Abstract
Objective:
To evaluate the relationship between detection of DNA viruses, ferritin, and outcomes in children with severe sepsis.
Study design:
We enrolled 75 pediatric patients with severe sepsis admitted to a tertiary care children’s hospital. Plasma ferritin was measured within 48h of diagnosis and subsequently twice weekly. HSV1, HHV6, EBV, CMV, and Adenovirus DNAemia was assessed by PCR.
Results:
The incidence of DNAemia was significantly increased in patients with ferritin ≥1,000ng/mL (78% vs 28%; P < .05). Patients with ferritin ≥1,000 ng/mL were more likely to have multiple DNA viruses detected in plasma (39% vs. 4%; p<0.001). The number of viruses detected in plasma directly correlated with the degree of hyperferritinemia and development of combined hepatobiliary and hematologic dysfunction after controlling for bacterial and fungal co-infections (p<0.05) as well as increased mortality after controlling for severity of illness and cancer diagnosis (OR: 2.77, 95%CI: 1.35-5.70, p<0.05).
Conclusions:
Viral DNAemia was associated with hyperferritinemia and adverse outcome in pediatric severe sepsis. Prospective studies are needed to determine whether hyperferritinemia may be used to identify patients at risk of occult DNAemia.
Keywords: Sepsis, DNA Viremia, Hyperferritinemia, Multiple organ dysfunction syndrome
The prevalence of pediatric severe sepsis, defined as sepsis plus failure of at least one organ, increased by 81% between 1995 and 2005 with a case-fatality of 8.9%.(1) In children who survive initial refractory hypotension, progression to multiple organ dysfunction syndrome (MODS) is the most common mode of death.(2) The bedside biomarkers procalcitonin and C-reactive protein are commonly used by clinicians to assess the evolution of bacterial infection in sepsis.(3-5) However, despite growing evidence that occult DNA viremia occurs in adult sepsis patients in association with increased mortality and in children with severe sepsis associated with increased secondary infection risk, there is no comparable clinical bedside biomarker in use to assess DNAemia.(6,7)
One promising candidate biomarker is ferritin, an acute phase reactant and iron binding protein that is released into the circulation in response to microbial infection and cell death.(8) Reference ferritin values (2.5th – 97.5th percentiles) are age dependent in children, ranging from 10-500 ng/mL in 13month – 3 year olds to 10-125 ng/mL in 15-18 year olds.(9) Several important cut-offs for circulating ferritin as a predictive biomarker have been reported in hospitalized children. In children with severe sepsis cared for in a resource-limited setting, ferritin values >500 ng/mL were associated with increased illness-severity-adjusted mortality.(10) In a study of hospitalized children admitted to a tertiary care center in the United States, serum ferritin values >1,000 and >3,000 ng/mL were associated with a stepwise increased adjusted risk of intensive care unit (ICU) admission and death.(11) The ferritin cutoff associated with mortality for children admitted to a quaternary care pediatric ICU has not been established.
Unlike many bacterial and fungal infections, viral infections are frequently associated with elevated levels of plasma ferritin.(12-15) For example, the median ferritin value in adults during acute Epstein-Barr virus (EBV) infection is 431 ng/mL.(13) Similar associations have been observed in adults with infection due to hepatitis B and C viruses as well as human immunodeficiency virus.(12) In children, plasma ferritin has been shown to predict disease progression in severe dengue and was associated with viremia and death in patients with ebola virus.(14,15) In the present study, we explored the hypothesis that detection of circulating viral DNA (DNAemia) in children with severe sepsis is associated with hyperferritinemia.
METHODS
This study was approved by the Institutional Review Board of the University of Pittsburgh and performed as an ancillary study to a single-center prospective study of children admitted to the Children’s Hospital of Pittsburgh of UPMC Pediatric Intensive Care Unit (PICU) with severe sepsis.(5) Patients were screened twice weekly for inclusion criteria. Sepsis was defined as suspicion of infection plus ≥2 criteria of systemic inflammatory response syndrome. Severe sepsis was defined as presence of sepsis and at least one organ failure. The presence or absence of organ failure was determined twice weekly for up to 28 days in the PICU as previously described.(16) Briefly, the following criteria for organ failure were used: cardiovascular - need for cardiovascular support; pulmonary - need for mechanical ventilation support with the ratio of the arterial partial pressure of oxygen and the fraction of inspired oxygen (PaO2/FiO2) < 300 without this support; hepatic – total bilirubin >1.0 mg/dL and alanine aminotransferase (ALT) > 100 units/L; renal – serum creatinine > 1.0 mg/dL and oliguria (urine output < 0.5 mL/kg/hr), hematologic - thrombocytopenia < 100,000/μL and prothrombin time INR > 1.5 × normal. Organ failure index (OFI) was tabulated for each timepoint by adding the number of failing organs, and the maximum OFI was used for analysis.
One hundred consecutive cases of severe sepsis were enrolled in the parent study between January and December 2014, of which 75 patients had sufficient stored plasma for analyses and were included. Additional inclusion criteria were arterial or central venous catheter for blood sampling, age >44 weeks gestation and <18 years, and desire for aggressive care. Vital status was determined at the time of discharge from PICU.
Viral DNA Detection
Viral detection was performed by polymerase chain reaction (PCR) assay of thawed plasma as previously described.(6,7) Briefly, the BioMerieux NucliSens easyMAG automated extractor (BioMerieux Durham, NC, USA) was used to extract total nucleic acids that was then amplified using primer sequences specific for herpes simplex type 1 (HSV1), human herpesvirus 6 (HHV6), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus.(6,17,18) PCR was performed on an Applied Biosystems 7300 real-time PCR instrument (Applied Biosystems, Foster City, CA, USA) using standard protocols.
Plasma Ferritin Measurement
Blood samples were obtained within 24-72h of development of severe sepsis and subsequently twice weekly until removal of the arterial and central venous catheters or 28d enrollment. Samples were centrifuged to obtain plasma and frozen for batch analysis. Ferritin measurement was performed according to the clinical laboratory practice at UPMC Children’s Hospital of Pittsburgh. Treating physicians were blinded to the results. Ferritin measurements from the original cohort of 100 patients were previously reported as part of a systemic inflammation mortality risk contingency table and as a component of the diagnosis of macrophage activation syndrome immune phenotype. (5,16) These data have been re-analyzed in the 75 patients with sufficient plasma available for detection of DNA viruses to evaluate the association between DNAemia and hyperferritinemia.
Statistical Analyses
Statistics were performed using STATA 14 (Statacorp LLC, College Station, TX, USA) and Prism 7 (GraphPad Software Inc, La Jolla, CA, USA). Associations between patient characteristic, DNAemia, and ferritin was determined using Fisher exact test or chi-square test for categorical variables and two-sided Wilcoxon rank-sum with normal approximation and continuity correction or unpaired t-test for continuous variables. Youden J statistic was used to determine optimal cutoff for sensitivity and specificity. Multivariate logistic regression model was constructed with independent variables with significance p <0.1 in univariate analysis. P <0.05 was considered statistically significant.
RESULTS
Between January 2014 – December 2014, 100 consecutive cases of severe sepsis admitted to the PICU were enrolled of which 75 had sufficient plasma available for analysis (Table I). For subjects with multiple septic episodes (n=2), both encounters were included for analysis. Clinical characteristics were compared between included and excluded patients (Table 2; available at www.jpeds.com). Patients excluded due to insufficient plasma were found to be less likely to have bacterial infection as the primary etiology for severe sepsis (p=0.01).
Table 1.
Baseline Characteristics according to DNA viremia status
| Total (n=75) |
+ DNA Viremia (n=30) |
− DNA Viremia (n=45) |
P-value | |
|---|---|---|---|---|
| Age (y) | 6.2±6.0 | 6.5±6.1 | 5.9±6.1 | 0.5311 |
| Female | 33 (44%) | 13 (43%) | 20 (44%) | 1.000 2 |
| Chronic Illness | 48 (64%) | 23 (77%) | 25 (56%) | 0.0622 |
| Transplant | 19 (25%) | 10 (33%) | 9 (20%) | 0.1932 |
| Cancer | 11 (15%) | 4 (13%) | 7 (16%) | 1.0002 |
| Bacterial Infection | 48 (64%) | 18 (60%) | 30 (67%) | 0.6272 |
| Fungal Infection | 5 (7%) | 4 (13%) | 1 (2%) | 0.1512 |
| Non-DNA Viral Infection | 15 (20%) | 10 (33%) | 5 (11%) | 0.0182 |
| PRISM | 9 [0 – 35] | 9.5 [1 – 35] | 9 [0 – 30] | 0.6301 |
| Maximum ferritin value (ng/mL) | 2025 ± 6233 | 3752 ± 9245 | 874 ± 2369 | < 0.011 |
Data presented as frequency, mean ± SD, or median [range] as appropriate.
Mann-Whitney or unpaired t-test
Chi-square or Fisher exact test
Table 2;
online only. Characteristics of Patients With and Without Sufficient Plasma for DNAemia Analysis
| Total (n=100) |
Sufficient Plasma for Analysis (n=75) |
Insufficient Plasma for Analysis (n=25) |
P-value | |
|---|---|---|---|---|
| Age (y) | 5.8±5.7 | 6.2±6.0 | 4.8±4.4 | 0.3001 |
| Female | 47 (47%) | 33 (44%) | 14 (56%) | 0.298 2 |
| Chronic Illness | 59 (59%) | 48 (64%) | 11 (44%) | 0.0782 |
| Transplant | 22 (22%) | 19 (25%) | 3 (12%) | 0.2642 |
| Cancer | 14 (14%) | 11 (15%) | 3 (12%) | 1.0002 |
| Bacterial Infection | 57 (57%) | 48 (64%) | 9 (36%) | 0.0142 |
| Fungal Infection | 9 (9%) | 5 (7%) | 4 (16%) | 0.2222 |
| Non-DNA Viral Infection | 22 (22%) | 15 (20%) | 7 (28%) | 0.4032 |
| PRISM | 9 [0 – 41] | 9 [0 – 35] | 7 [0 – 41] | 0.1731 |
| Maximum ferritin value (ng/mL) | 1679 ± 5519 | 2025 ± 6233 | 581 ± 1751 | 0.2571 |
Data presented as frequency, mean ± SD, or median [range] as appropriate.
Mann-Whitney or unpaired t-test
Chi-square or Fisher exact test
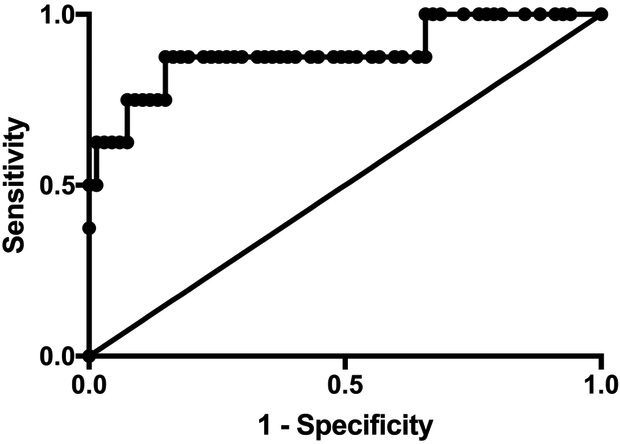
Overall mortality was 11% (8/75) among the 75 patients in this analysis. To extend previously reported cutoffs for ferritin associated with mortality risk to a quaternary care pediatric ICU, we divided plasma ferritin values into four groups (10,11): <500, 500-999, 1000-2999, and ≥ 3000 ng/mL and observed mortality in these groups of 2.2%, 0%, 22%, and 56% respectively. A ROC curve of maximum ferritin and mortality was found to have an area under the curve of 0.89 (95% CI 0.74 – 1.04; p<0.001) (Figure 1; available at www.jpeds.com). The optimal cutoff for ferritin to maximize sensitivity and specificity was 1210 ng/mL (sensitivity 88%, specificity 85%). A multivariate model was constructed which controlled for cancer diagnosis (association p<0.1 for mortality in univariate analysis) and illness severity using Pediatric Risk of Mortality (PRISM) score and determined that cases with ferritin ≥ 1210 ng/mL had 27.5-fold greater odds for mortality (95% CI 2.9 – 264.8; p=0.004).
Figure 1; online. Receiver operating characteristic (ROC) curve of maximum ferritin in patients based on vital status.
ROC was performed for plasma ferritin values in 8 patients with severe sepsis who died and 67 patients with severe sepsis who survived. Area under the curve is 0.881 (95%CI: 0.7379 – 1.038; p<0.001).
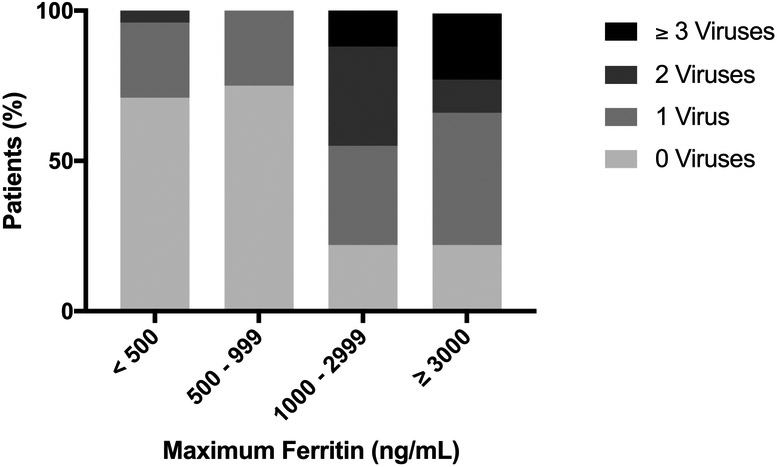
As previously reported, we tested all patients post hoc for the presence of five common DNA viruses by PCR and found that 40% (30/75) of sepsis cases had detectable DNAemia.(7) Standard of care testing by the ICU clinical team also identified at least one non-DNA respiratory virus in the nasopharynx in 20% (15/75) of cases. Of the thirty cases with DNAemia, only 10% (3/30) had DNA viral infection detected as part of routine care by the clinical team. As shown in Figure 2, cases with higher plasma ferritin levels were more likely to have viral DNAemia (p<0.01). Overall, 78% (14/18) of patients with ferritin ≥1000 ng/mL had viral DNAemia, versus 28% (16/57) of patients with ferritin <1,000 (p<0.05). The association between DNAemia and hyperferritinemia remained after controlling for sepsis severity by PRISM and cancer diagnosis (OR 9.6, 95% CI: 2.3 – 40.1; p< 0.01). Ferritin ≥1000 ng/mL was significantly associated with the presence of EBV, HHV6, and adenovirus (p <0.05 for all), but not HSV1 or CMV (Table 3; available at www.jpeds.com). Four patients with ferritin ≥1,000 ng/mL were not found to have DNAemia, however, each had another diagnosis which may be associated with hyperferritinemia: two patients were on extracorporeal membrane oxygenation (ECMO) circuits, which can cause hemolysis-induced hyperferritinemia by hemoglobin-haptoglobin complex binding to CD163 on macrophages; one patient was newly diagnosed with systemic onset juvenile idiopathic arthritis (JIA), and one patient was being treated with granulocyte-macrophage colony stimulating factor (GM-CSF).(8,19-21) Notably, however, hyperferritinemia was not present in all patients on ECMO (2/5 (40%) did not have hyperferritinemia) or patients treated with GM-CSF (6/13 (46%) did not have hyperferritinemia). The patient diagnosed with JIA was the only patient with this diagnosis in this cohort.
Figure 2. Maximum ferritin is associated with number of different circulating DNA viruses in plasma of children with severe sepsis.
Stacked bar graph of number of DNA viruses detected post hoc in testing of blood for 5 common DNA viruses: herpes simplex 1, human herpes virus 6, Epstein-Barr virus, cytomegalovirus, and adenovirus. Separating patients by maximum ferritin value, we observed: (median;IQR): ferritin < 500 (viruses = 0; 0 – 1), 500 – 999 (viruses = 0; 0 – 0.75), 1000 - 2999 (viruses=1; 0.5 – 2), and ≥ 3000 (viruses = 1; 0.5 – 3) (p < 0.01).
Table 3;
online only. DNAemia in severe sepsis patients with and without hyperferritinemia
| Total (N=75) |
Max Ferritin < 1,000 ng/mL (N=57) |
Max Ferritn ≥ 1,000 ng/mL (N=18) |
P-value | |
|---|---|---|---|---|
| HSV1 | 3 (4%) | 1 (1.8%) | 2 (11.1%) | NS1 |
| EBV | 10 (13.3%) | 4 (7.0%) | 6 (33.3%) | p<0.051 |
| CMV | 5 (6.7%) | 3 (5.3%) | 2 (11.1%) | NS1 |
| HHV6 | 6 (7.9%) | 1 (1.8%) | 5 (27.8%) | p<0.011 |
| Adenovirus | 20 (26.7%) | 9 (15.8%) | 11 (61.1%) | p<0.0011 |
| Any DNA Virus | 30 (40%) | 16 (28.1%) | 14 (77.8%) | p<0.0011 |
| More than 1 DNA virus | 9 (12%) | 2 (3.5%) | 7 (38.9%) | p<0.0011 |
Data presented as frequency
Chi-square or Fisher’s exact test, as appropriate
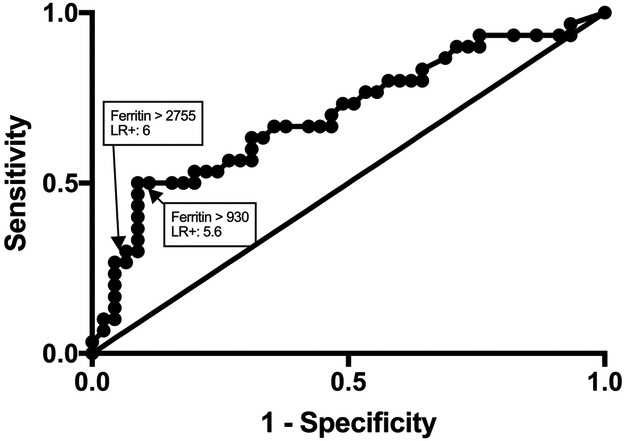
To determine optimal thresholds for ferritin to predict viral DNAemia we constructed a ROC curve of maximum ferritin values in patients with or without DNAemia (Figure 3) and determined a cutoff for ferritin of > 930 ng/mL using Youden J statistic (sensitivity 50%, specificity 91%) and > 2755 ng/mL using likelihood ratio (LR). With pre-test probability of DNAemia in this cohort of 0.4, and a LR of 6 using ferritin > 2755 ng/mL, the post-test probability of DNAemia is 0.8.
Figure 3. Receiver operating characteristic (ROC) curve of maximum ferritin in patients with or without viral DNAemia.
ROC was performed for plasma ferritin in 30 patients with severe sepsis and viral DNAemia and 45 patients with severe sepsis without DNAemia. Area under the curve is 0.7007 (95%CI: 0.576 – 0.8255; p<0.01). Identified are the points which correspond to optimal cutoffs identified by Youden J statistic (ferritin > 930 ng/mL) and likelihood ratio (ferritin > 2755 ng/mL).
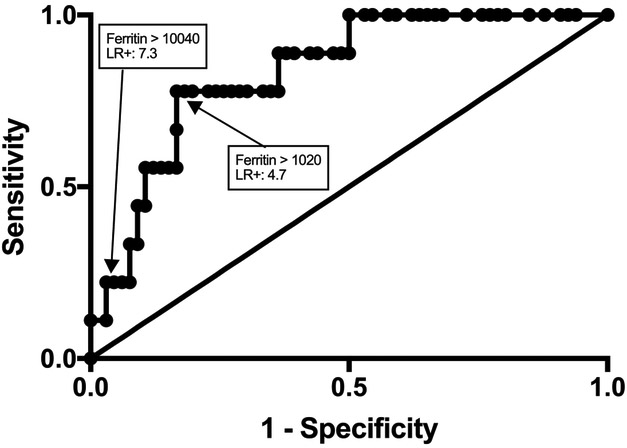
Thirty-nine percent of patients (7/18) with ferritin ≥1000 ng/mL had multiple DNA viruses detected compared with 3.5% (2/57) patients with ferritin < 1000 ng/mL (p<0.001) (Figure 2). Ferritin levels predicted the extend of DNAemia, 0-1 virus detected compared with 2 or more DNA viruses detected (AUC 0.8333 (95%CI: 0.7154 – 0.9513; p<0.01) (Figure 4;available at www.jpeds.com). In a multivariate regression model, plasma ferritin level was positively associated with the number of different circulating DNA viruses identified after controlling for bacterial and fungal co-infection (p <0.05) as well as controlling for clinical detection of non-DNA viruses. The most common DNA virus co-infection was EBV and adenovirus, which was present in 56% (5/9) of patients with multiple DNA viruses detected.
Figure 4; online. Receiver operating characteristic (ROC) curve of maximum ferritin level in patients with or without multiple-virus DNAemia.
ROC was performed for plasma ferritin in 9 patients with severe sepsis and multiple DNAemia and 66 patients with severe sepsis with 0 - 1 DNAemia. Area under the curve is 0.8333 (95%CI: 0.7154– 0.9513; p<0.01). Identified are the points that correspond to optimal cutoffs identified by Youden J statistic (ferritin > 1020 ng/mL; sensitivity 78%, specificity 83%) and likelihood ratio (ferritin > 2755 ng/mL; LR 7.3).
To evaluate the relationship between viral DNAemia and sepsis severity, OFI and vital status was determined. DNAemia was significantly associated with OFI ≥ 4 (30% (9/30) with DNAemia vs. 11% (5/45) without DNA viremia; p<0.05) even controlling for bacterial and fungal co-infection by logistic regression (OR 4.9, 95%CI 1.4-17.4, p<0.05). No significant differences were observed between patients with or without viral DNAemia with respect to individual organ failures after adjustment for multiple comparisons; however, hepatobiliary dysfunction and hematologic dysfunction in combination, clinical features of MAS(22,23), was associated with a significantly higher circulating ferritin (5714±2559 vs. 656±120; p<0.01) and total DNA viruses detected (1±1.3 vs. 0.4±0.7; p<0.05), after controlling for presence of bacterial and fungal co-infections (OR 1.9, 95% CI: 1.1 – 3.5; p<0.05). Mortality was 17% (5/30) in patients with DNAemia and 7% (3/45) in patients without viral DNAemia (p=0.25). The number of DNA viruses detected was greater in patients who died (1.6 vs. 0.5; p<0.05), and the association between number of DNA viruses detected and mortality risk remained significant after adjustment for bacterial and fungal co-infection and severity of illness using PRISM score and cancer diagnosis (OR: 2.6, 95%CI: 1.1-6.3; p<0.05). Patients with HHV6 detected in plasma had significantly higher mortality compared with HHV6-negative patients (67% (4/6) vs. 6% (4/69); p<0.001). The other viruses tested were not statistically associated with higher mortality when examined individually: EBV (30% (3/10) vs. 8% (5/65); p=0.07), adenovirus (20% (4/20) vs. 7% (4/55); p=0.10), HSV (33% (1/3) vs. 10% (7/72); p=0.29) and CMV (20% (1/5) vs. 10% (7/70); p=0.44).
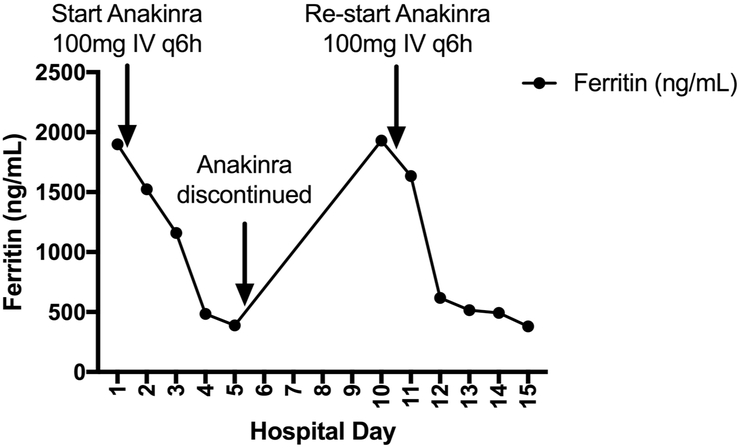
Figure 5 shows an illustrative case of a 17-year-old immune competent child admitted to the intensive care unit with severe sepsis and viral DNAemia. Plasma ferritin level was measured on admission and was 1,899 ng/mL. Subsequent testing confirmed acute EBV infection. In addition to supportive care, the patient was treated with the IL-1 receptor antagonist anakinra.(22) Plasma ferritin rapidly declined in conjunction with resolution of the patient’s organ failure. Anakinra was discontinued and the patient subsequently had recurrence of fever and serositis. Plasma ferritin was measured and found to again be elevated on hospital day 10. Anakinra was re-started and the patient subsequently improved and was discharged on hospital day 15.
Figure 5. Plasma ferritin values in a patient with viral DNAemia and hyperferritinemic multiorgan dysfunction syndrome treated with anakinra.
17-year old with EBV-associated hyperferritinemic multiorgan dysfunction syndrome. In addition to the recrudescent increase in ferritin values, this patient had worsening serositis and fever after discontinuation of anakinra.
DISCUSSION
Emerging data demonstrate that ferritin has a diverse array of functions in healthy and disease states.(24,25) The association between hyperferritinemia and sepsis severity in children was initially shown by Garcia et al in a resource limited setting in which ferritin level was independently associated with mortality.(10) Subsequently, Bennett et al determined a ferritin level cutoff of 1000ng/mL as being associated with increased risk of ICU admission and mortality in hospitalized children in the resource rich setting.(11) Our study corroborates these findings in a quaternary care pediatric ICU and finds that, in patients with severe sepsis, ferritin level is associated with occult viral DNAemia. We observed that DNAemia was present in 78% of patients with severe sepsis and plasma ferritin >1000 ng/mL. The association between DNAemia and plasma ferritin remained significant even when controlling for bacterial and fungal co-infection. Plasma ferritin was specifically associated with detection of EBV, HHV6, and adenoviral DNAemia.
Walton et al have demonstrated that adults with sepsis commonly have occult viral DNAemia, with a similar incidence to that seen in patients with bone marrow transplant or organ transplantation.(6) In the pediatric cohort used in this study, Davila et al previously reported an incidence of DNAemia for children with severe sepsis which was similarly high to that of adults.(7) Notably, we found that only 10% of the patients found post hoc to have viral DNAemia were identified by routine clinical testing, suggesting that the presence of DNAemia can be under-recognized with usual care. In our experience, DNA viral testing often is limited to patients with severe sepsis receiving immunosuppressive medications to treat cancer or to prevent transplant rejection. However, unlike plasma ferritin, we found that cancer diagnosis and history of organ transplant alone were not good predictors of DNAemia in children with severe sepsis. In part, children with severe sepsis and DNAemia may more generally have an immune-paralysis phenotype – an acquired and potentially treatable immune deficiency affecting both the innate and adaptive immune system.(7,16,26)
Adenovirus was the DNA virus identified most frequently in our patient cohort of children with severe sepsis. Although the majority of reported cases of severe adenoviral infection have occurred in children following solid organ or stem cell transplantation, adenovirus also can cause life-threatening disease in immunocompetent children.(27) Disseminated adenoviral infection occurs from new infection or reactivation of adenovirus residing in lymphoid tissues of the upper respiratory and gastrointestinal tracts. Viral reactivation in cancer and transplant patients is thought to occur via chemotherapeutic depletion of interferon-producing lymphoid cells.(28) In immunocompromised patients, the use of antiviral agents is associated with reduced viral load and improved survival when started earlier in the course of disease.(29) Treatment of immunocompetent children with disseminated adenoviremia using antiviral agents is controversial due to renal toxicity, poor antiviral activity of the available agents, and the typical self-limited nature of the disease.(28) However, patients with severe sepsis and acquired immune suppression with adenoviremia may represent a cohort of patients deserving further study for risks and benefits of anti-adenoviral agents.
Weiss et al previously reported that MODS was the most common mode of death in children that survived initial resuscitation and subsequently died three or more days after sepsis recognition.(2) Examining the association between viral DNAemia and MODS, we found that patients with severe sepsis and DNAemia were more likely to have failure of ≥ 4 organs compared with patients without DNAemia. In addition, each DNA virus type detected conferred a 2-fold increase in mortality risk adjusted for illness severity and cancer diagnosis. Thus, viral DNAemia may represent a risk factor for progression of organ failure in children with severe sepsis.
The production of ferritin is tightly regulated by the presence of intracellular iron as well as by cytokines and inflammation.(8) In response to infection, iron sequestration to macrophages and hepatocytes promotes translation of ferritin subunits by influencing regulatory protein binding to the iron responsive element. Cytokines may influence ferritin gene expression via NFκB and mRNA translation through induction of inducible nitric oxide synthase (iNOS) and production of NO. Increased storage of iron by ferritin intracellularly and extracellularly limits growth of bacterial and fungal pathogens that depend on the bioavailability of iron.(30) Interleukin-18 (IL-18) has been proposed to induce a marked elevation of plasma ferritin in response to viral infection as well as interferon-gamma production by T lymphocytes for defense against intracellular pathogens.(12,13) Slaats et al proposed a model of acute phase reactants characterized by IL-18 and ferritin response to viral infection and an IL-6 and CRP response to bacterial infection, although some bacteria are known to induce elevation of plasma IL-18 and some viruses are known to raise IL-6.(12) Kernan et al proposed a feed-forward mechanism in which DNAemia may induce hyperferritinemia by binding to pattern recognition receptors, such as Toll-like receptor 9, and amplify the inflammatory response in sepsis.(24)
Hemophagocytic lymphohistiocytosis (HLH) and MAS are hyperinflammatory syndromes characterized by cytokine storm, organ failure, and high mortality which may be due to rare genetic mutations or as complications of autoimmune / autoinflammatory disorders or infection.(31) Viral infections are the most common infectious triggers of HLH/MAS, with EBV being the most commonly reported.(32) Kryiazopoulou et al defined macrophage activation-like syndrome (MALS) in the Hellenic Sepsis Study Group, a large prospective cohort of sepsis patients, based on the presence of Sepsis-3 criteria and either hepatobiliary dysfunction and disseminated intravascular coagulation or a score based on HLH diagnostic criteria. MALS, which accounted for ~4% of the patients, was independently associated with early mortality. We observed that patients with MAS in our cohort had significantly higher circulating ferritin and number of circulating DNA viruses after controlling for bacterial and fungal co-infections. Patients with sepsis and high ferritin levels may be a marker of evolving MALS or MAS, and the high ferritin in this setting may allow for earlier recognition of and intervention for these life-threatening complications of sepsis.
Several therapeutic options that may target viral DNAemia induced hyperferritinemia and hyperinflammation are available and have been shown to be effective in specific sepsis groups. Demirkol et al found that children with hyperferritinemia associated failure of five and six organs who were treated with plasma exchange and intravenous immunoglobulin or methylprednisolone had improved survival compared with patients receiving a more immunosuppressive regimen that included dexamethasone and/or cyclosporine and/or etoposide.(33) Shakoory et al performed a secondary analysis of an adult Phase 3 trial of interleukin-1 receptor antagonist for severe sepsis and found survival was significantly increased in patients with concurrent hepatobiliary dysfunction and disseminated intravascular coagulation, an organ failure pattern of macrophage activation syndrome, if treated with anakinra.(22) We present the case of a previously healthy child with severe sepsis, acute EBV infection, and hyperferritimia who was treated at our institution with anakinra in addition to supportive care. Further study is needed to assess whether an approach that incorporates measuring plasma ferritin and testing for DNA viruses combined with targeted antiviral or immunomodulatory therapies can lead to improved outcomes for children with high mortality risk due to severe sepsis.
There are several important limitations to our study. First, this was a single center study with a relatively high incidence of patients with chronic medical conditions that may have contributed to the high prevalence of viral DNAemia in our cohort. A multiple center study is needed to corroborate our findings. Second, our post hoc DNAemia surveillance testing was performed on plasma, which suggests that active viral replication was occurring in patients who tested positive though we could not distinguish between primary infection and reactivation. Sufficient plasma was not available for serologies to study this further. Third, the current study is an association study which does not determine whether DNAemia was causative for hyperferritinemia, organ failure or mortality. Fourth, two patients each had two independent episodes of sepsis included in the final analysis. Our conclusions does not change if only the first episode of sepsis is included for each patient. Lastly, we tested for five common DNA viruses, but it is possible that we missed patients with other DNA viruses not included in our panel.
A prospective multicenter study is needed to evaluate the utility of ferritin levels as a biomarker for occult viral DNAemia in children with severe sepsis.
Acknowledgments
Supported by NICHD T32 HD40686 (to D.S.), R01 GM108618 (to J.C.), UG1 HD049983 (to J.C.), and U01 HD049934. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2013. September;14(7):686–93. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SL, Balamuth F, Hensley J, Fitzgerald JC, Bush J, Nadkarni VM, et al. The Epidemiology of Hospital Death Following Pediatric Severe Sepsis: When, Why, and How Children With Sepsis Die. Pediatr Crit Care Med. 2017. September;18(9):823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merker M, Bolliger R, Schuetz P. Procalcitonin-guided decision-making results in a significant reduction of antibiotic therapy and hospital stay in neonates with suspected early-onset sepsis. BMJ Evid-Based Med. 2018. August;23(4):154–5. [DOI] [PubMed] [Google Scholar]
- 4.Quadir AF, Britton PN. Procalcitonin and C-reactive protein as biomarkers for neonatal bacterial infection. J Paediatr Child Health. 2018. June;54(6): 695–9. [DOI] [PubMed] [Google Scholar]
- 5.Carcillo JA, Sward K, Halstead ES, Telford R, Jimenez-Bacardi A, Shakoory B, et al. A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis. Pediatr Crit Care Med J Soc Crit Care Med. 2017;18(2):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, et al. Reactivation of multiple viruses in patients with sepsis. PloS One. 2014;9(2):e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila S, Halstead ES, Hall MW, Doctor A, Telford R, Holubkov R, et al. Viral DNAemia and Immune Suppression in Pediatric Sepsis. Pediatr Crit Care Med. 2018;19(1):e14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002. May 15;99(10):3505–16. [DOI] [PubMed] [Google Scholar]
- 9.Soldin OP, Bierbower LH, Choi JJ, Choi JJ, Thompson-Hoffman S, Soldin SJ. Serum iron, ferritin, transferrin, total iron binding capacity, hs-CRP, LDL cholesterol and magnesium in children; new reference intervals using the Dade Dimension Clinical Chemistry System. Clin Chim Acta Int J Clin Chem. 2004. April;342(0):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia PCR, Longhi F, Branco RG, Piva JP, Lacks D, Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr Oslo Nor 1992. 2007. December;96(12):1829–31. [DOI] [PubMed] [Google Scholar]
- 11.Bennett TD, Hayward KN, Farris RWD, Ringold S, Wallace CA, Brogan TV. Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr Crit Care Med J Soc Crit Care Med. 2011. November;12(6):e233–236. [DOI] [PubMed] [Google Scholar]
- 12.Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL-1βIL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016. December;12(12):e1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Veerdonk FL, Wever PC, Hermans MHA, Fijnheer R, Joosten LAB, van der Meer JWM, et al. IL-18 serum concentration is markedly elevated in acute EBV infection and can serve as a marker for disease severity. J Infect Dis. 2012. July 15;206(2):197–201. [DOI] [PubMed] [Google Scholar]
- 14.McElroy AK, Erickson BR, Flietstra TD, Rollin PE, Nichol ST, Towner JS, et al. Ebola Hemorrhagic Fever: Novel Biomarker Correlates of Clinical Outcome. J Infect Dis. 2014. August 15;210(4):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soundravally R, Agieshkumar B, Daisy M, Sherin J, Cleetus CC. Ferritin levels predict severe dengue. Infection. 2015. February;43(1):13–9. [DOI] [PubMed] [Google Scholar]
- 16.Carcillo JA, Halstead ES, Hall MW, Nguyen TC, Reeder R, Aneja R, et al. Three Hypothetical Inflammation Pathobiology Phenotypes and Pediatric Sepsis-Induced Multiple Organ Failure Outcome. Pediatr Crit Care Med. 2017;18(6):513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez JL, Storch GA. Multiplex, quantitative, real-time PCR assay for cytomegalovirus and human DNA. J Clin Microbiol. 2002. July;40(7):2381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henke-Gendo C, Ganzenmueller T, Kluba J, Harste G, Raggub L, Heim A. Improved quantitative PCR protocols for adenovirus and CMV with an internal inhibition control system and automated nucleic acid isolation. J Med Virol. 2012. June;84(6):890–6. [DOI] [PubMed] [Google Scholar]
- 19.Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol Hoboken NJ. 2014. November;66(11):3160–9. [DOI] [PubMed] [Google Scholar]
- 20.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The Immunology of Macrophage Activation Syndrome. Front Immunol. 2019;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100(3):481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016. February;44(2):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, Dimopoulos G, Pantazi A, Orfanos SE, et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017. 18;15(1): 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017. 01;29(9):401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kernan KF, Ghaloul-Gonzalez L, Shakoory B, Kellum JA, Angus DC, Carcillo JA. Adults with septic shock and extreme hyperferritinemia exhibit pathogenic immune variation. Genes Immun. 2018. July 6; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall MW, Greathouse KC, Thakkar RK, Sribnick EA, Muszynski JA. Immunoparalysis in Pediatric Critical Care. Pediatr Clin North Am. 2017;64(5):1089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis Off Publ Infect Dis Soc Am. 1998. November;27(5):1194–200. [DOI] [PubMed] [Google Scholar]
- 28.Radke JR, Cook JL. Human adenovirus infections: update and consideration of mechanisms of viral persistence. Curr Opin Infect Dis. 2018;31(3):251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiwarkar P, Amrolia P, Sivaprakasam P, Lum SH, Doss H, O’Rafferty C, et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood. 2017. 06;129(14):2033–7. [DOI] [PubMed] [Google Scholar]
- 30.Parrow NL, Fleming RE, Minnick MF. Sequestration and scavenging of iron in infection. Infect Immun. 2013. October;81(10):3503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakike E, Giamarellos-Bourboulis EJ. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front Immunol. 2019. January 31;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maakaroun NR, Moanna A, Jacob JT, Albrecht H. Viral infections associated with haemophagocytic syndrome. Rev Med Virol. 2010. March;20(2):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demirkol D, Yildizdas D, Bayrakci B, Karapinar B, Kendirli T, Koroglu TF, et al. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care Lond Engl. 2012. December 12;16(2):R52. [DOI] [PMC free article] [PubMed] [Google Scholar]