Abstract
Traumatized women are more likely than traumatized men to develop post-traumatic stress disorder (PTSD). Still, the inclusion of females in animal models of PTSD has largely been avoided, likely due to the variable hormone profile of female rodents. Because a valid animal model of PTSD that incorporates females is still needed, we examined the influence of estrous stage and ovarian hormones on the female rat response to a predator-based psychosocial stress model of PTSD. Female Sprague-Dawley rats were exposed to psychosocial stress or control conditions for 31 days. Stressed rats were given two cat exposures and daily social instability; control rats were handled daily. Beginning on Day 32, rats underwent physiological or behavioral testing. In Experiment 1, vaginal smears were collected on days of the first and second cat exposures and each day of behavioral testing to determine estrous stage. In Experiments 2 and 3, ovariectomized or sham control rats were exposed to stress or control conditions. Then, they were given behavioral testing (Exp 2), or their hearts were isolated and subjected to ischemia/reperfusion on a Langendorff isolated heart system (Exp 3). Chronic stress increased anxiety-like behavior, irrespective of estrous stage or ovariectomy condition. Ovariectomized females displayed greater startle responses and anxiety-like behavior than sham rats. Stress had no impact on myocardial sensitivity to ischemic injury; however, ovariectomized females exhibited greater ischemia-induced infarction than sham rats. These findings suggest that ovarian hormones may prevent anxiety-like behavior and be cardioprotective in non-stressed controls, but they do not interact with chronic stress to influence the development of PTSD-like sequelae in female rats.
Keywords: stress, animal model, PTSD, ovariectomy, sex differences, estrous
INTRODUCTION
Post-traumatic stress disorder (PTSD) is a psychiatric condition that results following exposure to a traumatic event, such as sexual assault, combat, or a motor vehicle accident. Individuals who develop PTSD experience intrusive memories of the trauma, heightened anxiety, hyperarousal, cognitive impairments, and numerous deleterious physiological symptoms, including increased risk of cardiovascular disease (Zoladz and Diamond, 2013). Not all traumatized individuals develop PTSD, suggesting that certain susceptibility factors increase one’s likelihood of developing the disorder. One susceptibility factor for PTSD is biological sex. Although women have lower trauma exposure rates than men (Breslau et al., 1991; Breslau et al., 1997; Kessler et al., 1995), they are much more likely than men to develop PTSD when they do experience trauma (Tolin and Foa, 2006). One explanation for this sex difference is that women are more likely to experience interpersonal violence (e.g., rape), which is associated with remarkably high rates of PTSD (Breslau et al., 1997; Kessler et al., 1995; Perkonigg et al., 2000). Nevertheless, investigators have shown that traumatized women are more likely to develop PTSD than traumatized men even when controlling for sex differences in the type of trauma experienced (Tolin and Foa, 2006). Over the past couple of decades, advances in research have helped us understand why women are more susceptible to PTSD. For instance, investigators have emphasized the role of circulating sex hormones, such as estradiol and progesterone, in conferring increased susceptibility to PTSD in women (Ramikie and Ressler, 2018). However, our understanding of the neurobiological mechanisms that underlie sex differences in PTSD susceptibility is extremely limited, partly due to the lack of female subjects in PTSD-related preclinical research.
Animal models are vital to our understanding of psychiatric conditions. These models enable investigators to examine underlying physiological mechanisms of disorder-related behaviors and allow for pre-post designs that are practically impossible to perform in humans with psychiatric illnesses. Such models are also crucial for drug development, as they permit investigators to test the efficacy of novel experimental treatments. Perhaps most importantly, animal models allow investigators to perform such manipulations in a well-controlled environment. Although preclinical researchers have examined the impact of stress on physiology and behavior in non-human animals for many decades, the use of females in such work, especially work involving animal models of PTSD, has largely been avoided (Cohen and Yehuda, 2011; Richter-Levin et al., in press). A major reason for this avoidance might be due to the very short duration (4–5 days) of their estrous cycle, which has made it difficult for preclinical investigators to control for the influence of sex hormones on physiological and behavioral endpoints believed to be influenced by stress-related experimental manipulations.
Preclinical work that has examined the effects of animal models of PTSD on female physiology and behavior has produced equivocal results. Some studies have suggested that females are less susceptible than males to exhibiting PTSD-like symptoms following exposure to intense stress (Diehl et al., 2007; Keller et al., 2015; Koresh et al., 2016), while others have suggested that males and females respond similarly to such stress (Mazor et al., 2009; Viviani et al., 2012). For instance, the single prolonged stress paradigm, a well-validated animal model of PTSD, produces fear extinction retention deficits in males, but not females, suggesting that females may be less susceptible to the effects of this procedure (Keller et al., 2015). On the other hand, work from Hagit Cohen’s group has indicated that males and females respond to intense stress similarly. In one study, Cohen and colleagues reported that 3/19 (16%) female rats exposed to predator scent stress developed extreme behavioral responses (e.g., heightened anxiety, exaggerated startle response), compared to 6/15 (40%) male rats (Koresh et al., 2016); however, this difference was not statistically significant. In a separate study with greater statistical power, the same investigators reported nearly identical rates of extreme behavioral responses in male and female rats (14% and 12%, respectively) (Mazor et al., 2009). A consistent finding that has come out of the Hagit Cohen laboratory is that estrous phase does not appear to interact with intense stress to influence behavioral outcomes (Koresh et al., 2016; Mazor et al., 2009). Because some investigators have even reported that females are more susceptible than males to the effects of stress on behavior (e.g., McCormick et al., 2005; McCormick et al., 2008), the impact that stress has on female behavior, relative to that of males, likely depends on the type of stress and behavioral endpoints utilized in the specific study. Moreover, because male and female rodents exhibit baseline differences on many behavioral tests (e.g., Blizard et al., 1975; Johnston and File, 1991), it is difficult to make direct comparisons of susceptibility to stress-induced alterations of behavior in males and females.
Our laboratory uses a predator-based psychosocial stress model of PTSD that produces multiple physiological and behavioral changes in rats that are remarkably similar to those observed in people with PTSD. The model consists of a 31-day paradigm, during which rats are exposed to a predator (adult, female cat) on two occasions (separated by a period of 10 days) and experience chronic social instability by having their cage mates changed daily (Zoladz et al., 2008). The rats are given two cat exposures to mimic the re-experiencing symptoms that characterize PTSD, and the daily social instability provides a chronic mild stressor that is meant to mimic the inconsistent social environment experienced by PTSD patients. Male rats exposed to this model exhibit a powerful memory of the cat exposures, heightened anxiety on the elevated plus maze, an exaggerated startle response, cognitive impairments, elevated heart rate and blood pressure, increased myocardial sensitivity to ischemic injury, low baseline levels of corticosterone, enhanced negative feedback of the hypothalamus-pituitary-adrenal (HPA) axis, elevated levels of norepinephrine, reduced levels of serotonin, increased measures of inflammation and oxidative stress, and elevated methylation of hippocampal Bdnf DNA (Rorabaugh et al., 2015; Roth et al., 2011; Wilson et al., 2014a; Wilson et al., 2014b; Wilson et al., 2014c; Wilson et al., 2013; Zoladz et al., 2008; Zoladz et al., 2012, 2013; Zoladz et al., 2015).
Almost all of the previous work with this predator-based psychosocial stress model of PTSD has been conducted in males. In the only study that involved female rats, we found that stressed males, but not stressed females, exhibited heightened anxiety on the elevated plus maze and increased myocardial sensitivity to ischemic injury (Rorabaugh et al., 2015). However, we did not control for the stage of estrous in female rats in this study, which could interact with stress exposure to affect behavioral outcomes in this model. Therefore, in the present study, we examined the influence of estrous stage at the time of each predator exposure and at the time of behavioral testing on the responses of female rats to the predator-based psychosocial stress model of PTSD. We also assessed the influence of ovariectomy on the behavioral responses of female rats to the predator-based psychosocial stress model of PTSD, as well as their sensitivity to myocardial ischemic injury. We hypothesized that specific stages of estrous, as well as ovariectomy, might be associated with greater susceptibility to adverse physiological and behavioral sequelae following intense stress.
MATERIAL AND METHODS
Animals
Experimentally naïve adult female Sprague-Dawley rats from an established breeding colony at Ohio Northern University were used for all experiments. All rats were weaned on postnatal day 21 and housed 4 per cage until one week prior to the onset of stress (described below), at which point they were pair-housed. The rats were housed in standard Plexiglas cages on a 12-h light/dark schedule (lights on at 0700) with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at Ohio Northern University and followed the procedures recommended by the Guide for the Care and Use of Laboratory Animals provided by the National Institutes of Health.
Stress procedure
All rats were at least 8 weeks of age by the onset of the stress procedure. The stress procedure has been described at length in previous publications (Rorabaugh et al., 2015; Roth et al., 2011; Zoladz et al., 2008; Zoladz et al., 2012, 2013; Zoladz et al., 2015). Briefly, rats were assigned to “stress” or “no stress” groups. On Day 1, rats in the stress groups were immobilized in plastic DecapiCones (Braintree Scientific; Braintree, MA) and placed in a perforated wedgeshaped Plexiglas enclosure (Braintree Scientific; Braintree, MA; 20 × 20 × 8 cm). This enclosure was then taken to a cat housing room and placed in a metal cage (61 × 53 × 51 cm) with an adult female cat for 1 h. The Plexiglas enclosure prevented any contact between the cat and rats, but the rats were still exposed to all non-tactile sensory stimuli associated with the cat. Canned cat food was smeared on top of the enclosure to direct the cat’s attention toward the rats. An hour later, the rats were returned to their home cages. Rats in the no stress groups remained in their home cages during the 1-h stress period. Rats in the stress groups were given two stress sessions, which were separated by 10 days. The first stress session took place during the light cycle (between 0800 and 1200), and the second stress session took place during the dark cycle (between 1900 and 2100). The stress sessions took place during different times of the day to add an element of unpredictability as to when the rats might re-experience the predator exposure, as per our previously-employed methodology.
Beginning on the day of the first stress session (Day 1), rats in the stress groups were exposed to unstable housing conditions for 31 days. Rats in the stress groups were housed two per cage, but every day, their cohort pair combinations were changed. Rats in the no stress groups were housed with the same cohort pair throughout the experiment; these rats were handled daily to control for handling effects on stressed animals.
Body weights were measured on the days of the first stress session, the second stress session, and the first day of behavioral testing. Growth rates during the stress paradigm [g/day = (weight during behavioral testing – weight during the first stress session) / 31] were calculated for statistical analyses.
Behavioral testing
One day after the 31-day stress paradigm, all rats began three consecutive days of behavioral testing, unless otherwise noted below.
Elevated plus maze (EPM)
On Day 32, rats were tested on the EPM to assess anxiety-like behavior. A red light mounted above the EPM was used to provide low ambient lighting during testing. The rats were placed on the EPM for 5 min, and their behavior was videotaped with a JVC hard disk camera hanging above the EPM and scored offline by two separate investigators who were blind to the experimental conditions of the animals. The investigators scored the amount of time rats spent in the open arms of the EPM, as well as the number of entries rats made into the closed arms (a measure of overall locomotor activity).
Startle response
On Day 33, rats’ acoustic startle responses were measured via soundproof startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA) to measure rats’ acoustic startle responses. The rats were placed in Plexiglas cylinders within the startle chambers, and rat movement in response to the startle probes was detected by a piezoelectric accelerometer. The SR-LAB calibration unit was routinely used to ensure consistent startle response quantification. We utilized a startle response method that has been employed by Cohen and colleagues on numerous occasions (e.g., Cohen et al., 2011; Cohen et al., 2010; Cohen et al., 2006; Cohen et al., 2012; Manjoch et al., 2016). Each test session began with a 5-min acclimation period to background noise of 68 dB, followed by 30 acoustic startle probes presented in six trial blocks (each trial block included five presentations of 40-ms, 110 dB white noise bursts with 30–45 s inter-stimulus intervals). We quantified rats’ mean startle responses across all 30 trials.
Open field
On Day 34, the rats were tested in an open field to assess anxiety-like behavior and general locomotor activity. The open field (61 cm × 61 cm × 61 cm) was made of four Plexiglas sides and a gridded Plexiglas bottom, consisting of 16 grids (each grid was 15.25 cm × 15.25 cm). A red light mounted above the open field was used to provide low ambient lighting during testing. The rats were placed in the open field for 5 min, and their behavior was videotaped with a JVC hard disk camera hanging above the open field and scored offline by two separate investigators who were blind to the experimental conditions of the animals. The investigators scored the amount of time rats spent in the center of the open field (4 innermost grids), the amount of time rats spent in the periphery of the open field, the number of grid crossings, and the number of rearing episodes.
Experiments
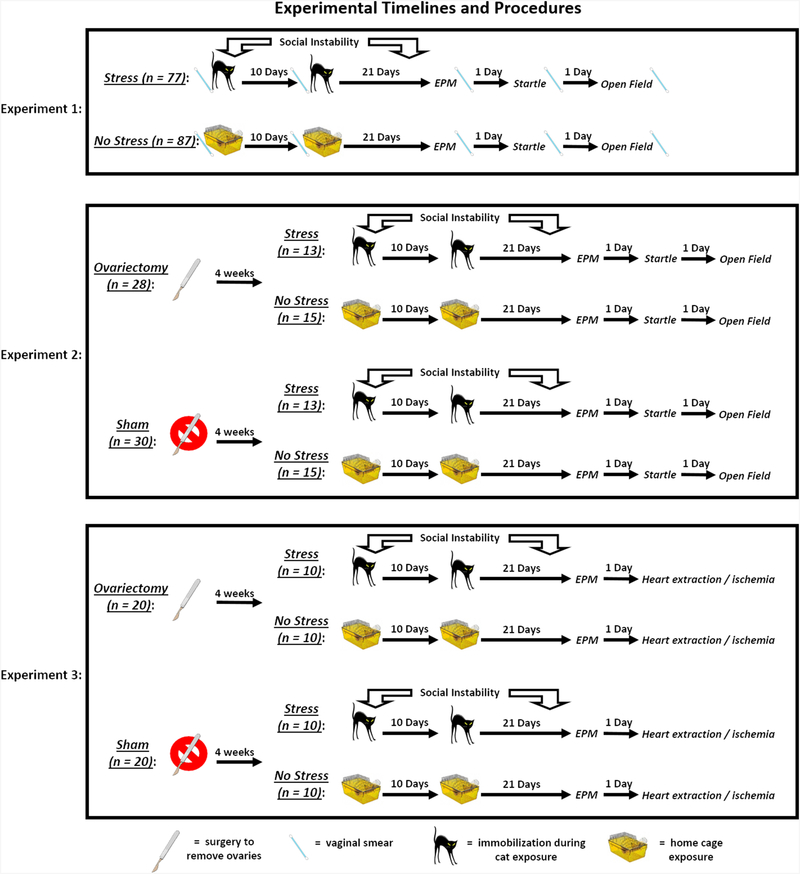
The timelines for each experiment described below can be found in Fig. 1.
Fig. 1.
Experimental timelines and procedures. In Experiment 1, rats in the stress group were exposed to a 31-day chronic stress paradigm that consisted of two cat exposures and daily social instability. Stressed rats were immobilized and exposed to an adult, female cat for 1 h on Days 1 (during the light cycle) and 11 (during the dark cycle), and throughout the 31-day paradigm, the cage mates of stressed rats were randomly changed on a daily basis. Rats in the no stress group remained in their home cages on Days 1 and 11 and remained with the same cage mate throughout the paradigm. On Day 32, all rats were tested for anxiety-like behavior on the elevated plus maze (EPM); on Day 33, all rats were tested for their acoustic startle responses; and, on Day 34, all rats were tested for anxiety-like behavior in an open field. Vaginal smears were collected from all rats on Days 1, 11, 32, 33, and 34 to determine the stage of estrous. In Experiment 2, rats underwent ovariectomy or sham surgery at 4 weeks of age, after which they were randomly assigned to stress or no stress groups. Four weeks after surgery, all rats underwent the same procedures as outlined in Experiment 1, except no vaginal smears were collected. In Experiment 3, rats underwent ovariectomy or sham surgery at 4 weeks of age, after which they were randomly assigned to stress or no stress groups. Four weeks after surgery, all rats underwent the same stress procedures as outlined in Experiment 2. On Day 32, all rats were tested for anxiety-like behavior on the EPM. On Day 33, all rats were anesthetized, and their hearts were removed and exposed to ischemia / reperfusion, as outlined in the methods.
Experiment 1 – Influence of estrous stage on behavioral responses to psychosocial stress
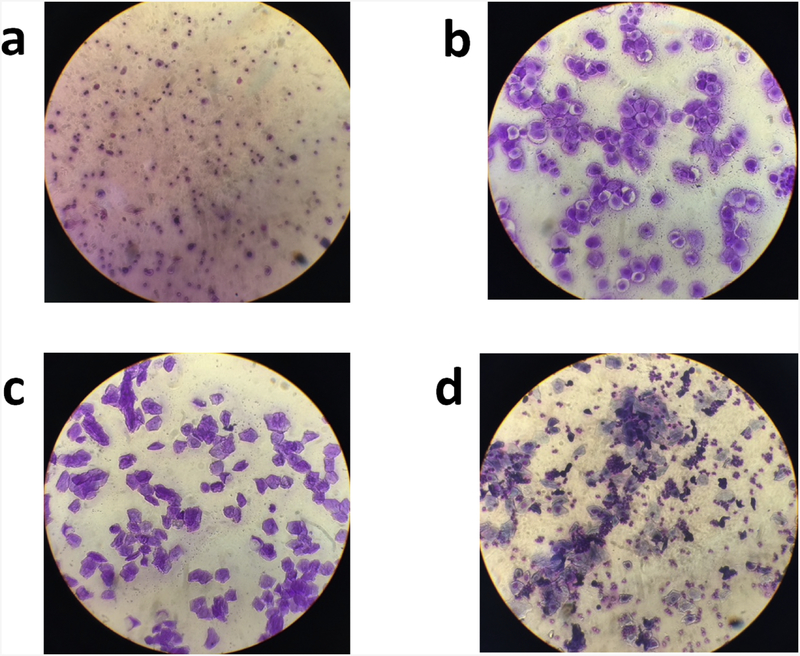
Experiment 1 was designed to examine whether estrous stage interacted with psychosocial stress to influence rat behavior. Female rats were exposed to the stress procedures outlined above (stress: N = 77; no stress: N = 87), and we determined their estrous stage at different points throughout the paradigm. Vaginal cells were collected from the rats on days of the first stress session, the second stress session, and each day of behavioral testing. The collected samples were stained and examined under a microscope to determine the stage of estrous. Representative slide images for each stage of estrous are presented in Fig. 2.
Fig. 2.
Representative images from each stage of estrous. A sterile micropipette was used to flush the vaginal cavity of female rats with distilled water (McLean et al., 2012). The collected sample was placed on a glass slide and allowed to dry at room temperature. The slides were then stained with crystal violet and examined under a microscope to determine the stage of estrous. The following guidelines were used to determine the stage of estrous: (a) diestrus (many leukocytes, occasional nucleated epithelial cells, no cornified cells); (b) proestrus (many nucleated epithelial cells, occasional cornified cells, and few leukocytes); (c) estrus (many cornified cells, few nucleated epithelial cells, no leukocytes); (d) metestrus (some cornified cells, nucleated epithelial cells, and leukocytes).
Experiment 2 – Influence of ovariectomy on behavioral responses to psychosocial stress
Experiment 2 was designed to examine whether ovariectomy interacted with psychosocial stress to influence rat behavior. Ovariectomized females underwent bilateral ovariectomy at 4 weeks of age under isoflurane anesthesia. Oviducts were cauterized through a 1-cm dorsal incision over each ovary, and the ovaries were removed with a pair of scissors. The incisions were closed with wound clips which were removed 10 days later. Control animals underwent a sham operation in which dorsal incisions were made over each ovary without ligation or removal of the ovary. Following surgery, ovariectomized and sham animals were left in their home cages to recover until being assigned to stress (ovariectomy: N = 13; sham: N = 15) or no stress (ovariectomy: N = 15; sham: N = 15) conditions at approximately 8 weeks of age.
Experiment 3 – Influence of ovariectomy on myocardial responses to psychosocial stress
Experiment 3 was designed to examine whether ovariectomy interacted with psychosocial stress to influence myocardial sensitivity to ischemic injury. Ovariectomy and sham operations, as well as the stress (ovariectomy: N = 10; sham: N = 10) or no stress (ovariectomy: N = 10; sham: N = 10) procedures, took place as described under Experiment 2. Similar to Experiments 1 and 2, all rats underwent EPM testing on Day 32; however, on Day 33, we assessed myocardial sensitivity to ischemic injury in a Langendorff isolated heart system. Rats were anesthetized with pentobarbital (75 mg/kg, i.p.), and their hearts were rapidly removed. Rat hearts were cannulated while bathed in ice-cold Krebs-Henseleit solution (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4, 0.5 mM Na2EDTA, 11 mM glucose, 2.5 mM CaCl2, pH 7.4). Krebs solution was perfused through the aortic cannula at a constant pressure of 80 mmHg. Contractile function of the left ventricle was measured using an intraventricular balloon connected to a pressure transducer. The balloon was inflated to an end diastolic pressure of 4–8 mmHg, and data were continually recorded using a Powerlab 4SP data acquisition system (AD Instruments, Colorado Springs, CO). The heart was submerged in Krebs solution to maintain the temperature of the heart at 37.5 ± 0.5°C throughout the experiment, and temperature was continuously monitored using a thermocouple placed on the surface of the heart. Hearts were equilibrated for 25 min prior to the onset of 20 min ischemia and 2 h of reperfusion. Hearts were excluded after the 25-min equilibration period if developed pressure was less than 100 mmHg, coronary flow rate was greater than 20 ml/min, or if there were persistent arrhythmias. Pre-ischemic contractile function was measured immediately prior to ischemia. Post-ischemic recovery of contractile function was measured following 1 h of reperfusion.
Following 20 min ischemia and 2 h reperfusion, hearts were perfused with 1% triphenyltetrazolium chloride for 8 min (at a rate of 7.5 ml/min) and then submerged in 1% triphenyltetrazolium chloride and incubated at 37°C for 15 min. Hearts were subsequently frozen at −80°C, sliced into approximately 1 mm sections, soaked in 10% neutral buffered formalin, and then photographed with a Nikon SMZ 800 microscope equipped with a Nikon DS-Fi1 digital camera. The infarcted surface area and total surface area of each slice was measured using NIH Image J software. Infarct size was expressed as a percentage of the area at risk (AAR). The AAR was defined as the entire ventricular myocardium since hearts were exposed to global ischemia. Uterine weights were measured at the completion of Experiments 2 and 3 to confirm the efficacy of each ovariectomy or sham operation.
Statistical analyses
In Experiment 1, growth rates were analyzed with an independent samples t-test, with stress serving as the between-subjects factor. Two-way ANOVAs were utilized to analyze EPM, startle, and open field behavior. For each behavioral assessment, we performed three separate ANOVAs. Each ANOVA included stress as one factor; the other factor was estrous phase during the first stress session, second stress session, or during the specific behavioral assessment. In Experiments 2 and 3, growth rates, all behavioral data, and infarct sizes were analyzed with two-way ANOVAs, with stress and ovariectomy serving as the between-subjects factors. For analyses of cardiac function, we used mixed-model ANOVAs, with stress and ovariectomy serving as the between-subjects factors and time point (pre-ischemia, post-ischemia) serving as the within-subjects factor. Alpha was set as 0.05 for all analyses, and Bonferroni-corrected post hoc tests were employed when the omnibus F test indicated the presence of a significant effect. Effect sizes were estimated by calculating partial eta squared (ηp2) for ANOVAs and Cohen’s d for pairwise comparisons.
RESULTS
Experiment 1
Growth rates
Stressed rats (0.44 ± 0.04 g/day) gained less weight during the 31-day stress paradigm than non-stressed rats (0.60 ± 0.06 g/day), t(164) = 2.14, p < 0.05, d = 0.33.
Elevated plus maze (EPM)
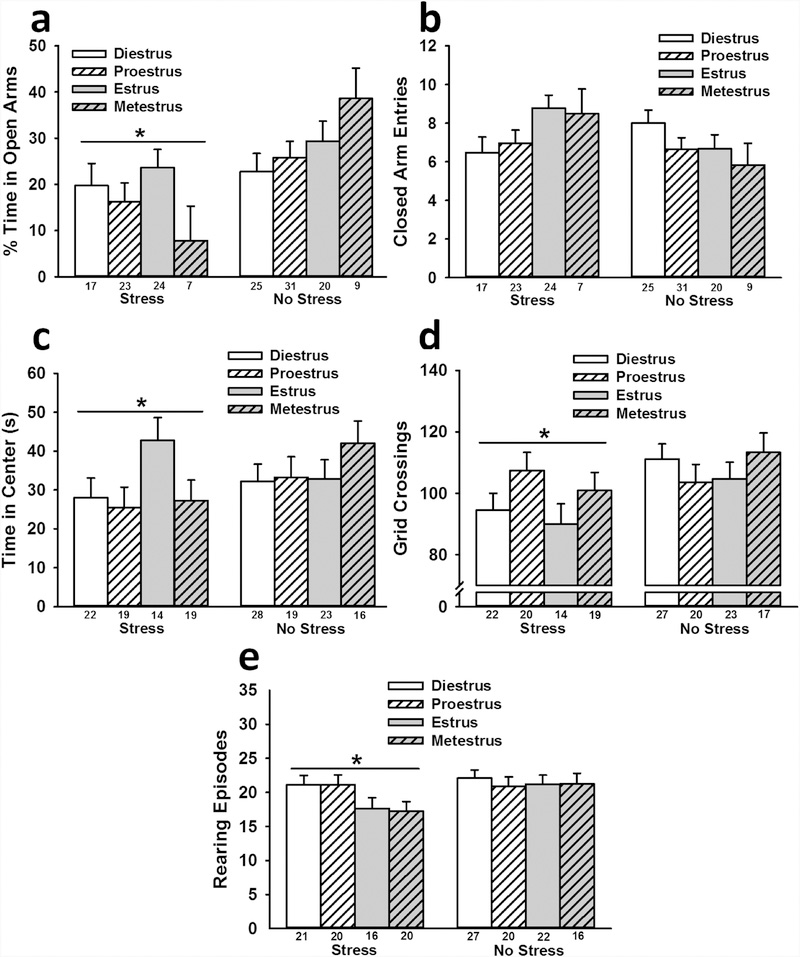
Stressed rats spent less time in the open arms of the EPM than non-stressed rats regardless of the time point of estrous phase [estrous phase during first stress session: F(1,146) = 10.93, ηp2 = 0.07; estrous phase during second stress session: F(1,139) = 11.60, ηp2 = 0.08; estrous phase during EPM testing: F(1,148) = 13.36, ηp2 = 0.08 (all p < 0.001); Fig. 3 a]. No significant effects were observed for closed arm entries, indicating that the observed effect of stress was not due to effects on locomotor activity (Fig. 3b). The phase of estrous at all three time points had no significant impact on open arm time, nor did it interact with stress to influence open arm time.
Fig. 3.
Interactive influence of stress and estrous phase (at the time of testing) on female rat behavior. Stressed rats spent less time in the open arms of the EPM than non-stressed rats (a), which was not attributable to stress-induced alterations of general locomotor activity on the EPM (b). Stressed rats also spent less time in the center of the open field (c), exhibited less locomotor activity in the open field (d), and displayed fewer rearing episodes in the open field (e) than non-stressed rats. Data are presented as means ± SEM. Sample sizes for each estrous phase are indicated by the number under each bar. * p < 0.05 relative to no stress.
Startle response
Regardless of the time point of estrous phase that was included as a between-subjects factor, analyses revealed no significant effects of stress or stage of estrous on startle responses.
Open field
Regardless of the time point of estrous phase that was included as a between-subjects factor, analyses revealed that stressed rats spent less time in the center of the open field (estrous phase during first stress session: F(1,149) = 5.22, p < 0.05, ηp2 = 0.03; estrous phase during second stress session: F(1,142) = 10.75, p < 0.001, ηp2 = 0.07; estrous phase during EPM testing: F(1,152) = 11.24, p < 0.001, ηp2 = 0.07; Fig. 3c) and made fewer grid crossings (estrous phase during first stress session: F(1,152) = 5.23, p < 0.05, ηp2 = 0.03; estrous phase during second stress session: F(1,144) = 8.60, p < 0.01, ηp2 = 0.06; estrous phase during EPM testing: F(1,154) = 7.39, p < 0.01, ηp2 = 0.05; Fig. 3d) than non-stressed rats. When stage of estrous during the second stress session or during behavioral testing was included as a between-subjects factor, analyses revealed that stressed rats also exhibited fewer rearing episodes than non-stressed rats (estrous phase during second stress session: F(1,144) = 6.80, p < estrous phase during open field testing: F(1,154) = 4.26, p < 0.05, ηp2 = 0.03; Fig. 3e). When stage of estrous during the second stress session was included as a between-subjects factor, there was a significant Stress x Estrous interaction for rearing episodes, F(3,144) = 3.36, p < 0.05, ηp2 = 0.07, indicating that stressed rats exhibited fewer rearing episodes if they were in metestrus during the second stress session (d = 1.52).
Experiment 2
Uterus weights
One ovariectomized rat from the stress group was removed from all data analyses because of evidence of an unsuccessful ovariectomy surgery (uterine weight = 0.412 g). Each of the remaining ovariectomized rats exhibited lower uterine weights than each of the sham rats. As a group, the ovariectomized rats (M = 0.048 g; SEM = 0.002; range = 0.032 – 0.068 g) exhibited lower uterine weights than the sham rats (M = 0.497 g; SEM = 0.007; range = 0.208 – 1.169 g), confirming that these ovariectomy surgeries were successful, F(1,54) = 111.18, p < 0.001, ηp2 = 0.67.
Growth rates
There was no significant effect of stress on growth rates, F(1,54) = 1.81, p > 0.05, ηp2 = 0.03. Ovariectomized rats (1.19 ± 0.07 g/day) tended to gain more weight during the 31-day stress paradigm than sham rats (1.00 ± 0.07 g/day), although this effect was not statistically significant, F(1,54) = 3.86, p = 0.054, ηp2 = 0.07.
Elevated plus maze
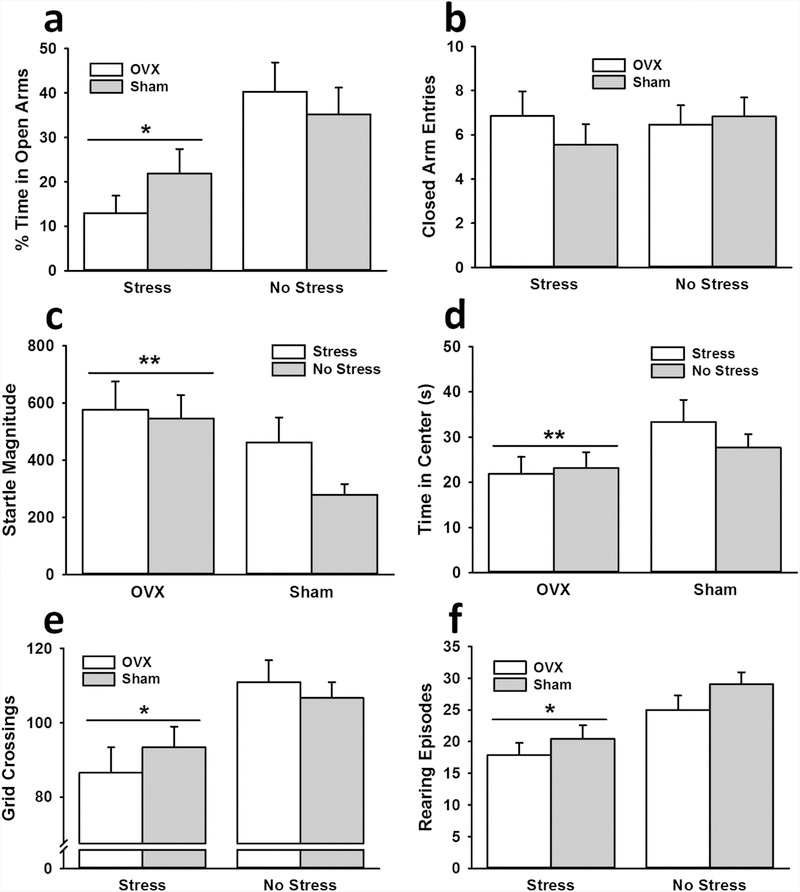
Stressed rats spent less time in the open arms of the EPM than non-stressed rats, F(1,53) = 12.58, p < 0.001, ηp2 = 0.19 (Fig. 4a). Ovariectomy condition had no significant impact on open arm time, nor did it interact with stress to influence open arm time. No significant differences were observed for closed arm entries, suggesting that the observed effect of stress was not due to effects on locomotor activity (Fig. 4b).
Fig. 4.
Interactive influence of stress and ovariectomy on female rat behavior. Stressed rats, independent of surgery condition, spent less time in the open arms of the EPM (a), exhibited less locomotor activity in the open field (e), and displayed fewer rearing episodes in the open field (f) than non-stressed rats. Ovariectomized (OVX) rats demonstrated greater startle responses (c) and spent less time in the center of the open field (d) than sham rats. Data are presented as means ± SEM. * p < 0.05 relative to no stress; ** p < 0.05 relative to sham.
Startle response
Ovariectomized rats exhibited greater startle responses than sham rats, F(1,54) = 5.84, p < 0.05, ηp2 = 0.10 (Fig. 4c). There was no significant effect of stress on startle response, nor did it significantly interact with ovariectomy condition to influence startle response.
Open field
Ovariectomized rats spent less time in the center of the open field than sham rats, F(1,54) = 4.33, p < 0.05, ηp2 = 0.07 (Fig. 4d). No other significant effects were observed for center time in the open field. Stressed rats made fewer grid crossings, F(1,54) = 11.03, p < 0.01, ηp2 = 0.17 (Fig. 4e), and exhibited fewer rearing episodes, F(1,54) = 14.29, p < 0.001, ηp2 = 0.21 (Fig. 4f), than non-stressed rats. There was no significant effect of ovariectomy on grid crossings or rearing episodes, nor did it significantly interact with stress to influence grid crossings or rearing episodes.
Experiment 3
Uterus weights
Each of the ovariectomized rats exhibited lower uterine weights than each of the sham rats. As a group, the ovariectomized rats (M = 0.067 g; SEM = 0.003; range = 0.043 – 0.099 g) exhibited lower uterine weights than the sham rats (M = 0.57 g; SEM = 0.009; range = 0.283 – 1.05 g), confirming that these ovariectomy surgeries were successful, F(1,36) = 178.41, p < 0.001, ηp2 = 0.83.
Growth rates
There was no significant effect of stress on growth rates, F(1,36) = 0.47, p > 0.05, ηp2 = 0.01. However, ovariectomized rats (2.43 ± 0.19 g/day) gained more weight during the 31-day stress paradigm than sham rats (1.53 ± 0.19 g/day), F(1,36) = 11.39, p < 0.01, ηp2 = 0.24.
Elevated plus maze
No significant effects were observed for time spent in the open arms (Fig. 5a) or for closed arm entries.
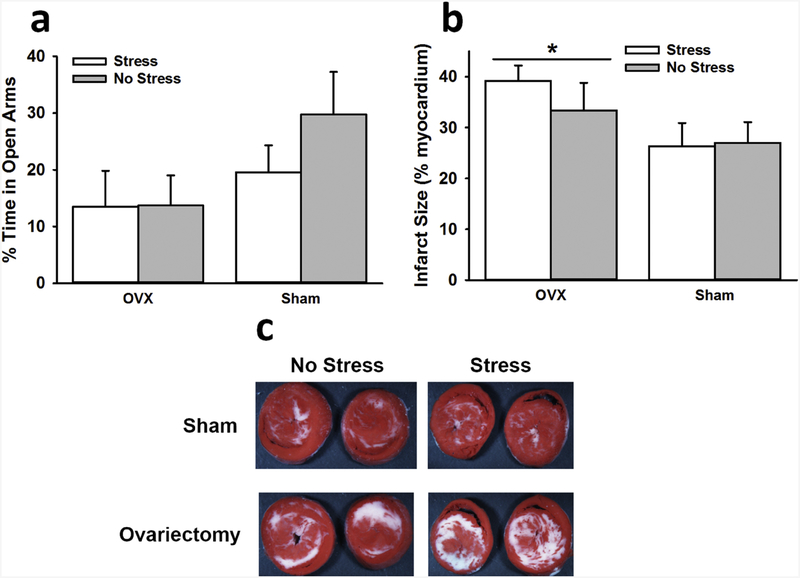
Fig. 5.
Interactive influence of stress and ovariectomy on EPM behavior and myocardial sensitivity to ischemic injury. No significant effects were observed for the amount of time spent in the open arms of the EPM (a). OVX rats exhibited significantly larger infarct sizes than sham rats following ischemia (b). Images depict representative myocardial slices with white areas indicative of infarction (c). Data are presented as means ± SEM. * p < 0.05 relative to sham.
Infarct Size
There was no significant effect of stress on infarct size, F(1,32) = 0.33, p > 0.05, ηp2 = 0.01. However, ovariectomized rats exhibited greater infarct sizes than sham rats, F(1,32) = 4.54, p < 0.05, ηp2 = 0.12 (Fig. 5b).
Myocardial function
The analyses of developed pressure [F(1,33) = 96.62, ηp2 = 0.75], +dP/dT [F(1,33) = 106.87, ηp2 = 0.76], −dP/dT [F(1,33) = 95.75, ηp2 = 0.74], end diastolic pressure [F(1,34) = 127.45, ηp2 = 0.79], and flow rate [F(1,33) = 14.31, ηp2 = 0.30] all revealed significant effects of time (all p < 0.001 before ischemia vs. after 1 hour of reperfusion); developed pressure, +dP/dT, −dP/dT, and flow rate decreased following ischemia. End diastolic pressure increased following ischemia (Table 1). No significant effects of stress or ovariectomy were observed on either presischemic contractile function or postischemic recovery of contractile function.
Table 1.
Impact of stress and ovariectomy (OVX) on measures of myocardial function (mean ± SEM)
| Coronary flow rate (ml/min/g) | Developed pressure (mmHg) | +dP/dT (mmHg/s) | −dP/dT (mmHg/s) | End diastolic pressure (mmHg) | Heart rate (bpm) | |
|---|---|---|---|---|---|---|
| Sham + no stress | ||||||
| Pre-ischemia | 93.22 ± 3.55 | 2038.78 ± 97.12 | −1635.44 ± 92.15 | 6.44 ± 0.41 | 293.78 ± 9.75 | 10.70 ± 0.59 |
| Recovery | 58.11 ± 4.15 | 1275.30 ± 94.95 | −1077.60 ± 83.66 | 44.00 ± 6.30 | 288.89 ± 12.25 | 9.22 ± 0.60 |
| Sham + stress | ||||||
| Pre-ischemia | 95.00 ± 4.77 | 2014.89 ± 102.66 | −1642.89 ± 84.86 | 5.67 ± 0.44 | 265.56 ± 14.19 | 11.86 ± 0.94 |
| Recovery | 56.11 ± 8.27 | 1223.80 ± 173.82 | −1027.30 ± 143.31 | 41.44 ± 6.27 | 271.22 ± 14.23 | 9.72 ± 0.50 |
| OVX + no stress | ||||||
| Pre-ischemia | 97.80 ± 3.13 | 2157.90 ± 77.44 | −1798.60 ± 86.12 | 7.10 ± 0.38 | 289.30 ± 12.06 | 12.56 ± 1.00 |
| Recovery | 57.30 ± 6.48 | 1265.10 ± 135.89 | −1070.50 ± 111.83 | 55.40 ± 7.83 | 278.70 ± 11.19 | 10.93 ± 0.53 |
| OVX + stress | ||||||
| Pre-ischemia | 91.30 ± 3.57 | 1993.40 ± 84.72 | −1659.20 ± 87.29 | 6.20 ± 0.39 | 287.60 ± 7.16 | 11.94 ± 0.67 |
| Recovery | 51.56 ± 5.98 | 1095.80 ± 113.98 | −920.78 ± 88.89 | 43.90 ± 7.37 | 265.10 ± 7.63 | 10.73 ± 0.62 |
DISCUSSION
We have shown that female rats exposed to our predator-based psychosocial stress model of PTSD exhibit long-term changes in behavior indicative of heightened anxiety. Independent of estrous phase, stressed females spent less time in the open arms of the EPM, exhibited reduced locomotor activity in the open field, spent less time in the center of the open field, and displayed less rearing in the open field than non-stressed females. Similar effects were observed in ovariectomized females exposed to the stress paradigm. These results indicate that females exhibit a PTSD-like behavioral phenotype, at least with regards to anxiety-like behavior, similar to that which was previously reported for males following exposure to our animal model of PTSD.
Some work in rodents has shown that estrous phase correlates with anxiety-like behavior. For instance, investigators have reported that females in proestrus spend more time in the open arms of the EPM, exhibit greater risk-taking behavior, and demonstrate more social interaction than females in other stages (Frye et al., 2000; Marcondes et al., 2001). However, estrous-dependent differences in such behavior have not always been observed (Hiroi and Neumaier, 2006; Plappert et al., 2005). The inconsistency in this area of research might be attributable to a lack of adequate statistical power in some studies to detect estrous-based differences in behavior. Because in previous work we failed to observe significant behavioral alterations in females exposed to our model of PTSD (Rorabaugh et al., 2015), we predicted that estrous stage might interact with the model to influence behavioral outcomes. Contrary to these expectations, in Experiment 1, stressed females exhibited heightened anxiety-like behavior on the EPM and in the open field, which was largely independent of estrous stage at the time of each cat exposure and behavioral testing. In fact, the only significant effect of estrous was observed for rearing episodes in the open field. Specifically, stressed rats exhibited fewer rearing episodes in the open field than non-stressed rats if they were in metestrus at the time of the second stress session. This effect should be interpreted cautiously, however, as our repeated analyses on the same behavioral data (due to including rats’ estrous stage from various time points) likely increased our risk of making a Type II error. The consistent finding from Experiment 1 of heightened anxiety in stressed females on the EPM does contrast with our previous results (Rorabaugh et al., 2015). It is possible that our previous study was underpowered to detect such behavioral effects in females, especially when considering that such females were likely in different stages of estrous at the time of testing.
The observation of PTSD-like behaviors in females is consistent with previous work in which investigators observed that the female response to stress models of PTSD was similar to that of males (Koresh et al., 2016; Mazor et al., 2009; Viviani et al., 2012). However, some investigators have observed that females are less susceptible to stress-induced alterations of physiology and behavior (e.g., Diehl et al., 2007). Indeed, studies have shown that female rats were resistant to SPS-induced impairments of fear extinction retention and HPA axis function (Keller et al., 2015; Pooley et al., 2018). Even if female rodents do exhibit PTSD-like sequelae, they may display some phenotypic differences from males. In the present set of experiments, stressed females did not exhibit enhanced acoustic startle responses, which we have observed in previous work with stressed males (Zoladz et al., 2008; Zoladz et al., 2013). Nevertheless, the absence of greater startle responses in stressed females is consistent with other preclinical work, in which both SPS and predator-based models of PTSD led to increased acoustic startle responses in males, but not females (Pooley et al., 2018). It is also consistent with work in PTSD patients, as some studies have shown that females with PTSD do not exhibit exaggerated startle responses like their male counterparts (e.g., Medina et al., 2001). Future work could utilize rodent models of PTSD to examine further the possible phenotypic differences between males and females exposed to intense stress.
In order to further assess the involvement of sex hormones in the female response to our stress model, we also exposed ovariectomized females to the paradigm. For the most part, these experiments revealed effects that were similar to those of Experiment 1. In Experiment 2, independent of surgery condition, stressed females spent less time in the open arms of the EPM, exhibited less locomotor activity in the open field, and displayed fewer rearing episodes in the open field than non-stressed females. Interestingly, we did not replicate the stress-induced increase in anxiety-like behavior on the EPM in Experiment 3. The inability to replicate this effect appeared to be driven by an unusually low amount of open arm exploration in ovariectomized controls (Fig. 5a), relative to the amount of open arm exploration they displayed in Experiment 2 (Fig. 4a). Indeed, sham animals behaved similarly on the EPM in both experiments. The discrepancy in the data from ovariectomized controls could be due to differential effects of the ovariectomy procedure across the two experiments or simply the manifestation of behavioral variability.
Consistent with Experiment 1, stressed females did not exhibit enhanced acoustic startle responses in Experiment 2. Ovariectomized females did exhibit increased weight gain, greater startle responses, and an increase in some anxiety-like behavior (e.g., decreased time spent in center of the open field), relative to sham rats. Previous work has consistently shown that ovariectomized females gain more weight than controls; however, studies have not always reported an increase in the startle response and/or anxiety-like behavior following ovary removal (Vaillancourt et al., 2002). Our results suggest that the presence of sex hormones may prevent anxiety-like behavior, but they do not interact with stress to influence the development of PTSD-like behavioral sequelae.
Our finding that ovariectomized rats develop significantly larger infarcts than sham rats is consistent with the well-established cardioprotective role of ovarian hormones (Deschamps et al., 2010; Kolodgie et al., 1997; Liu et al., 2004; Sivasinprasasn et al., 2016). We previously reported that male (but not female) rats exposed to this PTSD model develop myocardial hypersensitivity to ischemia (Rorabaugh et al., 2015). Thus, we hypothesized that the presence of ovarian hormones may protect the hearts of female rats from the development of stress-induced hypersensitivity to ischemic injury. However, the finding that stress produced similar effects on ovariectomized rats and sham rats suggests that the sex-dependent cardiac effect of this PTSD model is unrelated to ovarian hormones. Further work is needed to understand why females do not develop the myocardial hypersensitivity to ischemia that develops in male rats following exposure to this model (Rorabaugh et al., 2019; Rorabaugh et al., 2015).
There were some limitations of the present experiments that warrant consideration. First, Experiment 1 may have been underpowered to fully assess the impact of estrous stage on behavioral outcomes, as some stages had relatively smaller sample sizes (e.g., metestrus). It also would have been optimal to assess the interaction between stress and estrous stage in a single analysis, thus enabling an examination of interaction effects of estrous stages at different time points in the model. However, such analyses were precluded because of the necessary sample size. Second, the ovariectomy surgeries were performed on pre-pubertal females, which could have led to different effects on behavior than ovariectomies performed on adult females. Indeed, previous work has shown that pre-pubertal ovariectomies results in greater stress sensitivity than adulthood ovariectomies (Romeo et al., 2004). Thus, the results from Experiment 2 and 3 should be interpreted with this in mind. Finally, the startle response and open field data from Experiments 1 and 2 could have been influenced by prior testing on the rats. To mitigate such influences, we tested rats on the EPM first, given its robust sensitivity to anxiety-like behavior. We also separated each test by a period of 24 h. Still, the influence of the prior day’s testing could have had carryover effects that interacted with the influence of stress on behavioral outcomes.
Conclusion
Our results suggest that estrous stage (or the presence of ovarian hormones) does not significantly interact with stress to influence the development of PTSD-like anxiety in females. Furthermore, the results indicate that despite minor phenotypic differences, stressed females generally display anxiety-like behaviors that are comparable to those observed in stressed males. Thus far in our assessment of females, we have established that, similar to stressed males, stressed females exhibit reduced growth rate, heightened anxiety on the EPM, and heightened anxiety in the open field. We have also shown that, unlike stressed males, stressed females do not exhibit an exaggerated startle response, nor do they show greater myocardial sensitivity to ischemic injury (Rorabaugh et al., 2015). Future work will need to employ additional physiological and behavioral endpoints to determine whether females exhibit other PTSD-like alterations (e.g., cognitive impairments, enhanced negative feedback of HPA axis) that have been observed in males exposed to this model and to understand why females are protected from the development of myocardial hypersensitivity to ischemia that is observed in stressed males. As of now, our data suggest that this model may be useful for examining mechanisms and treatment of PTSD-like sequelae in female rodents.
Highlights.
Chronic stress led to PTSD-like behavioral alterations in female rats.
Estrous stage and ovariectomy did not impact the stress-induced changes in females.
Ovariectomized rats exhibited greater anxiety and startle responses than sham rats.
Ovariectomized rats displayed greater myocardial sensitivity to ischemic injury.
Acknowledgments
ROLE OF THE FUNDING SOURCE
The research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R15HL132322. The National Institutes of Health had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DECLARATION OF INTEREST
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blizard DA, Lippman HR, Chen JJ, 1975. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiology & behavior 14, 601–608. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson E, 1991. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Archives of general psychiatry 48, 216–222. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR, 1997. Sex differences in posttraumatic stress disorder. Archives of general psychiatry 54, 1044–1048. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Koresh O, Matar MA, Geva AB, Zohar J, 2011. Early post-stressor intervention with propranolol is ineffective in preventing posttraumatic stress responses in an animal model for PTSD. Eur Neuropsychopharmacol 21, 230–240. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J, 2010. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. Journal of neuroendocrinology 22, 889–904. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, Kozlovsky N, Zohar J, 2006. Anisomycin, a protein synthesis inhibitor, disrupts traumatic memory consolidation and attenuates posttraumatic stress response in rats. Biological psychiatry 60, 767–776. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA, 2012. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 37, 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Yehuda R, 2011. Gender differences in animal models of posttraumatic stress disorder. Disease markers 30, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E, Sun J, 2010. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med 20, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl LA, Silveira PP, Leite MC, Crema LM, Portella AK, Billodre MN, Nunes E, Henriques TP, Fidelix-da-Silva LB, Heis MD, Goncalves CA, Quillfeldt JA, Dalmaz C, 2007. Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain research 1144, 107–116. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME, 2000. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacology, biochemistry, and behavior 67, 587–596. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF, 2006. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behavioural brain research 166, 93–100. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE, 1991. Sex differences in animal tests of anxiety. Physiology & behavior 49, 245–250. [DOI] [PubMed] [Google Scholar]
- Keller SM, Schreiber WB, Staib JM, Knox D, 2015. Sex differences in the single prolonged stress model. Behavioural brain research 286, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Farb A, Litovsky SH, Narula J, Jeffers LA, Lee SJ, Virmani R, 1997. Myocardial protection of contractile function after global ischemia by physiologic estrogen replacement in the ovariectomized rat. J Mol Cell Cardiol 29, 2403–2414. [DOI] [PubMed] [Google Scholar]
- Koresh O, Kaplan Z, Zohar J, Matar MA, Geva AB, Cohen H, 2016. Distinctive cardiac autonomic dysfunction following stress exposure in both sexes in an animal model of PTSD. Behavioural brain research 308, 128–142. [DOI] [PubMed] [Google Scholar]
- Liu ML, Xu X, Rang WQ, Li YJ, Song HP, 2004. Influence of ovariectomy and 17beta-estradiol treatment on insulin sensitivity, lipid metabolism and post-ischemic cardiac function. Int J Cardiol 97, 485–493. [DOI] [PubMed] [Google Scholar]
- Manjoch H, Vainer E, Matar M, Ifergane G, Zohar J, Kaplan Z, Cohen H, 2016. Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behavioural brain research 306, 91–105. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, 2001. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & behavior 74, 435–440. [DOI] [PubMed] [Google Scholar]
- Mazor A, Matar MA, Kaplan Z, Kozlovsky N, Zohar J, Cohen H, 2009. Gender-related qualitative differences in baseline and post-stress anxiety responses are not reflected in the incidence of criterion-based PTSD-like behaviour patterns. World J Biol Psychiatry 10, 856–869. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE, 2005. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Hormones and behavior 48, 64–74. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ, 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural brain research 187, 228–238. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA, 2012. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp, e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina AM, Mejia VY, Schell AM, Dawson ME, Margolin G, 2001. Startle reactivity and PTSD symptoms in a community sample of women. Psychiatry research 101, 157–169. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen HU, 2000. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta psychiatrica Scandinavica 101, 46–59. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Rodenbucher AM, Pilz PK, 2005. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiology & behavior 84, 585–594. [DOI] [PubMed] [Google Scholar]
- Pooley AE, Benjamin RC, Sreedhar S, Eagle AL, Robison AJ, Mazei-Robison MS, Breedlove SM, Jordan CL, 2018. Sex differences in the traumatic stress response: PTSD symptoms in women recapitulated in female rats. Biol Sex Differ 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramikie TS, Ressler KJ, 2018. Mechanisms of Sex Differences in Fear and Posttraumatic Stress Disorder. Biological psychiatry 83, 876–885. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Stork O, Schmidt MV, in press. Animal models of PTSD: a challenge to be met. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS, 2004. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology 80, 387–393. [DOI] [PubMed] [Google Scholar]
- Rorabaugh BR, Bui AD, Seeley SL, Eisenmann ED, Rose RM, Johnson BL, Huntley MR, Heikkila ME, Zoladz PR, 2019. Myocardial hypersensitivity to ischemic injury is not reversed by clonidine or propranolol in a predator-based rat model of posttraumatic stress disorder. Progress in neuro-psychopharmacology & biological psychiatry 89, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorabaugh BR, Krivenko A, Eisenmann ED, Bui AD, Seeley S, Fry ME, Lawson JD, Stoner LE, Johnson BL, Zoladz PR, 2015. Sex-dependent effects of chronic psychosocial stress on myocardial sensitivity to ischemic injury. Stress (Amsterdam, Netherlands) 18, 645–653. [DOI] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM, 2011. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. Journal of psychiatric research 45, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasinprasasn S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N, 2016. Estrogenic Impact on Cardiac Ischemic/Reperfusion Injury. J Cardiovasc Transl Res 9, 23–39. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Foa EB, 2006. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological bulletin 132, 959–992. [DOI] [PubMed] [Google Scholar]
- Vaillancourt C, Cyr M, Rochford J, Boksa P, Di Paolo T, 2002. Effects of ovariectomy and estradiol on acoustic startle responses in rats. Pharmacology, biochemistry, and behavior 74, 103–109. [DOI] [PubMed] [Google Scholar]
- Viviani D, Haegler P, Strasser DS, Steiner MA, 2012. Sex comparison on long-lasting behavioral and physiological disturbances induced by single shock experience in rats. Physiology & behavior 107, 243–251. [DOI] [PubMed] [Google Scholar]
- Wilson CB, Ebenezer PJ, McLaughlin LD, Francis J, 2014a. Predator exposure/psychosocial stress animal model of post-traumatic stress disorder modulates neurotransmitters in the rat hippocampus and prefrontal cortex. PloS one 9, e89104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Dange R, Harre JG, Shaak TL, Diamond DM, Francis J, 2014b. Differential effects of sertraline in a predator exposure animal model of post-traumatic stress disorder. Frontiers in behavioral neuroscience 8, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Francis J, 2014c. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behavioural brain research 268, 72–80. [DOI] [PubMed] [Google Scholar]
- Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J, 2013. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PloS one 8, e76146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Conrad CD, Fleshner M, Diamond DM, 2008. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress (Amsterdam, Netherlands) 11, 259–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM, 2013. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neuroscience and biobehavioral reviews 37, 860–895. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Fleshner M, Diamond DM, 2012. Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology 37, 1531–1545. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Fleshner M, Diamond DM, 2013. Differential effectiveness of tianeptine, clonidine and amitriptyline in blocking traumatic memory expression, anxiety and hypertension in an animal model of PTSD. Progress in neuro-psychopharmacology & biological psychiatry 44, 1–16. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Park CR, Fleshner M, Diamond DM, 2015. Psychosocial predator-based animal model of PTSD produces physiological and behavioral sequelae and a traumatic memory four months following stress onset. Physiology & behavior 147, 183–192. [DOI] [PubMed] [Google Scholar]