Abstract
Eating tasty foods dampens responses to stress – an idea reflected in the colloquial term ‘comfort foods’. To study the neurobiological mechanisms by which palatable foods provide stress relief, we previously characterized a limited sucrose intake (LSI) paradigm in which male rats are given twice-daily access to 4 ml of 30% sucrose solution (vs. water as a control), and subsequently have reduced hypothalamic-pituitary-adrenocortical (HPA) axis responsivity and anxiety-related behaviors. Importantly, women may be more prone to ‘comfort feeding’ than men, and this may vary across the menstrual cycle, suggesting the potential for important sex and estrous cycle differences. In support of this idea, LSI reduces HPA axis responses in female rats during the proestrus/estrus (P/E), as opposed to the diestrus 1/diestrus 2 (D1/D2) estrous cycle stage. However, the effect of LSI on anxiety-related behaviors in females remains unknown. Here we show that LSI reduced stress-related behaviors in female rats in the elevated plus-maze and restraint tests, but not in the open field test, though only during P/E. LSI also decreased the HPA axis stress response primarily during P/E, consistent with prior findings. Finally, cFos immunolabeling (a marker of neuronal activation) revealed that LSI increased post-restraint cFos in the central amygdala medial subdivision (CeM) and the bed nucleus of the stria terminalis posterior subnuclei (BSTp) exclusively during P/E. These results suggest that in female rats, palatable food reduces both behavioral and neuroendocrine stress responses in an estrous-cycle dependent manner, and the CeM and BSTp are implicated as potential mediators of these effects.
Keywords: Sucrose, ACTH, corticosterone, estrous cycle, stress-related behavior
INTRODUCTION
The overall incidence of obesity among adults in the United States was 36% in 2014, and the Center for Disease Control reports the prevalence of obesity is slightly higher in women than men, affecting 38.3% and 34.3% of adult women and men, respectively (Flegal et al., 2012; Ogden et al., 2015). Daily life stress may be one contributor to these high obesity rates. In 40–70% of people, stress can increase food intake (Epel et al., 2004; Oliver and Wardle, 1999; Weinstein et al., 1997), particularly the intake of highly-palatable, calorically-dense foods that are often high in carbohydrates and/or fat (Cartwright et al., 2003; Epel et al., 2001; Groesz et al., 2012; Kim et al., 2013; Laugero et al., 2011; Oliver and Wardle, 1999; Zellner et al., 2006). These foods may be chosen for their ability to decrease feelings of stress or negative mood, an idea commonly thought of as ‘comfort food.’ In support of this idea, consumption of palatable foods can reduce stress responses both in humans (Anderson et al., 1987; Dube et al., 2005; Gibson, 2006; Macht and Mueller, 2007; Markus et al., 2000; Tomiyama et al., 2011; Tryon et al., 2013) and rodents (Bell et al., 2002; Dallman et al., 2005; Finger et al., 2011; Kinzig et al., 2008; la Fleur et al., 2005; Maniam and Morris, 2010; Pecoraro et al., 2004; Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007).
To investigate the mechanisms by which these highly-palatable ‘comfort foods’ can reduce stress responses, our lab has developed a limited sucrose intake (LSI) paradigm in rats (Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007). In this paradigm, adult, male rats with ad libitum access to standard chow are given twice-daily brief (up to 30 minutes (min)), limited (up to 4 ml/session; or 8 ml/day) access to a 30% sucrose solution (vs. water as a control). A history of LSI reduces numerous stress responses in male rats, including HPA axis responses to an acute stress, stress-induced tachycardia, and anxiety-like behavior (Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007).
Reports suggest that women may be more prone to ‘comfort food’ style eating than men (Grunberg and Straub, 1992; Klein et al., 2004; Oliver and Wardle, 1999; Oliver et al., 2000; Wansink et al., 2003; Wardle et al., 2000; Zellner et al., 2006), and this may vary across the menstrual cycle (Hildebrandt et al., 2015; Klump et al., 2013a; Klump et al., 2013b; Racine et al., 2013). Therefore, the effects of palatable food to reduce stress may differ between men and women, which could have important implications for prevention or treatment of obesity and its related metabolic disorders. While our LSI paradigm was initially developed and characterized in male rats, studying its effects in female rats could provide important insights into sex differences in palatable food-mediated stress relief. Indeed, an initial study of LSI in female rats showed that, while females have a similar metabolic response to the LSI paradigm as males, the ability of LSI to blunt the HPA axis stress response in females depends on estrous cycle stage (Egan et al., 2018). More specifically, LSI reduced HPA reactivity primarily during the proestrus/estrus (P/E) stage of their estrous cycle.
Importantly, altered HPA axis regulation is often accompanied by changes in mood and behavior (Gold, 2015; Jacobson, 2014; Packard et al., 2016; Spiga et al., 2014; Strohle and Holsboer, 2003). For example, LSI decreases HPA axis reactivity in male rats, in addition to reducing indices of anxiety-like behaviors in the elevated plus-maze, social interaction, and open field tests (Ulrich-Lai et al., 2010). This suggests that LSI-mediated HPA dampening may be concomitant with reduced anxiety-related behaviors in female rats. As LSI-induced HPA blunting primarily occurs during P/E, and anxiety-related behaviors can also vary throughout the estrous cycle (Frye et al., 2000; Frye and Walf, 2002; Galeeva and Tuohimaa, 2001; Gray and Cooney, 1982; Marcondes et al., 2001; Meziane et al., 2007; Mora et al., 1996; Pare and Redei, 1993; Walf et al., 2009), the present studies therefore test the hypothesis that LSI attenuates anxiety-related behaviors in females exclusively during P/E. Behavior was tested in the open field and elevated plus-maze – two widely-used tests of anxiety-related behaviors (Cruz et al., 1994; File et al., 2004; McCarthy et al., 1995; Pellow et al., 1985; Prut and Belzung, 2003; Ulrich-Lai et al., 2010; Walsh and Cummins, 1976). Struggling behavior was also assessed during a restraint test. Evidence indicates that the amount of struggling during restraint correlates with the degree of HPA activation (Grissom et al., 2008; Weinberg et al., 2010), and that the anxiolytic drug diazepam decreases behavioral struggling (Ushijima et al., 1986), suggesting the restraint test may be a useful approach to simultaneously measure stress-related behavior and HPA axis activation. Finally, the effect of LSI on post-restraint cFos immunolabeling, a common indirect marker of recent neuronal activation (Chan et al., 1993; Cullinan et al., 1995; Curran and Morgan, 1995; Kovacs and Sawchenko, 1996; Ulrich-Lai et al., 2007), was determined in multiple stress-activated brain regions to identify those that may contribute to the cycle-dependent anxiolytic and HPA-blunting effects of LSI.
MATERIALS AND METHODS
Animals
Adult, female Long-Evans rats weighing approximately 150–175 g (~7 weeks of age at time of arrival) were acquired from Harlan Laboratories (Indianapolis, IN). Rats were individually housed in a temperature- and humidity-controlled environment with a 12 hour (6:00–18:00) light-dark cycle. Rats were given ad libitum access to standard rat chow (LM-485; Harlan Teklad, Madison, WI) and water throughout the experiment. All animals were acclimated to the housing facilities for at least one week before beginning experimental protocols. Housing facilities were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC) and are consistent with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. To evaluate the overall effects of LSI on energy balance, food intake and body weight were monitored throughout the experiment, and body composition was measured with nuclear magnetic resonance (NMR, EchoMRI, Houston, TX) on experiment days 0 and 22. We have previously seen that both male (Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007) and female (Egan et al., 2018) LSI rats eat slightly less chow, presumably to compensate for the calories provided by the sucrose, and as a result LSI does not alter body weight and percent body fat.
Experimental overview
A schematic of the experimental timeline is shown in Figure 1. The LSI paradigm was performed as previously described (Christiansen et al., 2011; Egan et al., 2018; Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2016; Ulrich-Lai et al., 2011; Ulrich-Lai et al., 2007). Briefly, rats with free access to food and water were given additional twice-daily (approximately 8:30 h and 15:30 h), brief (up to 30 min), limited (up to 4 ml per session; maximum of 8 ml per day) access to a second drink bottle containing 30% sucrose solution (MP Biomedical, Solon, OH), or water as a control. Rats underwent the LSI paradigm for a total of 23 days, and received behavioral testing (as detailed below) in the open field test (OFT) and elevated plus-maze (EPM) on days 12 and 16 of LSI exposure, respectively. The OFT and EPM were performed midday between 10:00 h and 14:30 h, so as not to overlap with the times of LSI exposure. Rats also received a restraint stress (as detailed below) on the morning following the last LSI exposure (experiment day 24). Vaginal lavage was performed on each animal immediately following the OFT (day 12), EPM (day 16) and restraint (day 24) tests to determine estrous cycle stage as described below.
Figure 1. Experimental timeline of LSI exposure.
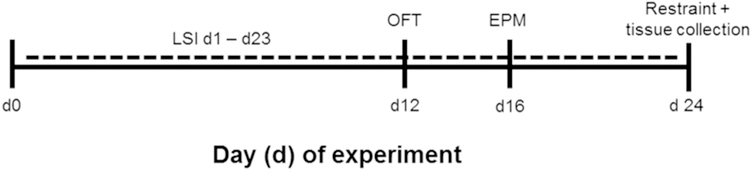
Female rats received twice-daily access to a limited amount of sucrose drink (vs. water as a control) on days 1–23. Rats were tested in the open field test (OFT) and elevated plus-maze (EPM) between the morning and afternoon LSI sessions on days 12 and 16, respectively. On the morning of day 24, rats received a restraint challenge with subsequent collection of brain tissue for cFos immunolabeling.
Open field test (OFT)
On experiment day 12, rats were individually placed into an open field apparatus (~1 m x ~1 m) for 5 min under dim light, and their behavior was video recorded for later analysis. Clever TopScan (CleverSys Inc., Reston, VA) software was used to determine the time spent in the center of the open field and total distance traveled. Grooming and rearing behaviors were scored by two observers unaware of the treatment groups, and the observers’ scores were averaged to obtain the mean value of each behavior for each rat. The values between the observers were typically within ~10% of each other. In rare instances, larger discrepancies occurred between the two observers’ scores, in which case the video for that rat was rescored to reach a consensus.
Elevated plus-maze (EPM)
On experiment day 16, rats were individually placed into the center of an EPM apparatus (approximate arm size of 4 in. wide x 40 in. long, 14-in. high walls on the closed arms) in a dark room under red lights, and were allowed to explore the maze for 5 minutes. The resulting behavior was video recorded for later analysis. Clever TopScan (CleverSys Inc., Reston, VA) software was used to measure the total time spent in the open arms, the total number of entries into the open arms, the total number of entries into all arms, and the total distance traveled. Head dipping behavior, defined as any time the animal moved its head off the EPM apparatus and looked over the edge of an open arm (Cruz et al., 1994), was scored by two observers unaware of treatment groups and the average of these scores was taken as the value for each rat. The observers’ values were typically within ~10% of each other. In the rare instance when larger discrepancies occurred, the video for that rat was rescored to reach consensus. Moreover, behavioral patterns in the EPM are known to vary across the duration of the test, with most open arm activity occurring during the first 1–2 minutes of the test (Arabo et al., 2014; Bertoglio and Carobrez, 2002; Carobrez and Bertoglio, 2005; Casarrubea et al., 2013; Holmes and Rodgers, 1998; Rodgers et al., 1996). Thus, to consider the temporal dynamics of each rat’s response to the test, behaviors were grouped into consecutive 1-min bins for analysis.
Restraint stress for behavioral struggling, HPA reactivity, and brain cFos responses
On the morning of experiment day 24, rats were given a 20-minute restraint stress challenge, and did not receive LSI on that day. Prior to restraint testing, rats were left undisturbed in their home cages in their normal housing room. The home cage for each rat was then individually carried to an adjacent procedure room, where each rat was placed into a clear, well-ventilated plastic restraint tube (i.e., a cylinder that is approximately 20 cm long, and 7 cm in diameter) with rapid collection of tail-clip blood samples (200 μl) into ice-cold EDTA-coated tubes. Care was taken to ensure this first blood collection (i.e., at 0 min) was completed within 3 min of first touching/moving each rat’s home cage, thereby ensuring assessment of basal, pre-stress hormone levels (Vahl et al., 2005). Rat behavior while in the restraint tube was video recorded for later analysis. Blood samples were again collected at 20, 40, 60, and 90 min after the onset of restraint, with each sample collection being completed within 3 min. Note that in our previous experiment assessing the impact of LSI on HPA reactivity to restraint stress in female rats (Egan et al., 2018), blood samples were collected through 60 min after stress onset, as we routinely use for analogous studies in male rats (Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2011). In that experiment, LSI reduced the plasma adrenocorticotropic hormone (ACTH) response at the 60-min time point, but did not impact the corresponding plasma corticosterone levels. However, this did not preclude the possibility that plasma corticosterone may have been affected at later post-stress time points, as there is an inevitable time-lag between changes in plasma ACTH and subsequent changes in plasma corticosterone. In the present experiment, therefore, post-stress blood collection sampling was extended to 90 min after stress onset to increase the opportunity to detect potential changes in plasma corticosterone in female rats. Blood samples were centrifuged (3000 x g, 15 min, 4°C) and plasma was stored at −80°C until measurement of plasma ACTH and corticosterone by radioimmunoassay (RIA). Struggling behaviors, defined as any behaviors that represent the animal trying to make or find a way out of the restrainer (e.g., biting, chewing, scratching or pushing at the walls of the restrainer, and/or attempting to turn or spin around in the restrainer (Grissom et al., 2008; Weinberg et al., 2010)), was scored by two observers unaware of the treatment groups. The values between observers were typically within 10% of each other, and in the rare instance in which larger discrepancies occurred, the video for that rat was rescored to reach a consensus. Since struggling behavior varies across the duration of the restraint, with most vigorous struggling occurring during the first 5 min of the test (Grissom et al., 2008; Weinberg et al., 2010), data were grouped into consecutive 5-min bins for analysis.
Immediately after the 90-min time point blood collection, rats were injected with an overdose of pentobarbital and vaginal lavage samples were taken to determine estrous cycle stage. Next, a cardiac blood sample was quickly collected and resulting plasma was stored at − 80°C until later measurement of plasma estradiol by RIA. Rats were then transcardially perfused with 0.9% saline followed by 3.7% paraformaldehyde for collection of brains for cFos immunolabeling. The 90-min post-stress time point was selected for brain collection as it approximates the peak of the cFos protein response to acute stress (Kovacs and Sawchenko, 1996; Sonnenberg et al., 1989). Brains were post-fixed in 3.7% paraformaldehyde overnight at room temperature, and were then stored in 30% sucrose in phosphate-buffered saline (PBS) at 4°C. Brains were sectioned (25 µm) in a 1-in-12 series on a freezing-stage microtome (Leica Biosystems, Wetzler, Germany) and slices were stored in cryoprotectant (PBS with 1% polyvinylpyrrolidone (PVP-40, Sigma Chemical, Perth, WA), 30% ethylene glycol (Fisher Scientific, Pittsburgh, PA) and 30% sucrose (Amaresco, Solon, OH)) at −20°C until immunolabeling for cFos protein expression.
Assessment of cFos immunolabeling in stress-related brain regions
Immunohistochemistry was performed as previously described (Ryan et al., 2018). Sections were washed in 50 mM potassium phosphate-buffered saline (KPBS), incubated in a 20% hydrogen peroxide solution for 20 min, and rinsed again in KPBS. Sections were then blocked in a solution of 0.1% bovine serum albumin (BSA, Sigma-Aldrich) with 0.2% Triton X-100 (Sigma-Aldrich) in KPBS for 1 hr at room temperature, and incubated overnight at 4°C with primary antibody (primary rabbit antisera against cFos, 1:200, product # sc-52, Santa Cruz, Dallas, TX) diluted in blocking solution. The following morning, sections were rinsed in KPBS, incubated in biotinylated goat anti-rabbit secondary antibody (1:500, Vector Laboratories, Burlingame, CA) for 1 h, rinsed in KPBS, and incubated in avidin-biotin-peroxidase (Vectastain ABC solution, Vector Laboratories, Burlingame, CA) for 1 hr. Following another rinse in KPBS, sections were reacted with 3,3’-diaminobenzidine (Sigma Chemical, Perth, WA). Immunolabeling for cFos was visualized using brightfield light microscopy on an Axio Imager.M2 microscope, with an AxioCam camera, and using Zen 2012 software (Carl Zeiss Microscopy, Jena, Germany).
The density of cFos-positive cells was measured bilaterally in all available, intact sections that contained the regions of interest, as defined by standard rat brain atlases (Paxinos and Watson, 1998; Swanson, 1998), using Scion Image software (Scion Corp, Frederick, MD). Immunolabeling for cFos was assessed in the following regions: basolateral amygdala (BLA); bed nucleus of the stria terminalis, posterior subnuclei (BSTp) (principal, transverse, and interfascicular); central amygdala (CeA), medial (CeM) and lateral (CeL) subdivisions; dorsomedial hypothalamus (DMH), dorsal and ventral subdivisions; medial amygdala, posterodorsal nucleus (MeApd); nucleus accumbens (NAc),core and shell subdivisions; prefrontal cortex (PFC), prelimbic (PL), infralimbic (IL) and anterior cingulate (Ant Cing) subdivisions; and paraventricular nucleus of the hypothalamus (PVN). All analyses were performed by an observer unaware of the treatment groups.
Hormone assays
Plasma ACTH levels were measured via RIA using a specific antiserum generously donated by Dr. William Engeland (University of Minnesota, Minneapolis, MN) as described previously (Jasper and Engeland, 1991). The minimum detection of this assay is 15.6 pg/ml, and the intra-assay and inter-assay coefficients of variance are both 13%. Plasma corticosterone levels were measured via an 125I corticosterone double antibody RIA kit (product #07120103, MP Biomedicals, Solon, OH). The minimum detection of this assay is 7.7 ng/ml, and the intra-assay and inter-assay coefficients of variance are both 7%, as indicated by the manufacturer. Plasma estradiol levels were measured via an 125I 17Β-estradiol (E2) double antibody RIA kit (product # 07138102, MP Biomedicals, Solon, OH). The minimum detection of this assay is 10 pg/ml, and the intra-assay and inter-assay coefficients of variance are 5 and 9%, respectively, as indicated by the manufacturer.
Determination of estrous cycle stage by cytology
To determine the impact of estrous cycle on behavioral, HPA, and brain measures, vaginal lavage samples were taken at the end of each behavioral test (on experiment days 12 and 16), and again just prior to animal perfusion and tissue collection (on experiment day 24). Estrous cycle stage assessments were limited to the days of sample collection due to concerns that the stress associated with a daily lavage procedure could impact the primary experimental outcomes (i.e., stress-related behavioral and HPA axis reactivity, and brain activation patterns). Vaginal cytology was then used to determine estrous cycle stage (Becker et al., 2005; Cora et al., 2015; Goldman et al., 2007; Marcondes et al., 2002). More specifically, diestrus 1 (D1) cytology was characterized by large numbers of leukocytes, mucous, and smaller amounts of non-nucleated epithelial cells. Diestrus 2 (D2) was very similar to D1, but could be distinguished by having a predominance of leukocytes. In contrast, proestrus (P) and estrus (E) had predominant clusters of nucleated non-cornified and non-nucleated cornified epithelial cells, respectively, with little-to-no leukocytes and mucous. Late proestrus was defined as a transitional stage between P and E, in which large numbers of nucleated non-cornified epithelial cells were accompanied by the appearance of some non-nucleated cornified epithelial cells. Animals were then classified into two different estrous cycle sub-groups: 1) those in D1 or D2 (D1/D2), when females would be expected to have lower levels of gonadal hormones, and 2) those in proestrus, late proestrus, or estrus (P/E), when females would be expected to have higher levels of gonadal hormones (Asarian and Geary, 2013; Butcher et al., 1974; Cecchini et al., 1983; Lu et al., 1985; Nequin et al., 1979; Smith et al., 1975). These two estrous cycle sub-groups were then compared to each other in all analyses (see below) to test for potential cycle-specific effects.
Statistical analysis
Data are presented as mean ± SEM. Intake from the second drink bottle, food intake, body weight, and percent body fat data were each analyzed by two-way repeated measures ANOVA with the factors DRINK and TIME. OFT data were analyzed via two-way ANOVA with the factors DRINK and CYCLE. EPM, restraint struggling, plasma ACTH and plasma corticosterone data were each analyzed by three-way repeated measures ANOVA with the factors DRINK, CYCLE, and TIME. Plasma estradiol levels were analyzed by one-way ANOVA with estrous cycle stage as the factor. Data sets with non-homogenous variance underwent a square-root transformation prior to ANOVA. ANOVAs were followed by a protected Neuman-Keuls post-hoc analysis. Potential outliers were tested as described previously (McClave and Dietrich, 1994; Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007) using two criteria: 1) outliers were values that were more than 1.96 times the standard deviation from the mean, and 2) outliers were values that were below the lower quartile or above the upper quartile by more than 1.5 times the interquartile range. Both criteria had to be met for a value to be removed as an outlier. Statistical significance was taken as p < 0.05. All main or interactive effects in the ANOVAs that reached statistical significance are described in the Results section; any main or interactive effects that are not discussed were p > 0.05.
When statistical differences were observed, effect size estimates were performed to indicate the strength of the treatment effects. For two-way and three-way ANOVAs, eta squared (η2, the proportion of total variation that can be attributed to each main or interactive effect) was determined using the formula: η2 = SSeffect / SStotal. In addition, as eta squared decreases as more variables are included in the experimental design, this complicates comparisons of effect sizes across experiments. To circumvent this difficulty, partial eta squared, (ηp2, the proportion of variation attributed to each main or interactive effect after excluding the variance explained by the other effects) was also determined using the formula: ηp2 = SSeffect / (SSeffect + SSerror(effect)).
RESULTS
Effects of LSI on energy balance
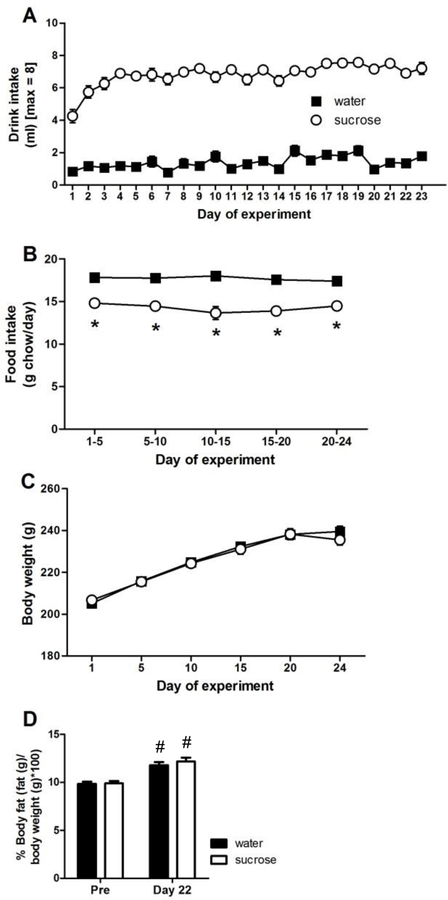
Rats given access to a 30% sucrose solution began to drink it in amounts approaching the maximum allowed within the first few days of LSI (Figure 2A), in accordance with its known high palatability to rats (Sclafani, 1987, 1991). In contrast, controls given water in the second drink bottle drank very little, as they had ad libitum access to water throughout, and thus had little-to-no incentive to drink additional water. Statistical analysis of drink intake showed a main effect of DRINK (F1,1103 = 476.22, p < 0.01, η2 = 0.78, ηp2 = 0.91), a main effect of TIME (F22,1103 = 10.28, p < 0.01, η2 = 0.024, ηp2 = 0.18), and a DRINK x TIME interaction (F22,1103 = 3.76, p < 0.01, η2 = 0.009, ηp2 = 0.075). Post-hoc analyses revealed greater drink intake among sucrose-fed rats on every day of LSI exposure.
Figure 2. The impact of LSI on energy balance in female rats.
(A) Drink intake from the second drink bottle, (B) food intake, (C) body weight, and (D) percent body fat. Not shown on panel (A) – all sucrose are greater (p < 0.05) than their respective water for every day of LSI exposure. *p < 0.05 vs. water, #p < 0.05 vs. Pre (experimental day 0). n = 23–24/group.
Food intake also showed a main effect of DRINK (F1,239 = 74.27, p < 0.01, η2 = 0.44, ηp2 = 0.62), and post-hoc analysis revealed that sucrose-fed rats reduced their food intake compared to water-fed controls (by ~15–20%) (Figure 2B). Body weight increased for both sucrose- and water-fed rats throughout the experiment (main effect of TIME F5,287 = 226.62, p < 0.01, η2 = 0.56, ηp2 = 0.83), with no difference between drink groups (Figure 2C). Percent body fat also showed a main effect of TIME (F1,95 = 57.16, p < 0.01, η2 = 0.61, ηp2 = 0.69), with increased body fat on experiment day 22 compared to experiment day 0 (Figure 2D); moreover, there were no differences between drink groups at either time point. Notably, the overall effects of LSI on energy balance are similar to those seen previously using both male (Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007) and female (Egan et al., 2018) rats.
Open field test
The percent of time spent in the center of the open field, a traditional measure of anxiety-like behavior in the OFT (File et al., 2004; McCarthy et al., 1995; Prut and Belzung, 2003; Walsh and Cummins, 1976), showed no differences among drink or cycle groups (Figure 3A). Similarly, grooming, an ethological index that positively correlates with anxiety-like behavior (Prut and Belzung, 2003; Walsh and Cummins, 1976), also showed no differences among groups (Figure 3B), nor did rearing, which can be used as a measure of vertical locomotion (Prut and Belzung, 2003) (Figure 3C). In contrast, total distance traveled showed a main effect of CYCLE (F1,45 = 4.65, p < 0.05, η2 = 0.082, ηp2 = 0.010), and a DRINK x CYCLE interaction (F1,45 = 5.85, p < 0.05, η2 = 0.10, ηp2 = 0.12). Post-hoc analyses revealed that sucrose-fed rats had increased total locomotion during the D1/D2 estrous cycle stage (Figure 3D). For consistency with the other behavioral tests utilized in this study, grooming and rearing were also analyzed as a time course after subdividing the data into consecutive 1-min intervals. That analysis also showed no effects of DRINK or CYCLE for either grooming or rearing (data not shown). Taken together, these data suggest that neither LSI nor estrous cycle affect anxiety-like behaviors in the OFT in female rats.
Figure 3. A history of LSI increases total locomotor activity but not anxiety-related behaviors in the OFT.
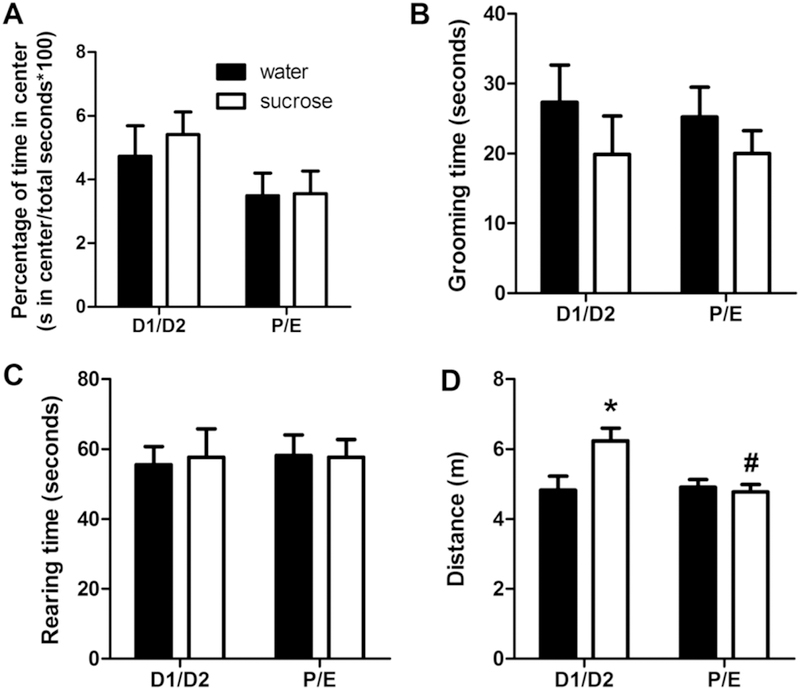
The impact of prior sucrose (vs. water) on the percentage of time spent in the center (A), time spent grooming (B) and rearing (C), and total distance traveled (D) in female rats tested during diestrus (D1/D2) and proestrus/estrus (P/E). *p < 0.05 vs. water, #p < 0.05 vs. D1/D2. n = 8–13/group.
Elevated plus-maze
Anxiety-like behaviors in the EPM were assessed via the time spent in open arms and the number of entries into open arms (Cruz et al., 1994; File et al., 2004; McCarthy et al., 1995; Pellow et al., 1985; Ulrich-Lai et al., 2010), as well as the number of head dips, which is considered an ethological index of exploratory behavior indicative of reduced anxiety (Cruz et al., 1994). Time spent in the open arms showed a main effect of TIME (F4,184 = 19.53, p < 0.01, η2 = 0.22, ηp2 = 0.37), with the highest amounts of open arm time occurring during the first minute of the EPM test. Open arm time also showed a DRINK x CYCLE x TIME interaction (F4,184 = 4.14, p < 0.01, η2 = 0.047, ηp2 = 0.11), and post-hoc analyses revealed that during minute 1, sucrose increased open arm time only when rats were in the P/E estrous cycle stage at the time of testing (Figure 4A).
Figure 4. A history of LSI decreases anxiety-like behaviors in the EPM only during P/E.
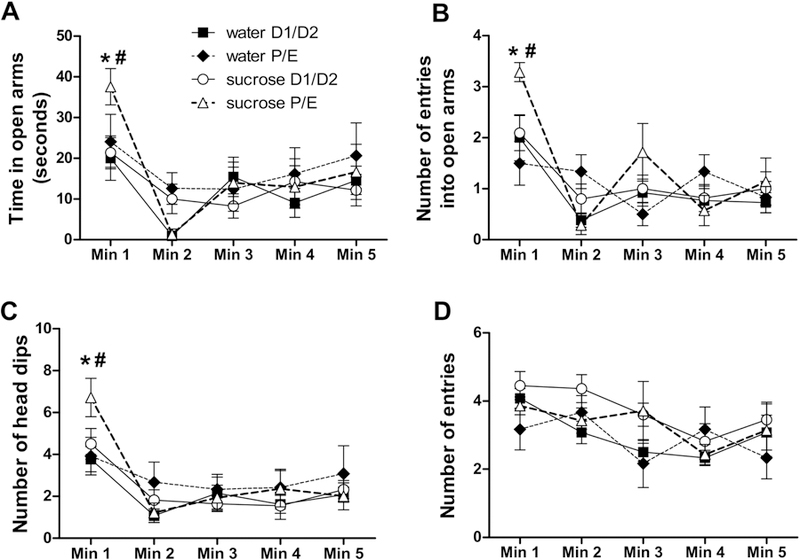
The impact of prior sucrose (vs. water) on the time spent in the open arms (A), and the number of entries into the open arms (B), head dips (C), and total (open and closed) arm entries (D) in female rats tested during diestrus (D1/D2) and proestrus/estrus (P/E). *p < 0.05 vs. water, #p < 0.05 vs. D1/D2. n = 6–12/group.
The number of entries into open arms also showed a main effect of TIME (F4,189 = 23.37, p < 0.01, η2 = 0.26, ηp2 = 0.41), with the greatest number occurring during the first minute of the test, as well as DRINK x TIME (F4,189 = 5.17, p < 0.01, η2 = 0.058, ηp2 = 0.13) and DRINK x CYCLE x TIME (F4,189 = 6.69, p < 0.05, η2 = 0.076, ηp2 = 0.16) interactions. Post-hoc analyses revealed that during minute 1, sucrose increased the number of open arm entries exclusively when rats were in the P/E estrous cycle stage at the time of testing (Figure 4B).
The number of head dips also showed a main effect of TIME (F4,184 = 27.61, p < 0.01, η2 = 0.29, ηp2 = 0.46), with the greatest number occurring during the first minute of the test, as well as DRINK x TIME (F4,184 = 4.33, p < 0.01, η2 = 0.045, ηp2 = 0.12) and DRINK x CYCLE x TIME (F4,184 = 3.25, p < 0.05, η2 = 0.033, ηp2 = 0.090) interactions. Similar to other measures, post-hoc analyses revealed that during minute 1, sucrose increased the number of head dips only when rats were in P/E (Figure 4C).
In contrast, when the total number of entries into the open and closed arms was used as a measure of locomotor activity (Cruz et al., 1994; File et al., 2004; McCarthy et al., 1995; Pellow et al., 1985), there were main effects of DRINK (F1,184 = 6.04, p < 0.05, η2 = 0.043, ηp2 = 0.16) and TIME (F4,184 = 8.99, p < 0.01, η2 = 0.019, ηp2 = 0.21), but no individual group differences were identified by post-hoc analysis at any time point (Figure 4D). Total distance traveled over the entire five minutes was also analyzed, with no main or interactive effects of either drink or cycle (data not shown). Collectively, these data suggest that LSI decreases anxiety-like behaviors in the EPM during the P/E stage of the estrous cycle. Moreover, open arm and head dipping behaviors were expressed at their highest levels during the first minute of the EPM test, and the LSI effects were most notable at this same time point.
Struggling during the restraint test
Time spent struggling during an acute restraint stress test showed main effects of DRINK (F1,140 = 4.11, p < 0.05, η2 = 0.034, ηp2 = 0.087) and TIME (F2,140 = 21.02, p < 0.01, η2 = 0.16, ηp2 = 0.33), with the time spent struggling generally decreasing over the duration of the test. Struggling time also showed DRINK x CYCLE (F1,140 = 4.63, p < 0.05, η2 = 0.038, ηp2 = 0.097), CYCLE x TIME (F2,140 = 4.02, p < 0.05, η2 = 0.030, ηp2 = 0.086) and DRINK x CYCLE x TIME (F2,140 = 3.21, p < 0.05, η2 = 0.024, ηp2 = 0.069) interactions. Post-hoc analyses revealed that during the first five minutes of the restraint test, sucrose decreased the time spent struggling only among rats tested in the P/E stage of the estrous cycle (Figure 5).
Figure 5. A history of LSI decreases struggling behavior during the restraint test only during P/E.
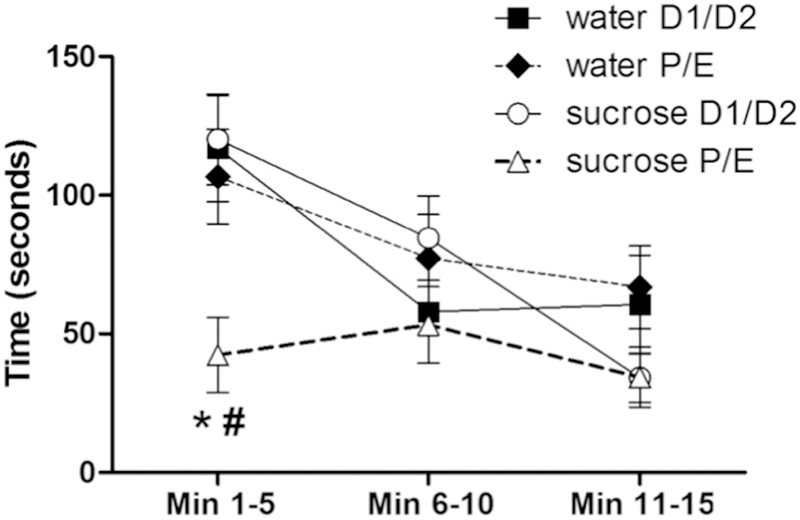
The impact of prior sucrose (vs. water) on the time spent struggling by female rats tested during diestrus (D1/D2) and proestrus/estrus (P/E). *p < 0.05 vs. water, #p < 0.05 vs. D1/D2. n = 9–15/group.
HPA axis response to restraint
To assess the HPA axis response to acute stress, we measured plasma ACTH and corticosterone prior to and following the restraint test. For the plasma ACTH response (Figure 6A,B), there were main effects of DRINK (F1,234 = 7.92, p < 0.01, η2 = 0.031, ηp2 = 0.16) with sucrose generally lowering plasma ACTH, and TIME (F4,234 = 197.48, p < 0.01, η2 = 0.63, ηp2 = 0.82) with restraint stress elevating plasma ACTH levels, as well as a DRINK x TIME interaction (F4,234 = 3.84, p < 0.01, η2 = 0.012, ηp2 = 0.082). Post-hoc analyses revealed sucrose decreased plasma ACTH primarily at 20, 40, and 60 min after restraint onset during the P/E estrous cycle stage.
Figure 6. A history of LSI dampens the plasma ACTH and corticosterone responses to restraint only during P/E.
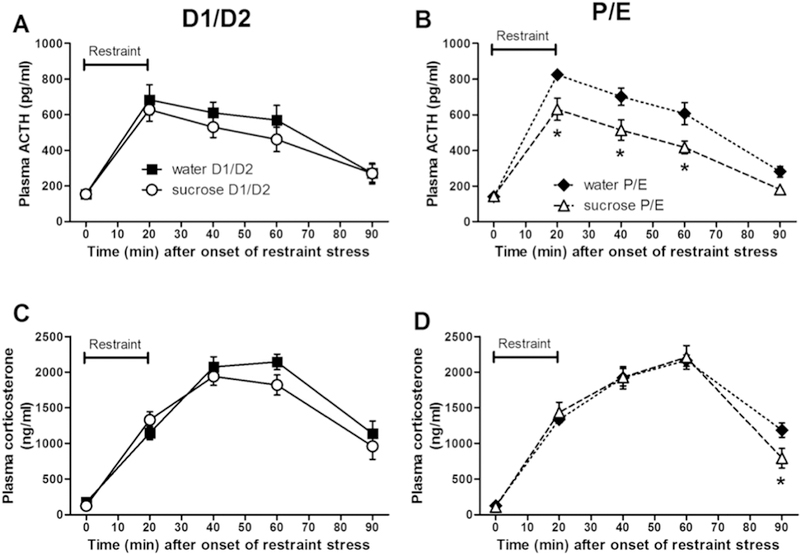
The impact of prior sucrose (vs. water) on the plasma ACTH (A, B) and corticosterone (C, D) responses to an acute restraint stress in female rats tested during diestrus (D1/D2) (A, C) and proestrus/estrus (P/E) (B, D). *p < 0.05 vs. water. n = 9–15/group. Note that for each hormone, data were analyzed via 3-way repeated measures ANOVA comparing DRINK, CYCLE, and TIME, but are graphed separated by estrous cycle stage to aid visualization.
The plasma corticosterone response (Figure 6C,D) showed a main effect of TIME (F4,234 = 402.48, p < 0.01, η2 = 0.85, ηp2 = 0.90) with restraint stress increasing plasma corticosterone levels, and a DRINK x TIME interaction (F4,234 = 3.33, p < 0.05, η2 = 0.007, ηp2 = 0.072). Post-hoc analyses revealed that sucrose decreased plasma corticosterone primarily at 90 min after restraint onset during the P/E stage of the estrous cycle.
Plasma estradiol
Plasma estradiol levels were measured on day 24 to corroborate the day 24 estrous cycle staging by lavage cytology, as in our prior study (Egan et al., 2018). While the experiment was designed to analyze behavioral, HPA, and immunolabeling endpoints in two broad estrous cycle stages (i.e., D1/D2 vs. P/E), plasma estradiol peaks specifically in proestrus (Asarian and Geary, 2013; Butcher et al., 1974; Cecchini et al., 1983; Lu et al., 1985; Nequin et al., 1979; Smith et al., 1975). Plasma estradiol levels were therefore compared after further subdividing rats into diestrus 1, diestrus 2, proestrus, late proestrus/estrus, and estrus based on their lavage cytology. This analysis (Table 1) revealed no differences between the cycle stages (one-way ANOVA, p > 0.05). However, unlike previous experiments (Egan et al., 2018), no rats in this particular cohort were identified as being in proestrus on experiment day 24. Thus, there was no opportunity to observe the specific stage characterized by peak estradiol levels, resulting in roughly equivalent plasma estradiol levels across the other stages (though plasma estradiol did trend higher during late proestrus/estrus relative to diestrus 2 when these two stages were directly compared (p = 0.05 by 2-tailed Mann-Whitney U test)).
Table 1. Rat plasma estradiol levels.
Rat plasma estradiol levels on experiment day 24 are shown divided by their respective estrous cycle stage, as determined by vaginal lavage cytology on experiment day 24. N/A= not available as no rats were in this estrous cycle stage at the time of assessment.
| Estrous cycle stage | Plasma estradiol (pg/ml) |
|---|---|
| Diestrus 1 (n = 14) | 133.5 ± 12.70 |
| Diestrus 2 (n = 7) | 109.9 ± 4.86 |
| Proestrus (n = 0) | N/A |
| Late proestrus/estrus (n = 6) | 161.5 ± 22.69 |
| Estrus (n = 19) | 145.9 ± 13.76 |
Post-restraint cFos immunolabeling in stress-associated brain regions
Quantification of cFos-positive cells was used as an index of restraint-induced activation in several brain regions known to be involved in stress regulation (Ulrich-Lai and Herman, 2009), including the BLA, BSTp, CeM, CeL, DMH (dorsal and ventral subdivisions), MeApd, NAc (core and shell subdivisions), PFC (PL, IL, Ant Cing subdivisions), and PVN.
As the medial and lateral subdivisions of the central amygdala can be differentially regulated by stress and reward (Ciocchi et al., 2010; Duvarci et al., 2011; Fudge and Haber, 2000; Gilpin et al., 2015; Haubensak et al., 2010; Martina et al., 1999), analysis of the CeA was sub-divided into its medial and lateral subneuclei. For the CeM, there was a main effect of RINK (F1,44 = 4.31, p < 0.05, η2 = 0.088, ηp2 = 0.095), with post-hoc analysis revealing that sucrose primarily increased cFos-positive immunolabeling among rats in P/E (Figure 7A,D). Of note, this sucrose effect appears to occur specifically in the medial subdivision of the CeA, since cFos-positive immunolabeling in the CeL was not altered by either drink or cycle (Figure 7B,D).
Figure 7. A history of LSI increases post-restraint cFos-immunolabeling in the CeM and BSTp only during P/E.
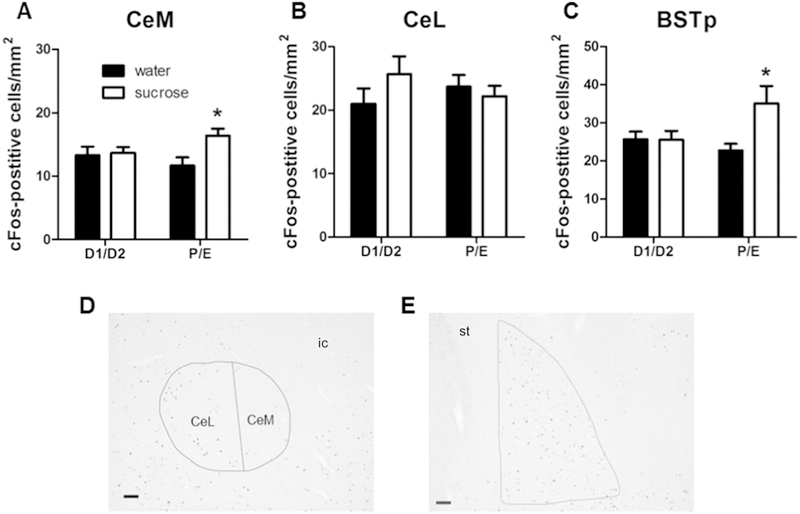
The impact of prior sucrose (vs. water) on the density of post-restraint cFos-positive cells in the CeM (A, D), CeL (B, D), and BSTp (C, E) in female rats tested during diestrus (D1/D2) and proestrus/estrus (P/E). Representative images shown in (D) and (E) are taken at 50x magnification from a water D1/D2 rat; scale bar = 100 µm. *p < 0.05 vs. water. n = 7–15/group. Abbreviations: BSTp, posterior subnuclei of the bed nucleus of the stria terminalis; CeM, medial subdivision of the central amygdala; CeL, lateral subdivision of the central amygdala; ic, internal capsule; st, stria terminalis.
For the BSTp, there was a main effect of DRINK (F1,35 = 4.34, p < 0.05, η2 = 0.10, ηp2 = 0.12) and a DRINK x CYCLE interaction (F1,35 = 4.60, p < 0.05, η2 = 0.11, ηp2 = 0.13). Post-hoc analyses revealed that sucrose increased cFos-positive immunolabeling only among rats in the P/E stage of the estrous cycle (Figure 7C,E).
In contrast, neither sucrose nor estrous cycle stage impacted post-restraint cFos immunolabeling in any of the other brain regions examined (Table 2), including the BLA, DMH (dorsal and ventral subdivisions), MeApd, NAc (core and shell subdivisions), PFC (PL, IL, and anterior cingulate subdivisions), and PVN.
Table 2. Post-restraint cFos-positive immunolabeling in stress- and reward-regulatory brain regions.
The impact of prior sucrose (vs. water) on the density of post-restraint cFos-positive cells (number of cells/mm2) in multiple stress- and reward-regulatory brain regions of female rats tested during diestrus (D1/D2) and proestrus/estrus (P/E). n = 7–15/group.
| Brain region | Water D1/D2 | Water P/E | Sucrose D1/D2 | Sucrose P/E |
|---|---|---|---|---|
| BLA | 78.06 ± 2.67 | 80.69 ± 3.77 | 86.08 ± 4.33 | 86.98 ± 4.28 |
| DMH dorsal | 83.94 ± 14.45 | 85.46 ± 8.53 | 70.68 ± 7.24 | 90.18 ± 4.18 |
| DMH ventral | 104.85 ± 9.90 | 118.58 ± 9.06 | 101.67 ± 9.88 | 116.09 ± 6.92 |
| MeApd | 118.93 ± 12.01 | 130.92 ± 9.82 | 133.16 ± 8.93 | 133.56 ± 9.91 |
| NAc core | 55.00 ± 6.57 | 50.86 ± 5.25 | 49.57 ± 4.66 | 46.89 ± 5.55 |
| NAc shell | 60.62 ± 5.48 | 55.10 ± 4.60 | 50.19 ± 2.16 | 50.34 ± 5.46 |
| PFC PL | 128.16 ± 9.78 | 147.41 ± 9.35 | 156.32 ± 10.98 | 139.73 ± 7.42 |
| PFC IL | 102.78 ± 5.82 | 111.49 ± 6.66 | 115.76 ± 6.89 | 112.77 ± 5.29 |
| PFC Ant Cing | 112.94 ± 8.43 | 128.65 ± 9.86 | 122.29 ± 9.02 | 123.35 ± 6.79 |
| PVN | 158.69 ± 11.14 | 163.72 ± 8.74 | 148.08 ± 9.09 | 143.34 ± 9.50 |
DISCUSSION
In this study, we found that a history of limited, intermittent sucrose reduces stress-related behaviors during the EPM and restraint tests during the P/E stage of the estrous cycle. This was accompanied by reduced HPA axis responses to stress in sucrose-fed rats during P/E. Furthermore, post-restraint cFos immunolabeling in the CeM and BSTp was also increased by sucrose during P/E. Collectively, these data support the hypothesis that palatable foods reduce anxiety-like behaviors in female rats in an estrous cycle-dependent manner.
Impact of LSI on stress-related behaviors
As there is evidence that there may be sex differences in anxiety-related behaviors (Frye et al., 2000; Frye and Walf, 2002; Johnston and File, 1991; Pare and Redei, 1993), and that anxiety-related behaviors may vary with estrous cycle (Frye et al., 2000; Frye and Walf, 2002; Galeeva and Tuohimaa, 2001; Gray and Cooney, 1982; Marcondes et al., 2001; Meziane et al., 2007; Mora et al., 1996; Pare and Redei, 1993; Walf et al., 2009), the present study tested whether LSI blunts anxiety-related behaviors in female rats, as seen previously for male rats (Ulrich-Lai et al., 2010). Furthermore, as we have previously shown that LSI dampens HPA axis responsivity primarily during the P/E stage of the estrous cycle (Egan et al., 2018), we hypothesized that LSI would similarly attenuate anxiety-like behaviors during P/E in female rats. We found that a history of LSI increased time in the open arms of the EPM and decreased struggling during restraint, but had no effect on time in the center of the OFT, in female rats during P/E. These findings generally support our hypothesis, and suggest that LSI reduces stress-related behaviors in female rats in an estrous cycle-dependent manner. It should be noted as a limitation that estrous cycle assessments by vaginal lavage were restricted to the days of sample collection, as opposed to occurring daily throughout the entire experiment. We chose to limit the number of vaginal lavages to avoid the possibility that the stress associated with repeated daily lavage could confound the behavioral and HPA axis responses to stress. Nonetheless, it is intriguing to speculate that reduced anxiety-related behavior during P/E may have ethological relevance for rodents, as it could promote exploration and the seeking of a mate. Moreover, the specificity of the LSI effects to the P/E estrous cycle stage may have important clinical implications, as it suggests that stress relief by ‘comfort foods’ may also vary across the menstrual cycle in women.
LSI reduced anxiety-related behaviors during the EPM in both male and female P/E rats (Ulrich-Lai et al., 2010), indicating that LSI may have similar anxiolytic effects in males and females. In contrast, LSI did not affect anxiety-related behaviors in the OFT in female rats, as it previously did in male rats (Ulrich-Lai et al., 2010). Moreover, LSI increased the distance travelled in the OFT during D1/D2, though vertical locomotor activity (rearing) in the OFT was not affected by drink or cycle, nor was total distance travelled in the EPM. This suggests LSI does not have widespread effects on overall locomotor activity, and complicates interpretation of the female OFT results. However, as LSI decreases anxiety-related behaviors in the OFT in males (with no effects on total locomotor activity) (Ulrich-Lai et al., 2010), the effects of LSI on behaviors in the OFT may differ between male and female rats. For example, a duration of LSI longer than 12 days may be needed to alter anxiety-related behaviors in the OFT in females. In support of this idea, reduced anxiety-related behaviors were observed in the EPM on day 16 of LSI exposure, indicating that a longer duration of LSI may be required for anxiolytic effects in females.
Finally, the effect of LSI on struggling during the restraint test has not been previously characterized in either male or female rats. As such, our finding that LSI reduced struggling only during P/E is a novel finding that supports the use of struggling as an approach to simultaneously measure stress-related behavior and HPA axis activation. Additionally, preliminary data shows LSI similarly reduces struggling during the restraint test in male rats (unpublished data), suggesting that LSI may impact stress coping behaviors during the restraint test in both sexes.
LSI reduces HPA axis reactivity to restraint stress
As we previously reported (Egan et al., 2018), LSI reduced plasma ACTH responses to restraint stress during the P/E stage of the estrous cycle. In the current study, we also found that LSI reduced plasma corticosterone during P/E at the 90-min post-stress time point. We had previously collected blood samples through 60-min post-restraint, as is routinely used in analogous studies in male rats, and as such, failed to detect LSI effects on post-restraint plasma corticosterone (Egan et al., 2018). By extending the sampling time out to 90 minutes post-restraint in the current experiment, we were able to detect changes in plasma corticosterone that are not apparent at earlier time points. This supports the need for extended sampling times in female rats, and suggests the time course of LSI effects of HPA axis reactivity may differ between male and female rats. For example, it is intriguing to speculate that LSI may act in P/E females, at least in part, by shortening the duration of the post-stress glucocorticoid response. This could occur via direct actions on HPA-regulatory brain regions, increased glucocorticoid negative feedback, and/or a faster rate of corticosterone metabolism and clearance. It is also interesting to note that plasma ACTH is reduced by LSI as early as 20 minutes after stress onset, whereas plasma corticosterone is not reduced until 90 minutes. It is possible that early after stress onset, plasma ACTH levels are above the threshold required to evoke maximal adrenal corticosterone release, and as a result, reduced plasma corticosterone by LSI can only be observed after plasma ACTH levels fall below this threshold at later post-stress time points. In support of this idea, estrogen enhances adrenal responsivity to ACTH (Figueiredo et al., 2007), implying that less plasma ACTH may be needed to evoke maximal rates of adrenal corticosterone production during P/E. Thus, during P/E, despite a clear reduction in plasma ACTH by sucrose at 20–60 min after stress onset, the reduction in plasma corticosterone may not be observed until plasma ACTH levels have waned (e.g., at 90 min).
Potential brain mechanisms underlying P/E-specific effects of LSI in female rats
We previously measured the effect of LSI on immunolabeling for two proteins that are associated with long-term changes in neural plasticity and neuroadaptation (pCREB and FosB/deltaFosB) in multiple stress- and reward-regulatory brain regions in the basal, unstressed state of female rats (Chen et al., 1997; Huang et al., 2000; McClung et al., 2004; Miyamoto, 2006; Nestler et al., 1999; Silva et al., 1998)). A Bayesian network analysis was then performed on this dataset, identifying a neurocircuit that includes the BLA, NAc, PFC and BST as likely being modified by LSI in female rats during P/E (Egan et al., 2018). As the BLA, NAc, and PFC do not have significant direct projections to the PVN (Ulrich-Lai and Herman, 2009), this suggests that they may act through one or more intermediary structures, such as the BSTp, to influence HPA reactivity. Importantly, the present post-stress cFos data extend and support this possibility, showing that restraint-induced cFos activation in the BSTp is enhanced by prior LSI only during P/E, thereby corroborating the BSTp as a potential candidate for this intermediary brain site. The BSTp provides direct inputs to the PVN that are largely GABAergic in nature (Boudaba et al., 1996; Choi et al., 2007; Dong and Swanson, 2004; Herman et al., 2003; Myers et al., 2014; Ulrich-Lai and Herman, 2009), and thus increased activation of the BSTp during P/E may act to blunt HPA axis responsivity by increasing inhibition of the PVN. The BLA, NAc, and PFC all have direct or indirect projections to the BSTp (Dong et al., 2001; McDonald, 1991; Myers et al., 2014; Pitkanen et al., 1997; Vertes, 2004), so there is anatomical evidence to support the idea of the BSTp as this intermediary. Moreover, the BSTp expresses both estrogen receptor alpha (ERα) and beta (ERβ) (Shughrue et al., 1997; Shughrue and Merchenthaler, 2001; Shughrue et al., 1998), and ER signaling is linked with altered BST neuronal activation (cFos) and anxiety-related behavior (Kudwa et al., 2014; Lund et al., 2005; Oyola et al., 2012; Walf and Frye, 2005). Therefore, a possible scenario is that LSI and estrous cycle interact to alter the functional relationships within a BLA-NAc-PFC circuit in the basal, unstressed state. Subsequently, when a stressor occurs during P/E, signals from the BLA, NAc, and PFC converge on the BSTp, which integrates these signals to provide greater inhibition of the PVN, therefore resulting in an overall blunted HPA axis response. Importantly, this same neurocircuit is well-positioned to modulate the effects of LSI on stress-related behaviors, including struggling during restraint and open arm exploration in the EPM (Dong and Swanson, 2004; Felix-Ortiz et al., 2016; Weinberg et al., 2010).
The present analysis of post-stress cFos immunolabeling indicated the CeM as another potential region mediating P/E-specific stress-dampening by LSI. The CeM is the main output nucleus of the CeA, and activation of this region is typically thought of as stress-excitatory (Beaulieu et al., 1986; Ciocchi et al., 2010; Duvarci et al., 2011; Feldman et al., 1994; Haubensak et al., 2010; Xu et al., 1999). Thus, the observed increase in cFos immunolabeling, coupled with decreased behavioral and HPA axis stress responses, seems contradictory. However, the role of the CeA in stress regulation may be more complex than originally thought. For instance, sucrose ingestion increases neuronal activation (cFos) in the CeA, (particularly the medial portion) (Yamamoto et al., 1997) as does administration of the anxiolytic drug diazepam (Lkhagvasuren et al., 2014), and LSI increases pCREB- and FosB/deltaFosB-immunolabeling in the CeA of unstressed female rats (Egan et al., 2018). This suggests the CeA may contribute to palatable feeding and/or anxiolytic responses. In support of this, rats given ad libitum sucrose in addition to chow had increased post-restraint corticotropin-releasing hormone (CRH) mRNA in the CeA, which was accompanied by decreased HPA axis responsivity to restraint (Foster et al., 2009). Furthermore, Logrip et al. showed that synaptic responses in the CeM can vary across the estrous cycle (Logrip et al., 2017). Taking this information together, we speculate that differential functioning in the CeM during P/E may make it more sensitive to the effects of previous sucrose intake, thus promoting greater stress-blunting effects of LSI during P/E.
Finally, the BLA generally promotes stress responses (Bhatnagar et al., 2004; Coover et al., 1973; Feldman et al., 1983; Goldstein et al., 1996; Szafarczyk et al., 1986), suggesting that LSI may act by decreasing its stress-excitatory output. Consistent with this idea, in male rats LSI alters indices of neuronal plasticity within the BLA in the basal, unstressed state, and also reduces post-stress BLA cFos expression (Christiansen et al., 2011; Ulrich-Lai et al., 2010; Ulrich-Lai et al., 2007). In female rats, LSI increases BLA FosB/deltaFosB immunolabeling in the basal, unstressed state during P/E, and Bayesian analyses identify the BLA as a member of the most likely neurocircuit that is modified by LSI in both males and female P/E rats (Egan et al., 2018; Ulrich-Lai et al., 2016). However, the present data indicate that LSI does not alter the number of post-stress cFos-positive cells in the BLA of female rats (Table 2). Thus, while our prior results strongly implicate a role for the BLA in the LSI stress-blunting in male rats, a role for the BLA in the LSI effects in female rats appears to be more nuanced. As there are strong functional relationships among the BLA, CeA and BST (Dong et al., 2001; Myers et al., 2014; Pitkanen et al., 1997; Swanson and Petrovich, 1998; Tye et al., 2011), this may indicate that for female rats, the BLA contributes to LSI stress-dampening primarily via its ability to influence the function of these other brain regions.
Conclusions
Women may be more prone to eating ‘comfort food’ than are men, and emotional eating can vary across the menstrual cycle (Greeno and Wing, 1994; Grunberg and Straub, 1992; Hildebrandt et al., 2015; Klein et al., 2004; Klump et al., 2013a; Klump et al., 2013b; Oliver and Wardle, 1999; Racine et al., 2013; Wansink et al., 2003; Wardle et al., 2000; Zellner et al., 2006). This suggests the potential for important sex- and cycle-dependent effects of ‘comfort feeding.’ We have previously shown that a limited, intermittent sucrose intake paradigm reduces anxiety-like behaviors in male rats (Ulrich-Lai et al., 2010), and the present work extended this line of research to examine the effects of LSI on anxiety-related behaviors in female rats across the estrous cycle. The results indicate that LSI reduces stress-related behaviors in the EPM and restraint tests (but not the OFT), while also blunting HPA axis activation in female rats during P/E. Furthermore, LSI increases post-restraint cFos immunolabeling in the CeM and BSTp only during P/E. Collectively, the results support the hypothesis that palatable foods reduce anxiety-related behavior and HPA axis responsivity, with the effects in females varying across the estrous cycle stage. Moreover, neuronal signaling in the CeM and BSTp may contribute to the stress-blunting effects of LSI in females during P/E.
HIGHLIGHTS.
Stress relief by “comfort foods” may vary across the estrous cycle.
Sucrose blunts anxiety-like behaviors in a cycle-specific manner.
Sucrose decreases the HPA axis response to restraint in a cycle-specific manner.
Sucrose increases post-restraint cFos in forebrain in a cycle-specific manner.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health [grant numbers R56 DK118292 (YMU), R01 DK091425 (YMU), F32 DK102334 (AEBP), and T32 DK059803 (AEE)] and an Albert J. Ryan Foundation Fellowship (AEE). We would like to thank Dr. William Engeland for generously providing the antiserum for the ACTH RIA, and Jody Caldwell for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
REFERENCES
- Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS, Kappas A, 1987. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life sciences 40, 1761–1768. [DOI] [PubMed] [Google Scholar]
- Arabo A, Potier C, Ollivier G, Lorivel T, Roy V, 2014. Temporal analysis of free exploration of an elevated plus-maze in mice. Journal of experimental psychology. Animal learning and cognition 40, 457–466. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N, 2013. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305, R1215–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Barden N, 1986. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology 44, 247–254. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E, 2005. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF, 2002. Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. Journal of neuroendocrinology 14, 330–342. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP, 2002. Behavioral profile of rats submitted to session 1-session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions. Behav Brain Res 132, 135–143. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K, 2004. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci 1032, 315–319. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG, 1996. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci 16, 7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW, 1974. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ, 2005. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29, 1193–1205. [DOI] [PubMed] [Google Scholar]
- Cartwright M, Wardle J, Steggles N, Simon AE, Croker H, Jarvis MJ, 2003. Stress and dietary practices in adolescents. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 22, 362–369. [DOI] [PubMed] [Google Scholar]
- Casarrubea M, Roy V, Sorbera F, Magnusson MS, Santangelo A, Arabo A, Crescimanno G, 2013. Temporal structure of the rat’s behavior in elevated plus maze test. Behav Brain Res 237, 290–299. [DOI] [PubMed] [Google Scholar]
- Cecchini DJ, Chattoraj SC, Fanous AS, Panda SK, Brennan TF, Edelin KC, 1983. Radioimmunoassay of 2-hydroxyestrone in plasma during the estrous cycle of the rat: interrelationships with estradiol, progesterone, and the gonadotropins. Endocrinology 112, 1122–1126. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE, 1993. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci 13, 5126–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ, 1997. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci 17, 4933–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP, 2007. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27, 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP, 2011. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav 103, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A, 2010. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282. [DOI] [PubMed] [Google Scholar]
- Coover G, Ursin H, Levine S, 1973. Corticosterone and avoidance in rats with basolateral amygdala lesions. J Comp Physiol Psychol 85, 111–122. [DOI] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, Travlos G, 2015. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicologic pathology 43, 776–793. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG, 1994. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49, 171–176. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ, 1995. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JI, 1995. Fos: an immediate-early transcription factor in neurons. J Neurobiol 26, 403–412. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE, 2005. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain, behavior, and immunity 19, 275–280. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW, 2001. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38, 192–246. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, 2004. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471, 396–433. [DOI] [PubMed] [Google Scholar]
- Dube L, LeBel JL, Lu J, 2005. Affect asymmetry and comfort food consumption. Physiol Behav 86, 559–567. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D, 2011. Central amygdala activity during fear conditioning. J Neurosci 31, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan AE, Thompson AMK, Buesing D, Fourman SM, Packard AEB, Terefe T, Li D, Wang X, Song S, Solomon MB, Ulrich-Lai YM, 2018. Palatable food affects HPA axis responsivity and forebrain neurocircuitry in an estrous cycle-specific manner in female rats. Neuroscience 384, 224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R, 2004. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci 1032, 208–210. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K, 2001. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26, 37–49. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J, 1994. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res 658, 21–26. [DOI] [PubMed] [Google Scholar]
- Feldman S, Siegel RA, Conforti N, 1983. Differential effects of medial forebrain bundle lesions on adrenocortical responses following limbic stimulation. Neuroscience 9, 157–163. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM, 2016. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP, 2007. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab 292, E1173–1182. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT, 2004. Animal tests of anxiety. Current protocols in neuroscience Chapter 8, Unit 8 3. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF, 2011. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience 192, 351–360. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL, 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama 307, 491–497. [DOI] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF, 2009. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology 150, 2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME, 2000. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav 67, 587–596. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, 2002. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Hormones and behavior 41, 306–315. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN, 2000. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience 97, 479–494. [DOI] [PubMed] [Google Scholar]
- Galeeva A, Tuohimaa P, 2001. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res 119, 41–47. [DOI] [PubMed] [Google Scholar]
- Gibson EL, 2006. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav 89, 53–61. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Herman MA, Roberto M, 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77, 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, 2015. The organization of the stress system and its dysregulation in depressive illness. Molecular psychiatry 20, 32–47. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL, 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth defects research. Part B, Developmental and reproductive toxicology 80, 84–97. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH, 1996. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci 16, 4787–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P, Cooney J, 1982. Stress-induced responses and open-field behavior in estrous and nonestrous mice. Physiol Behav 29, 287–292. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR, 1994. Stress-induced eating. Psychol Bull 115, 444–464. [DOI] [PubMed] [Google Scholar]
- Grissom N, Kerr W, Bhatnagar S, 2008. Struggling behavior during restraint is regulated by stress experience. Behav Brain Res 191, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B, Epel E, 2012. What is eating you? Stress and the drive to eat. Appetite 58, 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Straub RO, 1992. The role of gender and taste class in the effects of stress on eating. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 11, 97–100. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ, 2010. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE, 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in neuroendocrinology 24, 151–180. [DOI] [PubMed] [Google Scholar]
- Hildebrandt BA, Racine SE, Keel PK, Burt SA, Neale M, Boker S, Sisk CL, Klump KL, 2015. The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. The International journal of eating disorders 48, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ, 1998. Responses of Swiss-Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav 60, 473–488. [DOI] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER, 2000. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci 20, 6317–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, 2014. Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Comprehensive Physiology 4, 715–738. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC, 1991. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol 261, R1257–R1268. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE, 1991. Sex differences in animal tests of anxiety. Physiol Behav 49, 245–250. [DOI] [PubMed] [Google Scholar]
- Kim Y, Yang HY, Kim AJ, Lim Y, 2013. Academic stress levels were positively associated with sweet food consumption among Korean high-school students. Nutrition 29, 213–218. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Honors MA, 2008. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav 95, 108–113. [DOI] [PubMed] [Google Scholar]
- Klein LC, Faraday MM, Quigley KS, Grunberg NE, 2004. Gender differences in biobehavioral aftereffects of stress on eating, frustration, and cardiovascular responses. J Appl Soc Psych 34, 538–562. [Google Scholar]
- Klump KL, Keel PK, Burt SA, Racine SE, Neale MC, Sisk CL, Boker S, 2013a. Ovarian hormones and emotional eating associations across the menstrual cycle: an examination of the potential moderating effects of body mass index and dietary restraint. The International journal of eating disorders 46, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S, Hu JY, 2013b. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of abnormal psychology 122, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE, 1996. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci 16, 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, McGivern RF, Handa RJ, 2014. Estrogen receptor beta and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol Behav 129, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF, 2005. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology 146, 2193–2199. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Falcon LM, Tucker KL, 2011. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite 56, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lkhagvasuren B, Oka T, Nakamura Y, Hayashi H, Sudo N, Nakamura K, 2014. Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience 272, 34–57. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M, 2017. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JK, LaPolt PS, Nass TE, Matt DW, Judd HL, 1985. Relation of circulating estradiol and progesterone to gonadotropin secretion and estrous cyclicity in aging female rats. Endocrinology 116, 1953–1959. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ, 2005. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology 146, 797–807. [DOI] [PubMed] [Google Scholar]
- Macht M, Mueller J, 2007. Immediate effects of chocolate on experimentally induced mood states. Appetite 49, 667–674. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ, 2010. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology 35, 1553–1564. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP, 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian journal of biology = Revista brasleira de biologia 62, 609–614. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, 2001. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 74, 435–440. [DOI] [PubMed] [Google Scholar]
- Markus R, Panhuysen G, Tuiten A, Koppeschaar H, 2000. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav 70, 333–342. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Pare D, 1999. Physiological properties of central medial and central lateral amygdala neurons. J Neurophysiol 82, 1843–1854. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S, 1995. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Hormones and behavior 29, 279–295. [DOI] [PubMed] [Google Scholar]
- McClave J, Dietrich F, 1994. Statistics, 6 ed. Macmillan College Publishing Company, Inc, Englewood Cliffs, NJ. [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ, 2004. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132, 146–154. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, 1991. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44, 1–14. [DOI] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W, 2007. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes, brain, and behavior 6, 192–200. [DOI] [PubMed] [Google Scholar]
- Miyamoto E, 2006. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. Journal of pharmacological sciences 100, 433–442. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G, 1996. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 21, 609–620. [DOI] [PubMed] [Google Scholar]
- Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP, 2014. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct 219, 1287–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB, 1979. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biology of reproduction 20, 659–670. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J, 1999. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res 835, 10–17. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, Flegal KM, 2015. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief, 1–8. [PubMed]
- Oliver G, Wardle J, 1999. Perceived effects of stress on food choice. Physiol Behav 66, 511–515. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J, Gibson EL, 2000. Stress and food choice: a laboratory study. Psychosomatic medicine 62, 853–865. [DOI] [PubMed] [Google Scholar]
- Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK, 2012. Anxiolytic effects and neuroanatomical targets of estrogen receptor-beta (ERbeta) activation by a selective ERbeta agonist in female mice. Endocrinology 153, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard AE, Egan AE, Ulrich-Lai YM, 2016. HPA axis interactions with behavioral systems. Comprehensive Physiology 6, 1897–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP, Redei E, 1993. Sex differences and stress response of WKY rats. Physiol Behav 54, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The rat brain in stereotaxic coordinates, 4th ed. Academic Press, New York. [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF, 2004. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology 145, 3754–3762. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of neuroscience methods 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE, 1997. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20, 517–523. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL, 2013. Individual differences in the relationship between ovarian hormones and emotional eating across the menstrual cycle: a role for personality? Eating behaviors 14, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ, Cole JC, Dewar CV, Kidd GR, Kimpson PH, 1996. Plus-maze retest profile in mice: importance of initial stages of trial 1 and response to post-trial cholinergic receptor blockade. Pharmacol Biochem Behav 54, 41–50. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Packard AEB, Larson KR, Stout J, Fourman SM, Thompson AMK, Ludwick K, Habegger KM, Stemmer K, Itoh N, Perez-Tilve D, Tschop MH, Seeley RJ, Ulrich-Lai YM, 2018. Dietary Manipulations That Induce Ketosis Activate the HPA Axis in Male Rats and Mice: A Potential Role for Fibroblast Growth Factor-21. Endocrinology 159, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, 1987. Carbohydrate taste, appetite, and obesity: an overview. Neurosci Biobehav Rev 11, 131–153. [PubMed] [Google Scholar]
- Sclafani A, 1991. Starch and sugar tastes in rodents: an update. Brain research bulletin 27, 383–386. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I, 1997. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388, 507–525. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I, 2001. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol 436, 64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I, 1998. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology 139, 5267–5270. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S, 1998. CREB and memory. Annu Rev Neurosci 21, 127–148. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD, 1975. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96, 219–226. [DOI] [PubMed] [Google Scholar]
- Sonnenberg JL, Macgregor-Leon PF, Curran T, Morgan JI, 1989. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron 3, 359–365. [DOI] [PubMed] [Google Scholar]
- Spiga F, Walker JJ, Terry JR, Lightman SL, 2014. HPA axis-rhythms. Comprehensive Physiology 4, 1273–1298. [DOI] [PubMed] [Google Scholar]
- Strohle A, Holsboer F, 2003. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry 36 Suppl 3, S207–214. [DOI] [PubMed] [Google Scholar]
- Swanson LW, 1998. Brain maps: structure of the rat brain Elsevier, Amsterdam. [Google Scholar]
- Swanson LW, Petrovich GD, 1998. What is the amygdala? Trends Neurosci 21, 323–331. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Caracchini M, Rondouin G, Ixart G, Malaval F, Assenmacher I, 1986. Plasma ACTH and corticosterone responses to limbic kindling in the rat. Experimental neurology 92, 583–590. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES, 2011. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 36, 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon MS, DeCant R, Laugero KD, 2013. Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiol Behav 114-115, 32–37. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K, 2011. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP, 2010. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A 107, 20529–20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Wang X, Song S, Herman JP, 2016. Statistical modeling implicates neuroanatomical circuit mediating stress relief by ‘comfort’ food. Brain Struct Funct 221, 3141–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Herman JP, 2011. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav 103, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP, 2007. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology 148, 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima I, Mizuki Y, Hara T, Kudo R, Watanabe K, Yamada M, 1986. The role of adenosinergic, GABAergic and benzodiazepine systems in hyperemotionality and ulcer formation in stressed rats. Psychopharmacology (Berl) 89, 472–476. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP, 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289, E823–828. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2005. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology 30, 1288–1301. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA, 2009. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res 196, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA, 1976. The open-field test: a critical review. Psychol Bull 83, 482–504. [PubMed] [Google Scholar]
- Wansink B, Cheney MM, Chan N, 2003. Exploring comfort food preferences across age and gender. Physiol Behav 79, 739–747. [DOI] [PubMed] [Google Scholar]
- Wardle J, Steptoe A, Oliver G, Lipsey Z, 2000. Stress, dietary restraint and food intake. Journal of psychosomatic research 48, 195–202. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Grissom N, Paul E, Bhatnagar S, Maier SF, Spencer RL, 2010. Inescapable but not escapable stress leads to increased struggling behavior and basolateral amygdala c-fos gene expression in response to subsequent novel stress challenge. Neuroscience 170, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SE, Shide DJ, Rolls BJ, 1997. Changes in food intake in response to stress in men and women: psychological factors. Appetite 28, 7–18. [DOI] [PubMed] [Google Scholar]
- Xu Y, Day TA, Buller KM, 1999. The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1beta administration. Neuroscience 94, 175–183. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Sakai N, Iwafune A, 1997. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci Lett 226, 127–130. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A, 2006. Food selection changes under stress. Physiol Behav 87, 789–793. [DOI] [PubMed] [Google Scholar]