Abstract
Objective.
Neurostimulation technologies are important for studying neural circuits and the connections that underlie neurological and psychiatric disorders. However, current methods come with limitations such as the restraint on movement imposed by the wires delivering stimulation. The objective of this study was to assess whether the e-Particle (EP), a novel wireless neurostimulator, could sufficiently stimulate the brain to modify behavior without these limitations.
Approach.
Rats were implanted with the EP and a commercially available stimulating electrode. Animals received rewarding brain stimulation, and performance in a conditioned place preference (CPP) task was measured. To ensure stimulation-induced neuronal activation, immediate early gene c-fos expression was also measured.
Main results.
The EP was validated in a commonly used CPP task by demonstrating that (1) wireless stimulation via the EP induced preference behavior that was comparable to that induced by standard wired electrodes and (2) neuronal activation was observed in projection targets of the stimulation site.
Significance.
The EP may help achieve a better understanding of existing brain stimulation methods while overcoming their limitations. Validation of the EP in a behavioral model suggests that the benefits of this technology may extend to other areas of animal research and potentially to human clinical applications.
Keywords: deep brain stimulation, neurostimulator, conditioned place preference, neurostimulation, e-Particle, medial forebrain bundle
Introduction
Deep brain stimulation (DBS) technologies are commonly used for the treatment of neurological disorders such as Parkinson’s disease (Benabid et al 1987, Anderson et al 2017), essential tremor (Nazzaro et al 2013), dystonia (Cury et al 2017, Meoni et al 2017), and epilepsy (Loddenkemper et al 2001). Beyond these neurological conditions, DBS has gained increasing interest in the treatment of psychiatric diseases (Dougherty and Widge 2017, Graat et al 2017, Widge et al 2018). This increasing use of neurostimulation methods is due to an increased recognition of neurocircuit dysfunction as a cause of mental disorder. Neurostimulation techniques can target specific brain areas and alter their dysfunctional activity, an outcome that is often difficult to achieve through other means.
Despite the success of these treatments, their use is often accompanied by unpleasant side effects including infection, discomfort, lead misplacement or migration, and necessary corrective surgeries to repair hardware and replace batteries due to the relatively short battery life (Fenoy and Simpson 2014). Other brain stimulation technologies such as transcranial magnetic stimulation (TMS) are less invasive and avoid most of the issues surrounding lead-and battery-based implants. Unfortunately, TMS and other related technologies cannot reliably modulate deep brain structures (Wagner et al 2009, Sliwinska et al 2014). Given these conditions, there is a clear need for more targeted treatments like DBS and also a need for improved technology capable of supporting or supplementing these treatments (Kuhn et al 2009, Lo and Widge 2017). It would therefore be clinically valuable to have a wireless technology for precise yet non-invasive stimulation of deep brain areas.
In addition to their clinical potential, wireless technologies could also have a range of pre-clinical applications, including facilitating DBS testing in animals. Most animal models of DBS and related technologies involve connecting animals to a bulky head-mounted tether, which limits free behavior and makes social interaction experiments difficult to manage. As a result, numerous classes of wireless neurostimulators have emerged, and are still being developed, to address these issues (Li et al 2015, Khalifa et al 2018).
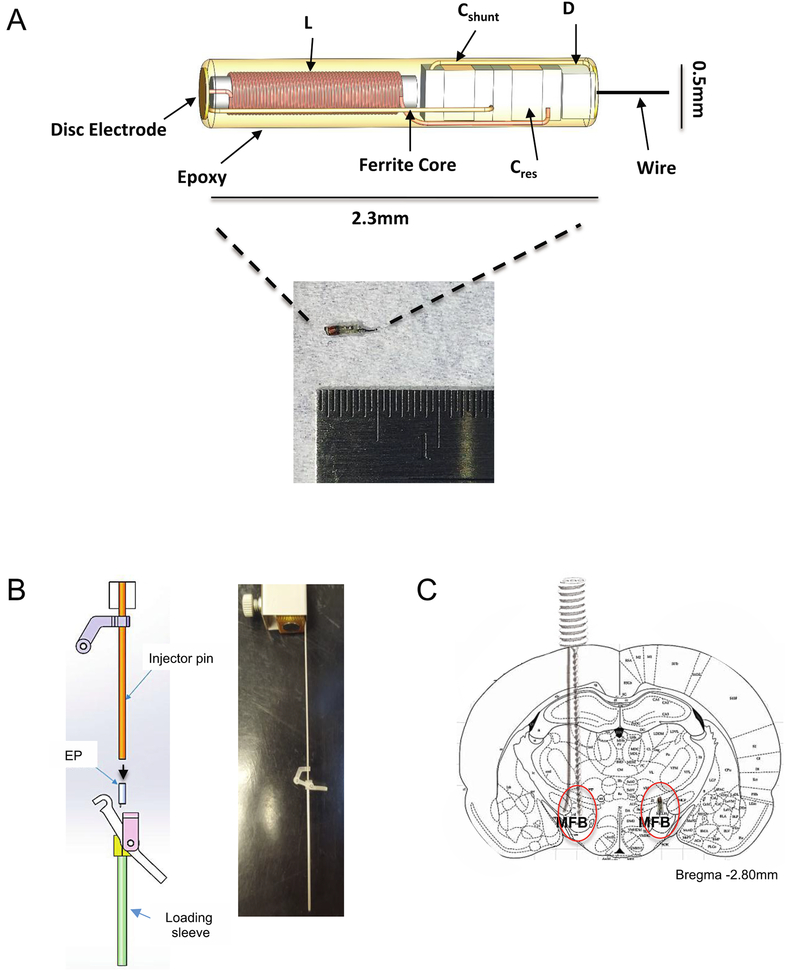
The e-Particle (EP) is a novel, sub-millimeter wireless neurostimulator that addresses and avoids some of the above-mentioned complications of wired neurostimulators. The EP is composed of a coil for inductive power, a capacitor to tune the coil to 10.7 MHz, a diode to rectify the received signals, and two electrodes (Freeman et al 2017) (figure 1(A)). A roughened platinum disc electrode is used as the anode, and a roughened platinum wire is used as the cathode. The platinum surface was mechanically roughened in order to increase the effective surface area, which decreases the electrode impedance. A wire is used in place of a disc electrode on the cathodic electrode to allow the tip of the cathode to penetrate the neural structure being targeted for stimulation and avoid disrupting that tissue. The disc side of the EP is comparable in size to implantable human stimulation leads, but is very large compared to rodent brain structures.
Figure 1.
E-particle (EP) design and implantation. (A) The EP is a wireless neurostimulator with a terminal platinum wire to penetrate the neural tissue. A coil (L) is used for inductive power, a capacitor (Cres) to tune the coil to 10.7 MHz, a diode (D) to rectify the received signals, a capacitor (Cshunt) to enhance rectification, and two electrodes (adapted from Freeman et al (2017)). A roughened platinum disc electrode is used as the anode, and a roughened platinum wire is used as the cathode. (B) The injector tool for EP implantation is used by loading the EP into the loading sleeve, and the injector pin is inserted and attached to complete the lever assembly (left). An assembled introducer attached to a stereotaxic cannula holder arm is illustrated on the right. The injector is lowered into the brain, and the lever is pressed to release the EP into the target brain tissue. (C) This diagram illustrates how each animal was implanted with a Plastics One electrode into the medial forebrain bundle (MFB) on one side of the brain and the EP on the other side of the brain.
Compared to other wireless devices, the EP provides a number of notable advantages. For one, it is smaller than microbattery-powered neurostimulators that offer similar functionality (Schulman 2008, Grill et al 2009). The EP also does not require a battery and is inductively powered. Injectable microbeads work in a similar fashion to the EP and offer the added ability to record as well as stimulate, but although this technology has been validated in sciatic nerve stimulation in a rodent model, it requires additional refining to be safe and effective for human use (Khalifa et al 2018). An injectable cellular-scale optoelectronic device was shown to modulate behavior via wireless optogenetics (Kim et al 2013, Montgomery et al 2015), but still requires a fairly bulky headstage. Nanoparticles are another class of injectable wireless neurostimulators that aim to reduce the invasiveness (damage to brain tissue) of current technologies by activating specific brain areas via magnetothermal stimulation (Chen et al 2015), but further behavioral testing is necessary to valid ate these wireless technologies and better understand their capabilities for modulating behavior.
The EP has been demonstrated to be sufficient for the stimulation of the sciatic nerve to successfully induce a motor response (Freeman et al 2017). However, this was performed as an open surgical preparation for direct stimulation. Implantation into the brain is more challenging. In a freely moving animal with a chronic brain implant, we cannot guarantee that the energy-delivering coil will be optimally aligned to the receiving coil. This is a critical factor, as the transmitting coil induces a voltage in the implanted coil, and the induced voltage is sensitive to the relative orientation of the two coils. The maximum voltage is achieved when the planes of the coils are parallel to each other. Since animals move their heads in uncontrolled ways, three transmitter coils positioned perpendicular to each other are used with the EP to maximize the induced voltage. Further testing is necessary to ensure that this positioning yields adequate voltage for any head orientation. Further, although the EP can clearly excite neurons in general, it is not yet proven to be able to excite a sufficient number of neurons in a DBS-like application to produce a behavioral effect. This is largely due to the fact that the device was designed to produce tens of microamperes (μA). Animal DBS experiments use currents in the hundreds of μA, and human DBS uses milliamperes. With these unknowns, confirming the EP’s efficacy in an animal model could provide valuable insight for studying DBS’ mechanisms of action and perhaps lead to improvements in how it is being used today to alter behaviors and neural functioning.
In this study, we validated the EP as a tool for brain stimulation that can effectively modify behavior. We used a conditioned place preference (CPP) task because the response to rewarding brain stimulation is clearly observable and robust, making it a good paradigm for evaluating CNS stimulation technologies. This validation included: (1) demonstrating that the EP can alter behavior in a common laboratory brain stimulation paradigm, (2) comparing the behavior changed by EP stimulation to that induced by traditional wired stimulation, and (3) verifying that the EP induced neuronal activation in brain regions that receive projections from the stimulation site.
Materials and methods
Subjects
Eight adult Sprague Dawley male rats 250–300 g were obtained from Harlan-Envigo Laboratories (Indianapolis, Indiana) and housed in the Massachusetts General Hospital Center for Comparative Medicine in Charlestown, MA. After acclimating to the colony room for 1 week, all animals were handled daily for at least another week. The sample size was based on the number of e-Particles available in the initial fabrication run, not on an a priori power calculation. However, a post hoc calculation (G * Power 3.1) using the observed Session effect (see below) indicates that we had 80% power to detect an effect size f of 0.05, much smaller than the actual effect size. Throughout the study, animals were weighed daily. They were maintained on a 12 h light/dark cycle and a diet of ad libitum rat chow and water. Experiments were performed during the light phase of the light/dark cycle. All procedures conducted in this study were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital and abide by the guidelines set forth by the Institutional Animal Care and Use Committee.
Surgery
A common stimulation target for proof-of-concept behavior experiments is the medial forebrain bundle (MFB; Carlezon and Chartoff 2007, McMurray et al 2017). MFB is a pathway of fibers that allows dopaminergic inputs from the ventral tegmental area to reach forebrain targets such as the medial prefrontal cortex and striatum (Nieuwenhuys et al 1982). This pathway plays a critical role in the rewarding effects of intracranial self-stimulation (ICSS) and CP paradigms (Carlezon and Chartoff 2007). Interestingly, DBS of the medial forebrain bundle has also been shown to induce acute antidepressant effects in individuals with treatment-resistant depression (Bewernick et al 2017), alleviating certain depressive symptoms in the acute and long-term. For these reasons, we targeted the MFB to evaluate the efficacy of the EP in this rodent model.
All rats were placed into an induction chamber and were anesthetized with isoflurane. Before induction, animals were injected with intraperitoneal (i.p.) atropine (0.01 mg kg–1) for respiratory support, at least 20 min before isoflurane. After induction animals were injected with subcutaneous (s.c.) narcotic pain reliever buprenorphine (0.05 mg kg−1), an anti-inflammatory non-narcotic pain reliever flunixin meglumine (2.5 mg kg−1, s.c.), and intramuscular (i.m.) antibiotic enrofloxacin (5 mg kg−1). Lidocaine (2%) was injected at the incision site before beginning surgery. To protect the eyes, lubricating eye ointment was also applied.
Each animal was implanted with a platinum twisted pair electrode (MS333/8-BIU/SPC, Plastics One, Roanoke, VA) on one side of the brain and an EP on the other. The EP was implanted using an insertion tool developed by Cirtec Medical Systems and Draper Laboratory for the implantation and delivery of the EP (figure 1(B)). The EP was first placed into the loading sleeve, and then the injector pin was inserted into the loading sleeve to push the EP down to the tip. Once the injector pin was in place, the upper portion of the lever connected to the bottom portion to complete the lever mechanism. The injector was then attached to a stereotaxic electrode holder that connected to the stereotax. The injector was then lowered into the hole in the skull at the appropriate coordinates for the MFB, and the lever was pressed to release the EP. After about 1 min, the injector was raised back up, and the hole was covered with bone wax.
We targeted the MFB at 2.8 mm posterior to bregma, 1.7 mm lateral from the midsagittal suture, and 7.8 mm below the dura, as per Paxinos and Watson (2007) and as noted in the MFB intracranial stimulation protocol by Carlezon and Chartoff (2007) (figure 1(C)). After implanting both the Plastics One electrode and the EP, 5–6 self-tapping bone screws, 0.85 mm in diameter and 4 mm in length (Fine Science Tools, Foster City, CA) were placed around the incision margins on the skull to anchor the electrode with dental acrylic (Durabase Fiber Powder and Repair Liquid, Patterson Dental, St. Paul, MN). Animals were allowed to recover for one week.
Behavioral testing paradigm
Stimulation: e-Particle.
The EP was activated for stimulation (monophasic, 50 Hz, 0.1 ms pulse width, and 0.25 s pulse train duration with a 1 s lockout) using three coils, each square in shape measuring 6 × 6 inches and activated simultaneously. The three coils were positioned orthogonal to one another and were mounted on the outside of the behavioral arena (approximately 18 inches high, 22 inches wide, 28 inches long), covering both walls and floor. This configuration was provided to induce sufficient stimulation within the EP and deliver monophasic pulses in trains of 1–50 pulses. The use of three orthogonal coils, transmitting in-phase simultaneously, made the harvested current insensitive to changes in the rat’s head orientation. Similarly, the large coil diameter kept the magnetic field nearly constant in any given quadrant, allowing the stimulation current to be less sensitive to variability due to the rat’s movement. The physical limits of the device maintained the amplitude under ~100 μA and could not be directly controlled by the experimenter. The output current levels for the EP stimulation were approximately tens of microamperes of current, as reported previously (Freeman et al 2017).
Stimulation: Plastics One.
The Plastics One electrodes were connected via their electrode pedestals to a commutator and stimulus isolator (A-M Systems 2200, A-M Systems, Sequim, WA), which delivered current-controlled stimulation. Parameters for the wired stimulation were determined via previously described bar-press titration methods (Carlezon and Chartoff 2007). In the titration procedure, the implanted animals first underwent food deprivation and were then trained to press two levers for food pellets. After shaping, the animals received MFB stimulation in place of food when they pressed one of the levers. Stimulation parameters for the experiment were reached when the rat was reliably pressing the lever that provided MFB stimulation, indicating that the expected rewarding effects had been achieved (as demonstrated previously in Widge and Moritz (2014)). Biphasic stimulation at 350 μA, 170 Hz, 0.1 ms pulse width and 0.5 s pulse train duration with a 1 s lockout produced a stimulation > food preference in all tested animals. Hence, this single set was used for all place preference experiments. It was not possible to perform the same titration with the EP, because there is no mechanism for controlling or limiting the output current and because the metal components of the operant chamber were not compatible with the applied magnetic field. To note, MFB stimulation with the commercial wired electrode set at lower parameters (closer to EP parameters) did not produce behavioral changes, indicating that the EP parameters were too low for use with traditional electrodes. The EP’s terminal wire, however, presents a very different surface area than the commercial electrode. Specifically, the Plastics One electrode is insulated and thus presents only a platinum-iridium disc at its cut tip, for a charge delivering area of πr2 = π(62.5 μm)2 = 12 272 μm2. The EP terminal wire is an un-insulated cylinder, giving an effective surface area of πr2 + 2πr * length = π(63.5 μm)2 + 2π(63.5 μm) * 1000 μm length of wire = 411 650 μm2. That is, although its total charge delivery and charge density are smaller, the EP could access a much larger volume of tissue. The MFB is a relatively large fiber bundle, and the tips of the Plastics One wires likely only accessed a fraction of its total area. The EP’s lower charge density likely activated only larger-diameter axons, but it was in physical proximity to more of those axons than was the wired stimulation. This may have produced sufficient activated axon counts to produce a perception of brain stimulation reward.
CPP.
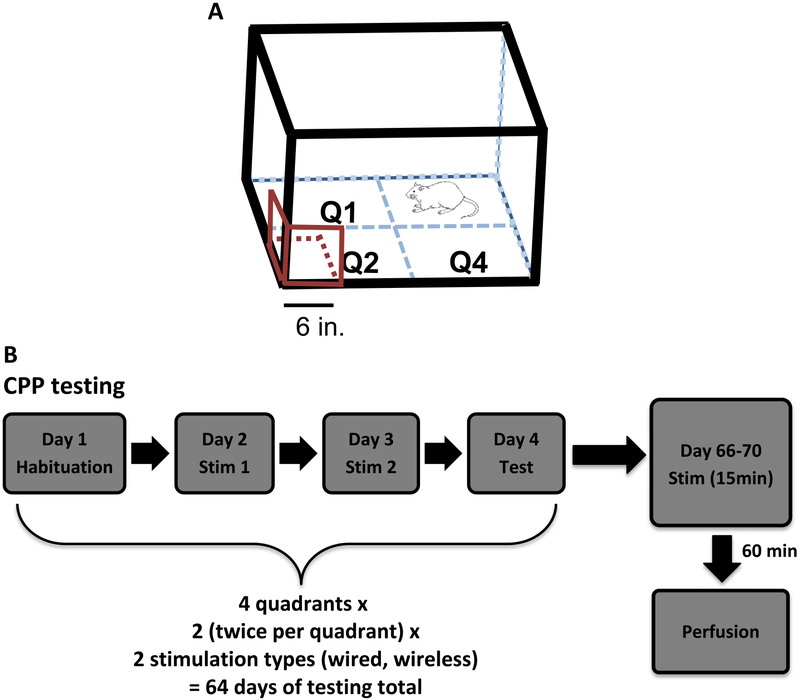
After recovery from surgery, animals were acclimated to an open field (OF) for five 15 min habituation sessions prior to CPP testing. Each round of CPP testing was performed over 4 consecutive days (figure 2). During the habituation sessions, the animals were placed in the open field without any stimulation, first unconnected and then connected to the stimulating wires. The average of the habituation sessions was taken and served as the baseline. We chose each animal’s least preferred quadrant to be the initial stimulation quadrant. This was done selectively in order to avoid the confounding effects of a naturally preferred quadrant. On the next two consecutive days, animals went through 15 min stimulation sessions. During these stimulation sessions, each animal was placed in the CPP field.
Figure 2.
CPP field and experimental timeline. (A) Rats in this study were placed into an open field with four quadrants. When the animals entered a predetermined stimulation quadrant (with coil attached), they received stimulation. The three square coils (in red) are attached to bottom left corner of the arena. The rat in the diagram is in quadrant 3 scale bar = 6 in. (B) The CPP experiment consisted of four sessions: habituation, stimulation day 1, stimulation day 2, and test. These sessions took place four times (each quadrant served as a stimulation quadrant). Two rounds of the four sessions were run for each of the two stimulation types for a total of 64 d of testing. Animals were sacrificed at the end of behavioral testing, following 60 min of continuous EP stimulation in the same arena.
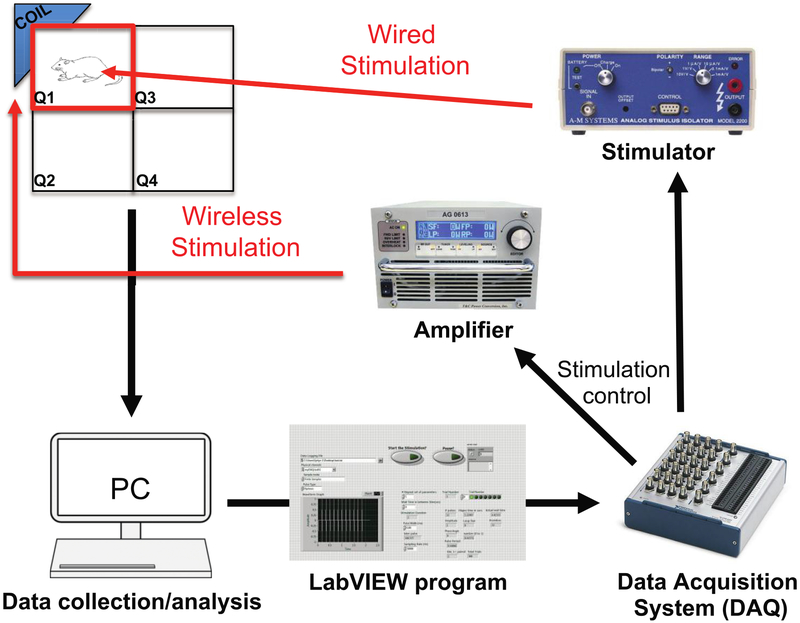
Using an automated video-tracking program coded in LabVIEW, a stimulation pulse was triggered every time the animal entered the stimulation quadrant that was specifically chosen for that session (figure 3). In each round of CPP testing, animals were randomly assigned to one of two types of stimulation: EP wireless stimulation or wired stimulation. It is important to note that although EP stimulation does not require the connection of wires, the animals were still tethered during EP stimulation in order to ensure that all conditions except for stimulation type were matched across the groups. In doing so, we eliminated the possible influence of the connecting wire itself on stimulation-induced behavior change. Stimulation parameters for both types of stimulation were set in LabVIEW. The type of stimulation triggered was defined as ‘wired’ or ‘wireless’, and it was selected by the experimenter before the start of the behavioral test. For the wired stimulation, analog pulse sequences were output from LabVIEW through the analog output of a data acquisition device (NI PCIe-6353; National Instruments, Austin, TX), to the input of a stimulus isolator (A-M Systems 2200, A-M Systems, Sequim, WA). The isolator converted the command voltage to constant-current stimulation sent via a tethering cable to the Plastics One electrode (see above regarding stimulation parameters). The stimulation was triggered every second that the rat remained in the stimulating quadrant (figure 3). For EP stimulation, the cable remained connected to the Plastics One pedestal, but was not used to deliver stimulation. Instead, a digital pulse train was generated by the LabVIEW program, which modulated the wireless power transfer carrier frequency in an on/off fashion via the DAQ. The wireless power transfer carrier frequency was set to match the resonant frequency of the EP and the transmit coil to ensure maximum power transfer. This modulated signal was then harvested by the EP and demodulated to the original digital pulse train, which was then delivered as stimulation. Again, the wireless EP stimulation was triggered every second as long as the rat remained in the selected stimulating quadrant.
Figure 3.
Block diagram of computer setup for behavior. A computer and attached video camera track rats in real time as they explore the arena. When the animal dwells in the target quadrant, stimulation occurs. Pulse sequences sent from LabVIEW through the DAQ are sent through an amplifier to a transmitter coil for EP stimulation and sent through a stimulator for wired stimulation.
The two consecutive days of stimulation sessions were followed by a test session. For the test session, animals were placed in the CPP field and allowed to explore for 15 min with no stimulation given. The test session also served as a recall test for the MFB stimulation-induced preferred quadrant. All animals received 32 sessions of stimulation (four sessions per quadrant × four quadrants × each quadrant served as the stimulation quadrant twice; figure 2). Only one quadrant was active for stimulation within a four-day block.
c-fos immunofluorescence.
In this study, a combination of behavioral and c-fos measurements was used to confirm activation of the MFB. This method was used instead of simultaneous MFB stimulation and recording of neuronal activity in its upstream/downstream targets to avoid any false signal created by electromagnetic interference between the transmitter and any electrophysiology amplifier. After completing all rounds of CPP stimulation, each animal was confined to one quadrant of the field. The animal then received 15 min of EP stimulation (50 Hz, 0.1 ms pulse width and 0.25 s pulse train duration with a 1 s offset) and wired stimulation (350 μA, 170 Hz, 0.1 ms pulse width and 0.5 s pulse train duration with a 1 s offset) triggered by the LabVIEW program as in the experiment (figure 3). The MFB has primarily ipsilateral connections so both stimulation types were tested, one type of electrode in each hemisphere (Nieuwenhuys et al 1982). After stimulation, the animal was returned to its home cage for 60 min. 60 min was chosen for peak immediate early gene c-fos expression, which has been reported to occur between 60–120 min after the behavior of interest (Cullinan et al 1995, Barros et al 2015). After 60 min, all rats were intraperitoneally injected with pentobarbital/phenytoin at 150 mg kg−1 pentobarbital (Beuthanasia®-D C IIIN, Merck Animal Health, Patterson Veterinary, Devens, MA). Once deeply anesthetized and unresponsive to toe pinch, animals were transcardially perfused with 0.9% saline for a 25 min exsanguination at a speed of 20 ml min–1, then fixed with 4% paraformaldehyde (PFA) for 25 min at the same speed. The euthanasia approach conforms to AVMA euthanasia guidelines.
The brains were extracted and stored in 4% PFA solution at 4 °C overnight, transferred to 20% sucrose phosphate-buffered saline (PBS) and stored at 4 °C for 24 h, and then in 30% sucrose PBS solution at 4 °C for at least another 24 h for cryoprotection. The brains were then prepared for cryostat sectioning by freezing them rapidly in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC). Tissue was sliced coronally at 40 μm thickness at −21 °C, with every third slice collected.
After sectioning, the tissue then began processing for fluorescent c-fos staining. The tissue sections were first blocked for 30 min on a low-speed orbital shaker, at room temperature, in a PBS solution consisting of 0.3% Triton-X 100 and 3% Normal Goat Serum (NGS). Tissue sections were then transferred into a 3% NGS PBS solution with a 1:50 concentration of polyclonal (Ab-2) [4–17] rabbit anti-c-fos antibody (PC05L, Millipore Sigma, Temecula, CA) and incubated at 4 °C overnight on a low-speed orbital shaker. After the incubation with the c-fos antibody, tissue sections were washed in PBS (15 min, three times). In low lighting, the tissue was then incubated for 3 h at room temperature (RT) in a PBS solution with Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (1:1000; Invitrogen, Thermo Fisher Scientific, Eugene, OR). Tissue was protected from photo bleaching by covering vials/well plates with aluminum foil, and all procedures were carried out in low lighting.
The tissue slices were washed in PBS (15 min, two times). After washing, the tissue was placed in a PBS solution with a concentration of 1:50 000 of 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, Saint Louis, MO) for 20 min at RT on a low-speed orbital shaker. The tissue was again washed in PBS (15 min, three times) and placed in fresh PBS after the third wash until the tissue could be mounted.
The tissue was mounted about 1–2 d after fluorescent c-fos staining onto subbed slides and allowed to dry for 1 d, continuing to follow measures to prevent photo bleaching. The tissue was rehydrated for 3 min in deionized water and cover slipped with Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL). The slides were allowed to dry, and the edges of each slide were sealed with clear nail polish and then stored in aluminum foil.
Nissl staining.
In addition to c-fos immunohistochemistry, extra tissue samples were also collected in the same manner described above for Nissl staining. The tissue was mounted on subbed slides the day after slicing. After allowing the mounted tissue to dry, it was dehydrated with a series of concentrations of ethanol/deionized water and ethanol/chloroform solutions. The tissue was stained with a 0.25% thionine stain for 3 min, rehydrated with ethanol/deionized water solutions (3 min each in 95%, 100%, 100%), and then cleared with Citrisolv (Fisher Scientific, Pittsburgh, PA) for at least 30 min. The slides were coverslipped with DPX medium (Electro Microscopy Sciences, Hatfield, PA) and then cleaned by removing the excess dried DPX medium on the slide with a razor and small amount of ethanol. The Nissl-stained tissue sections were visualized under light microscopy to confirm electrode placement. This was assessed based on the electrode tracks, confirming that each tract terminated within the MFB target region based on manual registration of each image to the atlas. For further confirmation of the EP location that might have been affected by brain extraction and slicing, a subset of animals were used to capture a CT-MRI coregistered image of an undisturbed EP in the MFB.
Imaging.
C-fos expression was assessed in cortical regions, one known to receive MFB projections and another that does not, confirming locations by visually comparing the neighboring Nissl-stained slices to the Paxinos and Watson atlas, in two animals. Brain slices from other animals did not stain adequately for c-fos quantification. The target regions we examined were the nucleus accumbens (NAcc; Bregma + 1.00 mm), an MFB projection site (Chergui et al 1996), and motor cortex (MC; Bregma + 1.00 mm-same section as nucleus accumbens). Although MFB innervates multiple brain areas, we chose to focus on the NAcc (core region) because of its well-studied involvement in reward circuitry and because many of the dopaminergic fibers terminate in this region (Nieuwenhuys et al 1982). The EP was implanted on the left side of the brain and the wired electrode on the right for both animals. Given that the MFB largely runs ipsilaterally, we evaluated c-fos expression in the NAcc and MC in the left hemisphere to assess evidence of neural activation via EP stimulation, as well as the right hemisphere to examine wired stimulation effects. The regions were imaged with a Nikon A1 SiR scanning confocal microscope at 40 × magnification. The same area was measured for each target structure. For quantification, c-fos positive cells were counted using ImageJ software (NIH, Bethesda, MD). Cells that met or were above an image-specific threshold for intensity of expression were counted. These thresholds were set by an operator who was not aware of the study’s a priori hypotheses, who did not participate in the behavioral data collection, and was blind to the experimental conditions. Two sections per brain area were counted, averaged, and converted to a percentage of c-fos positive cells compared to cells stained with DAPI.
Data analysis.
During each session the centroid data, mapping the exact position of the animal during the session, was recorded and saved by the tracker. The centroid trajectories were analyzed offline using Matlab. The percentage of time spent in the stimulation quadrants was analyzed for each session: baseline, stimulation day 1, stimulation day 2, and test session. Data were analyzed for both EP stimulation and Plastics One electrode stimulation for all animals and compared to determine the efficacy of the EP to induce place preference across the sessions.
Our a priori hypotheses were that CPP, as measured by time spent in the stimulation quadrant, would not differ between the EP and Plastics One stimulation in omnibus or in any given session. That is, we hypothesized that there would not be a significant interaction between session and stimulation type, but that there would be a significant effect of session. This would reflect equivalent CPP induction by the two stimulation methods. Repeated measures ANOVAs and post hoc paired t-tests were performed to assess these differences, using the individual session as the unit of analysis (SPSS 24, IBM). Given that these were pre-planned tests, we did not apply a Bonferroni correction. The a priori hypotheses were similar for the c-fos analysis. We hypothesized that there would be more c-fos positive cells in a MFB projection site (NAcc) compared to a non-projection site (MC). Paired t-tests were used to compare c-fos counts between the nucleus accumbens and motor cortex to confirm effective MFB stimulation.
Results
Histology
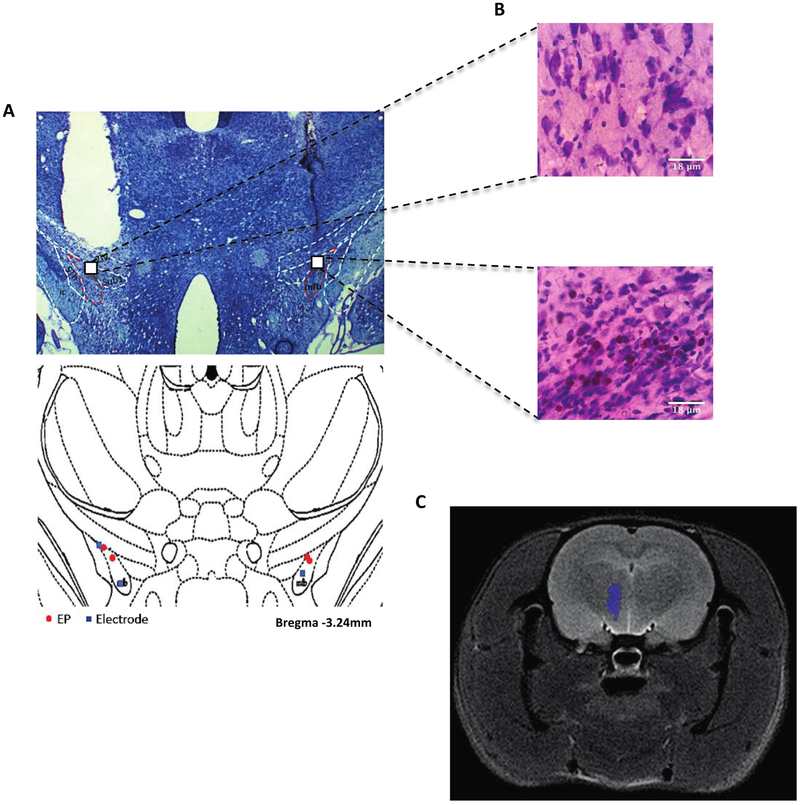
For confirmation that the stimulations were successfully inducing neuronal activation of the MFB, we verified successful placement of the EP and Plastics One electrodes using a thionine Nissl stain (figure 4(A)). Given that monophasic stimulation has the potential to damage neural tissue, we also confirmed that there was no substantial tissue damage due to the stimulation, noting that the surrounding tissue did not show gliosis or cell death (figure 4(B)). No animals displayed misplaced electrodes or EP implants, but four animals were excluded from further analysis due to head cap failure or illness that precluded behavioral testing (final n = 4 for behavior and histology analyses). A CT-MRI coregistered image also illustrates the successful placement of the EP (figure 4(C)).
Figure 4.
Histological results. (A) Thionine Nissl-stained section illustrating the location of the wired electrode and EP placement in the MFB. Placements of both types of electrodes are shown in the bottom illustration. Red: EP; blue: wired electrode. (B) Magnified image of tissue at the tip of the electrode (40×). Neither the EP-implanted tissue (left) nor the wired electrode (right) showed decreased cellular density suggestive of severe damage. There is some blood present in the right image, suggesting possible microtrauma from the wired electrode insertion; scale bar = 18 μm. (C) This CT-MRI coregistered image of the EP (purple) illustrates its placement in the MFB.
Behavior
All animals received MFB stimulations of both types: wireless stimulation (EP) and wired stimulation (Plastics One). All four quadrants served as the stimulation quadrant for two rounds of the four sessions. As predicted, there was no main effect of stimulation type (repeated measures ANOVA, F(1,57) =0.628, p = 0.431). There was a main effect of session, demonstrating successful place preference induction (F(3,171) =6.06, p = 0.001). There was no interaction between session and stimulation type (F(3,171) =1.14, p = 0.335). For the wired (Plastics One stimulation) group, the time spent in the stimulation quadrant significantly increased, indicating strong place preference conditioning, during the first stimulation session (paired t-test, t(31) =–2.21, p = 0.035), second stimulation session (t(31) =–2.26, p = 0.031), and test session (t(31) =–2.60, p = 0.014) compared to the baseline session. For the EP stimulation group, time spent in the stimulation quadrant was significantly less during the first day of stimulation compared to the baseline session (t(26) =2.09, p = 0.047), but showed no differences between the other sessions and baseline (data not shown).
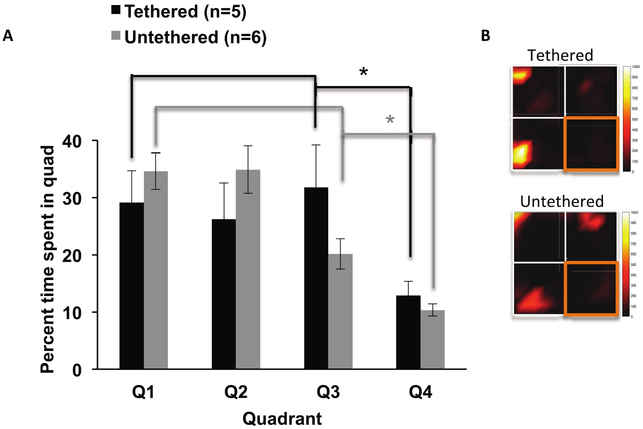
In further review of the behavioral data, the animals appeared to avoid quadrant 4 in all sessions. We noted that this quadrant was closer to the door of the room, and thus to light sources. It is therefore possible that quadrant 4 was slightly brighter than other quadrants, which the rats may have found aversive. To confirm this unanticipated place aversion, we placed a group of Plastics One implanted animals that were naïve to the experiment into the arena. We allowed each animal to explore the arena for 15 min, both while tethered and untethered, to control for effects of the tethering wire. There was a main effect of quadrant (F(3,27) =6.79, p = 0.001), main effect of tether (F(1,9) =8.18, p = 0.019), and no interaction between tether and quadrant (F(3,27) =1.622, p = 0.207). Two sample t-tests with unequal variances revealed significantly less time spent in quadrant 4 compared to the other quadrants in both the tethered (t(17.1) = 3.77, p = 0.002) and untethered groups (t(21.3) =7.23, p < 0.001) (figures 5(A) and (B)). This effect could have masked behavioral effects of the EP. We thus re-ran the main experiment’s ANOVA excluding all sessions where quadrant 4 was targeted for stimulation.
Figure 5.
Quadrant 4 aversion. (A) A subset of animals, new to the CPP field, spent less time in quadrant 4 compared to other quadrants in the absence of any stimulation regardless of tether, *p < 0.05. (B) Heatmaps illustrate the amount of time spent in other quadrants compared to the quadrant 4 (outlined in orange) with more time spent displayed as colors increasing in intensity from black to yellow. Error bars represent SEM.
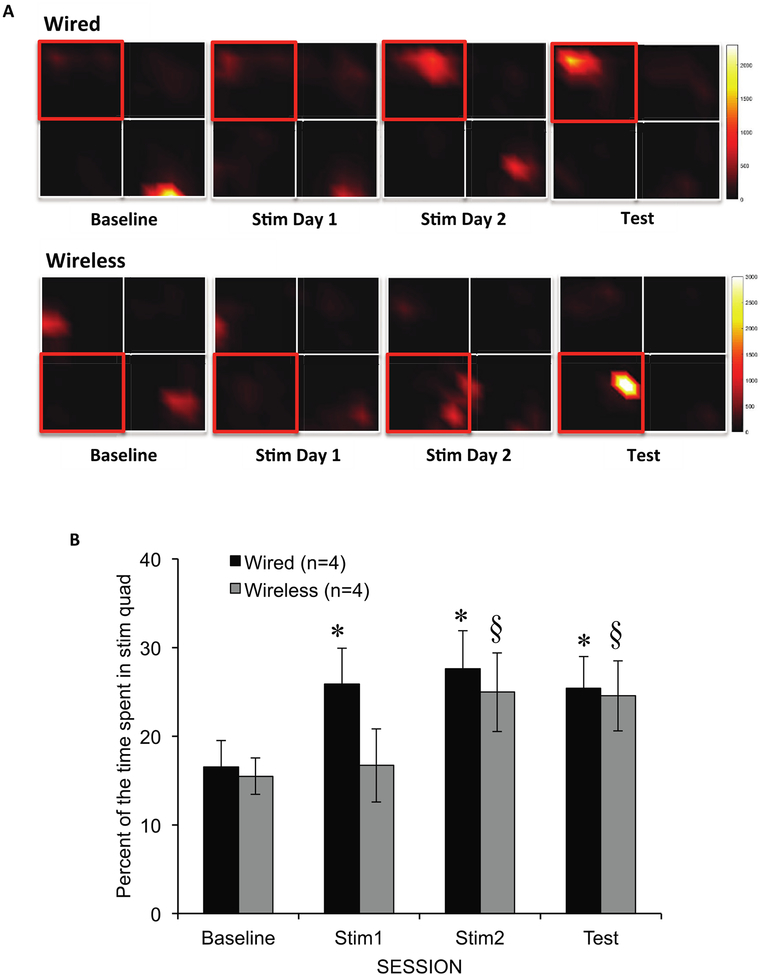
With the elimination of quadrant 4, there was a main effect of session (repeated measures ANOVA, F(3,129) =5.81, p = 0.001), no interaction between session and stimulation type (F(6,129) =1.13, p = 0.339), and no main effect of stimulation type (F(1, 43) =0.642, p = 0.427) (figure 6). The wired (Plastics One) group showed strong place preference conditioning, with significantly more time spent in the stimulation quadrant during stimulation session 1 (t(23) =−3.68, p = 0.001), stimulation session 2 (t(23) =–2.52, p = 0.019), and the test session (t(23) =−2.32, p = 0.029) compared to the baseline session. Animals in the wireless (EP) group spent more time in the stimulation quadrant during stimulation session 2 (t(20) =−2.47, p = 0.023] and the test session (t(20) =−2.56, p = 0.019), but not during stimulation session 1 (t(20) =−0.59, p = 0.564). There were no significant differences between the two stimulation types in any session (p > 0.05). These results indicate that the EP stimulated rats were achieved CPP as a result of MFB stimulation, although preference required more total exposures to stimulation than with wired stimulation (figure 6).
Figure 6.
CPP behavioral results. (A) Heatmaps demonstrate the formation of a CPP across sessions with time spent in the stimulation quadrant increasing as colors go from black to yellow. Quadrants outlined in red indicate the stimulation quadrant. (B) This bar graph illustrates the percentage of time the animals spent within the stimulation quadrant per session as a result of wired and wireless (EP) stimulation. With wired MFB stimulation, the rats preferred the stimulation quadrant (increased time spent in stimulation quadrant) during the first day of stimulation, and this preference remained during the test session. EP stimulation induced conditioned preference behavior by the second day of stimulation, which was also maintained during the test session, *p < 0.05, compared to baseline; §p < 0.05, compared to baseline. Error bars represent SEM.
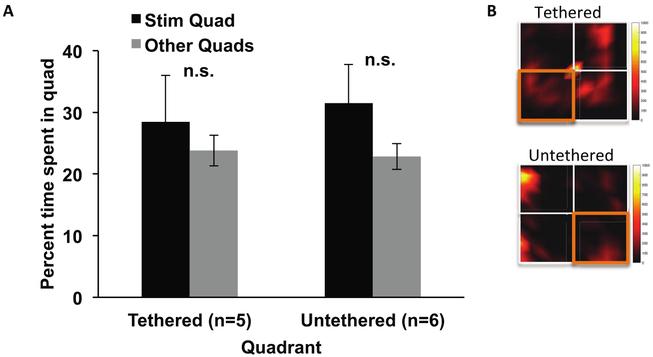
We considered the possibility that rats were able to detect heat emission from the EP transmitter coil. The supplemental warmth might be rewarding for the animal regardless of whether it received effective MFB stimulation. To test this possibility, a group of animals with Plastics One electrodes (and without EP implants) and naïve to the experiment were placed into the open field and were allowed to freely roam while the EP transmission coil was maintained continuously active in one designated stimulation quadrant. Animals underwent one 15 min session of exploration while tethered and another session untethered to account for any possible effects of the connecting wire. There was no difference in the time spent in the ‘stimulation’ quadrant and the other ‘unstimulated’ quadrants regardless of tether (tethered: t(4) =0.553, p = 0.61; untethered: t(5) = 0.727, p = 0.50), indicating that heat emission from the transmitter coil was not a factor in our results (figures 7(A) and (B)). It is important to note that in addition to checking for heat emission effects, we also considered any direct effects of transient changes in the magnetic field on the rodent that was independent of the EP. Because the field levels were tailored to be below the specific absorption rate (SAR) limit, any induced electric fields would be far below the perceptible level, for example, by inducing stimulation directly in somatosensory neurons or muscles of the rodent.
Figure 7.
Coil warmth tests. (A) To assess whether animals were attracted to heat potentially generated by the coil attached to a corner of the open field, a subset of animals without an implanted EP were placed into the open field with the trigger for the coil turned on as long as the rat remained in the selected stimulating quadrant. There was no difference between time spent in the stimulation quadrant compared to the non-stimulation quadrants. n.s. =not significant, p > 0.05. (B) Heatmaps illustrate the time spent in the quadrants and demonstrate that rats did not increase time spent in the stimulation quadrant. Quadrants outlined in orange indicate the stimulation quadrant. Error bars represent SEM.
Stimulation-induced activity
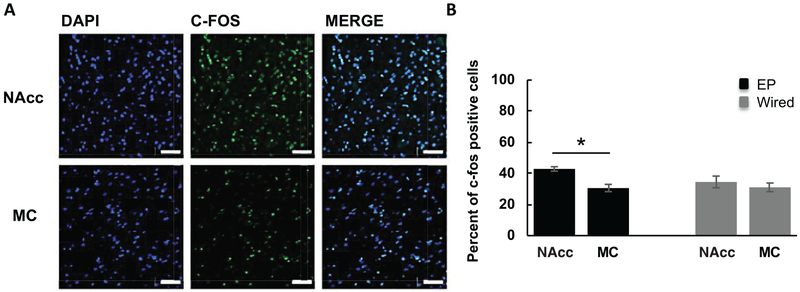
We also measured c-fos, a marker of recent neuronal activity, in regions of the brain that are known projection sites of the MFB, such as the nucleus accumbens (NAcc). We included the motor cortex, which does not have projections from the MFB, to demonstrate that neuronal activation was MFB-specific. Wired and EP stimulation showed comparable amounts of c-fos expression in the NAcc. However, there was greater c-fos activation within the NAcc compared to the MC (42.9% ± 1.4% versus 30.6% ± 2.4%; p < 0.05) on the side of the EP implant, suggesting that the medial forebrain bundle and its projections were successfully activated by the EP (figure 8). NAcc c-fos expression was also higher than MC expression (35.8% ± 3.8% versus 31.1% ± 2.8%; p > 0.05) with wired stimulation; however, this difference did not reach statistical significance. We note that given the small sample size (n = 2), both analyses are underpowered and should be interpreted accordingly.
Figure 8.
C-fos induced by EP and wired stimulation. (A) Representative confocal images (40×) of DAPI, c-fos, and merged DAPI and c-fos-stained images within the NAcc and MC in the left hemisphere (EP side) only; scale bar = 50 μm. (B) EP stimulation produced significantly more c-fos positive cells (shown here as a percentage of DAPI-stained cells) in the nucleus accumbens (NAcc), a brain region innervated by the MFB, compared to the motor cortex (MC), which does not have direct connections within the MFB. There was a slight increase in NAcc c-fos expression compared to MC with wired stimulation, but it was not statistically significant (n = 2, both animals received EP and wired stimulation). *p < 0.05. Error bars represent SEM.
Discussion
The e-Particle
The current study aimed to verify the efficacy of a novel, sub-millimeter wireless neurostimulator, the e-Particle (Freeman et al 2017), to (1) excite a neural pathway and its projection targets and thus (2) elicit behavior that depends on that pathway. We specifically sought to show that the EP could mimic the efficacy of standard neurostimulation methods, a necessary prerequisite for use in scientific or clinical applications. Here, we assessed whether MFB stimulation via EP could successfully condition place preference. We observed increased preference for the stimulation quadrant during both EP and wired stimulation. The EP stimulation was slightly slower to achieve CPP compared to wired stimulation, requiring a second stimulation day, but ultimately did not differ from wired stimulation in the degree of place preference. C-fos expression in the MFB-innervated region, nucleus accumbens, was significantly greater than c-fos expression in the motor cortex, as previously observed with MFB stimulation (Chergui et al 1996). Due to the electromagnetic interference that would occur between the transmitter and any electrophysiology amplifier, we measured c-fos activity within these brain areas in place of electrophysiological recordings. The lack of statistical significance between NAcc and MC c-fos expression with wired stimulation may have been due to being too underpowered for this analysis; small sample size due to EP availability is a limitation in this study. Despite these limitations, the pattern of c-fos expression we observed confirmed that the EP is capable of activating deep brain structures to a sufficient degree to shape behavior.
Potential clinical and research applications of EP
The EP wireless neurostimulator may improve how we study DBS in animal models, which can ultimately lead to a better understanding of the neurobiological mechanisms underlying the success (or failure) of clinical treatments. Current preclinical models require electrodes to be connected to stimulating wires. These tethers restrict natural movement, thereby limiting the types of behavioral assessments that could be conducted to investigate the effects of neurostimulation. For instance, it is difficult to conduct experiments where animals must enter confined spaces or where multiple animals interact socially. They also require head caps that are bulky and can fail, again leading to both change in animals’ natural behavior and the potential for significant loss of data. Removing the need to attach connecting leads could reduce the amount of tissue damage that occurs following implantation, as well as allowing animal subjects to behave more naturally, i.e. more like human patients with DBS implants.
EP limitations
One critical difference between standard electrodes and the EP is the limited range of stimulation parameters afforded by the design of the EP. Traditional clinical and preclinical stimulators, such as the isolator and wired electrode used in the current study, deliver biphasic constant current pulses. This is also true for human DBS applications. However, the EP can only produce monophasic pulses at an amplitude much lower than the output of the wired system. Moreover, DBS stimulation in humans is typically administered at high frequencies (e.g. 130 Hz). EP stimulation at only 50 Hz may not be sufficient for effective clinical application. While we realize this may be a setback in the EP’s design, the inability to control these parameters was the compromise that was made in order to make the EP small enough to implant deep inside the brain. Other more controllable devices are too large to be used in this way. Despite these parameter limitations, the EP demonstrated the ability to produce behavioral effects even with the small current levels generated (tens of microamperes). While methods of measuring the exact current output of the implantable device are currently being explored, one possible idea to address the EP’s stimulation intensity is to create a fully implantable transmitter coil that could increase the signal compared to an externally placed coil. For patients, this design would provide the advantage of eliminating having to apply a transmitter coil for every administration of DBS. However, that transmitter coil would lose a certain portion of its applied energy as heat, potentially creating tissue damage risk that exceeds implantable safety thresholds. To avoid this, the amplitude of the applied fields would have to be limited, a restriction which might actually reduce field levels at the site of the implant. Although the potential advantages and disadvantages of this idea require further exploration, a closer match to DBS-like stimulation should be possible with the addition of active components. On the other hand, we stress that these directly counteract the EP’s advantages as a passive device.
Conclusion
Implantable neurostimulators have shown great promise in the treatment of neurological/psychiatric disorders (Mayberg et al 2005, Lo and Widge 2017). However, they also suffer from limitations, particularly related to limited battery life and the fragility of interconnecting components. Existing wireless and non-invasive technologies are typically either limited to activating the superficial cortex (Camprodon et al 2016) or lack temporal precision (Fini and Tyler 2017). Recent work by Grossman et al (2017) demonstrated a new approach to noninvasive stimulation of neurons lying deep within the brain, by manipulating temporal interference between two applied electrical fields. However, the EP and similarly implanted transducer technologies provide notable advantages over the temporal interference approach. Transducers ensure that energy is more precisely applied to its target and may offer more controllability. Here we demonstrated that the EP can not only stimulate a specific target (MFB), but also that this method of stimulation can effectively modulate behavior. An additional and important advantage of the EP is that it can also be used to study social behaviors with multiple animals together. It is difficult to conduct studies like these with the connecting wires and bulky headstages because the animals become distracted by them or damage them instead, making it almost impossible to assess the behavior of interest. Although further validation and logistical evaluation is necessary before assessing the EP’s clinical applications, this study’s findings indicate that the EP could offer some unique advantages for animal neurostimulation studies.
Acknowledgments
This research was supported by funding from Draper Laboratory to D K Freeman and A S Widge, The Brain and Behavior Research Foundation, and the Harvard Brain Science Initiative Bipolar Disorder Seed Grant Program supported by Kent and Liz Dauten. Gregory R Wojtkiewicz of the Center for Systems Biology at MGH provided images produced by CT-MRI co-registration. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
The computer code used to run behavioral experiments and analyze data may be found at https://github.com/tne-lab after publication.
References
- Anderson D, Beecher G and Ba F 2017 Deep brain stimulation in Parkinson’s disease: new and emerging targets for refractory motor and nonmotor symptoms Parkinson’s Dis 2017. 5124328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros VN, Mundim M, Galindo LT, Bittencourt S, Porcionatto M and Mello LE 2015. The pattern of c-Fos expression and its refractory period in the brain of rats and monkeys Frontiers Cell. Neurosci 9 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Louveau A, Henry S and de Rougemont J 1987. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease Appl. Neurophysiol 50 344–6 [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Gippert SM, Switala C, Coenen VA and Schlaepfer TE 2017. Deep brain stimulation to the medial forebrain bundle for depression-long-term outcomes and a novel data analysis strategy Brain Stimul 10 664–71 [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Rauch SL, Greenberg BD and Dougherty DD 2016. Psychiatric Neurotherapeutics: Contemporary Surgical and Device-Based Treatments (New York, NY: Humana Press; ) [Google Scholar]
- Carlezon WA and Chartoff EH 2007. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation Nat. Protocols 2 2987–95 [DOI] [PubMed] [Google Scholar]
- Chen R, Romero G, Christiansen MG, Mohr A and Anikeeva P 2015. Wireless magnetothermal deep brain stimulation Science 347 1477–80 [DOI] [PubMed] [Google Scholar]
- Chergui K, Nomikos GG, Mathé JM, Gonon F and Svensson TH 1996. Burst stimulation of the medial forebrain bundle selectively increases Fos-like immunoreactivity in the limbic forebrain of the rat Neuroscience 72 141–56 [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H and Watson SJ 1995. Pattern and time course of immediate early gene expression in rat brain following acute stress Neuroscience 64 477–505 [DOI] [PubMed] [Google Scholar]
- Cury RG et al. 2017. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia Neurology 89 1416–23 [DOI] [PubMed] [Google Scholar]
- Dougherty DD and Widge AS 2017. Neurotherapeutic interventions for psychiatric illness Harv. Rev. Psychiatry 25 253–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoy AJ and Simpson RK 2014. Risks of common complications in deep brain stimulation surgery: management and avoidance J. Neurosurg 120 132–9 [DOI] [PubMed] [Google Scholar]
- Fini M and Tyler WJ 2017. Transcranial focused ultrasound: a new tool for non-invasive neuromodulation Int. Rev. Psychiatry 29 168–77 [DOI] [PubMed] [Google Scholar]
- Freeman DK. et al. A sub-millimeter, inductively powered neural stimulator. Frontiers Neurosci. 2017;11:659. doi: 10.3389/fnins.2017.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graat I, Figee M and Denys D 2017. The application of deep brain stimulation in the treatment of psychiatric disorders Int. Rev. Psychiatry 29 178–90 [DOI] [PubMed] [Google Scholar]
- Grill WM, Norman SE and Bellamkonda RV 2009. Implanted neural interfaces: biochallenges and engineered solutions Annu. Rev. Biomed. Eng 11 1–24 [DOI] [PubMed] [Google Scholar]
- Grossman N et al. 2017. Noninvasive deep brain stimulation via temporally interfering electric fields Cell 169 1029–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa A et al. 2018. The microbead: a highly miniaturized wirelessly powered implantable neural stimulating system IEEE Trans. Biomed. Circuits Syst 12 521–31 [DOI] [PubMed] [Google Scholar]
- Kim TI et al. 2013. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics Science 340 211–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Gaebel W, Klosterkoetter J and Woopen C 2009. Deep brain stimulation as a new therapeutic approach in therapy-resistant mental disorders: ethical aspects of investigational treatment Eur. Arch. Psychiatry Clin. Neurosci 259 135. [DOI] [PubMed] [Google Scholar]
- Li X, Serdijn WA, Zheng W, Tian Y and Zhang B 2015. The injectable neurostimulator: an emerging therapeutic device Trends Biotechnol 33 388–94 [DOI] [PubMed] [Google Scholar]
- Lo M-C and Widge AS 2017. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness Int. Rev. Psychiatry 29 191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddenkemper T et al. 2001. Deep brain stimulation in epilepsy J. Clin. Neurophysiol 18 514–32 [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM and Kennedy SH 2005. Deep brain stimulation for treatment-resistant depression Neuron 45 651–60 [DOI] [PubMed] [Google Scholar]
- McMurray MS, Conway SM and Roitman JD 2017. Brain stimulation reward supports more consistent and accurate rodent decision-making than food reward eNeuro 4 ENEURO.0015-7.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meoni S et al. 2017. Pallidal deep brain stimulation for dystonia: a long term study J. Neurol. Neurosurg. Psychiatry 88 960–7 [DOI] [PubMed] [Google Scholar]
- Montgomery KL et al. 2015. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice Nat. Methods 12 969–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro JM, Lyons KE and Pahwa R 2013. Deep brain stimulation for essential tremor Handbook Clin. Neurol 116 155–66 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Geeraedts LMG and Veening JG 1982. The medial forebrain bundle of the rat. I. General introduction J. Comp. Neurol 206 49–81 [DOI] [PubMed] [Google Scholar]
- Paxinos G and Watson C 2007. The Rat Brain in Stereotaxic Coordinates 6th edn (Cambridge, MA: Academic; ) [Google Scholar]
- Schulman JH 2008. The feasible FES system: battery powered BION stimulator Proc. IEEE 96 1226–39 [Google Scholar]
- Sliwinska MW, Vitello S and Devlin JT 2014. Transcranial magnetic stimulation for investigating causal brain-behavioral relationships and their time course J. Vis. Exp 89 51735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Rushmore J, Eden U and Cabre A-V 2009. Biophysical foundations underlying TMS: setting the stage for an effective use of neurostimulation in the cognitive neurosciences Cortex 45 1025–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS and Moritz CT 2014. Pre-frontal control of closed-loop limbic neurostimulation by rodents using a brain-computer interface J. Neural Eng 11 024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Malone DAJ and Dougherty DD 2018. Closing the loop on deep brain stimulation for treatment-resistant depression Frontiers Neurosci 12 175. [DOI] [PMC free article] [PubMed] [Google Scholar]