Abstract
Neural activation patterns in the ventral visual cortex in response to different categories of visual stimuli (e.g., faces vs. houses) are less selective, or distinctive, in older adults than in younger adults, a phenomenon known as age-related neural dedifferentiation. In this study, we investigated whether neural dedifferentiation extends to auditory cortex. Inspired by previous animal work, we also investigated whether individual differences in GABA are associated with individual differences in neural distinctiveness in humans. 20 healthy young adults (ages 18–29) and 23 healthy older adults (over 65) completed a functional magnetic resonance imaging (fMRI) scan, during which neural activity was estimated while they listened to music and foreign speech. GABA levels in the auditory, ventrovisual and sensorimotor cortex were estimated in the same individuals in a separate magnetic resonance spectroscopy (MRS) scan. Relative to the younger adults, the older adults exhibited both (1) less distinct activation patterns for music vs. speech stimuli and (2) lower GABA levels in the auditory cortex. Also, individual differences in auditory GABA levels (but not ventrovisual or sensorimotor GABA levels) were associated with individual differences in neural distinctiveness in the auditory cortex in the older adults. These results demonstrate that age-related neural dedifferentiation extends to the auditory cortex and suggest that declining GABA levels may play a role in neural dedifferentiation in older adults.
Keywords: aging, auditory, GABA, dedifferentiation
Introduction
Aging is often accompanied by declines in cognitive (Harada et al., 2013; Park et al., 2002; Salthouse, 1996) and sensory function (Fortunato et al., 2016). These declines have a significant negative impact on the daily lives of older individuals and are often early indicators of pathology. However, there are substantial individual differences in these declines: some older adults experience severe impairments while others do not (Christensen et al., 1999; Hultsch et al., 2002; Wilson et al., 2002). Understanding the neural bases of these individual differences may therefore be helpful in designing interventions that slow or halt some age-related impairments.
One neural factor that may play a role is an age-related decline in neural distinctiveness. Neuroimaging studies have repeatedly found that activation patterns evoked by different categories of visual stimulus are more similar (less distinctive or differentiated) in older adults than in younger adults (Carp et al., 2011b; Park et al., 2004; Voss et al., 2008), a phenomenon referred to as age-related neural dedifferentiation. For example, Park et al. (2004) reported that young adults exhibit much greater activation in the fusiform face area (FFA) when viewing faces than when viewing words or buildings. In contrast, older adults exhibited almost as much activity in the FFA when viewing words and buildings as they did when viewing faces. In other words, activity in the FFA was more specialized or distinctive in the young compared with the old. Likewise, activity in the parahippocampal place area was more specialized for buildings in the young compared with the old, and activity in the visual word form area was more specialized for words.
Similar results have been reported using multi-voxel pattern-based analysis (MVPA). For example, Park et al. (2010) trained a support vector machine (SVM) to distinguish fMRI activation patterns evoked by faces from activation patterns evoked by houses and then tested its accuracy in classifying activation patterns on which it had not been trained. The classifier was significantly more accurate in distinguishing face patterns from house patterns in young compared with older adults, providing additional evidence that neural distinctiveness declines with age. Following Haxby et al., (2001), Carp et al. (2011b) assessed the similarity (correlation) of activation patterns evoked by faces, houses, and words in young and old adults. In young adults, activation patterns evoked by the same stimulus category (e.g., different face blocks) were much more similar than patterns evoked by different stimulus categories (e.g., face vs. house blocks) suggesting high neural distinctiveness. However, this measure of neural distinctiveness declined significantly with age. We use both the SVM- and similarity-based measures of neural distinctiveness in this study.
Individual differences in neural distinctiveness have also been associated with individual differences in behavior in older adults. For example, Park et al. (2010) assessed behavioral performance on a range of fluid processing tasks that tend to decline with age (WAIS Digit Symbol task, Dot Comparison task, Trail-making tasks A and B, and the Controlled Oral Association Task (verbal-fluency)). They found that individual differences in neural distinctiveness accounted for over 30% of the variance in fluid processing ability over and above age. Likewise, Koen, Hauck, & Rugg (2019) reported that neural distinctiveness in the parahippocampal place area was significantly correlated with recognition memory performance and with a latent fluency factor derived from the neuropsychological test battery.
Most previous studies of neural dedifferentiation have focused on the visual cortex, and so it remains unclear the extent to which dedifferentiation occurs in other cortical regions, such as auditory cortex. There is evidence that the receptive fields of individual neurons in auditory cortex become less selective or differentiated with age. For example, Turner, Hughes, & Caspary (2005) reported that the receptive fields of auditory neurons are less selective to pure tones in older rats compared with younger rats. Frequency selective bandwidths of auditory neurons also get larger and receptive fields overlap more in older rats (Villers-Sidani et al., 2010). Likewise, neurons in primary and secondary auditory cortex are less spatially tuned in older compared with younger macaques (Juarez-Salinas et al., 2010) and auditory frequency selectivity also declines with age in mice (Leong et al., 2011). Together, these results suggest that in many mammals, the neural selectivity of single neurons declines in auditory cortex. Of course, effects of age on neural selectivity as measured at the level of single neurons in animals could be very different from effects on the selectivity of gross functional activation patterns measured by fMRI in humans. One goal of the present study is therefore to investigate whether activation patterns in auditory cortex are less selective or distinctive in older compared with younger adults, like they are in visual cortex.
Another neural factor that may contribute to age-related behavioral impairments is declines in the brain’s major inhibitory neurotransmitter, gamma-aminobutyric acid (GABA). GABA levels measured using magnetic resonance spectroscopy (MRS) are reduced in older adults compared to younger adults in the occipital cortex (Chalavi et al., 2018; Hermans et al., 2018; Simmonite et al., 2018), in frontal and parietal regions (Gao et al., 2013; Hermans et al., 2018), and in supplementary motor area and sensorimotor cortex (Cassady et al., 2019; Chalavi et al., 2018; Hermans et al., 2018). Furthermore, individual differences in GABA in specific cortical regions have been associated with individual differences in some aspects of cognitive (Hermans et al., 2018; Porges et al., 2017; Simmonite et al., 2018) and motor (Cassady et al., 2019) performance. However, the results of the small number of human studies investigating age-related changes in GABA levels in the auditory cortex are mixed (Chen et al., 2013; Profant et al., 2015). In the present study, we therefore also investigated whether older adults exhibit reduced GABA levels in the auditory cortex compared with young adults.
To date, age-related neural dedifferentiation and declines in GABA levels have been studied in isolation from one another. In the present study, we also test whether individual differences in GABA are associated with individual differences in neural distinctiveness and if this relationship is region specific. This work is motivated by previous studies in animals showing a causal link between GABA levels and neural selectivity. Leventhal et al. (2003) showed that the application of GABA or a GABA agonist increased the orientation selectivity of cells in the visual cortex of older rhesus monkeys. Conversely, application of a GABA antagonist decreased the orientation selectivity of cells in the visual cortex of young monkeys (Leventhal et al., 2003). GABA receptor antagonists have also been shown to broaden the frequency response of neurons in the inferior colliculus of chinchillas, making the cells’ response less selective (Caspary et al., 2002). These animal-based findings suggest that inhibitory GABA levels play a causal role in maintaining the neural selectivity of single neurons and that age-related declines in GABA levels might therefore mediate age-related declines in neural selectivity.
Of course, selectivity at the level of individual neurons is quite different from selectivity at the level of fMRI activation patterns. Nevertheless, age-related declines in GABA could plausibly influence both. For example, many models of cortical processing assume that neural representations compete with each other and that more active representations inhibit less active representations in a kind of winner-take-all competition (Desimone and Duncan,1995; O'Reilly, 1998). Such competition between neural representations is presumably mediated by inhibitory interneurons using GABA. And if GABA levels decline with age, then winning neural representations would be less able to inhibit other representations, potentially resulting in the kind of neural dedifferentiation observed with fMRI. We investigated this idea by measuring GABA levels and neural distinctiveness in the same individuals in the auditory cortex and assessing the relationship between these measures.
In sum, we combined fMRI and MRS to test the hypotheses that age-related dedifferentiation extends to the human auditory cortex, that auditory GABA levels decline with age, and that GABA levels are associated with neural distinctiveness in the auditory cortex of older adults.
Methods
Participants
Twenty young adults (8 males, mean age = 23.6, range 18 to 28 years) and 23 older adults (7 males, mean age = 69.91, range 65 to 81 years) adults participated in the study. Carp et al., 2011, found that the neural representations of visual stimuli are less distinct in older adults than in young adults (effect size: Cohen’s d = 1.06). Assuming a similar effect size in the auditory modality, a sample of approximately 20 subjects per group would be required to achieve 90% power to detect an effect. All participants were right-handed, native English speakers with normal or corrected to normal vision. We excluded participants who used hearing aids or scored lower than 23 on the Montreal Cognitive Assessment (MOCA) (Carson et al., 2018). We ensured that none of our participants knew any of the foreign languages that were used as auditory stimuli for the fMRI task. All sessions took place at the University of Michigan’s Functional MRI Laboratory, Ann Arbor, Michigan. Participants were recruited from Ann Arbor and the surrounding area.
Session Design
Eligible participants completed a functional MRI session and an MRS session on the same scanner on separate days within a few weeks of each other. These data were collected as a part of larger study called the Michigan Neural Distinctiveness or MiND study. Here, we only describe the portions of the study that are relevant to this experiment. Please refer to (Gagnon et al., 2019) for further details on the MiND study itself.
fMRI Session
We collected both structural and functional MRI data using a 3T General Electric Discovery Magnetic Resonance System with an 8-channel head coil at the Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, USA. We obtained T1-weighted images using an SPGR (3D BRAVO) sequence with the following parameters: Inversion Time (TI) = 500 ms; flip angle = 15°; Field of View (FOV) = 256 × 256 mm. While the structural scan was being collected, each participant heard a trial version of the auditory stimuli and the volume was adjusted to ensure that each participant could comfortably hear the stimuli presented during the scan.
During the functional scans, T2*-weighted images were collected with a 2D Gradient Echo spiral pulse sequence with the following parameters: TR = 2000 ms; TE = 30 ms; flip angle = 90°; FOV = 220 × 220 mm; 43 axial slices with thickness = 3 mm and no spacing, collected in an interleaved bottom-up sequence. The total acquisition time for the functional scan was 6 minutes and 10 seconds with 185 volumes. E-Prime software was used to present auditory stimuli, which consisted of six 20-second blocks of foreign speech clips, six 20-second blocks of instrumental music clips, and twelve 10-second blocks of fixation between every pair of auditory blocks. The order of the speech and music blocks was pseudorandomized.
Each speech block consisted of a 20-second news segment in one of the following foreign languages: Creole, Macedonian, Marathi, Persian, Swahili and Ukranian. Each music block consisted of a 20-second segment of instrumental music from one of the following pieces: Bach Sinfonia No. 5, Smokey by Mountain, Bamboula by L.M Gottschalk, Spagnoletta Nuova by Fabritio Caroso, Kuhlau: Fantaisie for Solo Flute in D major (Op. 38, No. 3), and a violin rendition of the country song “When the right one comes along”.
A fixation cross was presented on the screen for the entire duration of the task. To ensure that subjects were attending to the auditory presentation, target trials (guitar plucks) occurred randomly about once a minute during the task. The participants were instructed to press a button with their right index finger every time a target trial was presented. Sounds were presented through an MRI-compatible Avotec Conformal Headset.
MRS Session
MR Spectroscopy data was collected using the same scanner on a different day. During this second session, we first collected T1-weighted structural images using the same parameters as in the fMRI session. MRS data were acquired using a MEGA-PRESS sequence with the following parameters: TE=68ms (TE1=15ms, TE2=53ms), TR=1.8sec, 256 transients (128 ON interleaved with 128 OFF) of 4,096 data points; spectral width=5kHz, frequency selective editing pulses (14ms) applied at 1.9ppm (ON) and 7.46 ppm (OFF); total scan time about 8.5 minutes per voxel.
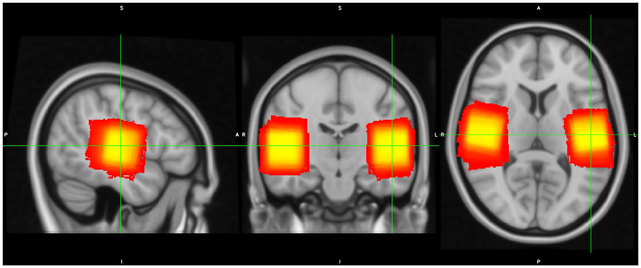
MRS data were collected from two 3cm × 3cm × 3cm voxels placed in the left and right auditory cortex (Figure 1), left and right ventrovisual cortex and left and right sensorimotor cortex (Figure S1). In order to ensure subject-level specificity, auditory voxels were placed to overlap maximally with each participant’s own functional activation maps (using a contrast of Speech + Music vs. Fixation) obtained from the fMRI run described previously.
Fig 1.
MRS voxel overlap across participants, with brighter (yellow) colors representing more participant overlap and darker (red) colors representing less overlap.
Quantification of GABA levels
We used the Gannet 3.0 MATLAB toolbox to estimate GABA levels in each of the two (left and right auditory) MRS voxels. The time domain data was frequency- and phase-corrected using spectral registration. It was filtered with 3-Hz exponential line broadening and zero-filled by a factor of 16. GABA levels were computed by fitting a Gaussian model to the 3-ppm peak in the difference spectrum and quantified relative to water (fit with a Gaussian-Lorentzian model) in institutional units (Figure S2). This editing scheme results in significant excitation of coedited macromolecule (MM) signal, that have been reported to contribute approximately 45% to the edited signal at 3-ppm. Thus, we report all GABA values as GABA+ (i.e., GABA + MM) in the present study. There are substantial differences in the relaxation constants and water visibility between white matter (WM), grey matter (GM) and cerebrospinal fluid (CSF). To account for these differences, a binary mask of the MRS voxels was created using Gannet’s integrated voxel-to-image co-registration. Next, segmentation of the anatomical image was performed using the Segment function in SPM12 and the voxel fractions containing CSF, GM and WM were computed. From this procedure, a tissue-corrected GABA+ value was calculated for each participant. Since the signal in GM and WM have different strengths, an alpha tissue-corrected (fully corrected) GABA+ value was also computed for each participant. We also estimated levels of N-acetylspartate (NAA) from LCModel (Provencher, 1993) to control for neural integrity differences within older adults.
fMRI Data Preprocessing
fMRI data were k-space despiked, reconstructed, and corrected for heart beat and breathing using the RETROICOR algorithm. The initial five volumes were deleted and the data were then slice time corrected using the spm_slice_timing function from SPM. Motion correction was performed using the Freesurfer FSFAST processing stream. Freesurfer was used to resample the data into two-dimensional cortical surfaces (one for the left hemisphere and one for the right hemisphere) based on a white/gray matter segmentation of each subject’s own high-resolution structural image computed using Freesurfer’s recon-all function. The data were then spatially smoothed within each cortical surface using a 5-mm two-dimensional smoothing kernel.
ROI Selection
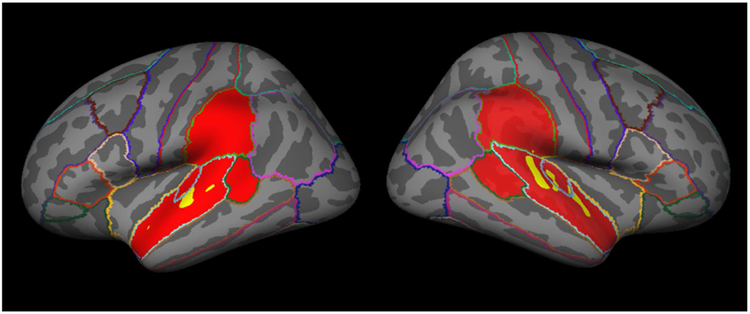
Because this was an auditory task we restricted our analysis to an anatomical mask containing the bilateral superior temporal gyrus, bank of the superior temporal sulcus, transverse temporal gyrus and supramarginal gyrus using cortical parcellation labels generated by FreeSurfer based on the Desikan-Killiany Atlas (aparc.annot). The resulting mask contained more than 37,000 vertices on the cortical surface (Figure 2). We obtained grey-matter thickness, volume and surface area estimates within this mask.
Fig 2.
Participant-specific example of structural (in red) and functional (in yellow) masks. The functional mask was based on the 1400 most activated vertices from the music vs. fixation and foreign speech vs. fixation contrasts, under the constraint that an equal number of vertices were included from each contrast.
In order to ensure that only subject-specific, task-relevant vertices were analyzed, we then created a functional mask for each subject. Neural activation was estimated using a General Linear Model, fit with two box-car regressors (music vs. fixation and speech vs. fixation), convolved with a standard hemodynamic function. Beta values for each of the two regressors were obtained at each vertex. The functional mask was generated by selecting the most active vertices from both conditions in an alternating order (e.g., the most highly activated vertex for the music vs. fixation contrast, then the most highly activated vertex for the speech vs. fixation contrast, then the next most activated vertex for the music vs. fixation contrast, etc.). If the next most active vertex for a contrast had already been included in the functional mask, then the next most active voxel that had not already been included in the functional mask was added. This approach ensured that both conditions were equally represented in the functional mask. The functional mask selection was blind to whether the chosen vertex was selective for one condition or was activated by both conditions (Figure 2).
Using a speech + music vs. fixation contrast, we calculated the total number of vertices across both hemispheres that were activated (p<0.001, uncorrected) during auditory perception for each subject. 95% of the subjects had greater than 1400 such vertices, so we chose an ROI-size of 1400 vertices as our default functional mask size. We also varied the ROI-size from small (1000 vertices) to very large (the entire anatomical mask) to ensure that any observed effects on neural distinctiveness did not depend on the size of the ROI.
Neural activation
In order to generate multiple independent activation patterns for use in multivoxel pattern analysis (MVPA), we then fit another General Linear Model that included separate box-car regressors for each of the 12 task-blocks (6 music and 6 speech), convolved with a standard hemodynamic function. Fitting the model produced beta values at each vertex separately for each of the 12 blocks. Neural distinctiveness was computed using these beta values (activation maps) as described below.
SVM-based calculation of distinctiveness
Machine learning classifiers, such as linear-SVMs (support vector machines), find a hyperplane that maximally separates multidimensional datapoints into different categories based on labeled training data. Classification accuracy can then be assessed on new, untrained activation patterns. Following previous work (Park et.al., 2010), we used SVM accuracy as a proxy for neural distinctiveness; if accuracy in classifying activation patterns is high, then those patterns are considered distinctive. Conversely, if accuracy is low, then the distinctiveness of the patterns is low. We used a leave-one-pair-out cross-validation approach, in which the classifier was trained to fit 10 of the 12 activation maps (5 music and 5 speech) within the functional ROI and then was tested on the two left-out activation maps (1 music and 1 speech). This process was repeated leaving out each of 36 different activation map pairs and the average classification accuracy was used as a measure of neural distinctiveness or specificity. Classification accuracy of 50% is chance.
Correlation-based calculation of distinctiveness
We also used a correlation-based approach that produces a more continuous measure of neural distinctiveness and that avoids ceiling effects (Haxby et al., 2001; Park et al., 2010). For each subject, correlations between the activation maps for all unique pairs of blocks of the same type were computed within the functional ROI (e.g., music block 1 with music block 2, music block 3 with music block 6, speech block1 with speech block4, etc.). These correlations were then averaged to produce a within-category correlation value. Likewise, correlations between activation maps for all unique pairs of blocks of different types were computed (e.g., music block 1 with speech block 2, music block 3 with speech block 6, speech block1 with music block4, etc.). These correlations were then averaged to produce a between-category correlation value. Neural distinctiveness was then defined as the difference between the average within-category correlation and average between-category correlation. This measure has a theoretical range of 2 to −2. This multivariate analysis reveals fine-grained differences in the distinctiveness of activation patterns rather than differences in the average activation between the two categories as a univariate method would.
Results
Neural Distinctiveness and Aging
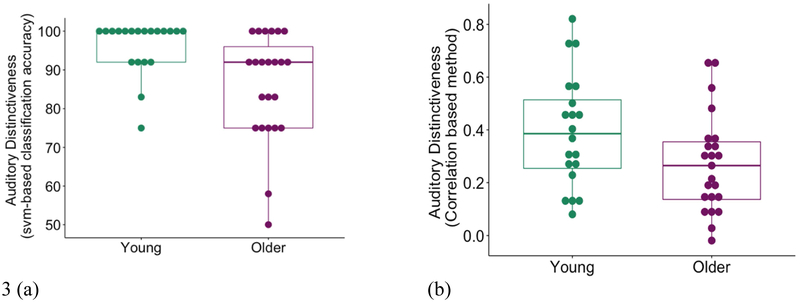
Neural distinctiveness as measured by SVM classifier accuracy (Figure 3a) was significantly lower in older adults (mean = 85.9%) compared to young adults (mean = 96.3%), (t (41) = −3.2, p = 0.005 (based on 10,000 bootstraps). Likewise, when neural distinctiveness was computed based on pattern similarity/dissimilarity using the difference between within-category and between-category correlations (Figure 3b), older adults exhibited less distinctive activation patterns (mean = 0.27) than did young adults (mean = 0.39) (t (41) = −2.04, p = 0.047). In other words, using both measures the activation patterns for music and speech were more similar or confusable in older adults than younger adults.
Fig 3.
(a) Neural distinctiveness based on the accuracy of an SVM classifier in distinguishing music from foreign speech (percent correct classifications). Distinctiveness was significantly lower in older adults (in purple) than young adults (in green) (t (41) = −3.2, p = 0.005 (based on 10,000 bootstraps)). (b) Neural distinctiveness based on the difference between within-condition similarity and between-condition similarity. Distinctiveness was again significantly lower in older adults (in purple) than young adults (in green) (t (41) = −2.04, p = 0.047).
One problem with the SVM-based measure of distinctiveness is that it is prone to ceiling effects. For example, the classifier was 100% accurate in classifying the activation patterns for 17 of the 43 participants. In contrast, the correlation-based measure can take on any real value between −2 and 2 and is much less susceptible to ceiling effects. The two measures were also significantly correlated (r (41) = 0.43, p = 0.004). We therefore used the correlation-based measure for subsequent analyses.
There was no significant difference between the number of activated vertices (p<0.001, uncorrected) within the anatomical mask for young and older adults in a music + speech vs. fixation contrast (t (41) = −0.64, p = 0.53). Furthermore, there was no significant difference in the mean (t (41) = −1.26, p=0.22) or peak (t (41) = −0.71, p=0.48) activation level between the two age groups for this contrast. Similarly, the total number of vertices activated during the music vs. fixation contrast (t (41) = −0.86, p = 0.39) and speech vs. fixation contrast (t (41) = 0.81, p =0.42) did not differ between the two age-groups. Differences in distinctiveness between the two age groups were therefore not driven by differential activation levels between the groups, but rather by differences in the similarity/dissimilarity of neural activation patterns elicited by music and speech.
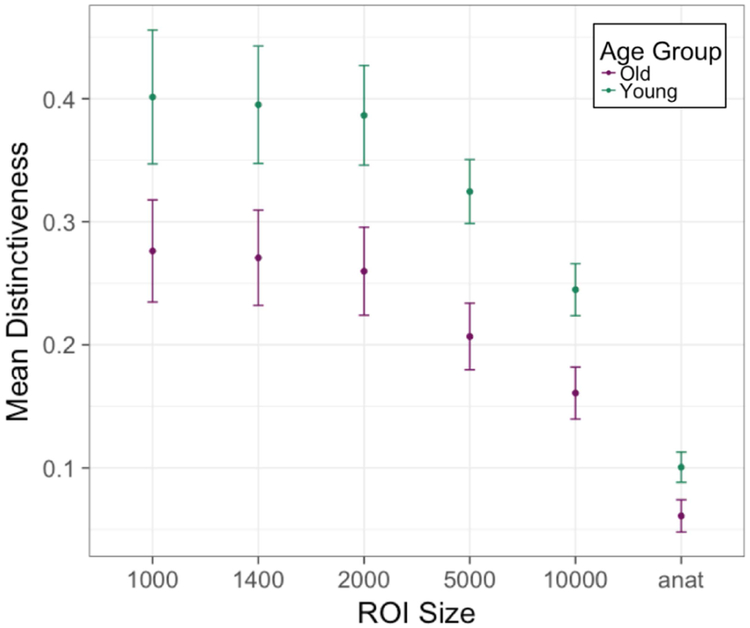
In order to ensure that the effect of aging on neural distinctiveness was not due to the selection of a particular ROI size, we computed a pairwise t-test at every ROI size and found that distinctiveness declined with age independent of ROI size selection (the effect was only marginally significant at the smallest ROI size) (Figure 4, Table S1). However, as ROI-size increased, the average distinctiveness values declined suggesting that the larger ROIs included task-irrelevant vertices that added noise to the distinctiveness measure.
Fig 4.
Neural distinctiveness in the two age groups as a function of ROI size. Distinctiveness was significantly lower in older adults (in purple) than younger adults (in green) for most ROI sizes (also see Table S1). The vertical axis is mean distinctiveness (measured as within-between difference) for each ROI size and group (young and older adults) with standard error bars. The horizontal axis is the ROI size (in number of vertices; “anat” refers to the entire anatomical mask of approximately 37,000 vertices).
Somewhat surprisingly, we did not find any significant differences in mean activation levels in the left vs. right hemisphere, either in the speech vs. fixation contrast (t speech (42) = 0.57, p = 0.57) or the music vs. fixation contrast (t music(42) = 1.4, p = 0.14). Likewise, we did not observe significant differences in peak activation levels (t music (42) = 1.01, p = 0.32; t speech (42) = −0.22, p = 0.82) or in the total number of activated vertices (t music (42) = 0.77, p = 0.44; t speech (42) = −0.4, p = 0.7) between right and left hemispheres.
The observed age-related decline in neural distinctiveness could be due to changes in the ear, to changes in the brain, or both. In particular, peripheral changes in the ear that reduce auditory sensitivity could lead to reduced neural distinctiveness, independent of age-related changes in auditory cortex itself. To explore this issue, we analyzed participants’ pure-tone threshold and its relationship to neural distinctiveness. The older adults exhibited higher pure-tone thresholds at frequencies above 2000 Hz (t4000 (41) = 6.3, p = 1.4e-07, t8000(41) = 5.9, p = 6.3e-07). Nevertheless, neural distinctiveness was still significantly lower in the older vs. younger participants after controlling for average pure tone threshold (t (41) = −2.04, p = 0.048) and average pure tone threshold was not significantly associated with neural distinctiveness, whether analyzed in the whole sample (r (41) = −0.04, p = 0.79) or in the older group alone (r (21) = −0.17, p = 0.43). Furthermore, when neural distinctiveness was correlated with pure tone threshold at each individual frequency (125,500,1000,2000,4000,8000), none of the associations was significant (all p’s > 0.25). One reason we may not have observed any associations is that greater than 90% of the power in our auditory stimuli were at frequencies below 2000 Hz (See Figure S6) where the effects of age on pure tone threshold were not significant (t125 (41) = 1.18, t500 (41) = 0.5, t1000 (41) = 1.8, t2000 (41) = 1.9) (See Figure S5). Although these results suggest that peripheral changes cannot completely explain age-related declines in auditory neural distinctiveness, pure tone threshold is just one (rather coarse) measure of peripheral hearing. So, it is still quite plausible that age-related changes in the ear contribute to age-related changes in auditory neural distinctiveness.
We also examined age-related changes in grey-matter thickness and surface area. Older adults exhibited significantly thinner grey-matter (t (37.4) =−6.82, p= 4.7e-08) and reduced surface area (t (39.6) =−3.49, p=0.001) within the anatomically defined mask. Neural distinctiveness was still significantly lower in the older adults even after controlling for changes in grey-matter thickness (r (41) =−0.32, p=0.037), but not after controlling for surface area (r (41) = −0.17, p= 0.28). These results indicate that changes in neural distinctiveness might be at least partially due to anatomical changes that accompany aging.
GABA+ Levels and Aging
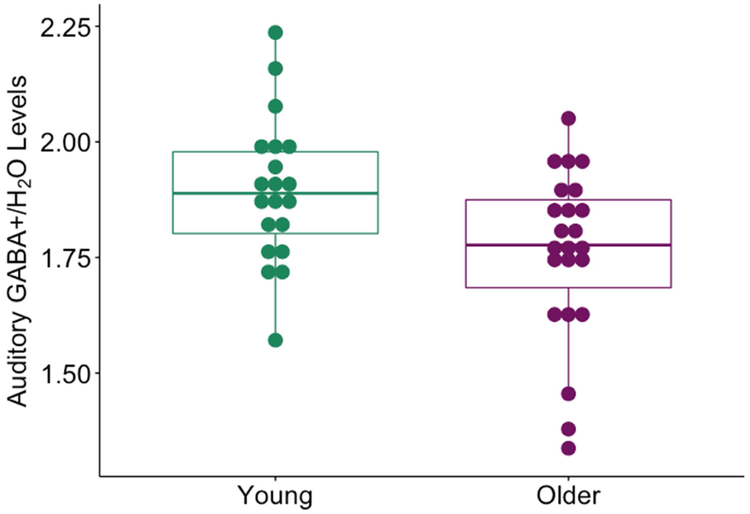
Raw GABA+ levels were significantly lower in the auditory cortex in older adults (mean = 1.75) than in young adults (mean = 1.89) (t (41) =−2.78, p=0.008) (Figure 5). Raw GABA+ levels were also significantly lower in the sensorimotor cortex (t (41) = −3.18, p = 0.002) (reported in Cassady et.al., 2019) and ventrovisual cortex (t (41) = −2.87, p = 0.006). Raw GABA measures in the auditory cortex were not significantly correlated with GABA levels in the ventrovisual (r (43) = 0.25, p = 0.1) or sensorimotor cortex (r (43) = 0.17, p = 0.3) after controlling for age. Auditory GABA levels were also not significantly associated with average pure-tone threshold average, whether analyzed in the entire sample (r (43) = −0.22, p = 0.16) or in older adults alone (r (21) = −0.26, p = 0.23), suggesting that individual differences in auditory GABA are not directly associated with peripheral auditory differences.
Fig 5.
Raw GABA+/Water levels in the auditory cortex estimated by MRS. GABA+ levels were significantly lower in older adults (in purple) than young adults (in green) (t (41) = −2.6, p = 0.01).
We also used an ANCOVA to investigate whether there were systematic differences between GABA levels across hemisphere and if this effect interacted with age. There was a 0.01). significant main effect of age on GABA+ independent of hemisphere (F (1,41) = 7.5, p=0.009) but no main effect of hemisphere (F (1,41) = 0.008, p=0.93). There was also no significant interaction between hemisphere and age (F (1,41) = 0.19, p=0.66). Because there were no significant differences between the GABA+ estimates in the two hemispheres and because the two estimates were significantly correlated (r (41) = 0.52, p=0.0003), we averaged the GABA+ estimates from each hemisphere for further analysis.
There are differences in T1 and T2 relaxation time in the GABA signal that are dependent on the tissue from which it is measured. Since, GABA+ levels are estimated from relatively large voxels which contain grey matter, white matter and cerebrospinal fluid (CSF), it is important to estimate and correct for tissue composition differences between voxels. Gannet uses SPM-based registration to estimate the tissue composition within the voxel and correct them (Edden et.al., 2014; Harris et.al., 2015). These tissue-composition corrected GABA+ estimates were significantly (t (41) = −3.18, p = 0.003) lower in older adults (mean = 1.99) than young adults (mean = 2.18). However, there are also substantial GABA concentration differences between different tissues: CSF contains negligible amounts of GABA, while white matter has half the concentration of GABA compared to grey matter. Gannet also computes fully tissue-composition corrected GABA estimates that account for these concentration differences. Age did not have a significant main effect on these fully corrected GABA+ estimates (t (41) = 0.25, p = 0.8). These results indicate that completely accounting for structural changes with age like tissue composition might explain age differences in GABA+ estimates in auditory cortex.
GABA and Distinctiveness
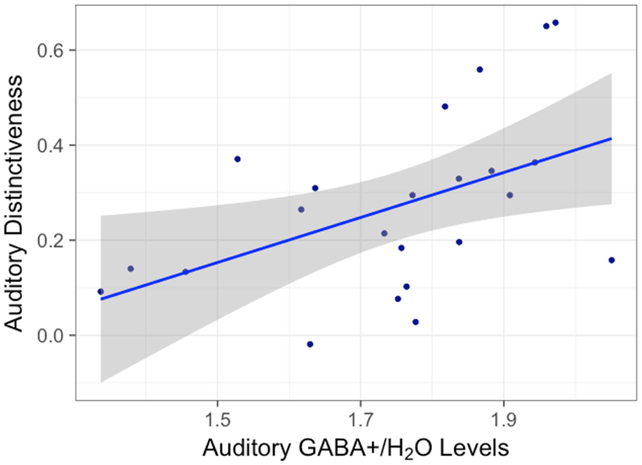
Average raw GABA+ levels in the auditory cortex were positively correlated with neural distinctiveness in the older adults (r (21) = 0.54, p = 0.008) (Figure 6), but not the younger adults (r (18) = −0.18, p = 0.45) (Figure S3). This GABA-distinctiveness relationship was also region-specific: neither ventrovisual GABA (r (21) = 0.25, p = 0.25) nor sensorimotor GABA (r (21) = 0.19, p = 0.38) were significantly correlated with auditory distinctiveness in the older adults.
Fig 6.
The relationship between raw auditory GABA+/Water levels and auditory neural distinctiveness in older adults. Individual differences in GABA+ were significantly correlated with individual differences in neural distinctiveness in older adults. (r (21) = 0.54, p = 0.008)
Our primary measure of GABA was quantified relative to water, but we also analyzed GABA quantified relative to Creatine (Cr) to confirm the reliability of the results. GABA/Cr levels were also significantly lower in older adults compared to young (t (41) =3.44, p = 0.001), and were also correlated with auditory distinctiveness levels within the older adults (r (21) = 0.42, p = 0.04) but not within young adults (r (18) = 0.03, p = 0.9).
We also performed a hierarchical regression to examine whether the fully tissue-composition corrected auditory GABA+ levels explained significant variance beyond that explained by differences in age and other anatomical changes like grey matter volume, NAA (associated with neural integrity) and average pure tone threshold (to account for peripheral hearing differences). GABA levels explained significant variance beyond these other factors within the older sample (F (1,17) = 5.37, p = 0.03), while in the entire sample there was a trend (F (1,37) = 3.75, p = 0.06).
Discussion
The age-related neural dedifferentiation hypothesis posits that the neural representations of different stimuli become less distinct with age (Li et al., 2001). Most of the previous evidence for this hypothesis in humans has come from studies of visual cortex. In the present study, we showed that neural distinctiveness also declines with age in the auditory cortex, extending the scope of age-related neural dedifferentiation. This age-related decline in distinctiveness was independent of ROI-size, was present after controlling for peripheral hearing performance (pure-tone threshold), and was still present after controlling for grey matter thickness.
We also examined GABA levels in the auditory cortex and the relationship between GABA and distinctiveness. Consistent with previous animal research, we found that GABA levels decline with age in the auditory cortex and showed for the first time that individual differences in GABA levels are associated with individual differences in neural distinctiveness.
Age-related dedifferentiation
Previous research in animals provides direct evidence for age-related declines in neural selectivity or distinctiveness at the level of single neurons (Juarez-Salinas et al., 2010; Khouri et al., 2011; Schmolesky et al., 2000). Neuroimaging studies in humans have also found that large-scale patterns of neural activation in ventral visual cortex become less distinct with age (Goh et al., 2010; Goh, 2011; Park et al., 2004). Similar findings have also been reported in motor cortex during left vs. right finger tapping (Carp et al., 2011a), in hippocampus during memory retrieval of different items (Giovanello and Schacter, 2012) and in posterior medial cortex for different emotion regulation strategies (Martins et al., 2015). Our study contributes to this growing body of literature by showing that age-related dedifferentiation also extends to auditory cortex.
A natural question is whether the observed declines in neural distinctiveness in auditory cortex are due to age-related changes in the peripheral auditory system, i.e. the ear, or whether they reflect more central changes in the cortex. Aging is accompanied by several changes in the ear, including the loss of hair cells, dysfunction of the stria vascularis, and stiffening of the basilar membrane (Ouda and Syka, 2012). Such changes in the peripheral auditory system could result in a noisier auditory input. And noisier information could plausibly produce less distinctive cortical representations, even if central auditory processing in the cortex itself has not changed dramatically.
To investigate these issues, we analyzed the pure tone thresholds of our young and old participants at frequencies ranging from 125 to 8000 Hz. And we did observe an increase in auditory thresholds at higher frequencies in the older adults (see Supplemental Figure S5). However, perhaps surprisingly, we did not find much evidence that this, admittedly coarse, measure of hearing influenced neural distinctiveness. Neural distinctiveness still declined with age after controlling for average pure tone threshold and the size of this effect was about the same as it had been without controlling for pure tone threshold. Also, average pure tone threshold was not significantly associated with neural distinctiveness. One reason we may not have observed any associations is that 90% of the power in our auditory stimuli were at frequencies below 2000 Hz where the effects of age on pure tone threshold were not significant. Of course, pure tone threshold is just one (rather coarse) measure of peripheral hearing and it is still quite plausible that age-related changes in the ear contribute to age-related changes in auditory neural distinctiveness.
Another important issue is that the low-level auditory characteristics of our two categories of auditory stimuli were different. Most notably, the music stimuli have significantly more power at frequencies between 1000 and 1500 Hz than does the speech (see supplemental figure S6). It is therefore quite plausible that neural distinctiveness was influenced by these low-level differences and not just by the difference in high-level category (speech vs. music).
Nevertheless, whether neural distinctiveness reflects low-level differences, high-level differences, or both, the critical findings in this paper are that neural distinctiveness declines with age and is significantly associated with GABA. And it seems difficult to attribute either of those between-subject effects to low-level differences between the categories (which contribute to within-subject differences). First, both effects were based on between-subject comparisons (old vs. young, lower GABA vs. higher GABA) and all the participants were presented with the same stimuli. And second, both stimulus categories were dominated by frequencies between 100 and 1500Hz (see supplemental figure S6), where we did not see significant age differences in pure tone threshold (see supplemental figure S5).
Age-related decline in GABA levels
Several animal studies have reported that levels of the inhibitory neurotransmitter GABA decline with age in the auditory system. For example, previous studies in animals have reported declines in GABA in the inferior colliculus (Caspary et al., 1990; Gutiérrez et al., 1994; Ouda and Syka, 2012) and auditory cortex (Ling et al., 2005) of aging rats, as well as in the cochlea of aging mice (Tang et al., 2014). There is also an age-related decrease in the protein and mRNA levels of the most abundant GABAA receptor subunits in inferior colliculus and auditory cortex of rats (Caspary et al., 1990; Gutiérrez et al., 1994; Caspary et al., 2013). GABAB receptor binding in the inferior colliculus also declines with age in rats (Milbrandt et al., 1994).
Only a few human studies have investigated age-related changes in auditory GABA levels, and the results are mixed. Profant et al. (2015) did not observe a significant effect of age on GABA levels in auditory cortex. In contrast, Chen et al. (2013) did report a significant decline in GABA levels: in the right (but not the left) hemisphere before pure tone stimulation, and in both hemispheres after stimulation. Likewise, Gao et al. (2015) reported that older adults suffering from age-related hearing loss exhibited lower GABA levels in auditory cortex compared to other older adults. Consistent with these results, our study provides further evidence that auditory GABA levels decline significantly with age in older adults compared to younger adults. However, fully tissue-composition and concentration corrected GABA estimates did not show an age-related decline. This suggests that observed declines in GABA levels with age may be mediated by age-related changes in tissue composition. These observations might account for some of the apparent discrepancies in the previous literature.
The observed age-related declines in GABA are also consistent with the view that some age-related behavioral impairments may reflect an underlying deficit in inhibition (Hasher and Zacks, 1988; Lustig et al., 2007). These theories suggest that older adults have greater difficulty preventing irrelevant information from gaining access to attention than young adults as a result of impaired inhibitory function. Thus, older adults may be more susceptible to distraction and more likely to choose a non-optimal response. Since GABA is the brain’s major inhibitory neurotransmitter, age-related reductions in GABA could naturally explain the observed inhibitory deficit.
Auditory GABA is associated with neural distinctiveness
Leventhal et al. (2003) showed that the neural selectivity of orientation-specific cells in visual cortex declines with age. They also showed that the selectivity of individual neurons can be experimentally manipulated by the application of GABA, a GABA agonist, or a GABA antagonist. Specifically, visual neurons in older macaques that were not orientation-selective became selective after the application of GABA or the GABA agonist muscimol. Conversely, visual neurons in young macaques that were orientation-selective, became non-selective after the application of the GABA antagonist bicuculline. Together these results demonstrate that changes in GABA activity can cause changes in neural selectivity, at least in individual neurons in visual cortex. Researchers have reported similar findings in the auditory system. For example, the application of a GABA antagonist reduces the selectivity of cells to sinusoidally amplitude modulated (SAM) stimuli in the inferior colliculus of rats (Caspary et al., 2002), as well as the rate and direction selectivity of cells to FM sweeps in the auditory system of bats (Razak and Fuzessery, 2009).
Obviously, the selectivity of individual receptive fields might be quite different from the selectivity of the large-scale neural representations that can be measured using fMRI in humans. Nevertheless, age-related declines in GABA could plausibly influence both and so we decided to test whether individual differences in GABA were associated with individual differences in neural distinctiveness, and the results confirmed the prediction. Older participants with higher levels of auditory GABA, as measured by MRS, had significantly greater neural distinctiveness than did older adults with lower GABA levels, even after controlling for age, NAA (a marker of neural integrity), and grey matter volume. These results are consistent with the hypothesis that age-related declines in GABA contribute to age-related neural dedifferentiation.
Furthermore, this relationship was region-specific: GABA estimates in ventrovisual and somatosensory cortex were not significantly associated with auditory distinctiveness. These results suggest that the observed GABA-distinctiveness relationship is probably not due to some confounding effect (increased variance with age, vascular changes with age) that would be present throughout the brain.
Animal research has shown a direct association between decline in auditory neural selectivity and age-related hearing loss (Khouri et al., 2011; Trujillo and Razak, 2013). If GABA levels influence neural distinctiveness, as our results suggest, then pharmacological treatments that target GABA could be a promising avenue for clinical research aimed at mitigating age-related hearing impairments.
Limitations
A key limitation of the current study is that it is correlational. We therefore cannot conclude that age-related changes in GABA cause changes in neural distinctiveness, only that they are related. Another limitation is that the study is cross-sectional rather than longitudinal. The observed age differences could therefore be influenced by cohort or period effects (Hofer et.al.,2002; Bowen et.al.,1999). Longitudinal studies also make it possible to observe the order of effects which can shed light on causal directionality. Finally, MRS estimates of GABA do not measure GABA activity, but GABA volume. Nor do they distinguish between intracellular and extracellular GABA. These shortcomings should presumably make it harder to observe relationships between auditory GABA and auditory distinctiveness, so the fact that we did find a significant relationship suggests that the relationship may be fairly strong.
Conclusions
In sum, our findings show that neural dedifferentiation extends to the auditory cortex. Furthermore, they demonstrate that GABA levels in auditory cortex decline with age and that individual differences in GABA are associated with individual differences in neural distinctiveness. Together these findings are consistent with the hypothesis that age-related declines in GABA contribute to age-related declines in neural distinctiveness.
Supplementary Material
Highlights.
Older adults have less distinct activation patterns for music vs. foreign speech in auditory cortex than young adults.
Older adults also exhibit lower levels of the neurotransmitter GABA in the auditory cortex.
Individual differences in auditory GABA levels (but not ventrovisual or sensorimotor GABA levels) are associated with individual differences in neural distinctiveness in the auditory cortex of older adults.
Acknowledgements
This work was supported by a grant from the National Institutes of Health to TAP (RA01AG050523). The authors would like to thank Dr. Cindy Lustig for valuable comments on previous drafts.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowen HP, Wiersema MF, 1999. Matching method to paradigm in strategy research: limitations of cross-sectional analysis and some methodological alternatives. Strateg. Manag. J. 20, 625–636. [Google Scholar]
- 2.Carp J, Park J, Hebrank A, Park DC, Polk TA, 2011a. Age-Related Neural Dedifferentiation in the Motor System. PLOS ONE 6(12): e29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carp J, Park J, Polk TA, Park DC, 2011b. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. NeuroImage 56, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson N, Leach L, Murphy KJ, 2018. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatry 33, 379–388. [DOI] [PubMed] [Google Scholar]
- 5.Caspary DM, Hughes LF, Ling LL, 2013. Age-related GABAA receptor changes in rat auditory cortex. Neurobiol. Aging 34, 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspary DM, Palombi PS, Hughes LF, 2002. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear. Res 168, 163–173. [DOI] [PubMed] [Google Scholar]
- 7.Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arnerić SP, 1990. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J. Neurosci. Off. J. Soc. Neurosci 10, 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Peltier SJ, Petrou M, Taylor SF, Weissman DH, Seidler RD, Polk TA, 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. NeuroImage 186, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalavi S, Pauwels L, Heise K-F, Zivari Adab H, Maes C, Puts NAJ, Edden RAE, Swinnen SP, 2018. The neurochemical basis of the contextual interference effect. Neurobiol. Aging 66, 85–96. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Liang Y, Deng Y, Li J, Chen S, Wang C, Luo P, 2013. Age-Associated Reduction of Asymmetry in Human Central Auditory Function: A 1H-Magnetic Resonance Spectroscopy Study. Neural Plasticity, vol. 2013, Article ID 735290, 7 pages, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, Rodgers B, 1999. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychol. Aging 14, 365–379. [DOI] [PubMed] [Google Scholar]
- 12.Desimone R, Duncan J, 1995. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci 18, 193–222. [DOI] [PubMed] [Google Scholar]
- 13.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ, 2014. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J. Magn. Reson. Imaging JMRI 40, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortunato S, Forli F, Guglielmi V, De Corso E, Paludetti G, Berrettini S, Fetoni AR, 2016. A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol Ital 2016;36(3):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagnon H, Simmonite M, Cassady K, Chamberlain J, Freiburger E, Lalwani P, Kelley S, Foerster B, Park DC, Petrou M, Seidler RD, Taylor SF, Weissman DH, Polk TA, 2019. Michigan Neural Distinctiveness (MiND) study protocol: investigating the scope, causes, and consequences of age-related neural dedifferentiation. BMC Neurol. 19, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao F, Edden RAE, Li M, Puts NAJ, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B, Edden RAE, 2015. Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. NeuroImage 106, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovanello KS, Schacter DL, 2012. Reduced Specificity of Hippocampal and Posterior Ventrolateral Prefrontal Activity during Relational Retrieval in Normal Aging. J Cogn Neurosci. 2012;24(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh JO, Suzuki A, Park DC, 2010. Reduced Neural Selectivity Increases fMRI Adaptation with Age during Face Discrimination. NeuroImage 51, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh JOS, 2011. Functional Dedifferentiation and Altered Connectivity in Older Adults: Neural Accounts of Cognitive Aging. Aging and disease, 2(1), 30–48. [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez A, Khan ZU, Morris SJ, De Blas AL, 1994. Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J. Neurosci. Off. J. Soc. Neurosci 14, 7469–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada CN, Natelson Love MC, Triebel K, 2013. Normal Cognitive Aging. Clin. Geriatr. Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris AD, Puts NAJ, Edden RAE, 2015. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation and tissue relaxations. J. Magn. Reson. Imaging JMRI 42, 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasher L, Zacks RT, 1988. Working Memory, Comprehension, and Aging: A Review and a New View, in: Bower GH (Ed.), Psychology of Learning and Motivation. Academic Press, pp. 193–225. [Google Scholar]
- 25.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P, 2001. Distributed and Overlapping Representations of Faces and Objects in Ventral Temporal Cortex. Science 293, 2425–2430. [DOI] [PubMed] [Google Scholar]
- 26.Hermans L, Leunissen I, Pauwels L, Cuypers K, Peeters R, Puts NAJ, Edden RAE, Swinnen SP, 2018a. Brain GABA levels are associated with inhibitory control deficits in older adults. Journal of Neuroscience 5 September 2018, 38 (36) 7844–7851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermans L, Levin O, Maes C, van Ruitenbeek P, Heise K-F, Edden RAE, Puts NAJ, Peeters R, King BR, Meesen RLJ, Leunissen I, Swinnen SP, Cuypers K, 2018b. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol. Aging 65, 168–177. [DOI] [PubMed] [Google Scholar]
- 28.Hofer SM, Sliwinski MJ, Flaherty BP, 2002. Understanding Ageing: Further Commentary on the Limitations of Cross-Sectional Designs for Ageing Research. Gerontology 48, 22–29. [Google Scholar]
- 29.Hultsch DF, MacDonald SWS, Dixon RA, 2002. Variability in reaction time performance of younger and older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci 57, P101–115. [DOI] [PubMed] [Google Scholar]
- 30.Juarez-Salinas DL, Engle JR, Navarro XO, Recanzone GH, 2010. Hierarchical and Serial Processing in the Spatial Auditory Cortical Pathway Is Degraded by Natural Aging. J. Neurosci 30, 14795–14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khouri L, Lesica NA, Grothe B, 2011. Impaired auditory temporal selectivity in the inferior colliculus of aged Mongolian gerbils. J. Neurosci. Off. J. Soc. Neurosci 31, 9958–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koen JD, Hauck N, Rugg MD, 2019. The Relationship between Age, Neural Differentiation, and Memory Performance. J. Neurosci. Off. J. Soc. Neurosci 39, 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leong U-C, Barsz K, Allen PD, Walton JP, 2011. Neural correlates of age-related declines in frequency selectivity in the auditory midbrain. Neurobiol. Aging 32, 168–178. [DOI] [PubMed] [Google Scholar]
- 34.Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y, 2003. GABA and its agonists improved visual cortical function in senescent monkeys. Science 300, 812–815. [DOI] [PubMed] [Google Scholar]
- 35.Li SC, Lindenberger U, Sikström S, 2001. Aging cognition: from neuromodulation to representation. Trends Cogn. Sci 5, 479–486. [DOI] [PubMed] [Google Scholar]
- 36.Ling LL, Hughes LF, Caspary DM, 2005. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience 132, 1103–1113. [DOI] [PubMed] [Google Scholar]
- 37.Lustig C, Hasher L, Zacks RT, 2007. Inhibitory deficit theory: Recent developments in a “new view,” in: Inhibition in Cognition. American Psychological Association, Washington, DC, US, pp. 145–162. [Google Scholar]
- 38.Martins B, Ponzio A, Velasco R, Kaplan J, Mather M, 2015. Dedifferentiation of emotion regulation strategies in the aging brain. Soc. Cogn. Affect. Neurosci 10, 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milbrandt JC, Albin RL, Caspary DM, 1994. Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol. Aging 15, 699–703. [DOI] [PubMed] [Google Scholar]
- 40.O’Reilly RC, 1998. Six principles for biologically based computational models of cortical cognition. Trends Cogn. Sci 2, 455–462. [DOI] [PubMed] [Google Scholar]
- 41.Ouda L, Syka J, 2012. Immunocytochemical profiles of inferior colliculus neurons in the rat and their changes with aging. Front. Neural Circuits 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK, 2002. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 17, 299–320. [PubMed] [Google Scholar]
- 43.Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR, 2004. Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. U. S. A 101, 13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J, Carp J, Hebrank A, Park DC, Polk TA, 2010. Neural specificity predicts fluid processing ability in older adults. J. Neurosci. Off. J. Soc. Neurosci 30, 9253–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porges EC, Woods AJ, Edden RAE, Puts NAJ, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA, 2017. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Profant O, Tintěra J, Balogová Z, Ibrahim I, Jilek M, Syka J, 2015. Functional Changes in the Human Auditory Cortex in Ageing. PLoS One. 2015;10(3):e0116692. Published 2015 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razak KA, Fuzessery ZM, 2009. GABA Shapes Selectivity for the Rate and Direction of Frequency-Modulated Sweeps in the Auditory Cortex. J. Neurophysiol 102, 1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salthouse TA, 1996. The processing-speed theory of adult age differences in cognition. Psychol. Rev 103, 403–428. [DOI] [PubMed] [Google Scholar]
- 49.Schmolesky MT, Wang Y, Pu M, Leventhal AG, 2000. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci 3, 384–390. [DOI] [PubMed] [Google Scholar]
- 50.Simmonite M, Carp J, Foerster BR, Ossher L, Petrou M, Weissman DH, Polk TA, (2018). Age-Related Declines in Occipital GABA are Associated with Reduced Fluid Processing Ability. Acad. Radiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang X, Zhu X, Ding B, Walton JP, Frisina RD, Su J, 2014. Age-related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience 259, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trujillo M, Razak KA, 2013. Altered cortical spectrotemporal processing with age-related hearing loss. J. Neurophysiol 110, 2873–2886. [DOI] [PubMed] [Google Scholar]
- 53.Turner JG, Hughes LF, Caspary DM, 2005. Affects of Aging on Receptive Fields in Rat Primary Auditory Cortex Layer V Neurons. J. Neurophysiol 94, 2738–2747. [DOI] [PubMed] [Google Scholar]
- 54.Villers-Sidani E. de, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM, 2010. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc. Natl. Acad. Sci 107, 13900–13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Doerksen S, Hu L, McAuley E, Kramer AF, 2008. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Res. 1244, 121–131. [DOI] [PubMed] [Google Scholar]
- 56.Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA, 2002. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging 17, 179–193. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.