Abstract
Reading disorder (RD) is characterized by deficient phonological processing, but children with RD also have cognitive control deficits, the neural correlates of which are not fully understood. We used fMRI to assess neural activity during the resolution of cognitive conflict on the Simon Spatial Incompatibility task and patterns of resting-state functional connectivity (RSFC) from task control (TC) regions in 7–12-year-old children with RD compared to their typically developing (TD) peers. Relative to TD children (n=17), those with RD (n=16) over-engaged a right superior/medial frontal cluster during the resolution of conflict (p=.05). Relative to TD children (n=18), those with RD (n=17) also showed reduced RSFC (voxel-wise p<.001; cluster-size p<.05, FDR corrected) from cingulo-opercular seeds to left hemisphere fronto-parietal and temporo-parietal reading-related regions, perhaps reflecting reduced organization of TC circuits and reduced integration with reading-related regions. Children with RD additionally showed reduced RSFC between fronto-parietal and default mode network regions. Follow-up analyses in a subset of children with both useable task and resting state data (RD=13; TD=17) revealed that greater conflict-related activation of the right frontal Simon task ROI associated with better word-reading, perhaps suggesting a compensatory role for this over-engagement. Connectivity from fronto-parietal seeds significantly associated with Simon task performance and word-reading accuracy in RD children. These findings suggest that altered functioning and connectivity of control circuits may contribute to cognitive control deficits in children with RD. Future studies should assess the utility of adding cognitive control training to reading remediation programs.
Keywords: conflict resolution, cognitive control, dyslexia, fMRI, reading disorder
Introduction
Reading disorder (RD) is characterized by a primary deficit in phonological processing (Melby-Lervag, Lyster, & Hulme, 2012; Vellutino & Fletcher, 2005 ) that likely derives from dysfunction in the left hemisphere neural circuit that subserves reading (Richlan, 2012). However, children with RD also show cognitive control deficits on neuropsychological (or behavioral) tasks (Bednarek et al., 2004; Booth, Boyle, & Kelly, 2010; Brosnan et al., 2002; Facoetti & Turatto, 2000; Mahe, Doignon-Camus, Dufour, & Bonnefond, 2014; Purvis & Tannock, 2000; Reiter, Tucha, & Lange, 2005; Varvara, Varuzza, Sorrentino, Vicari, & Menghini, 2014; Willcutt & Pennington, 2000; Willcutt et al., 2001; Willcutt, Pennington, Olson, Chhabildas, & Hulslander, 2005) that associate with their reading problems, including impairments in word reading, reading fluency, and reading comprehension (Borella, Carretti, & Pelegrina, 2010; Kibby, Lee, & Dyer, 2014). Thus, cognitive control deficits may underlie or exacerbate reading problems in children with RD. Prior findings suggest altered functional connectivity of TC circuits in individuals with RD (Horowitz-Kraus, Toro-Serey, & DiFrancesco, 2015; Horowitz-Kraus, Hershey, Kay, & DiFrancesco, 2019; Koyama et al., 2013; Schurz et al., 2015), but the functional neural correlates of cognitive control in children with RD are not fully understood.
The capacity for cognitive control typically develops gradually over childhood and adolescence, paralleling the functional development of TC circuits (Marek, Hwang, Foran, Hallquist, & Luna, 2015). Specifically, the fronto-parietal and cingulo-opercular TC networks have been identified and segregated through analysis and meta-analysis of task-based and resting state functional magnetic resonance imaging (fMRI) data from healthy adults and children (Dosenbach et al., 2007; Dosenbach et al., 2006; Fair et al., 2007; Nee, Wager, & Jonides, 2007; Sestieri, Corbetta, Spadone, Romani, & Shulman, 2014). Yet, resting state functional connectivity (RSFC) data suggest that the segregation of TC circuits may be disrupted in children and adolescents with RD (Horowitz-Kraus et al., 2019; Horowitz-Kraus et al., 2015; Koyama et al., 2013; Schurz et al., 2015).
Furthermore, typically developing (TD) children activate prefrontal and parietal regions of TC circuits to engage the control necessary to resolve conflict on Simon Spatial Incompatibility and Flanker tasks (Margolis et al., 2017; Rubia et al., 2006; Sheridan, Kharitonova, Martin, Chatterjee, & Gabrieli, 2014; Vaidya et al., 2005). Meta-analyses of fMRI data from individuals with RD during reading tasks suggest that under-engagement of left inferior frontal gyrus (IFG)/frontal operculum (FO) and inferior parietal lobule (IPL), as well as over-engagement of bilateral anterior insula (AI) are markers of RD (Maisog, Einbinder, Flowers, Turkeltaub, & Eden, 2008; Richlan, 2012; Richlan, Kronbichler, & Wimmer, 2009; Richlan et al., 2010; Wimmer et al., 2010). Such altered function of these cingulo-opercular and fronto-parietal regions may also reflect deficits in cognitive control processes, and specifically the resolution of cognitive conflict, which is impaired in children and adults with RD (Bednarek et al., 2004; Facoetti & Turatto, 2000; Mahe et al., 2014). Thus, we suspect that abnormalities in the function and connectivity of these circuits may, in part, underlie the cognitive control deficits detected in children with RD. Indeed, changes have been documented in the connectivity of TC circuits following intervention programs aimed at enhancing top-down executive processes required for fluent reading (Horowitz-Kraus et al., 2015). Studying both the functioning of TC circuits during the Simon task and connectivity at rest in children with RD may help us understand the neural correlates of their cognitive control deficits. Such understanding could set the stage for the development of other reading remediation strategies that incorporate cognitive control training to enhance the functioning of these circuits.
Importantly, the left lateralized neural circuit that subserves reading behavior shares several key regions with TC circuits: IFG in the cingulo-opercular network and middle frontal gyrus (MFG), IPL, and intraparietal sulcus (IPS) in the fronto-parietal circuit (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2007; Koyama et al., 2013; Richlan, 2012). Given this overlap across reading and control circuits, altered functional connectivity within or between control circuits may underlie or contribute to the co-occurrence of reading and control deficits in children with RD. Relative to typically developing children, those with RD show reduced lateralization of activity within the reading circuit (Finn et al., 2014), as well as reduced global efficiency within the cingulo-opercular circuit (Horowitz-Kraus et al., 2015) and reduced connectivity within the fronto-parietal circuit (Koyama et al., 2013; Schurz et al., 2015). However, these prior studies have not evaluated directly RSFC from bilateral TC regions in children with RD compared to their TD peers or how patterns of RSFC associate with deficits in cognitive control or reading impairment.
We used task-based and resting-state fMRI to assess the neural correlates of cognitive control processes in children with RD. Given that children with RD show patterns of right frontal over-engagement during reading tasks (Hoeft, McCandliss, et al., 2011; Hoeft et al., 2007; S. E. Shaywitz et al., 1998), we hypothesized that relative to TD children, children with RD would over-engage right frontal cortices during the resolution of cognitive conflict on the Simon task. We additionally hypothesized that compared to TD children, those with RD would show altered connectivity from cingulo-opercular and fronto-parietal TC regions and specifically reduced segregation (increased connectivity) between regions of these circuits, particularly from regions shared with the left hemisphere reading circuit (i.e., left IFG, MFG, IPL, IPS). Finally, we explored whether task-based activation or RSFC associated with word-reading accuracy, attentional symptoms, and Simon task performance.
Methods
Participants
Thirty-five children diagnosed with RD and 18 TD children were recruited from schools and clinics in New York City. Children were native English speakers between seven and 12 years old. Participants were excluded if they had a history of neurological illness, seizures, head trauma, mental retardation, or any lifetime diagnosis of a neurodevelopmental disorder other than Specific Learning Disorder (SLD) or Attention Deficit Hyperactivity Disorder (ADHD). TD children had no diagnoses. The presence or absence of lifetime diagnoses was determined using the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (Kaufman et al., 1997). Interviews were administered by trained research assistants and reviewed by licensed psychologists. Children were included in the RD group if they: 1) were referred to the study because of suspected reading problems and had a history of difficulty learning to read or a previously diagnosed SLD as defined in DSM 5 or a Learning Disorder (LD) as defined in DSM-IV TR, and 2) had poor performance (at or below the 25th percentile) on at least 3 measures of word-reading accuracy, pseudoword reading, encoding, rapid naming, or silent or oral reading comprehension (see supplement) administered within one month of the scan. Two independent licensed psychologists with expertise in the assessment of learning disabilities reviewed case information, including test performance and educational history, to confirm diagnoses and study inclusion. All of the children in the RD group met criteria for SLD in reading as defined in DSM5. Participants and their parents provided informed assent and consent in accordance with the rules and regulations of the Institutional Review Board of the New York State Psychiatric Institute.
Of the 53 children enrolled (35 RD, 18 TD), 13 children with RD were excluded for poor task accuracy (<65% accuracy) and 2 were excluded for excessive head motion during both Simon and resting state runs (motion criteria described below), leaving 20 children with RD. Subsequently, 4 children with RD and 1 TD were excluded from Simon task analyses due to excessive head motion, leaving 16 children with RD and 17 TD children in the task fMRI analysis. Three children with RD and no TD children were excluded from resting state analyses due to excessive head motion, leaving 17 children with RD and 18 TD children in the resting-state fMRI analyses. A subset of 30 children (n=13 RD and n=17 TD) who had both useable task and resting state data was used for follow-up analyses with behavioral measures. We also tested group differences in task-related activation and RSFC in this subset of children. Final groups were matched on age, sex, and in-scanner head motion on both modalities.
Word-Reading Accuracy Score
A word-reading accuracy score was created by converting scaled scores to standard scores and calculating the mean of all 3 standard scores for each child’s performance on WJ-III Letter-Word Identification, TOWRE-2 Sight Word Efficiency, GORT Accuracy.
Neuropsychological Measures
Children completed a measure of intellectual functioning (Wechsler Abbreviated Scale of Intelligence). Parents completed the DuPaul ADHD Rating Scale – Fourth Edition (DRS); total current symptoms were summed for each participant and used as an index of ADHD symptoms. Parents also completed the Hollingshead Four-Factor Index of Socioeconomic Status (see supplement).
Image Acquisition
Imaging was performed on a GE Signa 3-Tesla LX scanner (Milwaukee, WI) with a standard quadrature GE head coil. Head positioning was standardized using the canthometal line. A T1-weighted sagittal localizing scan was used to position the axial functional images parallel to anterior commissure-posterior commissure line. A 3D spoiled gradient recall (SPGR) image was acquired for coregistration with functional images and standard Montreal Neurological Institute (MNI) coordinates. Task-based and resting state functional images were obtained using a T2*-sensitive gradient-recalled, single-shot, echo-planar pulse sequence (3mm isotropic voxels) in runs of 140 images, plus 6 dummy frames discarded for steady state. Task imaging parameters were repetition time (TR) of 2,200ms, echo time (TE) of 30ms, 90° flip angle, single excitation per image, 24 × 24 cm field of view, no gap, covering the entire brain. Resting state imaging parameters were repetition time (TR) of 2,000ms, echo time (TE) of 30ms, 77° flip angle, single excitation per image, 24 × 24 cm field of view, no gap, covering the entire brain. Three runs of the Simon task (5 minutes, 21 seconds per run) and two resting state runs (4 minutes, 52 seconds per run) were collected.
Task-based fMRI
Simon Spatial Incompatibility Task:
Stimuli were presented with EPRIME software (Psychology Software Tools, Inc., Sharpsburg, Pennsylvania) and back-projected onto a screen in scanner. A series of white arrows pointing left or right were displayed against a black background to the left or right of a central white fixation cross-hair. Each white arrow stimulus subtended 1 degree of visual angle vertically and 3.92 degrees visual angle from left to right. The stimuli were centered vertically on all trials and the horizontal center of the arrow was placed at 38% or 62% of the width of the visual display for left and right presentations, respectively. Stimuli were “congruent” (pointing in the same direction as their position on the screen), “incongruent” (pointing opposite their position on the screen), or “blank” (central cross-hair). A button press recorded responses and reaction time (RT) for each trial. Stimulus duration was 1,300ms, with a jittered interstimulus interval (mean=5,352ms [SD=842]; range=4,009–6,857ms). Each run included 55 stimuli, with 22 congruent stimuli (11 left-pointing arrows presented left of midline and 11 right-pointing arrows presented right of midline), 22 incongruent stimuli (11 left-pointing arrows presented right of midline and 11 right-pointing arrows presented left of midline), and 11 blank stimuli (longer periods of fixation) presented pseudorandomly. Participants completed three scan runs, totaling 66 congruent and 66 incongruent stimuli.
Preprocessing
Image preprocessing was performed using Statistical Parametric Mapping (SPM) 12 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) and custom Matlab code. Despiking was used to replace spikes in each voxel’s timeseries with the average of the preceding and following non-spike timepoints. Slice-timing correction was applied using the middle slice of each volume as the reference image. Images were corrected for motion in three translational directions and three rotations (Friston et al., 1995). Task runs were visually inspected and discarded if artifacts were found or if participants moved >3mm total in any direction (total mean motion) across a run. Each participant’s functional images were first coregistered to their skull-stripped high-resolution SPGR structural image. The SPGR image was then segmented and normalized to the MNI template (colin27T1), and parameters determined for this normalization were applied to functional images. Normalized images were spatially smoothed with a Gaussian kernel of 8mm full-width half-maximum. Time series were high-pass filtered at 1⁄128 Hz to remove low-frequency drift.
Parametric Analyses
First-level analyses were performed for each participant using the general linear model (GLM) function in SPM12 with a weighted least-squares algorithm. Prior fMRI data from healthy individuals on this task show that frontal activation increases with more conflict (incongruent vs. congruent trials), with the greatest difference for stimuli following a prior congruent trial (i.e. correct responses to incongruent trials preceded by congruent [cI] trials relative to correct responses to congruent [cC] trials preceded by congruent trials) (Horga et al., 2011). Thus, we focused on the contrast modeling cI-cC, the post-congruent conflict effect. GLMs included predictors for each trial type: (1) congruent preceded by congruent (cC), (2) congruent preceded by incongruent (iC), (3) incongruent preceded by congruent (cI), and (4) incongruent preceded by incongruent (iI), (5) fixation trials, and (6) errors. These events were convolved with the canonical hemodynamic response function (Henson, Price, Rugg, Turner, & Friston, 2002). A first-order autoregression with restricted maximum likelihood algorithm was used remove serial correlations in the fMRI time series.
A region-of-interest (ROI) analysis was used to test our a priori hypothesis that children with RD would over-engage right frontal cortices during the resolution of post-congruent conflict relative to TD children. Specifically, we used MarsBar (Brett, Anton, Valabregue, & Poline, 2002) to mask a right frontal cluster (MFG, superior frontal gyrus (SFG), precentral gyrus [PreCG]) engaged by healthy children performing the Simon task in prior work (623 Voxels, peak = MNI 30, −7, 58) (Margolis et al., 2017). This cluster resides within the dorsal attention network (DAN; Gordon et al., 2016; Power et al., 2011). Beta values for the post-congruent conflict contrast averaging across this ROI were extracted for each participant and entered into a multiple regression to evaluate group differences, controlling for current ADHD symptoms, age, total mean motion, and Full-Scale Intelligence Quotient (FSIQ). In follow-up analyses, group differences in conflict-related activations were assessed in the subset of children who had both useable Simon and resting state data (n=13 RD, n=17 TD). We then explored associations of conflict-related activation with word-reading accuracy, controlling for ADHD symptoms, within the RD group only, to avoid artificially inflating correlations given that the groups were defined by reading ability. Exploratory whole brain and psychophysiological interaction analyses were conducted, the latter to explore group differences in task connectivity associated with conflict resolution (see supplement). We also tested whether Simon-related activation during the incongruent – congruent (I-C) contrast varied between groups (see supplement).
Resting State fMRI
Preprocessing
Preprocessing in SPM12 and the CONN-fMRI Functional Connectivity Toolbox v16.b (http://www.nitric.org/projects/conn/), with MATLAB version R2015a, included slice timing correction, motion correction using a six-parameter rigid body transformation, band-pass filtering (0.01Hz<f<0.1Hz), spatial coregistration to participant-specific structural scans, normalization to MNI space, and spatial smoothing using a 8mm full-width half-maximum Gaussian kernel. T1 structural scans were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using Voxel-Based Morphometry 8 toolbox (http://www.neuro.uni-jena.de/vbm/) and then normalized to MNI template. Frames exceeding a frame-wise displacement (FD) threshold of 0.5mm or change in global signal z-score>3 were identified as outliers using the Artifact Detection Tools and were regressed out of the data. Runs with >15% of frames identified as outliers were discarded.
Parametric Analyses
Seed-to-voxel connectivity maps were generated in CONN using spherical seeds (6mm radius) within cingulo-opercular and fronto-parietal circuits (Horowitz-Kraus et al., 2015) (Table 2). This method allowed a parsimonious approach to investigating connectivity from regions of TC circuits to the rest of the brain as well as connectivity between TC regions. Head motion realignment parameters were used as nuisance regressors and aCompCor (Behzadi, Restom, Liau, & Liu, 2007) was used to correct for physiological noise by regressing out five principal components from white matter and from CSF. This approach limits the influence of confounds, such as head motion, peripheral physiology, and other imaging artifact. Of note, mean motion during resting state scans and number of valid frames did not correlate with age, reading accuracy, or FSIQ (all p’s > .15). Given the debate regarding selection of confound regression methods (Ciric et al., 2017), supplemental analyses were conducted to also include global signal regression (GSR; see supplemental results and Table S2).
Table 2.
MNI Coordinates of RSFC Seeds and Between-group Differences in Connectivity: Follow-up Analyses in Children with Useable Task And Resting State Data.
| Seed | Direction | Seed x,y,z | Target | Target x,y,z | Cluster Size | b |
|---|---|---|---|---|---|---|
| Cingulo-opercular | ||||||
| L ant AI/FO | RD>TD | −51, 13, 18 | PostCG/ R SPL | 48,−32,60 | 238 | 0.210 |
| L med AI/FO | RD>TD | −33, 24, 1 | R SPL | 12,−56, 68 | 618 | 0.201 |
| L lat AI/FO | RD>TD | −34, 14, 5 | L Cerebellum | −4, −76, −26 | 523 | 0.227 |
| L aPFC | −28, 51, 15 | - | ||||
| L ant Thalamus | −12, −15, 8 | - | ||||
| R ant AI/FO | RD>TD | 36, 16, 4 | R SPL | 38,−52,70 | 212 | 0.277 |
| R med AI/FO | RD>TD | 33, 25, −1 | - | |||
| R lat AI/FO | RD>TD | 45, 23, −4 | Cerebellum | 0,−74,−22 | 1302 | 0.264 |
| RD>TD | L Cerebellum | −24,−58,−42 | 272 | 0.209 | ||
| RD>TD | L Cerebellum/FFG | −26,−64,−22 | 239 | 0.248 | ||
| TD>RD | L MTG | −52,−38,−8 | 556 | −0.292 | ||
| TD>RD | L AG | −62,−64,24 | 169 | −0.281 | ||
| TD>RD | L SFG | −4,30,58 | 162 | −0.281 | ||
| TD>RD | R IFG | 52,24,18 | 141 | −0.359 | ||
| R ant PFC | TD>RD | 27, 50, 23 | R PreCG/IFG | 48,0,28 | 613 | −0.282 |
| R ant Thalamus | 10, −15, 8 | - | ||||
| Dorsal ACC | TD>RD | −1, 10, 46 | L AG | −64,−58,28 | 259 | −0.279 |
| TD>RD | L SFG | −22,22,32 | 171 | −0.305 | ||
| Frontoparietal | ||||||
| L DLPFC | RD>TD | −43, 22, 34 | R SMG/PostCG | 48,−34,56 | 282 | 0.305 |
| RD>TD | L SMG | −58,−36,52 | 245 | 0.287 | ||
| Midcingulate | RD>TD | 0, −29, 30 | L ITG | −40,−48,−8 | 237 | 0.232 |
| L IPL * | TD>RD | −51, −51, 36 | R SFG | 28,−2,64 | 378 | −0.272 |
| TD>RD | L CentOpercular | −48,4,2 | 233 | −0.193 | ||
| TD>RD | ACC | 0,26,30 | 175 | −0.195 | ||
| L IPS ** | TD>RD | −31, −59, 42 | PCC | −4,−46,12 | 348 | −0.212 |
| L Precuneus | −9, −72, 37 | - | ||||
| R DLPFC | 43, 22, 34 | - | ||||
| R IPL | 51, 47, 42 | - | ||||
| R IPS | 30, −61, 39 | - | ||||
| R Precuneus | 10, −69, 39 | - |
indicates significant association with Simon task performance
significant association with reading accuracy. Direction indicates whether connectivity is greater in RD or TD groups; Seed x,y,z, provides the MNI coordinate for the center of each seed; Target identifies the location of the target cluster showing significant group differences in connectivity with the seed (all passing voxel-wise p<.001, FDR cluster size correction q<.05); Target x,y,z provides the MNI coordinate for the peak of each target cluster; Cluster size provides the number of significant voxels in the target cluster; b provides the unstandardized coefficient for group differences from regression analysis on average cluster connectivity, controlling for ADHD symptoms, age, FSIQ, and mean motion (all passing Bonferroni correction p<.0025). ACC, anterior cingulate cortex; AG, angular gyrus; AI/FO, anterior insula-frontal operculum; aPFC, anterior prefrontal cortex; CentOpercular, central operculum; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; IPS intraparietal sulcus; L, left; MTG, middle temporal gyrus, PCC, posterior cingulate; PreCG, precentral gyrus, PostCG, postcentral gyrus; SFG, superior frontal gyrus; SMG, supramarginal gyrus; SPL, superior parietal lobule.
Second-level independent t-test maps were generated to compare children with RD relative to TD children, controlling for ADHD symptoms, age, FSIQ, and mean motion. Maps were thresholded at voxel-level significance p<.001 and a false discovery rate (FDR) corrected cluster size threshold q<.05. Average connectivity from each cluster was extracted for post-hoc testing and group differences in each cluster were further corrected for testing across the 20 seeds of interest at p<.0025 (Bonferroni correction, p<.05/20 seeds), controlling for ADHD symptoms, age, FSIQ, and mean motion. In follow-up analyses, we tested group differences in RSFC in a subset of children who had useable Simon and resting state data (n=13 RD, n=17 TD). Associations of RSFC with word-reading accuracy were then explored, controlling for ADHD symptoms, within the RD group. We also explored associations of Simon task performance with RSFC from left hemisphere regions common to reading and control circuits across all children, controlling for group.
Results
Participants
Twenty children with RD and 18 TD children were included in the analyses (Table 1). Compared to TD children, those with RD had significantly lower scores on all reading and IQ measures, however, both groups had mean IQ scores greater than 100. Further, compared to TD children, those with RD had higher parent ratings of ADHD symptoms and 6 children with RD had co-morbid ADHD diagnosis. The groups did not differ in age, sex, race/ethnicity, SES, mean motion during task or resting state scan, or valid frames remaining after outlier regression in the resting state analysis (Table 1). These results were the same in the subset of children who had useable Simon and resting-state data (Table S1).
Table 1.
Clinical Characteristics of Participants in Imaging Analyses
| Participants |
|||||
|---|---|---|---|---|---|
| Characteristic | RD (n = 20) | TD (n=18) | Analysis | ||
| Mean/Count | SD/% | Mean/Count | SD/% | p | |
| Age, months | 124.70 | 20.02 | 117.72 | 15.35 | 0.24 |
| Sex (female) | 8 | 40% | 10 | 56% | 0.79 |
| Race/Ethnicity (caucasian) | 13 | 65% | 10 | 56% | 0.84 |
| Handedness (right) | 19 | 95% | 15 | 83% | 0.25 |
| SES | 54.19 | 6.98 | 55.91 | 4.36 | 0.38 |
| DuPaul ADHD Rating Scale, Current | 16.70 | 11.78 | 7.33 | 8.47 | <0.01 |
| WASI | |||||
| Full-4 IQ | 114.50 | 12.34 | 125.39 | 13.44 | 0.01 |
| Verbal IQ | 112.45 | 11.79 | 124.61 | 11.55 | <0.01 |
| Performance IQ | 113.40 | 14.65 | 120.06 | 13.99 | 0.16 |
| Word Reading Accuracy | 86.49 | 8.304 | 111.42 | 10.09 | <0.01 |
| GORT-5 Accuracy | 80.50 | 9.58 | 107.22 | 14.47 | <0.01 |
| TOWRE-2 Sight Word Efficiency | 84.61 | 10.29 | 105.17 | 13.38 | <0.01 |
| WJ-III Letter Word Identification | 94.95 | 9.23 | 118.44 | 9.52 | <0.01 |
| Resting State Data (n=35) | |||||
| Mean Motion (mm) | 0.285 | 0.185 | 0.212 | 0.208 | 0.28 |
| Invalid Frames | 10.00 | 14.44 | 8.00 | 12.82 | 0.67 |
| Rapid Simon Task Data (n=33) | |||||
| Average Accuracy | 82.3% | 14.9% | 87.8% | 13.3% | 0.10 |
| cI – cC Reaction Time (ms) | 35 | 47 | 79 | 47 | 0.84 |
| Total Mean Motion (mm) | 1.22 | 1.11 | 0.891 | 1.12 | 0.78 |
Standard Scores are reported. RD, Reading Disorder; TD, Typically Developing; WASI; Wechsler Abbreviated Intelligence Scale. SES; socio-economic status, Hollingshead total score. Simon task performance data were analyzed while controlling for current ADHD symptoms. Mean motion indicates the average frame-wise displacement. Invalid frames indicates the number of frames excluded as outliers for frame-wise displacement or changes in intensity. Total mean motion indicates average total displacement in each task run.
Simon Task Performance
Within the full sample of children recruited for the study, those with RD (n=35) performed less accurately than TD children (n=18) on the Simon task (t(50.83)=3.87, p<.001). To avoid analyzing data from children who responded at random during the task (chance performance), those who achieved <65% correct responses overall were excluded from the neuroimaging analyses. Thirteen out of 35 children with RD, but no TD children, performed below this accuracy cutoff. Children with RD excluded for poor performance (N=13) vs. those not excluded (N=22) did not differ in ADHD symptoms (t(33)=−0.267, p=.79), sex (χ2=2.087, p=.149), FSIQ (t(33)=−1.261, p=0.216), age (t(33)=1.450, p=0.157), or word-reading accuracy (t(33)=−0.032, p=0.974).
Among the children included in the task fMRI analyses (n=16 RD; n=17 TD), linear regression analyses controlling for ADHD symptoms, age, and FSIQ revealed that the groups did not differ significantly in accuracy or post-congruent conflict reaction time (Table 1).
Task-based fMRI
Relative to TD children (n=17), children with RD (n=16) showed significantly greater activation associated with the resolution of post-congruent conflict (cI-cC contrast) in the right frontal ROI (b=1.13, t=2.06, p=.05; Figure 1a-b), controlling for ADHD symptoms, age, FSIQ, and total mean motion. These results were similar (b=1.59, t=2.99, p=.006) in the subset of children with both usable task and resting state data (i.e. excluding 3 children with RD who exhibited excessive head motion during the resting state scans). Additionally, post-congruent conflict activation was positively associated with word-reading accuracy in children with RD (b=4.64, t=2.18, p=.05; Figure 1c). No significant group differences for the I-C contrast were detected (p=.17). Exploratory whole brain and psychophysiological interaction analyses with this ROI as a seed were not significant (see supplement).
Figure 1.
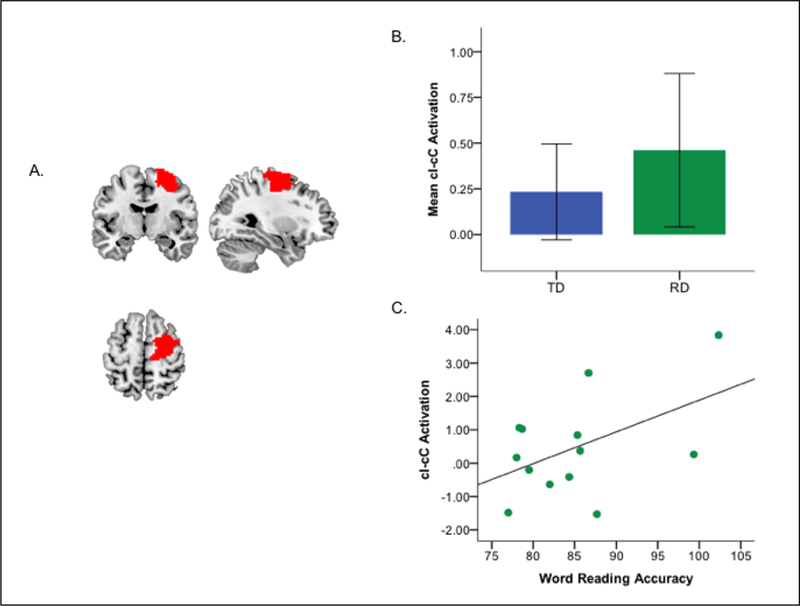
Brain activation associated with post-congruent conflict and associations with reading and attention problems. (A) Mask of the a priori defined right frontal cluster (middle frontal (MFG), superior frontal gyrus (SFG), precentral gyrus [PreCG]) engaged by healthy children performing the Simon task in prior work, 623 Voxels (Margolis et al., 2017). (B) Group differences in brain activation associated with correct responses during the resolution of post-congruent conflict (cI-cC) in the right frontal cluster (mean values per group, error bars indicate one standard error). (C) Scatterplot shows the association of post-congruent conflict-related neural activity in the right frontal cluster with word reading accuracy in children with RD (for ease of interpretation non-residualized values are shown). RD, reading disorder; TD, typically developing.
Resting State fMRI.
Relative to TD children (n=18), those with RD (n=17) showed reduced connectivity of cingulo-opercular regions with left hemisphere fronto-parietal and temporo-parietal reading-related regions (i.e., left IPL, angular gyrus [AG] and MTG) and increased connectivity with cerebellum. In addition, children with RD showed reduced and negative connectivity from fronto-parietal to DMN regions whereas TD children showed positive connectivity to DMN (i.e., left posterior cingulate cortex [PCC]; Table S2, Figure S1). These results were consistent in the subset of children with useable task and resting state data (i.e. excluding 1 TD child and 3 children with RD who had excessive head motion during the Simon task; Table 2). In this subset, connectivity from left IPL to a right frontal region that largely overlapped with the Simon Task ROI was inversely associated with Simon task performance (mean cI-cC RT; b = −1.02, t=−2.21, p=0.036; Figures 2a–b, S2). In the children with RD, connectivity from left IPS to PCC was positively associated with word-reading accuracy (b = 0.008, t=2.255, p=0.048, Figure 2a,c).
Figure 2.
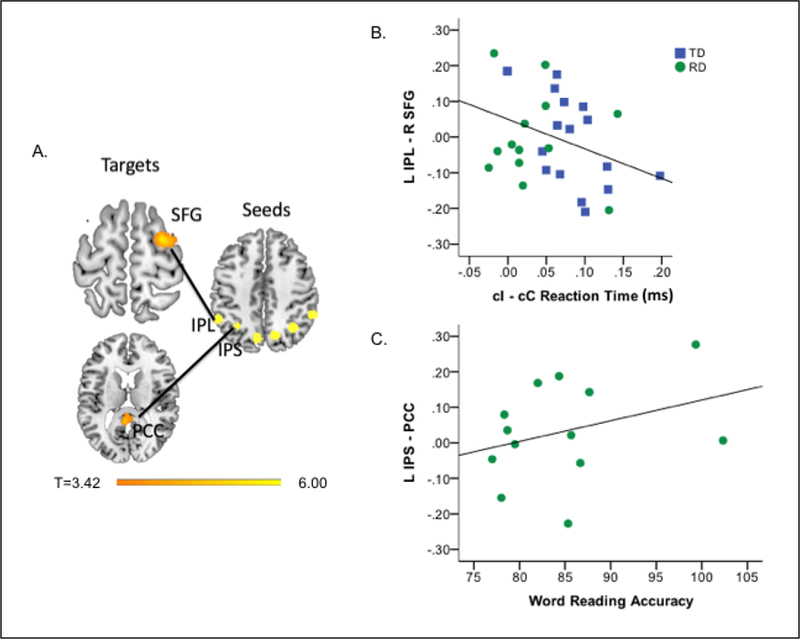
Group differences in resting state functional connectivity (RSFC) from fronto-parietal regions and associations with Simon performance and word reading accuracy. (A) Relative to TD children, children with RD showed reduced RSFC from left IPL to right SFG and from left IPS to PCC. Scatterplots show associations between connectivity and (B) Simon performance (cI-cC reaction time in milliseconds) across all children (connectivity values represent residual values controlling for group) and (C) word reading accuracy in children with RD (for ease of interpretation non-residualized values are shown). IPS, intraparietal sulcus; IPL, intraparietal lobule; PCC, posterior cingulate; RD, reading Disorder; SFG, superior frontal gyrus; TD, typically developing.
A supplemental analysis including GSR produced similar findings of reduced connectivity of cingulo-opercular regions with left hemisphere fronto-parietal and temporo-parietal reading-related regions, as well as from fronto-parietal to DMN regions, in children with RD (Table S3).
Discussion
Compared to TD children, children with RD demonstrated altered patterns of behavior and brain function associated with the resolution of cognitive conflict, as well as altered patterns of RSFC from cingulo-opercular and fronto-parietal TC regions. Consistent with deficits in conflict resolution documented in children with RD (Bednarek et al., 2004; Facoetti & Turatto, 2000; Mahe et al., 2014), one third of the children with RD performed too poorly on the Simon task to be included in the imaging analysis (<65% accuracy), whereas all of the TD children performed above this cutoff. The children with RD who were included in the imaging analyses over-engaged a right superior/medial frontal cluster when processing and resolving conflict. Activation of this cluster was associated with better word-reading, suggesting a compensatory role for this frontal over-engagement. Contrary to our hypothesis, children with RD did not show less segregation (greater connectivity) of TC circuits but, rather, reduced connectivity between cingulo-opercular and frontoparietal regions, and specifically between cingulo-opercular regions and left temporo-parietal regions that are shared across fronto-parietal and reading circuits. Children with RD also showed reduced connectivity from left IPL, a TC region, to right SFG, a region within the DAN that overlaps with our Simon task ROI. Such connectivity was associated their Simon task performance. Children with RD additionally showed reduced connectivity from cingulo-opercular and fronto-parietal to default mode network regions, and such connectivity from fronto-parietal to DMN regions was associated with word-reading accuracy. These findings suggest that altered connectivity between TC regions and across fronto-parietal and DMN regions may contribute to the cognitive control deficits observed in children with RD.
During the resolution of conflict on the Simon task, children with RD over-engaged a right frontal ROI (MFG, SFG, and PreCG) previously shown to support conflict resolution on this task in a larger sample of TD children (Margolis et al., 2017). In children with RD, engagement of this cluster was greatest in those with the best word-reading accuracy, perhaps suggesting a compensatory role for their over-engagement of this cluster. Engagement of this cluster may also have allowed these children with RD to perform as well as their TD peers, and well enough to be included in the imaging analyses. These findings align with prior fMRI data showing right frontal compensation in children with RD during reading-related tasks (Hoeft et al., 2007; Hoeft, Walter, et al., 2011; Shaywitz et al., 2002; Shaywitz et al., 1998; Temple et al., 2003). Thus, right frontal activation likely improves both cognitive control and reading ability in children with RD.
Relative to their TD peers, children with RD showed reduced connectivity between TC circuits, specifically FP and DAN regions, that associated inversely with Simon task performance. In addition, children with RD showed reduced connectivity of cingulo-opercular with left hemisphere regions that are shared across fronto-parietal and reading circuits. The cingulo-opercular circuit is integrated with other circuits in healthy children, and more integration is associated with age-related improvements in the capacity for cognitive control (Marek et al., 2015). Reduced connectivity between TC circuits and from cingulo-opercular to reading-related regions may reflect reduced integration of the cingulo-opercular circuit with the fronto-parietal circuit and with regions required for reading. Reduced connectivity between right IFG and aPFC and between right IFG and lateral AIFO in children with RD may further reflect reduced organization of the cingulo-opercular circuit that could underlie difficulty generalizing phonemic rules and monitoring errors when reading, consistent with the role of the cingulo-opercular circuit in implementing stable task control (Dosenbach et al., 2007). Reduced connectivity between TC regions may also contribute to difficulty learning from feedback as children try to learn the sound/symbol code, consistent with the role of the FP in supporting trial-to-trial adjustments in control in response to feedback (Dosenbach et al., 2007).
Relative to TD children, those with RD also showed reduced connectivity from regions shared across fronto-parietal and reading circuits (IPS) to default mode network regions. Although fronto-parietal and default mode networks are anti-correlated in healthy adults (Fox et al., 2005), they are positively correlated (i.e., connected) in healthy children (Chai, Ofen, Gabrieli, & Whitfield-Gabrieli, 2014; Fair et al., 2008), consistent with our findings from our healthy sample who showed positive connectivity between TC and DMN regions (IPS-PCC). In contrast, we detected reduced connectivity from TC to DMN regions in children with RD, and such connectivity was associated with their poor reading. The normative developmental shift from positive to negative connectivity across task positive and default mode networks has been observed across left hemisphere reading and the default mode networks in healthy individuals (Koyama et al., 2011). Whereas negative connectivity in adults is thought to reflect increased reading automaticity, positive connectivity may benefit healthy children since it associates with their reading ability (Koyama et al., 2011). Taken together, such findings suggest that altered connectivity between TC and DMN regions may contribute to both reading problems and cognitive control deficits in children with RD.
The engagement of cognitive control may be required for reading. For example, difficulty resolving conflict on the flanker task may reflect impaired visual attention and translate to difficulty resolving visual stimuli during reading (Bednarek et al., 2004; Facoetti & Turatto, 2000; Mahe et al., 2014). Difficulty resolving conflict may also affect phonological processing, at least in opaque orthographies such as English, which necessitates the application of competing phonemic rules to letter stimuli and flexible shifting between those rules in different contexts. For example, reading the consonant-vowel-consonant word r-a-t requires learning that the ‘a’ has a short vowel sound. Learning to read the word r-a-t-e requires identifying the silent ‘e’ and then inhibiting the short vowel sound for ‘a’ to instead activate the long vowel sound. Thus, accurate and efficient word reading may require the engagement of two cognitive control processes: identifying salient details and resolving conflict between two phonemic rules. Our findings suggest that these control processes are relevant to understanding the pathophysiology of RD.
Our study was limited by our relatively small sample size. One third of the children with RD had difficulty performing the Simon task, consistent with cognitive control deficits typically observed in children with RD (Purvis & Tannock, 2000; Willcutt & Pennington, 2000; Willcutt et al., 2005), and were therefore excluded from imaging analyses. Future research should assess whether these children with greater cognitive control deficits comprise a distinct RD phenotype. Further, seven were not included in our follow-up analyses due to excessive head motion during either resting-state or task runs, a common problem when imaging young children (Satterthwaite et al., 2012). Recent findings suggest that children with RD show changes in functional connectivity after executive function training that are distinct from those observed in children with RD and comorbid ADHD (Horowitz-Kraus et al., 2019). Thus, aberrant connectivity of regions of TC with reading and DMN circuits in the current study may be attributed to the presence of ADHD symptoms in the children with RD. Although, we controlled for ADHD symptoms in all analyses, future studies should examine these issues in larger samples of children with RD with and without ADHD. To address our small sample size, we used stringent methods to reduce false positives, including a hypothesis-driven ROI approach for the task-based analysis and a Bonferroni adjustment to cluster-size FDR-corrected results for the RSFC analyses. Exploratory analyses of RSFC with behavioral outcomes were not adjusted for multiple comparisons and require replication. Last, our findings are based on seed-based connectivity analyses, limiting our ability to make inferences about internetwork connectivity. Future studies of whole brain connectivity in larger samples are needed to further investigate such questions.
In summary, children with RD over-engage right superior/medial frontal cortex during the engagement of control necessary to resolve cognitive conflict, consistent with their known deficits in control processes. Their engagement of this cluster was associated with better word-reading accuracy suggesting a compensatory role for this over-engagement. TC connectivity to a cluster largely overlapping with this Simon task ROI was reduced in children with RD, and such reduced connectivity was associated with Simon task performance. Together, these findings point to a compensatory mechanism wherein children with RD overactivate right frontal cortex during the engagement of control in order to compensate for reduced connectivity with TC circuits. Further, children with RD showed reduced integration and organization of cingulo-opercular regions, and reduced integration of fronto-parietal and DMN regions that associated with performance on word-reading accuracy. Thus, aberrant connectivity between control, reading, and default mode network regions in children with RD may underlie their cognitive control deficits, thereby contributing to their inefficient processing or allocation of attentional resources during reading, as well difficulties applying the rules of phonics when learning to read or when reading novel words. Future studies should continue to assess the utility of adding cognitive control training to traditional reading remediation programs.
Supplementary Material
Acknowledgements:
This work was supported by NIEHS K23ES026239, Promise Project at Columbia, and the NVLD Project.
Funding:
This work was supported by the NVLD project, Promise Project at Columbia, and NIEHS 1K23ES026239–01A1.
Footnotes
Compliance with Ethical Standards
Conflict of Interest:
All authors (Amy E. Margolis, David Pagliaccio, Katie S. Davis, Lauren Thomas, Sarah M. Banker, Marilyn Cyr, Rachel Marsh) declare that they have no conflict of interest.
Ethical approval:
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation.
Informed consent:
Informed consent and assent was obtained from all legal guardians and participants in the study.
References
- Bednarek DB, Saldana D, Quintero-Gallego E, Garcia I, Grabowska A, & Gomez CM (2004). Attentional deficit in dyslexia: a general or specific impairment? Neuroreport, 15(11), 1787–1790. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JN, Boyle JM, & Kelly SW (2010). Do tasks make a difference? Accounting for heterogeneity of performance of children with reading difficulties on tasks of executive function: findings from a meta-analysis. British Journal of Developmental Psychology, 28(Pt 1), 133–176. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, & Pelegrina S (2010). The specific role of inhibition in reading comprehension in good and poor comprehenders. Journal of Learning Disabilities, 43(6), 541–552. doi: 10.1177/0022219410371676 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, & Poline JB (2002, June 2–6, 2002). Region of interest analysis using an SPM toolbox (MARSBAR). Paper presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Brosnan M, Demetre J, Hamill S, Robson K, Shepherd H, & Cody G (2002). Executive functioning in adults and children with developmental dyslexia. Neuropsychologia, 40(12), 2144–2155. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, & Whitfield-Gabrieli S (2014). Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of Cognitive Neuroscience, 26(3), 501–513. doi: 10.1162/jocn_a_00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, … Satterthwaite TD (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage, 154, 174–187. doi: 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, & Petersen SE (2008). A dual-networks architecture of top-down control. Trends in cognitive sciences, 12(3), 99–105. doi: 10.1016/j.tics.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, … Petersen SE (2007). Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A, 104(26), 11073–11078. doi: 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, … Petersen SE (2006). A Core System for the Implementation of Task Sets. Neuron, 50(5), 799–812. doi: 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, & Turatto M (2000). Asymmetrical visual fields distribution of attention in dyslexic children: a neuropsychological study. Neuroscience Letters, 290(3), 216–218. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, … Schlaggar BL (2008). The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A, 105(10), 4028–4032. doi: 10.1073/pnas.0800376105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, … Schlaggar BL (2007). Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A, 104(33), 13507–13512. doi: 10.1073/pnas.0705843104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, … Constable RT (2014). Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biological Psychiatry, 76(5), 397–404. doi: 10.1016/j.biopsych.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, & Frackowiak RSJ (1995). Spatial registration and normalization of images. Human Brain Mapping, 2, 165–189. [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, & Petersen SE (2016). Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cerebral cortex (New York, N.Y.: 1991), 26(1), 288–303. doi: 10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, & Friston KJ (2002). Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage, 15(1), 83–97. doi: 10.1006/nimg.2001.0940 [DOI] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, … Gabrieli JD (2011). Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A, 108(1), 361–366. doi: 10.1073/pnas.1008950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, … Gabrieli JD (2007). Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci U S A, 104(10), 4234–4239. doi: 10.1073/pnas.0609399104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, & Reiss AL (2011). Neuroanatomical differences in toddler boys with fragile x syndrome and idiopathic autism. Arch Gen Psychiatry, 68(3), 295–305. doi: 10.1001/archgenpsychiatry.2010.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G, Maia TV, Wang P, Wang Z, Marsh R, & Peterson BS (2011). Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. Journal of Neuroscience, 31(45), 16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Hershey A, Kay B, & DiFrancesco M (2019). Differential effect of reading training on functional connectivity in children with reading difficulties with and without ADHD comorbidity. Journal of Neurolinguistics, 49, 93–108. doi: 10.1016/j.jneuroling.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Toro-Serey C, & DiFrancesco M (2015). Increased Resting-State Functional Connectivity in the Cingulo-Opercular Cognitive-Control Network after Intervention in Children with Reading Difficulties. PloS One, 10(7), e0133762. doi: 10.1371/journal.pone.0133762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kibby MY, Lee SE, & Dyer SM (2014). Reading performance is predicted by more than phonological processing. Frontiers in Psychology, 5, 960. doi: 10.3389/fpsyg.2014.00960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, … Milham MP (2013). Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PloS One, 8(2), e55454. doi: 10.1371/journal.pone.0055454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, … Milham MP (2011). Resting-state functional connectivity indexes reading competence in children and adults. Journal of Neuroscience, 31(23), 8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe G, Doignon-Camus N, Dufour A, & Bonnefond A (2014). Conflict control processing in adults with developmental dyslexia: an event related potentials study. Clinical Neurophysiology, 125(1), 69–76. doi: 10.1016/j.clinph.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, & Eden GF (2008). A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences, 1145, 237–259. doi: 10.1196/annals.1416.024 [DOI] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, & Luna B (2015). The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biology, 13(12), e1002328. doi: 10.1371/journal.pbio.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Davis KS, Pao LS, Lewis A, Yang X, Tau G, … Marsh R (2017). Verbal-spatial IQ discrepancies impact brain activation associated with the resolution of cognitive conflict in children and adolescents. Dev Sci doi: 10.1111/desc.12550 [DOI] [PMC free article] [PubMed]
- Melby-Lervag M, Lyster SA, & Hulme C (2012). Phonological skills and their role in learning to read: a meta-analytic review. Psychological Bulletin, 138(2), 322–352. doi: 10.1037/a0026744 [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, & Jonides J (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience, 7(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, … Petersen SE (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi: 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis KL, & Tannock R (2000). Phonological processing, not inhibitory control, differentiates ADHD and reading disability. Journal of the American Academy of Child and Adolescent Psychiatry, 39(4), 485–494. doi: 10.1097/00004583-200004000-00018 [DOI] [PubMed] [Google Scholar]
- Reiter A, Tucha O, & Lange KW (2005). Executive functions in children with dyslexia. Dyslexia, 11(2), 116–131. doi: 10.1002/dys.289 [DOI] [PubMed] [Google Scholar]
- Richlan F (2012). Developmental dyslexia: dysfunction of a left hemisphere reading network. Frontiers in Human Neuroscience, 6, 120. doi: 10.3389/fnhum.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2009). Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping, 30(10), 3299–3308. doi: 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, & Wimmer H (2010). A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading? PloS One, 5(8), e12073. doi: 10.1371/journal.pone.0012073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, & Brammer M (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping, 27(12), 973–993. doi: 10.1002/hbm.20237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, … Gur RE (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage, 60(1), 623–632. doi: 10.1016/j.neuroimage.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, & Kronbichler M (2015). Resting-State and Task-Based Functional Brain Connectivity in Developmental Dyslexia. Cerebral Cortex, 25(10), 3502–3514. doi: 10.1093/cercor/bhu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Spadone S, Romani GL, & Shulman GL (2014). Domain-general signals in the cingulo-opercular network for visuospatial attention and episodic memory. Journal of Cognitive Neuroscience, 26(3), 551–568. doi: 10.1162/jocn_a_00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, … Gore JC (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 52(2), 101–110. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, … Gore JC (1998). Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A, 95(5), 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M, Kharitonova M, Martin RE, Chatterjee A, & Gabrieli JD (2014). Neural substrates of the development of cognitive control in children ages 5–10 years. Journal of Cognitive Neuroscience, 26(8), 1840–1850. doi: 10.1162/jocn_a_00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, & Gabrieli JD (2003). Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci U S A, 100(5), 2860–2865. doi: 10.1073/pnas.0030098100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, & Gabrieli JD (2005). Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry, 162(9), 1605–1613. doi: 10.1176/appi.ajp.162.9.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvara P, Varuzza C, Sorrentino AC, Vicari S, & Menghini D (2014). Executive functions in developmental dyslexia. Frontiers in Human Neuroscience, 8, 120. doi: 10.3389/fnhum.2014.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellutino FR, & Fletcher JM (2005. ). “Developmental dyslexia,”. In Snowling MJ & Hulme CJ (Eds.), The Science of Reading: A Handbook (pp. 362–378.). Oxford,UK: Blackwell. [Google Scholar]
- Willcutt EG, & Pennington BF (2000). Psychiatric comorbidity in children and adolescents with reading disability. Journal of Child Psychology and Psychiatry and Allied Disciplines, 41(8), 1039–1048. [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, & Olson RK (2001). A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology, 110(1), 157–172. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, & Hulslander J (2005). Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: in search of the common deficit. Developmental Neuropsychology, 27(1), 35–78. doi: 10.1207/s15326942dn2701_3 [DOI] [PubMed] [Google Scholar]
- Wimmer H, Schurz M, Sturm D, Richlan F, Klackl J, Kronbichler M, & Ladurner G (2010). A dual-route perspective on poor reading in a regular orthography: an fMRI study. Cortex, 46(10), 1284–1298. doi: 10.1016/j.cortex.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


