·3 – 44·1) | 26·6 (17·4 – 64·5) | 26·2 (19·2 – 59·5) |
| CD4–cell count (cells per μL)d | 687 (301 – 1941) | 623 (393 – 1,767) | 749 (343 – 1,941) | 664 (301 – 1,515) |
| Plasma HIV–1–RNA (copies per mL)e,f | <40 (<40 – 38,877) | 3417 (<40 – 38,877) | <40 (<40 – 2,071) | <40 (<40 – 327) |
| <400 copies per mL; n/N (%) | 52/69 (75) | 5/21 (24) | 23/24 (96) | 24/24 (100%) |
| <40 copies per mL; n/N (%) | 47/69 (68) | 3/21 (14) | 22/24 (92) | 22/24 (92) |
| Days from last menstrual period to Entry (days)g | 9 (3 – 157) | 9 (5 – 157) | 9 (4 – 57) | 9 (3 – 74) |
Data were collected at Entry (Day 0) and presented as median (range) unless indicated. No significant pairwise differences between each ART group compared to the Control group were observed, except for those noted (significance p<0.05). The Wilcoxon rank–sum test compared continuous variables and the Fisher’s exact test was used for categorical variables; all tests compared each antiretroviral group and the Control group. The p-value for each comparison is given in the Supplemental Appendix on page 6.
Participants in the Atazanavir/ritonavir group were older compared to the Control group; p=0.020 by Wilcoxon rank–sum.
Distribution of country of enrollment differed significantly between the Atazanavir/ritonavir group vs. Control group p=0.0010 by Fisher’s exact test.
For weight and BMI: n=72 total, n=23 for Efavirenz Group.
For CD4 cell count measured at screening: n=71 total, n=23 for Control Group, and n=24 for Efavirenz Group.
For plasma HIV–1 RNA: n=69 total, n=21 for Control Group, n=24 for Efavirenz Group, and n=24 for Atazanavir/ritonavir Group.
Participants in both ART groups had lower viral loads than the Control group; Efavirenz group vs Control group and Atazanavir/ritonavir group vs Control group both p<0.0001 by Wilcoxon Rank Sum.
For days from last menstrual period to Entry: n=71 total; n=25 for Control Group, n=25 for Efavirenz Group, and n=21 for Atazanavir/ritonavir Group.
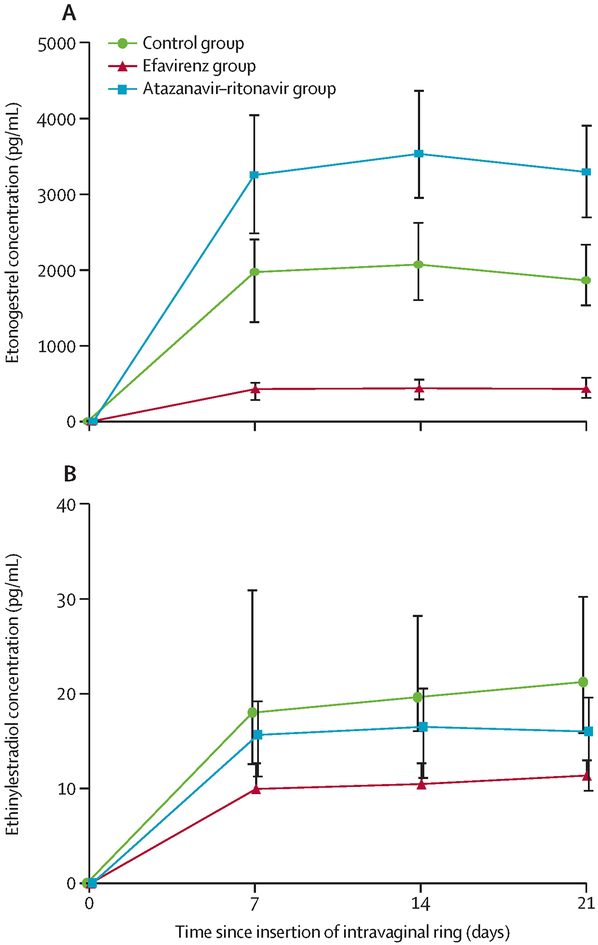
Etonogestrel PK results are described in Table 2 and Figure 2a. For the etonogestrel primary endpoint, Day 21 levels were 79% lower in participants receiving efavirenz compared to the Control group. The etonogestrel exposure over the entire dosing period is reflected by a 79% lower etonogestrel AUC0–21days. In contrast, in the atazanavir/ritonavir group, etonogestrel Day 21 concentrations were 71% higher and the AUC0–21days was 79% higher compared to the Control group.
Table 2.
Etonogestrel and ethinyl estradiol plasma pharmacokinetic parameters after release from a vaginal ring contraceptive device over 21 days
| Pharmacokinetic parameter | Group | N |
Median (IQR) |
Geometric Mean (95% CI) |
Geometric Mean Ratio (90% CI) |
Wilcoxon rank–sum p–value |
| Day 7 concentration (pg/mL) | Control (no ART) | 24 | 1,970 (1,310 – 2,400) | 1,774 (1,504 – 2,092) | ||
| Efavirenz | 25 | 427 (282 – 509) | 343 (267 – 437) | 0·19 (0·15 – 0·25) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 3,250 (2,480 – 4,040) | 3,065 (2,631 – 3,571) | 1·73 (1·44 – 2·07) | <0·0001 | |
| Day 14 concentration (pg/mL) | Control (no ART) | 23 | 2,070 (1,600 – 2,620) | 1,876 (1,557 – 2,262) | ||
| Efavirenz | 25 | 437 (292 – 550) | 371 (286 – 481) | 0·20 (0·15 – 0·26) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 3,530 (2,950 – 4,360) | 3,369 (2,817 – 4,030) | 1·80 (1·46 – 2·22) | <0·0001 | |
| Day 21 concentration (pg/mL) | Control (no ART) | 25 | 1,860 (1,530 – 2,330) | 1,812 (1,509 – 2,178) | ||
| Efavirenz | 25 | 429 (311 – 577) | 381 (288 – 503) | 0·21 (0·16 – 0·28) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 3,290 (2,690 – 3,900) | 3,105 (2,539 – 3,798) | 1·71 (1·37 – 2·14) | <0·0001 | |
| AUC0–21days (pg*week/mL)a | Control (no ART) | 23 | 33,600 (23,404 – 42,085) | 31,687 (26,687 – 37,624) | ||
| Efavirenz | 25 | 7,724 (5,251 – 9,064) | 6,503 (5,096 – 8,297) | 0·21 (0·16 – 0·26) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 58,745 (52,881 – 73,665) | 56,790 (48,604 – 66,354) | 1·79 (1·49 – 2·16) | <0·0001 | |
| Pharmacokinetic parameter | Group | N |
Median (IQR) |
Geometric Mean (95% CI) |
Geometric Mean Ratio (90% CI) |
Wilcoxon rank–sum p–value |
| Day 7 concentration (pg/mL) | Control (no ART) | 24 | 18 (13 – 31) | 20 (16 – 25) | ||
| Efavirenz | 25 | 10 (7 – 13) | 9 (7 – 11) | 0·44 (0·34 – 0·58) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 16 (11 – 19) | 14 (12 – 17) | 0·72 (0·58 – 0·91) | 0·066 | |
| Day 14 concentration (pg/mL) | Control (no ART) | 23 | 20 (16 – 28) | 21 (17 – 25) | ||
| Efavirenz | 25 | 11 (8 – 13) | 9 (7 – 11) | 0·42 (0·32 – 0·55) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 17 (11 – 21) | 15 (13 – 18) | 0·74 (0·59 – 0·91) | 0·032 | |
| Day 21 concentration (pg/mL) | Control (no ART) | 25 | 21 (16 – 30) | 22 (18 – 26) | ||
| Efavirenz | 25 | 11 (7 – 13) | 9 (7 – 11) | 0·41 (0·32 – 0·52) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 16 (10 – 20) | 14 (11 – 17) | 0·62 (0·49 – 0·79) | 0·0037 | |
| AUC0–21days (pg*week/mL)a | Control (no ART) | 23 | 324 (252 – 524) | 352 (287 – 433) | ||
| Efavirenz | 25 | 188 (146 – 227) | 154 (120 to 199) | 0·44 (0·34 – 0·57) | <0·0001 | |
| Atazanavir/ ritonavir | 24 | 268 (219 – 339) | 259 (221 – 303) | 0·73 (0·60 – 0·91) | 0·036 |
Abbreviations: ART; antiretroviral therapy, AUC, area under the concentration time curve.
Estimated value based on trapezoidal rule from Day 0–21 (SAS version 9.4, SAS Institute Inc, Cary, NC, USA)
Figure 2: Median (interquartile range) plasma concentrations (pg/mL) of (A) etonogestrel and (B) ethinyl estradiol from days 0 through 21 of continuous vaginal ring use.
Participants not yet receiving antiretroviral therapy (ART) are represented in green (Control Group), participants receiving efavirenz–based ART are represented in red (EFV Group), and participants receiving atazanavir/ritonavir–based ART are represented in blue (ATV/r group).
Ethinyl estradiol PK results are described in Table 2 and Figure 2b. Similar to etonogestrel, the ethinyl estradiol Day 21 concentrations were 59% lower with 56% lower AUC0–21days in participants receiving efavirenz compared to the Control group. Participants in the atazanavir/ritonavir group had 38% lower ethinyl estradiol Day 21 concentrations, and 27% lower AUC0–21days compared to the Control group.
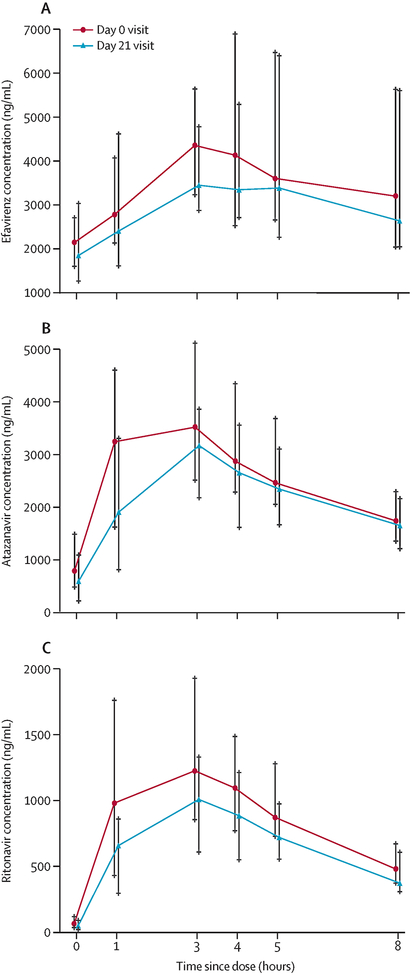
One participant in each ART group was excluded from the ART PK evaluation due to missed PK sampling related to poor venous access. Site investigators did not exclude any participants due to reported ART non–adherence. During hormone use (Day 21) the efavirenz Cmin was 36% lower and the AUC0–24h was 13% lower compared to the hormone–free period (Day 0); Table 3 and Figure 3a. Atazanavir exposure was lower on Day 21, but only the Cmax was statistically significant; Table 3 and Figure 3b. Compared to the hormone free period (Day 0), ritonavir AUC0–8h and AUC0–24h decreased 34 and 37%, respectively, due to a 41% lower Cmax; Table 3 and Figure 3c. For all other antiretroviral PK parameters, we observed lower summary concentrations on Day 21, but none were statistically different from Day 0. In a post hoc analysis, considering conservative thresholds for efavirenz and atazanavir Cmins that have been associated with effectiveness (1000ng/mL and 150ng/mL, respectively),28 three participants in the efavirenz group and six participants in the atazanavir/ritonavir group had at least one concentration below these thresholds. Eight of these nine participants remained virologically suppressed (<40 copies per mL); one participant in the efavirenz group, who had a HIV–1–RNA >1000 copies per mL at both entry and Day 21.
Table 3.
Efavirenz, atazanavir and ritonavir plasma pharmacokinetic parameters on study Day 0, before vaginal ring contraceptive insertion, and Day 21, in combination with the vaginal ring contraceptive
| Pharmacokinetic parameter | Study day | N |
Median (IQR) |
Geometric Mean (95% CI) |
Geometric Mean Ratio (90% CI) |
Wilcoxon signed rank p–value |
| Cmax (ng/mL) | 0 | 24 | 4,541 (3,321 – 6,720) | 4,868 (3,778 – 6,273) | ||
| 21 | 24 | 3,786 (3,289 – 6,724) | 4,717 (3,699 – 6,014) | 0·97 (0·85 – 1·11) | 0·72 | |
| Cmin (ng/mL) | 0 | 24 | 2,122 (1,435 – 2,703) | 2,353 (1,714 – 3,231) | ||
| 21 | 24 | 1,766 (1,174 – 2,351) | 1,504 (854 – 2,646) | 0·64 (0·42 – 0·97) | 0·020 | |
| AUC0–8h (ng*h/mL)a | 0 | 24 | 26,974 (21,035 – 42,834) | 30,768 (23,419 – 40,422) | ||
| 21 | 24 | 24,937 (17,731 – 41,999) | 27,551 (20,782 – 36,525) | 0·90 (0·79 – 1·01) | 0·14 | |
| AUC0–24h, (ng*h/mL)a | 0 | 24 | 68,949 (51,162 – 108,194) | 79,049 (59,545 – 104,941) | ||
| 21 | 24 | 57,796 (42,701 – 113,611) | 69,129 (51,318 – 93,120) | 0·87 (0·77 – 0·99) | 0·031 | |
| Pharmacokinetic parameter | Study day | N |
Median (IQR) |
Geometric Mean (95% CI) |
Geometric Mean Ratio (90% CI) |
Wilcoxon signed rank p–value |
| Cmax (ng/mL) | 0 | 23 | 4,291 (3,112 – 5,496) | 3,717 (2,891 – 4,780) | ||
| 21 | 23 | 3,583 (2,726 – 4,124) | 2,828 (1,932 – 4,139) | 0·76 (0·55 – 1·05) | 0·049 | |
| Cmin (ng/mL) | 0 | 23 | 797 (489 – 1,417) | 641 (379 – 1,084) | ||
| 21 | 23 | 599 (226 – 1,096) | 451 (256 – 794) | 0·70 (0·41 – 1·21) | 0·17 | |
| AUC0–8h (ng*h/mL)a | 0 | 22 | 20,560 (16,651 – 28,438) | 19,726 (15,367 – 25,320) | ||
| 21 | 22 | 18,324 (13,160 – 21,654) | 14,678 (9,805 – 21,973) | 0·75 (0·52 – 1·08) | 0·10 | |
| AUC0–24h, (ng*h/mL)a | 0 | 23 | 44,314 (31,525 – 51,744) | 38,992 (30,558 – 49,754) | ||
| 21 | 23 | 36,765 (22,246 – 49,836) | 29,882 (20,725 – 43,086) | 0·77 (0·57 – 1·03) | 0·077 | |
| Pharmacokinetic parameter | Study day | N |
Median (IQR) |
Geometric Mean (95% CI) |
Geometric Mean Ratio (90% CI) |
Wilcoxon signed rank p–value |
| Cmax (ng/mL) | 0 | 23 | 1,437 (982 – 2,274) | 1,351 (1,062 – 1,719) | ||
| 21 | 23 | 1,063 (742 – 1,472) | 790 (473 – 1,321) | 0·59 (0·38 – 0·91) | 0·0046 | |
| Cmin (ng/mL) | 0 | 23 | 70 (41 – 124) | 73 (42 – 126) | ||
| 21 | 23 | 52 (25 – 95) | 49 (29 – 81) | 0·67 (0·38 – 1·19) | 0·30 | |
| AUC0–8h (ng*h/mL)a | 0 | 22 | 6,746 (5,340 – 11,026) | 6,921 (5,466 – 8,764) | ||
| 21 | 22 | 5,352 (3,900 – 7,615) | 4,634 (2,997 – 7,165) | 0·66 (0·46 – 0·96) | 0·0018 | |
| AUC0–24h, (ng*h/mL)a | 0 | 23 | 10,740 (7,778 – 15,538) | 10,875 (8,534 – 13,856) | ||
| 21 | 23 | 7,211 (5,855 – 11,874) | 6,865 (4,524 – 10,419) | 0·63 (0·45 – 0·89) | 0·0082 |
Abbreviations: AUC, area under the concentration time curve; Cmax, maximum concentration; Cmin, minimum concentration.
Estimated value based on linear up, log down trapezoidal rule (Phoenix® WinNonLin® version 7.0; Certara USA, Inc., Princeton, NJ)
Figure 3: Plasma concentrations of (A) efavirenz, (B) atazanavir, and (C) ritonavir.
Line represents median (interquartile range) value in ng/mL per timepoint. Day 0 represents antiretroviral therapy alone, Day 21 represents antiretroviral therapy in combination with the contraceptive vaginal ring.
The endogenous progesterone concentrations from Day 0 through Day 28 are presented in the Appendix (pages 7–9). Six participants reported the IVR was outside of the vagina at any time during the study period; five reported <15 minutes per excursion, one participant in the efavirenz group reported 13 hours without the IVR in place 48 hours prior to the Day 14 evaluation; we excluded this participant from the progesterone analysis. All participants in the Control (n=25) and atazanavir/ritonavir groups (n=24) had endogenous progesterone values below the level of detection (0·5 ng/mL) by Day 14, and remained undetectable through day 28. In the efavirenz group, 4 of 24 participants had progesterone levels above the level of detection (values 0.6, 2.4, 4.3 and 5.4 ng/mL) on Day 14, all 24 had progesterone values below the level of detection on Day 21, and 2 additional participants had progesterone levels above the level of detection (values 0.8, and 1.7 ng/mL) on Day 28.
The IVR was inserted for all but one enrolled participant; thus 83 participants are included in the safety analyses. For both ART groups, all participants had HIV–1–RNA ≤400 copies per mL at screening, but by entry one participant in the efavirenz group had an HIV–1–RNA value of 2,071 copies per mL. On Day 21, the same efavirenz group participant was virologically detectable and one additional participant in the atazanavir/ritonavir group had HIV–1–RNA >400 copies per mL.
Overall, 23 of 83 (27·7%) participants reported 35 AEs related to the study therapy. Table 4 describes overall and group–specific occurrences of AEs; all AEs were Grade 1 or 2. Grade 2 AEs occurred in four of 83 participants. In the Control group, two participants (7.4% of 27) experienced Grade 2 symptoms (vaginal discharge, candidiasis, and/or bacterial vaginosis). In the efavirenz group, one participant (3.6% of 28) exhibited a Grade 2 symptom (metrorrhagia). In the atazanavir/ritonavir group, one participant (3.6% of 28) exhibited Grade 2 symptoms (lower abdominal pain and decreased libido). One participant in the efavirenz group discontinued IVR therapy due to intermenstrual bleeding (Grade 2; Figure 1: treatment discontinued per participant request). Adverse effects reported as unrelated to study procedures are in the Appendix (page 11).
Table 4:
Signs, symptoms, or diagnoses determined to be possibly, probably, or definitely related to study drug among enrolled participants who received the vaginal ring
| Total (n=83) |
Control Group (n=27) | Efavirenz Group (n=28) | Atazanavir/ritonavir Group (n=28) | |
|---|---|---|---|---|
| Participants experiencing an adverse effect | 23 (27·7) | 7 (25·9) | 8 (28·6) | 8 (28·6) |
| Grade 1 | 19 (23·0) | 5 (18·5) | 7 (25·0) | 7 (25·0) |
| Grade 2 | 4 (4·8) | 2 (7·4) | 1 (3·6) | 1 (3·6) |
| Adverse effects | ||||
| Abnormal vaginal dischargea | 12 (14·5) | 4 (14·8) | 4 (14·2) | 4 (14·2) |
| Menstrual bleeding irregularitiesb | 7 (8·4) | 1 (3·7) | 4 (14·2) | 2 (7·1) |
| Lower abdominal cramping | 4 (4·8) | 2 (7·4) | 0 (0·0) | 2 (7·1) |
| Vaginal pruritus/itching | 5 (6·0) | 2 (7·4) | 2 (7·1) | 1 (3·6) |
| Decreased libidoc | 2 (2·4) | 0 (0·0) | 0 (0·0) | 2 (7·1) |
| Bacterial vaginosisc | 1 (1·2) | 1 (3·7) | 0 (0·0) | 0 (0·0) |
| Bladder pressure | 1 (1·2) | 0 (0·0) | 1 (3·6) | 0 (0·0) |
| Breast tenderness | 1 (1·2) | 0 (0·0) | 1 (3·6) | 0 (0·0) |
| Vaginal Candidiasisc | 1 (1·2) | 1 (3·7) | 0 (0·0) | 0 (0·0) |
| Endocervical polyps | 1 (1·2) | 0 (0·0) | 1 (3·6) | 0 (0·0) |
| Lower abdominal painc | 1 (1·2) | 0 (0·0) | 0 (0·0) | 1 (3·6) |
| Vaginal dryness | 1 (1·2) | 0 (0·0) | 0 (0·0) | 1 (3·6) |
| Vaginal pain | 1 (1·2) | 0 (0·0) | 0 (0·0) | 1 (3·6) |
Data are presented as n(%). Comparing each antiretroviral group to the control group, post hoc two-sided Fisher’s exact test p values were 0.24 to >0.99 for both antiretroviral groups. The p-value for each comparison is given in the Supplemental Appendix on page 10.
Grade 2 (n=2)
Includes intermenstrual bleeding or spotting, metrorrhagia; normal menses excluded.
Grade 2 (n=1)
Discussion
We found that efavirenz–based ART significantly decreased the systemic exposure to both etonogestrel and ethinyl estradiol released from a IVR (79% and 59% lower, respectively) compared to women with HIV not receiving ART. Our findings raise concern for the effectiveness of the IVR contraceptive in women receiving efavirenz–based ART, and studies evaluating ovulation rates in the context of this DDI are necessary. In contrast, atazanavir–ritonavir containing ART resulted in variable influence on contraceptive hormone exposure compared to women not receiving ART (71% higher etonogestrel, 38% lower ethinyl estradiol). Hence, atazanavir/ritonavir–based ART is unlikely to influence the IVR contraceptive effectiveness, as progestin exposure was higher than controls. Despite these changes, no excess AEs were reported in the atazanavir/ritonavir group compared to controls (Table 4). The influence of ART on etonogestrel is likely mediated via CYP3A4, the enzyme primarily responsible for etonogestrel metabolism, due to CYP3A4 induction by efavirenz and inhibition by atazanavir/ritonavir. The effect of ART on ethinyl estradiol is also likely related to induction of multiple pathways of ethinyl estradiol metabolism by both efavirenz and atazanavir/ritonavir. Although NRTIs were administered in both ART arms, studies have not observed significant DDIs between NRTIs and hormonal contraceptives.18
The DDI observed between atazanavir/ritonavir and etonogestrel/ethinyl estradiol via an IVR were similar to data with oral contraceptive pills (norgestimate 85% higher, ethinyl estradiol 19% lower,),29 as well as other DDI studies of protease inhibitors boosted with ritonavir or cobicistat plus hormonal contraceptives (e.g. progestin increases and estrogen decreases).18 Our etonogestrel results are directionally similar to, but greater in magnitude than, efavirenz–based ART plus either oral norgestimate (64% lower),16 levonorgestrel subdermal implant (47% lower)15, or oral emergency contraception (58% lower).13 These results are similar to another non–oral DDI study evaluating efavirenz–based ART plus the etonogestrel subdermal implant (82% lower etonogestrel), supporting that induction of CYP3A influences etonogestrel metabolism despite avoiding oral first–pass metabolism.14 Unexpectedly, we observed a greater effect of efavirenz–based ART on vaginally administered ethinyl estradiol than previously described in a healthy volunteer study between efavirenz and oral ethinyl estradiol (10% lower).16 Differences in the magnitude of efavirenz–hormone DDIs may be influenced by study participant characteristics. Pharmacogenetic varients that influence efavirenz metabolism have been associated with the extent of the DDI observed between efavirenz and levonorgestrel implants,30 and our participants were enrolled in regions of the world with higher prevalence of these variants compared to many efavirenz–hormone DDI studies. Also, the duration of ART use prior to DDI assessment may influence the observed interaction; the inductive properties of efavirenz take weeks to reach maximal effect, which is challenging to achieve in healthy volunteer studies. Finally, there may be variable influence of CYP3A inducers/inhibitors on contraceptive hormones,31 suggesting the complex pathways of hormone metabolism are not yet fully elucidated.
The clinical impact of DDIs that decrease hormone exposure may vary based on the type of hormone, route of administration, resulting PK of hormones administered, and intended therapeutic goal (e.g. ovulation suppression or endometrial changes). For example, progestin–releasing IUDs prevent pregnancy by multiple mechanisms of action, which may include systemic effects on ovulation suppression, but are primarily local, so systemic hormone exposure is less likely to influence IUD effectiveness. In contrast, progestin–releasing subdermal implants prevent pregnancy through both ovulation suppression and mucosal effects, despite lower systemic progestin exposure compared to other routes of administration. In this context, DDIs that decrease progestin exposure, specifically efavirenz–based ART, are associated with higher rates of contraceptive failure for contraceptive implants in cohort studies compared to other routes of administration.15,32,33 Further, IVRs are in development as multipurpose preventive technologies to provide local antimicrobial protection from infection and systemic hormone exposure for contraception.11,12 In these products, each drug and device may result in different pharmacologic properties. For example, dapivirine released from an IVR achieves local concentrations several–folds higher than systemic concentrations,34 while levonorgestrel released from an IVR reach systemic concentrations that may suppress ovulation.11,12 In addition, the local vaginal milieu may influence the PK properties of some drugs.35 Given these considerations, DDI potential from both systemic and local influences on drug metabolism and transport (e.g. expression of local and systemic drug transporters, drug metabolizing enzymes, and the microbiome) must be considered in the context of desired therapeutic concentrations, which are often unknown for hormonal therapies.
Similar to other routes of hormone administration, we observed modestly lower efavirenz and ritonavir exposure during IVR use, which is proposed to be related to hormone modulation of drug metabolizing enzymes.18 The Cmin during a dosing interval, and therefore the ability to remain above the inhibitory concentration, is often the PK parameter evaluated during therapeutic drug monitoring.28 The efavirenz Cmin was 36% lower during hormone use in our study, but remained above the concentration associated with efavirenz effectiveness.28 Ritonavir and atazanavir Cmins were were within the expected range for both drugs on Day 21.28 Given these considerations, vaginally–administered hormones may not have a clinically significant influence on ART effectiveness. In the setting of incomplete adherence to ART or dose reduction of efavirenz to 400mg daily, these changes may become clinically relevant.
In our study, adherence was assessed by self–report, which could be further influenced by study procedures that required rescheduling PK visits with non–adherence. Our adherence self–report form is commonly used in ACTG studies and correlates with virologic success.25 We also measured HIV–RNA at Days 0 and 21, and one participant in each ART group was virologically detectable, which may indicate ART non–adherence. However, if participants were non–adherent to ART, the effect of ART on hormone concentrations would be biased to the null. Instead, we saw significant changes in hormone exposure with either ART regimen. Unmeasured non–adherence to the IVR is also a possibility. Our findings of higher etonogestrel concentrations in the atazanavir/ritonavir group, yet significantly lower hormone exposure in the efavirenz group, supports adequate adherence in both the atazanavir/ritonavir and Control groups, and there was no indication of disparate adherence in the efavirenz group. Finally, prior studies of similar ART with both oral and non–oral hormonal contraceptives support our findings.18
Other limitations of our study include the potential for residual confounding due to the lack of participant randomization. In addition to unmeasured confounding, we observed differences in age and country of enrollment between the atazanavir/ritonavir and Control groups. Our study was also not powered to evaluate the contraceptive effectiveness of, or AEs related to, the IVR; all participants agreed to use a second form of non–hormonal contraception during the study which may have influenced the rate of AEs. In addition, most participants did not start the IVR in relationship to their menses as advised by product labeling,8 which may influence ovulation suppression. In addition, consistent with other PK studies evaluating the IVR contraceptive,9 we used sparse PK monitoring of hormones, and intensive PK monitoring was not conducted after the IVR was removed. Therefore, we could not calculate hormone clearance or half–life, and the observed Cmax may not be reflective of the true Cmax due to sampling frequency. Finally, efavirenz, atazanavir, or ritonavir are all administered once daily, but our intensive PK sampling occurred over 8–hours and the pre–dose sample was imputed to infer the expected concentration 24–hours after observed dosing. Despite this, the observed results are consistent with expected concentrations.28
In summary, we observed significant differences in vaginally administered hormone concentrations when contraceptive IVRs were used in combination with common oral ART regimens. Our results raise concern for the possibility of compromised effectiveness of vaginally administered hormonal contraceptives in women receiving efavirenz–based ART. In contrast, we predict that atazanavir/ritonavir–based ART would be unlikely to influence the contraceptive effectiveness of IVR hormonal contraception, but may lead to more unscheduled bleeding related to lower ethinyl estradiol concentrations with concomitant use. These differences were similar to or greater as compared to prior drug interaction studies in women using both oral hormonal contraceptives and ART regimens, highlighting that hormone related drug–drug interactions are still of concern with non–orally administered hormone contraceptive methods. Further understanding of therapeutic hormone concentrations and DDI potential of intravaginal drugs in the context of pharmacodynamic assessment of ovulation will help optimize this important route of drug delivery for persons living with, or at risk for, HIV infection.
Contributors:
KKS, CG and SEC contributed to the literature search, study design, data collection and interpretation, and manuscript drafting and revision. YSC, SLR contributed to the study design, data analysis and interpretation, figures, and manuscript drafting and revision. FA contributed to the study design, pharmacokinetic data analysis and interpretation, and manuscript revision. BB contributed to the study design, data interpretation, and manuscript revision. RWC contributed to the study design, laboratory data analysis and interpretation, and manuscript revision. KC, LM contributed to study design, data management and manuscript revision. CDZ contributed to the literature search, study design, participant enrollment, data interpretation, and manuscript revision.VA, MA, RKF, and SS contributed to participant recruitment, data collection and interpretation, and manuscript revision. DG contributed to the pharmacokinetic data analysis and interpretation, and manuscript revision. The members of the study group were responsible for study oversight and played other important roles for the study at their sites, reviewed the study results, manuscript, and provided other intellectual contributions.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the AIDS Clinical Trials Group (ACTG) through the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UMI AI068636; UMI AI068634; UMI AI106701. This work was also supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network through the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1AI068632; UM1AI068616; UM1AI106716 with cofounding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health under award number HHSN275201300003C. This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award number 1R01HD085887 (PI Scarsi). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We gratefully acknowledge the support of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, for providing NuvaRing® for use in this study.
We gratefully acknowledge the patients who participated in this research, and site personnel who contributed to this work, including: Dr. Angela Cristina Andrade and Vânia Stiepanowez Regina de Oliveira Rocha, Instituto de Pesquisa Clínica Evandro Chagas (IPEC) – Fiocruz (Site 12101; 5UM1AI069476); Maureen McNichols, Rush University Medical Center/Ruth M Rothstein CORE Center (Site 5083; HHSN275201800001I); Johnson Ondiek, KISUMU CRS (Site 31460; UM1AI069418); Nila Dharan and Christie Lyn Costanza, Rutgers New Jersey Medical School (Site 31786; AI069419–12); Dr. Carmel Ganoza and Maria Esther Guevara, Barranco CRS (Site 11301; 1U01AI069438–01); San Miguel CRS (Site 11302; 1U01AI069438–01); Gaborone CRS (Site 12701; UM1 AI069456–08); Dr. Karen Tashima and Deborah Perez, The Miriam Hospital (Site 2951; 2UM1A1069412–08); Professor Kiat Ruxrungtham, The Thai Red Cross AIDS Research Centre (TRC–ARC) CRS (Site 31802; 5UM1AI069399); Suwat Chariyalertsak and Daralak Tavornprasit, Chiang Mai University HIV Treatment CRS (Site 31784; 5UM1AI069399–10); USC LA NICHD CRS (Site 5048); Dr. Michael S. Saag, Alabama CRS (Site 31788; UM1 AI069452–08); Dr. Emily Barr and Dr. Adriana Weinberg, University of Colorado CRS (Site 5052; Westat ID–6101–S035); Dr. Jaime G. Deville and Dr. Carla Janzen, David Geffen School of Medicine at UCLA CRS (Site 5112; NICHD Contract HHSN275201300003C, Westat ID–6101–S059, UL1TR001881); Dr. Mhleli Masango and Dr. Lee Fairlie, Wits RHI Shandukani Research Centre (Site 8051; 5UM1A1068632); Columbia Physicians & Surgeons (P & S) CRS (Site 30329; UM1 AI069470–08); Raphaelle Auguste and Marlene Burey, Jacobi Medical Center (Site 5013; HHSN275201300003C); San Juan City Hospital (Site 5031); Dr. Mobeen H Rathore and Saniyyah Mahmoudi, University of Florida Center for HIV/AIDS Research, Education and Service (UF CARES) (Site 5051; HHSN275201300003C); Dr. Jorge L Santana and Marielly Lopez–Rivera, Puerto Rico AIDS Clinical Trails Unit (PR–AIDS) CRS (Site 5401; 5 UM1 AI069415–13); The University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (Site 6601; UM1 AI069415–09).
Funding:
National Institutes of Health, through the AIDS Clinical Trials Group and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network, National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health.
Footnotes
Additional AIDS Clinical Trials Group A5316 Study Team members:
| First Name (middle initial) | Last Name |
|---|---|
| Liz | Barr |
| Christina | Blanchard–Horan |
| Elizabeth | Connick |
| Mary Allegra | Cermak |
| Nahida | Chakhtoura |
| Cecelia | Chang–Ching |
| Andee | Fox |
| David W. | Haas |
| Alan | Landay |
| Mey | Leon |
| Jeong–Gun | Park |
| Kristine | Patterson |
| Thucuma | Sise |
| Greg | Spear |
| David | Shugarts |
| Pamela | Tshandu |
| Charles R. | Wira |
Declaration of Interests:
KKS, YSC, SLR, FA, BB, RWC, KC, LEM, CDZ, VA, MA, RKF, DG, and CG report no competing interests.
SS received personal fees and grants to her institution from Gilead Sciences and personal fees from Thera Technologies outside of the submitted work. SEC received personal fees from Merck & Co. outside of the submitted work.
The author from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (CG) had a role in the design, data interpretation, manuscript revision, and intellectual contribution; however, her views were her own and did not represent the funder’s views.
Data Sharing
The authors confirm that all data underlying the findings are fully available without restriction. Due to ethical restrictions, study data are available upon request from sdac.data@sdac.harvard.edu with the written agreement of the AIDS Clinical Trials Group.
Prior presentation: These data were presented in part as oral abstracts at the 2018 Conference on Retroviruses and Opportunistic Infections, Oral Abstract #141, and the 2018 International Workshop on Clinical Pharmacology of Antiviral Therapy, Oral Abstract #10.
References
- 1.Feyissa TR, Harris ML, Melka AS, Loxton D. Unintended Pregnancy in Women Living with HIV in Sub–Saharan Africa: A Systematic Review and Meta–analysis. AIDS and behavior 2018. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global health sector response to HIV, 2000–2015: Focus on innovations in Africa: progress report. Available at: https://apps.who.int/iris/bitstream/handle/10665/198065/9789241509824_eng.pdf?ua=1?sequence=1 Accessed: 11 February 2019.
- 3.Adeniyi OV, Ajayi AI, Moyaki MG, Goon DT, Avramovic G, Lambert J. High rate of unplanned pregnancy in the context of integrated family planning and HIV care services in South Africa. BMC Health Serv Res 2018; 18(1): 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton MY, Zhou W, Frazier EL. Unplanned pregnancies and contraceptive use among HIV–positive women in care. PloS one 2018; 13(5): e0197216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Juneja S, Vitoria M, et al. Projected Uptake of New Antiretroviral (ARV) Medicines in Adults in Low–and Middle–Income Countries: A Forecast Analysis 2015–2025. PloS one 2016; 11(10): e0164619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrader SP, Ragucci KR. Contraception. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach Ninth Edition. New York: McGaw Hill Education, 2014: 1271–86. [Google Scholar]
- 7.Johansson ED, Sitruk–Ware R. New delivery systems in contraception: vaginal rings. American journal of obstetrics and gynecology 2004; 190(4 Suppl): S54–9. [DOI] [PubMed] [Google Scholar]
- 8.NuvaRing® [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; June 2018. [Google Scholar]
- 9.Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clinical pharmacokinetics 2000; 39(3): 233–42. [DOI] [PubMed] [Google Scholar]
- 10.Institute Guttmacher. Contraceptive Use in the United States. July 2018. Available at: https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states Accessed: 11 February 2019.
- 11.Achilles SL, Hendrix CW, Poloyac SM, et al. Safety and pharmacokinetics of dapivirine and levonorgestrel vaginal rings for multipurpose prevention of HIV and pregnancy (MTN–030/IPM 041). HIV Research for Prevention; 2018. Madrid, Spain: Abstract OA12.02LB. [Google Scholar]
- 12.Thurman AR, Schwartz JL, Brache V, et al. Randomized, placebo controlled phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PloS one 2018; 13(6): e0199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carten ML, Kiser JJ, Kwara A, Mawhinney S, Cu–Uvin S. Pharmacokinetic interactions between the hormonal emergency contraception, levonorgestrel (Plan B), and Efavirenz. Infectious diseases in obstetrics and gynecology 2012; 2012: 137192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappell CA, Lamorde M, Nakalema S, et al. Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS 2017; 31(14): 1965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarsi KK, Darin KM, Nakalema S, et al. Unintended Pregnancies Observed With Combined Use of the Levonorgestrel Contraceptive Implant and Efavirenz–based Antiretroviral Therapy: A Three–Arm Pharmacokinetic Evaluation Over 48 Weeks. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016; 62(6): 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevinsky H, Eley T, Persson A, et al. The effect of efavirenz on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy HIV–negative women. Antivir Ther 2011; 16(2): 149–56. [DOI] [PubMed] [Google Scholar]
- 17.Vieira CS, Bahamondes MV, de Souza RM, et al. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel–releasing implant pharmacokinetics in HIV–positive women. J Acquir Immune Defic Syndr 2014; 66(4): 378–85. [DOI] [PubMed] [Google Scholar]
- 18.Scarsi KK, Darin KM, Chappell CA, Nitz SM, Lamorde M. Drug–Drug Interactions, Effectiveness, and Safety of Hormonal Contraceptives in Women Living with HIV. Drug safety 2016; 39(11): 1053–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Back DJ, Houlgrave R, Tjia JF, Ward S, Orme ML. Effect of the progestogens, gestodene, 3–keto desogestrel, levonorgestrel, norethisterone and norgestimate on the oxidation of ethinyloestradiol and other substrates by human liver microsomes. The Journal of steroid biochemistry and molecular biology 1991; 38(2): 219–25. [DOI] [PubMed] [Google Scholar]
- 20.Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K. Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clinical pharmacology and therapeutics 2003; 74(4): 326–33. [DOI] [PubMed] [Google Scholar]
- 21.Dogterom P, van den Heuvel MW, Thomsen T. Absence of pharmacokinetic interactions of the combined contraceptive vaginal ring NuvaRing with oral amoxicillin or doxycycline in two randomised trials. Clinical pharmacokinetics 2005; 44(4): 429–38. [DOI] [PubMed] [Google Scholar]
- 22.Verhoeven CH, van den Heuvel MW, Mulders TM, Dieben TO. The contraceptive vaginal ring, NuvaRing, and antimycotic co–medication. Contraception 2004; 69(2): 129–32. [DOI] [PubMed] [Google Scholar]
- 23.Cohn SE, Park JG, Watts DH, et al. Depo–medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clinical pharmacology and therapeutics 2007; 81(2): 222–7. [DOI] [PubMed] [Google Scholar]
- 24.Vogler MA, Patterson K, Kamemoto L, et al. Contraceptive efficacy of oral and transdermal hormones when co–administered with protease inhibitors in HIV–1–infected women: pharmacokinetic results of ACTG trial A5188. J Acquir Immune Defic Syndr 2010; 55(4): 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self–reported adherence with the ACTG Adherence Questionnaire: a cross–protocol analysis. J Acquir Immune Defic Syndr 2007; 46(4): 402–9. [DOI] [PubMed] [Google Scholar]
- 26.United States Food and Drug Administration. Bioanalytical Method Validation: Guidance for Industry. May 2018. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf Accessed 14 February 2019.
- 27.DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clinical pharmacology and therapeutics 2013; 93(6): 479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punyawudho B, Singkham N, Thammajaruk N, et al. Therapeutic drug monitoring of antiretroviral drugs in HIV–infected patients. Expert Rev Clin Pharmacol 2016; 9(12): 1583–95. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Chung E, Yones C, et al. The effect of atazanavir/ritonavir on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy women. Antiviral therapy 2011; 16(2): 157–64. [DOI] [PubMed] [Google Scholar]
- 30.Neary M, Lamorde M, Olagunju A, et al. The Effect of Gene Variants on Levonorgestrel Pharmacokinetics When Combined With Antiretroviral Therapy Containing Efavirenz or Nevirapine. Clinical pharmacology and therapeutics 2017; 102(3): 529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang N, Shon J, Kim MJ, et al. Role of CYP3A in Oral Contraceptives Clearance. Clin Transl Sci 2018; 11(3): 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel RC, Onono M, Gandhi M, et al. Pregnancy rates in HIV–positive women using contraceptives and efavirenz–based or nevirapine–based antiretroviral therapy in Kenya: a retrospective cohort study. Lancet HIV 2015; 2(11): e474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry SH, Swamy P, Preidis GA, Mwanyumba A, Motsa N, Sarero HN. Implementing the Jadelle implant for women living with HIV in a resource–limited setting: concerns for drug interactions leading to unintended pregnancies. AIDS 2014; 28(5): 791–3. [DOI] [PubMed] [Google Scholar]
- 34.Chen BA, Panther L, Marzinke MA, et al. Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double–Blind Randomized Trial. J Acquir Immune Defic Syndr 2015; 70(3): 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurman AR, Chandra N, Yousefieh N, et al. Differences in Local and Systemic TFV PK Among Premenopausal Versus Postmenopausal Women Exposed to TFV 1% Vaginal Gel. J Acquir Immune Defic Syndr 2018; 78(1): 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.